Genetic Variability in Child Growth Among South American Populations: A Perspective Integrating Population Genetics, Growth Standards, and Precision Growth Medicine
Abstract
1. Introduction
2. The Background of Population Genetics in South America
3. The Genetic Landscape of Child Growth and Its Implications for Precision Growth Medicine
3.1. Polygenic Regulation of Height
3.2. Key Genes and Biological Pathways Influencing Stature
- ○
- Fibroblast growth factor (FGF) signaling pathway
- ○
- The Growth Hormone (GH)-Insuline- like growth factor-I (IGF-I) axis
- ○
- Wnt/β-Catenin Signaling
- ○
- Hedgehog signaling pathway
- ○
- BMP/TGF-β Pathway
| Gene | Pathway | Correlated with Height | Mechanism | Reference |
|---|---|---|---|---|
| FGFR3 | FGF signaling | Gain of function variants are associated with short stature. | Inhibits chondrocyte proliferation in growth plate | [51,53] |
| FGFR1 | FGF signaling | Limb and nervous system development | Short stature and variants in the gene are rare events. | [55,56,57] |
| FGFR4 | FGF signaling | rs351855 and rs4752570 are associated with height | Effect on embryonic development | [53,58] |
| FGFRL1 | FGF signaling | Height variation | Modulates cardiovascular system and bone formation | [59,60,61,62] |
| GH1 | GH-IGF-I axis | Variants are linked to sever short stature. Other SNPs are normal height variants. | GH1 encodes pituitary growth hormone. | [66] |
| GHR | GH-IGF-I axis | Variants identified in Laron syndrome and severe to mild growth failure | GHR mediates GH signaling in liver and growth plate; receptor defects impair JAK2–STAT5B activation | [66,68] |
| STAT5B | GH-IGF-I axis | Variants are linked to growth failure, IGF-I deficiency, and GH insensitivity. | Role in GH, essential for IGF-1 transcription | [66,67,68] |
| IGF1 and IGF1R | GH-IGF-I axis | Mutations have benn associated with intra and post- natal growth retardation | Effect on the GH-IGF-I axis | [66] |
| IGFALS | GH-IGF-I axis | Variants result in growth failure. | Stabilizes the IGF-I -IGFBP3 complex, prolonging IGD-I half-life. | [66] |
| CTNNB1 | Wnt/β-Catenin signaling | Neurodevelopmental disorders resulting in postnatal short stature | Encodes for the β-Catenin protein. | [70,71] |
| LPR5 | Wnt/β-Catenin signaling | Osteoporosis-pseudoglioma and high-bone-mass syndromes and short stature | Wnt transduction in the signaling pathway | [72,73] |
| WNT1 | Wnt/β-Catenin signaling | Osteogeneses imperfecta which cause short height. | Activates the signaling pathway. | [74,75] |
| IHH | Hedgehog signaling | Height associated | Role in endochondral ossification, major Hh input. | [77,78] |
| PTCH1 | Hedgehog signaling | Height associated | Inhibits Smo and regulates Hh activation threshold. | [77,78] |
| HHIP | Hedgehog signaling | Height associated | Encodes for negative regulator of the Hh signaling pathway. | [77,78] |
| GDF5 | BMP/TGF-β | Variants have been associated with symphalangism, brachydactyly, skeletal dysplasia and reduced stature | Encodes a protein that is member of the BMP ligand. | [81] |
| BMPR1B | BMP/TGF-β | Variants have been associated with symphalangism, brachydactyly and short stature | Encodes a protein that is member of the BMP ligand involved in cartilage formation | [81] |
3.3. Growth-Related Genetic Variants in South American Population
4. Clinical Growth Patterns in South American Children
4.1. Growth Hormone Therapy and Precision Growth Medicine: Genetic Modulators of Response
4.1.1. GH Therapy Indications
4.1.2. Genetic Predictors of GH Response
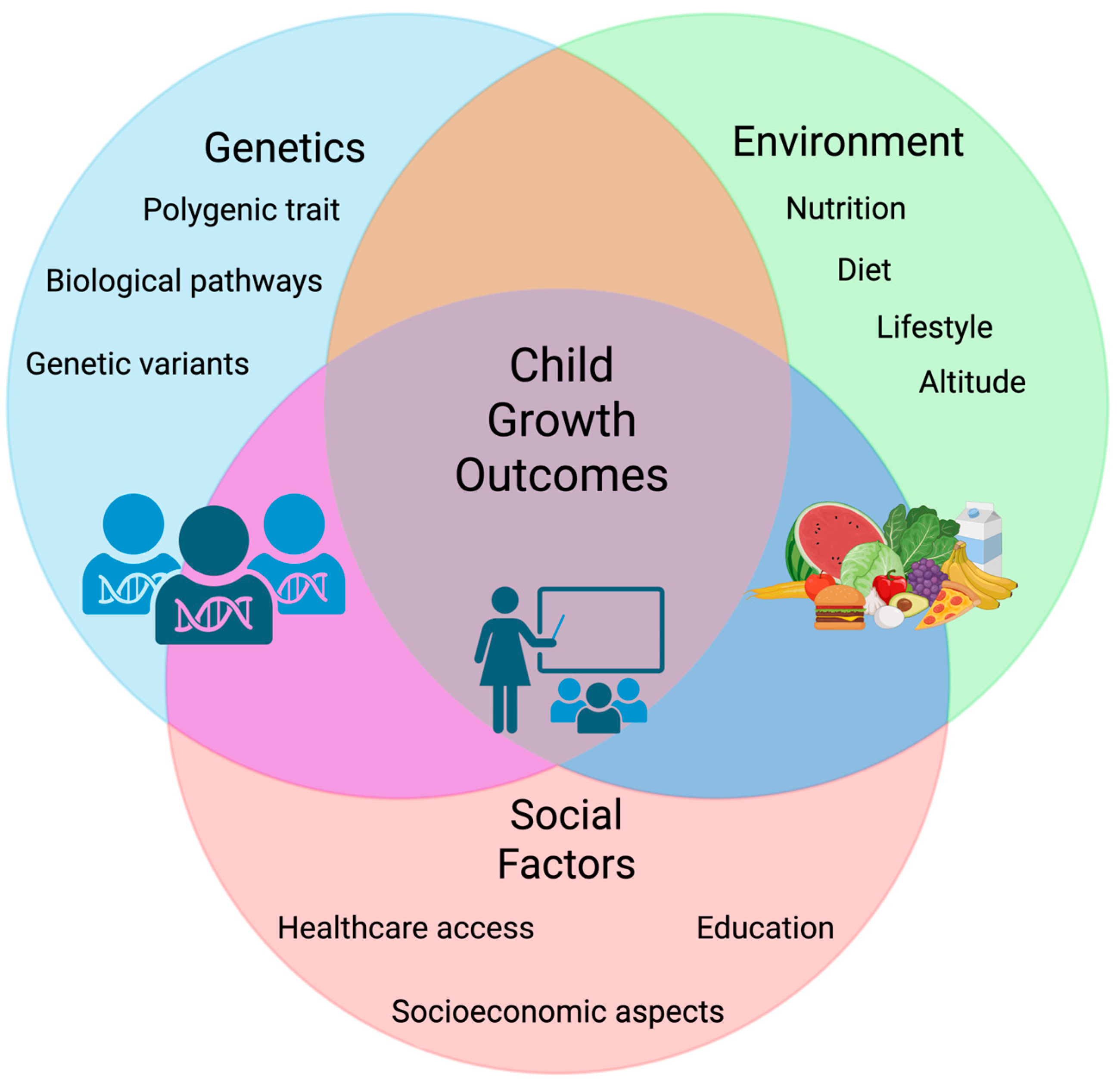
5. Environmental and Socioeconomic Modifiers of Growth
5.1. Infectious Disease Burden
5.2. Gene–Environment Interactions in Child Growth
6. Limitations
7. Future Directions
- Addressing these limitations requires a combination of immediate and long-term strategies that prioritize both biomedical and structural determinants of growth. The following areas emerge as the most actionable: Tackling malnutrition and its structural drivers: Public health policies must reduce socioeconomic inequities while integrating nutrition into healthcare delivery. Key priorities include reducing stunting, anemia in women of reproductive age, and low birth weight; preventing increases in childhood obesity; promoting breastfeeding; and decreasing child wasting [140]. The WHO also emphasizes the need for supportive environments, integration of nutrition into health interventions, adequate resource allocation, and systematic evaluation of intervention effectiveness.
