Estimation the Change in Liver Fibrosis Stage with Serial Measurement of Wisteria Floribunda Agglutinin-Positive Mac-2 Binding Protein in Metabolic Dysfunction-Associated Steatotic Liver Disease Patients
Abstract
1. Introduction
2. Results
2.1. Patients Characteristics
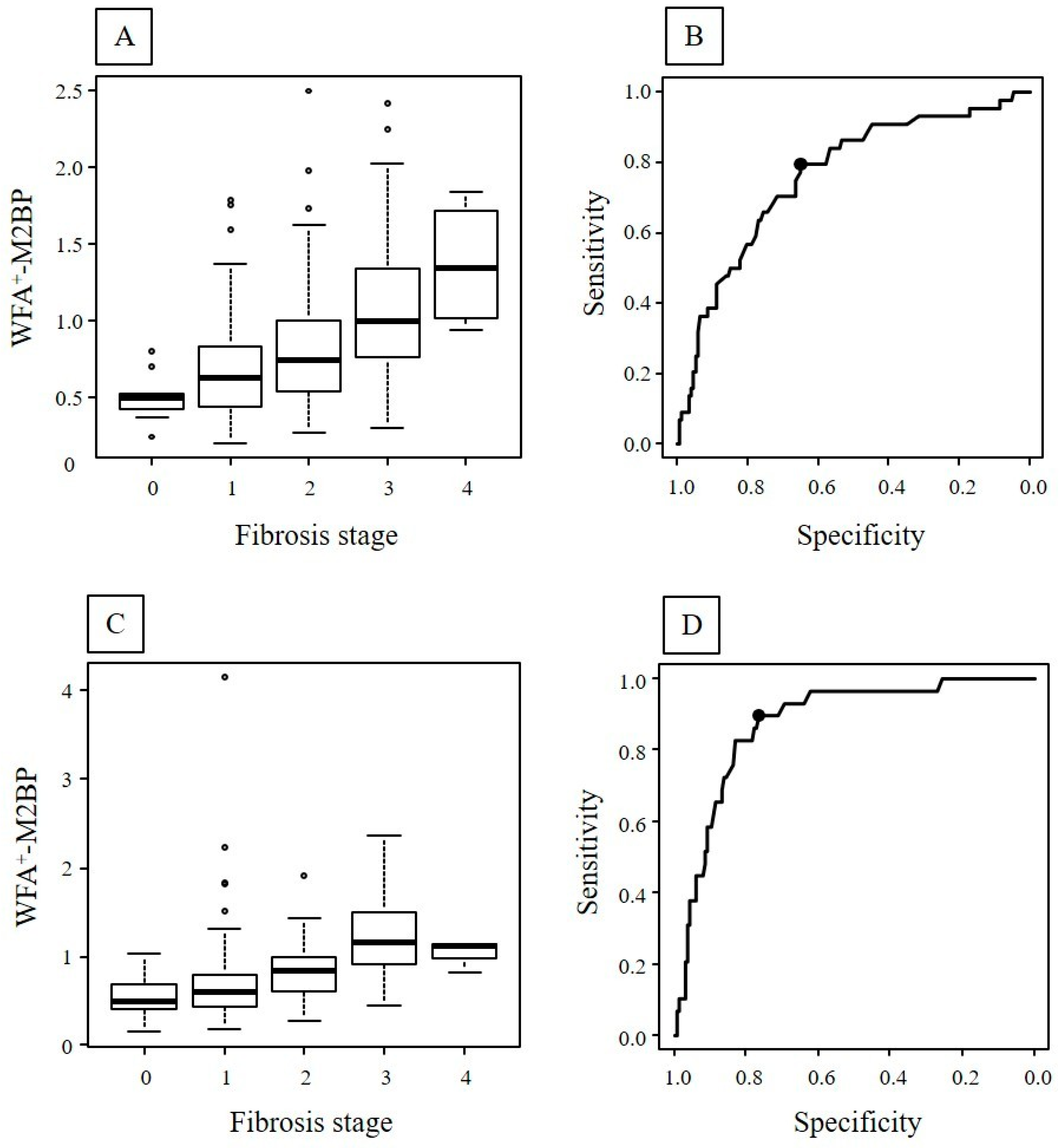
2.2. Fibrosis Stage and WFA+-M2BP in the First and Second Biopsy
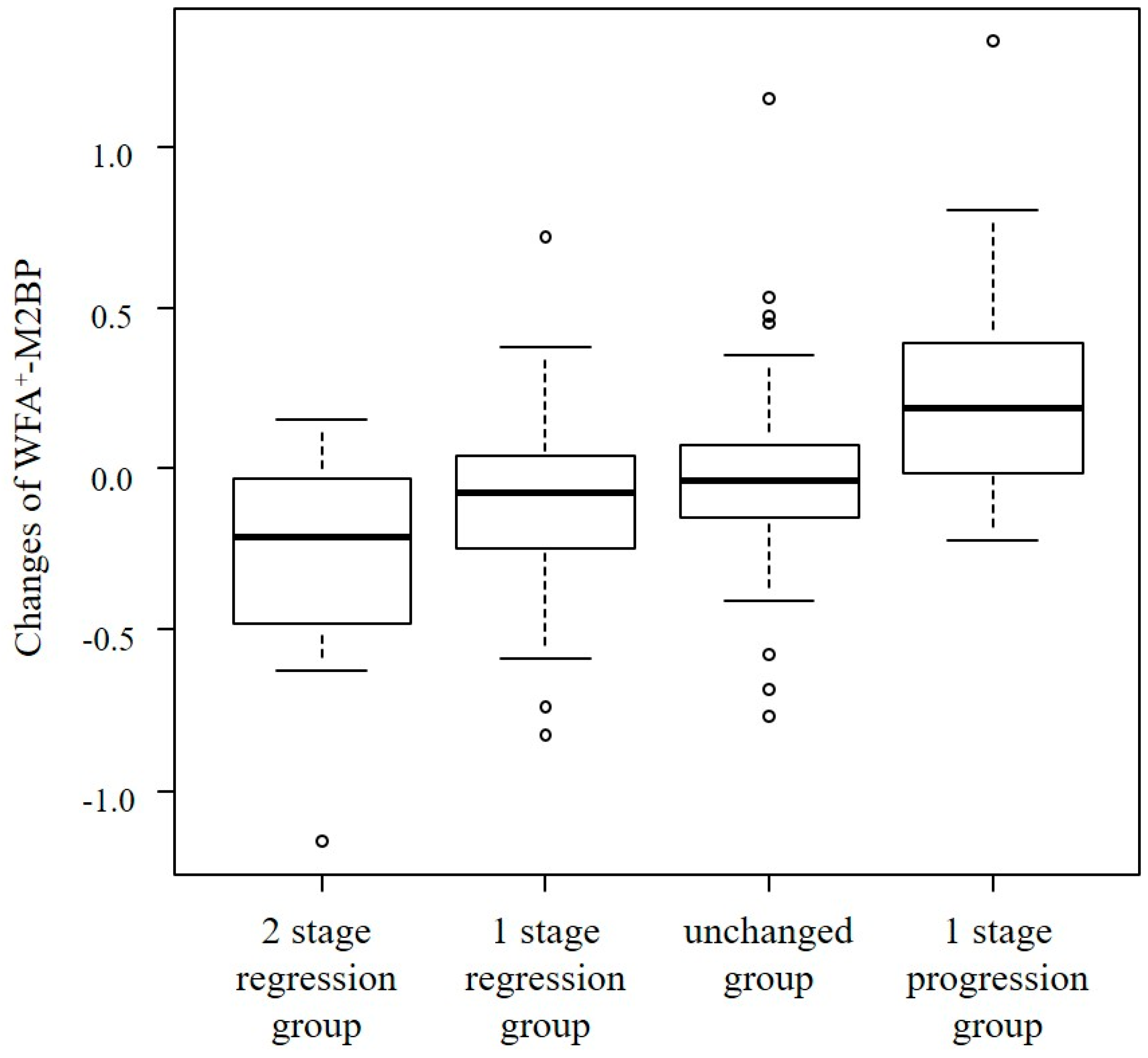
2.3. Change in Fibrosis Stage and WFA+-M2BP
2.4. Change in Fibrosis Stage and Fibrosis Markers
2.5. Factors Associated with Improvement of Liver Fibrosis
3. Discussion
4. Materials and Methods
4.1. Patients Characteristics
4.2. Assessment of Liver Fibrosis Stage
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Miao, L.; Targher, G.; Byrne, C.D.; Cao, Y.Y.; Zheng, M.H. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 2024, 35, 697–707. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, F.; Yuan, Y.; Gao, J.; Xiao, L.; Yan, C.; Guo, F.; Zhong, J.; Che, Z.; Li, W.; et al. Epidemiology of liver diseases: Global disease burden and forecasted research trends. Sci. China Life Sci. 2025, 68, 541–557. [Google Scholar]
- Tamaki, N.; Higuchi, M.; Kurosaki, M.; Loomba, R.; Izumi, N.; MRCHLiver Study Group. Risk Difference of Liver-Related Cardiovascular Events by Liver Fibrosis Status in Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2022, 20, 1171–1173.e2. [Google Scholar]
- Ajmera, V.; Kim, B.K.; Yang, K.; Majzoub, A.M.; Nayfeh, T.; Tamaki, N.; Izumi, N.; Nakajima, A.; Idilman, R.; Gumussoy, M.; et al. Liver Stiffness on Magnetic Resonance Elastography and the MEFIB Index and Liver-Related Outcomes in Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Individual Participants. Gastroenterology 2022, 163, 1079–1089.e5. [Google Scholar] [CrossRef]
- Tamaki, N.; Kimura, T.; Wakabayashi, S.I.; Umemura, T.; Kurosaki, M.; Loomba, R.; Izumi, N. Long-term clinical outcomes in steatotic liver disease and incidence of liver-related events, cardiovascular events and all-cause mortality. Aliment. Pharmacol. Ther. 2024, 60, 61–69. [Google Scholar] [CrossRef]
