Identification of miR136, miR155, and miR183 in Vascular Calcification in Human Peripheral Arteries
Abstract
1. Introduction
2. Results
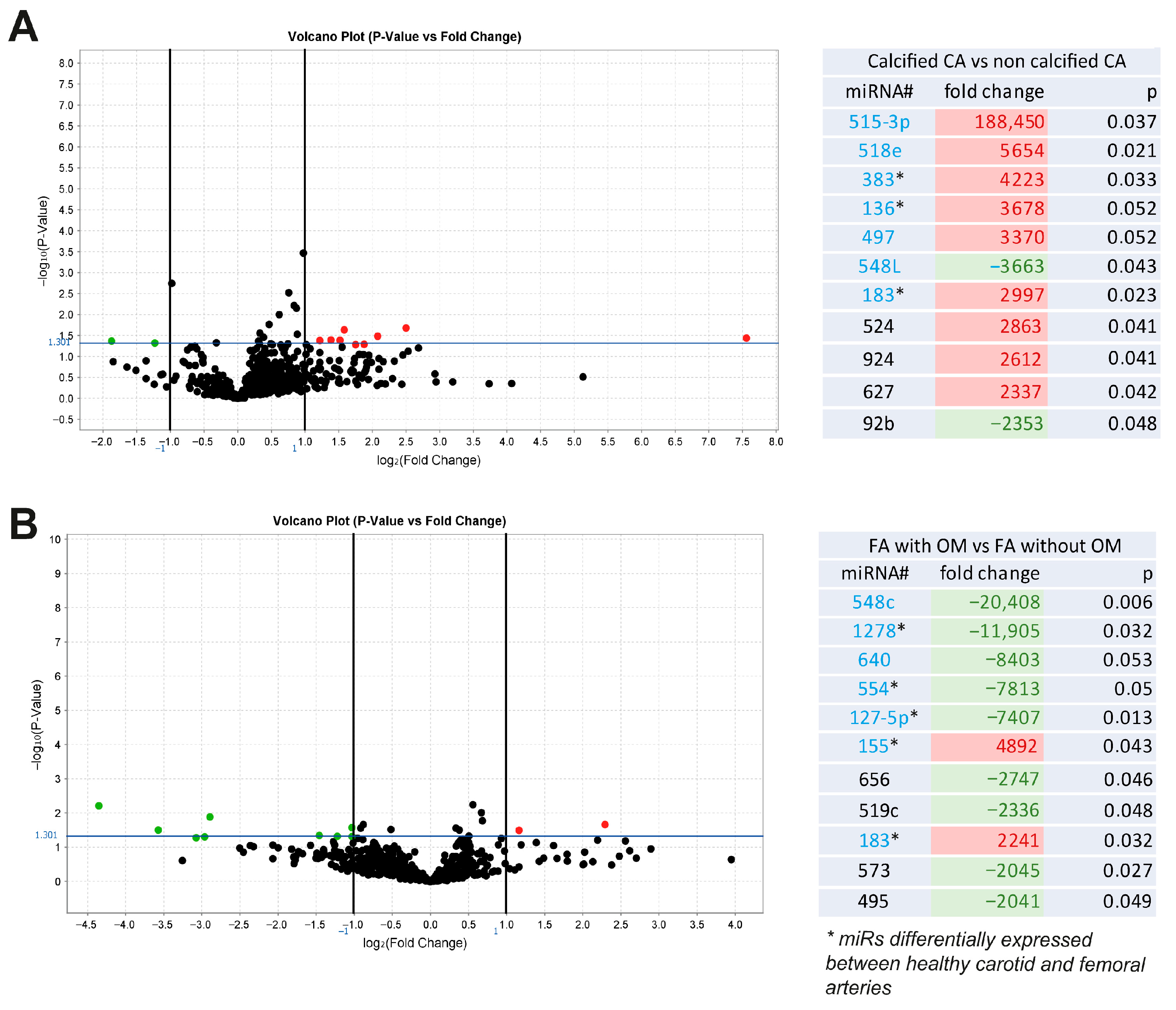
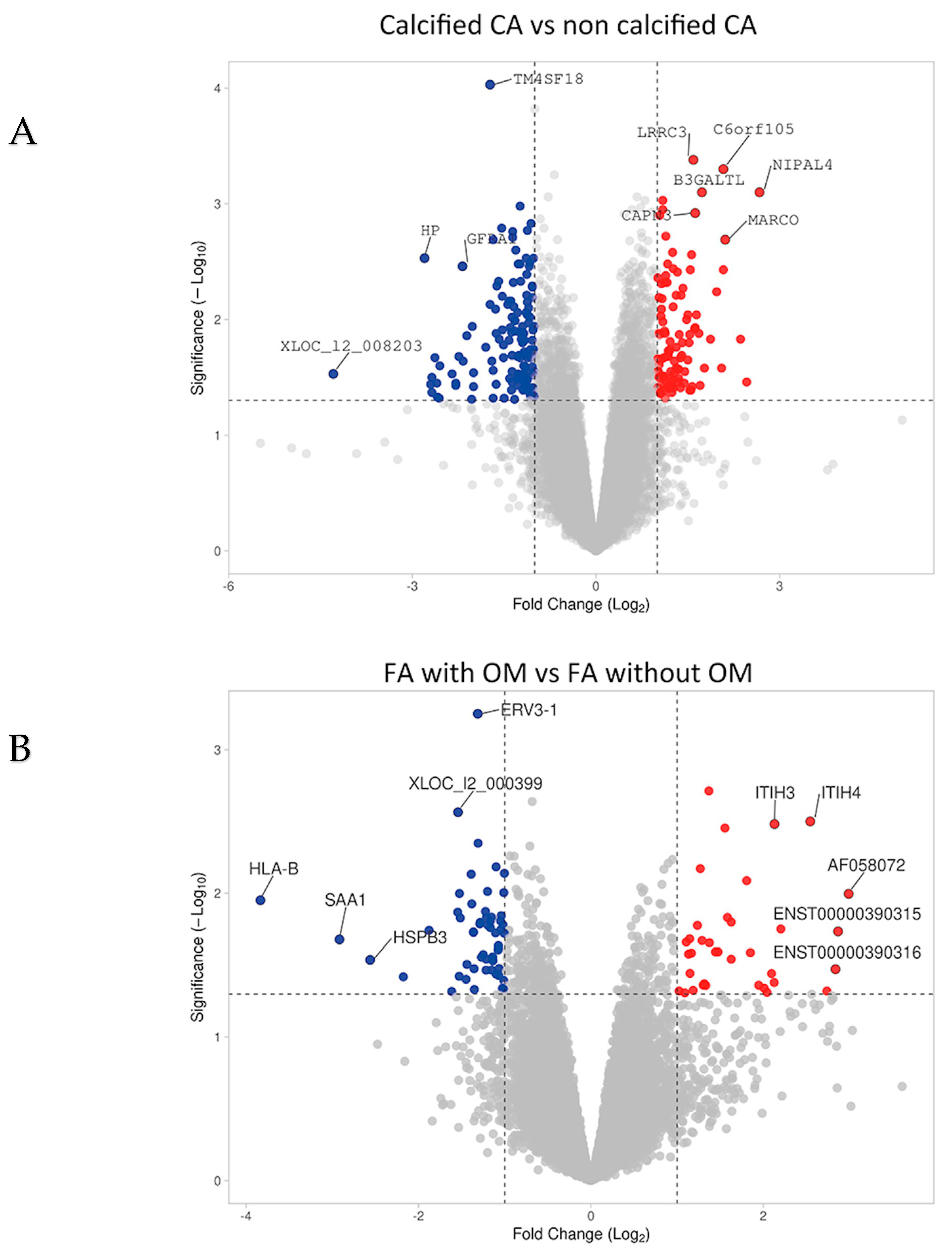
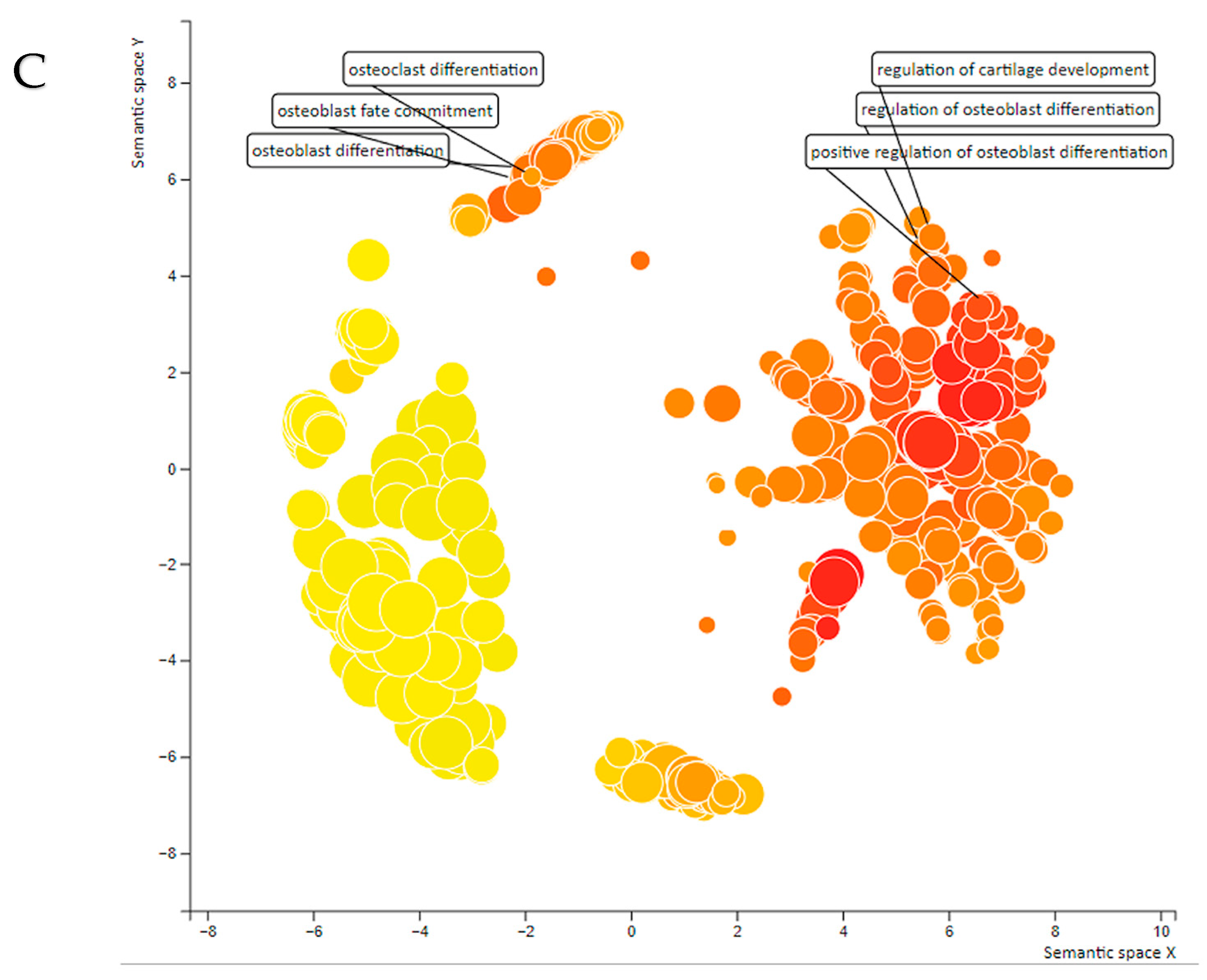
2.1. miRs Associated with Vascular Calcification and Ossification in Human Atherosclerotic Lesions
2.2. Selection of the Most Promising miRs Regulated During VSMC Mineralization
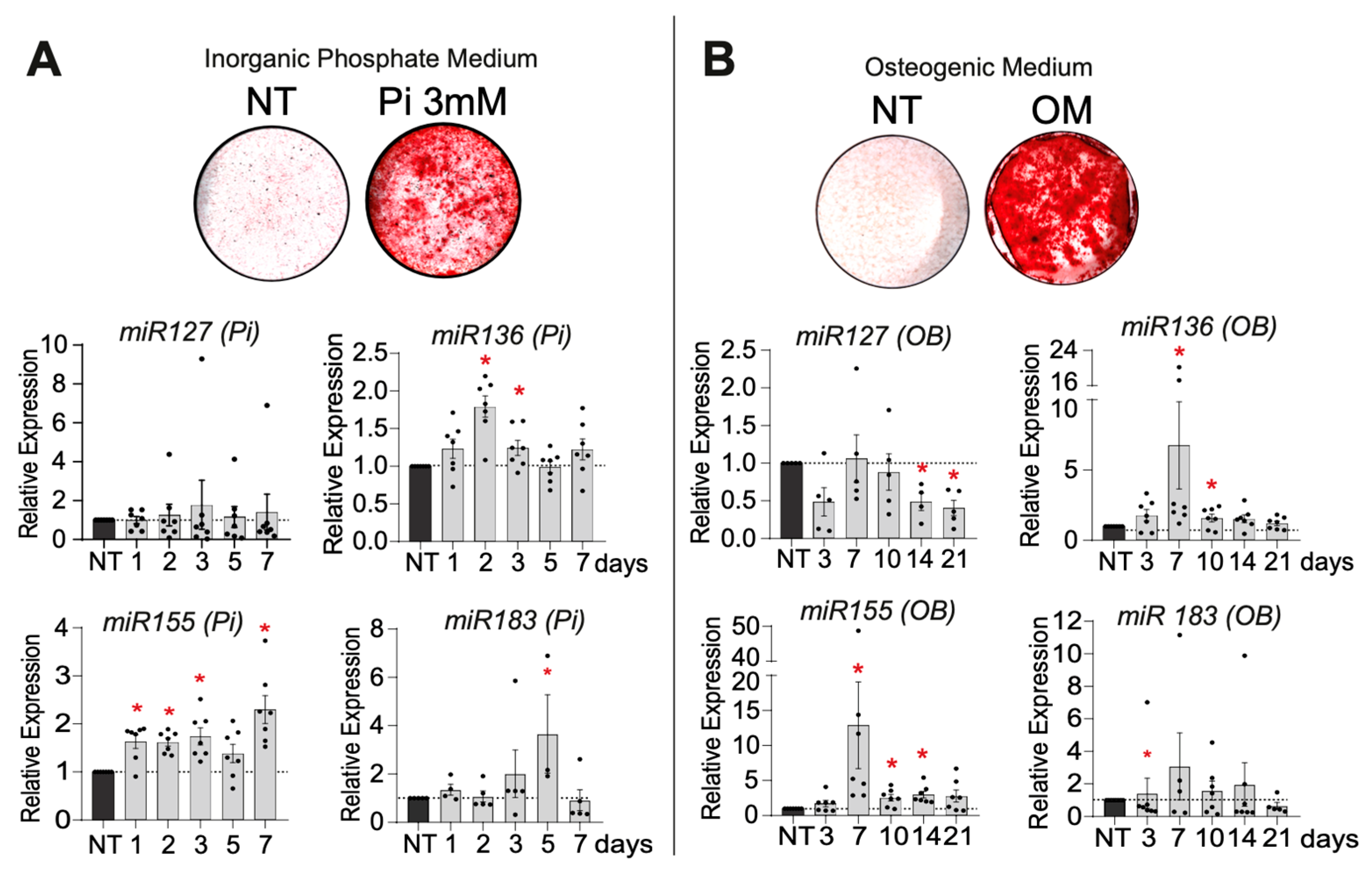
2.2.1. VSMC Mineralization
2.2.2. Selection of the miR Candidates According to Their Expression and Regulation During VSMC Mineralization
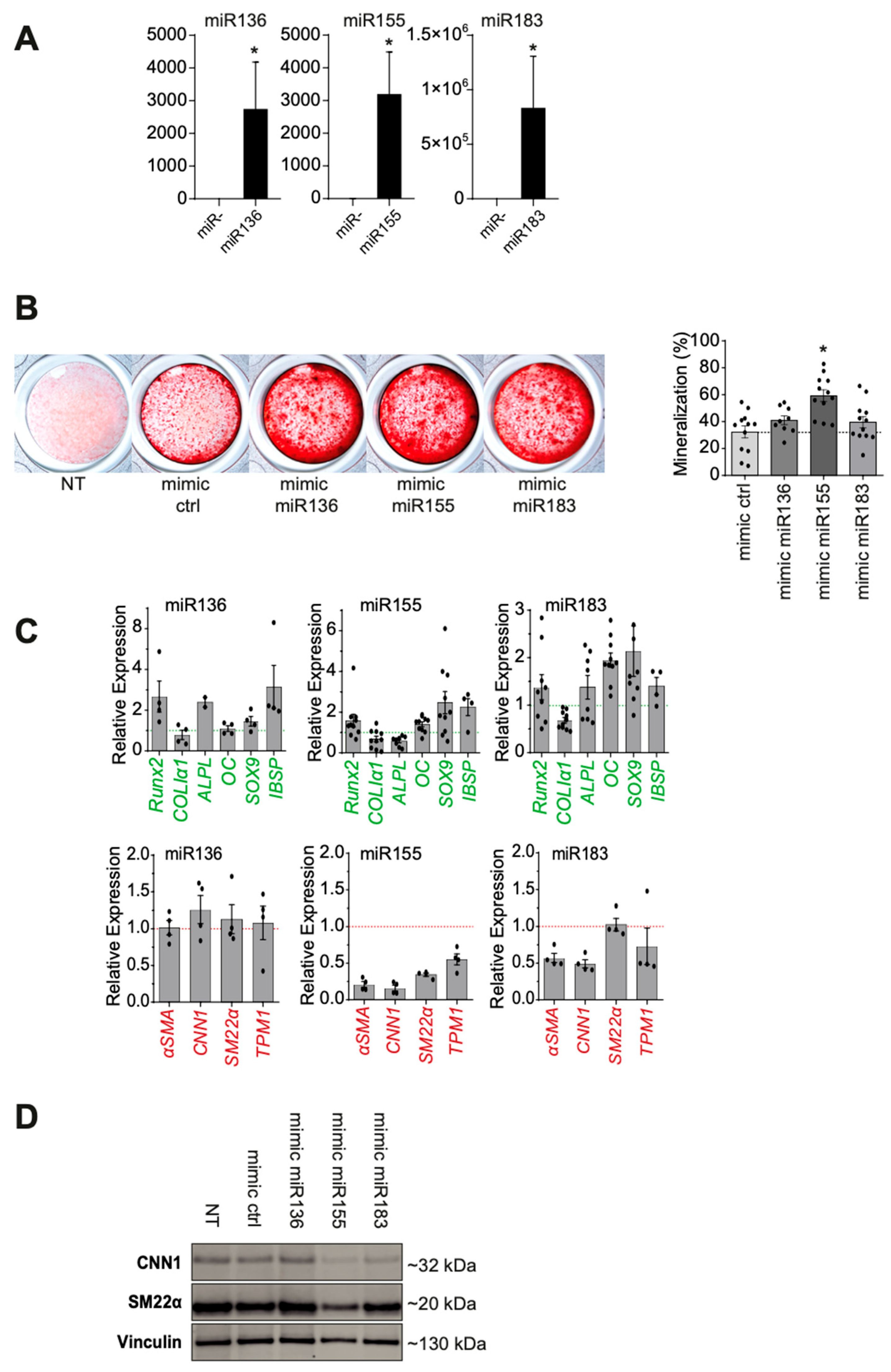
2.2.3. Functional Analysis After miR Candidate Transfection
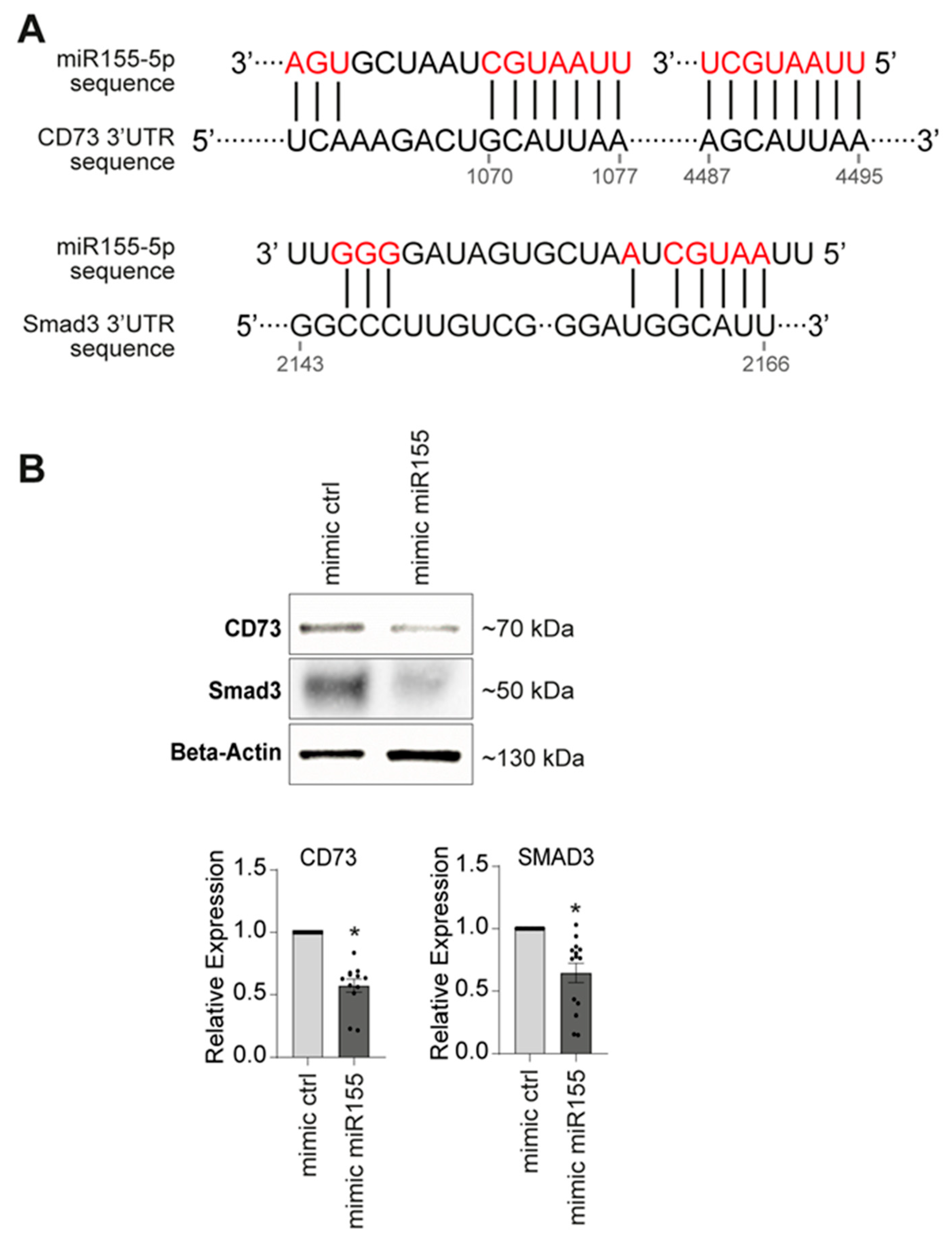
2.3. miR155 Targeted Gene Identification
3. Discussion
4. Materials and Methods
4.1. Biological Samples
4.2. RNA Extraction, miR Microfluidic Arrays, and Transcriptomic Analysis
4.3. Cell Culture and Mineralization
4.4. Cell Transfection and Functional Validation of miRs of Interest
4.5. Gene and miR Expression Analysis
4.6. In Silico Analysis of the miR Candidates
4.7. Protein Expression Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VC | Vascular Calcification |
| PAD | Peripheral Artery Disease |
| VSMCs | Vascular Smooth Muscle Cells |
References
- Sadat, U.; Mariam, N.B.G.; Usman, A.; Chowdhury, M.M.; El Nakhal, T.; DaCosta, O.F.; Gillard, J.H.; Hayes, P.D.; Varty, K. Association Between Abdominal Visceral Artery Calcification and All-Cause Mortality-A Computerized Tomography Imaging-Based Longitudinal Follow-Up Study. Angiology 2019, 70, 237–243. [Google Scholar] [CrossRef]
- Chowdhury, M.M.; Zieliński, L.P.; Sun, J.J.; Lambracos, S.; Boyle, J.R.; Harrison, S.C.; Rudd, J.H.; Coughlin, P.A. Editor’s Choice—Calcification of Thoracic and Abdominal Aneurysms is Associated with Mortality and Morbidity. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 101–108. [Google Scholar] [CrossRef]