- Expanding ethnically inclusive genomic research: Given the overwhelming reliance on European-derived evidence, large-scale initiatives such as the Latin American Genomics Consortium (LAGC), JAGUAR, and the Genetics of Latin American Diversity (GLAD) project are critical. These efforts aim to create population-specific databases and atlases, uncover genetic diversity, and identify disease-relevant markers. Importantly, increased inclusion of Native American and Afro-descendant populations is essential to improve predictive models, reduce bias in diagnostics, and ensure equitable clinical translation of genomic advances [159]. Developing region-specific growth references. Growth standards tailored to the genetic, nutritional, and environmental contexts of South American subpopulations would improve diagnostic precision and reduce misclassification. While WHO charts will continue to serve as a global benchmark, complementary regional charts could support improved assessments, better identification of nutritional deficiencies, and more targeted interventions.
- Integrating pharmacogenetics into clinical practice: Validated markers such as d3-GHR and SHOX can guide growth hormone therapy, while emerging candidates (e.g., ACAN, NPR2) require replication in admixed populations before routine use.
- Improving treatment adherence monitoring: Expanding the use of digital devices (e.g., easy pod) to monitor adherence in GH therapy can optimize height outcomes and reduce variability in treatment response.
- Ensuring equity in healthcare access: Policies must guarantee that underserved populations—particularly Native American and rural communities—have access to diagnostics, growth monitoring, and therapy, aligning with the Sustainable Development Goals (SDGs).
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bogin, B. Genetic and Neuroendocrine Regulation of Human Growth. In Patterns of Human Growth; Cambridge University Press: Cambridge, UK, 2020; pp. 339–402. [Google Scholar]
- Mummert, A.; Schoen, M.; Lampl, M. Growth and Life Course Health Development. In Handbook of Life Course Health Development; Springer: Cham, Switzerland, 2017; pp. 405–429. [Google Scholar]
- La Importancia de la Nutrición en los Primeros 1.000 Días de la Vida. Available online: https://www.actapediatrica.com/index.php/secciones/nutricion-infantil/1462-la-importancia-de-la-nutricion-en-los-primeros-1-000-dias-de-la-vida (accessed on 12 August 2025).
- Campoy Folgoso, C.; Martinón Torres, N.; Martín Martínez, B. Nutrición durante los primeros 1.000 días de vida. In Tratamiento en Gastroenterología, Hepatología y Nutrición Pediátrica; Ergón Creación, S.A.: Madrid, Spain, 2021; pp. 739–754. ISBN 9788417844998. [Google Scholar]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. Suppl. 2006, 450, 76–85. [Google Scholar]
- Papageorghiou, A.T.; Kennedy, S.H.; Salomon, L.J.; Altman, D.G.; Ohuma, E.O.; Stones, W.; Gravett, M.; Barros, F.C.; Victora, C.; Purwar, M.; et al. The INTERGROWTH-21 st fetal growth standards: Toward the global integration of pregnancy and pediatric care. Am. J. Obs. Gynecol. 2018, 218, S630–S640. [Google Scholar] [CrossRef]
- Falero-Gallego, M.P.; Redondo-González, O.; González-González, A.; Muñoz-Serrano, A.; Arias-Arias, A.; Moreno-Manzanaro-Fernández-Montes, I. Valoración antropométrica de escolares del área de salud de La Mancha-Centro. Comparación con el Estudio Transversal Español de Crecimiento 2010. Gac. Med. Mex. 2022, 158, 281–292. [Google Scholar] [CrossRef]
- Tarupi, W.; Lepage, Y.; Felix, M.L.; Monnier, C.; Hauspie, R.; Roelants, M.; Hidalgo, R.; Vercauteren, M. Growth references for weight, height, and body mass index for Ecuadorian children and adolescents aged 5–19 years. Arch. Argent. Pediatr. 2020, 118, 117–124. [Google Scholar] [PubMed]
- Venezolana De Endocrinología, S.; Venezuela, M. Estudio comparativo de las curvas de crecimiento nchs y oms en la evaluación del estado nutricional en niños menores de 5 AÑOS. AÑOS Rev. Venez. Endocrinol. Metab. 2021, 19, 149–161. [Google Scholar]
- Vallejo, G.M.; Uriel Calvo, M.; Hoz-Valle, J.D.L.; Romero, X.C. Crecimiento fetal en Bogotá Colombia: Una comparación con curvas internacionales y latinoamericanas. Rev. Bras. Saúde Matern. Infant. 2024, 24, e20230326. [Google Scholar] [CrossRef]
- Pediatría Integral Publica el Número 4 de 2025 Dedicado a Endocrinología I—SEPEAP. Available online: https://sepeap.org/pediatria-integral-publica-el-numero-4-de-2025-dedicado-a-endocrinologia/ (accessed on 12 August 2025).
- Piña Borrego, C.E. Variantes de la Normalidad del Crecimiento Infantil Versus Fallo de Medro. Rev. Cubana Pediatr. 2022, 94. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0034-75312022000400010&lng=es&nrm=iso&tlng=es (accessed on 12 August 2025).
