Proteomic Profiling of Limited-Stage Follicular Lymphoma Reveals Differentially Expressed Proteins Linked to Disease Progression Post-Radiation Therapy
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
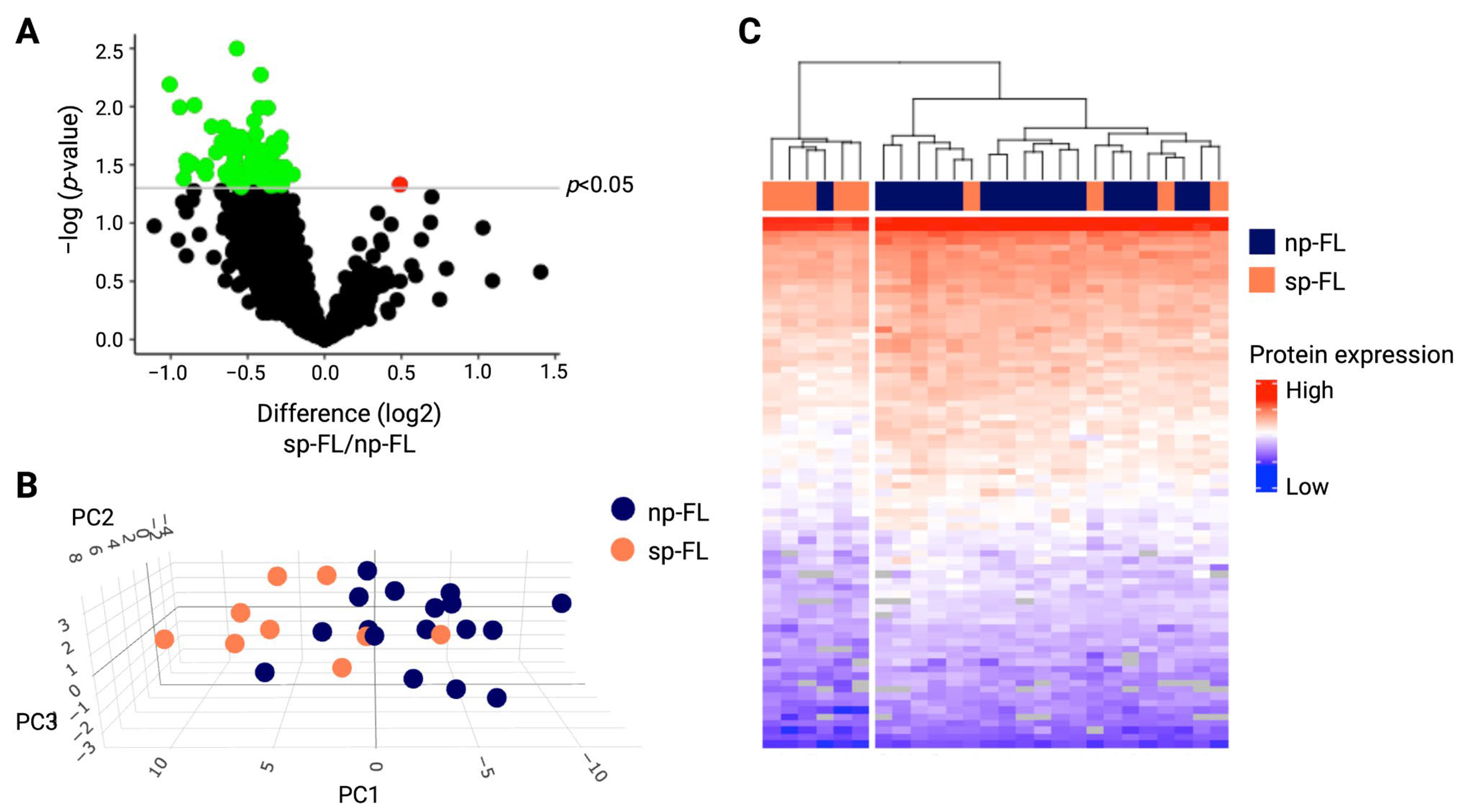
2.2. Proteomic Profiling Identifies Differentially Expressed Proteins Correlated to Subsequent Disease Progression
2.3. Differentially Expressed Proteins Suggest Alterations Within Metabolism and the Innate Immune System
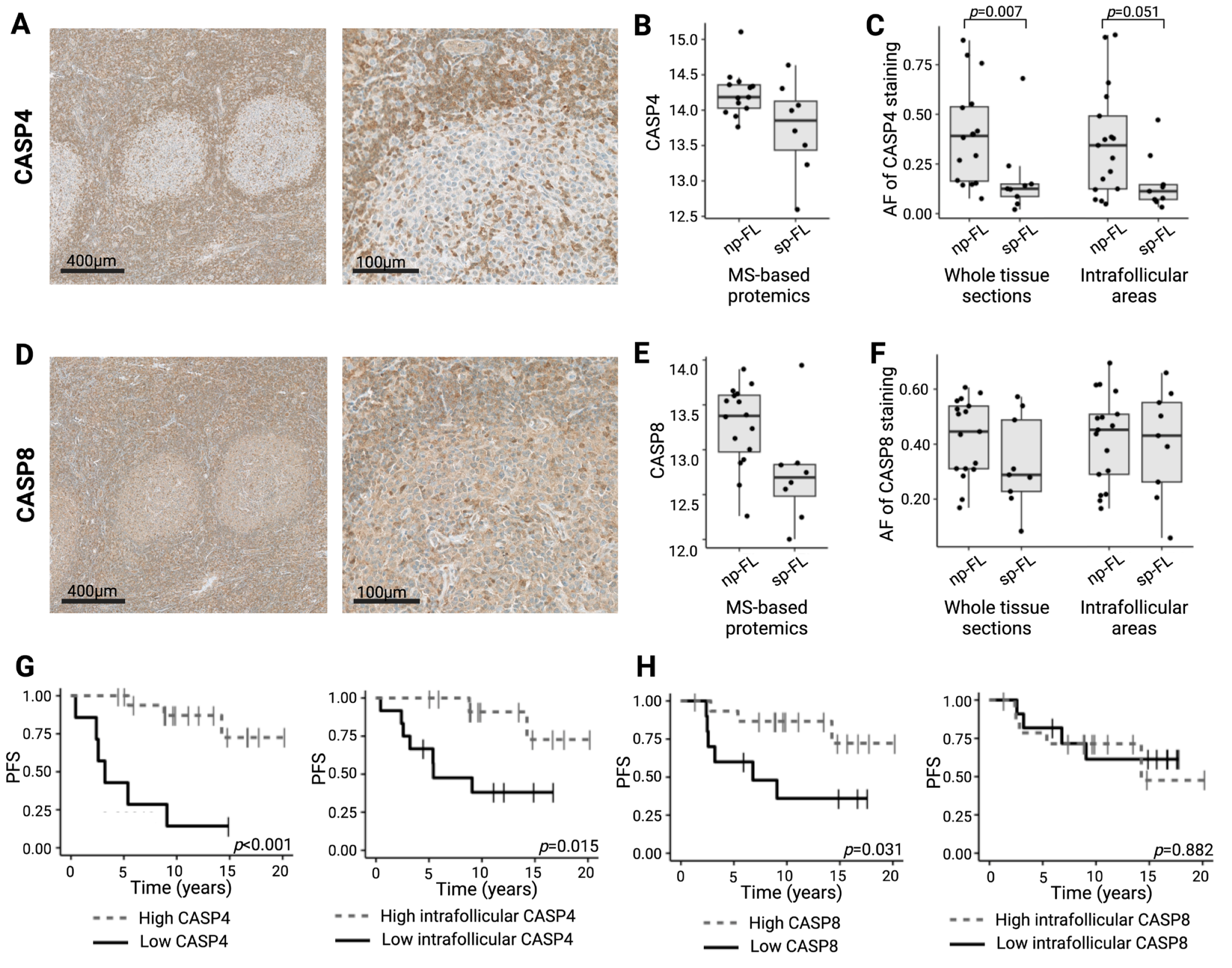
2.4. Low Caspase 4 and Caspase 8 Correlates to Risk of Disease Progression in Limited-Stage FL
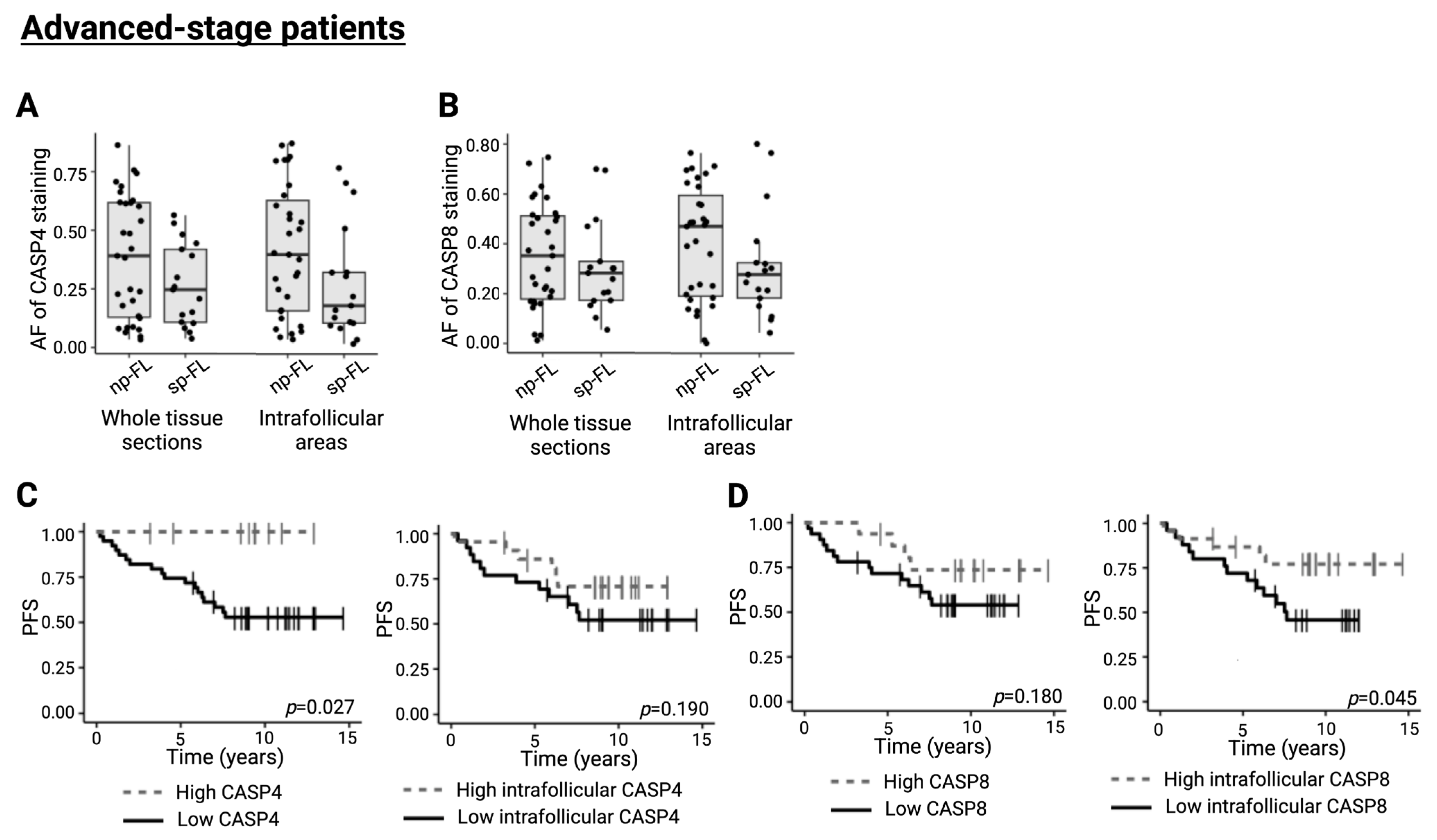
2.5. CASP4 and CASP8 Trend Towards an Implication in Disease Progression in Advanced-Stage FL
