TIGIT Expression and Its Implications in Non-Small-Cell Lung Cancer Progression and Therapy: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Review Design and Search Strategy
- -
- Population: The primary population included patients diagnosed with NSCLC at any stage who received TIGIT-targeted therapy, either alone or in combination with other immunotherapies.
- -
- Intervention: Interventions of interest included monoclonal antibodies targeting TIGIT (e.g., tiragolumab, vibostolimab) administered as monotherapy or in combination with PD-1/PD-L1 inhibitors. These therapies were selected based on their mechanism of action in modulating immune checkpoints to enhance antitumor immune responses.
- -
- Comparison: Comparative analysis included standard-of-care treatments (e.g., PD-1/PD-L1 inhibitors alone, chemotherapy) or placebo, depending on the study design.
- -
- Outcome: Outcomes analyzed focused on efficacy (objective response rate [ORR], progression-free survival [PFS], overall survival [OS], duration of response 450544 [DOR]) and safety (treatment-related adverse events [TRAEs], tolerability). Additionally, studies analyzing PD-L1 and TIGIT expression were included to evaluate prognostic and predictive biomarker potential.
2.2. Collecting Data
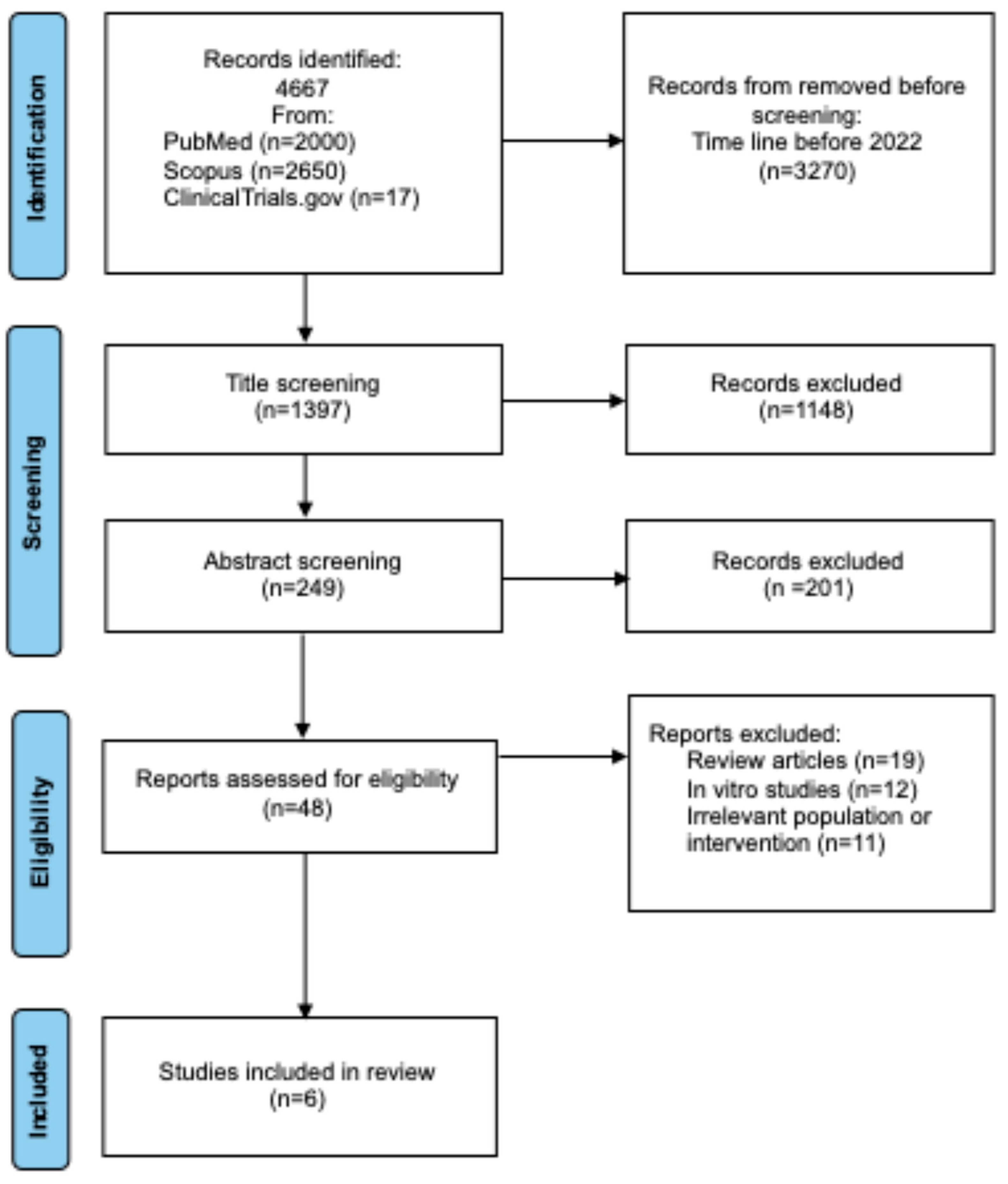
2.3. Selection and Identifications of Studies
2.4. Assessment of Risk of Bias in the Included Studies
3. TIGIT Expression in Non-Small-Cell Lung Cancer
4. Mechanism of Action of TIGIT
4.1. Direct Intracellular Signaling
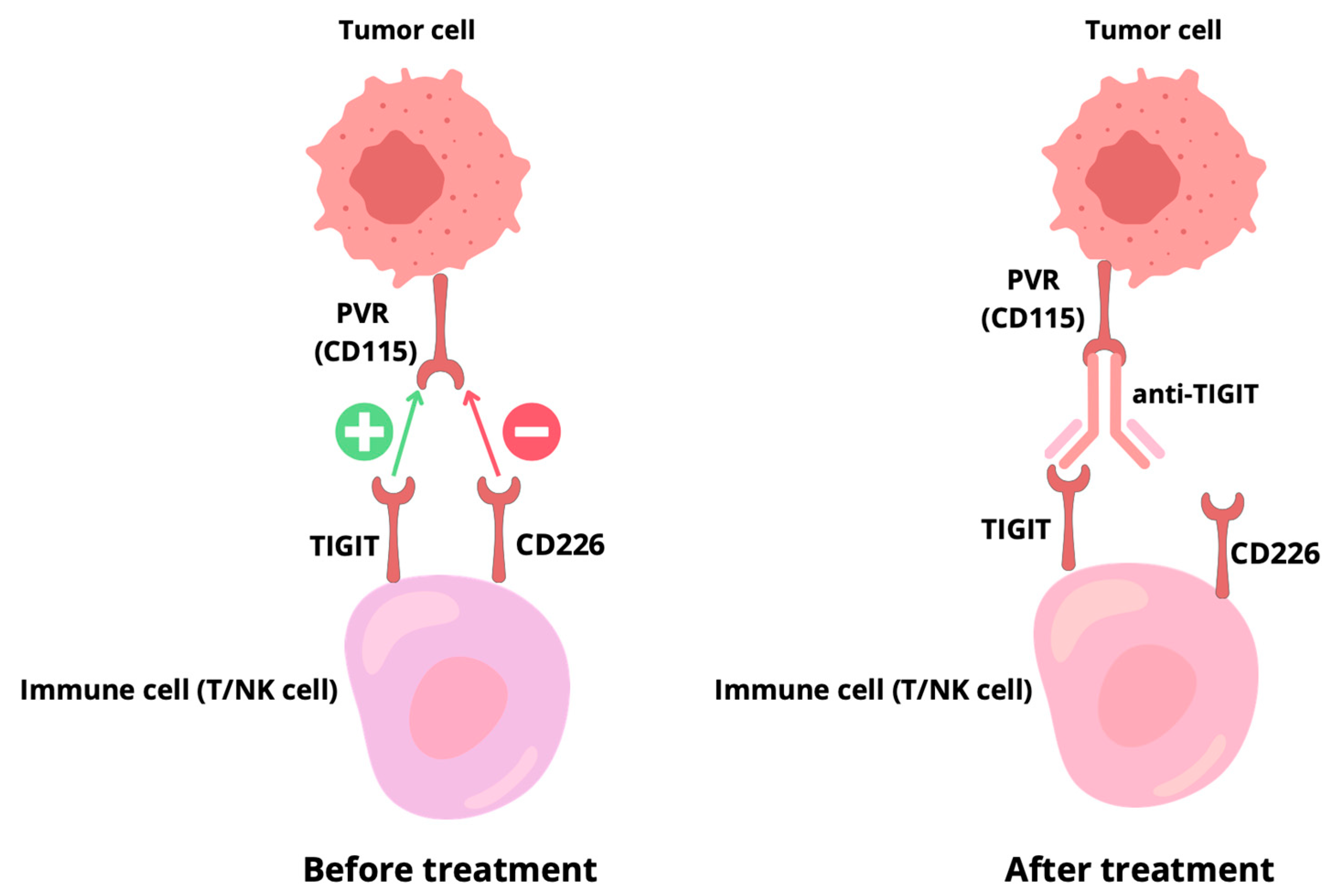
4.2. Regulation of CD226 Activity
4.3. Reverse Signaling by PVR Can Modulate the Immune Microenvironment
5. TIGIT-Targeted Drugs
6. Clinical Use of Anti-TIGIT in Non-Small-Cell Lung Cancer
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALK | Anaplastic Lymphoma Kinase |
| BRAF | B-Raf Proto-Oncogene |
| Cbl-b | Ubiquitin–Protein Ligase |
| cCRT | Concurrent Chemoradiotherapy |
| CTLA-4 | Cytotoxic T-Lymphocyte–Associated Protein 4 |
| DOR | Duration of Response |
| EOMES | Eomesodermin |
| ERK 1/2 | Extracellular Signal-Regulated Kinases 1 and 2 |
| FDA | Food and Drug Administration |
| FOXO1 | Forkhead Box O1 |
| FOXP3 | Forkhead Box P3 |
| Grb2 | Growth Factor Receptor-Bound Protein 2 |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HR | Hazard Ratio |