- Bravo, A.A.; Sheth, S.G.; Chopra, S. Liver biopsy. N. Engl. J. Med. 2001, 344, 495–500. [Google Scholar] [CrossRef]
- Ratziu, V.; Charlotte, F.; Heurtier, A.; Gombert, S.; Giral, P.; Bruckert, E.; Grimaldi, A.; Capron, F.; Poynard, T.; LIDO Study Group. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005, 128, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Calès, P.; Oberti, F.; Michalak, S.; Hubert-Fouchard, I.; Rousselet, M.C.; Konaté, A.; Gallois, Y.; Ternisien, C.; Chevailler, A.; Lunel, F. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatol. Baltim. Md. 2005, 42, 1373–1381. [Google Scholar] [CrossRef]
- Adams, L.A.; Sterling, R.K. Developing a new algorithm to diagnose advanced liver fibrosis: A lift or a nudge in the right direction? J. Hepatol. 2017, 66, 1111–1113. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Miyake, T.; Kuno, A.; Imai, Y.; Sawai, Y.; Hino, K.; Hara, Y.; Hige, S.; Sakamoto, M.; Yamada, G.; et al. Association between Wisteria floribunda agglutinin-positive Mac-2 binding protein and the fibrosis stage of non-alcoholic fatty liver disease. J. Gastroenterol. 2015, 50, 776–784. [Google Scholar] [CrossRef]
- Tamaki, N.; Higuchi, M.; Kurosaki, M.; Kirino, S.; Osawa, L.; Watakabe, K.; Wang, W.; Okada, M.; Shimizu, T.; Takaura, K.; et al. Wisteria floribunda agglutinin-positive mac-2 binding protein as an age-independent fibrosis marker in nonalcoholic fatty liver disease. Sci. Rep. 2019, 9, 10109. [Google Scholar] [CrossRef] [PubMed]
- Tapper, E.B.; Lai, M. Weight loss results in significant improvements in quality of life for patients with nonalcoholic fatty liver disease: A prospective cohort study. Hepatol. Baltim. Md. 2016, 63, 1184–1189. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5. [Google Scholar] [CrossRef] [PubMed]
