Abstract
Candida species are the primary fungal pathogens of invasive infections associated with high morbidity and mortality. The identification of these microorganisms is critical for therapeutic management and control of hospital infection. Herein, assays targeting the Intergenic Spacer 2 (IGS2) and Internal Transcribed Spacer 1 (ITS1) from the rDNA locus were developed to differentiate Candida species. Based on consensus nucleotide sequences, specific primers and positive controls were designed, and standard PCR and real-time PCR (qPCR) assays were performed. All primers resulted in specific amplification of the molecular targets of each species with no amplifications of the negative template control. Furthermore, the primers were highly specific when tested with a range of fungal DNAs and no cross-reactivity was observed among Candida species. The assays presented a limit of detection (LoD) of 10 copies of positive control per reaction for all specific primers designed. Overall, our results showed that qPCR assays employing primers targeting the regions IGS2 and ITS1 completely differentiated between Candida albicans, Candida auris, Candida parapsilosis, Candida tropicalis, and Nakazeomyces glabratus, with great accuracy and no amplification of DNA from other fungal species.
1. Introduction
Rapid diagnosis of Candida infections is crucial. Studies have shown that in-patients subjected to a delayed fluconazole treatment following the onset of the initial signs are more likely to experience fatal outcomes [1]. Therefore, efficient diagnoses to avoid postponing an appropriate antifungal therapy are highly significant [1].
Healthy individuals may harbor Candida as a member of their microbiome. However, under certain conditions, this ordinarily harmless microorganism can act as an opportunistic pathogen, leading to infections. In fact, such opportunistic fungi can lead to serious health problems, particularly in immunocompromised individuals, underscoring the urgent need for effective prevention and treatment strategies [2,3,4,5]. In recent years, the incidence of candidemia has been steadily increasing in hospital settings around the world, which highlights the growing concern over its impact on vulnerable populations [6,7,8,9]. The risk factors include broad-spectrum antibiotic and immunosuppressive therapy, stem cell or solid organ transplantation, prolonged stays in intensive care units (ICUs), multiple invasive medical procedures, and parenteral nutrition [10,11,12,13].
Among the fungal species that cause human infections, approximately 80% of cases are attributed to C. albicans, followed by non-albicans Candida (NAC) species. Worldwide, approximately 2,000,000 new cases of oral candidiasis, 1,300,000 of esophageal candidiasis, and approximately 134,000,000 of recurrent vulvovaginal candidiasis are reported yearly. Regarding severe candidiasis (bloodstream infections and invasive candidiasis), approximately 1,565,000 new cases are estimated, with 995,000 deaths (63.6%). Particularly in bloodstream infection, approximately 939,000 have negative results in culture-based identification [14]. In Brazil, the most common NAC species are Candida parapsilosis, Candida tropicalis, Nakaseomyces glabratus (formerly known as Candida glabrata), Pichia kudriavzevii (formerly known as Candida krusei), Candida auris, and Candida dubliniensis [15].
Although C. albicans is the predominant species, a significant increase in the isolation of other NAC species as causative agents of candidiasis has been reported. For instance, C. tropicalis is the leading cause of infection in neutropenic individuals and in those patients staying in ICUs. In addition, C. tropicalis is associated with high mortality rates, ranging from 55 to 60% in adults and 26 to 40% in pediatric patients [16]. N. glabratus is associated with oral infections in the elderly and denture wearers, is the second most frequent causative agent of vulvovaginitis, and is related to cases of candidemia, especially in immunocompromised patients and in ICUs. Neonatal, transplant, and parenteral nutrition patients are at higher risk of infections by C. parapsilosis. P. kudriavzevii, in turn, is frequently isolated from patients undergoing stem cell transplantation, due to the use of azoles [17].
Traditional microbiological methods for identifying Candida species require several days and rely on the isolation of yeasts from different biological sources [18]. In fact, this process involves several steps, including the assessment of germ tube and chlamydospore formation, optimal growth temperatures, as well as the ability to utilize different carbon and nitrogen sources [18,19,20].
Several DNA-based methods have emerged as powerful tools for the detection of Candida species. Among these, PCR-based techniques enable rapid and accurate identification, significantly improving sensitivity and specificity [18,19,20,21,22]. Moreover, molecular techniques such as multi-locus sequence typing (MLST) provides a robust framework for analyzing genetic variations across multiple loci, enhancing the precision of species identification [23]. Similarly, randomly amplified polymorphic DNA (RAPD) employs a unique amplification process that reveals polymorphisms in DNA sequences, thus offering valuable insights into the genetic diversity of Candida strains [23]. Collectively, these DNA-based approaches represent a significant advancement in the diagnosis of candidiasis and contribute to more effective monitoring and treatment strategies.
Several PCR-based assays target the ribosomal RNA (rRNA) gene cluster, which plays a crucial role in the identification and differentiation of various microorganisms. This gene cluster comprises four essential ribosomal genes: 18S, 5.8S, and 25–28S, along with the 5S gene. Additionally, it includes the Internal Transcribed Spacers (ITSs) 1 and 2, as well as the Intergenic Spacers (IGSs) 1 and 2 [24,25]. The ITS regions are particularly remarkable due to their high conservation across Candida species, which has established them as reliable targets for a wide range of molecular applications. These regions can be explored using multiple approaches, such as ITS length polymorphism [26,27], DNA sequencing [28], restriction fragment length polymorphism [29], and DNA hybridization probes [29,30]. Furthermore, PCR followed by restriction fragment length polymorphism assays, targeting either IGS2 or the full-length IGS, has also been successfully utilized to differentiate closely related Candida species and other fungi [31,32]. Each of these methods investigates the genetic features of the rDNA gene cluster, providing a robust framework for research in the context of clinical diagnostics. These results indicate that these regions are reliable targets for detecting different Candida species.
Systems based on hydrolysis probes, such as TaqMan, are widely used for the detection of Candida spp., but may present some important issues. The use of fluorogenic probes specific to each species increases the cost of the method. In addition to the system itself being more expensive, it also requires thermocyclers with multiple detection channels. This may restrict the application of probe-based qPCR, especially in places with limited resources. In contrast, the melting curve analysis allows species to be differentiated based on their different melting temperatures (Tm), using only intercalating dyes such as SYBR Green [33], thus eliminating the need for probes and multi-channel equipment. This approach not only reduces operating costs, but also requires only one detection channel (green), which is present in most conventional real-time thermal cyclers.