- Thomas, I.C.; Thompson, C.A.; Yang, M.; Allison, M.A.; Forbang, N.I.; Michos, E.D.; McClelland, R.L.; Budoff, M.J.; Criqui, M.H. Thoracic Aorta Calcification and Noncardiovascular Disease-Related Mortality. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1926–1932. [Google Scholar] [CrossRef]
- Allison, M.A.; Hsi, S.; Wassel, C.L.; Morgan, C.; Ix, J.H.; Wright, C.M.; Criqui, M.H. Calcified atherosclerosis in different vascular beds and the risk of mortality. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Vengrenyuk, Y.; Cardoso, L.; Weinbaum, S. Micro-CT based analysis of a new paradigm for vulnerable plaque rupture: Cellular microcalcifications in fibrous caps. Mol. Cell Biomech. 2008, 5, 37–47. [Google Scholar]
- Mujaj, B.; Bos, D.; Selwaness, M.; Leening, M.J.G.; Kavousi, M.; Wentzel, J.J.; van der Lugt, A.; Hofman, A.; Stricker, B.H.; Vernooij, M.W.; et al. Statin use is associated with carotid plaque composition: The Rotterdam Study. Int. J. Cardiol. 2018, 260, 213–218. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef]
- Megale, A.; Wolosker, N.; Kalil, V.; Nigro, J.; Wakisaka, C.; Dias, B.; Teivelis, M.; Rocha, M.; Mendes, C. Calcium Score Predicts Mortality After Revascularization in Critical Limb Ischemia. J. Endovasc. Ther. 2022, 29, 438–443. [Google Scholar] [CrossRef]
- Skolnik, J.; Weiss, R.; Meyr, A.J.; Dhanisetty, R.; Choi, E.T.; Cunningham-Hill, M.; Rubin, D.; Oresanya, L. Evaluating the Impact of Medial Arterial Calcification on Outcomes of Infrageniculate Endovascular Interventions for Treatment of Diabetic Foot Ulcers. Vasc. Endovasc. Surg. 2021, 55, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, T.; Oba, Y.; Koshida, R.; Suzuki, Y.; Murata, A.; Ito, T. The Impact of Femoropopliteal Artery Calcium Score after Endovascular