Minocycline Treatment Improves Memory and Reduces Anxiety by Lowering Levels of Brain Amyloid Precursor Protein and Indoleamine 2,3-Dioxygenase in a Rat Model of Streptozotocin-Induced Alzheimer’s Disease
Abstract
1. Introduction
2. Results
2.1. Behavioral Activity
2.1.1. Reference Memory Performance in the Probe Test on Day 4 of the Morris Water Maze (MWM) Test
2.1.2. Anxiety Behavior in the Elevated Plus Maze (EPM) Test
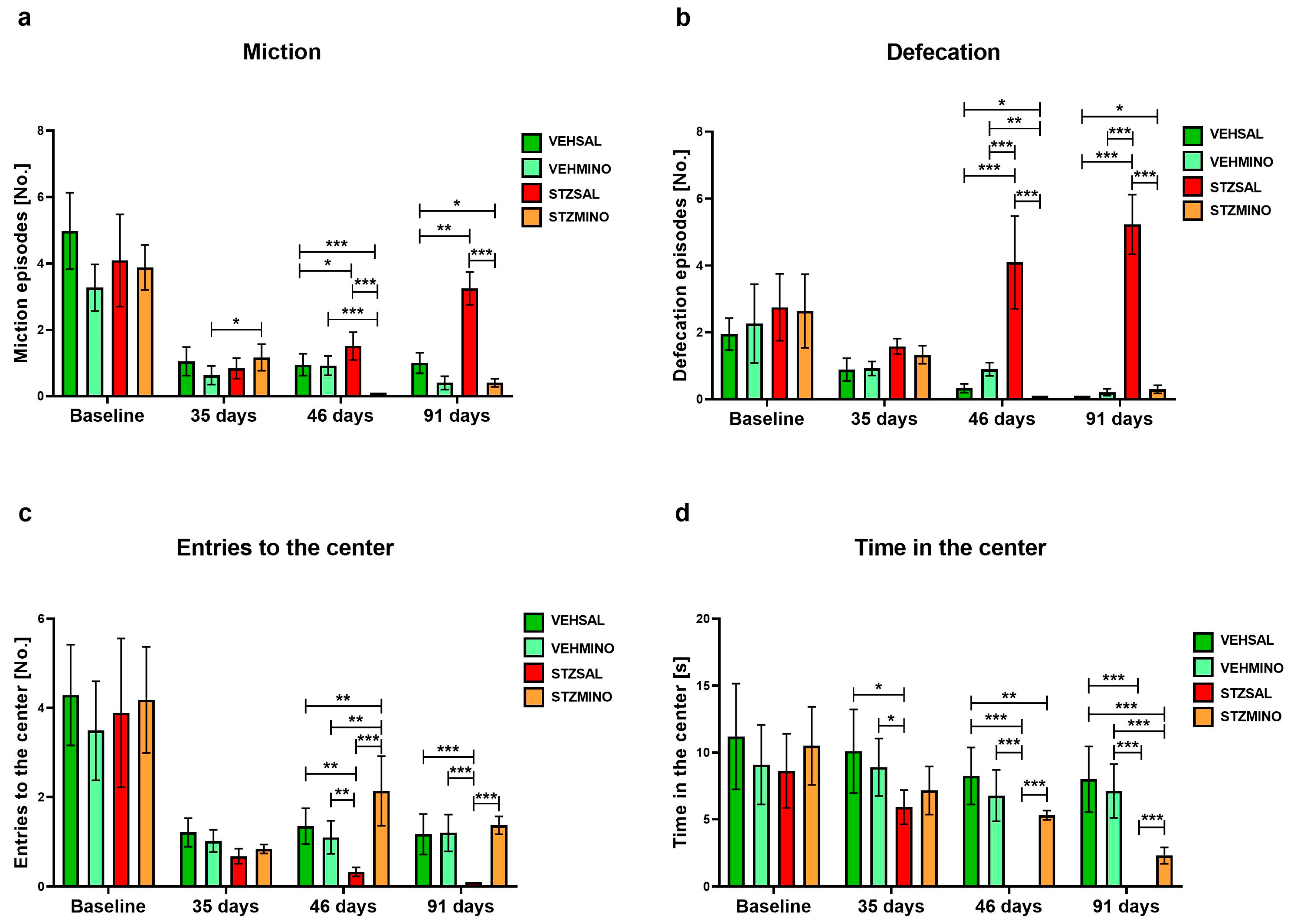
2.1.3. Anxiety Behavior in the White and Light Illuminated Open Field (OF) Test
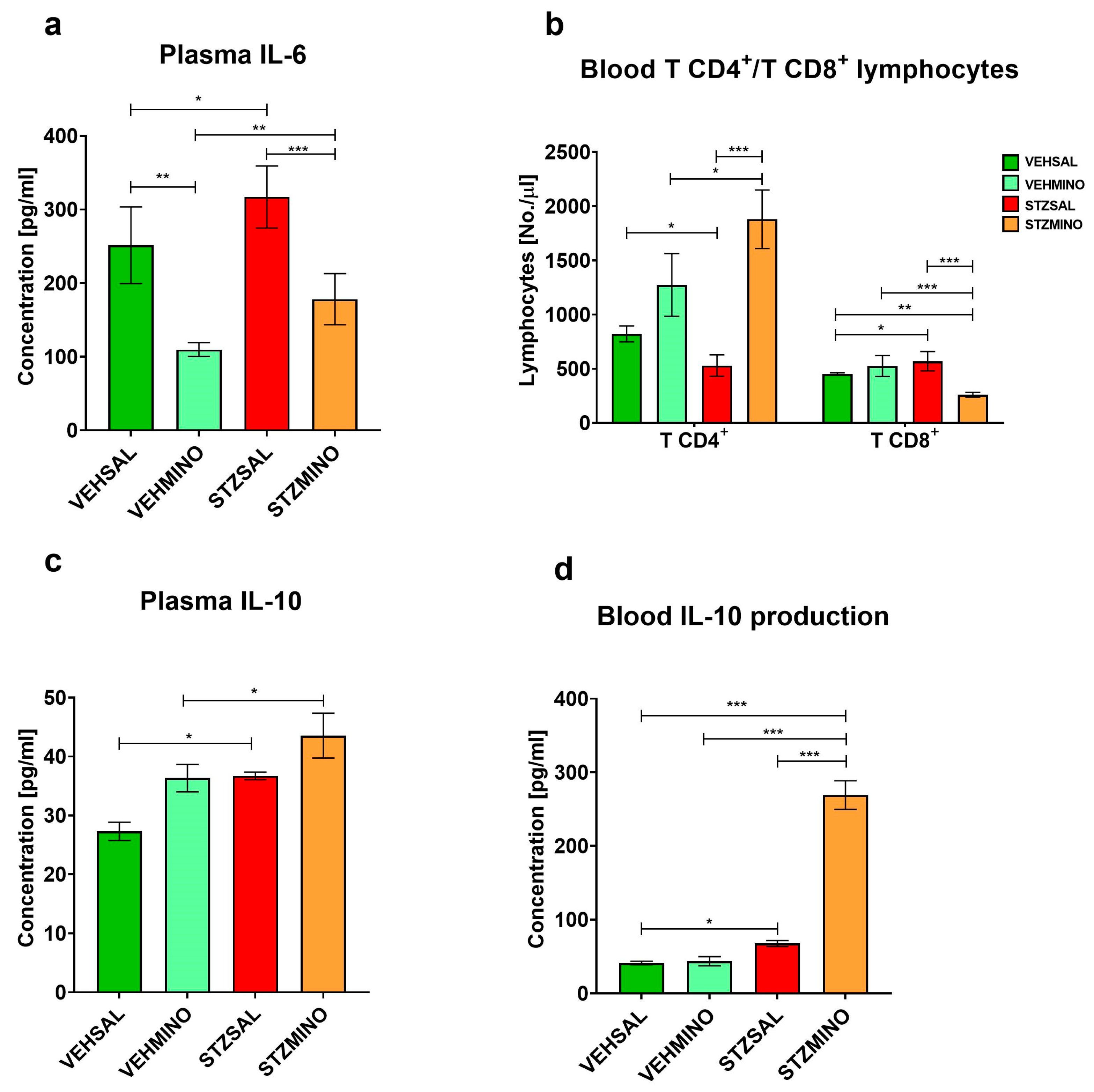
2.2. Plasma Cytokine Concentration and Production, and TCD4+/TCD8+ Lymphocyte Number
2.3. Plasma Corticosterone Concentration
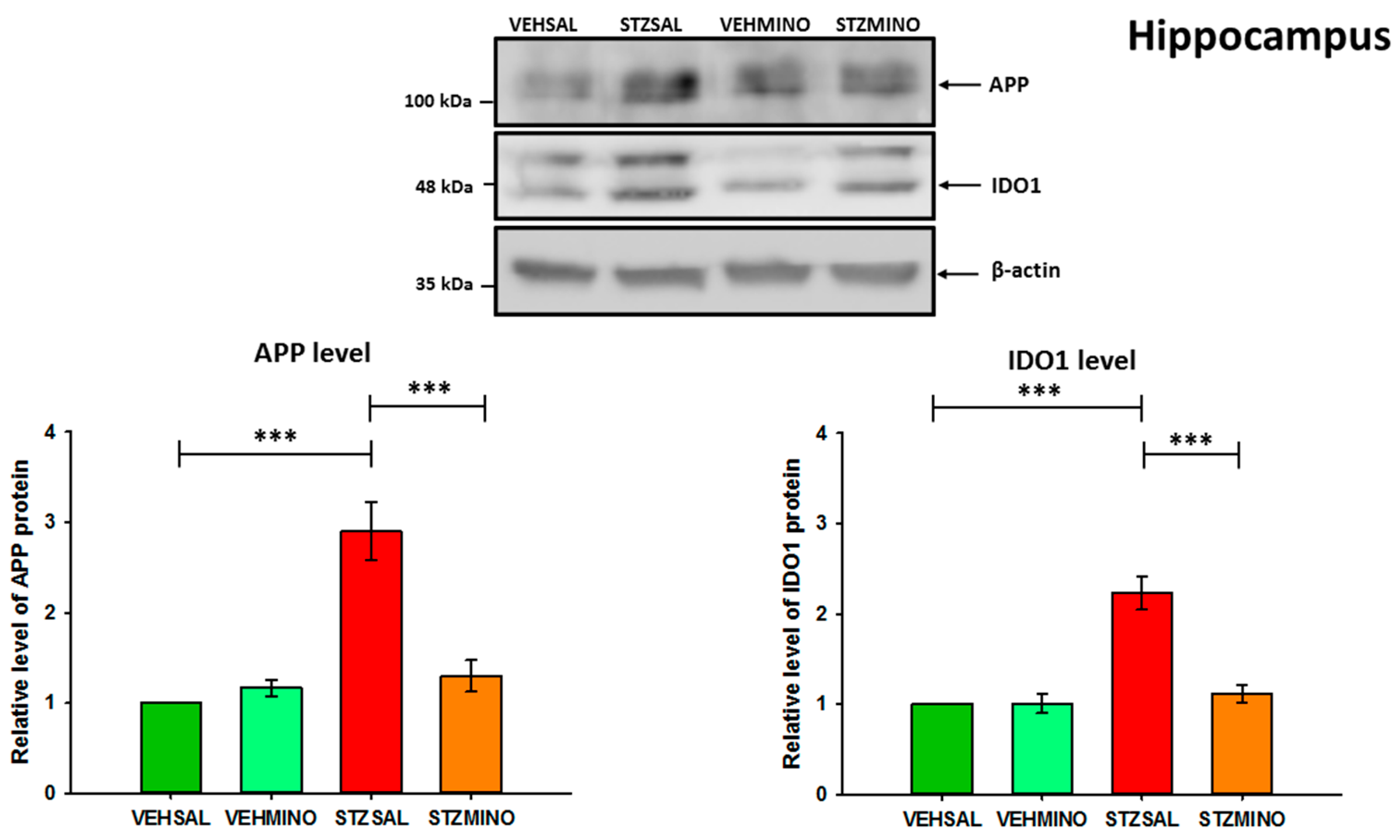
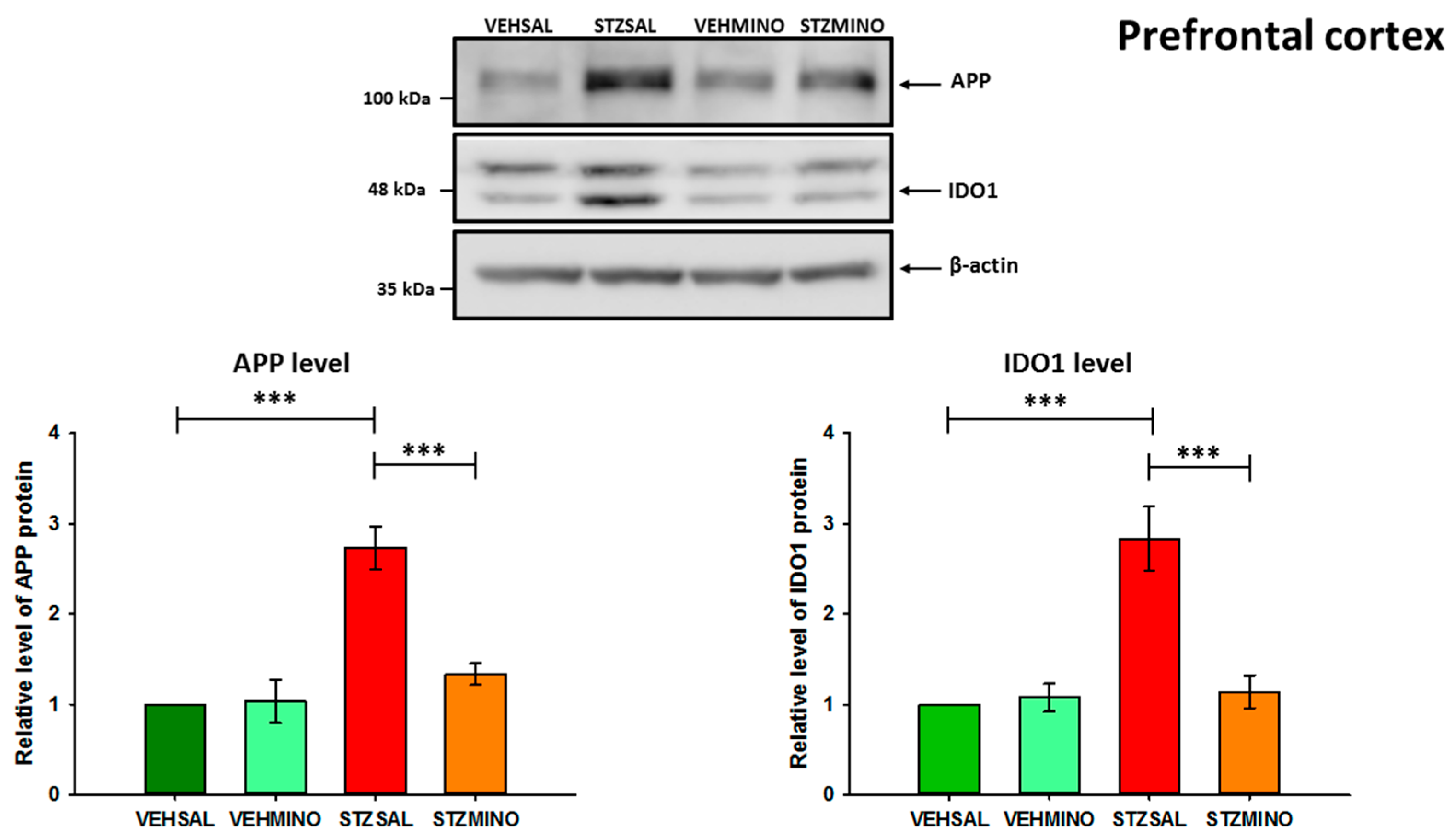
2.4. Amyloid Beta Precursor Protein (APP) and Indoleamine 2,3-Dioxygenase (IDO1) Protein Levels in the Hippocampus and Prefrontal Cortex
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Behavior in the Morris Water Maze (MWM)
4.3. Behavior in the Elevated Plus Maze Test (EPM)
4.4. Behavior in the White and Illuminated Open Field (OF) Test
4.5. Intracerebroventricular (ICV) Injections of Streptozotocin (STZ)—A Model of Sporadic Alzheimer’s Disease (sAD)
4.6. Minocycline (MINO) Treatment
4.7. Measurement of Interleukin (IL)-6, IL-10, and Corticosterone Concentrations in Plasma and TCD4+/TCD8+ Lymphocyte Number in Blood
4.7.1. Determination of Plasma Pro-Inflammatory Interleukin (IL)-6 and Antiinflammatory IL-10 Concentration and Peripheral Blood Mononuclear Cells (PBMC)-Derived Production of IL-6 and IL-10
4.7.2. Flow Cytometry Analysis of T Helper (CD3+CD4+) and T Cytotoxic (CD3+CD8+) Lymphocyte Subpopulations
4.8. Plasma Corticosterone Measurement
4.9. Isolation of Brain Structures
4.10. Reagents and Antibodies for the Measurement of IDO1 and APP Levels in the Brain
4.11. Sample Preparation and Western Blotting
4.12. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savitz, J. The Kynurenine Pathway: A Finger in Every Pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Qin, Z.-S.; Zheng, Y.; Xie, J.-Y.; Liang, S.-S.; Zhang, J.-L.; Feng, Y.-B.; Zhang, Z.-J. Minocycline, a Classic Antibiotic, Exerts Psychotropic Effects by Normalizing Microglial Neuroinflammation-Evoked Tryptophan-Kynurenine Pathway Dysregulation in Chronically Stressed Male Mice. Brain Behav. Immun. 2023, 107, 305–318. [Google Scholar] [CrossRef]
- Tao, X.; Yan, M.; Wang, L.; Zhou, Y.; Wang, Z.; Xia, T.; Liu, X.; Pan, R.; Chang, Q. Homeostasis Imbalance of Microglia and Astrocytes Leads to Alteration in the Metabolites of the Kynurenine Pathway in LPS-Induced Depressive-Like Mice. Int. J. Mol. Sci. 2020, 21, 1460. [Google Scholar] [CrossRef]
- Wang, H.; He, Y.; Sun, Z.; Ren, S.; Liu, M.; Wang, G.; Yang, J. Microglia in depression: An overview of microglia in the pathogenesis and treatment of depression. J. Neuroinflamm. 2022, 19, 132. [Google Scholar] [CrossRef]
- Souza, L.C.; Jesse, C.R.; de Gomes, M.G.; Del Fabbro, L.; Goes, A.T.R.; Donato, F.; Boeira, S.P. Activation of Brain Indoleamine-2,3-dioxygenase Contributes to Depressive-Like Behavior Induced by an Intracerebroventricular Injection of Streptozotocin in Mice. Neurochem. Res. 2017, 42, 2982–2995. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, Z.; Firouzi, M.; Hashemi-Monfared, A.; Zahednasab, H.; Harirchian, M.H. The Effect of Minocycline on Indolamine 2, 3 Dioxygenase Expression and the Levels of Kynurenic Acid and Quinolinic Acid in LPS-Activated Primary Rat Microglia. Cytokine 2018, 107, 125–129. [Google Scholar] [CrossRef]
