Enhanced Collagen Prolyl 4-Hydroxylase Activity and Expression Promote Cancer Progression via Both Canonical and Non-Canonical Mechanisms
Abstract
1. Structure of Collagen Prolyl 4-Hydroxylase
2. Function of C-P4H
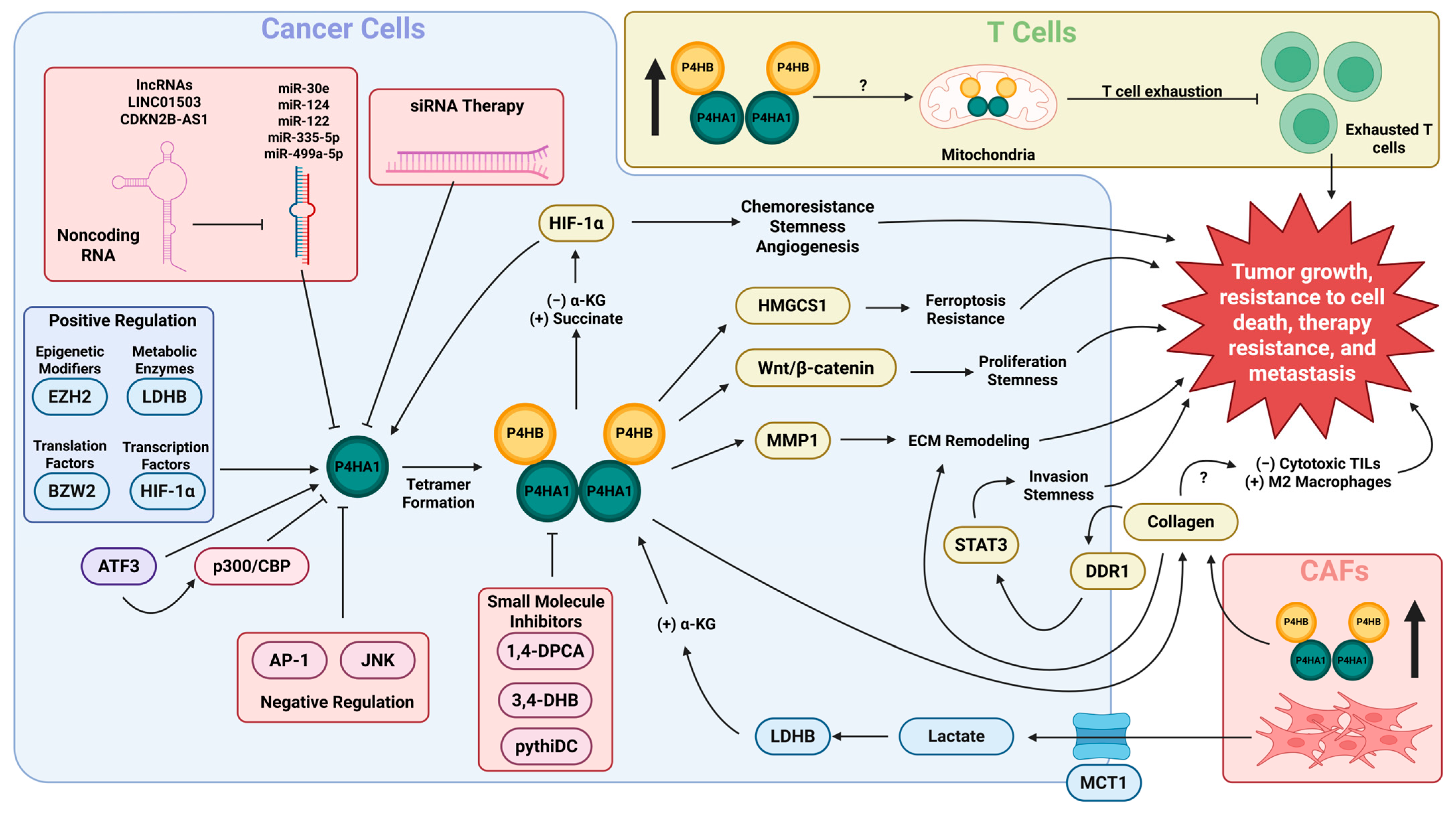
3. Regulation of P4HA1 Expression and C-P4H Enzyme Activity in Cancer
| Transcription Factors | Up-/Down-Regulated | Cancer or Cell Type/Reference(s) |
|---|---|---|
| HIF-1α | Up | Triple-negative breast cancer [35] |
| Colorectal adenocarcinoma [40,52] | ||
| Glioblastoma [37,39] | ||
| Pancreatic adenocarcinoma [45,72] | ||
| Head and neck squamous cell carcinoma [68] | ||
| Uveal melanoma [38] | ||
| Human embryonic kidney cells [41] | ||
| Human gingivial fibroblasts and human periodontal ligament cells [32] | ||
| USP5 and HIF-2α | Up | ER-positive and triple-negative breast cancer [46] |
| HIF-2α | Up | Colorectal adenocarcinoma [52] |
| STAT1 | Up | Esophageal squamous cell carcinoma [47] |
| SP1 | Up | Human aortic smooth muscle cells [48] |
| ATF3 | Up | Breast cancer [51] |
| Down | Glioblastoma [39] | |
| NonO | Down | Human aortic smooth muscle cells [49] |
| AP-1 | Down | Human myocardial fibroblasts [50] |
| Translation Factors and Non-coding RNAs | ||
| BZW2 | Up | Colorectal adenocarcinoma [53] |
| RBM4 | Up | Colorectal adenocarcinoma [52] |
| EIF4E2 | Up | Colorectal adenocarcinoma [52] |
| Nucleolin | Up | Fibrosarcoma [54] |
| Thyroid cancer [57] | ||
| miR-30e | Down | Hepatocellular carcinoma [55] |
| miR-122 | Down | Ovarian cancer [56,57,58,73] |
| Thyroid cancer [57] | ||
| miR-124 | Down | Prostate adenocarcinoma [44,59] |
| Lung adenocarcinoma [59] | ||
| miR-335-5p | Down | Pancreatic adenocarcinoma [60] |
| miR-499a-5p | Down | Head and neck squamous cell carcinoma [61] |
| Others | ||
| STT3B/MAGT1 | Up | Mouse embryonic fibroblasts [64] |
| SLC16A1/MCT1 | Up | Prostate adenocarcinoma [67] |
| Head and neck squamous cell carcinoma [68] | ||
| LDHB | Up | Head and neck squamous cell carcinoma [68] |
| PTRF | Up | Glioblastoma [39] |
| CHDH | Up | Colorectal adenocarcinoma [70] |
| FABP7 | Up | HER2+ breast cancer [71] |
4. P4HA1 Promotes Cell Proliferation, Invasiveness, Cell Stemness, and Chemoresistance
| Cancer Type | Downstream Effectors | Function(s) | References |
|---|---|---|---|
| Colorectal Cancer | Collagen I and IL17RB/c-Jun | Associated with increased metastasis in vitro and in vivo. | [70] |
| HIF-1α | Associated with increased proliferation and stemness. | [40] | |
| Wnt/β-catenin (Canonical Wnt pathway) | Increases proliferation and stemness while reducing susceptibility to cell death. | [40,53] | |
| Head and Neck Cancer | Collagen I | Increased collagen deposition is associated with altered cell cycle dynamics and increased cell stemness. | [68] |
| HMGCS1 | Increased proliferation and resistance to ferroptosis. | [85] | |
| Breast Cancer | Collagen I | Increased deposition is associated with increased invasiveness via alteration of the ECM. | [51] |
| HIF-1α | Positive feedback loop resulting in increased stemness and resistance to therapy. | [35] | |
| Glioma | HIF-1α | Positive feedback loop associated with increased succinate production, altered glycolytic metabolism, and chemoresistance. | [39] |
| PGK1 | Succinylated via increased succinate production, increasing its stability and leading to altered glycolysis and lactate secretion. | [39] | |
| CD31 and VEGFA | Increased expression contributing to cell stemness, phenotypic plasticity, and shift towards endothelioid phenotype. | [86] | |
| YAP/Collagen I | Increase in hydroxylation of YAP, stabilizing it and leading to an increase in collagen I transcription and deposition. Increased collagen I deposition is associated with chemoresistance. | [87] | |
| Pancreatic Cancer | HIF-1α | Positive feedback loop resulting in increased cell proliferation, resistance to chemotherapy, and stemness. | [45] |
| Prostate Cancer | MMP1 | Increased invasion in vitro. | [44] |
| Collagen I/DDR1/STAT3 | Increased collagen secretion leads to autocrine activation of DDR1, activating downstream STAT3 to enhance cell invasion and stemness. | [67] |
5. P4HA1 Contributes to Cancer Progression by Altering the Cellular Immune Response and Promoting Remodeling of the ECM
6. Prospects and Future Directions
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECM | Extracellular matrix |
| TME | Tumor microenvironment |
| C-P4H | Collagen prolyl 4-hydroxylase |
| TILs | Tumor-infiltrating lymphocytes |
| α-KG | α-ketoglutarate-dependent |