- Ruiz-Linares, A.; Adhikari, K.; Acuña-Alonzo, V.; Quinto-Sanchez, M.; Jaramillo, C.; Arias, W.; Fuentes, M.; Pizarro, M.; Everardo, P.; De Avila, F.; et al. Admixture in Latin America: Geographic Structure, Phenotypic Diversity and Self-Perception of Ancestry Based on 7,342 Individuals. PLoS Genet. 2014, 10, e1004572. [Google Scholar] [CrossRef]
- Borda, V.; Loesch, D.P.; Guo, B.; Laboulaye, R.; Veliz-Otani, D.; French, J.N.; Leal, T.P.; Gogarten, S.M.; Ikpe, S.; Gouveia, M.H.; et al. Genetics of Latin American Diversity Project: Insights into population genetics and association studies in admixed groups in the Americas. Cell Genom. 2024, 4, 100692. [Google Scholar] [CrossRef]
- Eslami, P.; Sayarifard, F.; Safdari, R.; Shahmoradi, L.; Karbasi, Z. Global perspective on pediatric growth hormone registries: A systematic review. J. Pediatr. Endocrinol. Metab. 2022, 35, 709–726. [Google Scholar] [CrossRef]
- Isabella, S.; Claudia, H.; Hoover, Q.; Héctor, P.; Sonia, R.; Diana, M.; Esperanza, G.C. Costo-efectividad del tratamiento con hormona del crecimiento recombinante humana en niños con talla baja. Repositorio Institucional; Universidad Militar Nueva Granada, Granada, España. Available online: https://repository.umng.edu.co/server/api/core/bitstreams/9c05ac21-3640-480d-a5c9-985f60186d1d/content (accessed on 8 August 2025).
- Cardoso Ore, C.A.; Flores Parra, J.R. Determinantes de la Mortalidad Infantil en Paises de América Latina y el Caribe Para el Periodo 2006–2020; Repositorio Institucional—Ulima; Universidad de Lima: Lima, Peru, 2024; Available online: https://repositorio.ulima.edu.pe/handle/20.500.12724/20695 (accessed on 12 August 2025).
- De Oliveira, T.C.; Secolin, R.; Lopes-Cendes, I. A review of ancestrality and admixture in Latin America and the caribbean focusing on native American and African descendant populations. Front. Genet. 2023, 14, 1091269. [Google Scholar] [CrossRef]
- Zambrano, A.K.; Gaviria, A.; Cobos-Navarrete, S.; Gruezo, C.; Rodríguez-Pollit, C.; Armendáriz-Castillo, I.; García-Cárdenas, J.M.; Guerrero, S.; López-Cortés, A.; Leone, P.E.; et al. The three-hybrid genetic composition of an Ecuadorian population using AIMs-InDels compared with autosomes, mitochondrial DNA and Y chromosome data. Sci. Rep. 2019, 9, 9247. [Google Scholar] [CrossRef] [PubMed]
- Tarazona-Santos, E.; Lavine, M.; Pastor, S.; Fiori, G.; Pettener, D. Hematological and pulmonary responses to high altitude in Quechuas: A multivariate approach. Am. J. Phys. Anthr. 2000, 111, 165–176. [Google Scholar] [CrossRef]
- Vicuña, L.; Fernandez, M.I.; Vial, C.; Valdebenito, P.; Chaparro, E.; Espinoza, K.; Ziegler, A.; Bustamante, A.; Eyheramendy, S. Adaptation to Extreme Environments in an Admixed Human Population from the Atacama Desert. Genome Biol. Evol. 2019, 11, 2468–2479. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, T.; Bergström, A.; Geppert, M.; Bawn, M.; Ohasi, D.; Shi, W.; Lacerda, D.R.; Solli, A.; Norstedt, J.; Reed, K.; et al. Y Chromosome Sequences Reveal a Short Beringian Standstill, Rapid Expansion, and early Population structure of Native American Founders. Curr. Biol. 2019, 29, 149–157e3. [Google Scholar] [CrossRef]
- Chacón-Duque, J.C.; Adhikari, K.; Fuentes-Guajardo, M.; Mendoza-Revilla, J.; Acuña-Alonzo, V.; Barquera, R.; Quinto-Sánchez, M.; Gómez-Valdés, J.; Everardo Martínez, P.; Villamil-Ramírez, H.; et al. Latin Americans show wide-spread Converso ancestry and imprint of local Native ancestry on physical appearance. Nat. Commun. 2018, 9, 5388. [Google Scholar] [CrossRef]
- Roca-Rada, X.; Souilmi, Y.; Teixeira, J.C.; Llamas, B. Ancient DNA Studies in Pre-Columbian Mesoamerica. Genes 2020, 11, 1346. [Google Scholar] [CrossRef]
- Peloso, V.C. Race and Ethnicity in Latin American History. In Race and Ethnicity in Latin American History; Taylor & Francis: London, UK, 2014; pp. 1–206. [Google Scholar]
- Homburger, J.R.; Moreno-Estrada, A.; Gignoux, C.R.; Nelson, D.; Sanchez, E.; Ortiz-Tello, P.; Pons-Estel, B.A.; Acevedo-Vasquez, E.; Miranda, P.; Langefeld, C.D.; et al. Genomic Insights into the Ancestry and Demographic History of South America. PLoS Genet. 2015, 11, e1005602. [Google Scholar]
- Torres, J.B.; Stone, A.C.; Kittles, R. An anthropological genetic perspective on creolization in the anglophone caribbean. Am. J. Phys. Anthropol. 2013, 151, 135–143. [Google Scholar] [CrossRef]
- Marcheco-Teruel, B.; Parra, E.J.; Fuentes-Smith, E.; Salas, A.; Buttenschøn, H.N.; Demontis, D.; Torres-Español, M.; Marín-Padrón, L.C.; Gómez-Cabezas, E.J.; Alvarez-Iglesias, V.; et al. Cuba: Exploring the History of Admixture and the Genetic Basis of Pigmentation Using Autosomal and Uniparental Markers. PLoS Genet. 2014, 10, e1004488. [Google Scholar] [CrossRef]
- Mathias, R.A.; Taub, M.A.; Gignoux, C.R.; Fu, W.; Musharoff, S.; O’Connor, T.D.; Vergara, C.; Torgerson, D.G.; Pino-Yanes, M.; Shringarpure, S.S.; et al. A continuum of admixture in the Western Hemisphere revealed by the African Diaspora genome. Nat. Commun. 2016, 7, 12522. [Google Scholar] [CrossRef] [PubMed]
- Fortes-Lima, C.; Gessain, A.; Ruiz-Linares, A.; Bortolini, M.C.; Migot-Nabias, F.; Bellis, G.; Moreno-Mayar, J.V.; Restrepo, B.N.; Rojas, W.; Avendaño-Tamayo, E.; et al. Genome-wide Ancestry and Demographic History of African-Descendant Maroon Communities from French Guiana and Suriname. Am. J. Hum. Genet. 2017, 101, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.R.; Gignoux, C.R.; Walters, R.K.; Wojcik, G.L.; Neale, B.M.; Gravel, S.; Daly, M.J.; Bustamante, C.D.; Kenny, E.E. Human Demographic History Impacts Genetic Risk Prediction across Diverse Populations. Am. J. Hum. Genet. 2017, 100, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues de Moura, R.; Coelho, A.V.C.; de Queiroz Balbino, V.; Crovella, S.; Brandão, L.A. Meta-analysis of Brazilian genetic admixture and comparison with other Latin America countries. Am. J. Hum. Biol. 2015, 27, 674–680. [Google Scholar] [CrossRef]
- Kivisild, T. Maternal ancestry and population history from whole mitochondrial genomes. Investig. Genet. 2015, 6, 3. [Google Scholar] [CrossRef]
- Kumar, S.; Bellis, C.; Zlojutro, M.; Melton, P.E.; Blangero, J.; Curran, J.E. Large scale mitochondrial sequencing in Mexican Americans suggests a reappraisal of Native American origins. BMC Evol. Biol. 2011, 11, 293. [Google Scholar] [CrossRef]
- Calafell, F.; Larmuseau, M.H.D. The Y chromosome as the most popular marker in genetic genealogy benefits interdisciplinary research. Hum. Genet. 2016, 136, 559–573. [Google Scholar] [CrossRef]
- Schurr, T.G.; Sherry, S.T. Mitochondrial DNA and Y chromosome diversity and the peopling of the Americas: Evolutionary and demographic evidence. Am. J. Hum. Biol. 2004, 16, 420–439. [Google Scholar] [CrossRef]
- Lell, J.T.; Sukernik, R.I.; Starikovskaya, Y.B.; Su, B.; Jin, L.; Schurr, T.G.; Underhill, P.A.; Wallace, D.C. The dual origin and Siberian affinities of Native American Y chromosomes. Am. J. Hum. Genet. 2002, 70, 192–206. [Google Scholar] [CrossRef]
- Phillips, C.; Fernandez-Formoso, L.; Gelabert-Besada, M.; Garcia-Magariños, M.; Santos, C.; Fondevila, M.; Carracedo, A.; Lareu, M.V. Development of a novel forensic STR multiplex for ancestry analysis and extended identity testing. Electrophoresis 2013, 34, 1151–1162. [Google Scholar] [CrossRef]
- Casals, F.; Rasal, R.; Anglada, R.; Tormo, M.; Bonet, N.; Rivas, N.; Vásquez, P.; Calafell, F. A forensic population database in El Salvador: 58 STRs and 94 SNPs. Forensic Sci. Int. Genet. 2022, 57, 102646. [Google Scholar] [CrossRef]
- Sanger Institute. Project Jaguar; Sanger Institute: Cambridge, UK, 2024; Available online: https://www.sanger.ac.uk/collaboration/project-jaguar/ (accessed on 24 July 2025).
