Theaflavins as Electrolyte Additives for Inhibiting Zinc Dendrites and Hydrogen Evolution in Aqueous Zinc-Ion Batteries
Abstract
1. Introduction
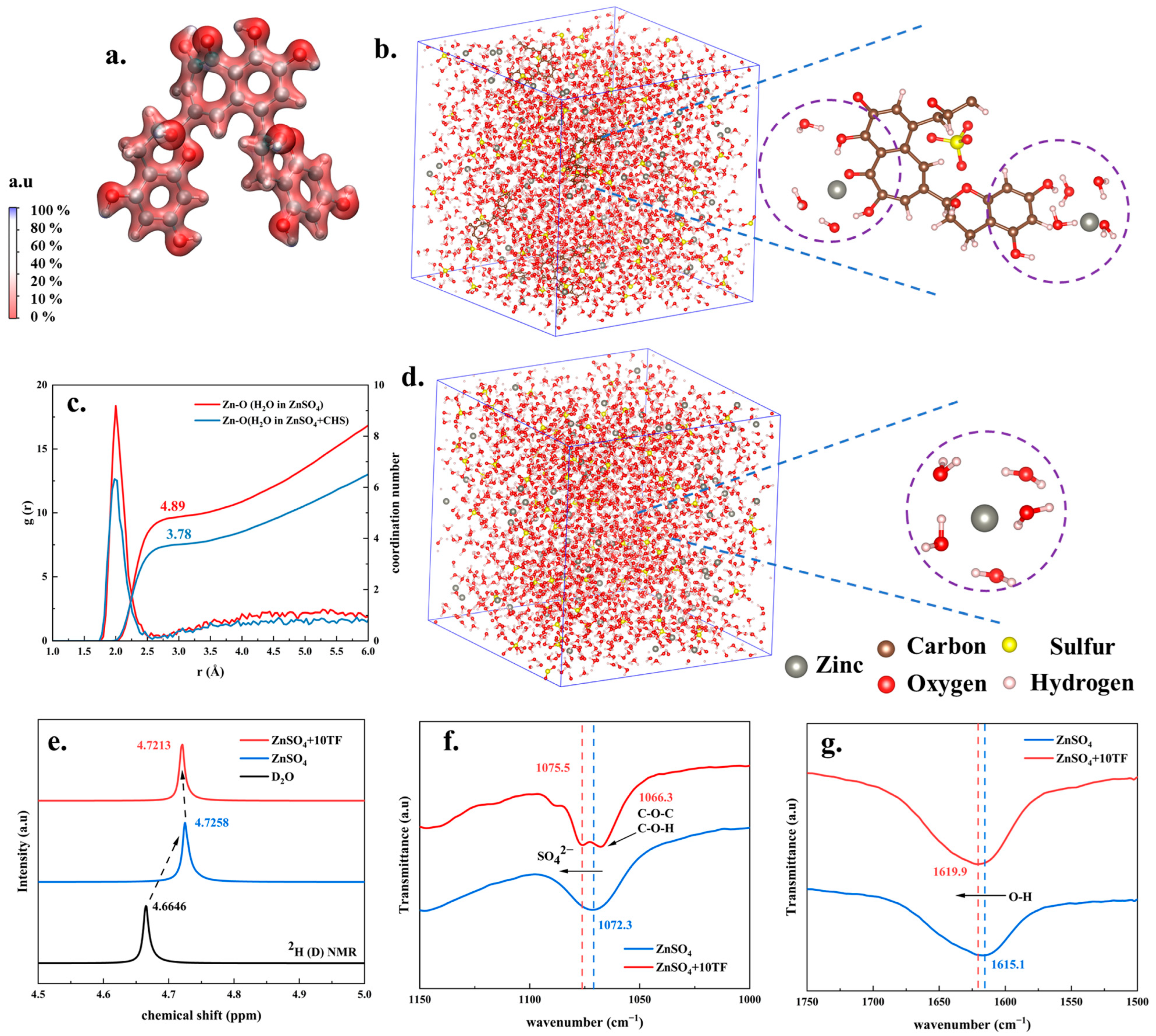
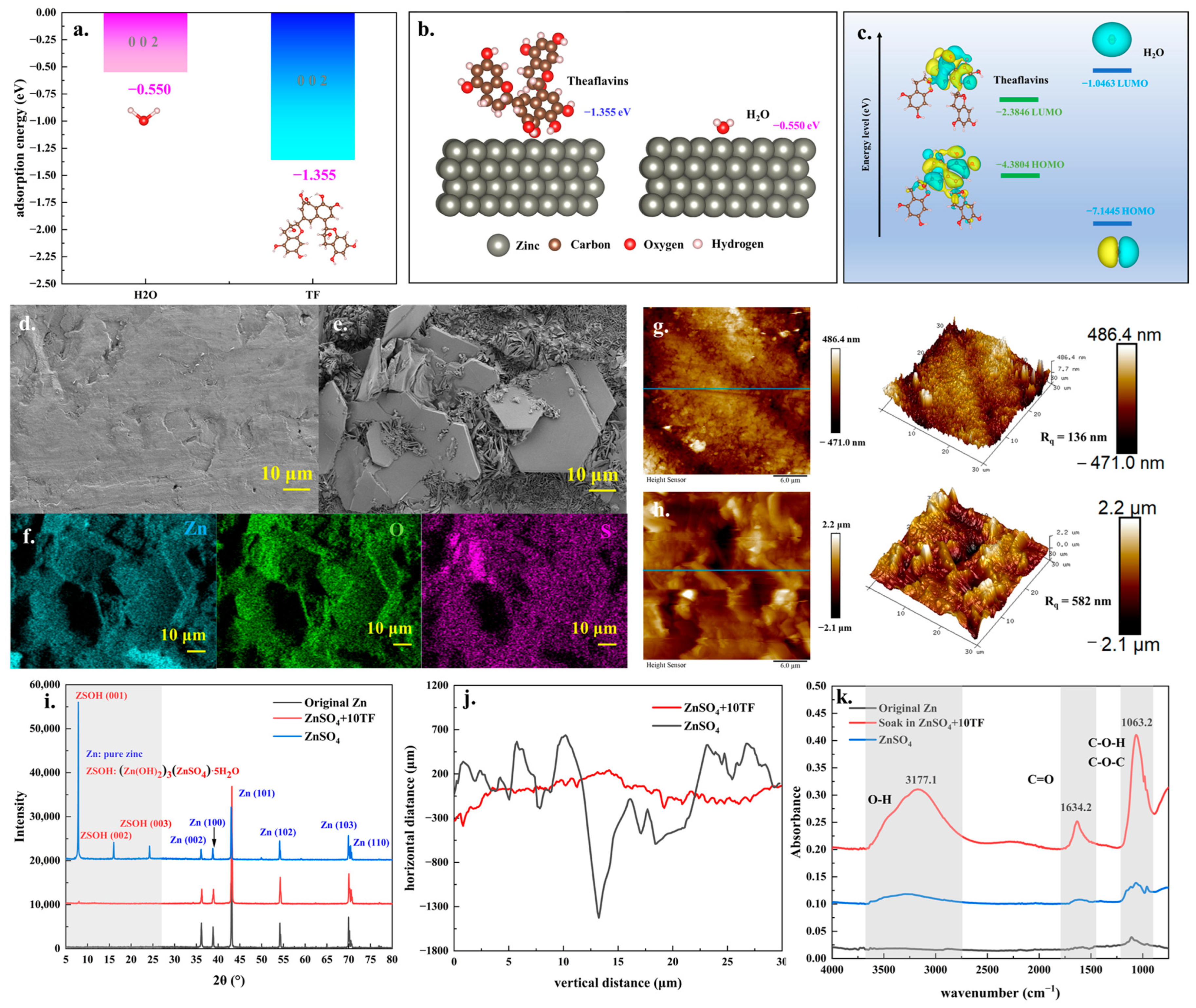
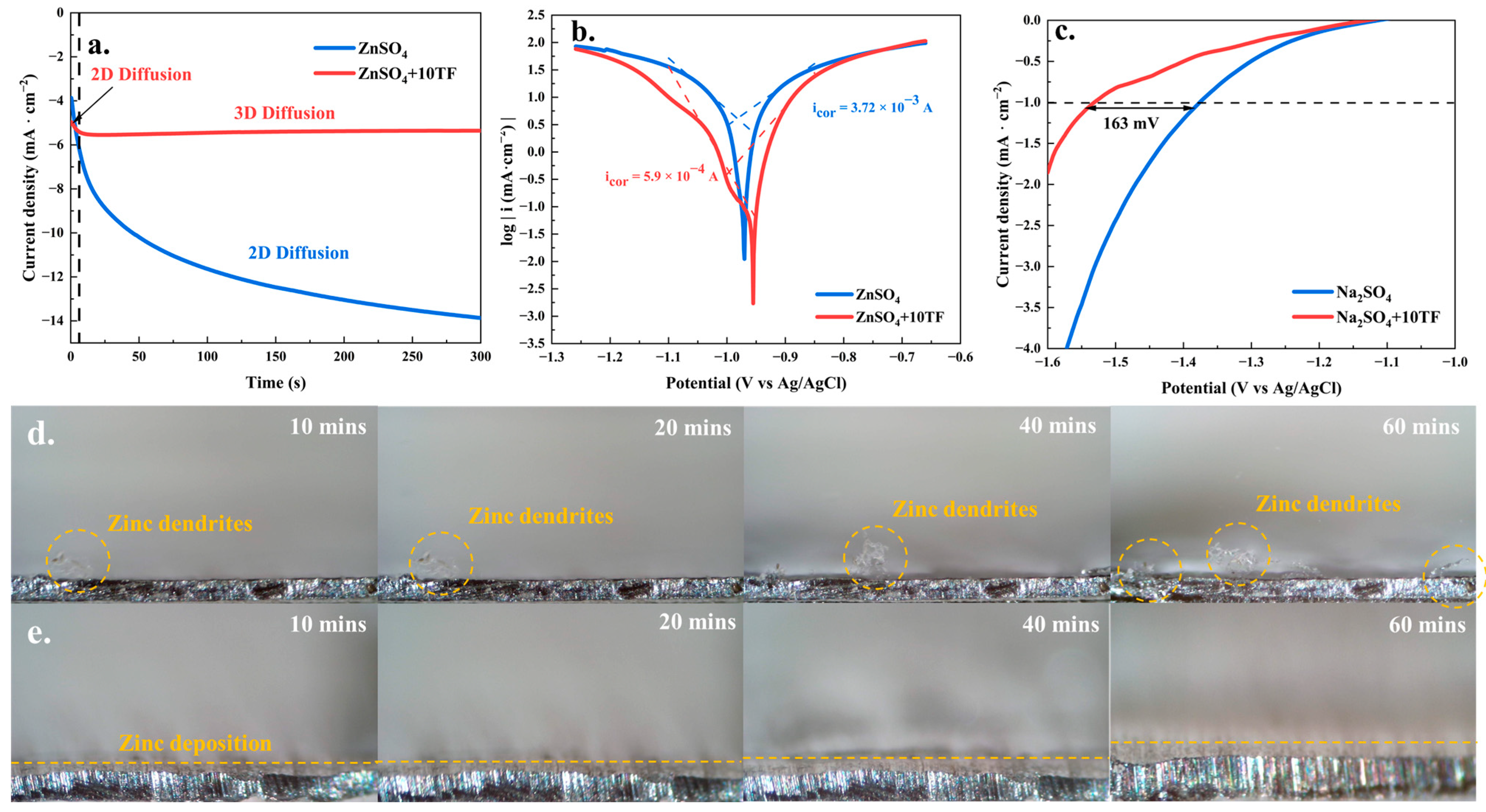
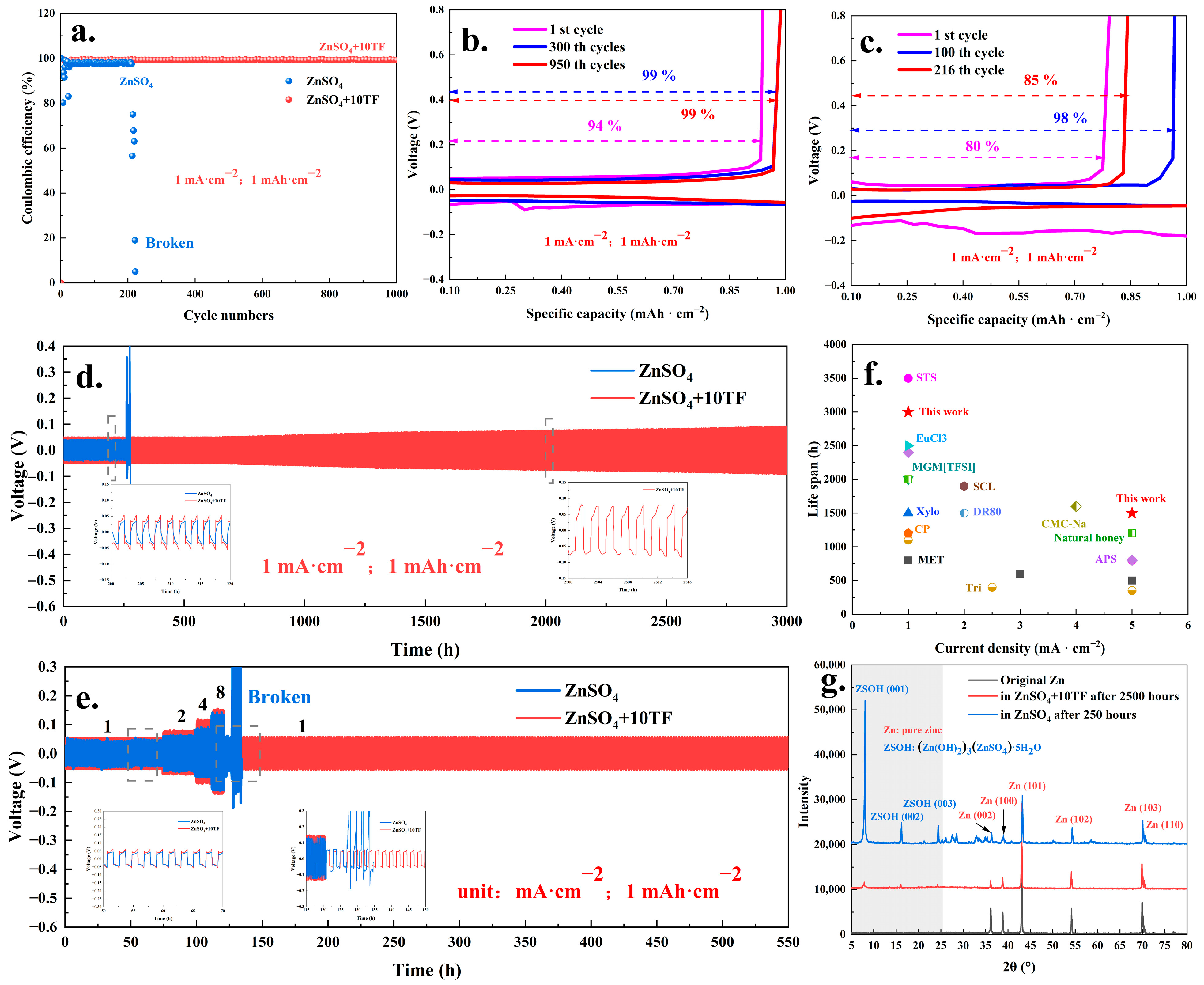
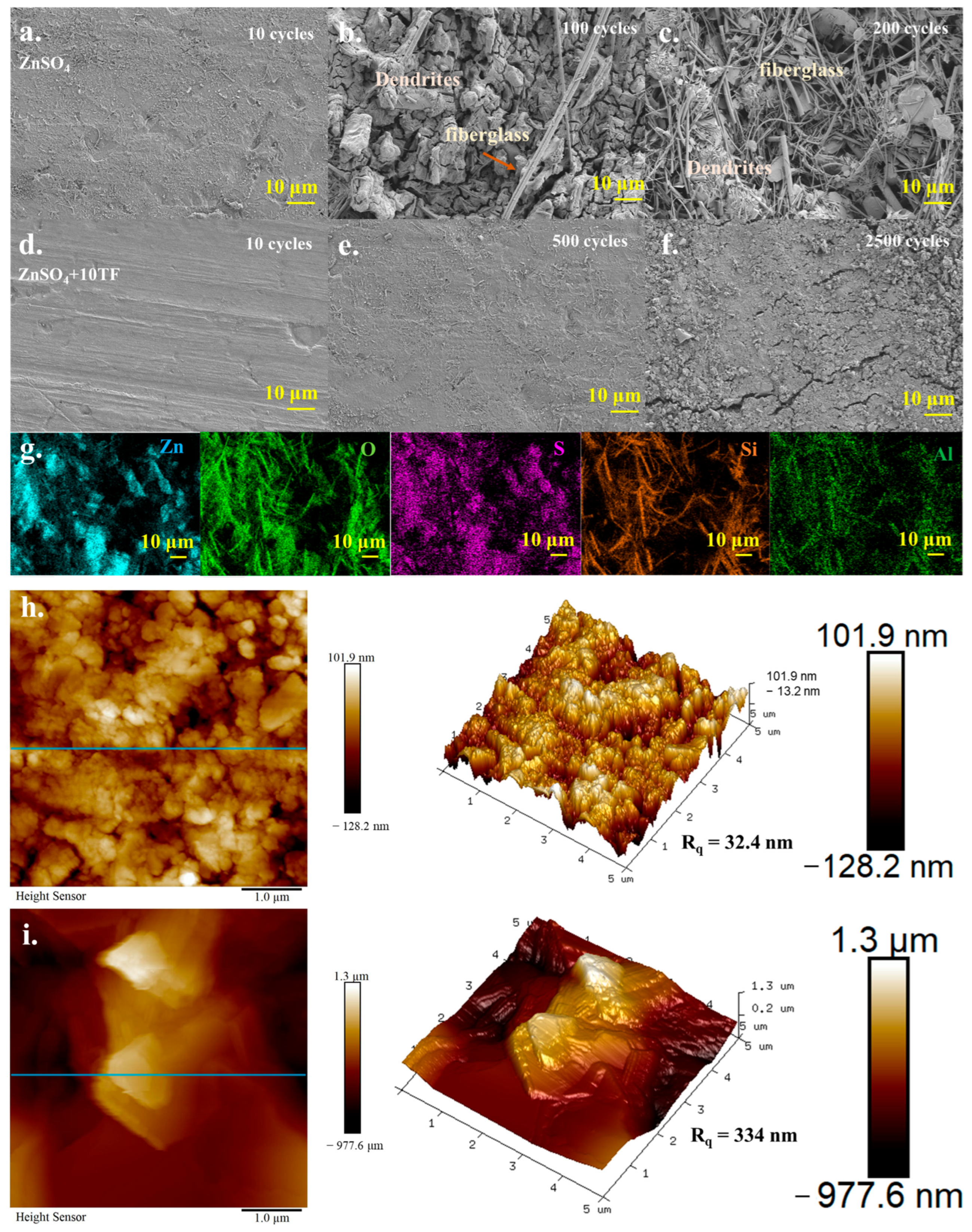
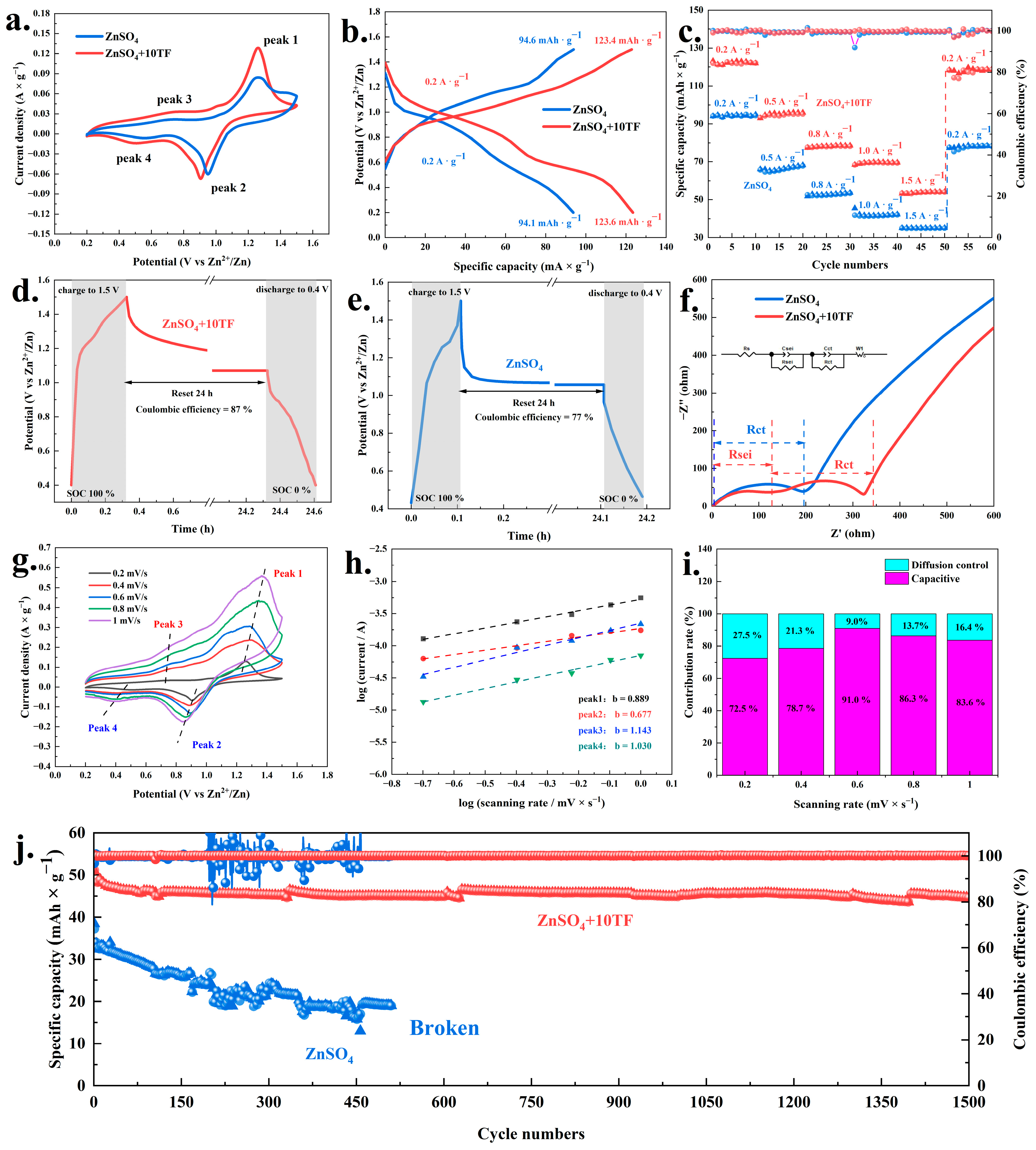
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zou, C.; Ma, F.; Pan, S.; Zhao, Q.; Fu, G.; Zhang, G.; Yang, Y.; Yu, H.; Liang, Y.; Lin, M.; et al. Global energy transition revolution and the connotation and pathway of the green and intelligent energy system. Pet. Explor. Dev. 2023, 50, 722–740. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, H.; Chen, Z.; Wu, Z.; Chen, Y.; Zeng, H.; Huang, J.; Yang, H.; Guo, D. In-situ amorphous transformation of V2O5 in V2O5/V6O13 for aqueous zinc ion batteries with high capacity and long cycles. J. Colloid Interface Sci. 2025, 697, 137999. [Google Scholar] [CrossRef]
- Jiang, F.; Yuan, X.; Hu, L.; Xie, G.; Zhang, Z.; Li, X.; Hu, J.; Wang, C.; Wang, H. A comprehensive review of energy storage technology development and application for pure electric vehicles. J. Energy Storage 2024, 86, 111159. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Mei, B.; Liu, J.; Niu, H.; Hou, Y. Imide Polymers with Bipolar-Type Redox-Active Centers for High-Performance Aqueous Zinc Ion Battery Cathodes and Electrochromic Materials. Int. J. Mol. Sci. 2025, 26, 3838. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Luo, L.; Fan, Y.; Du, Z. A review on thermal management of lithium-ion batteries for electric vehicles. Energy 2022, 238, 121652. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, J.; Liu, Q.; Wang, F.; Liu, X.; Chen, M. The Introduction of a BaTiO3 Polarized Coating as an Interface Modification Strategy for Zinc-Ion Batteries: A Theoretical Study. Int. J. Mol. Sci. 2024, 25, 11172. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wu, B.; Mu, Y.; Zhou, B.; Zhang, G.; Zeng, L. Metal-Organic Framework-Based Materials in Aqueous Zinc-Ion Batteries. Int. J. Mol. Sci. 2023, 24, 6041. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, H.; Zhang, Z.; Wang, Q.; Jin, C.; Wu, C.; Xu, C.; Hao, J.; Sun, L.; Du, Z.; et al. Fire and explosion characteristics of vent gas from lithium-ion batteries after thermal runaway: A comparative study. eTransportation 2022, 13, 100190. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Z.-H.; Yang, Z.-Q.; Yu, Y.-X.; Yang, Y. In situ synthesis of VO2 containing high-valent vanadium via surface oxidation of V2C MXene for robust near-interface reactions in aqueous zinc-ion batteries. Chem. Eng. J. 2025, 511, 162146. [Google Scholar] [CrossRef]
- Li, X.; Wu, S.; Qiu, L.; Xiang, J.; Zeng, P.; Wang, X.; Xiao, D.; Li, J.; Li, P. Engineering water-lean solvation structure to modulate interfacial chemistry for high-performance aqueous zinc-ion battery. J. Colloid Interface Sci. 2025, 686, 960–969. [Google Scholar] [CrossRef]
- Khamsanga, S.; Nguyen, M.T.; Yonezawa, T.; Thamyongkit, P.; Pornprasertsuk, R.; Pattananuwat, P.; Tuantranont, A.; Siwamogsatham, S.; Kheawhom, S. MnO2 Heterostructure on Carbon Nanotubes as Cathode Material for Aqueous Zinc-Ion Batteries. Int. J. Mol. Sci. 2020, 21, 4689. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Lee, J.; Park, Y.; Choi, J.W. Aqueous zinc ion batteries: Focus on zinc metal anodes. Chem. Sci. 2020, 11, 2028–2044. [Google Scholar] [CrossRef]
- Tan, L.; Li, Z.; Wang, L.; Shang, Y.; Wang, Z.; Tong, Z.; Li, Y.; Li, X. Enhancing ion shuttling through hydrogen bonding effect in ZnV2O4 aqueous zinc ion battery cathode. Chem. Eng. J. 2025, 513. [Google Scholar] [CrossRef]
- Innocenti, A.; Bresser, D.; Garche, J.; Passerini, S. A critical discussion of the current availability of lithium and zinc for use in batteries. Nat. Commun. 2024, 15, 1–6. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X. Review of vanadium-based electrode materials for rechargeable aqueous zinc ion batteries. J. Energy Chem. 2021, 56, 223–237. [Google Scholar] [CrossRef]
- Xu, H.; Yang, W.; Li, M.; Liu, H.; Gong, S.; Zhao, F.; Li, C.; Qi, J.; Wang, H.; Peng, W.; et al. Advances in Aqueous Zinc Ion Batteries based on Conversion Mechanism: Challenges, Strategies, and Prospects. Small 2024, 20, e2310972. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Fu, Y.; Dang, H.; Wang, D.; Ran, F. Optimization strategies toward advanced aqueous zinc-ion batteries: From facing key issues to viable solutions. Nano Energy 2023, 116, 108858. [Google Scholar] [CrossRef]
