Effect of Nickel Stress on Nitrogen Metabolism in Cucumber Plants
Abstract
1. Introduction
2. Results
2.1. Growth and Ni Accumulation
2.2. and Contents and NR and NiR Activity
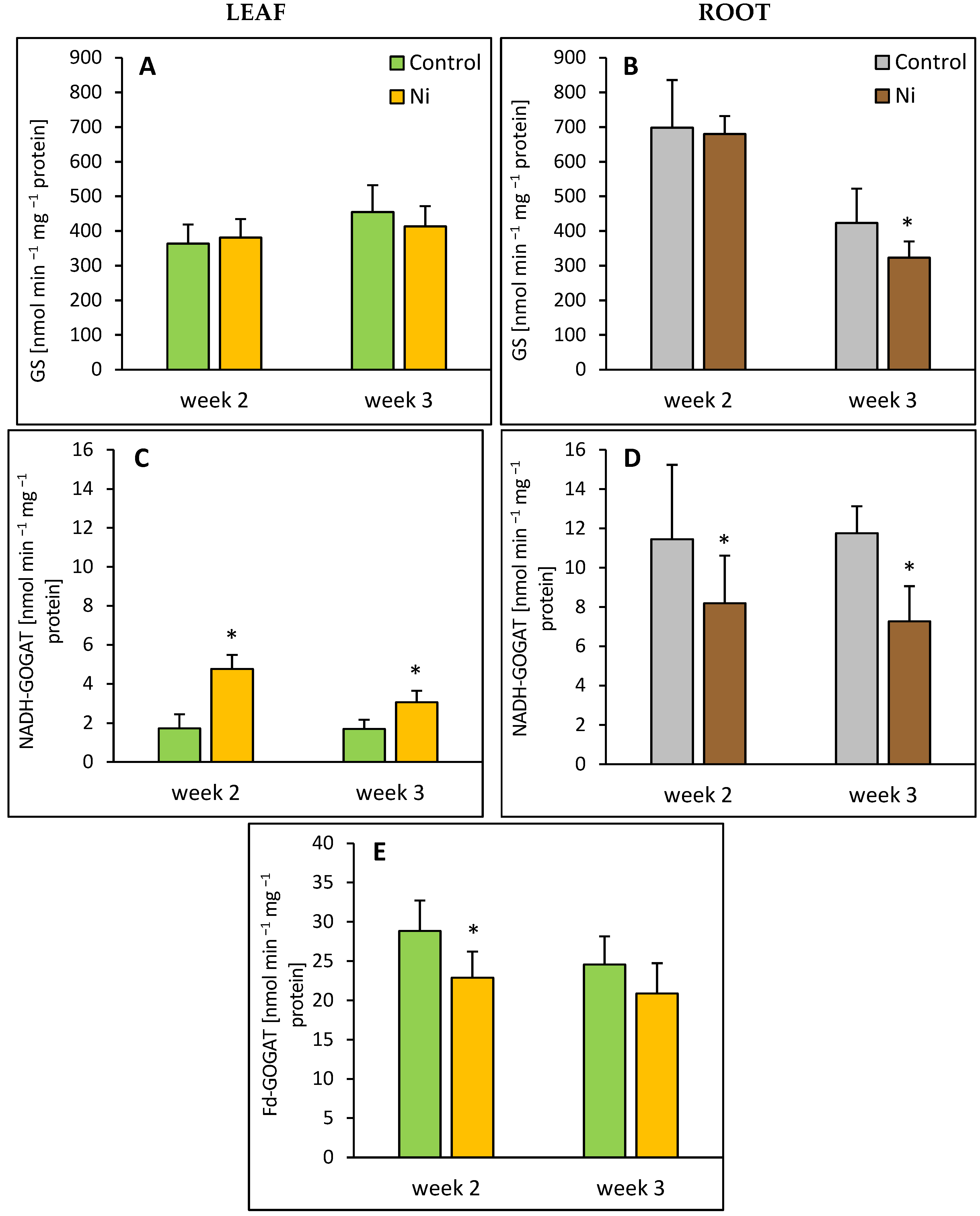
2.3. GS and GOGAT Activities
2.4. GDH and Aminotransferase Activities
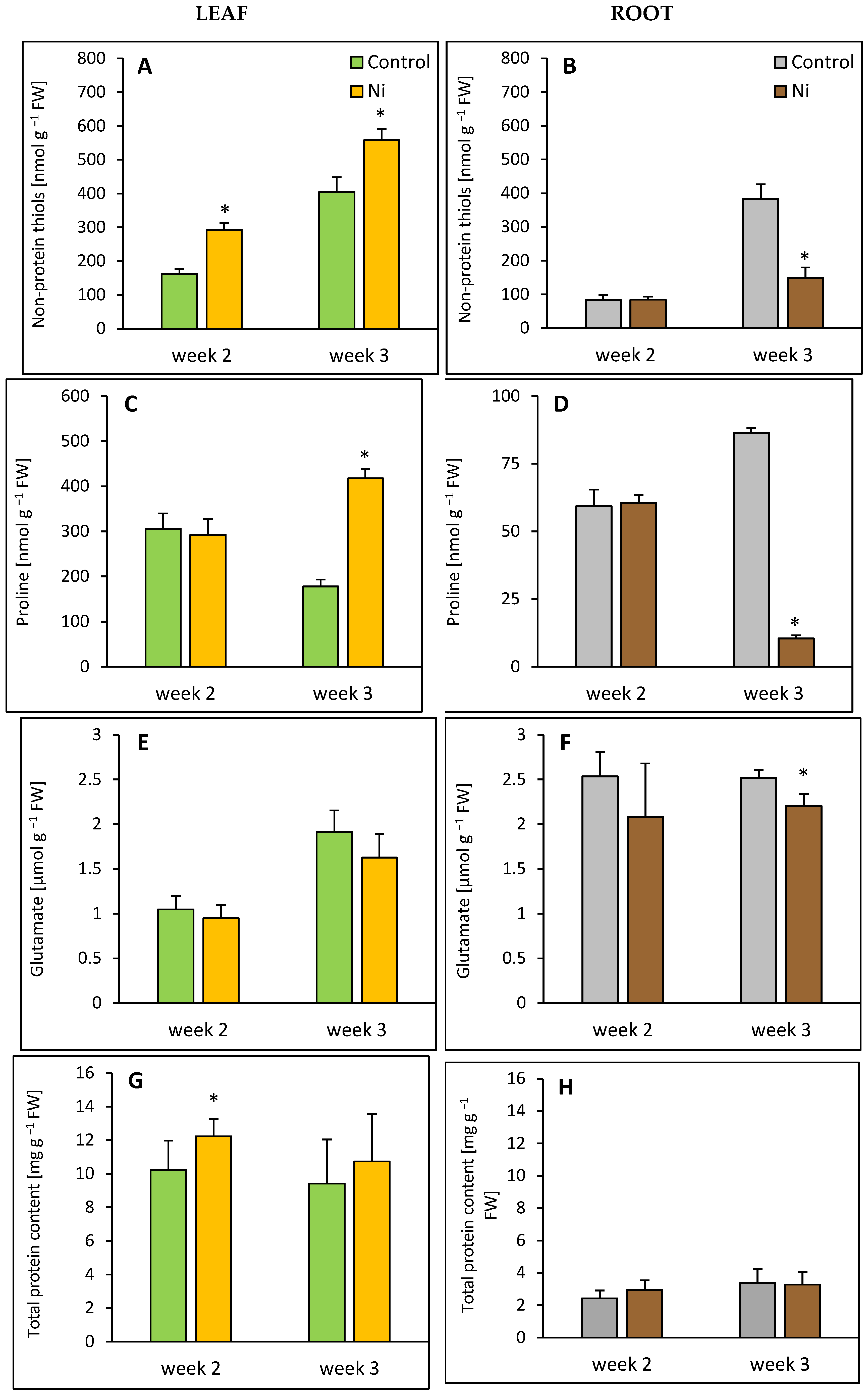
2.5. Non-Protein Thiol, Proline, Glutamate, and Total Protein Contents
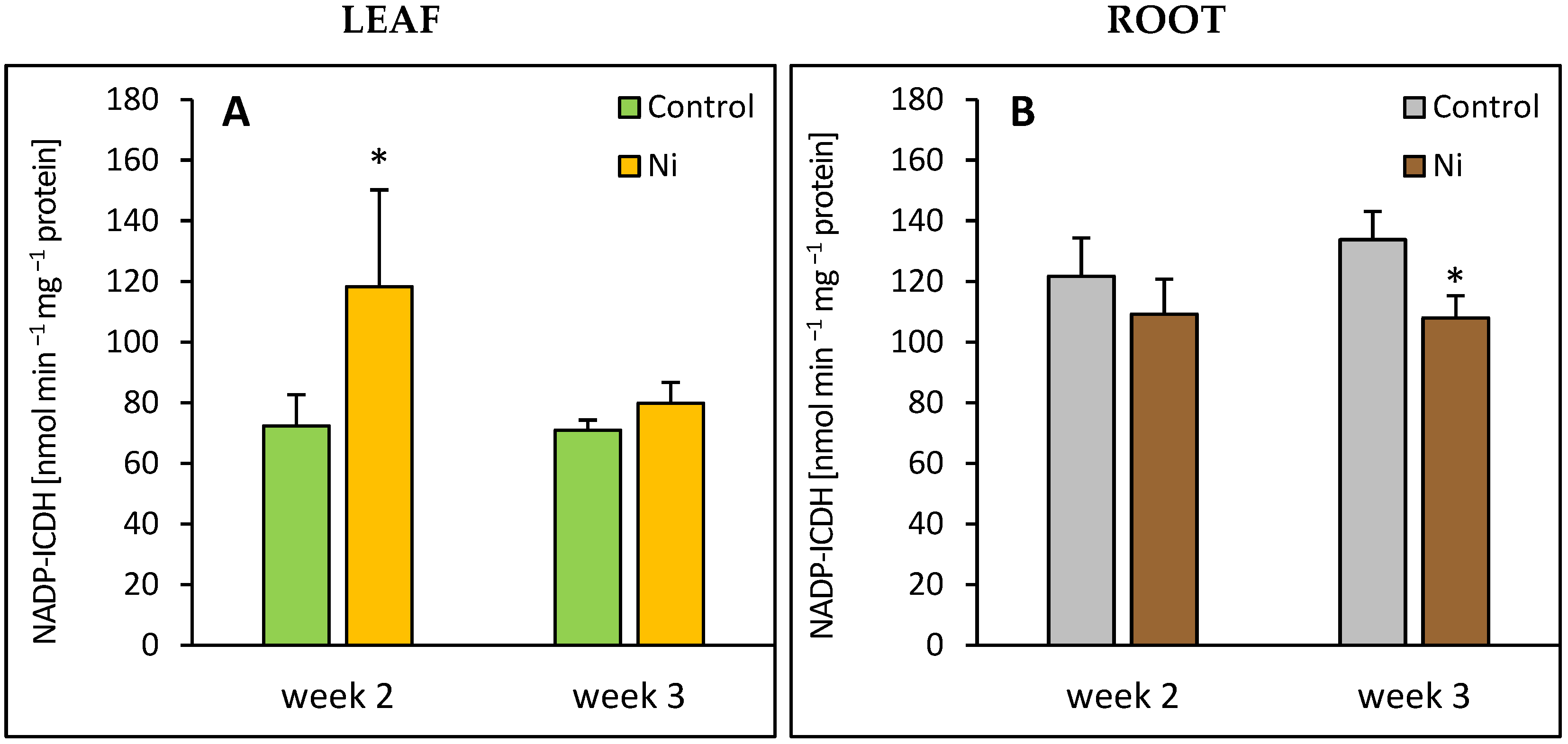
2.6. NADP-ICDH Activity
2.7. N-Metabolism-Related Enzymes Gene Expression
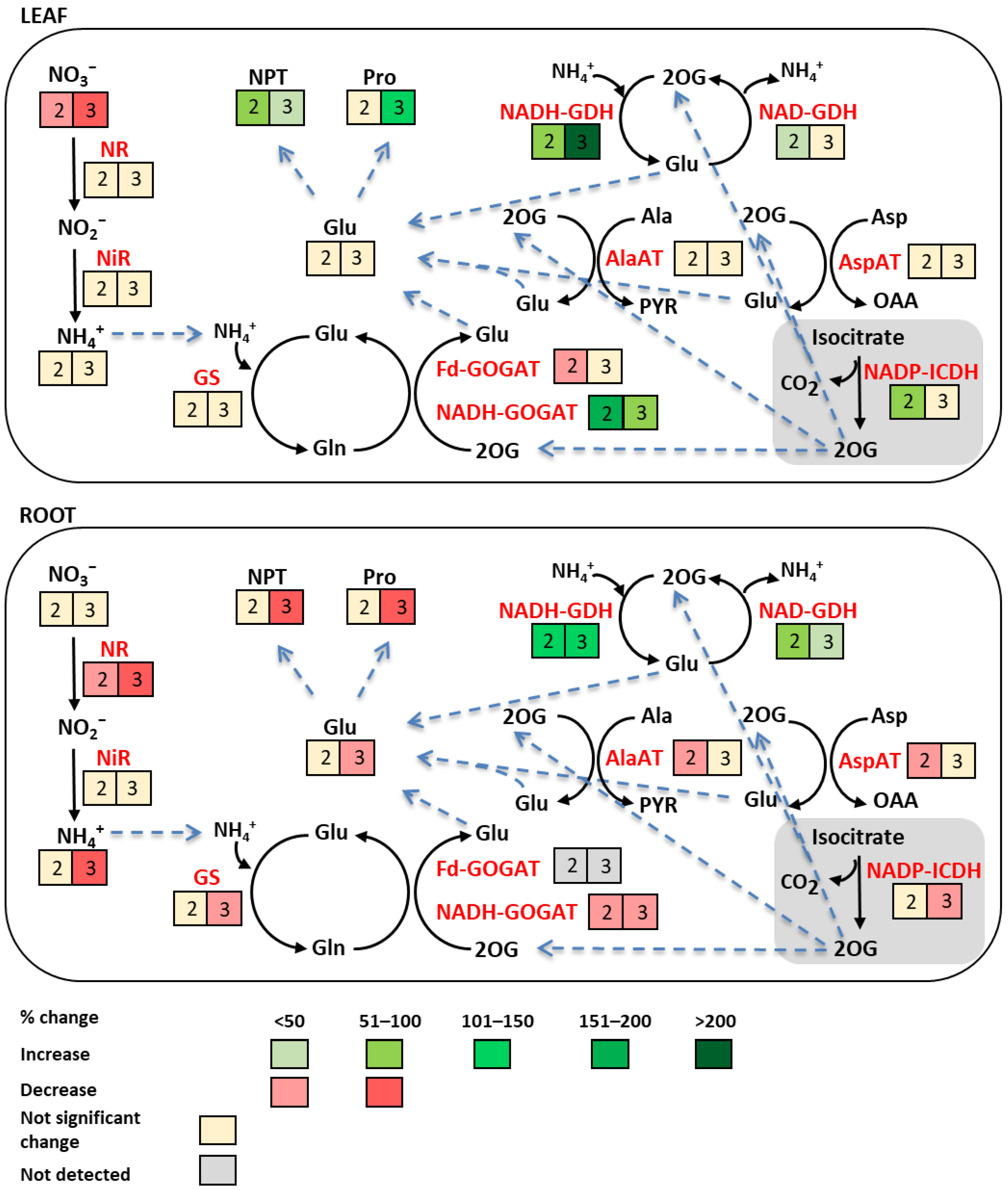
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Growth Parameters and Ni Contents
4.3. Nitrate, Ammonium, Glutamate, Proline, Non-Protein Thiols, and Total Protein Concentrations
4.4. Enzyme Extraction and Assays
4.4.1. Nitrate Reductase and Nitrite Reductase
4.4.2. Glutamate Synthase
4.4.3. Glutamine Synthetase, Glutamate Dehydrogenase, and Aminotransferases
4.4.4. Isocitrate Dehydrogenase
4.5. Gene Expression Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ala | Alanine |
| AlaAT | Alanine aminotransferase |
| Asp | Aspartate |
| AspAT | Aspartate aminotransferase |
| DTNB | 5,5′-dithiobis-(2-nitrobenzoic acid) |
| DW | Dry weight |
| EDTA | Ethylenediaminetetraacetic acid |
| FW | Fresh weight |
| GDH | Glutamate dehydrogenase |
| Gln | Glutamine |
| Glu | Glutamate |
| GOGAT | Glutamate synthase |
| GS | Glutamine synthetase |
| GSH | Glutathione |
| NADP-ICDH | Isocitrate dehydrogenase |
| NEA | Naphthylenediamine dihydrochloride |
| NiR | Nitrite reductase |
| NPT | Non-protein thiols |
| NR | Nitrate reductase |
| OAA | Oxaloacetate |
| 2-OG | 2-Oxoglutarate |
| PMSF | Phenylmethylsulphonyl fluoride |
| Pro | Proline |
| PVP | Polyvinylpyrrolidone |
| PYR | Pyruvate |
| SA | Sulfanilamide |
References
- Chen, C.; Huang, D.; Liu, J. Functions and Toxicity of Nickel in Plants: Recent Advances and Future Prospects. Clean 2009, 37, 304–313. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Rehman, A.; Cheema, S.A.; Fahad, S.; Rehman, S.; Sharma, A. Nickel; Whether Toxic or Essential for Plants and Environment—A Review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Aamer, M.; Nawaz, M.; Ali, A.; Khan, M.A.U.; Khan, T.A. Nickel Toxicity in Plants: Reasons, Toxic Effects, Tolerance Mechanisms, and Remediation Possibilities—A Review. Environ. Sci. Pollut. Res. 2019, 26, 12673–12688. [Google Scholar] [CrossRef] [PubMed]