- Mbongue, J.C.; Nicholas, D.A.; Torrez, T.W.; Kim, N.-S.; Firek, A.F.; Langridge, W.H.R. The Role of Indoleamine 2, 3-Dioxygenase in Immune Suppression and Autoimmunity. Vaccines 2015, 3, 703–729. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Role of the kynurenine metabolism pathway in inflammation-induced depression: Preclinical approaches. Curr. Top. Behav. Neurosci. 2017, 31, 117–138. [Google Scholar]
- Haroon, E.; Welle, J.; Woolwine, B.; Goldsmith, D.; Baer, W.; Patel, T.; Felger, J.; Miller, A. Associations among peripheral and central kynurenine pathway metabolites and inflammation in depression. Neuropsychopharmacology 2020, 45, 998–1007. [Google Scholar] [CrossRef]
- Salminen, A. Role of Indoleamine 2,3-Dioxygenase 1 (IDO1) and Kynurenine Pathway in the Regulation of the Aging Process. Ageing Res. Rev. 2022, 75, 101573. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.W.; Kim, Y.-K. Inflammation-Induced Depression: Its Pathophysiology and Therapeutic Implications. J. Neuroimmunol. 2017, 313, 92–98. [Google Scholar] [CrossRef]
- Höglund, E.; Øverli, Ø.; Winberg, S. Tryptophan Metabolic Pathways and Brain Serotonergic Activity: A Comparative Review. Front. Endocrinol. 2019, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Comai, S.; Bertazzo, A.; Brughera, M.; Crotti, S. Tryptophan in Health and Disease. Adv. Clin. Chem. 2020, 95, 165–218. [Google Scholar] [CrossRef]
- Dursun, E.; Gezen-Ak, D.; Hanağası, H.; Bilgiç, B.; Lohmann, E.; Ertan, S.; Atasoy, İ.L.; Alaylıoğlu, M.; Araz, Ö.S.; Gündüz, A.; et al. The interleukin 1 alpha, interleukin 1 beta, interleukin 6 and alpha-2-macroglobulin serum levels in patients with early or late onset Alzheimer’s disease, mild cognitive impairment or Parkinson’s disease. J. Neuroimmunol. 2015, 283, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.; Kummer, M.; Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014, 1, 463–477. [Google Scholar] [CrossRef]
- Baglietto-Vargas, D.; Shi, J.; Yaeger, D.M.; Ager, R.; LaFerla, F.M. Diabetes and Alzheimer’s Disease Crosstalk. Neurosci. Biobehav. Rev. 2016, 64, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The Amyloid Hypothesis of Alzheimer’s Disease at 25 Years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Lim, Y.-A.; Rhein, V.; Baysang, G.; Meier, F.; Poljak, A.; Raftery, M.J.; Guilhaus, M.; Ittner, L.M.; Eckert, A.; Götz, J. Abeta and Human Amylin Share a Common Toxicity Pathway via Mitochondrial Dysfunction. Proteomics 2010, 10, 1621–1633. [Google Scholar] [CrossRef]
- Moloney, A.M.; Griffin, R.J.; Timmons, S.; O’Connor, R.; Ravid, R.; O’Neill, C. Defects in IGF-1 Receptor, Insulin Receptor and IRS-1/2 in Alzheimer’s Disease Indicate Possible Resistance to IGF-1 and Insulin Signalling. Neurobiol. Aging 2010, 31, 224–243. [Google Scholar] [CrossRef]
- Yang, Y.; Song, W. Molecular Links between Alzheimer’s Disease and Diabetes Mellitus. Neuroscience 2013, 250, 140–150. [Google Scholar] [CrossRef]
- Puzzo, D.; Gulisano, W.; Arancio, O.; Palmeri, A. The keystone of Alzheimer pathogenesis might be sought in Abeta physiology. Neuroscience 2015, 307, 26–36. [Google Scholar] [CrossRef]
- Neth, B.J.; Craft, S. Insulin Resistance and Alzheimer’s Disease: Bioenergetic Linkages. Front. Aging Neurosci. 2017, 9, 345. [Google Scholar] [CrossRef]
- Li, L.; Hölscher, C. Common Pathological Processes in Alzheimer Disease and Type 2 Diabetes: A Review. Brain Res. Rev. 2007, 56, 384–402. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, G.D.; Bild, V.; Ababei, D.C.; Rusu, R.N.; Cobzaru, A.; Paduraru, L.; Bulea, D. Link Between Diabetes and Alzheimer’s Disease Due to the Shared Amyloid Aggregation and Deposition Involving Both Neurodegenerative Changes and Neurovascular Damages. J. Clin. Med. 2020, 9, 1713. [Google Scholar] [CrossRef]
