Combination Therapy with Human Chorionic Villi MSCs and Secretory Factors Enhances Cutaneous Wound Healing in a Rat Model
Abstract
1. Introduction
2. Results
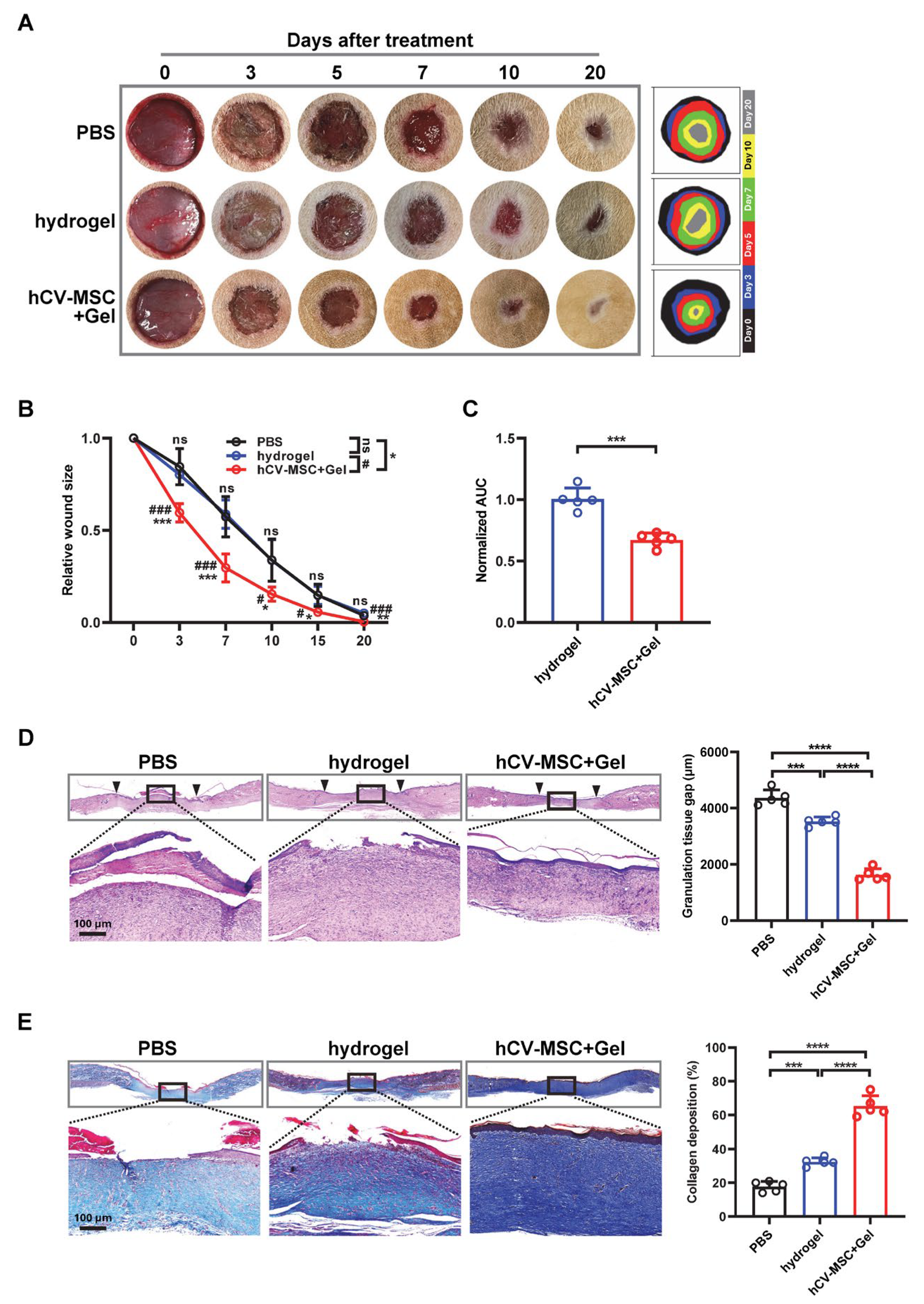
2.1. hCVMSCs Enhance Cutaneous Wound Healing in a Rat Model
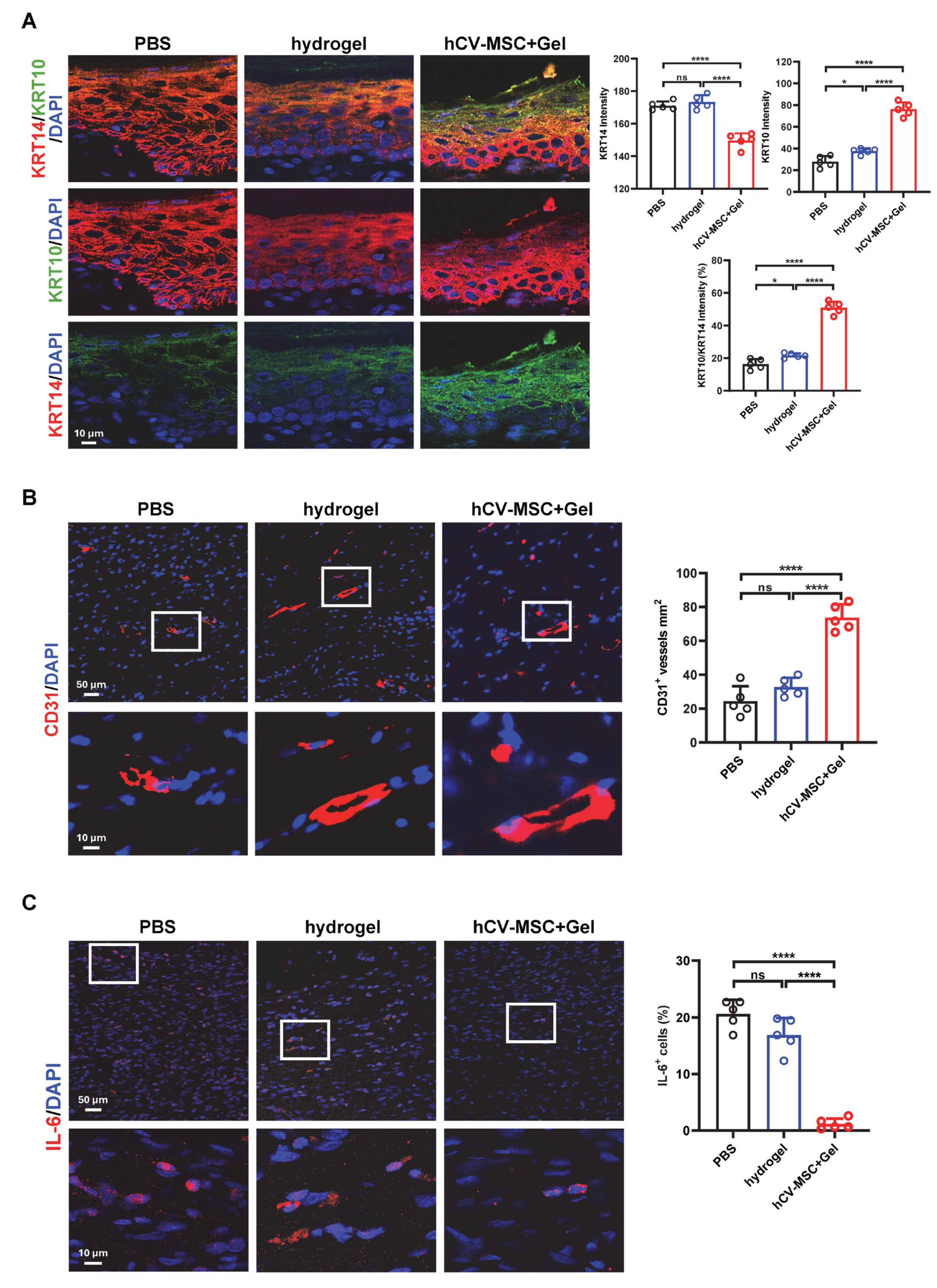
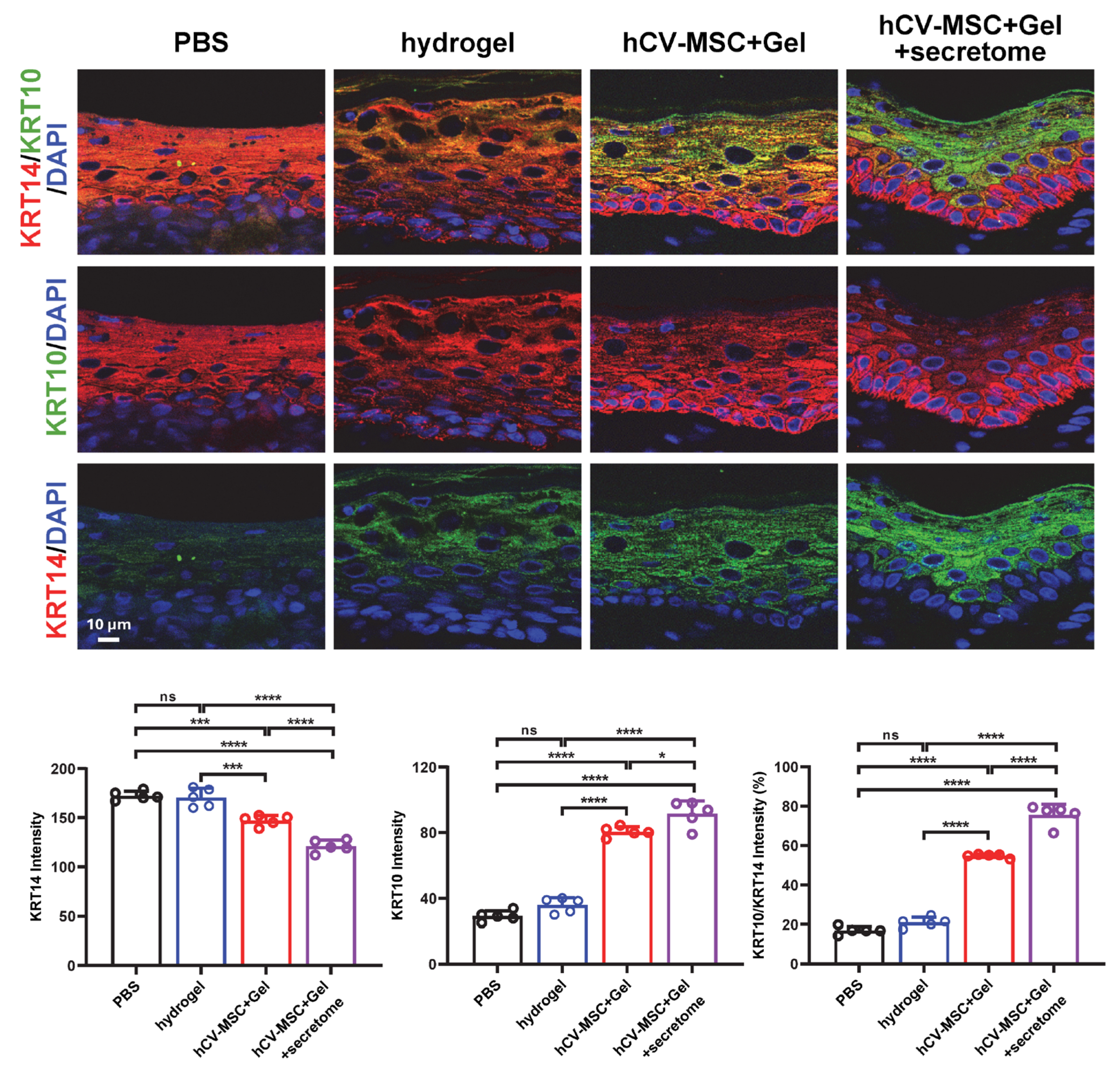
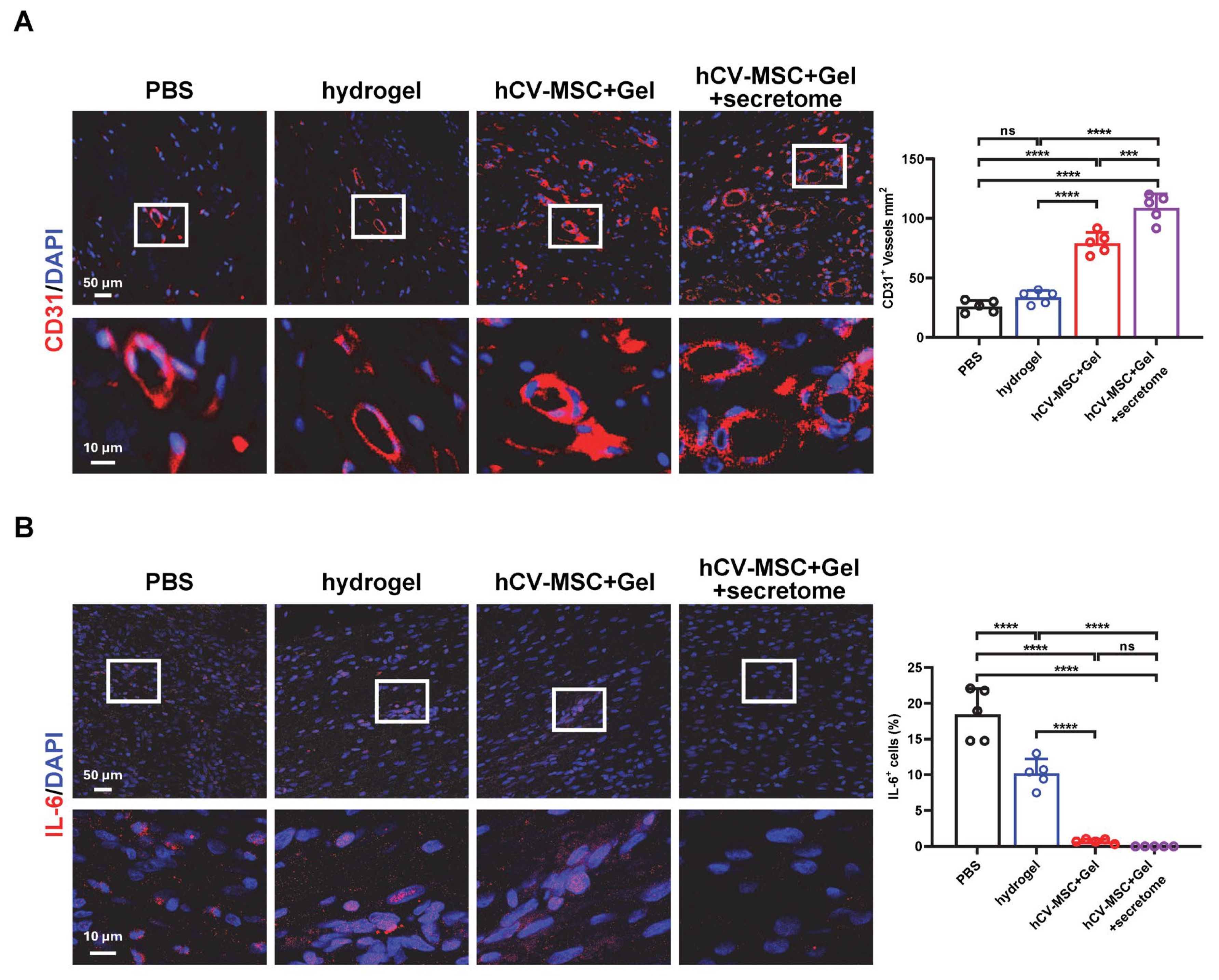
2.2. hCVMSCs Promote Re-Epithelization and Angiogenesis While Ameliorating Inflammation
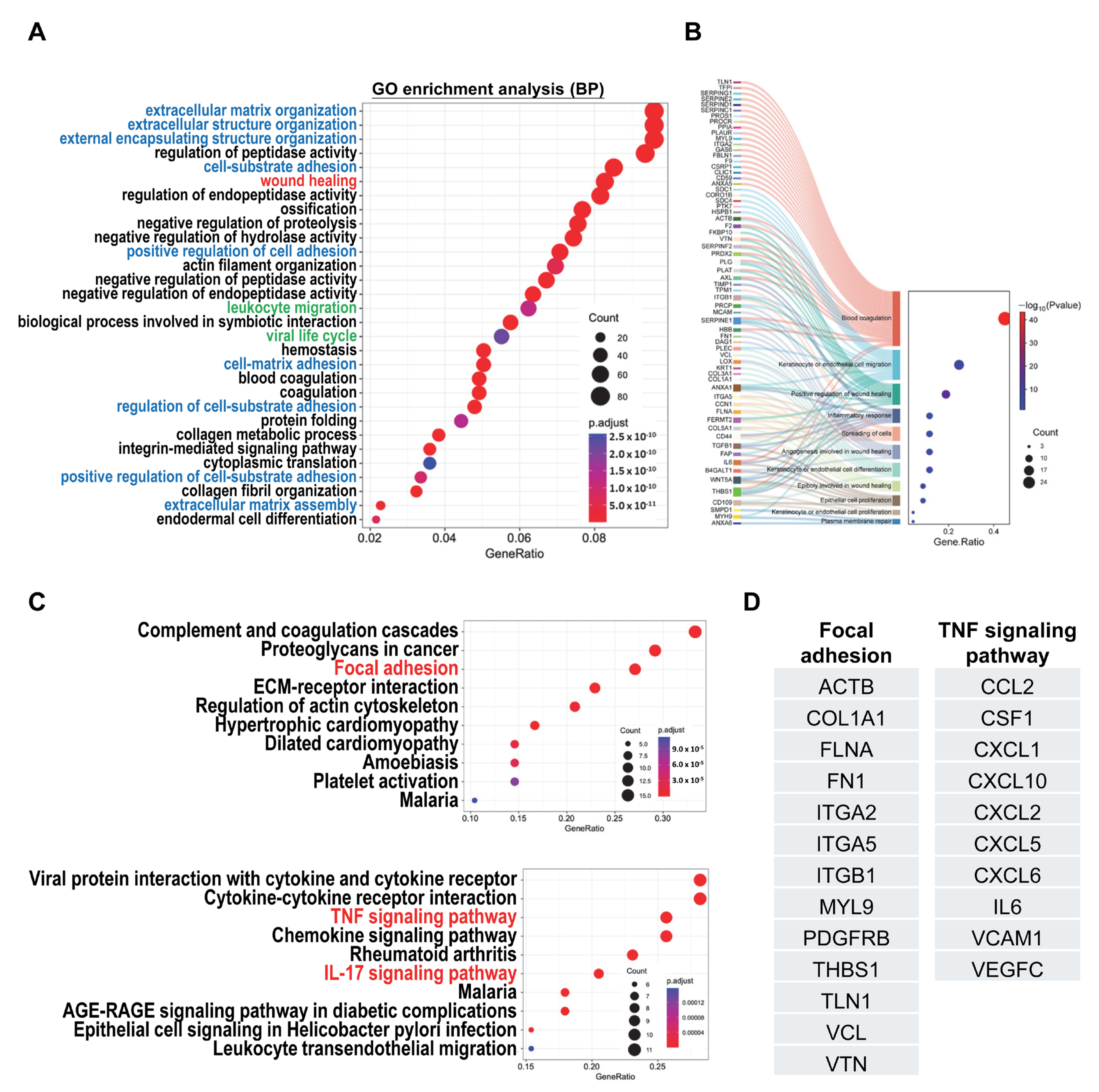
2.3. Analysis of Secretome Profiles of hCVMSCs Reveals Biological Pathways Associated with Wound Healing and Inflammatory Response
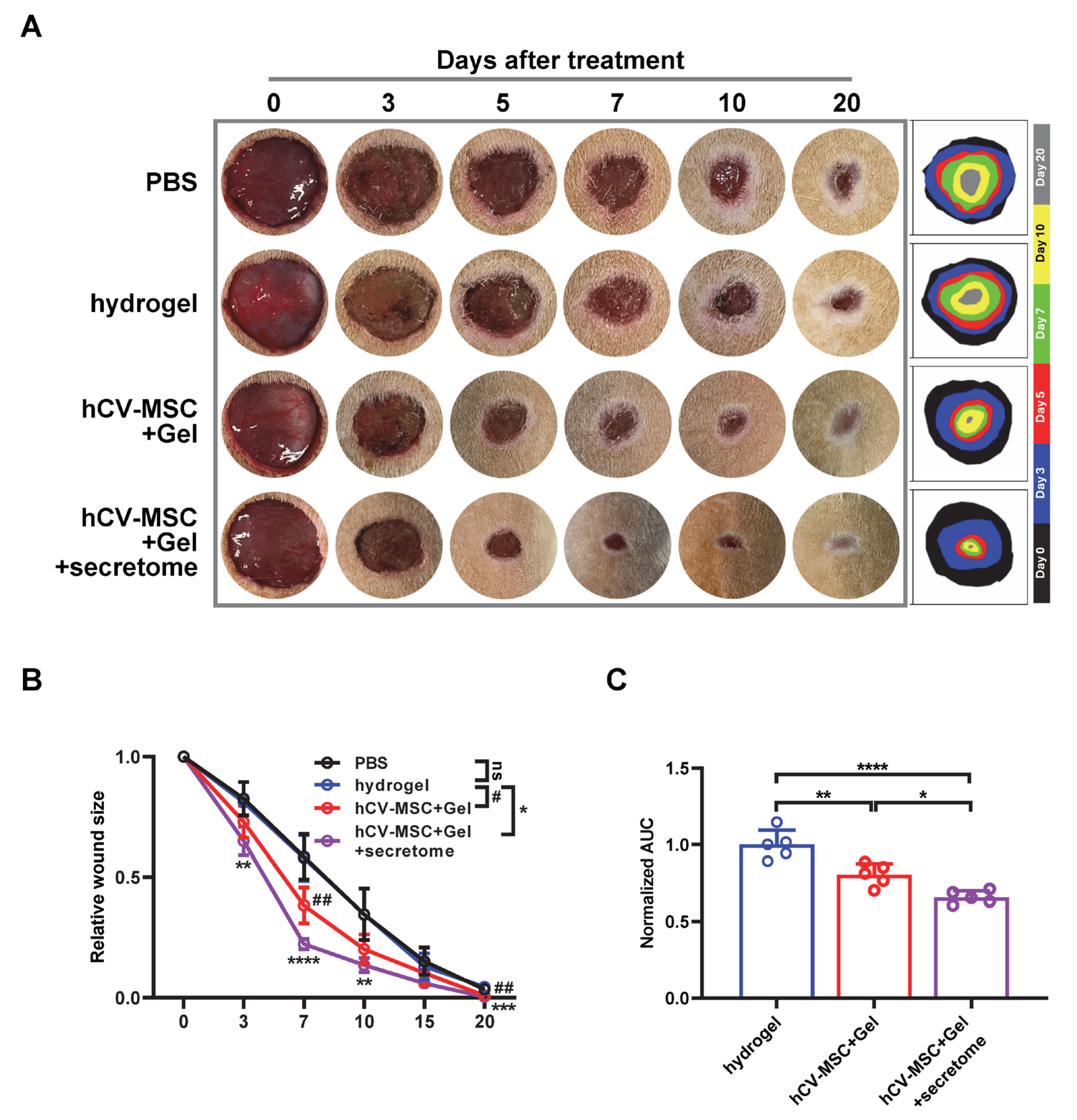
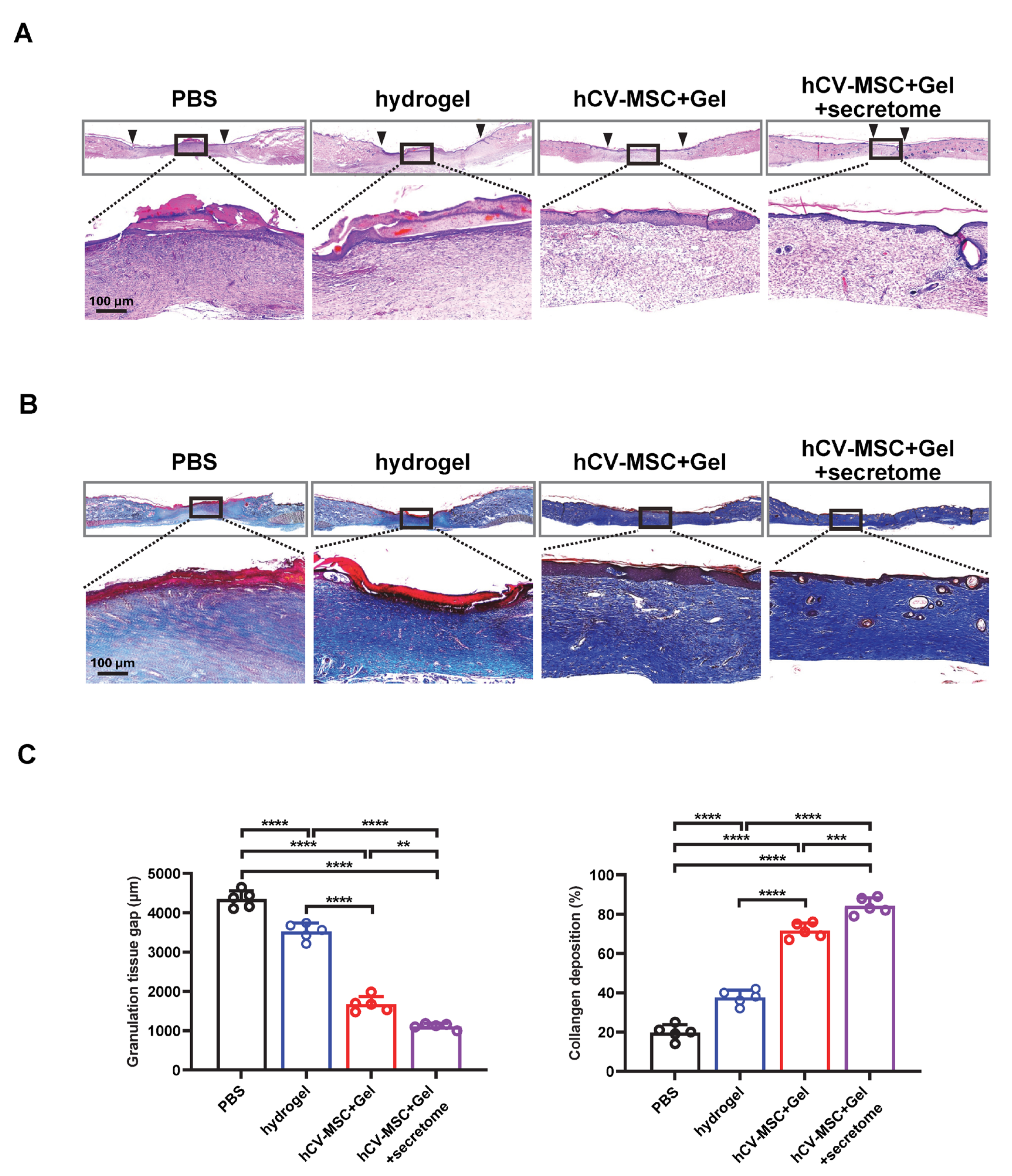
2.4. Therapeutic Effects of hCVMSC-Laden Hydrogel and Secretome Administration on Wound Healing
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Flow Cytometry
4.3. Multilineage Differentiation
4.4. Preparation of Stem Cell Secretome
4.5. Cell Viability Assay
4.6. Mass Spectrometry Sample Preparation and Analysis
4.7. Preparation of PEGDA/SA/Col-I Hydrogel
4.8. Fabrication of hCVMSC-Laden PEGDA/SA/Col-I Hydrogel
4.9. Live/Dead Assay
4.10. Protein Secretion Assay
4.11. Cutaneous Wound Healing Model in Rats and Treatment Strategy
4.12. Histology, Immunohistochemistry, and Immunofluorescence
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | one-way analysis of variance |
| ANXA1 | annexin A1 |
| AUC | Area Under the Curve |
| CCL2 | C-C Motif Chemokine Ligand 2 |
| CM | conditional media |
| CSF1 | Colony-Stimulating Factor 1 |
| ECM | Extracellular Matrix |
| FBS | Fetal Bovine Serum |
| FN1 | Fibronectin 1 |
| GO | Gene ontology |
| GRO | Growth-Regulated Oncogene |
| hCV-MSCs | Human Chorionic Villus Mesenchymal Stem Cells |
| IL | Interleukin |
| ITGB1 | integrin beta-1 |
| KRT14 | keratin 14 |
| KRT10 | keratin 10 |
| MMPs | Matrix Metalloproteinases |