- Kłobus, G.; Burzyński, M.; Buczek, J. Heavy Metals and Nitrogen Metabolism. In Physiology and Biochemistry of Metal Toxicity and Tolerance in Plants; Prasad, M.N.V., Strzałka, K., Eds.; Springer: Dordrecht, The Netherlands, 2002; ISBN 978-90-481-5952-9. [Google Scholar]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen Uptake, Assimilation and Remobilization in Plants: Challenges for Sustainable and Productive Agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Miflin, B.J.; Habash, D.Z. The Role of Glutamine Synthetase and Glutamate Dehydrogenase in Nitrogen Assimilation and Possibilities for Improvement in the Nitrogen Utilization of Crops. J. Exp. Bot. 2002, 53, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Kishorekumar, R.; Bulle, M.; Wany, A.; Gupta, K.J. An Overview of Important Enzymes Involved in Nitrogen Assimilation of Plants. In Nitrogen Metabolism in Plants. Methods in Molecular Biology; Gupta, K., Ed.; Humana: New York, NY, USA, 2020; Volume 2057, pp. 1–13. [Google Scholar]
- Brechlin, P.; Unterhalt, A.; Tischner, R.; Mäck, G. Cytosolic and Chloroplastic Glutamine Synthetase of Sugarbeet (Beta vulgaris) Respond Differently to Organ Ontogeny and Nitrogen Source. Physiol. Plant. 2000, 108, 263–269. [Google Scholar] [CrossRef]
- Xin, M.; Qin, Z.; Yang, J.; Zhou, X.; Wang, L. Functional Analysis of the Nitrogen Metabolism-Related Gene CsGS1 in Cucumber. J. Integr. Agric. 2021, 20, 1515–1524. [Google Scholar] [CrossRef]
- Dubois, F.; Tercé-Laforgue, T.; Gonzalez-Moro, M.-B.; Estavillo, J.-M.; Sangwan, R.; Gallais, A.; Hirel, B. Glutamate Dehydrogenase in Plants: Is There a New Story for an Old Enzyme? Plant Physiol. Biochem. 2003, 41, 565–576. [Google Scholar] [CrossRef]