- Hoogmartens, J.; Cacace, R.; Van Broeckhoven, C. Insight into the Genetic Etiology of Alzheimer’s Disease: A Comprehensive Review of the Role of Rare Variants. Alzheimer’s Dement. 2021, 13, e12155. [Google Scholar] [CrossRef] [PubMed]
- Wildsmith, K.R.; Holley, M.; Savage, J.C.; Skerrett, R.; Landreth, G.E. Evidence for Impaired Amyloid β Clearance in Alzheimer’s Disease. Alzheimer’s Res. Ther. 2013, 5, 33. [Google Scholar] [CrossRef]
- Marr, R.; Hafez, D. Amyloid-beta and Alzheimer’s disease: The role of neprilysin-2 in amyloid-beta clearance. Front. Aging Neurosci. 2014, 6, 187. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Scofield, V.L.; Yan, M.; Stoica, G.; Liu, N.; Wong, P.K. Attenuation of oxidative stress, inflammation and apoptosis by minocycline prevents retrovirus-induced neurodegeneration in mice. Brain Res. 2009, 1286, 174–184. [Google Scholar] [CrossRef]
- Budni, J.; Garcez, M.L.; de Medeiros, J.; Cassaro, E.; Bellettini-Santos, T.; Mina, F.; Quevedo, J. The Anti-Inflammatory Role of Minocycline in Alzheimer’s Disease. Curr. Alzheimer Res. 2016, 13, 1319–1329. [Google Scholar] [CrossRef]
- Sharma, V.; Goyal, A.; Subrahmanya, G. Effect of minocycline on oxidative stress induced by Intracerebroventricular Streptozotocin in rats. J. Pharm. Res. 2010, 3, 2198–2200. [Google Scholar]
- Choi, Y.; Kim, H.; Shin, K.; Kim, E.M.; Kim, M.; Kim, H.S.; Park, C.H.; Jeong, Y.H.; Yoo, J.; Lee, J.P.; et al. Minocycline Attenuates Neuronal Cell Death and Improves Cognitive Impairment in Alzheimer’s Disease Models. Neuropsychopharmacology 2007, 32, 2393–2404. [Google Scholar]
- Smith, D.; Woodman, B.; Mahal, A.; Sathasivam, K.; Ghazi-Noori, S.; Lowden, P.A.S.; Bates, G.P.; Hockly, E. Minocycline and Doxycycline are not beneficial in a model of huntington’s disease. Ann. Neurol. 2003, 54, 186–196. [Google Scholar]
- Naderi, Y.; Sabetkasaei, M.; Parvardeh, S.; Zanjani, T. Neuroprotective effects of pretreatment with minocycline on memory impairment following cerebral ischemia in rats. Behav. Pharmacol. 2017, 28, 214–222. [Google Scholar] [CrossRef]
- Parvardeh, S.; Sheikholeslami, M.A.; Ghafghazi, S.; Pouriran, R.; Mortazavi, S.E. Minocycline Improves Memory by Enhancing Hippocampal Synaptic Plasticity and Restoring Antioxidant Enzyme Activity in a Rat Model of Cerebral Ischemia-Reperfusion. Basic Clin. Neurosci. 2022, 13, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fan, Y.; Won, S.; Neumann, M.; Hu, D.; Zhou, L.; Weinstein, P.; Liu, J. Chronic Treatment with Minocycline Preserves Adult New Neuron and Reduces Functional Impairment After Focal Cerebral Ischemia. Stroke 2007, 38, 146–152. [Google Scholar] [CrossRef]
- Panizzutti, B.; Skvarc, D.; Lin, S.; Croce, S.; Alcy Meehan, A.; Bortolasci, C.; Marx, W.; Walker, A.; Hasebe, K.; Bianca, E.; et al. Minocycline as Treatment for Psychiatric and Neurological Conditions: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 5250. [Google Scholar] [CrossRef] [PubMed]
- Salkovic-Petrisic, M.; Hoyer, S. Central insulin resistance as a trigger for sporadic Alzheimer-like pathology: An experimental approach. J. Neural. Transm. Suppl. 2007, 72, 217–233. [Google Scholar] [CrossRef]
- Agrawal, R.; Tyagi, E.; Shukla, R.; Nath, C. Insulin receptor signaling in rat hippocampus: A study in STZ (ICV) induced memory deficit model. Eur. Neuropsychopharmacol. 2011, 21, 261–273. [Google Scholar] [CrossRef]