- Zhang, Q.; Luan, J.; Tang, Y.; Ji, X.; Wang, H.-Y. Interfacial Design of Dendrite-Free Zinc Anodes for Aqueous Zinc-Ion Batteries. Angew. Chem. Int. Ed. Engl. 2020, 59, 13180–13191. [Google Scholar] [CrossRef]
- Wen, Q.; Fu, H.; Cui, R.-D.; Chen, H.-Z.; Ji, R.-H.; Tang, L.-B.; Yan, C.; Mao, J.; Dai, K.-H.; Zhang, X.-H.; et al. Recent advances in interfacial modification of zinc anode for aqueous rechargeable zinc ion batteries. J. Energy Chem. 2023, 83, 287–303. [Google Scholar] [CrossRef]
- Khan, Z.; Kumar, D.; Crispin, X. Does Water-in-Salt Electrolyte Subdue Issues of Zn Batteries? Adv. Mater. 2023, 35, e2300369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Huang, S.; Yuan, Z.; Zhu, J.; Zhao, Z.; Niu, Z. Direct Self-Assembly of MXene on Zn Anodes for Dendrite-Free Aqueous Zinc-Ion Batteries. Angew. Chem. Int. Ed. Engl. 2020, 60, 2861–2865. [Google Scholar] [CrossRef]
- Wang, T.; Wang, P.; Pan, L.; He, Z.; Dai, L.; Wang, L.; Liu, S.; Jun, S.C.; Lu, B.; Liang, S.; et al. Stabling Zinc Metal Anode with Polydopamine Regulation through Dual Effects of Fast Desolvation and Ion Confinement. Adv. Energy Mater. 2022, 13, 2203523. [Google Scholar] [CrossRef]
- Zhu, J.; Bie, Z.; Cai, X.; Jiao, Z.; Wang, Z.; Tao, J.; Song, W.; Fan, H.J. A Molecular-Sieve Electrolyte Membrane enables Separator-Free Zinc Batteries with Ultralong Cycle Life. Adv. Mater. 2022, 34, e2207209. [Google Scholar] [CrossRef]
- Lin, Y.; Mai, Z.; Liang, H.; Li, Y.; Yang, G.; Wang, C. Dendrite-free Zn anode enabled by anionic surfactant-induced horizontal growth for highly-stable aqueous Zn-ion pouch cells. Energy Environ. Sci. 2023, 16, 687–697. [Google Scholar] [CrossRef]
- Xing, Z.; Huang, C.; Hu, Z. Advances and strategies in electrolyte regulation for aqueous zinc-based batteries. Co-ord. Chem. Rev. 2022, 452, 214299. [Google Scholar] [CrossRef]