Treatment. Ann. Vasc. Surg. 2020, 66, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Espitia, O.; Chatelais, M.; Steenman, M.; Charrier, C.; Maurel, B.; Georges, S.; Houlgatte, R.; Verrecchia, F.; Ory, B.; Lamoureux, F.; et al. Implication of molecular vascular smooth muscle cell heterogeneity among arterial beds in arterial calcification. PLoS ONE 2018, 13, e0191976. [Google Scholar] [CrossRef]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of smooth muscle cells in vascular calcification: Implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef]
- Albright, R.A.; Stabach, P.; Cao, W.; Kavanagh, D.; Mullen, I.; Braddock, A.A.; Covo, M.S.; Tehan, M.; Yang, G.; Cheng, Z.; et al. ENPP1-Fc prevents mortality and vascular calcifications in rodent model of generalized arterial calcification of infancy. Nat. Commun. 2015, 6, 10006. [Google Scholar] [CrossRef]
- Goettsch, C.; Hutcheson, J.D.; Aikawa, E. Erratum: Goettsch et al. MicroRNA in cardiovascular calcification: Focus on targets and extracellular vesicle delivery mechanisms. Circ. Res. 2013, 112, 1073–1084. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, X.; Shen, Y.; Chen, L.; Xu, C.; Zhao, H.; Wu, Y.; Zhang, Q.; Zhong, J.; Tang, Z.; et al. MicroRNA-32 promotes calcification in vascular smooth muscle cells: Implications as a novel marker for coronary artery calcification. PLoS ONE 2017, 12, e0174138. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, L.; Cong, G.; Ren, L.; Hao, L. MicroRNA-34b/c inhibits aldosterone-induced vascular smooth muscle cell calcification via a SATB2/Runx2 pathway. Cell Tissue Res. 2016, 366, 733–746. [Google Scholar] [CrossRef]
- Qiao, W.; Chen, L.; Zhang, M. MicroRNA-205 regulates the calcification and osteoblastic differentiation of vascular smooth muscle cells. Cell Physiol. Biochem. 2014, 33, 1945–1953. [Google Scholar] [CrossRef]