| MSC | Mesenchymal Stem Cell |
| PAI-1 | Plasminogen Activator Inhibitor-1 |
| PBS | phosphate-buffered saline |
| PEGDA | Poly (ethylene glycol) Diacrylate |
| ROI | region of interest |
| SA | Sodium Alginate |
| SERPINE1 | serpin family E member 1 |
| TNF-α | Tumor Necrosis Factor-alpha |
| WNT5A | Wingless Int-1 Family Member 5A |
References
- Martin, P. Wound healing—Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef]
- Santoro, M.M.; Gaudino, G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp. Cell Res. 2005, 304, 274–286. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A.F. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte-fibroblast interactions in wound healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef]
- Pena, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef]
- Saifullah, Q.; Sharma, A. Current Trends on Innovative Technologies in Topical Wound Care for Advanced Healing and Management. Curr. Drug Res. Rev. 2024, 16, 319–332. [Google Scholar] [CrossRef]
- Yu, X.; Liu, P.; Li, Z.; Zhang, Z. Function and mechanism of mesenchymal stem cells in the healing of diabetic foot wounds. Front. Endocrinol. 2023, 14, 1099310. [Google Scholar] [CrossRef]
- Guillamat-Prats, R. The Role of MSC in Wound Healing, Scarring and Regeneration. Cells 2021, 10, 1729. [Google Scholar] [CrossRef]
- Hsieh, M.W.; Wang, W.T.; Lin, C.Y.; Kuo, Y.R.; Lee, S.S.; Hou, M.F.; Wu, Y.C. Stem Cell-Based Therapeutic Strategies in Diabetic Wound Healing. Biomedicines 2022, 10, 2085. [Google Scholar] [CrossRef]
- Gandolfi, S.; Sanouj, A.; Chaput, B.; Coste, A.; Sallerin, B.; Varin, A. The role of adipose tissue-derived stromal cells, macrophages and bioscaffolds in cutaneous wound repair. Biol. Direct 2024, 19, 85. [Google Scholar] [CrossRef]
- Saadh, M.J.; Ramirez-Coronel, A.A.; Saini, R.S.; Arias-Gonzales, J.L.; Amin, A.H.; Gavilan, J.C.O.; Sarbu, I. Advances in mesenchymal stem/stromal cell-based therapy and their extracellular vesicles for skin wound healing. Hum. Cell 2023, 36, 1253–1264. [Google Scholar] [CrossRef]