- Robinson, S.A.; Slade, A.P.; Fox, G.G.; Phillips, R.; Ratcliffe, R.G.; Stewart, G.R. The Role of Glutamate Dehydrogenase in Plant Nitrogen Metabolism. Plant Physiol. 1991, 95, 509–516. [Google Scholar] [CrossRef]
- Romero-Cruz, M.d.C.; Leon-Vaz, A.; Vega, J.M.; Vigara, J. Alterations in Nitrogen Metabolism Caused by Heavy Metals in the Acid-Tolerant Microalga Coccomyxa onubensis. Algal Res. 2024, 84, 103784. [Google Scholar] [CrossRef]
- Forde, B.G.; Lea, P.J. Glutamate in Plants: Metabolism, Regulation, and Signalling. J. Exp. Bot. 2007, 58, 2339–2358. [Google Scholar] [CrossRef]
- Mhamdi, A.; Mauve, C.; Houda, G.; Saindrenan, P.; Hodges, M.; Noctor, G. Cytosolic NADP-Dependent Isocitrate Dehydrogenase Contributes to Redox Homeostasis and the Regulation of Pathogen Responses in Arabidopsis Leaves. Plant Cell Environ. 2010, 33, 1112–1123. [Google Scholar] [CrossRef]
- Baslam, M.; Mitsui, T.; Sueyoshi, K.; Ohyama, T. Recent Advances in Carbon and Nitrogen Metabolism in C3 Plants. Int. J. Mol. Sci. 2021, 22, 318. [Google Scholar] [CrossRef]
- Xin, M.; Wang, L.; Liu, Y.; Feng, Z.; Zhou, X.; Qin, Z. Transcriptome Profiling of Cucumber Genome Expression in Response to Long-Term Low Nitrogen Stress. Acta Physiol. Plant. 2017, 39, 130. [Google Scholar] [CrossRef]
- Gajewska, E.; Skłodowska, M. Nickel-Induced Changes in Nitrogen Metabolism in Wheat Shoots. J. Plant Physiol. 2009, 166, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, E.; Wielanek, M.; Bergier, K.; Skłodowska, M. Nickel-Induced Depression of Nitrogen Assimilation in Wheat Roots. Acta Physiol. Plant. 2009, 31, 1291–1300. [Google Scholar] [CrossRef]
- Hosseini, H.; Khoshgoftarmanesh, A.H. The Effect of Foliar Application of Nickel in the Mineral Form and Urea-Ni Complex on Fresh Weight and Nitrogen Metabolism of Lettuce. Sci. Hortic. 2013, 164, 178–182. [Google Scholar] [CrossRef]
- Rizwan, M.; Usman, K.; Alsafran, M.; Jabri, H.A.; Samreen, T.; Saleem, M.H.; Tu, S. Nickel Toxicity Interferes with /. Uptake and Nitrogen Metabolic Enzyme Activity in Rice (Oryza sativa L.). Plants 2022, 11, 1401. [Google Scholar] [CrossRef]