- Mishra, S.K.; Singh, S.; Shukla, S.; Shukla, R. Intracerebroventricular streptozotocin impairs adult neurogenesis and cognitive functions via regulating neuroinflammation and insulin signaling in adult rats. Neurochem. Int. 2018, 113, 56–68. [Google Scholar] [CrossRef]
- Peng, D.; Pan, X.; Cui, J.; Ren, Y.; Zhang, J. Hyperphosphorylation of tau protein in hippocampus of central insulin-resistant rats is associated with cognitive impairment. Cell. Physiol. Biochem. 2013, 32, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Lester-Coll, N.; Rivera, E.J.; Soscia, S.J.; Doiron, K.; Wands, J.R.; de la Monte, S.M. Intracerebral Streptozotocin Model of Type 3 Diabetes: Relevance to Sporadic Alzheimer’s Disease. J. Alzheimer’s Dis. 2006, 9, 13–33. [Google Scholar] [CrossRef]
- de la Monte, S.M. Contributions of Brain Insulin Resistance and Deficiency in Amyloid-Related Neurodegeneration in Alzheimer’s Disease. Drugs 2012, 72, 49–66. [Google Scholar] [CrossRef]
- Santos, T.O.; Mazucanti, C.H.; Xavier, G.F.; Torrão, A.S. Early and late neurodegeneration and memory disruption after intracerebroventricular streptozotocin. Physiol. Behav. 2012, 107, 401–413. [Google Scholar] [CrossRef]
- Souza, L.C.; Jesse, C.R.; de Gomes, M.G.; Viana, C.E.; Mattos, E.; Silva, N.C.; Boeira, S.P. Intracerebroventricular Administration of Streptozotocin as an Experimental Approach to Depression: Evidence for the Involvement of Proinflammatory Cytokines and Indoleamine-2,3-Dioxygenase. Neurotox. Res. 2017, 31, 464–477. [Google Scholar] [CrossRef]
- Souza, L.C.; Andrade, M.K.; Azevedo, E.M.; Ramos, D.C.; Bail, E.L.; Vital, M. Andrographolide Attenuates Short-Term Spatial and Recognition Memory Impairment and Neuroinflammation Induced by a Streptozotocin Rat Model of Alzheimer’s Disease. Neurotox. Res. 2022, 40, 1440–1454. [Google Scholar] [CrossRef]
- Qin, Y.; Hu, X.; Zhao, H.; Kurban, N.; Chen, X.; Yi, J.; Zhang, Y.; Cui, S.; Zhang, Y. Inhibition of Indoleamine 2,3-Dioxygenase Exerts Antidepressant-like Effects through Distinct Pathways in Prelimbic and Infralimbic Cortices in Rats under Intracerebroventricular Injection with Streptozotocin. Int. J. Mol. Sci. 2024, 25, 7496. [Google Scholar] [CrossRef]
- Belyaev, N.D.; Kellett, K.A.B.; Beckett, C.; Makova, N.Z.; Revett, T.J.; Nalivaeva, N.N.; Hooper, N.M.; Turner, A.J. The Transcriptionally Active Amyloid Precursor Protein (APP) Intracellular Domain Is Preferentially Produced from the 695 Isoform of APP in a β-Secretase-Dependent Pathway. J. Biol. Chem. 2010, 285, 41443–41454. [Google Scholar] [CrossRef]
- Nowakowska-Gołacka, J.; Czapiewska, J.; Sominka, H.; Sowa-Rogozińska, N.; Słomińska-Wojewódzka, M. EDEM1 Regulates Amyloid Precursor Protein (APP) Metabolism and Amyloid-β Production. Int. J. Mol. Sci. 2022, 23, 117. [Google Scholar] [CrossRef]
- Wolf, B.; Posnick, D.; Fisher, J.L.; Lewis, L.D.; Ernstoff, M.S. Indoleamine-2,3-Dioxygenase Enzyme Expression and Activity in Polarized Dendritic Cells. Cytotherapy 2009, 11, 1084–1089. [Google Scholar] [CrossRef]
- Nahomi, R.B.; Sampathkumar, S.; Myers, A.M.; Elghazi, L.; Smith, D.G.; Tang, J.; Lee, C.A.; Kern, T.S.; Nagaraj, R.H.; Fort, P.E. The Absence of Indoleamine 2,3-Dioxygenase Inhibits Retinal Capillary Degeneration in Diabetic Mice. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2042–2053. [Google Scholar] [CrossRef]
- Markulin, I.; Matasin, M.; Turk, V.; Salković-Petrisic, M. Challenges of repurposing tetracyclines for the treatment of Alzheimer’s and Parkinson’s disease. J. Neural Transm. 2022, 129, 773–804. [Google Scholar] [CrossRef]
- Sharma, V.; Goyal, A. Minocycline improves memory in Morris water maze task in inreacerebroventricular streptozotocin infused rats. Int. J. Pharm. Sci. Rev. Res. 2010, 5, 005. [Google Scholar]