- Wu, W.; Shang, Y.Q.; Dai, S.L.; Yi, F.; Wang, X.C. MiR-26a regulates vascular smooth muscle cell calcification in vitro through targeting CTGF. Bratisl. Lek. Listy 2017, 118, 499–503. [Google Scholar] [CrossRef]
- Cui, R.-R.; Li, S.-J.; Liu, L.-J.; Yi, L.; Liang, Q.-H.; Zhu, X.; Liu, G.-Y.; Liu, Y.; Wu, S.-S.; Liao, X.-B.; et al. MicroRNA-204 regulates vascular smooth muscle cell calcification in vitro and in vivo. Cardiovasc. Res. 2012, 96, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, J.; Gu, C.; Zhang, H. MiR-140-5p upregulation suppressed β-glycerophosphate-induced vascular smooth muscle cell calcification via targeting TLR4. Immunopharmacol. Immunotoxicol. 2022, 44, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Tan, J.; Lu, J.; Huang, J.; Li, X.; Xu, J.; Wang, X. MicroRNA-17-5p promotes vascular calcification by targeting ANKH. Curr. Neurovasc. Res. 2022, 19, 108–116. [Google Scholar] [CrossRef]
- Sudo, R.; Sato, F.; Azechi, T.; Wachi, H. MiR-29-mediated elastin down-regulation contributes to inorganic phosphorus-induced osteoblastic differentiation in vascular smooth muscle cells. Genes Cells 2015, 20, 1077–1087. [Google Scholar] [CrossRef]
- Mackenzie, N.C.W.; Staines, K.A.; Zhu, D.; Genever, P.; Macrae, V.E. miRNA-221 and miRNA-222 synergistically function to promote vascular calcification. Cell Biochem. Funct. 2014, 32, 209–216. [Google Scholar] [CrossRef]
- Herisson, F.; Heymann, M.F.; Chétiveaux, M.; Charrier, C.; Battaglia, S.; Pilet, P.; Rouillon, T.; Krempf, M.; Lemarchand, P.; Heymann, D.; et al. Carotid and femoral atherosclerotic plaques show different morphology. Atherosclerosis 2011, 216, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Goueffic, Y.; Davaine, J.M.; Merlini, T.; Rimbert, A.; Herisson, F.; Heymann, M.F.; Heymann, D.; Steenman, M.; Lambert, G. Arterial heterogeneity. Rev. Med. Interne 2013, 34, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Steenman, M.; Espitia, O.; Maurel, B.; Guyomarch, B.; Heymann, M.F.; Pistorius, M.A.; Ory, B.; Heymann, D.; Houlgatte, R.; Gouëffic, Y.; et al. Identification of genomic differences among peripheral arterial beds in atherosclerotic and healthy arteries. Sci. Rep. 2018, 8, 3940. [Google Scholar] [CrossRef] [PubMed]
- Shimokado, A.; Sun, Y.; Nakanishi, M.; Sato, F.; Oikawa, K.; Akasaka, T.; Muragaki, Y. Smad3 plays an inhibitory role in phosphate-induced vascular smooth muscle cell calcification. Exp. Mol. Pathol. 2014, 97, 458–464. [Google Scholar] [CrossRef]
- St Hilaire, C.; Ziegler, S.G.; Markello, T.C.; Brusco, A.; Groden, C.; Gill, F.; Carlson-Donohoe, H.; Lederman, R.J.; Chen, M.Y.; Yang, D.; et al. NT5E mutations and arterial calcifications. N. Engl. J. Med. 2011, 364, 432–442. [Google Scholar] [CrossRef]
- Raitoharju, E.; Lyytikäinen, L.-P.; Levula, M.; Oksala, N.; Mennander, A.; Tarkka, M.; Klopp, N.; Illig, T.; Kähönen, M.; Karhunen, P.J.; et al. miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis 2011, 219, 211–217. [Google Scholar] [CrossRef]
- Badi, I.; Mancinelli, L.; Polizzotto, A.; Ferri, D.; Zeni, F.; Burba, I.; Milano, G.; Brambilla, F.; Saccu, C.; Bianchi, M.E.; et al. miR-34a Promotes Vascular Smooth Muscle Cell Calcification by Downregulating SIRT1 (Sirtuin 1) and Axl (AXL Receptor Tyrosine Kinase). Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2079–2090. [Google Scholar] [CrossRef]
- Koide, T.; Mandai, S.; Kitaoka, R.; Matsuki, H.; Chiga, M.; Yamamoto, K.; Yoshioka, K.; Yagi, Y.; Suzuki, S.; Fujiki, T.; et al. Circulating Extracellular Vesicle-Propagated microRNA Signature as a Vascular Calcification Factor in Chronic Kidney Disease. Circ. Res. 2023, 132, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhong, J.-Y.; Lin, X.; Shan, S.-K.; Guo, B.; Zheng, M.-H.; Wang, Y.; Li, F.; Cui, R.-R.; Wu, F.; et al. Melatonin alleviates vascular calcification and ageing through exosomal miR-204/miR-211 cluster in a paracrine manner. J. Pineal Res. 2020, 68, e12631. [Google Scholar] [CrossRef]