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Identification of Differentially Expressed Proteins
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, E. Follicular lymphoma: 2023 update on diagnosis and management. Am. J. Hematol. 2022, 97, 1638–1651. [Google Scholar] [CrossRef] [PubMed]
- Enemark, M.B.H.; Wolter, K.; Campbell, A.J.; Andersen, M.D.; Sørensen, E.F.; Hybel, T.E.; Madsen, C.; Lauridsen, K.L.; Plesner, T.L.; Hamilton-Dutoit, S.J. Proteomics identifies apoptotic markers as predictors of histological transformation in patients with follicular lymphoma. Blood Adv. 2023, 7, 7418–7432. [Google Scholar] [CrossRef] [PubMed]
- Freedman, A.; Jacobsen, E. Follicular lymphoma: 2020 update on diagnosis and management. Am. J. Hematol. 2020, 95, 316–327. [Google Scholar] [CrossRef]
- Carbone, A.; Roulland, S.; Gloghini, A.; Younes, A.; von Keudell, G.; López-Guillermo, A.; Fitzgibbon, J. Follicular lymphoma. Nat. Rev. Dis. Prim. 2019, 5, 83. [Google Scholar] [CrossRef]
- Hübel, K.; Ghielmini, M.; Ladetto, M.; Gopal, A.K. Controversies in the Treatment of Follicular Lymphoma. HemaSphere 2020, 4, e317. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Kaseb, H.; Ali, M.A.; Gasalberti, D.P.; Koshy, N.V. Follicular Lymphoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538206/ (accessed on 13 September 2025).