| PDI | Protein disulfide isomerase |
| ER | Endoplasmic reticulum |
| PSB | Peptide-substrate-binding domain |
| CAT | C-terminal catalytic domain |
| DSBH | Double-stranded β-helix |
| HIF-1α | Hypoxia-inducible factor 1α |
| HREs | Hypoxia response elements |
| HAS | HIF ancillary sequence |
| PHDs | HIF-prolyl hydroxylases |
| STAT1 | Signal transducer and activator of transcription 1 |
| SP1 | Sp1 transcription factor |
| NonO | Non-POU domain containing octamer binding |
| AP-1 | Activator protein 1 |
| ERK1/2 | Extracellular signal-regulated kinases 1 and 2 |
| TNF-α | Tumor necrosis factor-α |
| ATF3 | Activating transcription factor 3 |
| GBM | Glioblastoma multiforme |
| RBM4 | RNA-binding motif protein 4 |
| eIF4E2 | Eukaryotic translation initiation factor 4E family member 2 |
| BZW2 | Basic leucine zipper and W2 domains 2 |
| COAD | Colorectal adenocarcinoma |
| EZH2 | Enhancer of zeste 2 polycomb repressive complex 2 subunit |
| PRC2 | Polycomb repressive complex 2 |
| PRAD | Prostate adenocarcinoma |
| CTBP1 | C-terminal binding protein 1 |
| PTMs | Post-translational modifications |
| MEF | Mouse embryonic fibroblast |
| STT3B | STT3 oligosaccharyltransferase complex catalytic subunit B |
| MAGT1 | Magnesium transporter 1 |
| SLC16A1 | Solute carrier family 16 member 1 |
| LDHB | Lactate dehydrogenase B |
| PTRF | Polymerase I and transcript release factor |
| CHDH | Choline dehydrogenase |
| CRC | Colorectal cancer |
| TRIM21 | Tripartite motif-containing protein 21 |
| FABP7 | Fatty acid binding protein 7 |
| OVCAR | Ovarian carcinoma |
| HMGCS1 | 3-hydroxy-3-methylglutaryl-CoA synthase 1 |
| MMP1 | Matrix metalloprotease 1 |
| PGK1 | Phosphoglycerate kinase 1 |
| TNBC | Triple-negative breast cancer |
| PDAC | Pancreatic ductal adenocarcinoma |
| HNSCC | Head and neck squamous carcinoma |
| DDR1 | Discoidin domain receptor tyrosine kinase 1 |
| YAP | Yes-associated protein |
| CAFs | Cancer-associated fibroblasts |
| 1,4-DPCA | 1,4-dihydrophenonthrolin-4-one-3-carboxylic acid |
| CTHRC1 | Collagen triple helix repeat containing 1 |
| 3,4-DHB | 3,4-dihydroxybenzoic acid |
| MBLs | Mannose-binding lectins |
References
- Myllyharju, J. Prolyl 4-hydroxylases, the key enzymes of collagen biosynthesis. Matrix Biol. 2003, 22, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Kukkola, L.; Hieta, R.; Kivirikko, K.I.; Myllyharju, J. Identification and Characterization of a Third Human, Rat, and Mouse Collagen Prolyl 4-Hydroxylase Isoenzyme*. J. Biol. Chem. 2003, 278, 47685–47693. [Google Scholar] [CrossRef] [PubMed]
- Annunen, P.; Helaakoski, T.; Myllyharju, J.; Veijola, J.; Pihlajaniemi, T.; Kivirikko, K.I. Cloning of the Human Prolyl 4-Hydroxylase α Subunit Isoform α(II) and Characterization of the Type II Enzyme Tetramer: THE α(I) AND α(II) SUBUNITS DO NOT FORM A MIXED α(I)α(II)β2 TETRAMER*. J. Biol. Chem. 1997, 272, 17342–17348. [Google Scholar] [CrossRef] [PubMed]
- Annunen, P.; Autio-Harmainen, H.; Kivirikko, K.I. The Novel Type II Prolyl 4-Hydroxylase Is the Main Enzyme Form in Chondrocytes and Capillary Endothelial Cells, whereas the Type I Enzyme Predominates in Most Cells. J. Biol. Chem. 1998, 273, 5989–5992. [Google Scholar] [CrossRef]
- Helaakoski, T.; Vuori, K.; Myllylä, R.; Kivirikko, K.I.; Pihlajaniemi, T. Molecular cloning of the alpha-subunit of human prolyl 4-hydroxylase: The complete cDNA-derived amino acid sequence and evidence for alternative splicing of RNA transcripts. Proc. Natl. Acad. Sci. USA 1989, 86, 4392–4396. [Google Scholar] [CrossRef]
- Rappu, P.; Salo, A.M.; Myllyharju, J.; Heino, J. Role of prolyl hydroxylation in the molecular interactions of collagens. Essays Biochem. 2019, 63, 325–335. [Google Scholar] [CrossRef]
- Ma, S.; Ong, L.T.; Jiang, Z.; Lee, W.C.; Lee, P.L.; Yusuf, M.; Ditzel, H.J.; Wang, Y.; Chen, Q.; Wang, W.; et al. Targeting P4HA1 promotes CD8(+) T cell progenitor expansion toward immune memory and systemic anti-tumor immunity. Cancer Cell 2024, 43, 213–231.e9. [Google Scholar] [CrossRef] [PubMed]
- Pihlajaniemi, T.; Myllylä, R.; Kivirikko, K.I. Prolyl 4-hydroxylase and its role in collagen synthesis. J. Hepatol. 1991, 13 (Suppl. 3), S2–S7. [Google Scholar] [CrossRef]
- Kivirikko, K.I.; Myllyharju, J. Prolyl 4-hydroxylases and their protein disulfide isomerase subunit. Matrix Biol. 1998, 16, 357–368. [Google Scholar] [CrossRef]
- Vuori, K.; Pihlajaniemi, T.; Myllylä, R.; Kivirikko, K.I. Site-directed mutagenesis of human protein disulphide isomerase: Effect on the assembly, activity and endoplasmic reticulum retention of human prolyl 4-hydroxylase in Spodoptera frugiperda insect cells. EMBO J. 1992, 11, 4213–4217. [Google Scholar] [CrossRef]