- 1000 Genomes Project Consortium; Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; et al. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef] [PubMed]
- 1000 Genomes Project Consortium; Abecasis, G.R.; Auton, A.; Brooks, L.D.; DePristo, M.A.; Durbin, R.M.; Handsaker, R.E.; Kang, H.M.; Marth, G.T.; McVean, G.A. An integrated map of genetic variation from 1,092 human genomes. Nature 2012, 491, 56. [Google Scholar] [CrossRef] [PubMed]
- Boyle, E.A.; Li, Y.I.; Pritchard, J.K. An expanded view of complex traits: From polygenic to omnigenic. Cell 2017, 169, 1177. [Google Scholar] [CrossRef] [PubMed]
- Silventoinen, K.; Sammalisto, S.; Perola, M.; Boomsma, D.I.; Cornes, B.K.; Davis, C.; Dunkel, L.; De Lange, M.; Harris, J.R.; Hjelmborg, J.V.; et al. Heritability of Adult Body Height: A Comparative Study of Twin Cohorts in Eight Countries. Twin Res. Hum. Genet. 2003, 6, 399–408. [Google Scholar] [CrossRef]
- Guo, M.H.; Hirschhorn, J.N.; Dauber, A. Insights and Implications of Genome-Wide Association Studies of Height. J. Clin. Endocrinol. Metab. 2018, 103, 3155. [Google Scholar] [CrossRef]
- Weedon, M.N.; Lettre, G.; Freathy, R.M.; Lindgren, C.M.; Voight, B.F.; Perry, J.R.; Elliott, K.S.; Hackett, R.; Guiducci, C.; Shields, B.; et al. A common variant of HMGA2 is associated with adult and childhood height in the general population. Nat. Genet. 2007, 39, 1245–1250. [Google Scholar] [CrossRef]
- Allen, H.L.; Estrada, K.; Lettre, G.; Berndt, S.I.; Weedon, M.N.; Rivadeneira, F.; Willer, C.J.; Jackson, A.U.; Vedantam, S.; Raychaudhuri, S.; et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature 2010, 467, 832–838. [Google Scholar] [CrossRef]
- Wood, A.R.; Esko, T.; Yang, J.; Vedantam, S.; Pers, T.H.; Gustafsson, S.; Chu, A.Y.; Estrada, K.; Luan, J.; Kutalik, Z.; et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat. Genet. 2014, 46, 1173–1186. [Google Scholar] [CrossRef]
- Yengo, L.; Sidorenko, J.; Kemper, K.E.; Zheng, Z.; Wood, A.R.; Weedon, M.N.; Frayling, T.M.; Hirschhorn, J.; Yang, J.; Visscher, P.M.; et al. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum. Mol. Genet. 2018, 27, 3641–3649. [Google Scholar] [CrossRef]
- Yengo, L.; Vedantam, S.; Marouli, E.; Sidorenko, J.; Bartell, E.; Sakaue, S.; Graff, M.; Eliasen, A.; Jiang, Y.; Raghavan, S.; et al. A saturated map of common genetic variants associated with human height. Nature 2022, 610, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Teven, C.M.; Farina, E.M.; Rivas, J.; Reid, R.R. Fibroblast growth factor (FGF) signaling in development and skeletal diseases. Genes Dis. 2014, 1, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Tomé, D.; Dias, M.S.; Correia, J.; Almeida, R.D. Fibroblast growth factor signaling in axons: From development to disease. Cell Commun. Signal. 2023, 21, 290. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Su, N.; Yang, J.; Tan, Q.; Huang, S.; Jin, M.; Ni, Z.; Zhang, B.; Zhang, D.; Luo, F.; et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target. Ther. 2020, 5, 181. [Google Scholar] [CrossRef]
- Fayad, C.; Legeai-Mallet, L. FGF Signaling: A Key Pathway During Skeletal Development. Biol. Extracell. Matrix 2024, 16, 247–285. [Google Scholar]
- Mustafa, S.; Akhtar, Z.; Asif, M.; Amjad, M.; Latif, M.; Hassan, M.; Faisal, M.; Faisal, M.; Iqbal, F. Novel missense variants in FGFR1 and FGFR3 causes short stature in enrolled families from Pakistan. Meta Gene 2020, 26, 100778. [Google Scholar] [CrossRef]
- Kress, W.; Petersen, B.; Collmann, H.; Grimm, T. An unusual FGFR1 mutation (fibroblast growth factor receptor 1 mutation) in a girl with non-syndromic trigonocephaly. Cytogenet. Cell Genet. 2000, 91, 138–140. [Google Scholar] [CrossRef]
- White, K.E.; Cabral, J.M.; Davis, S.I.; Fishburn, T.; Evans, W.E.; Ichikawa, S.; Fields, J.; Yu, X.; Shaw, N.J.; McLellan, N.J.; et al. Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. Am. J. Hum. Genet. 2005, 76, 361–367. [Google Scholar] [CrossRef]
- Cho, H.W.; Jin, H.S.; Eom, Y.B. Genetic variants of FGFR family associated with height, hypertension, and osteoporosis. Ann. Hum. Biol. 2023, 50, 187–195. [Google Scholar] [CrossRef]
- Cho, H.W.; Jin, H.S.; Eom, Y.B. FGFRL1 and FGF genes are associated with height, hypertension, and osteoporosis. PLoS ONE 2022, 17, e0273237. [Google Scholar] [CrossRef]
- Kichaev, G.; Bhatia, G.; Loh, P.R.; Gazal, S.; Burch, K.; Freund, M.K.; Schoech, A.; Pasaniuc, B.; Price, A.L. Leveraging Polygenic Functional Enrichment to Improve GWAS Power. Am. J. Hum. Genet. 2019, 104, 65–75. [Google Scholar] [CrossRef]
- Rieckmann, T.; Zhuang, L.; Flück, C.E.; Trueb, B. Characterization of the first FGFRL1 mutation identified in a craniosynostosis patient. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2009, 1792, 112–121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trueb, B. Biology of FGFRL1, the fifth fibroblast growth factor receptor. Cell. Mol. Life Sci. 2011, 68, 951–964. [Google Scholar] [PubMed][Green Version]
- Yuen, K.C.J.; Hjortebjerg, R.; Ganeshalingam, A.A.; Clemmons, D.R.; Frystyk, J. Growth hormone/insulin-like growth factor I axis in health and disease states: An update on the role of intra-portal insulin. Front. Endocrinol. 2024, 15, 1456195. [Google Scholar][Green Version]