| IFN-γ | Interferon Gamma |
| ICI | Immune Checkpoint Inhibitor |
| ITIM | Immunoreceptor Tyrosine-Based Inhibitory Motif |
| KRAS | Kirsten Rat Sarcoma Viral Oncogene Homolog |
| LAG-3 | Lymphocyte Activation Gene 3 |
| LC | Lung Cancer |
| MAPK | Mitogen-Activated Protein Kinase |
| MET | Mesenchymal–Epithelial Transition Factor |
| MHC | Major Histocompatibility Complex |
| NK | Natural Killer |
| NKT | Natural Killer T |
| NSCLC | Non-Small-Cell Lung Cancer |
| ORR | Objective Response Rate |
| OX | Tumor necrosis factor receptor superfamily member 4 |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Death-Ligand 1 |
| PFS | Progression-Free Survival |
| PVR | Poliovirus Receptor |
| RCT | Randomized Controlled Trials |
| RET | REarranged during Transfection |
| ROS1 | C-Ros Oncogene 1 |
| SCLC | Small-Cell-Lung Cancer |
| SHIP-1–Src | Homology 2 Domain-Containing Inositol 5-Phosphatase 1 |
| SH-2–Src | Homology 2 |
| T-BET | T-box Expressed in T Cells |
| TCE7 | Transcription factor 7 |
| T EFF | T Effector Cells |
| T EM | Effector Memory T Cells |
| TILs | Tumor-Infiltrating Lymphocytes |
| TIGIT | T-cell Immunoreceptor with Ig and ITIM Domains |
| TIM-3 | T-cell Immunoglobulin and Mucin Domain-Containing Protein 3 |
| T RM | T tissue-resident memory cells |
| TRAEs | Treatment-Related Adverse Events |
| TP53 | Tumor Protein p53 |
| ZAP70/Syk | Zeta-Chain-Associated Protein Kinase 70/Spleen Tyrosine Kinase |
| HPV | Human Papillomavirus |
| EGFR | Epidermal Growth Factor Receptor |
References
- Srivastava, S.; Mohanty, A.; Nam, A.; Singhal, S.; Salgia, R. Chemokines and NSCLC: Emerging role in prognosis, heterogeneity, and therapeutics. Semin. Cancer Biol. 2022, 86, 233–246. [Google Scholar] [CrossRef]
- Kehrle, K.; Hetjens, M.; Hetjens, S. Risk factors and preventive measures for lung cancer in the European Union. Epidemiologia 2024, 5, 539–546. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Gao, Y.; Chen, Y.; Qin, L.; Wu, I.X. Risk factors for the development of lung cancer among never smokers: A systematic review. Cancer Epidemiol. 2022, 81, 102274. [Google Scholar] [CrossRef]