- Vuori, K.; Pihlajaniemi, T.; Marttila, M.; Kivirikko, K.I. Characterization of the human prolyl 4-hydroxylase tetramer and its multifunctional protein disulfide-isomerase subunit synthesized in a baculovirus expression system. Proc. Natl. Acad. Sci. USA 1992, 89, 7467–7470. [Google Scholar] [CrossRef]
- John, D.C.; Grant, M.E.; Bulleid, N.J. Cell-free synthesis and assembly of prolyl 4-hydroxylase: The role of the beta-subunit (PDI) in preventing misfolding and aggregation of the alpha-subunit. EMBO J. 1993, 12, 1587–1595. [Google Scholar] [CrossRef]
- Koski, M.K.; Anantharajan, J.; Kursula, P.; Dhavala, P.; Murthy, A.V.; Bergmann, U.; Myllyharju, J.; Wierenga, R.K. Assembly of the elongated collagen prolyl 4-hydroxylase α2β2 heterotetramer around a central α2 dimer. Biochem. J. 2017, 474, 751–769. [Google Scholar] [CrossRef]
- Myllyharju, J.; Kivirikko, K.I. Identification of a novel proline-rich peptide-binding domain in prolyl 4-hydroxylase. EMBO J. 1999, 18, 306–312. [Google Scholar] [CrossRef]
- Anantharajan, J.; Koski, M.K.; Kursula, P.; Hieta, R.; Bergmann, U.; Myllyharju, J.; Wierenga, R.K. The Structural Motifs for Substrate Binding and Dimerization of the α Subunit of Collagen Prolyl 4-Hydroxylase. Structure 2013, 21, 2107–2118. [Google Scholar] [CrossRef]
- Pekkala, M.; Hieta, R.; Bergmann, U.; Kivirikko, K.I.; Wierenga, R.K.; Myllyharju, J. The peptide-substrate-binding domain of collagen prolyl 4-hydroxylases is a tetratricopeptide repeat domain with functional aromatic residues. J. Biol. Chem. 2004, 279, 52255–52261. [Google Scholar] [CrossRef]
- Murthy, A.V.; Sulu, R.; Koski, M.K.; Tu, H.; Anantharajan, J.; Sah-Teli, S.K.; Myllyharju, J.; Wierenga, R.K. Structural enzymology binding studies of the peptide-substrate-binding domain of human collagen prolyl 4-hydroxylase (type-II): High affinity peptides have a PxGP sequence motif. Protein Sci. 2018, 27, 1692–1703. [Google Scholar] [CrossRef]
- Koski, M.K.; Hieta, R.; Böllner, C.; Kivirikko, K.I.; Myllyharju, J.; Wierenga, R.K. The Active Site of an Algal Prolyl 4-Hydroxylase Has a Large Structural Plasticity*. J. Biol. Chem. 2007, 282, 37112–37123. [Google Scholar] [CrossRef] [PubMed]
- Ellgaard, L.; Ruddock, L.W. The human protein disulphide isomerase family: Substrate interactions and functional properties. EMBO Rep. 2005, 6, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, W.; Ren, J.; Fang, J.; Ke, H.; Gong, W.; Feng, W.; Wang, C.C. Structural insights into the redox-regulated dynamic conformations of human protein disulfide isomerase. Antioxid. Redox Signal 2013, 19, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Pirneskoski, A.; Ruddock, L.W.; Klappa, P.; Freedman, R.B.; Kivirikko, K.I.; Koivunen, P. Domains b′ and a′ of Protein Disulfide Isomerase Fulfill the Minimum Requirement for Function as a Subunit of Prolyl 4-Hydroxylase: THE N-TERMINAL DOMAINS a AND b ENHANCE THIS FUNCTION AND CAN BE SUBSTITUTED IN PART BY THOSE OF ERp57*. J. Biol. Chem. 2001, 276, 11287–11293. [Google Scholar] [CrossRef]
- Murthy, A.V.; Sulu, R.; Lebedev, A.; Salo, A.M.; Korhonen, K.; Venkatesan, R.; Tu, H.; Bergmann, U.; Jänis, J.; Laitaoja, M.; et al. Crystal structure of the collagen prolyl 4-hydroxylase (C-P4H) catalytic domain complexed with PDI: Toward a model of the C-P4H α2β2 tetramer. J. Biol. Chem. 2022, 298, 102614. [Google Scholar] [CrossRef]
- Kivirikko, K.I.; Helaakoski, T.; Tasanen, K.; Vuori, K.; Myllylä, R.; Parkkonen, T.; Pihlajaniemi, T. Molecular Biology of Prolyl 4-Hydroxylase. Ann. N. Y. Acad. Sci. 1990, 580, 132–142. [Google Scholar] [CrossRef]
- Vasta, J.D.; Raines, R.T. Collagen Prolyl 4-Hydroxylase as a Therapeutic Target. J. Med. Chem. 2018, 61, 10403–10411. [Google Scholar] [CrossRef]
- Gorres, K.L.; Raines, R.T. Prolyl 4-hydroxylase. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 106–124. [Google Scholar] [CrossRef]
- Rahman, M.M.; Sulu, R.; Adediran, B.; Tu, H.; Salo, A.M.; Murthy, S.; Myllyharju, J.; Wierenga, R.K.; Koski, M.K. Binding Differences of the Peptide-Substrate–Binding Domain of Collagen Prolyl 4-Hydroxylases I and II for Proline- and Hydroxyproline-Rich Peptides. Proteins Struct. Funct. Bioinform. 2025, 93, 1732–1746. [Google Scholar] [CrossRef]