- Rampazzo, M.V.; Cunha, M.L.O.; de Oliveira, L.C.A.; Silva, V.M.; Lanza, M.G.D.B.; de Melo, A.A.R.; dos Reis, A.R. Physiological Roles of Nickel on Antioxidant and Nitrogen Metabolism Increasing the Yield of Sugarcane Plants. J. Soil Sci. Plant Nutr. 2022, 22, 4438–4448. [Google Scholar] [CrossRef]
- Kevrešan, S.; Petrović, N.; Popović, M.; Kandrač, J. Effect of Heavy Metals on Nitrate and Protein Metabolism in Sugar Beet. Biol. Plant. 1998, 41, 235–240. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Khan, N.A.; Masood, A.; Per, T.S.; Asgher, M. Hydrogen Peroxide Alleviates Nickel-Inhibited Photosynthetic Responses through Increase in Use-Efficiency of Nitrogen and Sulfur, and Glutathione Production in Mustard. Front. Plant Sci. 2016, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Chaffei, C.; Pageau, K.; Suzuki, A.; Gouia, H.; Ghorbel, M.H.; Masclaux-Daubresse, C. Cadmium Toxicity Induced Changes in Nitrogen Management in Lycopersicon esculentum Leading to a Metabolic Safeguard through an Amino Acid Storage Strategy. Plant Cell Physiol. 2004, 45, 1681–1693. [Google Scholar] [CrossRef] [PubMed]
- Chaffei, C.; Gouia, H.; Ghorbel, M.H. Nitrogen Metabolism in Tomato Plants Under Cadmium Stress. J. Plant Nutr. 2003, 26, 1617–1634. [Google Scholar] [CrossRef]
- Attia, H.; Alamer, K.H. Supplementation of Jasmonic Acid Mitigates the Damaging Effects of Arsenic Stress on Growth, Photosynthesis and Nitrogen Metabolism in Rice. Rice 2024, 17, 31. [Google Scholar] [CrossRef]
- Zhang, L.-L.; He, X.-J.; Chen, M.; An, R.-D.; An, X.-L.; Li, J. Responses of Nitrogen Metabolism to Copper Stress in Luffa cylindrica Roots. J. Soil Sci. Plant Nutr. 2014, 14, 616–624. [Google Scholar] [CrossRef]
- Eprintsev, A.T.; Anokhina, G.B.; Selivanova, P.S.; Moskvina, P.P.; Igamberdiev, A.U. Biochemical and Epigenetic Regulation of Glutamate Metabolism in Maize (Zea mays L.) Leaves under Salt Stress. Plants 2024, 13, 2651. [Google Scholar] [CrossRef]
- León, A.M.; Palma, J.M.; Corpas, F.J.; Gómez, M.; Romero-Puertas, M.C.; Chatterjee, D.; Mateos, R.M.; del Río, L.A.; Sandalio, L.M. Antioxidative Enzymes in Cultivars of Pepper Plants with Different Sensitivity to Cadmium. Plant Physiol. Biochem. 2002, 40, 813–820. [Google Scholar] [CrossRef]