- Wang, B.; Pang, M.; Song, Y.; Wang, H.; Qi, P.; Bai, S.; Lei, X.; Wei, S.; Zong, Z.; Lin, S.; et al. Human fetal mesenchymal stem cells secretome promotes scarless diabetic wound healing through heat-shock protein family. Bioeng. Transl. Med. 2023, 8, e10354. [Google Scholar] [CrossRef]
- Saheli, M.; Bayat, M.; Ganji, R.; Hendudari, F.; Kheirjou, R.; Pakzad, M.; Najar, B.; Piryaei, A. Human mesenchymal stem cells-conditioned medium improves diabetic wound healing mainly through modulating fibroblast behaviors. Arch. Dermatol. Res. 2020, 312, 325–336. [Google Scholar] [CrossRef]
- Fui, L.W.; Lok, M.P.W.; Govindasamy, V.; Yong, T.K.; Lek, T.K.; Das, A.K. Understanding the multifaceted mechanisms of diabetic wound healing and therapeutic application of stem cells conditioned medium in the healing process. J. Tissue Eng. Regen. Med. 2019, 13, 2218–2233. [Google Scholar] [CrossRef] [PubMed]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Siu, W.S.; Leung, P.C. The Potential of MSC-Based Cell-Free Therapy in Wound Healing—A Thorough Literature Review. Int. J. Mol. Sci. 2023, 24, 9356. [Google Scholar] [CrossRef] [PubMed]
- Fahey, M.J.; Harman, R.M.; Thomas, M.A.; Pugliese, B.R.; Peters-Kennedy, J.; Delco, M.L.; Van de Walle, G.R. Preliminary in vivo investigation of the mesenchymal stromal cell secretome as a novel treatment for methicillin-resistant Staphylococcus aureus in equine skin wounds. Vet. Surg. 2024, 53, 1377–1389. [Google Scholar] [CrossRef]
- Kuncorojakti, S.; Pratama, A.Z.A.; Antujala, C.A.; Harijanto, C.T.B.; Arsy, R.K.; Kurniawan, P.A.; Tjahjono, Y.; Hendriati, L.; Widodo, T.; Aswin, A.; et al. Acceleration of wound healing using adipose mesenchymal stem cell secretome hydrogel on partial-thickness cutaneous thermal burn wounds: An in vivo study in rats. Vet. World 2024, 17, 1545–1554. [Google Scholar] [CrossRef]