- Salo, A.M.; Rappu, P.; Koski, M.K.; Karjalainen, E.; Izzi, V.; Drushinin, K.; Miinalainen, I.; Käpylä, J.; Heino, J.; Myllyharju, J. Collagen prolyl 4-hydroxylase isoenzymes I and II have sequence specificity towards different X-Pro-Gly triplets. Matrix Biol. 2024, 125, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Helaakoski, T.; Annunen, P.; Vuori, K.; MacNeil, I.A.; Pihlajaniemi, T.; Kivirikko, K.I. Cloning, baculovirus expression, and characterization of a second mouse prolyl 4-hydroxylase alpha-subunit isoform: Formation of an alpha 2 beta 2 tetramer with the protein disulfide-isomerase/beta subunit. Proc. Natl. Acad. Sci. USA 1995, 92, 4427–4431. [Google Scholar] [CrossRef] [PubMed]
- Salo, A.M.; Myllyharju, J. Prolyl and lysyl hydroxylases in collagen synthesis. Exp. Dermatol. 2021, 30, 38–49. [Google Scholar] [CrossRef]
- Tolonen, J.-P.; Salo, A.M.; Finnilä, M.; Aro, E.; Karjalainen, E.; Ronkainen, V.-P.; Drushinin, K.; Merceron, C.; Izzi, V.; Schipani, E.; et al. Reduced Bone Mass in Collagen Prolyl 4-Hydroxylase P4ha1+/−; P4ha2−/− Compound Mutant Mice. JBMR Plus 2022, 6, e10630. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, K.H.; Gess, B.; Lohaus, C.; Meyer, H.E.; Katschinski, D.; Kurtz, A. Oxygen tension regulates the expression of a group of procollagen hydroxylases. Eur. J. Biochem. 2003, 270, 4515–4522. [Google Scholar] [CrossRef]
- Morimoto, C.; Takedachi, M.; Kawasaki, K.; Shimomura, J.; Murata, M.; Hirai, A.; Kawakami, K.; Sawada, K.; Iwayama, T.; Murakami, S. Hypoxia stimulates collagen hydroxylation in gingival fibroblasts and periodontal ligament cells. J. Periodontol. 2021, 92, 1635–1645. [Google Scholar] [CrossRef]
- Kappler, M.; Kotrba, J.; Kaune, T.; Bache, M.; Rot, S.; Bethmann, D.; Wichmann, H.; Güttler, A.; Bilkenroth, U.; Horter, S.; et al. P4HA1: A single-gene surrogate of hypoxia signatures in oral squamous cell carcinoma patients. Clin. Transl. Radiat. Oncol. 2017, 5, 6–11. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, Y.; Ma, H.; Wang, J.; Guo, H.; Liu, H. A four-hypoxia-genes-based prognostic signature for oral squamous cell carcinoma. BMC Oral. Health 2021, 21, 232. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Stewart, R.L.; Chen, J.; Gao, T.; Scott, T.L.; Samayoa, L.M.; O’Connor, K.; Lane, A.N.; Xu, R. Collagen prolyl 4-hydroxylase 1 is essential for HIF-1α stabilization and TNBC chemoresistance. Nat. Commun. 2018, 9, 4456. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jin, G.; Mi, R.; Zhang, J.; Zhang, J.; Xu, H.; Cheng, S.; Zhang, Y.; Song, W.; Liu, F. Knockdown of P4HA1 inhibits neovascularization via targeting glioma stem cell-endothelial cell transdifferentiation and disrupting vascular basement membrane. Oncotarget 2017, 8, 35877–35889. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, S.; Yang, X.; Wang, W.; Shao, W.; Ji, T. P4HA1 as an unfavorable prognostic marker promotes cell migration and invasion of glioblastoma via inducing EMT process under hypoxia microenvironment. Am. J. Cancer Res. 2021, 11, 590–617. [Google Scholar]
- Kaluz, S.; Zhang, Q.; Kuranaga, Y.; Yang, H.; Osuka, S.; Bhattacharya, D.; Devi, N.S.; Mun, J.; Wang, W.; Zhang, R.; et al. Targeting HIF-activated collagen prolyl 4-hydroxylase expression disrupts collagen deposition and blocks primary and metastatic uveal melanoma growth. Oncogene 2021, 40, 5182–5191. [Google Scholar] [CrossRef]
- Yang, S.; Zhan, Q.; Su, D.; Cui, X.; Zhao, J.; Wang, Q.; Hong, B.; Ju, J.; Cheng, C.; Yang, E.; et al. HIF1α/ATF3 partake in PGK1 K191/K192 succinylation by modulating P4HA1/succinate signaling in glioblastoma. Neuro-Oncology 2024, 26, 1405–1420. [Google Scholar] [CrossRef]
- Zhang, Q.; Yin, Y.; Zhao, H.; Shi, Y.; Zhang, W.; Yang, Z.; Liu, T.; Huang, Y.; Yu, Z. P4HA1 regulates human colorectal cancer cells through HIF1α-mediated Wnt signaling. Oncol. Lett. 2021, 21, 145. [Google Scholar] [CrossRef]
- Rosell-Garcia, T.; Rivas-Muñoz, S.; Kin, K.; Romero-Albillo, V.; Alcaraz, S.; Fernandez-Tornero, C.; Rodriguez-Pascual, F. Multimerization of HIF enhances transcription of target genes containing the hypoxia ancillary sequence. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2023, 1866, 194963. [Google Scholar] [CrossRef]
- Takahashi, Y.; Takahashi, S.; Shiga, Y.; Yoshimi, T.; Miura, T. Hypoxic Induction of Prolyl 4-Hydroxylase α(I) in Cultured Cells*. J. Biol. Chem. 2000, 275, 14139–14146. [Google Scholar] [CrossRef]
- Bentovim, L.; Amarilio, R.; Zelzer, E. HIF1α is a central regulator of collagen hydroxylation and secretion under hypoxia during bone development. Development 2012, 139, 4473–4483. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthi, B.V.; Pathi, S.S.; Goswami, M.T.; Cieślik, M.; Zheng, H.; Nallasivam, S.; Arekapudi, S.R.; Jing, X.; Siddiqui, J.; Athanikar, J.; et al. The miR-124-prolyl hydroxylase P4HA1-MMP1 axis plays a critical role in prostate cancer progression. Oncotarget 2014, 5, 6654–6669. [Google Scholar] [CrossRef]