- Kuno, A.; Ikehara, Y.; Tanaka, Y.; Ito, K.; Matsuda, A.; Sekiya, S.; Hige, S.; Sakamoto, M.; Kage, M.; Mizokami, M.; et al. A serum “sweet-doughnut” protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci. Rep. 2013, 3, 1065. [Google Scholar] [CrossRef]
- Toshima, T.; Shirabe, K.; Ikegami, T.; Yoshizumi, T.; Kuno, A.; Togayachi, A.; Gotoh, M.; Narimatsu, H.; Korenaga, M.; Mizokami, M.; et al. A novel serum marker, glycosylated Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA(+)-M2BP), for assessing liver fibrosis. J. Gastroenterol. 2015, 50, 76–84. [Google Scholar] [CrossRef]
- Fujiyoshi, M.; Kuno, A.; Gotoh, M.; Fukai, M.; Yokoo, H.; Kamachi, H.; Kamiyama, T.; Korenaga, M.; Mizokami, M.; Narimatsu, H.; et al. Clinicopathological characteristics and diagnostic performance of Wisteria floribunda agglutinin positive Mac-2-binding protein as a preoperative serum marker of liver fibrosis in hepatocellular carcinoma. J. Gastroenterol. 2015, 50, 1134–1144. [Google Scholar] [CrossRef]
- Hanai, T.; Shiraki, M.; Ohnishi, S.; Miyazaki, T.; Ideta, T.; Kochi, T.; Imai, K.; Suetsugu, A.; Takai, K.; Shimizu, M.; et al. Impact of serum glycosylated Wisteria floribunda agglutinin positive Mac-2 binding protein levels on liver functional reserves and mortality in patients with liver cirrhosis. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2015, 45, 1083–1090. [Google Scholar] [CrossRef]
- Hayashi, T.; Tamaki, N.; Kurosaki, M.; Wang, W.; Okada, M.; Higuchi, M.; Takaura, K.; Takada, H.; Yasui, Y.; Tsuchiya, K.; et al. Use of the Serum Wisteria floribunda Agglutinin-Positive Mac2 Binding Protein as a Marker of Gastroesophageal Varices and Liver-Related Events in Chronic Hepatitis C Patients. Diagnostics 2020, 10, 173. [Google Scholar] [CrossRef]
- Yamasaki, K.; Tateyama, M.; Abiru, S.; Komori, A.; Nagaoka, S.; Saeki, A.; Hashimoto, S.; Sasaki, R.; Bekki, S.; Kugiyama, Y.; et al. Elevated serum levels of Wisteria floribunda agglutinin-positive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatol. Baltim. Md. 2014, 60, 1563–1570. [Google Scholar] [CrossRef]