- Zhang, Y.; Vaccarella, S.; Morgan, E.; Li, M.; Etxeberria, J.; Chokunonga, E.; Shunker Manraj, S.; Kamate, B.; Omonisi, A.; Bray, F. Global variations in lung cancer incidence by histological subtype in 2020: A population-based study. Lancet Oncol. 2023, 24, 1206–1218. [Google Scholar] [CrossRef]
- Wang, Z.; Xing, Y.; Li, B.; Li, X.; Liu, B.; Wang, Y. Molecular pathways, resistance mechanisms and targeted interventions in non-small-cell lung cancer. Mol. Biomed. 2022, 3, 42. [Google Scholar] [CrossRef]
- Kumar, M.; Sarkar, A. Current therapeutic strategies and challenges in NSCLC treatment: A comprehensive review. Exp. Oncol. 2022, 44, 7–16. [Google Scholar] [CrossRef]
- Jachowski, A.; Marcinkowski, M.; Szydłowski, J.; Grabarczyk, O.; Nogaj, Z.; Marcin, Ł.; Pławski, A.; Piotr Jagodziński, P.; Kazimierz Słowikowski, B. Modern therapies of nonsmall cell lung cancer. J. Appl. Genet. 2023, 64, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Hiltbrunner, S.; Cords, L.; Kasser, S.; Freiberger, S.N.; Kreutzer, S.; Toussaint, N.C.; Grob, L.; Opitz, I.; Messerli, M.; Zoche, M.; et al. Acquired resistance to anti-PD1 therapy in patients with NSCLC associates with immunosuppressive T cell phenotype. Nat. Commun. 2023, 14, 5154. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Liu, C.; Wang, Z.; Wu, W.; Zhang, N.; Zhang, L.; Hu, J.; Luo, P.; Zhang, J.; et al. Immune checkpoint modulators in cancer immunotherapy: Recent advances and emerging concepts. J. Hematol. Oncol. 2022, 15, 111. [Google Scholar] [CrossRef]
- Rishiq, A.; Bsoul, R.; Pick, O.; Mandelboim, O. Studying TIGIT activity against tumors through the generation of knockout mice. OncoImmunology 2023, 12, 2217735. [Google Scholar] [CrossRef]
- Jeong, B.S.; Nam, H.; Lee, J.; Park, H.Y.; Cho, K.J.; Sheen, J.H.; Song, E.; Oh, M.; Lee, S.; Choi, H.; et al. Structural and functional characterization of a monoclonal antibody blocking TIGIT. mAbs 2022, 14, 2013750. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, A.; Parisi, C.; Barlesi, F. Anti-TIGIT therapies for solid tumors: A systematic review. ESMO Open 2023, 8, 101184. [Google Scholar] [CrossRef]
- Schöpf, F.; Marongiu, G.L.; Milaj, K.; Sprink, T.; Kikhney, J.; Moter, A.; Roderer, D. Structural basis of Fusobacterium nucleatum adhesin Fap2 interaction with receptors on cancer and immune cells. Nat. Commun. 2025, 16, 8104. [Google Scholar] [CrossRef]