- Cao, X.P.; Cao, Y.; Li, W.J.; Zhang, H.H.; Zhu, Z.M. P4HA1/HIF1α feedback loop drives the glycolytic and malignant phenotypes of pancreatic cancer. Biochem. Biophys. Res. Commun. 2019, 516, 606–612. [Google Scholar] [CrossRef]
- Huang, W.; Liu, X.; Zhang, Y.; Deng, M.; Li, G.; Chen, G.; Yu, L.; Jin, L.; Liu, T.; Wang, Y.; et al. USP5 promotes breast cancer cell proliferation and metastasis by stabilizing HIF2α. J. Cell Physiol. 2022, 237, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Gou, W.; Yang, Y.; Shan, Q.; Xia, S.; Ma, Y. P4HA1, transcriptionally activated by STAT1, promotes esophageal cancer progression. Pathol. Int. 2023, 73, 147–158. [Google Scholar] [CrossRef]
- Li, L.; Zhang, K.; Cai, X.-J.; Feng, M.; Zhang, Y.; Zhang, M. Adiponectin Upregulates Prolyl-4-Hydroxylase α1 Expression in Interleukin 6-Stimulated Human Aortic Smooth Muscle Cells by Regulating ERK1/2 and Sp1. PLoS ONE 2011, 6, e22819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, M.-X.; Shen, Y.H.; Burks, J.K.; Zhang, Y.; Wang, J.; LeMaire, S.A.; Yoshimura, K.; Aoki, H.; Coselli, J.S.; et al. TNF-α Suppresses Prolyl-4-Hydroxylase α1 Expression via the ASK1–JNK–NonO Pathway. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1760–1767. [Google Scholar] [CrossRef][Green Version]
- Zhao, T.; Chen, H.; Cheng, C.; Zhang, J.; Yan, Z.; Kuang, J.; Kong, F.; Li, C.; Lu, Q. Liraglutide protects high-glucose-stimulated fibroblasts by activating the CD36-JNK-AP1 pathway to downregulate P4HA1. Biomed. Pharmacother. 2019, 118, 109224. [Google Scholar] [CrossRef]
- Dhamdhere, S.G.; Bansal, A.; Singh, P.; Kakani, P.; Agrawal, S.; Samaiya, A.; Shukla, S. Hypoxia-induced ATF3 escalates breast cancer invasion by increasing collagen deposition via P4HA1. Cell Death Dis. 2025, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-H.; Hung, H.-Y.; Yu, J.-S.; Liao, Y.-C.; Lai, M.-C. Hypoxia-induced translation of collagen-modifying enzymes PLOD2 and P4HA1 is dependent on RBM4 and eIF4E2 in human colon cancer HCT116 cells. FEBS J. 2025, 292, 881–898. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Afaq, F.; Bajpai, P.; Behring, M.; Kim, H.-G.; Varambally, A.; Chandrashekar, D.S.; Peter, S.; Diffalha, S.A.; Khushman, M.D.; et al. BZW2 Inhibition Reduces Colorectal Cancer Growth and Metastasis. Mol. Cancer Res. 2023, 21, 698–712. [Google Scholar] [CrossRef]
- Fähling, M.; Mrowka, R.; Steege, A.; Nebrich, G.; Perlewitz, A.; Persson, P.B.; Thiele, B.J. Translational Control of Collagen Prolyl 4-Hydroxylase-α(I) Gene Expression under Hypoxia*. J. Biol. Chem. 2006, 281, 26089–26101. [Google Scholar] [CrossRef]
- Feng, G.; Shi, H.; Li, J.; Yang, Z.; Fang, R.; Ye, L.; Zhang, W.; Zhang, X. MiR-30e suppresses proliferation of hepatoma cells via targeting prolyl 4-hydroxylase subunit alpha-1 (P4HA1) mRNA. Biochem. Biophys. Res. Commun. 2016, 472, 516–522. [Google Scholar] [CrossRef]
- Duan, Y.; Dong, Y.; Dang, R.; Hu, Z.; Yang, Y.; Hu, Y.; Cheng, J. MiR-122 inhibits epithelial mesenchymal transition by regulating P4HA1 in ovarian cancer cells. Cell Biol. Int. 2018, 42, 1564–1574. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; He, Y.; Liu, X.; Luo, F.; Jiang, Y.; Xiang, M.; Zhao, R. Cancer stem cell-like cells-derived exosomal lncRNA CDKN2B-AS1 promotes biological characteristics in thyroid cancer via miR-122-5p/P4HA1 axis. Regen. Ther. 2022, 22, 19–29. [Google Scholar] [CrossRef]
- Langhe, R.; Norris, L.; Saadeh, F.A.; Blackshields, G.; Varley, R.; Harrison, A.; Gleeson, N.; Spillane, C.; Martin, C.; O’Donnell, D.M.; et al. A novel serum microRNA panel to discriminate benign from malignant ovarian disease. Cancer Lett. 2015, 356, 628–636. [Google Scholar] [CrossRef]
- Robinson, A.D.; Chakravarthi, B.V.S.K.; Agarwal, S.; Chandrashekar, D.S.; Davenport, M.L.; Chen, G.; Manne, U.; Beer, D.G.; Edmonds, M.D.; Varambally, S. Collagen modifying enzyme P4HA1 is overexpressed and plays a role in lung adenocarcinoma. Transl. Oncol. 2021, 14, 101128. [Google Scholar] [CrossRef]
- Hu, Z.; Song, F.; Hu, Y.; Liao, T. Systematic Analysis of the Expression and Prognostic Significance of P4HA1 in Pancreatic Cancer and Construction of a lncRNA-miRNA-P4HA1 Regulatory Axis. BioMed Res. Int. 2020, 2020, 8877334. [Google Scholar] [CrossRef]