- Prado-Yupanqui, J.W.; Ramirez-Orrego, L.; Cortez, D.; Vera-Ponce, V.J.; Chenet, S.M.; Tejedo, J.R.; Tapia-Limonchi, R. The Hidden Power of the Secretome: Therapeutic Potential on Wound Healing and Cell-Free Regenerative Medicine—A Systematic Review. Int. J. Mol. Sci. 2025, 26, 1926. [Google Scholar] [CrossRef]
- Sipos, F.; Muzes, G. Disagreements in the therapeutic use of mesenchymal stem cell-derived secretome. World J. Stem Cells 2022, 14, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Bork, S.; Horn, P.; Krunic, D.; Walenda, T.; Diehlmann, A.; Benes, V.; Blake, J.; Huber, F.X.; Eckstein, V.; et al. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS ONE 2009, 4, e5846. [Google Scholar] [CrossRef]
- Kretlow, J.D.; Jin, Y.Q.; Liu, W.; Zhang, W.J.; Hong, T.H.; Zhou, G.; Baggett, L.S.; Mikos, A.G.; Cao, Y. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008, 9, 60. [Google Scholar] [CrossRef]
- Moll, G.; Rasmusson-Duprez, I.; von Bahr, L.; Connolly-Andersen, A.M.; Elgue, G.; Funke, L.; Hamad, O.A.; Lonnies, H.; Magnusson, P.U.; Sanchez, J.; et al. Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells 2012, 30, 1565–1574. [Google Scholar] [CrossRef]
- Li, C.H.; Zhao, J.; Zhang, H.Y.; Wang, B. Banking of perinatal mesenchymal stem/stromal cells for stem cell-based personalized medicine over lifetime: Matters arising. World J. Stem Cells 2023, 15, 105–119. [Google Scholar] [CrossRef]
- Abbaspanah, B.; Momeni, M.; Ebrahimi, M.; Mousavi, S.H. Advances in perinatal stem cells research: A precious cell source for clinical applications. Regen. Med. 2018, 13, 595–610. [Google Scholar] [CrossRef]
- Abumaree, M.H.; Al Jumah, M.A.; Kalionis, B.; Jawdat, D.; Al Khaldi, A.; Abomaray, F.M.; Fatani, A.S.; Chamley, L.W.; Knawy, B.A. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Rev. Rep. 2013, 9, 620–641. [Google Scholar] [CrossRef]