- Mireștean, C.C.; Iancu, R.I.; Iancu, D.P.T. LAG3, TIM3 and TIGIT: New targets for immunotherapy and potential associations with radiotherapy. Curr. Oncol. 2025, 32, 230. [Google Scholar] [CrossRef]
- Guégan, J.P.; Peyraud, F.; Dadone-Montaudie, B.; Teyssonneau, D.; Palmieri, L.J.; Clot, E.; Cousin, S.; Roubaud, G.; Cabart, M.; Leroy, L.; et al. Analysis of PD1, LAG3, TIGIT, and TIM3 expression in human lung adenocarcinoma reveals a 25-gene signature predicting immunotherapy response. Cell Rep. Med. 2024, 5, 101831. [Google Scholar] [CrossRef]
- Krzyżanowska, N.; Wojas-Krawczyk, K.; Milanowski, J.; Krawczyk, P. Future prospects of immunotherapy in non-small-cell lung cancer patients: Is there hope in other immune checkpoints targeting molecules? Int. J. Mol. Sci. 2022, 23, 3087. [Google Scholar] [CrossRef]
- Patel, A.J.; Middleton, G.W. TIGIT-based immunotherapeutics in lung cancer. Immunother. Adv. 2023, 3, ltad009. [Google Scholar] [CrossRef] [PubMed]
- Joller, N.; Anderson, A.C.; Kuchroo, V.K. LAG-3, TIM-3, and TIGIT: Distinct functions in immune regulation. Immunity 2024, 57, 206–222. [Google Scholar] [CrossRef]
- Reches, A.; Ophir, Y.; Stein, N.; Kol, I.; Isaacson, B.; Amikam, Y.C.; Elnekave, A.; Tsukerman, P.; Kucan Brlic, P.; Lenac, T.; et al. Nectin4 is a novel TIGIT ligand which combines checkpoint inhibition and tumor specificity. Front. Immunol. 2024, 15, 1430163. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Chen, J.; Ji, T.; Cong, X. TIGIT, a novel immune checkpoint therapy for melanoma. Cell Death Dis. 2023, 14, 466. [Google Scholar] [CrossRef]
- Worboys, J.D.; Vowell, K.N.; Hare, R.K.; Ambrose, A.R.; Bertuzzi, M.; Conner, M.A.; Patel, F.P.; Zammit, W.H.; Gali-Moya, J.; Hazime, K.S.; et al. TIGIT can inhibit T cell activation via ligation-induced nanoclusters, independent of CD226 co-stimulation. Nat. Commun. 2023, 14, 5016. [Google Scholar] [CrossRef] [PubMed]
- Banta, K.L.; Xu, X.; Chitre, A.S.; Au-Yeung, A.; Takahashi, C.; O’Gorman, W.E.; Wu, T.D.; Mittman, S.; Cubas, R.; Comps-Agrar, L.; et al. Mechanistic convergence of the TIGIT and PD-1 inhibitory pathways necessitates co-blockade to optimize anti-tumor CD8+ T cell responses. Immunity 2022, 55, 512–526.e9. [Google Scholar] [CrossRef] [PubMed]