- Filippi, A.R.; Ciammella, P.; Ricardi, U. Limited Stage Follicular Lymphoma: Current Role of Radiation Therapy. Mediterr. J. Hematol. Infect. Dis. 2016, 8, e2016041. [Google Scholar] [CrossRef]
- Dada, R. Diagnosis and management of follicular lymphoma: A comprehensive review. Eur. J. Haematol. 2019, 103, 152–163. [Google Scholar] [CrossRef]
- Casulo, C.; Dixon, J.G.; Le-Rademacher, J.; Hoster, E.; Hochster, H.S.; Hiddemann, W.; Marcus, R.; Kimby, E.; Herold, M.; Sebban, C.; et al. Validation of POD24 as a robust early clinical end point of poor survival in FL from 5225 patients on 13 clinical trials. Blood 2022, 139, 1684–1693. [Google Scholar] [CrossRef]
- Hershenfeld, S.A.; Tobin, J.W.; Shelton, V.; Calvente, L.; Lajkosz, K.; Liu, T.; Brodtkorb, M.; D’AMore, F.A.; Ludvigsen, M.; Baetz, T.; et al. Single gene mutations and prognosis in limited-stage follicular lymphoma treated with radiation therapy. Br. J. Haematol. 2024, 205, 1810–1814. [Google Scholar] [CrossRef] [PubMed]
- Sortais, C.; Lok, A.; Tessoulin, B.; Gastinne, T.; Mahé, B.; Dubruille, V.; Blin, N.; Touzeau, C.; Moreau, A.; Bossard, C.; et al. Progression of disease within 2 years (POD24) is a clinically relevant endpoint to identify high-risk follicular lymphoma patients in real life. Ann. Hematol. 2020, 99, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Devan, J.; Janikova, A.; Mraz, M. New concepts in follicular lymphoma biology: From BCL2 to epigenetic regulators and non-coding RNAs. Semin. Oncol. 2018, 45, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Enemark, M.H.; Hemmingsen, J.K.; Jensen, M.L.; Kridel, R.; Ludvigsen, M. Molecular Biomarkers in Prediction of High-Grade Transformation and Outcome in Patients with Follicular Lymphoma: A Comprehensive Systemic Review. Int. J. Mol. Sci. 2024, 25, 11179. [Google Scholar] [CrossRef]
- Hemmingsen, J.K.; Enemark, M.H.; Sørensen, E.F.; Lauridsen, K.L.; Hamilton-Dutoit, S.J.; Kridel, R.; Honoré, B.; Ludvigsen, M. Proteomic Profiling Identifies Predictive Signatures for Progression Risk in Patients with Advanced-Stage Follicular Lymphoma. Cancers 2024, 16, 3278. [Google Scholar] [CrossRef]
- Cartron, G.; Trotman, J. Time for an individualized approach to first-line management of follicular lymphoma. Haematologica 2022, 107, 7–18. [Google Scholar] [CrossRef]
- Iacoboni, G.; Morschhauser, F. Building the future management of follicular lymphoma with T-cell-redirecting strategies. Blood 2025, 145, 170–175. [Google Scholar] [CrossRef]
- Platnich, J.M.; Muruve, D.A. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch. Biochem. Biophys. 2019, 670, 4–14. [Google Scholar] [CrossRef]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2015, 7, a026716. [Google Scholar] [CrossRef]