- Chuaypen, N.; Chittmittraprap, S.; Pinjaroen, N.; Sirichindakul, B.; Poovorawan, Y.; Tanaka, Y.; Tangkijvanich, P. Serum Wisteria floribunda agglutinin-positive Mac-2 binding protein level as a diagnostic marker of hepatitis B virus-related hepatocellular carcinoma. Hepatol. Res. Off. J. JPN. Soc. Hepatol. 2018, 48, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Umemura, T.; Joshita, S.; Sekiguchi, T.; Usami, Y.; Shibata, S.; Kimura, T.; Komatsu, M.; Matsumoto, A.; Ota, M.; Tanaka, E. Serum Wisteria floribunda agglutinin-positive Mac-2 binding protein predicts hepatocellular carcinoma incidence and recurrence in nucleos(t)ide analogue therapy for chronic hepatitis B. J. Gastroenterol. 2018, 53, 740–751. [Google Scholar]
- Uojima, H.; Yamasaki, K.; Sugiyama, M.; Kage, M.; Ishii, N.; Shirabe, K.; Hidaka, H.; Kusano, C.; Murakawa, M.; Asahina, Y.; et al. Quantitative measurements of M2BPGi depend on liver fibrosis and inflammation. J. Gastroenterol. 2024, 59, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Oe, S.; Miyagawa, K.; Kusanaga, M.; Ogino, N.; Honma, Y.; Harada, M. Excess glucose alone induces hepatocyte damage due to oxidative stress and endoplasmic reticulum stress. Exp. Cell Res. 2024, 442, 114264. [Google Scholar] [CrossRef]
- Yoshitomi, K.; Hayashi, T.; Oe, S.; Shibata, M.; Honma, Y.; Harada, M.; Kooka, Y. Child-Pugh grade deterioration stratified by the etiology after transcatheter arterial chemoembolization as initial treatment for hepatocellular carcinoma. Sci. Rep. 2024, 14, 3707. [Google Scholar] [CrossRef]
- Hayashi, T.; Shibata, M.; Oe, S.; Miyagawa, K.; Honma, Y.; Harada, M. Antiplatelet Therapy Improves the Prognosis of Patients with Hepatocellular Carcinoma. Cancers 2020, 12, 3215. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatol. Baltim. Md. 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