- Conner, M.; Zhang, Y.; Liu, X. Emergence of the CD226 axis in cancer immunotherapy. Front. Immunol. 2022, 13, 914406. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Sim, H.I.; Yun, B.; Park, Y.; Jin, H. Revisiting T-cell adhesion molecules as potential targets for cancer immunotherapy: CD226 and CD2. Exp. Mol. Med. 2024, 56, 2113–2126. [Google Scholar] [CrossRef]
- Cui, H.; Hamad, M.; Elkord, E. TIGIT in cancer: From mechanism of action to promising immunotherapeutic strategies. Cell Death Dis. 2025, 16, 664. [Google Scholar] [CrossRef]
- Meng, F.; Xiang, M.; Liu, Y.; Zeng, D. TIGIT/PVR axis regulates anti-tumor immunity in hematologic malignancies. Ann. Hematol. 2025, 104, 1415–1426. [Google Scholar] [CrossRef]
- Nutsch, K.; Banta, K.L.; Wu, T.D.; Tran, C.W.; Mittman, S.; Duong, E.; Nabet, B.Y.; Qu, Y.; Williams, K.; Müller, S.; et al. TIGIT and PD-L1 co-blockade promotes clonal expansion of multipotent, non-exhausted antitumor T cells by facilitating co-stimulation. Nat. Cancer 2024, 5, 1834–1851. [Google Scholar] [CrossRef]
- Chiang, E.Y.; Mellman, I. TIGIT-CD226-PVR axis: Advancing immune checkpoint blockade for cancer immunotherapy. J. Immunother. Cancer 2022, 10, e004711. [Google Scholar] [CrossRef]
- Lee, B.R.; Chae, S.; Moon, J.; Kim, M.J.; Lee, H.; Ko, H.W.; Chul Cho, B.; Sup Shim, H.; Hwang, D.; Ryun Kim, H.; et al. Combination of PD-L1 and PVR determines sensitivity to PD-1 blockade. JCI Insight 2020, 5, e128633. [Google Scholar] [CrossRef]
- Moon, J.; Oh, Y.M.; Ha, S.J. Perspectives on immune checkpoint ligands: Expression, regulation, and clinical implications. BMB Rep. 2021, 54, 403–412. [Google Scholar] [CrossRef]
- Yue, C.; Gao, S.; Li, S.; Xing, Z.; Qian, H.; Hu, Y.; Wang, W.; Hua, C. TIGIT as a promising therapeutic target in autoimmune diseases. Front. Immunol. 2022, 13, 911919. [Google Scholar] [CrossRef]
- Chu, X.; Tian, W.; Wang, Z.; Zhang, J.; Zhou, R. Co-inhibition of TIGIT and PD-1/PD-L1 in cancer immunotherapy: Mechanisms and clinical trials. Mol. Cancer 2023, 22, 93. [Google Scholar] [CrossRef] [PubMed]
- Kraehenbuehl, L.; Weng, C.H.; Eghbali, S.; Wolchok, J.D.; Merghoub, T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat. Rev. Clin. Oncol. 2022, 19, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, L.; Yin, H.; Feng, X.; Lu, Q. TIGIT: An emerging immune checkpoint target for immunotherapy in autoimmune disease and cancer. Int. Immunopharmacol. 