- Coronado, R.E.; Stavenschi Toth, E.; Somaraki-Cormier, M.; Krishnegowda, N.; Dallo, S. Infusion of Some but Not All Types of Human Perinatal Stromal Cells Prevent Organ Fibrosis in a Humanized Graft versus Host Disease Murine Model. Biomedicines 2023, 11, 415. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, S.; Qing, Q.; Wang, P.; Ma, H.; Ma, Q.; Zhao, W.; Tang, H.; Lu, M. Distinct biological characteristics of mesenchymal stem cells separated from different components of human placenta. Biochem. Biophys. Rep. 2024, 39, 101739. [Google Scholar] [CrossRef] [PubMed]
- Gorodetsky, R.; Aicher, W.K. Allogenic Use of Human Placenta-Derived Stromal Cells as a Highly Active Subtype of Mesenchymal Stromal Cells for Cell-Based Therapies. Int. J. Mol. Sci. 2021, 22, 5302. [Google Scholar] [CrossRef] [PubMed]
- Volinsky, E.; Lazmi-Hailu, A.; Cohen, N.; Adani, B.; Faroja, M.; Grunewald, M.; Gorodetsky, R. Alleviation of acute radiation-induced bone marrow failure in mice with human fetal placental stromal cell therapy. Stem Cell Res. Ther. 2020, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Deng, Q.; Tsang, L.L.; Chang, G.; Guo, J.; Ruan, Y.C.; Wang, C.C.; Li, G.; Chan, H.F.; Zhang, X.; et al. Mesenchymal stem cells from perinatal tissues promote diabetic wound healing via PI3K/AKT activation. Stem Cell Res. Ther. 2025, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Woodley, D.T.; Chen, J.D.; Kim, J.P.; Sarret, Y.; Iwasaki, T.; Kim, Y.H.; O’Keefe, E.J. Re-epithelialization: Human keratinocyte locomotion. Dermatol. Clin. 1993, 11, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chiu, S.M.; Motan, D.A.L.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, J.; Ding, L.; Cai, R.; Cheng, J.; Liang, P.; Zhu, Y.; Zhang, Z. Autophagy inhibition enhances antifibrotic potential of placental mesenchymal stem cells of fetal origin via regulating TGF-β1 mediated protein degradation of HGF. Sci. Rep. 2025, 15, 13805. [Google Scholar] [CrossRef]