- Zeng, P.; Yang, J.; Liu, L.; Yang, X.; Yao, Z.; Ma, C.; Zhu, H.; Su, J.; Zhao, Q.; Feng, K.; et al. ERK1/2 inhibition reduces vascular calcification by activating miR-126-3p-DKK1/LRP6 pathway. Theranostics 2021, 11, 1129–1146. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, J.; Huang, S.; Cheng, N.; Zhang, C.; Li, Y.; Wang, X.; Liu, J.; You, B.; Du, J. MicroRNA-223-3p inhibits vascular calcification and the osteogenic switch of vascular smooth muscle cells. J. Biol. Chem. 2021, 296, 100483. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-T.; Yuan, T.-H.; Yeh, H.-Y.; Chen, H.-Y.; Huang, J.-W.; Chen, H.-W. Risk Factors Associated with Altered Circulating Micro RNA -125b and Their Influences on Uremic Vascular Calcification Among Patients with End-Stage Renal Disease. J. Am. Heart Assoc. 2019, 8, e010805. [Google Scholar] [CrossRef]
- Heuschkel, M.A.; Babler, A.; Heyn, J.; van der Vorst, E.P.C.; Steenman, M.; Gesper, M.; Kappel, B.A.; Magne, D.; Gouëffic, Y.; Kramann, R.; et al. Distinct role of mitochondrial function and protein kinase C in intimal and medial calcification in vitro. Front. Cardiovasc. Med. 2022, 9, 959457. [Google Scholar] [CrossRef]
- Li, Y.; Sun, W.; Saaoud, F.; Wang, Y.; Wang, Q.; Hodge, J.; Hui, Y.; Yin, S.; Lessner, S.M.; Kong, X.; et al. MiR155 modulates vascular calcification by regulating Akt-FOXO3a signalling and apoptosis in vascular smooth muscle cells. J. Cell Mol. Med. 2021, 25, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, Z.; Chang, Z. Osteogenic differentiation and calcification of human aortic smooth muscle cells is induced by the RCN2/STAT3/miR-155-5p feedback loop. Vascul. Pharmacol. 2021, 136, 106821. [Google Scholar] [CrossRef]