2023, 120, 110358. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.; Agrawal, M.Y.; Kaushik, I.; Ramachandran, S.; Srivastava, S.K. Immune checkpoint proteins: Signaling mechanisms and molecular interactions in cancer immunotherapy. Semin. Cancer Biol. 2022, 86, 137–150. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, X.; Gu, Z.; Jiang, Z.; Zhao, S.; Song, Y.; Yu, J. Targeting TIGIT for cancer immunotherapy: Recent advances and future directions. Biomark. Res. 2024, 12, 7. [Google Scholar] [CrossRef]
- Mu, S.; Liang, Z.; Wang, Y.; Chu, W.; Chen, Y.L.; Wang, Q.; Wang, G.; Wang, C. PD-L1/TIGIT bispecific antibody showed survival advantage in animal model. Clin. Transl. Med. 2022, 12, e754. [Google Scholar] [CrossRef]
- Brazel, D.; Ou, S.H.I.; Nagasaka, M. Tiragolumab (anti-TIGIT) in SCLC: Skyscraper-02, a towering inferno. Lung Cancer 2023, 14, 1–9. [Google Scholar] [CrossRef]
- Shapira-Frommer, R.; Niu, J.; Perets, R.; Peters, S.; Shouse, G.; Lugowska, I.; Garassion, M.C.; Sands, J.; Keenan, T.; Zhao, B.; et al. The KEYVIBE program: Vibostolimab and pembrolizumab for the treatment of advanced malignancies. Future Oncol. 2024, 20, 1983–1991. [Google Scholar] [CrossRef]
- Mettu, N.B.; Ulahannan, S.V.; Bendell, J.C.; Garrido-Laguna, I.; Strickler, J.H.; Moore, K.N.; Stagg, R.; Kapoun, A.M.; Faoro, L.; Sharma, S. A phase 1a/b open-label, dose-escalation study of etigilimab alone or in combination with nivolumab in patients with locally advanced or metastatic solid tumors. Clin. Cancer Res. 2022, 28, 882–892. [Google Scholar] [CrossRef]
- Hsiehchen, D.; Kainthla, R.; Kline, H.; Siglinsky, E.; Ahn, C.; Zhu, H. Dual TIGIT and PD-1 blockade with domvanalimab plus zimberelimab in hepatocellular carcinoma refractory to anti-PD-1 therapies: The phase 2 LIVERTI trial. Nat. Commun. 2025, 16, 5819. [Google Scholar] [CrossRef]
- Niu, J.; Maurice-Dror, C.; Lee, D.H.; Kim, D.W.; Nagrial, A.; Voskoboynik, M.; Chung, H.C.; Mileham, K.; Vaishampayan, U.; Rasco, D.; et al. First-in-human phase 1 study of the anti-TIGIT antibody vibostolimab as monotherapy or with pembrolizumab for advanced solid tumors, including non-small-cell lung cancer. Ann. Oncol. 