- Vicente, M.; Paneghini, V.; Stabile, A.; Amorim, M.; Silva, C.; Patrone, L.; Cunha, T.; Bícego, K.; Almeida, M.; Carrettiero, D.; et al. Inhibition of Pro-Inflammatory Microglia with Minocycline Improves Cognitive and Sleep-Wake Dysfunction Under Respiratory Stress in a Sporadic Model for Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 95, 317–337. [Google Scholar] [CrossRef]
- Mahmoudian, Z.; Ghanbari, A.; Rashidi, I.; Amiri, I.; Komaki, A. Minocycline effects on memory and learning impairment in the beta-amyloid-induced Alzheimer’s disease model in male rats using behavioral, biochemical, and histological methods. Eur. J. Pharmacol. 2023, 953, 175784. [Google Scholar] [CrossRef] [PubMed]
- Garcez, M.; Mina, F.; Bellettini-Santos, T.; Carneiro, F.; Luz, A.; Schiavo, G.; Andrighetti, M.; Scheid, A.; Renan Pereira Bolfe, R.; Budni, J. Minocycline reduces inflammatory parameters in the brain structures and serum and reverses memory impairment caused by the administration of amyloid β (1-42) in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 77, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.A.; Leon, W.C.; Fragoso, G.; Mushynski, W.E.; Almazan, G.; Cuello, A.C. Amyloid beta-induced nerve growth factor dysmetabolism in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2009, 68, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Parachikova, A.; Vasilevko, V.; Cribbs, D.H.; LaFerla, F.M.; Green, K.N. Reductions in amyloid-beta-derived neuroinflammation, with minocycline, restore cognition but do not significantly affect tau hyperphosphorylation. J. Alzheimer’s Dis. 2010, 21, 527–542. [Google Scholar] [CrossRef]
- Biscaro, B.; Lindvall, O.; Tesco, G.; Ekdahl, C.T.; Nitsch, R.M. Inhibition of microglial activation protects hippocampal neurogenesis and improves cognitive deficits in a transgenic mouse model for Alzheimer’s disease. Neurodegener. Dis. 2012, 9, 187–198. [Google Scholar] [CrossRef]
- Seabrook, T.J.; Jiang, L.; Maier, M.; Lemere, C.A. Minocycline affects microglia activation, Abeta deposition, and behavior in APP-tg mice. Glia 2006, 53, 776–782. [Google Scholar] [CrossRef]
- Pierzynowska, K.; Podlacha, M.; Gaffke, L.; Majkutewicz, I.; Mantej, J.; Węgrzyn, A.; Osiadły, M.; Myślińska, D.; Węgrzyn, G. Autophagy-Dependent Mechanism of Genistein-Mediated Elimination of Behavioral and Biochemical Defects in the Rat Model of Sporadic Alzheimer’s Disease. Neuropharmacology 2019, 148, 332–346. [Google Scholar] [CrossRef]
- Andrade, M.; Souza, L.; Azevedo, E.; Bail, E.; Zanata, S.; Andreatini, R.; Vital, M. Melatonin reduces β-amyloid accumulation and improves short-term memory in streptozotocin-induced sporadic Alzheimer’s disease model. IBRO Neurosci. Rep. 2023, 14, 264–272. [Google Scholar] [CrossRef]
- Ferretti, M.; Allard, S.; Partridge, V.; Ducatenzeiler, A.; Cuello, A. Minocycline corrects early, pre-plaque neuroinflammation and inhibits BACE-1 in a transgenic model of Alzheimer’s disease-like amyloid pathology. J. Neuroinflamm. 2012, 9, 62. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Huang, J.; Huang, S.; Bai, Y.; Li, Y.; Huang, W. Efficacy of antidepressant drugs in the treatment of depression in Alzheimer disease patients: A systematic review and network meta-analysis. J. Psychopharmacol. 2021, 35, 901–909. [Google Scholar] [CrossRef]
- Hurley, L.; Tizabi, Y. Neuroinflammation, neurodegeneration, and depression. Neurotox. Res. 2013, 23, 131–144. [Google Scholar] [CrossRef]
- Khajah, M.; Hawai, S. Efect of minocycline, methyl prednisolone, or combination treatment on the colonic bacterial population in a state of colonic infammation using the murine dextran sulfate sodium model. Microb. Cell Factories 2023, 22, 232. [Google Scholar] [CrossRef]