- Li, J.; Long, Y.; Yu, X.; Li, J.; Li, N.; Han, J.; Wang, J.; Yang, Z. Polymeric acid additive strategy for long-lifetime aqueous zinc-ion batteries. Energy Storage Mater. 2025, 76, 104154. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, Y.; Shi, Z.; Zhou, H.; Wang, H.; Wu, F.; Zhao, L.; Zeng, Y.; Kong, B.; Gong, W.; et al. Revealing the multifunctional nature of surfactant electrolyte additive in aqueous Zinc Ion batteries. Electrochimica Acta 2025, 514. [Google Scholar] [CrossRef]
- Qiu, J.; Liu, Z.; Yuan, T.; Bai, S.; Zhang, X.; Chen, J.; Zhang, Y. Novel organic additives with high dipole moments: Improving the anode interface structure to enhance the performance of zinc ion aqueous batteries. J. Colloid Interface Sci. 2024, 683, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Uzunoglu, G.Y.; Yuksel, R. Toward Green and Sustainable Zinc-Ion Batteries: The Potential of Natural Solvent-Based Electrolytes. Small 2025, 21, e2411478. [Google Scholar] [CrossRef]
- Tan, F.; Cai, X.; Yan, W.; Wang, Q.; Zhao, J.; Zhang, W. Natural honey helps stabilize zinc anode for dendrite-free aqueous zinc ion batteries. Energy Storage Mater. 2024, 67. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, J.; Wang, X.; Cheng, J.; Wang, B. Synergistic effects of modulating electrical double layer structure and facilitating uniform deposition of Zn2+ by biocompatible polysaccharide additive for high-performance aqueous zinc ion batteries. Colloids Surf. A Physicochem. Eng. Asp. 2025, 711, 136323. [Google Scholar] [CrossRef]
- Xiong, Y.; Teng, W.; Zhao, Z.; Xu, S.; Ma, Y.; Gong, Y.; Li, D.; Wang, X.; Shen, Y.; Shen, Z.; et al. Effective control of the solution environment in aqueous Zinc-ion batteries for promoting (002)-textured zinc growth by a Bio-electrolyte additive. Energy Storage Mater. 2024, 74, 103959. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, Y.; Guo, B.; Wang, X.; Zhang, Y.; Li, X.; Wang, M.; Lin, Y.; Cao, H. Malic acid additive with a dual regulating mechanism for high-performances aqueous zinc-ion batteries. Chem. Eng. J. 2024, 500, 157431. [Google Scholar] [CrossRef]
- Sun, P.; Ma, L.; Zhou, W.; Qiu, M.; Wang, Z.; Chao, D.; Mai, W. Simultaneous Regulation on Solvation Shell and Electrode Interface for Dendrite-Free Zn Ion Batteries Achieved by a Low-Cost Glucose Additive. Angew. Chem. Int. Ed. Engl. 2021, 60, 18247–18255. [Google Scholar] [CrossRef]