- Papoff, G.; Presutti, D.; Lalli, C.; Bolasco, G.; Santini, S.; Manelfi, C.; Fustaino, V.; Alemà, S.; Ruberti, G. CASP4 gene silencing in epithelial cancer cells leads to impairment of cell migration, cell-matrix adhesion and tissue invasion. Sci. Rep. 2018, 8, 17705. [Google Scholar] [CrossRef]
- Tian, G.; Li, Q.; Niu, L.; Luo, Y.; Wang, H.; Kang, W.; Fang, X.; Bai, S.; Yuan, G.; Pan, Y. CASP4 can be a diagnostic biomarker and correlated with immune infiltrates in gliomas. Front. Oncol. 2022, 12, 1025065. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Salvesen, G.S.; Walsh, C.M. Functions of caspase 8: The identified and the mysterious. Semin. Immunol. 2014, 26, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.P.; Khong, P.-L.; Tse, K.Y.; Chan, K.K.L.; Chu, M.M.Y.; Ngan, H.Y.S. Differentiation of aggressive and indolent subtypes of uterine sarcoma using maximum standardized uptake value. Nucl. Med. Commun. 2013, 34, 1185–1189. [Google Scholar] [CrossRef]
- Ludvigsen, M.; Madsen, C.; Kamper, P.; Hamilton-Dutoit, S.J.; Bendix, K.; D’AMore, F.; Honoré, B. Histologically transformed follicular lymphoma exhibits protein profiles different from both non-transformed follicular and de novo diffuse large B-cell lymphoma. Blood Cancer J. 2015, 5, e293. [Google Scholar] [CrossRef]
- Monrad, I.; Madsen, C.; Lauridsen, K.L.; Honoré, B.; Plesner, T.L.; Hamilton-Dutoit, S.; D’aMore, F.; Ludvigsen, M.; Lee, J.W. Glycolytic biomarkers predict transformation in patients with follicular lymphoma. PLoS ONE 2020, 15, e0233449. [Google Scholar] [CrossRef]
- Enemark, M.H.; Hemmingsen, J.K.; Andersen, M.D.; Hybel, T.E.; Bjørn, M.E.; Josefsson, P.L.; Pedersen, L.M.; Juul, M.B.; Pedersen, R.S.; Thorsgaard, M.; et al. Progression of disease within 24 months (POD24) in follicular lymphoma in the rituximab era: Incidence, clinicopathological risk factors, and outcome in a population-based Danish cohort. Blood Cancer J. 2024, 14, 167. [Google Scholar] [CrossRef]
- Xu, T.; Zheng, Z.; Zhao, W. Advances in the multi-omics landscape of follicular lymphoma. Int. J. Biol. Sci. 2023, 19, 1955–1967. [Google Scholar] [CrossRef]
- Arboe, B.; El-Galaly, T.C.; Clausen, M.R.; Munksgaard, P.S.; Stoltenberg, D.; Nygaard, M.K.; Klausen, T.W.; Christensen, J.H.; Gørløv, J.S.; de Nully Brown, P.; et al. The Danish National Lymphoma Registry: Coverage and Data Quality. PLoS ONE 2016, 11, e0157999. [Google Scholar] [CrossRef]
- Honoré, B. Proteomic Protocols for Differential Protein Expression Analyses. In Xenotransplantation; Methods in Molecular Biology; Springer Nature: Cham, Switzerland, 2020; Volume 2110, pp. 47–58. [Google Scholar] [CrossRef]
- Zougman, A.; Selby, P.J.; Banks, R.E. Suspension trapping (STrap) sample preparation method for bottom-up proteomics analysis. Proteomics 2014, 14, 1006–1000. [Google Scholar] [CrossRef]
- Cehofski, L.J.; Kojima, K.; Terao, N.; Kitazawa, K.; Thineshkumar, S.; Grauslund, J.; Vorum, H.; Honoré, B. Aqueous Fibronectin Correlates With Severity of Macular Edema and Visual Acuity in Patients With Branch Retinal Vein Occlusion: A Proteome Study. Investig. Ophthalmol. Vis. Sci. 2020, 61, 6. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