- Chaffei-Haouari, C.; Carrayol, E.; Ghorbel, M.H.; Gouia, H. Physiological and Biochemical Effects of Cadmium Toxicity in Enzymes Involved in Nitrogen and Amino—Acid Metabolism in Tomato Plants. Acta Bot. Gall. 2009, 156, 477–486. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, A.; Li, S.; Chai, T.; Liu, T.; Wang, J.; Qiao, K. A Novel NADP+-Isocitrate Dehydrogenase Contributes to Cadmium/Lead Detoxification and Tolerance in Plants. Int. J. Biol. Macromol. 2025, 312, 144094. [Google Scholar] [CrossRef]
- Lin, Y.C.; Kao, C.H. Proline Accumulation Induced by Excess Nickel in Detached Rice Leaves. Biol. Plant. 2007, 51, 351–354. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.-J. The Significance of Amino Acids and Amino Acid-Derived Molecules in Plant Responses and Adaptation to Heavy Metal Stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, E.; Skłodowska, M. Antioxidative Responses and Proline Level in Leaves and Roots of Pea Plants Subjected to Nickel Stress. Acta Physiol. Plant. 2005, 27, 329–340. [Google Scholar] [CrossRef]
- Kumar, S.; Wang, M.; Liu, Y.; Fahad, S.; Qayyum, A.; Jadoon, S.A.; Chen, Y.; Zhu, G. Nickel Toxicity Alters Growth Patterns and Induces Oxidative Stress Response in Sweetpotato. Front. Plant Sci. 2022, 13, 1054924. [Google Scholar] [CrossRef] [PubMed]
- Spormann, S.; Nadais, P.; Sousa, F.; Pinto, M.; Martins, M.; Sousa, B.; Fidalgo, F.; Soares, C. Accumulation of Proline in Plants under Contaminated Soils—Are We on the Same Page? Antioxidants 2023, 12, 666. [Google Scholar] [CrossRef]
- Morzyk-Ociepa, B.; Zelichowicz, N. The Stability Constants of Complexes of L-Proline with Nickel(II), Cobalt(II), and Copper(II) Determined by the Potentiometric Method. Chem. Pap. 1992, 46, 84–87. [Google Scholar]
- Atta, N.; Shahbaz, M.; Farhat, F.; Maqsood, M.F.; Zulfiqar, U.; Naz, N.; Ahmed, M.M.; Hassan, N.U.; Mujahid, N.; Mustafa, A.E.-Z.M.A.; et al. Proline-Mediated Redox Regulation in Wheat for Mitigating Nickel-Induced Stress and Soil Decontamination. Sci. Rep. 2024, 14, 456. [Google Scholar] [CrossRef]