Given the critical concerns regarding Candida infections, this study aimed to develop a specific and highly sensitive multiplex real-time PCR (m-qPCR) assay, based on melting curve analysis, designed for the accurate differentiation of five Candida species. These assays utilize primers specifically designed to target the IGS2 region in C. albicans, C. tropicalis, C. parapsilosis, and N. glabratus (formerly C. glabrata), and the ITS1 region in C. auris. This approach seeks to enhance diagnostic accuracy and facilitate rapid fungal identification within clinical laboratory settings.
2. Results
Initially, the IGS2 and ITS nucleotide sequences of Candida species and C. auris, respectively—available in the GenBank nucleotide database—were analyzed (Supplementary Figure S1), leading to the design of specific primer pairs for C. albicans, C. auris, C. parapsilosis, C. tropicalis, and N. glabratus (Table 1). In addition, a synthetic plasmid harboring all targets (positive control) was also designed (Supplementary Figure S2). Each primer pair was checked for similarity with other nucleotide sequences using the BLAST algorithm (v.2.15.0), and the results showed no matches other than the aforementioned Candida species. Furthermore, matches were not detected within the human genome. These results indicate that cross-reactivity with non-target sequences is unlikely to occur.

Table 1.
Characteristics of oligonucleotide primers utilized in the amplification assays.
Following in silico analyses, monoplex PCR assays were performed using the positive control and 1 µM of each designed primer. These reactions successfully generated amplicons of the expected lengths: 303 bp for C. albicans and C. tropicalis, 240 bp for C. auris, 218 bp for C. parapsilosis, and 295 bp for N. glabratus (Table 1/Supplementary Figure S3). The annealing temperature was set at 62 °C to establish the appropriate amplification conditions for target detection.
Additionally, a second round of monoplex PCR assays was conducted using 100 ng of genomic DNA obtained from C. albicans ATCC 26790, C. auris CBS 10913, C. parapsilosis ATCC 1975, C. tropicalis ATCC 28707, and N. glabratus ATCC 518. The amplicons generated from these PCR runs matched the expected lengths, confirming the specificity of the primers designed in this study (Table 2).

Table 2.
Positive control and microorganisms used to assess the specificity of the melting-curve-based real-time PCR.
Thereafter, we performed a monoplex qPCR assay using the synthetic positive control and 1 µM of each primer pair in a Rotor Gene Q-5Plex HRM platform to determine the melting temperature (Tm) peak of the specific amplicons. These results are shown in Figure 1a–e. Each graph represents the fluorescence signal as a function of temperature, showing the characteristic Tm of each specific amplicon. The observed Tm values were as follows: 80.3 ± 0.5 º C, 83.5 ± 0.5 °C, 80.4 ± 0.5 °C, 81.2 ± 0.5 °C, and 88.5 ± 0.5 °C, for C. albicans, C. auris, C. parapsilosis, C. tropicalis, and N. glabratus, respectively.
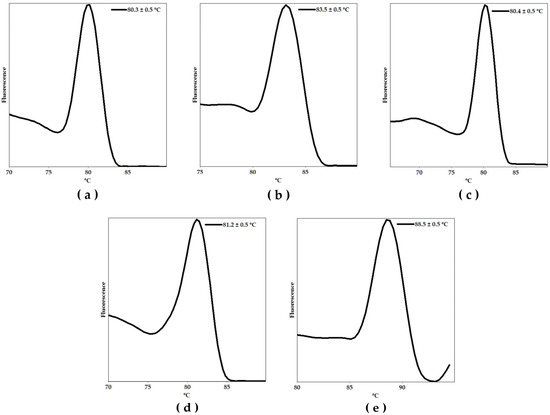
Figure 1.
Melting curve analyses showing the melting temperature (Tm) peaks of the amplicons associated with the identification of (a) Candida albicans, (b) Candida auris, (c) Candida parapsilosis, (d) Candida tropicalis, and (e) Nakaseomyces glabratus amplicons using the positive (plasmid) controls harboring all targets.
Moreover, a qPCR assay using the primers for the species studied herein was carried out individually within a single run. The results, presented in Figure 2, showed melting peak temperatures equivalent to those observed in the monoplex qPCR system.
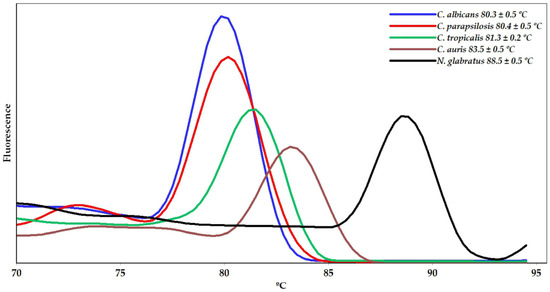
Figure 2.
Melting curve analysis from qPCR assays performed individually in a single run showing the melting temperature (Tm) peaks of the amplicons corresponding to the identification of Candida and Nakazeomyces species.
The analytical performance of the qPCR assays was also evaluated using DNA purified from cultures of Candida species. Amplification signals were detected for all yeasts tested, generating dissociation curves with single peaks, similar to those obtained with the positive controls.
To further assess primer specificity beyond in silico analysis, qPCR was performed using nucleic acids from a panel of bacterial and fungal organisms (Table 2). Amplification signals were exclusively observed for the targeted Candida species, thereby confirming the absence of cross-reactivity with non-target sequences.
To determine the analytical sensitivity of the qPCR assay and establish the limit of detection (LoD), a series of ten-fold serial dilutions of positive controls was carried out (ranging from 100 to 106 copies per reaction). The sensitivity assays were conducted in triplicate across five separate days, yielding a total of 15 replicates to ensure the robustness of the results. The LoD for each target gene was established at 10 copies per reaction (Figure 3a–e). The mean CT values for the target genes, which correspond to the smallest detectable quantities, were <26 for C. parapsilosis, <27 for C. albicans and C. auris, and <28 for C. tropicalis and N. glabratus. Furthermore, the efficiency of the reactions was assessed using the slopes of the standard curves, with values that ranged from 100% to 110% (Figure 4a–e).
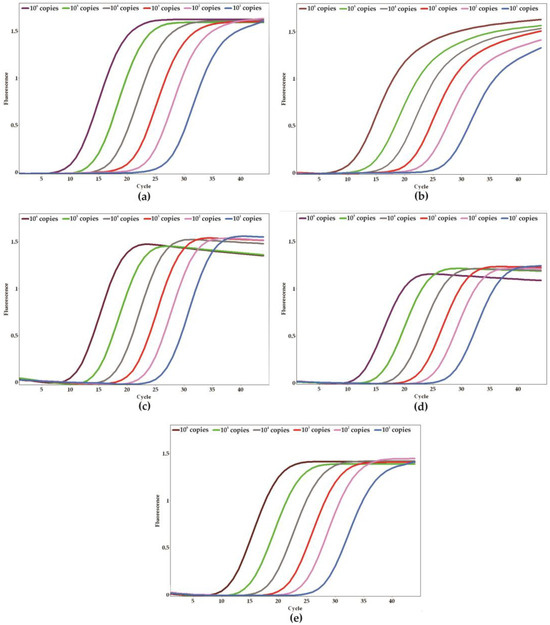
Figure 3.