- Suzuki, S.; Saitoh, A.; Ohashi, M.; Yamada, M.; Oka, J.; Yamada, M. The infralimbic and prelimbic medial prefrontal cortices have differential functions in the expression of anxiety-like behaviors in mice. Behav. Brain Res. 2016, 304, 120–124. [Google Scholar] [CrossRef]
- Mozafari, M.; Amiri, S.; Mehr, E.; Momeny, M.; Amini-Khoei, H.; Bijani, S.; Hosseini, M. Minocycline attenuates depressive-like behaviors in mice treated with the low dose of intracerebroventricular streptozotocin; the role of mitochondrial function and neuroinflammation. Mol. Biol. Rep. 2020, 478, 6143–6153. [Google Scholar] [CrossRef]
- Amani, M.; Shokouhi, G.; Salari, A. Minocycline prevents the development of depression-like behavior and hippocampal inflammation in a rat model of Alzheimer’s disease. Psychopharmacology 2019, 236, 1281–1292. [Google Scholar] [CrossRef]
- Kwidzinski, E.; Bechmann, I. IDO expression in the brain: A double-edged sword. J. Mol. Med. 2007, 85, 1351–1359. [Google Scholar] [CrossRef]
- Listowska, M.; Glac, W.; Grembecka, B.; Grzybowska, M.; Wrona, D. Change in blood CD4+T and CD8+T lymphocytes in stressed rats pretreated chronically with desipramine are more pronounced after chronic open field stress challenge. J. Neuroimmunol. 2015, 282, 54–62. [Google Scholar] [CrossRef]
- Wrona, D.; Listowska, M.; Kubera, M.; Majkutewicz, I.; Glac, W.; Wojtyła-Kuchta, B.; Plucińska, K.; Grembecka, B.; Podlacha, M. Chronic antidepressant desipramine treatment increases open field-induced brain expression and spleen production of interleukin 10 in rats. Brain Res. Bull. 2013, 99, 117–131. [Google Scholar] [CrossRef]
- Wrona, D.; Listowska, M.; Kubera, M.; Glac, W.; Grembecka, B.; Plucińska, K.; Majkutewicz, I.; Podlacha, M. Effects of chronic desipramine pretreatment on open field-induced suppression of blood natural killer cell activity and cytokine response depend on the rat’s behavioral characteristics. J. Neuroimmunol. 2014, 268, 13–24. [Google Scholar] [CrossRef]
- Pietrzak, R.H.; Laws, S.M.; Lim, Y.Y.; Bender, S.J.; Porter, T.; Doecke, J.; Ames, D.; Fowler, C.; Masters, C.L.; Milicic, L.; et al. Plasma Cortisol, Brain Amyloid-β, and Cognitive Decline in Preclinical Alzheimer’s Disease: A 6-Year Prospective Cohort Study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2017, 2, 45–52. [Google Scholar] [CrossRef]
- Gómez-Gallego, M.; Gómez-García, J. Stress and verbal memory in patients with Alzheimer’s disease: Different role of cortisol and anxiety. Aging Ment. Health 2019, 23, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Brureau, A.; Zussy, C.; Delair, B.; Ogier, C.; Ixart, G.; Maurice, T.; Givalois, L. Deregulation of hypothalamic-pituitary-adrenal axis functions in an Alzheimer’s disease rat model. Neurobiol. Aging 2013, 5, 1426–1439. [Google Scholar] [CrossRef]
- Mendez, M.F. The relationship between anxiety and Alzheimer’s disease. J. Alzheimer’s Dis. Rep. 2021, 5, 171–177, Erratum in J. Alzheimer’s Dis. Rep. 2021, 5, 563. [Google Scholar] [CrossRef]
- Good, M.; Bannerman, D. Hippocampal Synaptic Plasticity: Integrating Memory and Anxiety Impairments in the Early Stages of Alzheimer’s Disease. Curr. Top. Behav. Neurosci. 2025, 69, 27–48. [Google Scholar] [CrossRef]
- Lopez, D.; White, Z.; Hall, S. Anxiety in Alzheimer’s disease rats is independent of memory and impacted by genotype, age, sex, and exercise. Alzheimer’s Dement. 2024, 20, 3543–3550. [Google Scholar] [CrossRef]
- Likhtik, E.; Stujenske, J.M.; Topiwala, M.A.; Harris, A.Z.; Gordon, J.A. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat. Neurosci. 2014, 17, 106–113. [Google Scholar] [CrossRef]