- De Sousa, A.; AbdElgawad, H.; Fidalgo, F.; Teixeira, J.; Matos, M.; Hamed, B.A.; Selim, S.; Hozzein, W.N.; Beemster, G.T.S.; Asard, H. Al Exposure Increases Proline Levels by Different Pathways in an Al-Sensitive and an Al-Tolerant Rye Genotype. Sci. Rep. 2020, 10, 16401. [Google Scholar] [CrossRef]
- Kısa, D. Responses of Phytochelatin and Proline-Related Genes Expression Associated with Heavy Metal Stress in Solanum lycopersicum. Acta Bot. Croat. 2019, 78, 9–16. [Google Scholar] [CrossRef]
- Kumar, H.; Sharma, D.; Kumar, V. Nickel-Induced Oxidative Stress and Role of Antioxidant Defense in Barley Roots and Leaves. Int. J. Environ. Biol. 2012, 2, 121–128. [Google Scholar]
- Maheshwari, R.; Dubey, R.S. Nickel-Induced Oxidative Stress and the Role of Antioxidant Defence in Rice Seedlings. Plant Growth Regul. 2009, 59, 37–49. [Google Scholar] [CrossRef]
- Prajapati, D.H.; Ausma, T.; de Boer, J.; Hawkesford, M.J.; De Kok, L.J. Nickel Toxicity in Brassica Rapa Seedlings: Impact on Sulfur Metabolism and Mineral Nutrient Content. J. Fur Kult. 2020, 72, 473–478. [Google Scholar] [CrossRef]
- Maleva, M.G.; Nekrasova, G.F.; Malec, P.; Prasad, M.N.V.; Strzałka, K. Ecophysiological Tolerance of Elodea canadensis to Nickel Exposure. Chemosphere 2009, 77, 392–398. [Google Scholar] [CrossRef]
- Zagorchev, L.; Seal, C.; Kranner, I.; Odjakova, M. A Central Role for Thiols in Plant Tolerance to Abiotic Stress. Int. J. Mol. Sci. 2013, 14, 7405–7432. [Google Scholar] [CrossRef] [PubMed]
- Salbitani, G.; Maresca, V.; Cianciullo, P.; Bossa, R.; Carfagna, S.; Basile, A. Non-Protein Thiol Compounds and Antioxidant Responses Involved in Bryophyte Heavy-Metal Tolerance. Int. J. Mol. Sci. 2023, 24, 5302. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, R.; Ozawa, T.; Naito, T.; Kameda, Y.; Takenaga, H. Interactions between Cadmium and Nickel in Phytochelatin Biosynthesis and the Detoxification of the Two Metals in Suspension-Cultured Tobacco Cells. Biol. Plant. 2001, 44, 627–630. [Google Scholar] [CrossRef]