Analytical sensitivity of the qPCR system and the limit of detection for each specific target. (a) Candida albicans, (b) Candida auris, (c) Candida parapsilosis, (d) Candida tropicalis, and (e) Nakaseomyces glabratus. Amplification plot of serial dilutions resulting in 101 to 106 copies of the positive (plasmid) control harboring all targets.
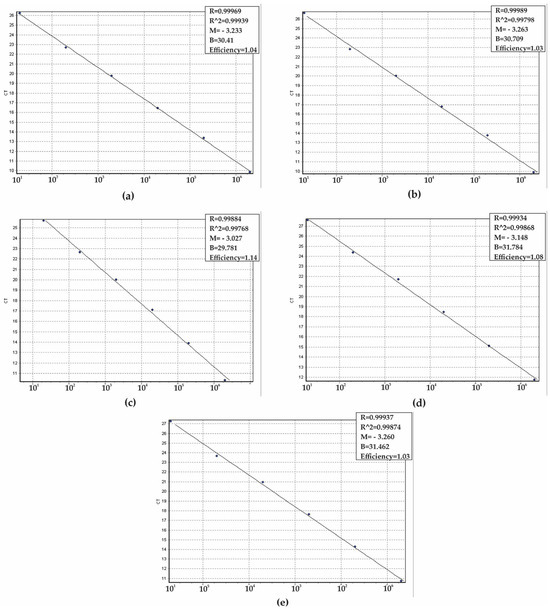
Figure 4.
Standard curves obtained by linear regression of the threshold cycle (CT) versus copy numbers to determine the efficiency of the qPCR system. (a) Candida albicans, (b) Candida auris, (c) Candida parapsilosis, (d) Candida tropicalis, and (e) Nakaseomyces glabratus. Slope (M), regression coefficient (R), and efficiency of the real-time PCR method are shown (a–e).
To evaluate the quality of the nucleic acid purification and the presence of potential interfering substances, primers targeting the human tRNA processing ribonuclease P (RNase P) gene were also included in the qPCR, resulting in a Tm value of 82.5 ± 0.50 °C (Supplementary Figure S3).
To evaluate the effect of simplified DNA extraction methods on qPCR efficiency and turnaround time, genomic DNA obtained through the boiling method and colony qPCR was directly used in the assays (Figure 5a,b). The results were consistent with the amplification assays whose template DNA was purified using the phenol–chloroform DNA extraction method. Furthermore, the efficiency of the system for detecting yeasts from blood samples was assessed using the assay (Figure 5c–g), which resulted in characteristic amplification patterns for all primers corresponding to the species included in the study.

Figure 5.
Amplification profiles of target genes using DNA obtained by the boiling method (a) and direct colony qPCR (b). Melting temperature peaks of Candida albicans (c), Candida auris (d), Candida parapsilosis (e), Candida tropicalis (f), and Nakaseomyces glabratus (g) amplicons using the blood samples spiked with each target Candida species.
3. Discussion
Nucleic acid amplification tests (NAATs) have been widely used in clinical laboratories to detect etiological agents of infectious diseases, owing to their high sensitivity and specificity, as well as their ability to deliver rapid results [36]. In contrast, traditional culture-based tests for identifying microbial agents are often time-consuming, labor-intensive, and prone to displaying limited sensitivity [37].
In this study, a new method for detecting the most common causative species of candidiasis is presented and can be applied for both diagnosis and research. We utilized the IGS2 [C. albicans, C. tropicalis, C. parapsilosis and N. glabratus (formerly C. glabrata)] and ITS1 (C. auris) regions of ribosomal DNA genes for the development of a qPCR assay for the differentiation of five causative agents of candidiasis. Notably, the ITS, IGS, and NTS regions of ribosomal DNA genes have been widely used for the diagnosis and phylogenetic analysis of etiological agents of infectious diseases [38,39,40,41,42].
To ensure the specificity and accuracy of our assays, we meticulously selected target nucleotide sequences based on the following criteria: (i) gene essentiality; (ii) conserved sequences within the species to eliminate any risk of false-negative or false-positive results due to genetic variations; (iii) non-overlapping melting temperatures; and (iv) limited presence of secondary structures—such as homodimers and heterodimers—up to 10% of the Tm and total ΔG values of the primers.
The results shown in Table 1 represent a detailed view of the specific primers—CaNTS2, CauITS1, CpNTS2, CtNTS2, and NgNTS2—for the specific identification of C. albicans, C. auris, C. parapsilosis, C. tropicalis, and N. glabratus—using the NTS region. A crucial step relied on the amplification profile using CaITS1, specifically designed for C. auris identification. This specificity is extremely relevant, considering the emergence of antifungal resistance in this species, representing a significant challenge for public health [43,44,45]. Our findings confirmed that NgNTS2 led to specific amplification of N. glabratus, a pathogen that holds significant importance due to its antifungal resistance profile. Accurate identification of this pathogen is crucial, and it allows for the implementation of suitable therapeutic procedures for infection treatment, contributing to reducing the mortality and morbidity rates [46]. As for C. parapsilosis, using CpNTS2 primers, specific identification was also achieved. Considering the impact of this species on nosocomial infections, particularly among immunocompromised individuals, which rank just after C. albicans, it is imperative to ensure precise identification [47,48]. Finally, the assay using CtNTS2 demonstrated specificity for C. tropicalis. This species presents clinical relevance, due to the prevalence of systemic infections and azole resistance. Therefore, this highlights the necessity for the development of rapid methodologies for detection and monitoring of C. tropicalis [16,49].
Furthermore, no amplification was observed for fungal species other than those for which the primers were designed, including other yeasts and filamentous fungi. Amplifications were also not observed when bacterial DNA was used as a template in the assays. These results ensure the high specificity of the method proposed in this study, highlighting its methodological robustness.
The assay designed and optimized in our study offers numerous advantages, such as reduced time for accurate diagnosis with specific identification of the causative agents. The method is based on the use of an intercalating dye, which reduces the overall cost of qPCR [33], eliminating the need for additional probes and requiring only a single channel for fluorescence detection. Furthermore, the test can be performed using conventional PCR equipment and allows for simultaneous detection of multiple Candida species, in different reactions, and in a single run, resulting in significant savings in time and laboratory resources.