- Fakhry, M.; Skafi, N.; Fayyad-Kazan, M.; Kobeissy, F.; Hamade, E.; Mebarek, S.; Habib, A.; Borghol, N.; Zeidan, A.; Magne, D.; et al. Characterization and assessment of potential microRNAs involved in phosphate-induced aortic calcification. J. Cell Physiol. 2018, 233, 4056–4067. [Google Scholar] [CrossRef]
- Kordaß, T.; Chao, T.Y.; Osen, W.; Eichmüller, S.B. Novel microRNAs modulating ecto-5’-nucleotidase expression. Front. Immunol. 2023, 14, 1199374. [Google Scholar]
- Hu, J.; Huang, S.; Liu, X.; Zhang, Y.; Wei, S.; Hu, X. miR-155: An Important Role in Inflammation Response. J. Immunol. Res. 2022, 2022, 7437281. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ma, C.; Zheng, X. MicroRNA-155 in the pathogenesis of atherosclerosis: A conflicting role? Heart Lung Circ. 2013, 22, 811–818. [Google Scholar] [CrossRef]
- Wei, Y.; Nazari-Jahantigh, M.; Neth, P.; Weber, C.; Schober, A. MicroRNA-126, -145, and -155: A therapeutic triad in atherosclerosis? Arterioscler. Thromb. Vasc. Biol. 2013, 33, 449–454. [Google Scholar] [CrossRef]
- Bruen, R.; Fitzsimons, S.; Belton, O. miR-155 in the Resolution of Atherosclerosis. Front. Pharmacol. 2019, 10, 463. [Google Scholar] [CrossRef]
- Zuo, J.; Yu, Y.; Zhu, M.; Jing, W.; Yu, M.; Chai, H.; Liang, C.; Tu, J. Inhibition of miR-155, a therapeutic target for breast cancer, prevented in cancer stem cell formation. Cancer Biomark. 2018, 21, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Su, L.-C.; Huang, A.-F.; Jia, H.; Liu, Y.; Xu, W.-D. Role of microRNA-155 in rheumatoid arthritis. Int. J. Rheum. Dis. 2017, 20, 1631–1637. [Google Scholar] [CrossRef]
- Davaine, J.M.; Quillard, T.; Brion, R.; Laperine, O.; Guyomarch, B.; Merlini, T.; Chatelais, M.; Guilbaud, F.; Brennan, M.Á.; Charrier, C.; et al. Osteoprotegerin, pericytes and bone-like vascular calcification are associated with carotid plaque stability. PLoS ONE 2014, 9, e107642. [Google Scholar] [CrossRef]