- Wu, Z.-H.; Zhong, Y.; Zhou, T.; Xiao, H.-J. miRNA biomarkers for predicting overall survival outcomes for head and neck squamous cell carcinoma. Genomics 2021, 113, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Behring, M.; Kim, H.-G.; Bajpai, P.; Chakravarthi, B.V.S.K.; Gupta, N.; Elkholy, A.; Diffalha, S.A.; Varambally, S.; Manne, U. Targeting P4HA1 with a Small Molecule Inhibitor in a Colorectal Cancer PDX Model. Transl. Oncol. 2020, 13, 100754. [Google Scholar] [CrossRef]
- Hornbeck, P.V.; Zhang, B.; Murray, B.; Kornhauser, J.M.; Latham, V.; Skrzypek, E. PhosphoSitePlus, 2014: Mutations, PTMs and recalibrations. Nucleic Acids Res. 2015, 43, D512–D520. [Google Scholar] [CrossRef]
- Shi, R.; Hu, W.; Zhang, Y.; Gao, S.; Smith, A.H.; Ye, J.; Cai, L.; Graham, L.M.; Li, C. Ascorbate inducible N259 glycans on prolyl 4-hydroxylase subunit α1 promote hydroxylation and secretion of type I collagen. Cell Mol. Life Sci. 2019, 76, 3449–3464. [Google Scholar] [CrossRef]
- Lamberg, A.; Pihlajaniemi, T.; Kivirikko, K.I. Site-directed mutagenesis of the alpha subunit of human prolyl 4-hydroxylase. Identification of three histidine residues critical for catalytic activity. J. Biol. Chem. 1995, 270, 9926–9931. [Google Scholar] [CrossRef]
- Xu, Y.; Xia, D.; Huang, K.; Liang, M. Hypoxia-induced P4HA1 overexpression promotes post-ischemic angiogenesis by enhancing endothelial glycolysis through downregulating FBP1. J. Transl. Med. 2024, 22, 74. [Google Scholar] [CrossRef]
- Ippolito, L.; Duatti, A.; Iozzo, M.; Comito, G.; Pardella, E.; Lorito, N.; Bacci, M.; Pranzini, E.; Santi, A.; Sandrini, G.; et al. Lactate supports cell-autonomous ECM production to sustain metastatic behavior in prostate cancer. EMBO Rep. 2024, 25, 3506–3531. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Y.; Zhao, H.; Wang, J.; Liu, Y.; Bai, J.; Hu, C.; Shang, Z. Lactate-driven type I collagen deposition facilitates cancer stem cell-like phenotype of head and neck squamous cell carcinoma. iScience 2024, 27, 109340. [Google Scholar] [CrossRef]
- Wu, F.; Liu, Q.; Zhang, J.; Xu, D.; Jiang, X.; Zhang, K.; Chen, Y.; Xia, X.; Jiang, Z.; Shi, Y.; et al. Prolyl 4-hydroxylase subunit alpha-2 acts as a TRIM21 ubiquitination substrate to promote papillary thyroid cancer progression via the glycolytic pathway. Cell Death Dis. 2025, 16, 395. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Shen, X.; Wang, S.; Zhang, Z.; Du, W.; Yang, C.; Jiang, X.; Zhang, X.; Huang, Y.; et al. Prolyl 4-hydroxylase α-subunit family regulation of type I collagen deposition and IL17RB/c-Jun activation synergistically mediate choline dehydrogenase promotion of colorectal cancer metastasis. MedComm 2025, 6, e70007. [Google Scholar] [CrossRef] [PubMed]
- Cordero, A.; Kanojia, D.; Miska, J.; Panek, W.K.; Xiao, A.; Han, Y.; Bonamici, N.; Zhou, W.; Xiao, T.; Wu, M.; et al. FABP7 is a key metabolic regulator in HER2+ breast cancer brain metastasis. Oncogene 2019, 38, 6445–6460. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, Y.; Ng, S.; Deng, Z.; Lyon, C.J.; Koay, E.J.; Ning, B.; Katz, M.H.; Chiao, P.J.; Fan, J.; et al. Circulating levels of hydroxylated bradykinin function as an indicator of tissue HIF-1α expression. Sci. Bull. 2020, 65, 1570–1579. [Google Scholar] [CrossRef]
- Li, J.; Ghazwani, M.; Zhang, Y.; Lu, J.; Li, J.; Fan, J.; Gandhi, C.R.; Li, S. miR-122 regulates collagen production via targeting hepatic stellate cells and suppressing P4HA1 expression. J. Hepatol. 2013, 58, 522–528. [Google Scholar] [CrossRef]
- Rømer, A.M.A.; Thorseth, M.L.; Madsen, D.H. Immune Modulatory Properties of Collagen in Cancer. Front. Immunol. 2021, 12, 791453. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, Z.; Zhu, A.; Xiong, X.; Zhang, J.; Xu, J.; Sy, M.S.; Li, C. Targeting type I collagen for cancer treatment. Int. J. Cancer 2022, 151, 665–683. [Google Scholar] [CrossRef]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef]