- Wang, R.; Liu, L.; Huang, S.; Wu, Y.; Chen, X.; Liang, Z.; Xu, J. An efficient electrolyte additive of 1,3,6-hexanetricarbonitrile for high performance aqueous zinc-ion batteries. J. Colloid Interface Sci. 2023, 646, 950–958. [Google Scholar] [CrossRef]
- Yin, J.; Liu, H.; Li, P.; Feng, X.; Wang, M.; Huang, C.; Li, M.; Su, Y.; Xiao, B.; Cheng, Y.; et al. Integrated electrolyte regulation strategy: Trace trifunctional tranexamic acid additive for highly reversible Zn metal anode and stable aqueous zinc ion battery. Energy Storage Mater. 2023, 59, 102800. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Orenstein, R.; Lu, X.; Wang, C.; Yanilmaz, M.; Peng, M.; Dong, Y.; Liu, Y.; Zhang, X. Zincophilic and hydrophobic groups of surfactant-type electrolyte additive enabled stable anode/electrolyte interface toward long-lifespan aqueous zinc ion batteries. Energy Storage Mater. 2024, 70, 103500. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Li, Y.; Zhao, C.; Zou, C.; Zhao, Z.; Li, X.; Wang, M.; Lin, Y.; Huang, Y.; et al. A zwitterionic-type multifunctional electrolyte additive of trigonelline hydrochloride stabilizes zinc anodes for advanced aqueous zinc-ion batteries. J. Energy Storage 2024, 97, 112834. [Google Scholar] [CrossRef]
- Li, X.; Xiang, J.; Qiu, L.; Chen, X.; Zhao, Y.; Wang, Y.; Yue, Q.; Gao, T.; Liu, W.; Xiao, D.; et al. Unlocking the stable interface in aqueous zinc-ion battery with multifunctional xylose-based electrolyte additives. J. Energy Chem. 2024, 100, 770–778. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, J.; Yuan, Y.; Ma, C.; Jia, S.; Zhang, X.; Zhang, G.; Zhou, X. A versatile electrolyte additive enabling highly reversible Zn anode in aqueous zinc-ion batteries. J. Energy Storage 2024, 102. [Google Scholar] [CrossRef]
- Tan, H.; Wang, P.; Yuan, G.; Yang, H.; Ye, J.; Lu, K.; Chen, G.; Peng, B.; Zhang, Q. Pyrrole as a multi-functional additive to concurrently stabilize Zn anode and cathode via interphase regulation towards advanced aqueous zinc-ion battery. J. Colloid Interface Sci. 2024, 676, 582–593. [Google Scholar] [CrossRef]