- Robinson, O.J.; Krimsky, M.; Lieberman, L.; Allen, P.; Vytal, K.; Grillon, C. Towards a mechanistic understanding of pathological anxiety: The dorsal medial prefrontal-amygdala ‘aversive amplification’ circuit in unmedicated generalized and social anxiety disorders. Lancet Psychiatry 2014, 1, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Z.; Zhang, W.H.; Zheng, Z.H.; Zou, J.X.; Liu, X.X.; Huang, S.H.; You, W.J.; He, Y.; Zhang, J.Y.; Wang, X.D.; et al. Identification of a prefrontal cortex-to-amygdala pathway for chronic stress-induced anxiety. Nat. Commun. 2020, 11, 2221. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Xu, Q.; Liu, J.; Guo, S.; Borgland, S.L.; Liu, S. Corticosterone Attenuates Reward-Seeking Behavior and Increases Anxiety via D2 Receptor Signaling in Ventral Tegmental Area Dopamine Neurons. J. Neurosci. 2021, 41, 1566–1581. [Google Scholar] [CrossRef]
- Russo, S.; Nestler, E. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013, 14, 609–625, Erratum in Nat. Rev. Neurosci. 2013, 14, 736. [Google Scholar] [CrossRef]
- Bananej, M.; Karimi-Sori, A.; Zarrindast, M.R.; Ahmadi, S. D1 and D2 dopaminergic systems in the rat basolateral amygdala are involved in anxiogenic-like effects induced by histamine. J. Psychopharmacol. 2012, 26, 564–574. [Google Scholar] [CrossRef]
- Wall, P.M.; Blanchard, R.J.; Markham, C.; Yang, M.; Blanchard, D.C. Infralimbic D1 receptor agonist effects on spontaneous novelty exploration and anxiety-like defensive responding in CD-1 mice. Behav. Brain Res. 2004, 152, 67–79. [Google Scholar] [CrossRef]
- Nasehi, M.; Mafi, F.; Oryan, S.; Nasri, S.; Zarrindast, M.R. The effects of dopaminergic drugs in the dorsal hippocampus of mice in the nicotine induced anxiogenic-like response. Pharmacol. Biochem. Behav. 2011, 98, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Rom, S.; Heldt, N.; Gajghate, S.; Seliga, A.; Reichenbach, N.; Persidsky, Y. Hyperglycemia and advanced glycation end products disrupt BBB and promote occludin and claudin-5 protein secretion on extracellular microvesicles. Sci. Rep. 2020, 10, 7274, Erratum in Sci. Rep. 2020, 10, 18828. [Google Scholar]
- Rom, S.; Zuluaga-Ramirez, V.; Gajghate, S.; Seliga, A.; Winfeld, M.; Heldt, N.; Kolpakov, M.A.; Bashkirova, Y.V.; Sabri, A.K.; Persidsky, Y. Hyperglycemia-driven neuroinfammation compromises BBB leading to memory loss in both diabetes mellitus (DM) type 1 and type 2 mouse models. Mol. Neurobiol. 2019, 56, 1883–1896. [Google Scholar] [CrossRef]
- Brown, R.; Tozer, D.; Loubière, L.; Hong, Y.; Hong, Y.; Fryer, T.; Williams, G.; Graves, M.; Aigbirhio, F.; O’Brien, J.; et al. Minocycline to reduce inflammation and blood brain barrier leakage in small vessel disease (MINERVA) trial study protocol. Eur. Stroke J. 2022, 7, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Mao, X.; Chunhui Tian, C.; Yang, Y.; Yang, K.; Canfield, S.; Zhu, D.; Gu, M.; Guo, F. Engineering blood-brain barrier microphysiological systems to model Alzheimer’s disease monocyte penetration and infiltration. Biomater. Sci. 2025, 25, 3650–3661. [Google Scholar] [CrossRef]
- Wrona, D.; Majkutewicz, I.; Świątek, G.; Dunacka, J.; Grembecka, B.; Glac, W. Dimethyl Fumarate as the Peripheral Blood Inflammatory Mediators Inhibitor in Prevention of Streptozotocin-Induced Neuroinflammation in Aged Rats. J. Inflamm. Res. 2022, 15, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Dunacka, J.; Świątek, G.; Wrona, D. High Behavioral Reactivity to Novelty as a Susceptibility Factor for Memory and Anxiety Disorders in Streptozotocin-Induced Neuroinflammation as a Rat Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 11562. [Google Scholar] [CrossRef]