- Chu, Y.; Zhu, C.; Yue, C.; Peng, W.; Chen, W.; He, G.; Liu, C.; Lv, Y.; Gao, G.; Yao, K.; et al. Chorionic villus-derived mesenchymal stem cell-mediated autophagy promotes the proliferation and invasiveness of trophoblasts under hypoxia by activating the JAK2/STAT3 signalling pathway. Cell Biosci. 2021, 11, 182. [Google Scholar] [CrossRef]
- Bo, Y.; Yang, L.; Liu, B.; Tian, G.; Li, C.; Zhang, L.; Yan, Y. Exosomes from human induced pluripotent stem cells-derived keratinocytes accelerate burn wound healing through miR-762 mediated promotion of keratinocytes and endothelial cells migration. J. Nanobiotechnol. 2022, 20, 291. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, D.H.; Noh, K.H.; Jung, C.R.; Kang, H.M. The proliferative and multipotent epidermal progenitor cells for human skin reconstruction in vitro and in vivo. Cell Prolif. 2022, 55, e13284. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, X.; Zhao, J.; Bie, F.; Liu, Y.; Xie, J.; Wang, P.; Zhu, J.; Xiong, Y.; Qin, S.; et al. Single-cell profiling reveals transcriptomic signatures of vascular endothelial cells in non-healing diabetic foot ulcers. Front. Endocrinol. 2023, 14, 1275612. [Google Scholar] [CrossRef]
- Agren, M.S.; Litman, T.; Eriksen, J.O.; Schjerling, P.; Bzorek, M.; Gjerdrum, L.M.R. Gene Expression Linked to Reepithelialization of Human Skin Wounds. Int. J. Mol. Sci. 2022, 23, 15746. [Google Scholar] [CrossRef]
- Simone, T.M.; Longmate, W.M.; Law, B.K.; Higgins, P.J. Targeted Inhibition of PAI-1 Activity Impairs Epithelial Migration and Wound Closure Following Cutaneous Injury. Adv. Wound Care 2015, 4, 321–328. [Google Scholar] [CrossRef]
- Whyte, J.L.; Smith, A.A.; Helms, J.A. Wnt signaling and injury repair. Cold Spring Harb. Perspect. Biol. 2012, 4, a008078. [Google Scholar] [CrossRef]