| First Biopsy | Second Biopsy | p-Value | |
|---|---|---|---|
| Age (years) | 59 (47–67) | 61 (49–69) | <0.01 |
| Gender (male/female) | 115/81 | ||
| AST (U/L) | 48 (34–73) | 25 (21–34) | <0.01 |
| ALT (U/L) | 74 (53–117) | 29 (21–50) | <0.01 |
| GGT (U/L) | 64 (43–90) | 32 (22–51) | <0.01 |
| TG (mg/dL) | 133 (103–194) | 118 (89–162) | <0.01 |
| HbA1c | 5.9 (5.6–6.5) | 5.8 (5.5–6.1) | <0.01 |
| Platelet counts (×109/L) | 23.3 (18.9–27.1) | 23.1 (18.8–27.1) | 0.23 |
| Fibrosis stage: 0/1/2/3/4 | 10/85/57/38/6 | 41/105/21/26/3 | <0.01 |
| Body Weight (kg) | 75.0 (64.6–87.1) | 71.2 (59.9–82.8) | <0.01 |
| BMI (kg/m2) | 28.0 (25.8–31.0) | 26.5 (23.9–29.8) | <0.01 |
| WFA+-M2BP | 0.72 (0.51–1.01) | 0.65 (0.47–0.90) | <0.01 |
| FIB-4 index | 1.39 (0.93–2.23) | 1.24 (0.84–1.77) | <0.01 |
| NFS | −2.01 (−3.05–0.90) | −1.91 (−2.83–0.79) | <0.01 |
| Type IV Collagen | 118.5 (96.8–154.0) | 110.0 (93.0–129.5) | <0.01 |
| Hyaluronic acid | 26.1 (12.2–46.8) | 23.8 (12.2–43.4) | <0.01 |
| Fibrosis Stage at Second Biopsy | ||||||
|---|---|---|---|---|---|---|
| Fibrosis Stage at First Biopsy | Stage 0 | Stage 1 | Stage 2 | Stage 3 | Stage 4 | Total |
| (A) | ||||||
| Stage 0 | 5 | 5 | 10 | |||
| Stage 1 | 29 | 48 | 8 | 85 | ||
| Stage 2 | 7 | 38 | 7 | 5 | 57 | |
| Stage 3 | 14 | 7 | 16 | 1 | 38 | |
| Stage 4 | 4 | 2 | 6 | |||
| (B) | ||||||
| Stage 0 | −0.06 | 0.19 | ||||
| Stage 1 | −0.08 | −0.03 | 0.21 | |||
| Stage 2 | −0.21 | 0.10 | −0.10 | 0.30 | ||
| Stage 3 | −0.24 | −0.07 | 0.03 | 0.14 | ||
| Stage 4 | −0.18 | −0.39 | ||||
| Changes in Fibrosis Stage | |||||
|---|---|---|---|---|---|
| 2 Stage Regression | 1 Stage Regression | Unchanged | 1 Stage Progression | p-Value | |
| FIB-4 index | −0.37 | −0.19 | 0.11 | −0.14 | 0.31 |
| NFS | 0.11 | 0.16 | 0.21 | 0.13 | 0.88 |
| WFA+-M2BP | −0.21 | −0.08 | −0.04 | 0.19 | <0.01 |
| Type IV Collagen | −29.00 | −6.50 | −10.00 | 10.00 | <0.01 |
| Hyaluronic acid | 0.00 | 0.00 | −1.05 | 0 | 0.60 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p-Value | OR | 95%CI | p-Value | |