- Ji, H.; Han, Z.; Lin, Y.; Yu, B.; Wu, D.; Zhao, L.; Wang, M.; Chen, J.; Ma, Z.; Guo, B.; et al. Stabilizing zinc anode for high-performance aqueous zinc ion batteries via employing a novel inositol additive. J. Alloy. Compd. 2022, 914, 165231. [Google Scholar] [CrossRef]
- Quan, Y.; Yang, M.; Chen, M.; Zhou, W.; Han, X.; Chen, J.; Liu, B.; Shi, S.; Zhang, P. Electrolyte additive of sorbitol rendering aqueous zinc-ion batteries with dendrite-free behavior and good anti-freezing ability. Chem. Eng. J. 2023, 458, 141392. [Google Scholar] [CrossRef]
- Sun, R.; Luo, D.; Zhou, H.; Zhang, Z.; Gao, Y.; Ma, S.; Li, Z.; Kang, X. Synchronous regulation of V2O5 cathode and Zn anode using sodium gluconate as an additive for long-life aqueous zinc-ion batteries. J. Energy Chem. 2024, 103, 703–713. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, J.; Bi, S.; Wang, R.; Niu, Z. A Binary Hydrate-Melt Electrolyte with Acetate-Oriented Cross-Linking Solvation Shells for Stable Zinc Anodes. Adv. Mater. 2022, 34, e2201744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ying, M.; Wang, Z.; Feng, W.; Lu, X.; Zhou, W.; Dai, J.; Lu, T.; Li, D.; Zhang, K.; et al. Physicochemical characterization of the fraction components of the theabrownin isolates from Pu’er tea. LWT 2024, 200, 116147. [Google Scholar] [CrossRef]
- Yang, T.; Tao, G.; Li, L.; Ma, Q. Study of the interaction mechanism between theaflavin and Zein. J. Food Eng. 2023, 359, 111700. [Google Scholar] [CrossRef]
- Zhang, R.; Cui, Y.; Liu, L.; Chen, S. A dual-functional rare earth halide additive for high-performance aqueous zinc ion batteries. J. Power Sources 2024, 602, 234351. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, Z.; Sun, R.; Ma, J.; Li, Z.; Wang, D.; Kang, X. Environmentally friendly additives for crystal surface modulation and suppression of dendrites for aqueous zinc-ion batteries. J. Energy Storage 2024, 87, 111375. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Wang, X.; Weng, Q.; Sun, W. Bifunctional electrolyte additive ammonium persulfate for high-performance aqueous zinc-ion batteries. Mater. Today Sustain. 2024, 28, 100948. [Google Scholar] [CrossRef]