- Blum, W.F.; Alherbish, A.; Alsagheir, A.; El Awwa, A.; Kaplan, W.; Koledova, E.; Savage, M.O. The growth hormone–insulin-like growth factor-I axis in the diagnosis and treatment of growth disorders. Endocr. Connect. 2018, 7, R212. [Google Scholar] [CrossRef]
- Vasques, G.A.; Andrade, N.L.M.; Correa, F.A.; Jorge, A.A.L. Update on new GH-IGF axis genetic defects. Arch. Endocrinol. Metab. 2019, 63, 608. [Google Scholar]
- Savage, M.O.; Hwa, V.; David, A.; Rosenfeld, R.G.; Metherell, L.A. Genetic Defects in the Growth Hormone–IGF-I Axis Causing Growth Hormone Insensitivity and Impaired Linear Growth. Front. Endocrinol. 2011, 2, 95. [Google Scholar]
- Nadeau, K.; Hwa, V.; Rosenfeld, R.G. STAT5b deficiency: An unsuspected cause of growth failure, immunodeficiency, and severe pulmonary disease. J. Pediatr. 2011, 158, 701–708. [Google Scholar] [CrossRef]
- Hwa, V. Human Growth Disorders Associated with Impaired GH action: Defects in STAT5B and JAK2. Mol. Cell. Endocrinol. 2020, 519, 111063. [Google Scholar] [CrossRef]
- Duan, P.; Bonewald, L.F. The Role of the Wnt/β-catenin Signaling Pathway in Formation and Maintenance of Bone and Teeth. Int. J. Biochem. Cell Biol. 2016, 77, 23. [Google Scholar]
- Hu, L.; Chen, W.; Qian, A.; Li, Y.P. Wnt/β-catenin signaling components and mechanisms in bone formation, homeostasis, and disease. Bone Res. 2024, 12, 39. [Google Scholar] [CrossRef]
- Ho, S.K.; Tsang, M.H.; Lee, M.; Cheng, S.S.W.; Luk, H.; Lo, I.F.M.; Chung, B.H.Y. CTNNB1 Neurodevelopmental Disorder. In GeneReviews®; University of Washington: Seattle, WA, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK580527/ (accessed on 5 August 2025).
- Ren, Q.; Chen, J.; Liu, Y. LRP5 and LRP6 in Wnt Signaling: Similarity and Divergence. Front. Cell Dev. Biol. 2021, 9, 670960. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.L.; Deutsch, S.; Choudhury, U.; Chevalley, T.; Bonjour, J.P.; Dermitzakis, E.T.; Rizzoli, R.; Antonarakis, S.E. Polymorphisms in the Low-Density Lipoprotein Receptor-Related Protein 5 (LRP5) Gene Are Associated with Variation in Vertebral Bone Mass, Vertebral Bone Size, and Stature in Whites. Am. J. Hum. Genet. 2004, 74, 866–875. [Google Scholar] [PubMed][Green Version]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Turin, C.G.; Joeng, K.S.; Kallish, S.; Raper, A.; Asher, S.; Campeau, P.M.; Khan, A.N.; Al Mukaddam, M. Heterozygous variant in WNT1 gene in two brothers with early onset osteoporosis. Bone Rep. 2021, 15, 101118. [Google Scholar] [CrossRef]
- Jing, J.; Wu, Z.; Wang, J.; Luo, G.; Lin, H.; Fan, Y.; Zhou, C. Hedgehog signaling in tissue homeostasis, cancers, and targeted therapies. Signal Transduct. Target. Ther. 2023, 8, 315. [Google Scholar] [CrossRef]
- Ohba, S. Hedgehog Signaling in Skeletal Development: Roles of Indian Hedgehog and the Mode of Its Action. Int. J. Mol. Sci. 2020, 21, 6665. [Google Scholar] [CrossRef]
- Weedon, M.N.; Lango, H.; Lindgren, C.M.; Wallace, C.; Evans, D.M.; Mangino, M.; Freathy, R.M.; Perry, J.R.; Stevens, S.; Hall, A.S.; et al. Genome-wide association analysis identifies 20 loci that influence adult height. Nat. Genet. 2008, 40, 575. [Google Scholar] [CrossRef]
- Costantini, A.; Guasto, A.; Cormier-Daire, V. TGF-β and BMP Signaling Pathways in Skeletal Dysplasia with Short and Tall Stature. Ann. Rev. Genom. Hum. Genet. 2025, 24, 225–253. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.F. Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res. 2009, 19, 71–88. [Google Scholar] [CrossRef]
- Wu, M.; Wu, S.; Chen, W.; Li, Y.P. The roles and regulatory mechanisms of TGF-β and BMP signaling in bone and cartilage development, homeostasis and disease. Cell Res. 2024, 34, 101–123. [Google Scholar] [CrossRef]
- Chi, C.; He, J.; Du, Z.; Zheng, Y.; D’Alessandro, E.; Chen, C.; Moawad, A.S.; Asare, E.; Song, C.; Wang, X. Two Retrotransposon Elements in Intron of Porcine BMPR1B Is Associated with Phenotypic Variation. Life 2022, 12, 1650. [Google Scholar] [CrossRef] [PubMed]
- Asgari, S.; Luo, Y.; Akbari, A.; Belbin, G.M.; Li, X.; Harris, D.N.; Selig, M.; Bartell, E.; Calderon, R.; Slowikowski, K.; et al. A positively selected FBN1 missense variant reduces height in Peruvians. Nature 2020, 582, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rhodes, L.; Graff, M.; Buchanan, V.L.; Justice, A.E.; Highland, H.M.; Guo, X.; Zhu, W.; Chen, H.H.; Young, K.L.; Adhikari, K.; et al. Ancestral diversity improves discovery and fine-mapping of genetic loci for anthropometric traits—The Hispanic/Latino Anthropometry Consortium. Hum. Genet. Genom. Adv. 2022, 3, 100099. [Google Scholar]
- Lejarraga, H.; Mariana del Pino, D.; Virginia Fano, D.; Caino, S.; Cole, T.J. Referencias de peso y estatura desde el nacimiento hasta la madurez para niñas y niños argentinos. Incorporación de datos de la OMS de 0 a 2 años, recálculo de percentilos para obtención de valores LMS. Arch. Argent. Pediatr. 2009, 107, 126–133. [Google Scholar]
- López de Blanco, M.; Macias de Tomei, C.; Landaeta-Jiménez, M.; Izaguirre de Espinoza, I.; Méndez de Pérez, B. Referencias internacional y nacional, su uso en el estudio del crecimiento y la maduración física. An. Venez. Nutr. 2021, 34, 37–48. [Google Scholar] [CrossRef]
- Maduración Sexual y Ósea Según Ritmo en Niños y Jóvenes Del Estudio Longitudinal de Caracas. Available online: https://www.analesdenutricion.org.ve/ediciones/2000/1/art-4/ (accessed on 14 August 2025).