- Wei, J.; Huang, K.; Chen, Z.; Hu, M.; Bai, Y.; Lin, S.; Du, H. Characterization of Glycolysis-Associated Molecules in the Tumor Microenvironment Revealed by Pan-Cancer Tissues and Lung Cancer Single Cell Data. Cancers 2020, 12, 1788. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, J. P4HA1, a Prognostic Biomarker that Correlates With Immune Infiltrates in Lung Adenocarcinoma and Pan-Cancer. Front. Cell Dev. Biol. 2021, 9, 754580. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, F.; Zheng, Q.; Wu, Y.; Wu, Y.a. Identification of Potential Diagnostic and Prognostic Values of P4HA1 Expression in Lung Cancer, Breast Cancer, and Head and Neck Cancer. DNA Cell Biol. 2020, 39, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Chai, H.; Zhou, C.; Xue, Z.; Zhang, M.; Qiao, L.; Wang, J.; Lu, F. Development and validation of a novel risk stratification signature derived from migrasome and tumor microenvironment-related genes for molecular subtyping and improving clinical outcomes in head and neck squamous cell carcinoma. Ann. Med. 2025, 57, 2558121. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Wu, J.; Cui, H.; Dai, L.; Ma, L.; Zhou, Z.; Liang, B.; Zhang, S.; Lin, S. A Novel Glycolysis and Hypoxia Combined Gene Signature Predicts the Prognosis and Affects Immune Infiltration of Patients with Colon Cancer. Int. J. Gen. Med. 2022, 15, 1413–1427. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, S.; Bai, H.; Wang, K.; Hao, J.; Zhang, J.; Li, J. Identification of Five Glycolysis-Related Gene Signature and Risk Score Model for Colorectal Cancer. Front. Oncol. 2021, 11, 588811. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Schnettler, E.; Venkatachalam, A.; Wang, Y.; Feldman, L.; Argenta, P.; Rodriguez-Rodriguez, L.; Ramakrishnan, S. Increased expression of collagen prolyl hydroxylases in ovarian cancer is associated with cancer growth and metastasis. Am. J. Cancer Res. 2023, 13, 6051–6062. [Google Scholar]
- Wang, H. A mitochondrial ferroptosis-related gene signature predicts prognosis and immune landscape in colon cancer. Front. Med. 2025, 12, 1614012. [Google Scholar] [CrossRef]
- Zhou, R.; Qiu, L.; Zhou, L.; Geng, R.; Yang, S.; Wu, J. P4HA1 activates HMGCS1 to promote nasopharyngeal carcinoma ferroptosis resistance and progression. Cell Signal 2023, 105, 110609. [Google Scholar] [CrossRef]
- Han, X.; Wang, Q.; Fang, S.; Wang, J.; Liu, F.; Zhang, J.; Jin, G. P4HA1 Regulates CD31 via COL6A1 in the Transition of Glioblastoma Stem-Like Cells to Tumor Endothelioid Cells. Front. Oncol. 2022, 12, 836511. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, G.; Zhong, X.; Zhong, J.; Chen, X.; Chen, Q.; Xue, J.; Yang, X.; Zhang, X.; Ling, Y.; et al. P4HA1 mediates YAP hydroxylation and accelerates collagen synthesis in temozolomide-resistant glioblastoma. Chin. Med. J. 2025, 138, 1991–2005. [Google Scholar] [CrossRef]
- Eriksson, J.; Joncour, V.L.; Jahkola, T.; Juteau, S.; Laakkonen, P.; Saksela, O.; Hölttä, E. Prolyl 4-hydroxylase subunit alpha 1 (P4HA1) is a biomarker of poor prognosis in primary melanomas, and its depletion inhibits melanoma cell invasion and disrupts tumor blood vessel walls. Mol. Oncol. 2020, 14, 742–762. [Google Scholar] [CrossRef]
- Piltti, J.; Bygdell, J.; Qu, C.; Lammi, M.J. Effects of long-term low oxygen tension in human chondrosarcoma cells. J. Cell. Biochem. 2018, 119, 2320–2332. [Google Scholar] [CrossRef]
- Cao, X.; Cao, Y.; Zhao, H.; Wang, P.; Zhu, Z. Prolyl 4-hydroxylase P4HA1 Mediates the Interplay Between Glucose Metabolism and Stemness in Pancreatic Cancer Cells. Curr. Stem Cell Res. Ther. 2023, 18, 712–719. [Google Scholar] [CrossRef]
- Li, Z.; Cai, H.; Zheng, J.; Chen, X.; Liu, G.; Lv, Y.; Ye, H.; Cai, G. Mitochondrial-related genes markers that predict survival in patients with head and neck squamous cell carcinoma affect immunomodulation through hypoxia, glycolysis, and angiogenesis pathways. Aging 2023, 15, 10347–10369. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ma, X.; Wang, Z.; Zhang, T.; Hua, Y.; Cai, Z. Identification of a novel glycolysis-related gene signature for predicting the prognosis of osteosarcoma patients. Aging 2021, 13, 12896–12918. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; He, Y.; Li, L.; Wu, C.; Hu, G. Overexpression of P4HA1 Is Correlated with Poor Survival and Immune Infiltrates in Lung Adenocarcinoma. BioMed Res. Int. 2020, 2020, 8024138. [Google Scholar] [CrossRef]
- Han, W.J.; He, P. A novel tumor microenvironment-related gene signature with immune features for prognosis of lung squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2023, 149, 13137–13154. [Google Scholar] [CrossRef]
- Gao, H.; Xing, F. A novel signature model based on mitochondrial-related genes for predicting survival of colon adenocarcinoma. BMC Med. Inform. Decis. Mak. 2022, 22, 277. [Google Scholar] [CrossRef] [PubMed]
- Belotti, Y.; Lim, S.B.; Iyer, N.G.; Lim, W.-T.; Lim, C.T. Prognostic Matrisomal Gene Panel and Its Association with Immune Cell Infiltration in Head and Neck Carcinomas. Cancers 2021, 13, 5761. [Google Scholar] [CrossRef]