- Cui, S.; Wang, X.; Miao, W.; Wang, X.; Li, X.; Xun, M.; Sun, K.; Peng, H.; Ma, G. Alleviating zinc dendrite growth by versatile sodium carboxymethyl cellulose electrolyte additive to boost long-life aqueous Zn ion capacitors. Energy Storage Mater. 2024, 68, 103356. [Google Scholar] [CrossRef]
- Yan, Q.; Hu, Z.; Liu, Z.; Wu, F.; Zhao, Y.; Chen, R.; Li, L. Synergistic interaction between amphiphilic ion additive groups for stable long-life zinc ion batteries. Energy Storage Mater. 2024, 67, 103299. [Google Scholar] [CrossRef]
- Bai, S.; Wu, Z.; Zhang, X.; Qiu, J.; Chen, J.; Liu, Z.; Zhang, Y. Bifunctional electrolyte additive enabling long cycle life of vanadium-based cathode aqueous zinc ion batteries. Chem. Eng. J. 2024, 500, 157281. [Google Scholar] [CrossRef]
- Li, S.; Xu, M.; Chen, K.; Wu, Q.; Li, Y.; Xie, C.; Li, Y.; Xu, Q.; Huang, J.; Xie, H. Rational design of epoxy functionalized ionic liquids electrolyte additive for hydrogen-free and dendrite-free aqueous zinc batteries. J. Colloid Interface Sci. 2024, 678, 934–947. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.; Chen, R.; Yang, H.; Zhang, W.; Miao, Y.; Liu, T.; Huang, J.; He, G. Induced Anionic Functional Group Orientation-Assisted Stable Electrode-Electrolyte Interphases for Highly Reversible Zinc Anodes. Adv. Sci. 2024, 11, e2402821. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, W.; Zhu, J. Stabilization of cathode electrolyte interphase for aqueous zinc-ion batteries. J. Energy Chem. 2024, 96, 359–386. [Google Scholar] [CrossRef]
- Pang, X.; Ji, S.; Zhang, P.; Feng, W.; Zhang, L.; Li, K.; Tang, Y.; Liu, Y. Interlayer doping of pseudocapacitive hydrated vanadium oxide via Mn2+ for high-performance aqueous zinc-ion battery. Electrochimica Acta 2022, 441, 141810. [Google Scholar] [CrossRef]
- Xiao, H.; Li, R.; Zhu, L.; Chen, X.; Xie, L.; Han, Q.; Qiu, X.; Yi, L.; Cao, X. Mn-containing heteropolyvanadate nanoparticles as a high-performance cathode material for aqueous zinc-ion batteries. J. Energy Storage 2024, 89, 111640. [Google Scholar] [CrossRef]
- Tan, Y.; Niu, X.; Chen, J. Phase regulation of manganese vanadium oxide and its effects on capacity for aqueous zinc-ion battery. J. Energy Storage 2024, 99, 113230. [Google Scholar] [CrossRef]