Dye-based qPCR assays coupled with the melting curve strategy to detect infectious microorganisms have been used in some studies. For example, Tavares et al. [42] used the IGS1 region of the ribosomal DNA (rDNA) as the target to differentiate Cryptococcus gattii sensu lato and Cryptococcus neoformans sensu lato using qPCR followed by melting curve analysis. Otaguiri et al. [50] proposed a melting-curve-based multiplex qPCR assay targeting the cfb gene to detect Streptococcus agalactiae in rectal–vaginal samples from pregnant women. Van Bergen et al. [51] implemented a melting-curve-based qPCR assay for the detection of Plasmodium in a routine clinical laboratory. Wan et al. [52] developed melting-curve-based RT-qPCR for the simultaneous detection of four human coronaviruses (HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1). Additionally, Tavares et al. [53] developed melting-curve-based RT-qPCR for the simultaneous detection of SARS-CoV-2, Influenza A virus, Human Respiratory Syncytial Virus, and Human Rhinovirus B. The method led to efficient amplification of the mentioned viruses, with parameters of sensitivity and specificity similar to those of standard commercial tests.
Rapid and accurate detection and identification of Candida species are critical for timely treatment and optimal patient outcomes, as different species exhibit distinct antifungal susceptibility profiles that can directly influence therapeutic decisions. The results highlight that the simplified purification method maintains qPCR efficiency despite its procedural simplicity [54]. Thus, the use of the boiling method as a viable and low-cost alternative for DNA extraction in molecular protocols, and colony qPCR, may represent a relevant advance for clinical laboratories, especially in resource-limited settings [54].
Moreover, we assessed the qPCR performance using human blood samples artificially contaminated with different Candida species. Bloodstream candidiasis is commonly diagnosed through culture, which is considered the gold standard. However, the application of this method can directly impact prognosis and mortality, often delaying the initiation of optimized antifungal therapy, especially in infections caused by multidrug-resistant species such as C. albicans and C. auris. The concept of time to positivity (TTP) refers to the amount of time required for a culture to yield a positive result; Candida species exhibit slower growth rates compared to most bacterial species, prolonging TTP and influencing patient prognosis [55]. Therefore, the implementation of rapid methods for the identification of specific etiological agents directly from the bloodstream facilitates faster decision-making, ensures the selection of the appropriate antifungal therapy, and contributes to the effective management of infections caused by resistant Candida species.
A system that combines magnetic resonance with PCR [T2 Magnetic Resonance (T2MR) assay] to detect five species of Candida—C. albicans, C. tropicalis, C. parapsilosis, P. kudriavzevii (formerly C. krusei), and N. glabratus) [56]—was developed by T2 Biosystems and approved by the FDA (Food and Drug Administration). This test provides results within 3 to 5 h from bloodstream samples for routine clinical use. However, some estimates suggest that each test can cost up to USD 150, apart from the cost of the equipment [57]. Assuming that Brazil is classified as a developing country, these costs represent a significant barrier to the effective implementation of this method within the Brazilian healthcare system.
Despite the high specificity of the proposed method, we recognize some limitations inherent to the approach used. In conventional PCR, differentiation between Candida albicans, C. tropicalis, and N. glabratus can be challenging due to the similar size of the amplified fragments, requiring high-resolution electrophoretic analysis to avoid ambiguous interpretations. Similarly, in qPCR with SYBR Green, we observed that the melting profiles of C. albicans and C. parapsilosis exhibit highly similar dissociation temperatures, which can compromise the unequivocal distinction between these species when amplified simultaneously. To overcome this limitation, we propose that the reactions be performed individually in the same run, which maintains the simplicity and speed of the technique, in addition to preserving its applicability in laboratories with basic infrastructure. Finally, we acknowledge that the present study did not evaluate the performance of the method in clinical samples, since the main objective was to validate the approach using reference strains. Nevertheless, the results obtained demonstrate the potential of the methodology as an accessible and efficient tool for screening and preliminary identification of yeasts of medical importance, with future applicability in clinical contexts after additional validations.
In conclusion, Candida IGS2 and ITS2 primers were designed to specifically amplify the target of each species in a single PCR run. These reactions reduce the cost and time required for Candida identification due to their simplicity and low cost, facilitating their application in routine clinical laboratory testing. The Candida identification primers showed great sensitivity and specificity, allowing DNA amplification exclusively for the target species. Overall, this study showed that the designed specific primers were able to differentiate medically important yeasts, including C. albicans, C. auris, C. tropicalis, C. parapsilosis, and N. glabratus. Finally, these molecular tests for Candida identification represent a new, sensitive, and specific method that enhances diagnostic efficiency. This improvement is important to optimize the management of patients at risk of systemic candidiasis, as it contributes to the rapid and early diagnosis of these infections and supports cost-effective treatment strategies.
4. Materials and Methods
4.1. PCR Primers and Positive Control Design
The nucleotide sequences of the IGS2 region of the ribosomal DNA gene of C. albicans (12 sequences), C. parapsilosis (12 sequences), C. tropicalis (3 sequences), and N. glabratus (formerly C. glabrata) (47 sequences), and the ITS1 region of the ribosomal DNA gene of C. auris (50 sequences), were retrieved from the GenBank/EMBL databases (available at http://www.ncbi.nlm.nih.gov, accessed on 10 July 2025). All the sequencies were subjected to multiple alignments by the ClustalW tool using BioEdit software (v.7.2.0) (Supplementary Table S1) to obtain a consensus sequence for each species. Specific primers were designed based on the consensus sequences of each species using the PrimerQuestTM and OligoAnalyzerTM tools (both available at http://www.idtdna.com, accessed on 6 March 2023). Moreover, the other yeast species were included in the alignment—C. dubliniensis, Debaryomyces hansenii (formerly Candida famata), Meyerozyma carpophila (formerly Candida carpophila), Pichia guilliermondii, Pichia caribbica, Pichia norvegensis, Candida zeylanoides, and Candida inconspicua. To ensure specificity, the NTS2 and ITS1 sequences from each Candida species and N. glabratus, the primer sequences targeting the designated regions of the ribosomal DNA locus, were compared with nucleotide sequences available in the GenBank database of the National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov, accessed on 10 July 2025) using the BLAST algorithm (blastn). Detailed information regarding the primer sequences, melting temperatures, and expected amplicon lengths is shown in Table 1. The amplicon sequences generated by each specific primer were inserted into the pUC57 plasmid to serve as a positive control (Supplementary Figure S1).