| Age (<60 years) | 0.75 | 0.43–1.31 | 0.31 | |||
| Sex (male) | 0.91 | 0.52–1.61 | 0.75 | |||
| BMI (>30kg/m2) | 0.61 | 0.33–1.11 | 0.11 | |||
| Advanced fibrosis (stage > 2) | 1.39 | 0.71–2.73 | 0.34 | |||
| Reduction in body weight (>10%) | 1.46 | 0.75–2.86 | 0.27 | |||
| ΔWFA+-M2BP (<−0.24) | 4.16 | 1.91–9.05 | <0.01 | 3.54 | 1.55–8.12 | <0.01 |
| ΔNAS (>2) | 5.48 | 2.96–10.20 | <0.01 | 5.05 | 2.69–9.51 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, T.; Kooka, Y.; Kanazawa, J.; Matsuda, T. Estimation the Change in Liver Fibrosis Stage with Serial Measurement of Wisteria Floribunda Agglutinin-Positive Mac-2 Binding Protein in Metabolic Dysfunction-Associated Steatotic Liver Disease Patients. Int. J. Mol. Sci. 2025, 26, 9410. https://doi.org/10.3390/ijms26199410
Hayashi T, Kooka Y, Kanazawa J, Matsuda T. Estimation the Change in Liver Fibrosis Stage with Serial Measurement of Wisteria Floribunda Agglutinin-Positive Mac-2 Binding Protein in Metabolic Dysfunction-Associated Steatotic Liver Disease Patients. International Journal of Molecular Sciences. 2025; 26(19):9410. https://doi.org/10.3390/ijms26199410
Chicago/Turabian StyleHayashi, Tsuguru, Yohei Kooka, Jo Kanazawa, and Tomoki Matsuda. 2025. "Estimation the Change in Liver Fibrosis Stage with Serial Measurement of Wisteria Floribunda Agglutinin-Positive Mac-2 Binding Protein in Metabolic Dysfunction-Associated Steatotic Liver Disease Patients" International Journal of Molecular Sciences 26, no. 19: 9410. https://doi.org/10.3390/ijms26199410
APA StyleHayashi, T., Kooka, Y., Kanazawa, J., & Matsuda, T. (2025). Estimation the Change in Liver Fibrosis Stage with Serial Measurement of Wisteria Floribunda Agglutinin-Positive Mac-2 Binding Protein in Metabolic Dysfunction-Associated Steatotic Liver Disease Patients. International Journal of Molecular Sciences, 26(19), 9410. https://doi.org/10.3390/ijms26199410