- Wang, L.; Lai, Y.; Tian, H.; Wang, J.; Zhao, W.; Wang, Y.; Li, L.; Zhang, L. Cooperative energy storage behaviors derived from PANI and V2CTx MXene for advanced aqueous zinc-ion batteries. J. Alloy. Compd. 2023, 945, 169366. [Google Scholar] [CrossRef]
- Ye, S.; Sheng, S.; Chen, Q.; Meng, L.; Yao, W.; Yao, H.; Wu, Z.; Zhang, F. Layer-by-layer assembled binder-free hydrated vanadium oxide-acetylene black electrode for flexible aqueous zinc ion battery. J. Electroanal. Chem. 2024, 964, 118334. [Google Scholar] [CrossRef]
- Páll, S.; Zhmurov, A.; Bauer, P.; Abraham, M.; Lundborg, M.; Gray, A.; Hess, B.; Lindahl, E. Heterogeneous parallelization and acceleration of molecular dynamics simulations in GROMACS. J. Chem. Phys. 2020, 153, 134110. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T. A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Cheng, T.; Chen, C.; Liu, F.; Wu, F.; Song, L.; Hou, B.; Tian, Y.; Zhao, X.; Ullah, S.; et al. Theaflavins as Electrolyte Additives for Inhibiting Zinc Dendrites and Hydrogen Evolution in Aqueous Zinc-Ion Batteries. Int. J. Mol. Sci. 2025, 26, 9399. https://doi.org/10.3390/ijms26199399
Zhang X, Cheng T, Chen C, Liu F, Wu F, Song L, Hou B, Tian Y, Zhao X, Ullah S, et al. Theaflavins as Electrolyte Additives for Inhibiting Zinc Dendrites and Hydrogen Evolution in Aqueous Zinc-Ion Batteries. International Journal of Molecular Sciences. 2025; 26(19):9399. https://doi.org/10.3390/ijms26199399
Chicago/Turabian StyleZhang, Xiao, Ting Cheng, Chen Chen, Fuqiang Liu, Fei Wu, Li Song, Baoxuan Hou, Yuan Tian, Xin Zhao, Safi Ullah, and et al. 2025. "Theaflavins as Electrolyte Additives for Inhibiting Zinc Dendrites and Hydrogen Evolution in Aqueous Zinc-Ion Batteries" International Journal of Molecular Sciences 26, no. 19: 9399. https://doi.org/10.3390/ijms26199399
APA StyleZhang, X., Cheng, T., Chen, C., Liu, F., Wu, F., Song, L., Hou, B., Tian, Y., Zhao, X., Ullah, S., & Li, R. (2025). Theaflavins as Electrolyte Additives for Inhibiting Zinc Dendrites and Hydrogen Evolution in Aqueous Zinc-Ion Batteries. International Journal of Molecular Sciences, 26(19), 9399. https://doi.org/10.3390/ijms26199399