- Lourenço, B.H.; Rodrigues, C.Z.; de Araújo Damasceno, A.A.; Cardoso, M.A.; Castro, M.C. Birth-to-childhood tracking of linear growth and weight gain in the MINA-Brazil Study. Rev. Saude Publica 2024, 57, 3s. [Google Scholar] [CrossRef]
- Jaime Castaño Castrillón, J.; Alberto Villegas Arenas, O. Crecimiento y desarrollo, modelos estadísticos, parámetros, pediatría. Arch. Med. 2012, 12, 18–30. [Google Scholar]
- Tarupi, W.; Lepage, Y.; Hauspie, R.; Félix, M.L.; Monnier, C.; Campbell, J.; Roelants, M.; Hidalgo, R.; Vercauteren, M. Cross-sectional study of child and adolescent growth in ecuador. Rev. Argent. Antropol. Biol. 2019, 21, 1–17. [Google Scholar] [CrossRef]
- Felix, M.L.; Basantes, C.; Nicola, S.; Hidalgo, S.; Guevara-Ramírez, P.; Cadena-Ullauri, S.; Zambrano, A.K. Nutritional Status Assessment of Newborns: Comparison of the CAN Score (Metcoff Methodology), Growth Curves, Anthropometry, and Plicometry. Nutrients 2025, 17, 1642. [Google Scholar] [CrossRef]
- Iannotti, L.L.; Zavaleta, N.; León, Z.; Caulfield, L.E. Growth and body composition of Peruvian infants in a periurban setting. Food Nutr. Bull. 2009, 30, 245. [Google Scholar] [CrossRef]
- Gatica-Domínguez, G.; Mesenburg, M.A.; Barros, A.J.D.; Victora, C.G. Ethnic inequalities in child stunting and feeding practices: Results from surveys in thirteen countries from Latin America. Int. J. Equity Health 2020, 19, 53. [Google Scholar] [CrossRef]
- Mena-Meléndez, L. Ethnoracial child health inequalities in Latin America: Multilevel evidence from Bolivia, Colombia, Guatemala, and Peru. SSM Popul. Health 2020, 12, 100673. [Google Scholar] [CrossRef] [PubMed]
- Mrejen, M.; Cruz, M.V.; Rosa, L. El Sistema de Vigilancia Alimentaria y Nutricional (SISVAN) como herramienta de seguimiento del estado nutricional de niños y adolescentes en Brasil. Cad. Saude Publica 2023, 39, e00169622. [Google Scholar] [CrossRef] [PubMed]
- Gatica-Domínguez, G.; Victora, C.; Barros, A.J.D. Ethnic inequalities and trends in stunting prevalence among Guatemalan children: An analysis using national health surveys 1995–2014. Int. J. Equity Health 2019, 18, 110. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Dommarco, J.A.; Cuevas-Nasu, L.; Bonvecchio-Arenas, A.; Unar-Munguía, M.; Gómez-Acosta, L.M.; Muñoz-Espinosa, A.; García-Feregrino, R.; Ávila-Arcos, M.A.; Méndez-Gómez-Humarán, I.; Ávila-Curiel, A.; et al. Mala nutrición en menores de cinco años. Salud Publica Mex. 2024, 66, 395–403. [Google Scholar]
- Esparza, B.; Moyano, E.; Mesa Cano, I.C. Estado nutricional según referencias OMS 2007 y local en escolares de la provincia de Morona Santiago, Ecuador—2024. In Pacha Revista de Estudios Contemporáneos del Sur Globa; Universidad Católica de Cuencal: Cuenca, Ecuador, 2024; Volume 5, p. e240317. [Google Scholar]
- Ramírez, E.; Ramos Salas, J.E.; Bustillos, M.B.; González Franco, L.R.; Guillen, E.F.; Jacome, A.P.; Valencia, M.E. WHO body mass index for age charts overestimate thinness and overweight compared to international and US charts applied to indigenous and non-indigenous Mexican children. Soc. Latinoam. Nutr. 2017, 67, 159–168. [Google Scholar]
- Ticona-Rendón, M.; Huanco-Apaza, D. Curva de referencia peruana del peso de nacimiento para la edad gestacional y su aplicación para la identificación de una nueva población neonatal de alto riesgo. Rev. Peru. Med. Exp. Salud Publica 2007, 24, 325–335. [Google Scholar]
- Pipman, V.; Alonso, G.; Escobar, M.E.; Pasqualini, T.; Keselman, A.; Boulgourdjian, E.; Arcari, A.; Bengolea, S.V.; D´Amato, S. Actualización. Indicaciones actuales para el uso de la hormona de crecimiento. Arch. Argent. Pediatr. 2014, 12, 89–95. [Google Scholar]
- Pérez Pérez, A.; Alonso Alonso, A.; González García, A.; Riaño Galán, I. Tratamiento con hormona de crecimiento en pediatría, ¿qué podemos mejorar? Endocrinol. Diabetes Nutr. 2023, 70, 313–318. [Google Scholar]
- Al-Beltagi, M.; Bediwy, A.S.; Saeed, N.K. Insulin-resistance in paediatric age: Its magnitude and implications. World J. Diabetes 2022, 13, 282. [Google Scholar] [CrossRef]
- Passone, C.D.G.B.; Franco, R.R.; Ito, S.S.; Trindade, E.; Polak, M.; Damiani, D.; Bernardo, W.M. Growth hormone treatment in Prader-Willi syndrome patients: Systematic review and meta-analysis. BMJ Paediatr. Open 2020, 4, e000630. [Google Scholar] [CrossRef]
- Houk, C.P.; Lee, P.A. Early diagnosis and treatment referral of children born small for gestational age without catch-up growth are critical for optimal growth outcomes. Int. J. Pediatr. Endocrinol. 2012, 2012, 11. [Google Scholar] [CrossRef]
- Sotos, J.F.; Tokar, N.J. Growth hormone significantly increases the adult height of children with idiopathic short stature: Comparison of subgroups and benefit. Int. J. Pediatr. Endocrinol. 2014, 2014, 15. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.J.; Wooh, J.W.; Tunny, K.A.; Waters, M.J. Growth hormone receptor; mechanism of action. Int. J. Biochem. Cell Biol. 2008, 40, 1984–1989. [Google Scholar] [CrossRef] [PubMed]
- Binder, G.; Rappold, G.A. SHOX Deficiency Disorders. In GeneReviews®; University of Washington: Seattle, WA, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1215/ (accessed on 31 July 2025).