- Afonseca, A.; Mota, I.; Vasques, G.; Soares, L.; Flores, M.; Azenha, M.; Teixeira, J. Nickel-Induced Differential Expression of Metallothioneins and Phytochelatin Synthase 1 in Arabidopsis thaliana: Organ-Specific Responses. Agronomy 2024, 14, 3026. [Google Scholar] [CrossRef]
- Mesci, S.; Çatal, M.İ. Effects of Nickel Sulphate and Lead Acetate Trihydrate on Heavy Metal Stress-Related Gene Activities in Forage Pea (Pisum sativum ssp. arvense L.) in Türkiye. Front. Plant Sci. 2025, 16, 1549488. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in Plants: Biosynthesis and Physiological Role in Environmental Stress Tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Kukkola, E.; Rautio, P.; Huttunen, S. Stress Indications in Copper- and Nickel-Exposed Scots Pine Seedlings. Environ. Exp. Bot. 2000, 43, 197–210. [Google Scholar] [CrossRef]
- Gajewska, E.; Witusińska, A.; Bernat, P. Nickel-Induced Oxidative Stress and Phospholipid Remodeling in Cucumber Leaves. Plant Sci. 2024, 348, 112229. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Haroon, M.H.; Schrader, L.E.; Youngs, V.L. Rapid Colorimetric Determination of Nitrate in Plant Tissue by Nitration of Salicylic Acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Molins-Legua, C.; Meseguer-Lloret, S.; Moliner-Martinez, Y.; Campíns-Falcó, P. A Guide for Selecting the Most Appropriate Method for Ammonium Determination in Water Analysis. TrAC Trends Anal. Chem. 2006, 25, 282–290. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Israr, M.; Sahi, S.V.; Jain, J. Cadmium Accumulation and Antioxidative Responses in the Sesbania drummondii Callus. Arch. Environ. Contam. Toxicol. 2006, 50, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Debouba, M.; Gouia, H.; Suzuki, A.; Ghorbel, M.H. NaCl Stress Effects on Enzymes Involved in Nitrogen Assimilation Pathway in Tomato “Lycopersicon esculentum” Seedlings. J. Plant Physiol. 2006, 163, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Groat, R.G.; Vance, C.P. Root Nodule Enzymes of Ammonia Assimilation in Alfalfa (Medicago sativa L.). Developmental Patterns and Response to Applied Nitrogen. Plant Physiol. 1981, 67, 1198–1203. [Google Scholar] [CrossRef]