- Dunacka, J.; Grembecka, B.; Wrona, D. Central Insulin-Like Growth Factor-1-Induced Anxiolytic and Antidepressant Effects in a Rat Model of Sporadic Alzheimer’s Disease Are Associated with the Peripheral Suppression of Inflammation. Cells 2025, 14, 1189. [Google Scholar] [CrossRef]
- Dunacka, J.; Grembecka, B.; Majkutewicz, I.; Wrona, D. Central insulin-like growth factor-1 treatment enhances working and reference memory by reducing neuroinflammation and amyloid beta deposition in a rat model of sporadic Alzeimer’s disease. Pharmaceuticals 2025, 18, 527. [Google Scholar] [CrossRef]
- Podlacha, M.; Glac, W.; Listowska, M.; Grembecka, B.; Majkutewicz, I.; Myślińska, D.; Plucińska, K.; Jerzemowska, G.; Grzybowska, M.; Wrona, D. Medial septal NMDA glutamate receptors are involved in modulation of blood natural killer cell activity in rats. J. Neuroimmune Pharmacol. 2016, 11, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Wrona, D.; Jurkowski, M.; Tokarski, J. Blood and spleen natural killer cell cytotoxicity after exposure to open field stress in rats: The effect of spontaneous locomotor activity. J. Neuroimmunol. 2004, 150, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: San Diego, CA, USA, 2007; ISBN 978-0-0804-7515-8. [Google Scholar]
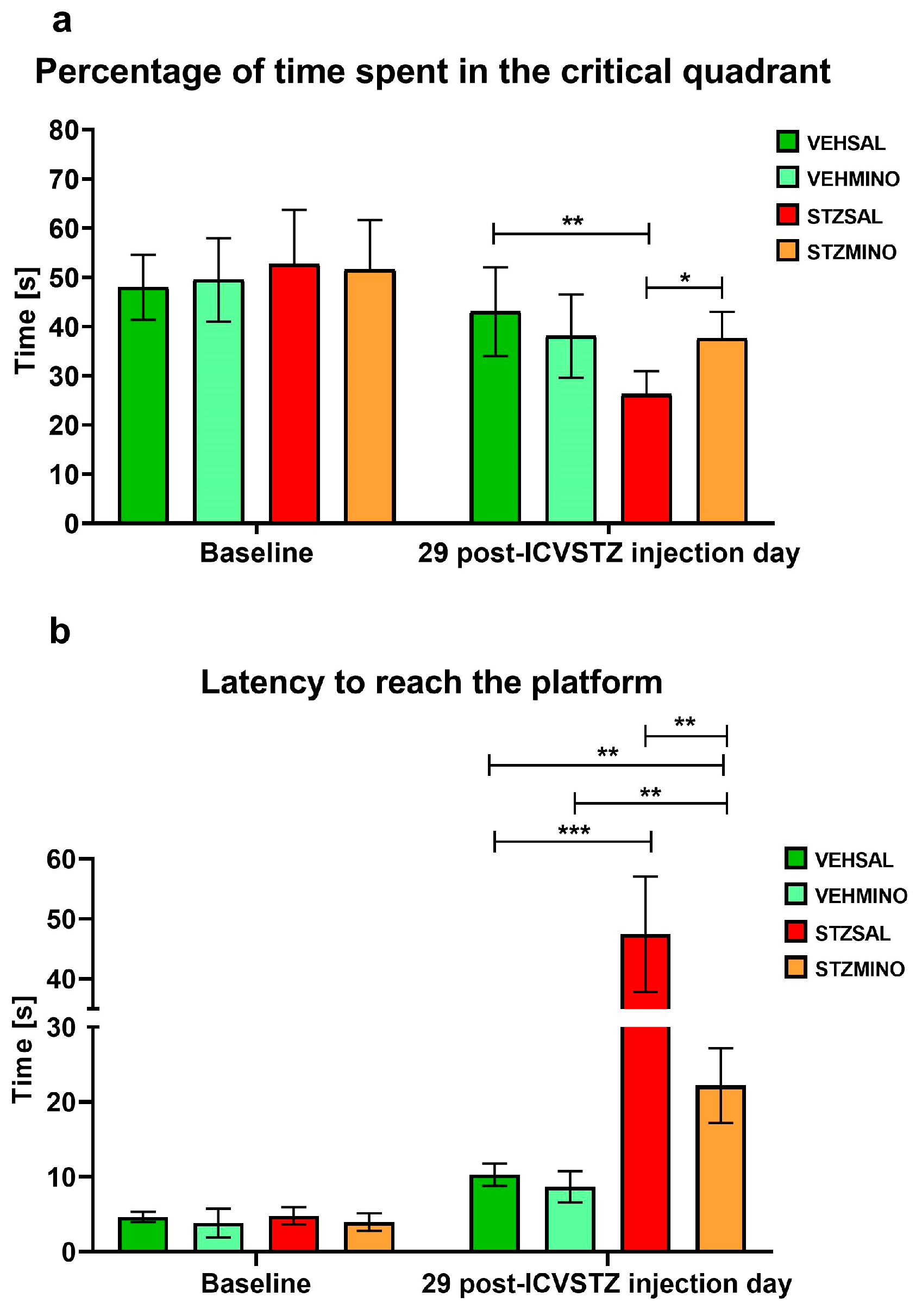
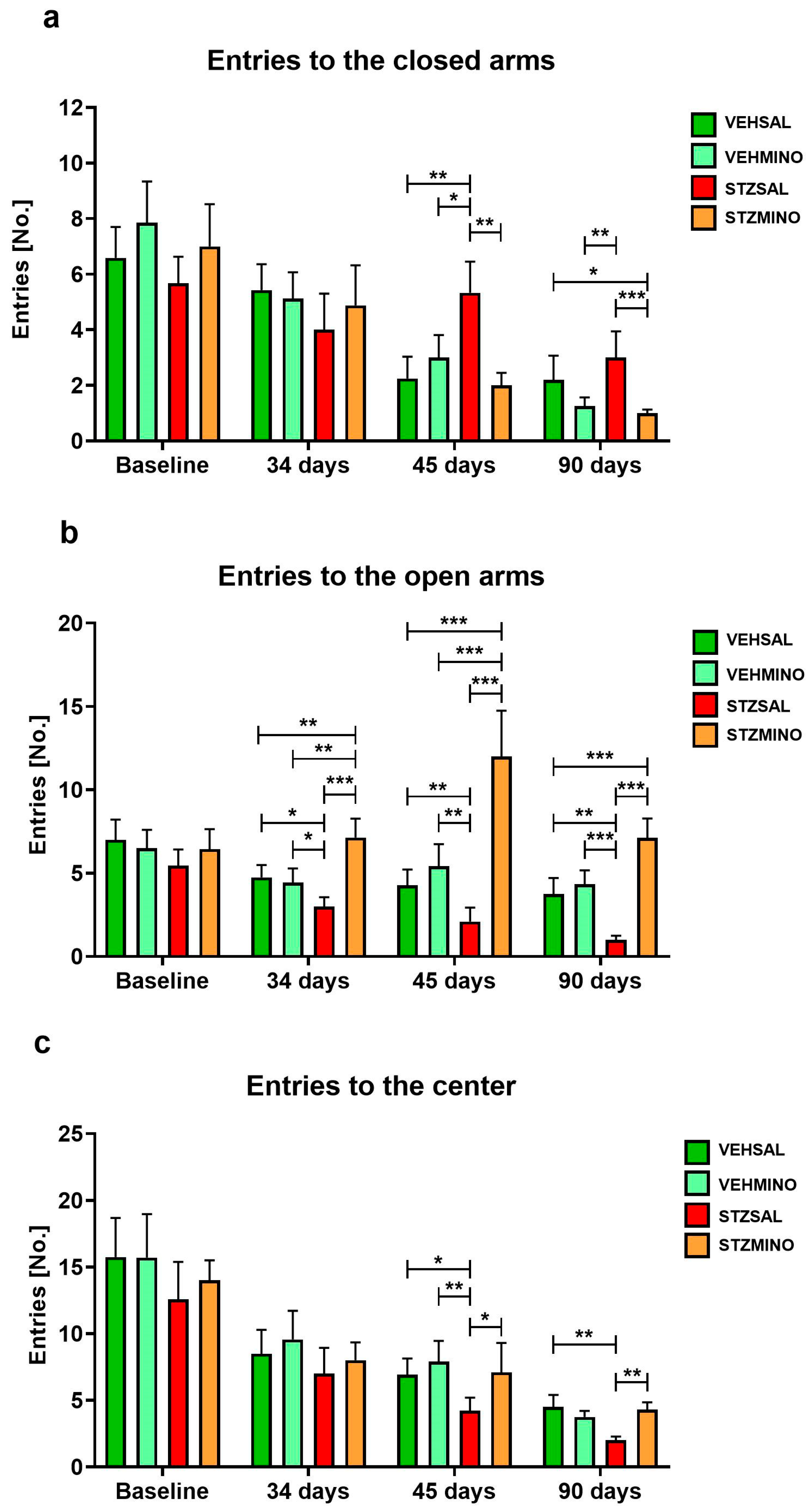
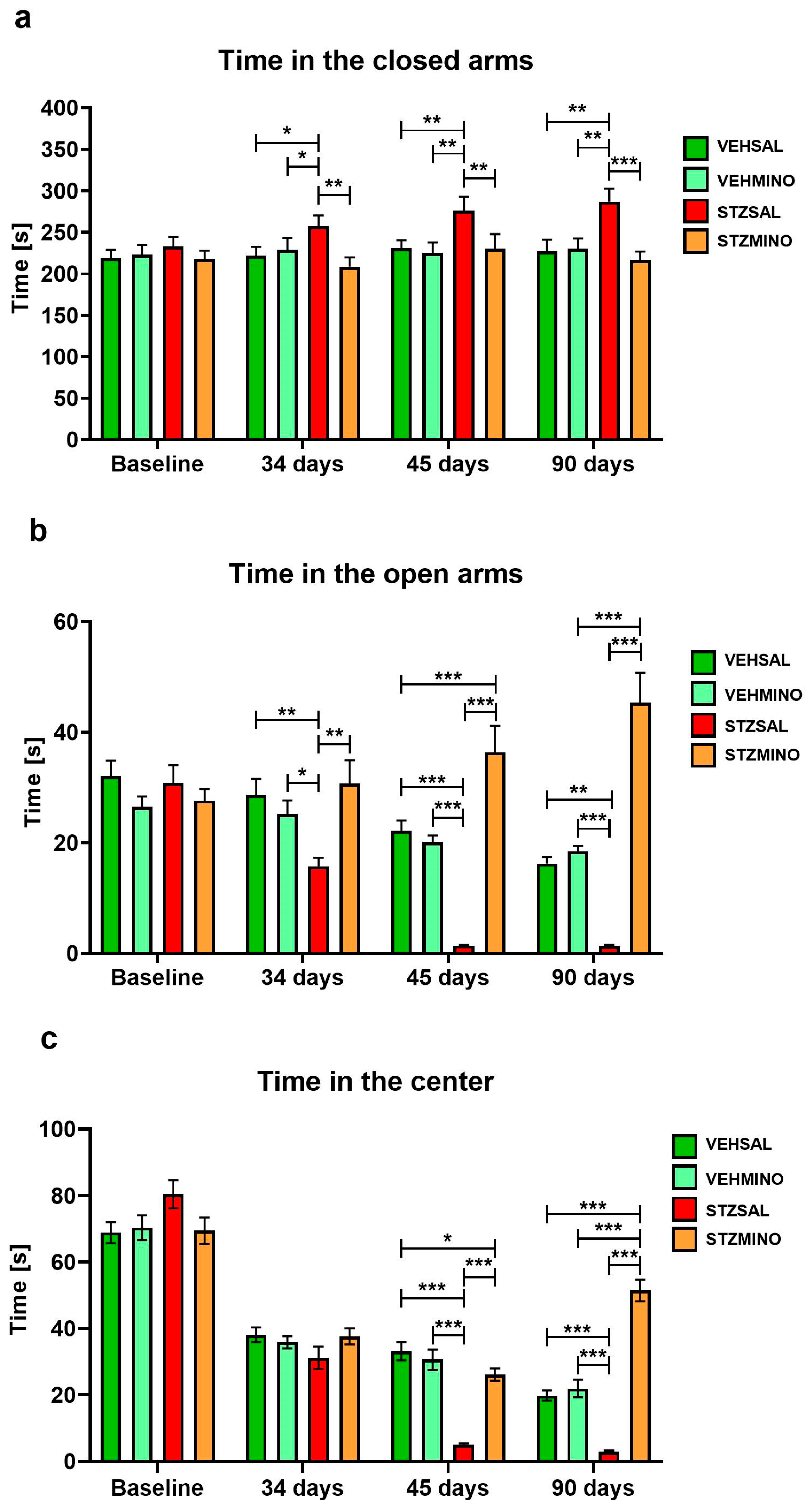
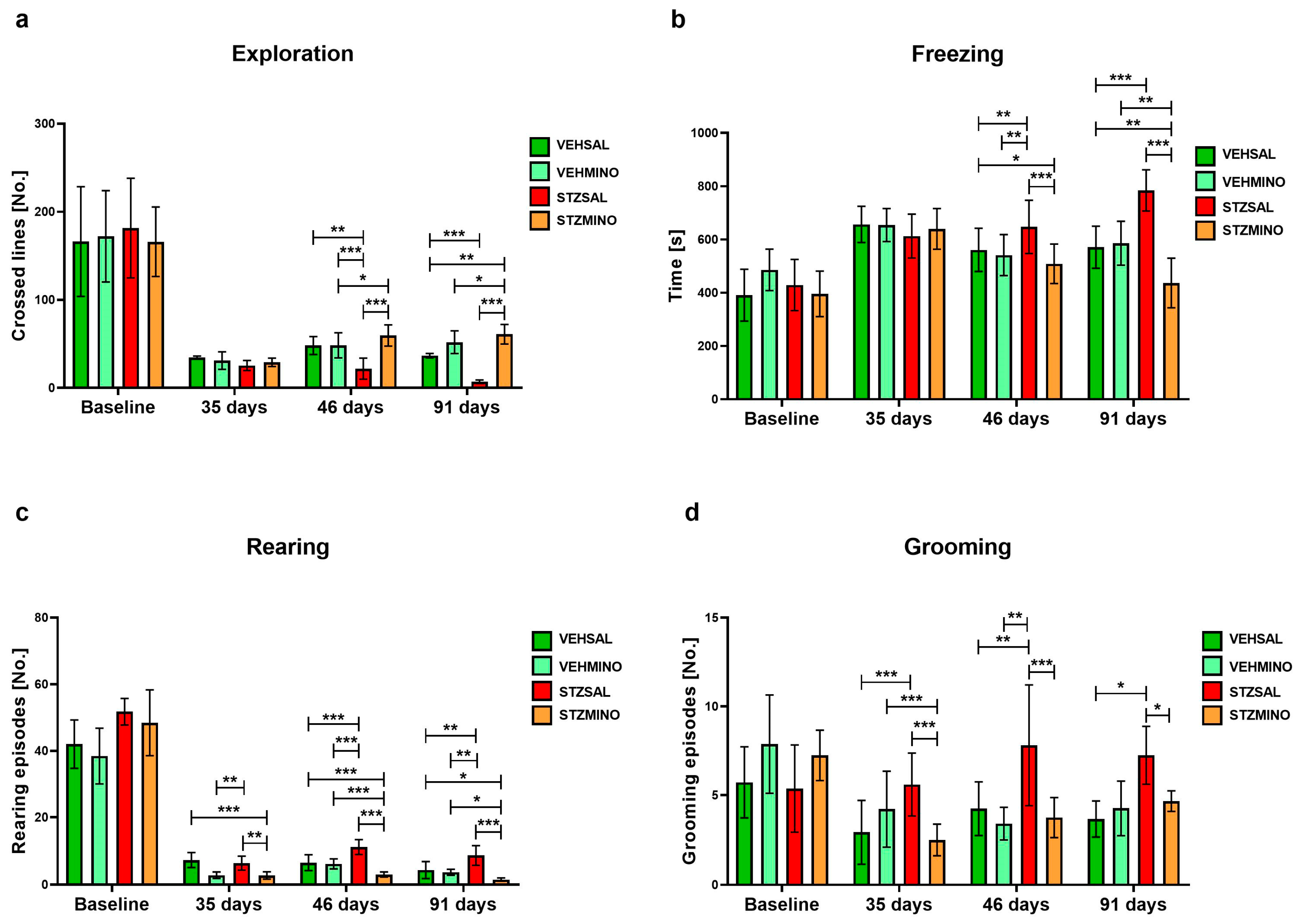


| Group | Treatment | |
|---|---|---|
| VEHSAL n = 10 | citrate buffer (VEH) icv | saline (SAL) i.p. |
| VEHMINO n = 10 | citrate buffer (VEH) icv | minocycline (MINO) i.p. |
| STZSAL n = 10 | streptozotocin (STZ) icv | saline (SAL) i.p. |
| STZMINO n = 10 | streptozotocin (STZ) icv | minocycline (MINO) i.p. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świątek, G.; Nowakowska-Gołacka, J.; Słomińska-Wojewódzka, M.; Glac, W.; Harackiewicz, O.; Kurowska-Rucińska, E.; Wrona, D. Minocycline Treatment Improves Memory and Reduces Anxiety by Lowering Levels of Brain Amyloid Precursor Protein and Indoleamine 2,3-Dioxygenase in a Rat Model of Streptozotocin-Induced Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 9397. https://doi.org/10.3390/ijms26199397
Świątek G, Nowakowska-Gołacka J, Słomińska-Wojewódzka M, Glac W, Harackiewicz O, Kurowska-Rucińska E, Wrona D. Minocycline Treatment Improves Memory and Reduces Anxiety by Lowering Levels of Brain Amyloid Precursor Protein and Indoleamine 2,3-Dioxygenase in a Rat Model of Streptozotocin-Induced Alzheimer’s Disease. International Journal of Molecular Sciences. 2025; 26(19):9397. https://doi.org/10.3390/ijms26199397
Chicago/Turabian StyleŚwiątek, Grzegorz, Jowita Nowakowska-Gołacka, Monika Słomińska-Wojewódzka, Wojciech Glac, Oliwia Harackiewicz, Ewelina Kurowska-Rucińska, and Danuta Wrona. 2025. "Minocycline Treatment Improves Memory and Reduces Anxiety by Lowering Levels of Brain Amyloid Precursor Protein and Indoleamine 2,3-Dioxygenase in a Rat Model of Streptozotocin-Induced Alzheimer’s Disease" International Journal of Molecular Sciences 26, no. 19: 9397. https://doi.org/10.3390/ijms26199397
APA StyleŚwiątek, G., Nowakowska-Gołacka, J., Słomińska-Wojewódzka, M., Glac, W., Harackiewicz, O., Kurowska-Rucińska, E., & Wrona, D. (2025). Minocycline Treatment Improves Memory and Reduces Anxiety by Lowering Levels of Brain Amyloid Precursor Protein and Indoleamine 2,3-Dioxygenase in a Rat Model of Streptozotocin-Induced Alzheimer’s Disease. International Journal of Molecular Sciences, 26(19), 9397. https://doi.org/10.3390/ijms26199397