- Plachy, L.; Dusatkova, P.; Maratova, K.; Petruzelkova, L.; Zemkova, D.; Elblova, L.; Kucerova, P.; Toni, L.; Kolouskova, S.; Snajderova, M.; et al. NPR2 Variants Are Frequent among Children with Familiar Short Stature and Respond Well to Growth Hormone Therapy. J. Clin. Endocrinol. Metab. 2020, 105, e746–e752. [Google Scholar] [CrossRef] [PubMed]
- Little, M.A. Evolutionary Strategies for Body Size. Front. Endocrinol. 2020, 11, 513525. [Google Scholar] [CrossRef]
- Stevens, A.; Perchard, R.; Garner, T.; Clayton, P.; Murray, P. Pharmacogenomics applied to recombinant human growth hormone responses in children with short stature. Rev. Endocr. Metab. Disord. 2021, 22, 135. [Google Scholar] [CrossRef]
- Wassenaar, M.J.E.; Dekkers, O.M.; Pereira, A.M.; Wit, J.M.; Smit, J.W.; Biermasz, N.R.; Romijn, J.A. Impact of the Exon 3-Deleted Growth Hormone (GH) Receptor Polymorphism on Baseline Height and the Growth Response to Recombinant Human GH Therapy in GH-Deficient (GHD) and Non-GHD Children with Short Stature: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2009, 94, 3721–3730. [Google Scholar] [CrossRef]
- Jorge, A.A.L.; Marchisotti, F.G.; Montenegro, L.R.; Carvalho, L.R.; Mendonca, B.B.; Arnhold, I.J. Growth hormone (GH) pharmacogenetics: Influence of GH receptor exon 3 retention or deletion on first-year growth response and final height in patients with severe GH deficiency. J. Clin. Endocrinol. Metab. 2006, 91, 1076–1080. [Google Scholar] [CrossRef]
- Sodero, G.; Arzilli, F.; Malavolta, E.; Lezzi, M.; Comes, F.; Villirillo, A.; Rigante, D.; Cipolla, C. Efficacy and Safety of Growth Hormone (GH) Therapy in Patients with SHOX Gene Variants. Children 2025, 12, 325. [Google Scholar] [CrossRef] [PubMed]
- González, M.G.; Navarrete, D.G.; Navarro, F.T.; Tolosa Navarro, F.; López Cuevas, P.; Rodríguez Convertino, F.; Román Reyes, R. Growth Hormone treatment in children with Growth Hormone deficiency, idiopathic short stature, SHOX gene mutation, small for gestational age and Turner syndrome. Andes Pediatr. Rev. Chil. Pediatr. 2024, 95, 151–158. [Google Scholar]
- Wu, X.; Wu, J.; Yuan, Y.; Yang, L.; Yu, L. Noonan syndrome: rhGH treatment and PTPN11 mutation. Mol. Genet. Genom. Med. 2023, 11, e2266. [Google Scholar] [CrossRef]
- Howell, S.J.; Wilton, P.; Lindberg, A.; Shalet, S.M. Growth hormone replacement and the risk of malignancy in children with neurofibromatosis. J. Pediatr. 1998, 133, 201–205. [Google Scholar] [CrossRef]
- Wu, J.; Wang, M.; Jiao, Z.; Dou, B.; Li, B.; Zhang, J.; Zhang, H.; Sun, Y.; Tu, X.; Kong, X.; et al. Novel Loss-of-Function Mutations in NPR2 Cause Acromesomelic Dysplasia, Maroteaux Type. Front. Genet. 2022, 13, 823861. [Google Scholar] [CrossRef]
- Stavber, L.; Gaia, M.J.; Hovnik, T.; Jenko Bizjan, B.; Debeljak, M.; Kovač, J.; Omladič, J.Š.; Battelino, T.; Kotnik, P.; Dovč, K. Heterozygous NPR2 Variants in Idiopathic Short Stature. Genes 2022, 13, 1065. [Google Scholar] [CrossRef]
- Ke, X.; Liang, H.; Miao, H.; Yang, H.; Wang, L.; Gong, F.; Pan, H.; Zhu, H. Clinical Characteristics of Short-Stature Patients with an NPR2 Mutation and the Therapeutic Response to rhGH. J. Clin. Endocrinol. Metab. 2021, 106, 431–441. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, L.; Liu, G.; Ma, C.; Zheng, J.; Niu, L. Evaluation of Growth Hormone Therapy in Seven Chinese Children With Familial Short Stature Caused by Novel ACAN Variants. Front. Pediatr. 2022, 10, 819074. [Google Scholar] [CrossRef]
- Çelik, N.B.; Losekoot, M.; Işık, E.; Gönç, E.N.; Alikaşifoğlu, A.; Kandemir, N.; Özön, Z.A. Long-term Growth Hormone Therapy in a Patient with IGF1R Deletion Accompanied by Delayed Puberty and Central Hypothyroidism. JCRPE J. Clin. Res. Pediatr. Endocrinol. 2024, 16, 481–488. [Google Scholar] [PubMed]
- Scaglia, P.A.; Keselman, A.C.; Braslavsky, D.; Martucci, L.C.; Karabatas, L.M.; Domené, S.; Gutiérrez, M.L.; Ballerini, M.G.; Ropelato, M.G.; Spinola-Castro, A.; et al. Characterization of four Latin American families confirms previous findings and reveals novel features of acid-labile subunit deficiency. Clin. Endocrinol. 2017, 87, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Assefi, A.; van Dommelen, P.; Arnaud, L.; Otero, C.; Fernandez-Luque, L.; Koledova, E.; Calliari, L.E. Adherence to Growth Hormone Treatment Using a Connected Device in Latin America: Real-World Exploratory Descriptive Analysis Study. JMIR Mhealth Uhealth 2022, 10, e32626. [Google Scholar] [CrossRef]
- Villela, T.R.; Freire, B.L.; Braga, N.T.P.; Arantes, R.R.; Funari, M.F.; Alexander, J.A.; Silva, I.N. Growth Hormone insensitivity (Laron syndrome): Report of a new family and review of Brazilian patients. Genet. Mol. Biol. 2020, 42, e20180197. [Google Scholar] [CrossRef]
- Boro, H.; Rahman, S.H.; Khatiwada, S.; Alam, S.; Khadgawat, R. Laron syndrome: An experience of treatment of two cases. J. Clin. Transl. Endocrinol. Case Rep. 2021, 19, 100076. [Google Scholar] [CrossRef]
- Alfaro Velásquez, J.M.; Maria Vásquez Trespalacios, E.; Urrego, R.; Arroyave Toro, M.C.; Montilla Velásquez, M.D.P.; Soto, C.M.D.; Vélez, J.C.Z.; Jaramillo Henríquez, V.; Flórez, J.E.S.; Monroy, F.P.; et al. Effect of Recombinant Human Growth Hormone (rhGH) Use on Genetic Methylation Patterns and Their Relationship with Body Composition in Small-for-Gestational-Age (SGA) Newborns. Biomedicines 2025, 13, 1288. [Google Scholar] [CrossRef]
- Haas-Lude, K.; Nagel, C.; Schwarze, C.; Mautner, V.F. Growth hormone treatment of patients with neurofibromatosis type 1. Neuropediatrics 2010, 41, P1332. [Google Scholar] [CrossRef]
- Vurallı, D.; Gönç, N.; Vidaud, D.; Özön, A.; Alikaşifoğlu, A.; Kandemir, N. Growth Hormone Deficiency in a Child with Neurofibromatosis-Noonan Syndrome. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Jorge, A.A.L.; Edouard, T.; Maghnie, M.; Pietropoli, A.; Kelepouris, N.; Romano, A.; Zenker, M.; Horikawa, R. Outcomes in growth hormone-treated Noonan syndrome children: Impact of PTPN11 mutation status. Endocr. Connect. 2022, 11, e210615. [Google Scholar] [CrossRef] [PubMed]
- Revista Española Endocrinología Pediátrica—Different Responses to rhGH Treatment in SGA Children: Patients with Mutations in Their IGF-1 Receptor, a View from the Research Lab to the Clinic. Available online: https://www.endocrinologiapediatrica.org/modules.php?name=articulos&idarticulo=633&idlangart=ES (accessed on 4 August 2025).