2022, 33, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Bedard, P.L.; LoRusso, P.; Gordon, M.S.; Bendell, J.; Oh, D.Y.; Myung-Ju, A.; Garralda, E.; D’Angelo, S.P.; Desai, J.; et al. Anti-TIGIT antibody tiragolumab alone or with atezolizumab in patients with advanced solid tumors: A phase 1a/1b nonrandomized controlled trial. JAMA Oncol. 2023, 9, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.C.; Abreu, D.R.; Hussein, M.; Cobo, M.; Patel, A.J.; Secen, N.; Lee, K.H.; Massuti, B.; Hiret, S.; Yang, J.C.H.; et al. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): Primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 2022, 23, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Herbst, R.; Horinouchi, H.; Paz-Ares, L.; Johnson, M.; Solomon, B.; Gumus, M.; Erman, M.; Bondarenko, I.; Kim, D.-W.; et al. SKYSCRAPER-01: A phase III, randomized trial of tiragolumab + atezolizumab versus placebo + atezolizumab in patients with previously-untreated PD-L1-high, locally advanced unresectable/metastatic NSCLC. Cancer Res. 2025, 85, CT051. [Google Scholar] [CrossRef]
- Mori, M.; Kanayama, M.; Kuwata, T.; Manabe, T.; Nemoto, Y.; Nishizawa, N.; Oyama, R.; Matsumiya, H.; Nabe, Y.; Taira, A.; et al. Prognostic impact of PD-L1 and TIGIT expression in non-small cell lung cancer following concurrent chemo-radiotherapy. Sci. Rep. 2023, 13, 3270. [Google Scholar] [CrossRef]
- Rodriguez-Abreu, D.; Bosch-Barrera, J.; Gray, J.E.; Ahn, M.J.; Johnson, M.; Yu, X.; Mohammad, S.; Chen, X.; Todd, T.; Kim, J.; et al. STAR-121: A phase III randomized study of domvanalimab and zimberelimab in combination with chemotherapy versus pembrolizumab with chemotherapy in untreated metastatic non–small cell lung cancer with no actionable gene alterations. Clin. Lung Cancer 2024, 25, 274–279. [Google Scholar] [CrossRef]
- Molla Desta, G.; Birhanu, A.G. Advancements in single-cell RNA sequencing and spatial transcriptomics: Transforming biomedical research. Acta Biochim. Pol. 2025, 72, 13922. [Google Scholar] [CrossRef]

| Antibody | IgG Subclass | Clinical Phase | Combination Partner | FDA Status |
|---|---|---|---|---|
| Tiragolumab | IgG1, Fc-competent | II-III | Atezolizumab (PD-L1) | Breakthrough Therapy Designation |
| Vibostolimab | IgG1, Fc-competent | Ib-II | Pembrolizumab (PD-1) | Orphan Drug Designation (SCLC) withdrawn |
| Etigilimab | IgG1, Fc-engineered | I-Ib | Nivolumab (PD-1) | FDA IND clearance (early stage) |