- Shah, R.; Amador, C.; Poe, A.J.; Spektor, T.M.; Bhandary, P.; Wang, Y.; Wang, Z.P.; Weisenberger, D.J.; Borges, V.F.; Sawant, O.B.; et al. Identification of Wnt-5a Receptors Important in Diabetic and Non-Diabetic Corneal Epithelial Wound Healing. Investig. Ophthalmol. Vis. Sci. 2025, 66, 64. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Spektor, T.M.; Weisenberger, D.J.; Ding, H.; Patil, R.; Amador, C.; Song, X.Y.; Chun, S.T.; Inzalaco, J.; Turjman, S.; et al. Reversal of dual epigenetic repression of non-canonical Wnt-5a normalises diabetic corneal epithelial wound healing and stem cells. Diabetologia 2023, 66, 1943–1958. [Google Scholar] [CrossRef] [PubMed]
- Kanchanawong, P.; Calderwood, D.A. Organization, dynamics and mechanoregulation of integrin-mediated cell—ECM adhesions. Nat. Rev. Mol. Cell Biol. 2023, 24, 142–161. [Google Scholar] [CrossRef] [PubMed]
- Pajic-Lijakovic, I.; Milivojevic, M.; McClintock, P.V.E. Physical aspects of epithelial cell-cell interactions: Hidden system complexities. Eur. Biophys. J. 2024, 53, 355–372. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef]
- Gillitzer, R. Role of chemokines in wound healing of human skin. Zentralbl Chir. 1996, 121, 29–30. [Google Scholar]
- Gillitzer, R.; Toksoy, A.; Voss, A. Role of chemokines in human skin wound healing. Zentralbl Chir. 2000, 125 (Suppl. S1), 56–59. [Google Scholar]
- Zaja-Milatovic, S.; Richmond, A. CXC chemokines and their receptors: A case for a significant biological role in cutaneous wound healing. Histol. Histopathol. 2008, 23, 1399–1407. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, L.; Du, C.; Wang, J.; Zhang, C.; Hu, L.; Wang, S. Dental pulp stem cells accelerate wound healing through CCL2-induced M2 macrophages polarization. iScience 2023, 26, 108043. [Google Scholar] [CrossRef]
- Wang, K.; Song, B.; Zhu, Y.; Dang, J.; Wang, T.; Song, Y.; Shi, Y.; You, S.; Li, S.; Yu, Z.; et al. Peripheral nerve-derived CSF1 induces BMP2 expression in macrophages to promote nerve regeneration and wound healing. npj Regen. Med. 2024, 9, 35. [Google Scholar] [CrossRef]