4.2. Microorganisms and Culture Conditions
A panel of 49 fungal and 8 bacterial strains (Table 2) was used for the development of the assay. Five colonies from each fungal and bacterial strain were inoculated into Sabouraud Dextrose Broth (SDB, Himedia, Thane, India) and Tryptic Soy Broth (TSB, Oxoid, São Paulo, Brazil), respectively. The microbial cultures were incubated at 37 °C for 48 h for fungi and 24 h for bacteria. Following incubation, the cells were harvested by centrifugation (10,000× g, 5 min), washed twice with sterile saline (NaCl 0.85%), and processed for DNA purification. Fungal and bacterial strains were stored at −80 °C, in SDB and TSB, respectively, and supplemented with 20% glycerol.
4.3. DNA Extraction
To extract genomic DNA, the cells were resuspended in a lysis buffer containing 10 mM Tris-HCl (pH 8.0), 2% Triton X-100, 1% SDS, 100 mM NaCl, and 1 mM EDTA, along with phenol solution (Sigma-Aldrich, St. Louis, MO, USA). Glass beads were added, and the mixture was vigorously stirred to disrupt cells. After centrifugation and recovery of the aqueous phase, the DNA was purified with a phenol:chloroform:isoamyl alcohol solution (25:24:1), followed by precipitation using a 2.5-fold volume of ice-cold absolute ethanol (J.T. Baker, NJ, USA). The resulting DNA pellet was washed with 70% ethanol, dried at room temperature, and resuspended in ultrapure water. DNA concentration was determined by absorbance at 260 nm using a spectrophotometer (Synergy HT, Biotek, VT, USA). After quantification, the genomic DNA of each isolate was adjusted to a concentration of 50 ng/µL.
4.4. PCR Design
The PCR conditions were determined through a two-step process. First, the designed oligonucleotides were tested in conventional PCR with a final volume of 20 μL containing 1× PCR buffer, 2.5 mM MgCl2, 1.25 µM of each dNTP, 0.65 U Taq DNA polymerase (Invitrogen, Waltham, MA, USA), 0.5 to 2 μM of each primer, and 1 × 106 copies of the positive control, to establish optimal annealing temperatures and primer concentrations. The amplification reactions were performed in a Veriti® 96-well Thermal Cycler (Applied Biosystems, Singapore) using an initial denaturation at 95 °C for 2 min. This was followed by 35 cycles, each consisting of 95 °C for 30 s (denaturation), 30 s at a temperature gradient from 60 °C to 70 °C (for optimal annealing temperature determination; Supplementary Figure S3), and 72 °C for 30 s (extension). A final extension step was carried out at 72 °C for 10 min. Reactions without nucleic acid templates were included as a negative template control (NTC). Afterwards, amplicons were analyzed in 2% agarose gel electrophoresis and stained with ethidium-bromide (0.5 µg/mL) (Sigma-Aldrich, St. Louis, MO, USA).
An annealing temperature of 62 °C and primer concentration of 1 μM, along with 100 ng of DNA from C. albicans ATCC26790, C. auris CBS 10913, N. glabrata ATCC518, C. parapsilosis ATCC1975, and C. tropicalis ATCC28707, were selected and used to validate the amplification conditions. Subsequently, all qPCR assays were performed in a Rotor Gene Q-5Plex HRM (Qiagen, NW, Germany) in a final volume of 20 µL containing 1 × 106 copies of the positive control, and 1 µM each of oligonucleotide and QuantiNova SYBR Green PCR mix (Qiagen, NW, Germany), according to the manufacturer’s recommendation. The cycling profile was as follows: 2 min at 50 °C, initial denaturation at 95 °C for 10 min, followed by 45 cycles of 30 s at 95 °C, 30 s at 62 °C, and 30 s at 72 °C. Melting curves were acquired with an increase in temperature ranging from 60 to 99 °C, with a time holding of 60 s at 0.5 °C at each step. NTC reactions were carried out simultaneously. Data were analyzed using the Rotor-Gene Q series software version 2.1.0.9.
4.5. Analytical Specificity, Sensitivity, and Validation
To determine the sensitivity of the qPCR assay, positive controls ranging from 100 to 106 copies per reaction were used to create a standard curve for each primer pair, based on the CT values as a function of the plasmid copy number. All amplification reactions were executed in duplicate in three separate experiments. The number of plasmid copies was determined by dividing the total mass of plasmids by the individual mass of each plasmid copy, as proposed by Thermo Fisher Scientific (2008) [58], according to the equation m = (n).(1.096 × 10−21 g/bp) [where m = mass and n = size of the plasmid plus insert, in base pairs], thus obtaining the number of copies per μL. Furthermore, the R2 values were calculated to evaluate the efficiency of the reactions. The efficiency (E) of each reaction was determined using the slope of standard curves according to the following equation: E = 10−1/slope. Each assay was carried out in triplicate on five consecutive days. A quantity of 100 ng of genomic DNA purified from the microorganisms listed in Table 2 was used for specificity analyses.
4.6. Analytical Validation with DNA-Boiling Purification, Culture-qPCR and Blood Samples
An experiment was carried out to assess the amplification performance and the efficiency of the system using DNA obtained by boiling. C. albicans ATCC 26790, C. auris CBS 10913, C. parapsilosis ATCC 22019, C. tropicalis ATCC 28707, and N. glabratus ATCC 2001) were cultivated on Sabouraud Dextrose Agar (SDA) for 48 h at 37 °C. After cultivation, one colony-forming unit (CFU) of each species was collected and added to 100 µL of Phosphate Buffered Saline (PBS) and boiled for 10 min. Afterwards, the system was centrifuged at 14,000× g, and the supernatant was recovered. Five microliters of the recovered material were used for qPCR amplification, according to the conditions described above.
Also, a second colony was recovered from cultivation and submitted directly to amplification, following the same protocol of amplification as described above. Additionally, a third experiment was carried out to simulate the detection performance in blood samples. Whole blood samples (n = 3) were obtained from adult men; the sample collection was approved by the Research Ethics Committee of the State University of Londrina (CEP/UEL) under the document number 47784621.2.0000.5231 and approval number 4.862.243. The participants signed an informed consent form, expressing their agreement on the use of their samples and the publication of the results described herein. A quantity of 200 µL of human blood was artificially contaminated with one CFU of each cultured yeast and used to purify the total genomic DNA using the QIAamp® DNA mini Kit (Qiagen) according to the manufacturer’s recommendations. The purified total DNA was used in the qPCR assays as previously described. Positive controls containing 106 copies of the plasmid were used in three different assays, and reactions without the addition of template DNA were used as negative controls. All amplification reactions were carried out in duplicate and in three independent experiments.
5. Patents
This study resulted in a patent application to the Brazilian National Institute of Intellectual Property, deposit number BR 10 2024 015 279 4.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26199411/s1.