| Total, n = 26 n (%) | sp-FL, n = 9 n (%) | np-FL, n = 17 n (%) | p-Value | |
|---|---|---|---|---|
| Sex | NS | |||
| Male | 10 (38) | 5 (56) | 5 (29) | |
| Female | 16 (62) | 4 (44) | 12 (71) | |
| Age at diagnosis, y | NS | |||
| Median | 69 | 71 | 69 | |
| Range | 23–86 | 34–83 | 23–86 | |
| FL grade | NS | |||
| 1 | 11 (42) | 5 (56) | 6 (35) | |
| 1/2 | 4 (16) | 0 (0) | 4 (24) | |
| 2 | 11 (42) | 4 (44) | 7 (41) | |
| Ann Arbor Stage | NS | |||
| I | 24 (92) | 9 (100) | 15 (88) | |
| II | 2 (8) | 0 (0) | 2 (12) | |
| B-symptoms | NS | |||
| No | 26 (100) | 9 (100) | 17 (100) | |
| Yes | 0 (100) | 0 (0) | 0 (0) | |
| Bulky disease | ||||
| No | 20 (77) | 6 (67) | 14 (82) | NS |
| Yes | 1 (4) | 1 (11) | 0 (0) | |
| Unknown | 5 (19) | 2 (22) | 3 (18) | |
| LDH-elevation | ||||
| No | 22 (85) | 7 (78) | 15 (88) | NS |
| Yes | 3 (12) | 1 (11) | 2 (12) | |
| Unknown | 1 (4) | 1 (11) | 0 (0) | |
| FLIPI | ||||
| Low | 23 (88) | 7 (78) | 16 (94) | NS |
| Intermediate | 2 (18) | 1 (11) | 1 (6) | |
| Unknown | 1 (4) | 1 (11) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemmingsen, J.K.; Enemark, M.H.; Sørensen, E.F.; Dohrmann, C.; Lauridsen, K.L.; Hamilton-Dutoit, S.J.; Kridel, R.; Honoré, B.; Ludvigsen, M. Proteomic Profiling of Limited-Stage Follicular Lymphoma Reveals Differentially Expressed Proteins Linked to Disease Progression Post-Radiation Therapy. Int. J. Mol. Sci. 2025, 26, 9306. https://doi.org/10.3390/ijms26199306
Hemmingsen JK, Enemark MH, Sørensen EF, Dohrmann C, Lauridsen KL, Hamilton-Dutoit SJ, Kridel R, Honoré B, Ludvigsen M. Proteomic Profiling of Limited-Stage Follicular Lymphoma Reveals Differentially Expressed Proteins Linked to Disease Progression Post-Radiation Therapy. International Journal of Molecular Sciences. 2025; 26(19):9306. https://doi.org/10.3390/ijms26199306
Chicago/Turabian StyleHemmingsen, Jonas Klejs, Marie Hairing Enemark, Emma Frasez Sørensen, Cecilie Dohrmann, Kristina Lystlund Lauridsen, Stephen Jacques Hamilton-Dutoit, Robert Kridel, Bent Honoré, and Maja Ludvigsen. 2025. "Proteomic Profiling of Limited-Stage Follicular Lymphoma Reveals Differentially Expressed Proteins Linked to Disease Progression Post-Radiation Therapy" International Journal of Molecular Sciences 26, no. 19: 9306. https://doi.org/10.3390/ijms26199306
APA StyleHemmingsen, J. K., Enemark, M. H., Sørensen, E. F., Dohrmann, C., Lauridsen, K. L., Hamilton-Dutoit, S. J., Kridel, R., Honoré, B., & Ludvigsen, M. (2025). Proteomic Profiling of Limited-Stage Follicular Lymphoma Reveals Differentially Expressed Proteins Linked to Disease Progression Post-Radiation Therapy. International Journal of Molecular Sciences, 26(19), 9306. https://doi.org/10.3390/ijms26199306