- Davaine, J.M.; Quillard, T.; Chatelais, M.; Guilbaud, F.; Brion, R.; Guyomarch, B.; Brennan, M.; Heymann, D.; Heymann, M.-F.; Gouëffic, Y. Bone Like Arterial Calcification in Femoral Atherosclerotic Lesions: Prevalence and Role of Osteoprotegerin and Pericytes. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 259–267. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Goedhart, J.; Luijsterburg, M.S. VolcaNoseR is a web app for creating, exploring, labeling and sharing volcano plots. Sci. Rep. 2020, 10, 20560. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Corvec, T.; Burgaud, M.; Steenman, M.; Tesfaye, R.A.; Gouëffic, Y.; Maurel, B.; Quillard, T. Identification of miR136, miR155, and miR183 in Vascular Calcification in Human Peripheral Arteries. Int. J. Mol. Sci. 2025, 26, 9349. https://doi.org/10.3390/ijms26199349
Le Corvec T, Burgaud M, Steenman M, Tesfaye RA, Gouëffic Y, Maurel B, Quillard T. Identification of miR136, miR155, and miR183 in Vascular Calcification in Human Peripheral Arteries. International Journal of Molecular Sciences. 2025; 26(19):9349. https://doi.org/10.3390/ijms26199349
Chicago/Turabian StyleLe Corvec, Tom, Mathilde Burgaud, Marja Steenman, Robel A. Tesfaye, Yann Gouëffic, Blandine Maurel, and Thibaut Quillard. 2025. "Identification of miR136, miR155, and miR183 in Vascular Calcification in Human Peripheral Arteries" International Journal of Molecular Sciences 26, no. 19: 9349. https://doi.org/10.3390/ijms26199349
APA StyleLe Corvec, T., Burgaud, M., Steenman, M., Tesfaye, R. A., Gouëffic, Y., Maurel, B., & Quillard, T. (2025). Identification of miR136, miR155, and miR183 in Vascular Calcification in Human Peripheral Arteries. International Journal of Molecular Sciences, 26(19), 9349. https://doi.org/10.3390/ijms26199349