| Domvanalimab | IgG1, Fc-competent | III | Zimberelimab (PD-1) | No FDA approval |
| Resistance to PD-1/PDL-1 | No Resistance | Resistance | |
|---|---|---|---|
| Type of therapy: | Combination therapy | Monotherapy | Combination therapy |
| Itching | 38% | 9% | 36% |
| Hypoalbuminemia | 31% | 3% | 0% |
| Fever | 21% | 6% | 3% |
| Lymphopenia | 18% | 0% | 0% |
| Rash | 15% | 21% | 21% |
| Fatigue | 13% | 21% | 24% |
| Joint pain | 5% | 12% | 0% |
| Reduced appetite | 5% | 9% | 12% |
| Nausea | 3% | 12% | 6% |
| Year | Phase | Population | PD-L1 Requirement | Intervention | Results | Citation |
|---|---|---|---|---|---|---|
| 2022 | I | 182 | - | Vibostolimab | Safety assessed; most common TRAEs: pruritus, hypoalbuminemia, fever, pain, lymphopenia, rash | [42] |
| 2023 | Ia/Ib | 24/49 | - | Tiragolumab | ORR 46% DOR not reached | [43] |
| 2022 | II | 135 | PD-L1 ≥ 1% | Tiragolumab | ORR 31.3% Median PFS 5.4 month | [44] |
| 2025 | III | 534 | PD-L1≥ 50% | Tiragolumab | Median PFS 7.0 month OS 23.1 month | [45] |
| 2023 | Retrospective | 55 | - | cCRT | Elevated PD-L1 and TIGIT expression post-cCRT associated | [46] |
| 2024 | III | 720 | - | Domvanalimab | Ongoing | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piekarz, J.; Picheta, N.; Szklener, K.; Mańdziuk, S. TIGIT Expression and Its Implications in Non-Small-Cell Lung Cancer Progression and Therapy: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 9307. https://doi.org/10.3390/ijms26199307
Piekarz J, Picheta N, Szklener K, Mańdziuk S. TIGIT Expression and Its Implications in Non-Small-Cell Lung Cancer Progression and Therapy: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(19):9307. https://doi.org/10.3390/ijms26199307
Chicago/Turabian StylePiekarz, Julia, Natalia Picheta, Katarzyna Szklener, and Sławomir Mańdziuk. 2025. "TIGIT Expression and Its Implications in Non-Small-Cell Lung Cancer Progression and Therapy: A Systematic Review" International Journal of Molecular Sciences 26, no. 19: 9307. https://doi.org/10.3390/ijms26199307
APA StylePiekarz, J., Picheta, N., Szklener, K., & Mańdziuk, S. (2025). TIGIT Expression and Its Implications in Non-Small-Cell Lung Cancer Progression and Therapy: A Systematic Review. International Journal of Molecular Sciences, 26(19), 9307. https://doi.org/10.3390/ijms26199307