- Bastidas, J.G.; Maurmann, N.; Scholl, J.N.; Weber, A.F.; Silveira, R.P.; Figueiro, F.; Stimamiglio, M.A.; Marcon, B.; Correa, A.; Pranke, P. Secretome of stem cells from human exfoliated deciduous teeth (SHED) and its extracellular vesicles improves keratinocytes migration, viability, and attenuation of H2O2-induced cytotoxicity. Wound Repair Regen. 2023, 31, 827–841. [Google Scholar] [CrossRef]
- Gnecchi, M.; Zhang, Z.; Ni, A.; Dzau, V.J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 2008, 103, 1204–1219. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Q.; Huang, J.; Tsang, L.L.; Guo, J.; Wang, C.C.; Zhang, X.; Jiang, X. Combination Therapy with Human Chorionic Villi MSCs and Secretory Factors Enhances Cutaneous Wound Healing in a Rat Model. Int. J. Mol. Sci. 2025, 26, 6888. https://doi.org/10.3390/ijms26146888
Deng Q, Huang J, Tsang LL, Guo J, Wang CC, Zhang X, Jiang X. Combination Therapy with Human Chorionic Villi MSCs and Secretory Factors Enhances Cutaneous Wound Healing in a Rat Model. International Journal of Molecular Sciences. 2025; 26(14):6888. https://doi.org/10.3390/ijms26146888
Chicago/Turabian StyleDeng, Qingwen, Jiawei Huang, Lai Ling Tsang, Jinghui Guo, Chi Chiu Wang, Xiaohu Zhang, and Xiaohua Jiang. 2025. "Combination Therapy with Human Chorionic Villi MSCs and Secretory Factors Enhances Cutaneous Wound Healing in a Rat Model" International Journal of Molecular Sciences 26, no. 14: 6888. https://doi.org/10.3390/ijms26146888
APA StyleDeng, Q., Huang, J., Tsang, L. L., Guo, J., Wang, C. C., Zhang, X., & Jiang, X. (2025). Combination Therapy with Human Chorionic Villi MSCs and Secretory Factors Enhances Cutaneous Wound Healing in a Rat Model. International Journal of Molecular Sciences, 26(14), 6888. https://doi.org/10.3390/ijms26146888