- Zhou, H.; Lei, Y.; Luo, J.; Wang, J.; Peng, L.; Mou, K.; Xiang, L.; Luo, Y. Comprehensive analysis revealed P4Hs as new biomarkers for prognosis and immunotherapy in head and neck cancer. Sci. Rep. 2024, 14, 12234. [Google Scholar] [CrossRef]
- Ramirez, J.A.Z.; Romagnoli, G.G.; Falasco, B.F.; Gorgulho, C.M.; Fogolin, C.S.; Santos, D.C.D.; Junior, J.P.A.; Lotze, M.T.; Ureshino, R.P.; Kaneno, R. Blocking drug-induced autophagy with chloroquine in HCT-116 colon cancer cells enhances DC maturation and T cell responses induced by tumor cell lysate. Int. Immunopharmacol. 2020, 84, 106495. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Fang, S.; Wang, J.; Han, X.; Liu, F.; Jin, G. P4HA1 Down-Regulation Inhibits Glioma Invasiveness by Promoting M1 Microglia Polarization. Onco Targets Ther. 2021, 14, 1771–1782. [Google Scholar] [CrossRef]
- Cai, R.; Tressler, C.M.; Cheng, M.; Sonkar, K.; Tan, Z.; Paidi, S.K.; Ayyappan, V.; Barman, I.; Glunde, K. Primary breast tumor induced extracellular matrix remodeling in premetastatic lungs. Sci. Rep. 2023, 13, 18566. [Google Scholar] [CrossRef]
- Li, M.; Wang, Q.; Zheng, Q.; Wu, L.; Zhao, B.; Wu, Y. Prognostic and diagnostic roles of prolyl 4-hydroxylase subunit α members in breast cancer. Biomark. Med. 2021, 15, 1085–1095. [Google Scholar] [CrossRef]
- Cencioni, C.; Malatesta, S.; Vigiano Benedetti, V.; Licursi, V.; Perfetto, L.; Conte, F.; Ranieri, D.; Bartolazzi, A.; Kunkl, M.; Tuosto, L.; et al. The GLP-1R agonist semaglutide reshapes pancreatic cancer associated fibroblasts reducing collagen proline hydroxylation and favoring T lymphocyte infiltration. J. Exp. Clin. Cancer Res. 2025, 44, 18. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Huang, L.; Liu, J.; Chen, K.; Xu, R.; Wu, W. Single-cell analysis of gene regulatory networks in the mammary glands of P4HA1-knockout mice. PLoS Genet. 2025, 21, e1011505. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Serer, B.; Wissler, M.F.; Glover, B.K.; Lerner, M.G.; Oza, H.H.; Wang, V.; Knutsdottir, H.; Shojaeian, F.; Noller, K.; Baskaran, S.G.; et al. P4HA1 Mediates Hypoxia-Induced Invasion in Human Pancreatic Cancer Organoids. Cancer Res. Commun. 2025, 5, 881–895. [Google Scholar] [CrossRef]
- Pyagay, P.; Heroult, M.; Wang, Q.; Lehnert, W.; Belden, J.; Liaw, L.; Friesel, R.E.; Lindner, V. Collagen triple helix repeat containing 1, a novel secreted protein in injured and diseased arteries, inhibits collagen expression and promotes cell migration. Circ. Res. 2005, 96, 261–268. [Google Scholar] [CrossRef]
- Bhute, V.J.; Harte, J.; Houghton, J.W.; Maxwell, P.H. Mannose Binding Lectin Is Hydroxylated by Collagen Prolyl-4-hydroxylase and Inhibited by Some PHD Inhibitors. Kidney360 2020, 1, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Peng, R.; Liang, X.; Lan, Z.; Tang, M.; Hou, P.; Song, J.H.; Mak, C.S.L.; Park, J.; Zheng, S.-e.; et al. P4HA2-induced prolyl hydroxylation suppresses YAP1-mediated prostate cancer cell migration, invasion, and metastasis. Oncogene 2021, 40, 6049–6056. [Google Scholar] [CrossRef]
- Sato, K.; Sano, D.; Takahashi, H.; Kuwahara, T.; Aizawa, Y.; Aoyama, J.; Nojima, Y.; Hatano, T.; Arai, Y.; Nishimura, G.; et al. P4HA1 Promotes Cell Migration and Colonization in Hypopharyngeal Squamous Cell Carcinoma. Anticancer Res. 2023, 43, 2571–2582. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, J.; Zhu, Y.; Zhang, Y.; Zhao, X. Multilevel chitosan–gelatin particles loaded with P4HA1 siRNA suppress glioma development. Drug Deliv. Transl. Res. 2024, 14, 665–677. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hironaka, D.; Xiong, G. Enhanced Collagen Prolyl 4-Hydroxylase Activity and Expression Promote Cancer Progression via Both Canonical and Non-Canonical Mechanisms. Int. J. Mol. Sci. 2025, 26, 9371. https://doi.org/10.3390/ijms26199371
Hironaka D, Xiong G. Enhanced Collagen Prolyl 4-Hydroxylase Activity and Expression Promote Cancer Progression via Both Canonical and Non-Canonical Mechanisms. International Journal of Molecular Sciences. 2025; 26(19):9371. https://doi.org/10.3390/ijms26199371
Chicago/Turabian StyleHironaka, Dalton, and Gaofeng Xiong. 2025. "Enhanced Collagen Prolyl 4-Hydroxylase Activity and Expression Promote Cancer Progression via Both Canonical and Non-Canonical Mechanisms" International Journal of Molecular Sciences 26, no. 19: 9371. https://doi.org/10.3390/ijms26199371
APA StyleHironaka, D., & Xiong, G. (2025). Enhanced Collagen Prolyl 4-Hydroxylase Activity and Expression Promote Cancer Progression via Both Canonical and Non-Canonical Mechanisms. International Journal of Molecular Sciences, 26(19), 9371. https://doi.org/10.3390/ijms26199371