Author Contributions
Conceptualization, E.R.T., S.F.Y.-O. and L.M.Y.; methodology, V.P.S., I.M.d.C., P.H.G.B., G.S.-R., A.P.S.O., G.B.-G. and J.P.S.; validation, E.R.T., A.T.M. and L.M.Y.; formal analysis, E.R.T., K.I., S.F.Y.-O. and L.M.Y.; investigation, E.R.T., V.P.S. and L.M.Y.; resources, K.I., S.F.Y.-O. and L.M.Y.; data curation, E.R.T., K.I. and S.F.Y.-O.; writing—original draft preparation, E.R.T. and L.M.Y.; writing—review and editing, E.R.T., G.B.-G. and S.F.Y.-O.; visualization, E.R.T., K.I., S.F.Y.-O. and L.M.Y.; supervision, S.F.Y.-O. and L.M.Y.; project administration, S.F.Y.-O. and L.M.Y.; funding acquisition, K.I., S.F.Y.-O. and L.M.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Process 402387/2020-1), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Financial Code 01), Fundação Araucária-PR agreement 219/2023 PDI, and Superintendência de Ciência, Tecnologia e Ensino Superior (SETI)/UEL (Edital PROPPG 06/2025).
Institutional Review Board Statement
The study protocol was approved by the Ethics Committee of the State University of Londrina (Document 47784621.2.0000.5231, Opinion Number 4.862.243-CEP/UEL).
Informed Consent Statement
Written informed consent was obtained from all participants for the publication of this report and any accompanying images.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
V.P.S.: I.M.d.C., P.H.G.B., G.S.-R., and G.B.-G. were funded by graduate scholarships from CAPES; K.I. and S.F.Y.-O were funded by research fellowships from CNPq.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Garey, K.W.; Rege, M.; Pai, M.P.; Mingo, D.E.; Suda, K.J.; Turpin, R.S.; Bearden, D.T. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: A multi-institutional study. Clin. Infect. Dis. 2006, 43, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Braga, P.R.; Cruz, I.L.; Ortiz, I.; Barreiros, G.; Nouér, S.A.; Nucci, M. Secular trends of candidemia at a Brazilian tertiary care teaching hospital. Braz. J. Infect. Dis. 2018, 22, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Motta, F.A.; Dalla-Costa, L.M.; Muro, M.D.; Cardoso, M.N.; Picharski, G.L.; Jaeger, G.; Burger, M. Risk factors for candidemia mortality in hospitalized children. J. Pediatr. 2017, 93, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Vieira de Melo, A.P.; Zuza-Alves, D.L.; da Silva-Rocha, W.P.; Ferreira, L.B.C.S.; Francisco, E.C.; Salles de Azevedo Melo, A.; Maranhão Chaves, G. Virulence factors of Candida spp. obtained from blood cultures of patients with candidemia attended at tertiary hospitals in Northeast Brazil. J. Mycol. Med. 2019, 29, 132–139. [Google Scholar] [CrossRef]
- Khan, S.; Cai, L.; Bilal, H.; Khan, M.N.; Fang, W.; Zhang, D.; Yao, F.; Wang, X.; Wang, Q.; Hou, B.; et al. An 11-year retrospective analysis of candidiasis epidemiology, risk factors, and antifungal susceptibility in a tertiary care hospital in China. Sci. Rep. 2025, 15, 7240. [Google Scholar] [CrossRef]
- Enoch, D.A.; Yang, H.; Aliyu, S.H.; Micallef, C. The changing epidemiology of invasive fungal infections. Methods Mol. Biol. 2017, 1508, 17–65. [Google Scholar]
- Pfaller, M.A.; Castanheira, M. Nosocomial candidiasis: Antifungal stewardship and the importance of rapid diagnosis. Med. Mycol. 2016, 54, 1–22. [Google Scholar] [CrossRef]
- Kumamoto, C.A.; Gresnigt, M.S.; Hube, B. The gut, the bad and the harmless: Candida albicans as a commensal and opportunistic pathogen in the intestine. Curr. Opin. Microbiol. 2020, 56, 7–15. [Google Scholar] [CrossRef]
- Romo, J.A.; Kumamoto, C.A. On commensalism of Candida. J. Fungi 2020, 6, 16. [Google Scholar] [CrossRef]
- Doi, A.M.; Pignatari, A.C.; Edmond, M.B.; Marra, A.R.; Camargo, L.F.; Siqueira, R.A.; da Mota, V.P.; Colombo, A.L. Epidemiology and microbiologic characterization of nosocomial candidemia from a Brazilian National Surveillance Program. PLoS ONE 2016, 11, e0146909. [Google Scholar] [CrossRef]
- Ghrenassia, E.; Mokart, D.; Mayaux, J.; Demoule, A.; Rezine, I.; Kerhuel, L.; Calvet, L.; De Jong, A.; Azoulay, E.; Darmon, M. Candidemia in critically ill immunocompromised patients: Report of a retrospective multicenter cohort study. Ann. Intensive Care 2019, 9, 62. [Google Scholar] [CrossRef]
- Wu, P.F.; Liu, W.L.; Hsieh, M.H.; Hii, I.M.; Lee, Y.L.; Lin, Y.T.; Ho, M.W.; Liu, C.E.; Chen, Y.H.; Wang, F.D. Epidemiology and antifungal susceptibility of candidemia isolates of non-albicans Candida species from cancer patients. Emerg. Microbes Infect. 2017, 6, e87. [Google Scholar] [CrossRef]
- Lass-Flörl, C.; Kanj, S.S.; Govender, N.P.; Thompson, G.R., 3rd; Ostrosky-Zeichner, L.; Govrins, M.A. Invasive candidiasis. Nat. Rev. Dis. Primers 2024, 10, 20. [Google Scholar] [CrossRef]
- Denning, D.W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef]
- Agnelli, C.; Guimarães, T.; Sukiennik, T.; Lima, P.R.P.; Salles, M.J.; Breda, G.L.; Queiroz-Telles, F.; Chaves Magri, M.M.; Mendes, A.V.; Camargo, L.F.A.; et al. Prognostic trends and current challenges in candidemia: A comparative analysis of two multicenter cohorts within the past decade. J. Fungi 2023, 9, 468. [Google Scholar] [CrossRef]