- Agbaria, H.; Heuer, B.; Zieslin, N. Rootstock-Imposed Alterations in Nitrate Reductase and Glutamine Synthetase Activities in Leaves of Rose Plants. Biol. Plant. 1998, 41, 85–91. [Google Scholar] [CrossRef]
- De Sousa, C.A.F.; Sodek, L. Alanine Metabolism and Alanine Aminotransferase Activity in Soybean (Glycine max) during Hypoxia of the Root System and Subsequent Return to Normoxia. Environ. Exp. Bot. 2003, 50, 1–8. [Google Scholar] [CrossRef]
- Yang, Z.-M.; Yang, H.; Wang, J.; Wang, Y.-S. Aluminum Regulation of Citrate Metabolism for Al-Induced Citrate Efflux in the Roots of Cassia tora L. Plant Sci. 2004, 166, 1589–1594. [Google Scholar] [CrossRef]
- Ma, C.; Ban, T.; Yu, H.; Li, Q.; Li, X.; Jiang, W.; Xie, J. Urea Addition Promotes the Metabolism and Utilization of Nitrogen in Cucumber. Agronomy 2019, 9, 262. [Google Scholar] [CrossRef]



| Treatment | Fresh Weight [mg] | Ni Content [μg g −1 DW] | |||
|---|---|---|---|---|---|
| Week 2 | Week 3 | Week 2 | Week 3 | ||
| Leaf | Control | 284.3 ± 58.9 | 360 ± 55.33 | 1.98 ± 0.75 | 2.29 ± 0.25 |
| Ni | 133.6 ± 43.3 * | 214.23 ± 39.68 * | 292.85 ± 65.81 * | 286.45 ± 41.87 * | |
| Root | Control | 533.47 ± 104.87 | 927.92 ± 260.7 | 4.38 ± 0.42 | 3.25 ± 0.44 |
| Ni | 221.24 ± 58.77 * | 465.11 ± 167.58 * | 592.89 ± 82.12 * | 502.28 ± 194.27 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajewska, E.; Witusińska, A. Effect of Nickel Stress on Nitrogen Metabolism in Cucumber Plants. Int. J. Mol. Sci. 2025, 26, 9327. https://doi.org/10.3390/ijms26199327
Gajewska E, Witusińska A. Effect of Nickel Stress on Nitrogen Metabolism in Cucumber Plants. International Journal of Molecular Sciences. 2025; 26(19):9327. https://doi.org/10.3390/ijms26199327
Chicago/Turabian StyleGajewska, Ewa, and Aleksandra Witusińska. 2025. "Effect of Nickel Stress on Nitrogen Metabolism in Cucumber Plants" International Journal of Molecular Sciences 26, no. 19: 9327. https://doi.org/10.3390/ijms26199327
APA StyleGajewska, E., & Witusińska, A. (2025). Effect of Nickel Stress on Nitrogen Metabolism in Cucumber Plants. International Journal of Molecular Sciences, 26(19), 9327. https://doi.org/10.3390/ijms26199327