- Keighley, C.; Kim, H.Y.; Kidd, S.; Chen, S.C.; Alastruey, A.; Dao, A.; Bongomin, F.; Chiller, T.; Wahyuningsih, R.; Forastiero, A.; et al. Candida tropicalis-A systematic review to inform the World Health Organization of a fungal priority pathogens list. Med. Mycol. 2024, 62, myae040. [Google Scholar] [CrossRef]
- de Oliveira, C.S.; Colombo, A.L.; Francisco, E.C.; de Lima, B.; Gandra, R.F.; de Carvalho, M.C.P.; Carrilho, C.M.D.M.; Petinelli, R.; Pelison, M.; Helbel, C.; et al. Clinical and epidemiological aspects of candidemia in eight medical centers in the state of Parana, Brazil: Parana Candidemia Network. Braz. J. Infect. Dis. 2021, 25, 101041. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.Z.; Alam, Q.; Jiman-Fatani, A.; Kamal, M.A.; Abuzenadah, A.M.; Chaudhary, A.G.; Akram, M.; Haque, A. Candida identification: A journey from conventional to molecular methods in medical mycology. World J. Microbiol. Biotechnol. 2014, 30, 1437–1451. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Nguyen, M.H. Diagnosing invasive candidiasis. J. Clin. Microbiol. 2018, 56, e01909-17. [Google Scholar] [CrossRef] [PubMed]
- Neppelenbroek, K.H.; Seó, R.S.; Urban, V.M.; Silva, S.; Dovigo, L.N.; Jorge, J.H.; Campanha, N.H. Identification of Candida species in the clinical laboratory: A review of conventional, commercial, and molecular techniques. Oral Dis. 2014, 20, 329–344. [Google Scholar] [CrossRef]
- Higashi, Y.; Niimi, H.; Sakamaki, I.; Yamamoto, Y.; Kitajima, I. Rapid identification of Candida species in candidemia directly from blood samples using imperfect match probes. Sci. Rep. 2020, 10, 5828. [Google Scholar] [CrossRef]
- Asadzadeh, M.; Ahmad, S.; Al-Sweih, N.; Khan, Z. Rapid and accurate identification of Candida albicans and Candida dubliniensis by Real-Time PCR and melting curve analysis. Med. Princ. Pract. 2018, 27, 543–548. [Google Scholar] [CrossRef]
- Alkhars, N.; Al Jallad, N.; Wu, T.T.; Xiao, J. Multilocus sequence typing of Candida albicans oral isolates reveals high genetic relatedness of mother-child dyads in early life. PLoS ONE 2024, 19, e0290938. [Google Scholar] [CrossRef]
- Wengenack, N.L.; Binnicker, M.J. Fungal molecular diagnostics. Clin. Chest Med. 2009, 30, 391–408. [Google Scholar] [CrossRef] [PubMed]
- Torres-Machorro, A.L.; Hernández, R.; Cevallos, A.M.; López-Villaseñor, I. Ribosomal RNA genes in eukaryotic microorganisms: Witnesses of phylogeny? FEMS Microbiol. Rev. 2010, 34, 59–86. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Eisner, J.D.; Kattar, M.M.; Rassoulian-Barrett, S.L.; LaFe, K.; Yarfitz, S.L.; Limaye, A.P.; Cookson, B.T. Identification of medically important yeasts using PCR-based detection of DNA sequence polymorphisms in the internal transcribed spacer 2 region of the rRNA genes. J. Clin. Microbiol. 2000, 38, 2302–2310. [Google Scholar] [CrossRef][Green Version]
- Turenne, C.Y.; Sanche, S.E.; Hoban, D.J.; Karlowsky, J.A.; Kabani, A.M. Rapid identification of fungi by using the ITS2 genetic region and an automated fluorescent capillary electrophoresis system. J. Clin. Microbiol. 1999, 37, 1846–1851, Erratum in: J. Clin. Microbiol. 2000, 38, 944.. [Google Scholar] [CrossRef]
- Gharizadeh, B.; Norberg, E.; Löffler, J.; Jalal, S.; Tollemar, J.; Einsele, H.; Klingspor, L.; Nyrén, P. Identification of medically important fungi by the Pyrosequencing technology. Mycoses 2004, 47, 29–33. [Google Scholar] [CrossRef]
- De Llanos Frutos, R.; Fernández-Espinar, M.T.; Querol, A. Identification of species of the genus Candida by analysis of the 5.8S rRNA gene and the two ribosomal internal transcribed spacers. Antonie Van Leeuwenhoek 2004, 85, 175–185. [Google Scholar] [CrossRef]
- Einsele, H.; Hebart, H.; Roller, G.; Löffler, J.; Rothenhofer, I.; Müller, C.A.; Bowden, R.A.; van Burik, J.; Engelhard, D.; Kanz, L.; et al. Detection and identification of fungal pathogens in blood by using molecular probes. J. Clin. Microbiol. 1997, 35, 1353–1360. [Google Scholar] [CrossRef]
- Cornet, M.; Sendid, B.; Fradin, C.; Gaillardin, C.; Poulain, D.; Nguyen, H.V. Molecular identification of closely related Candida species using two ribosomal intergenic spacer fingerprinting methods. J. Mol. Diagn. 2011, 13, 12–22. [Google Scholar] [CrossRef]
- Cebeci Güler, N.; Tosun, I.; Aydin, F. The identification of Meyerozyma guilliermondii from blood cultures and surveillance samples in a university hospital in Northeast Turkey: A ten-year survey. J. Mycol. Med. 2017, 27, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Gabaldón, T.; Consortium OPATHY. Recent Trends in Molecular Diagnostics of Yeast Infections: From PCR to NGS. FEMS Microbiol. Rev. 2019, 43, 517–547. [Google Scholar] [CrossRef]
- World Health Organization. Chapter 6: Data Analysis. In Measles and Rubella Laboratory Network Manual; WHO: Geneva, Switzerland, 2018; Available online: https://www.who.int/docs/default-source/immunization/vpd_surveillance/lab_networks/measles_rubella/manual/chapter-6.pdf (accessed on 11 July 2025).
- Pinhata, J.M.; Cergole-Novella, M.C.; Carmo, A.M.S.; Silva, R.R.F.; Ferrazoli, L.; Sacchi, C.T.; Oliveira, R.S. Rapid Detection of Mycobacterium tuberculosis Complex by Real-Time PCR in Sputum Samples and Its Use in the Routine Diagnosis of a Reference Laboratory. J. Med. Microbiol. 2015, 64, 1040–1045. [Google Scholar] [CrossRef]
- Liu, W.; Lee, L.P. Toward rapid and accurate molecular diagnostics at home. Adv. Mater. 2023, 35, e2206525. [Google Scholar] [CrossRef] [PubMed]
- Kozel, T.R.; Wickes, B. Fungal diagnostics. Cold Spring Harb. Perspect. Med. 2014, 4, a019299. [Google Scholar] [CrossRef] [PubMed]
- Sidiq, F.; Hoostal, M.; Rogers, S.O. Rapid identification of fungi in culture-negative clinical blood and respiratory samples by DNA sequence analyses. BMC Res. Notes 2016, 9, 293. [Google Scholar] [CrossRef]
- Garcia Garces, H.; Hrycyk, M.F.; Giacobino, J.; Capela Machado, G.; Domingos Arantes, T.; Theodoro, R.C.; Bosco, S.M.G.; Bagagli, E. Molecular identification and phylogenetical analysis of dermatophyte fungi from Latin America. Mycoses 2016, 59, 787–797. [Google Scholar] [CrossRef]
- Trabelsi, H.; Neji, S.; Hadrich, I.; Khemakhem, N.; Sellami, H.; Makni, F.; Ayadi, A. Contribution of the internal transcribed spacer regions to the detection and identification of human fungal pathogens. Curr. Res. Transl. Med. 2019, 67, 100–106. [Google Scholar] [CrossRef]
- Wickes, B.L.; Wiederhold, N.P. Molecular diagnostics in medical mycology. Nat. Commun. 2018, 9, 5135. [Google Scholar] [CrossRef]
- Tavares, E.R.; Azevedo, C.S.; Panagio, L.A.; Pelisson, M.; Pinge-Filho, P.; Venancio, E.J.; Barros, T.F.; Yamada-Ogatta, S.F.; Yamauchi, L.M. Accurate and sensitive real-time PCR assays using intergenic spacer 1 region to differentiate Cryptococcus gattii sensu lato and Cryptococcus neoformans sensu lato. Med. Mycol. 2016, 54, 89–96. [Google Scholar]
- Bidaud, A.L.; Chowdhary, A.; Dannaoui, E. Candida auris: An emerging drug resistant yeast—A mini-review. J. Mycol. Med. 2018, 28, 568–573. [Google Scholar] [CrossRef]
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020, 16, e1008921. [Google Scholar] [CrossRef] [PubMed]
- Tsay, S.; Kallen, A.; Jackson, B.R.; Chiller, T.M.; Vallabhaneni, S. Approach to the investigation and management of patients with Candida auris, an emerging multidrug-resistant yeast. Clin. Infect. Dis. 2018, 66, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Granada, M.; Cook, E.; Sherlock, G.; Rosenzweig, F. Microbe profile: Candida glabrata—A master of deception. Microbiology 2024, 170, 001518. [Google Scholar] [CrossRef]
- Dai, Z.; Lan, X.; Cai, M.; Liao, Y.; Zhang, J.; Ye, N.; Lu, X.; Wang, J.; Xiao, Y.; Zhang, Y.; et al. Nineteen years retrospective analysis of epidemiology, antifungal resistance and a nomogram model for 30-day mortality in nosocomial candidemia patients. Front. Cell. Infect. Microbiol. 2025, 15, 1504866. [Google Scholar] [CrossRef]
- Peixoto, P.H.; Silva, M.L.; Portela, F.V.; da Silva, B.; Milanez, E.; de Oliveira, D.; Ribeiro, A.; de Almeida, H.; Lima-Neto, R.; Guedes, G.M.; et al. Clinical, epidemiological and laboratory features of invasive Candida parapsilosis complex infections in a brazilian pediatric reference hospital during the COVID-19 pandemic. J. Fungi 2023, 9, 844. [Google Scholar] [CrossRef]
- Lima, R.; Ribeiro, F.C.; Colombo, A.L.; de Almeida, J.N., Jr. The emerging threat antifungal-resistant Candida tropicalis in humans, animals, and environment. Front. Fungal Biol. 2022, 3, 957021. [Google Scholar] [CrossRef]
- Otaguiri, E.S.; Morguette, A.E.B.; Morey, A.T.; Tavares, E.R.; Kerbauy, G.; de Almeida Torres, R.S.L.; Chaves Júnior, M.; Tognim, M.C.B.; Góes, V.M.; Krieger, M.A.; et al. Development of a melting-curve based multiplex real-time PCR assay for simultaneous detection of Streptococcus agalactiae and genes encoding resistance to macrolides and lincosamides. BMC Pregn. Childbirth 2018, 18, 126. [Google Scholar] [CrossRef]
- Van Bergen, K.J.M.; Stuitje, A.R.; Akkers, R.C.; Vermeer, H.J.; Castel, R.; Mank, T.G. Performance of a novel melting curve-based qPCR assay for malaria parasites in routine clinical practice in non-endemic setting. Malar. J. 2023, 22, 191. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, Y.; He, Z.; Liu, J.; Lan, K.; Hu, Y.; Zhang, C. A melting curve-based multiplex RT-qPCR assay for simultaneous detection of four Human Coronaviruses. Int. J. Mol. Sci. 2016, 17, 1880. [Google Scholar] [CrossRef] [PubMed]
- Tavares, E.R.; de Lima, T.F.; Bartolomeu-Gonçalves, G.; de Castro, I.M.; de Lima, D.G.; Borges, P.H.G.; Nakazato, G.; Kobayashi, R.K.T.; Venancio, E.J.; Tarley, C.R.T.; et al. Development of a melting-curve-based multiplex real-time PCR assay for the simultaneous detection of viruses causing respiratory infection. Microorganisms 2023, 11, 2692. [Google Scholar] [CrossRef] [PubMed]
- Dubey, D.; Rath, S.; Subhadarshini, S.S.; Purohit, G.K.; Tripathy, D.; Panigrahi, R.; Palai, S.; Singhsamanta, D. Exploring rapid molecular methods for diagnosing Candida species infecting humans: A narrative review. Med. Infect. Dis. 2023, 5, 336–346. [Google Scholar] [CrossRef]
- Maffezzoli, P.; Kestler, M.; Burillo, A.; Corcione, S.; De Rosa, F.G.; Muñoz, P.; Bouza, E. Diagnostic and prognostic value of time to positivity in blood cultures. An opinion paper. Rev. Esp. Quimioter. 2025, 38, 8–20. [Google Scholar] [CrossRef]
- Zacharioudakis, I.M.; Zervou, F.N.; Mylonakis, E. T2 Magnetic Resonance Assay: Overview of Available Data and Clinical Implications. J. Fungi 2018, 4, 45. [Google Scholar] [CrossRef]
- Zervou, F.N.; Zacharioudakis, I.M.; Kurpewski, J.; Mylonakis, E. T2 Magnetic Resonance for Fungal Diagnosis. Methods Mol. Biol. 2017, 1508, 305–319. [Google Scholar]
- Thermo Fisher Scientific. Creating Standard Curves with Genomic DNA or Plasmid DNA Templates for Use in Quantitative PCR; Application Note; Thermo Fisher Scientific: Waltham, MA, USA, 2008; Available online: https://assets.thermofisher.com/TFS-Assets/LSG/Application-Notes/cms_042486.pdf (accessed on 10 July 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).