- Zaitoon, H.; Yackobovitch-Gavan, M.; Midlej, E.; Uretzky, A.; Laurian, I.; Dorfman, A.; Interator, H.; Lebenthal, Y.; Brener, A. The role of IGF1 in determining body composition in children and adolescents with growth hormone deficiency and those with idiopathic short stature. Endocrine 2024, 86, 1110. [Google Scholar] [CrossRef]
- Stavber, L.; Dovc, K.; Debeljak, M.; Jasna Suput, O.; Battelino, T.; Kotnik, P. Efficacy of growth hormone therapy in children with npr2 and acan gene variants: A comparative study. Endocr. Abstr. 2025, 110, 583. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.; Sun, Y.; Chen, J.; Yuan, K.; Zhang, Y.; Yang, X.; Lin, X.; Chen, R. Novel pathogenic NPR2 variants in short stature patients and the therapeutic response to rhGH. Orphanet J. Rare Dis. 2023, 18, 221. [Google Scholar] [CrossRef]
- Polidori, N.; Castorani, V.; Mohn, A.; Chiarelli, F. Deciphering short stature in children. Ann. Pediatr. Endocrinol. Metab. 2020, 25, 69–79. [Google Scholar] [CrossRef]
- Dipasquale, V.; Cucinotta, U.; Romano, C. Acute Malnutrition in Children: Pathophysiology, Clinical Effects and Treatment. Nutrients 2020, 12, 2413. [Google Scholar] [CrossRef]
- Merchán-Villafuerte, D.; Maricela, K.; Daniela, K. Impacto de la desnutrición en el desarrollo infantil de América Latina: Implicaciones para la salud y el desarrollo integral. MQRInvestigar 2024, 8, 3566–3586. [Google Scholar] [CrossRef]
- Espejo, J.P.; Tumani, M.F.; Aguirre, C.; Sanchez, J.; Parada, A. Nutritional food education: Strategies to improve adherence to a diet therapy plan. Rev. Chil. Nutr. 2022, 49, 391–398. [Google Scholar] [CrossRef]
- Correia, M.I.T.D.; Sulo, S.; Brunton, C.; Sulz, I.; Rodriguez, D.; Gomez, G.; Tarantino, S.; Hiesmayr, M. Prevalence of malnutrition risk and its association with mortality: NutritionDay Latin America survey results. Clin. Nutr. 2021, 40, 5114–5121. [Google Scholar] [CrossRef] [PubMed]
- Galicia, L.; López De Romaña, D.; Harding, K.B.; De-Regil, L.M.; Grajeda, R. Tackling malnutrition in Latin America and the Caribbean: Challenges and Opportunities. Rev. Panam Salud Publica 2016, 40, 138–146. [Google Scholar]
- Organización Panamericana de la Salud. Informe ONU: 131 Millones de Personas en América Latina y el Caribe no Pueden Acceder a una Dieta Saludable—OPS/OMS|Organización Panamericana de la Salud. 2023. Available online: https://www.paho.org/es/noticias/19-1-2023-informe-onu-131-millones-personas-america-latina-caribe-no-pueden-acceder-dieta (accessed on 11 August 2025).
- Domínguez Bucheli, O.; Arias Guevara, P.; Mejía Acosta, A.; Bucheli, K.M.; Armijos, L.; Acosta, A.M.; Bucheli, K.M.; Ocampo, M.B.; Villar, M.; Manthra Comunicación. Reporte de Nutrición 2022: La Desnutrición Crónica infantil en el Ecuador. 2023. Available online: https://consejoconsultivodci.com.ec/wp-content/uploads/2023/08/CRISFE-final-WEB.pdf (accessed on 11 August 2025).
- UNICEF Reporte 2023: Crece la Ola de Sobrepeso en la Niñez. ¿Demasiado Tarde para Revertir la Marea en América Latina y el Caribe? Ciudad de Panamá. 2023. Available online: https://www.unicef.org/lac/media/43026/file/Reporte%20sobrepeso%20ninez%20america%20latina%20caribe%202023%20UNICEF.pdf%20.pdf (accessed on 11 August 2025).
- Carrero, C.M.; Oróstegui, M.A.; Linda, R.E.; Barros Arrieta, D. Anemia infantil: Desarrollo cognitivo y rendimiento académico. In Archivos Venezolanos de Farmacología y Terapéutica; Sociedad Venezolana de Farmacología Clínica y Terapéutica: Caracas, Venezuela, 2018; Volume 37, Available online: https://www.redalyc.org/articulo.oa?id=55963209020 (accessed on 11 August 2025).
- Batrouni, L.; Piran, M.F.; Eandi, M.; Dasbul, G.; Toledo, S. Parámetros Bioquimicos y de ingesta de hierro, en niños de 12 a 24 meses de edad de cordoba, argentina. Rev. Chil. Nutr. 2004, 31, 330–335. [Google Scholar] [CrossRef]
- Parrales Toala, J.A.; Pilco Romero, T.J.; Pin Guerra, A.I.; Durán Pincay, Y.E. Study of the prevalence of intestinal parasitoses in latin america. MQRInvestigar 2022, 6, 1373–1395. [Google Scholar] [CrossRef]
- Nakandakari, M.D.; De la Rosa, D.N.; Beltrán-Fabián, M. Enteroparasitosis en niños de una comunidad rural de Lima-Perú. Rev. Medica Hered. 2016, 27, 96–99. [Google Scholar] [CrossRef]
- Navone, G.T.; Zonta, M.L.; Cociancic, P.; Garraza, M.; Gamboa, M.I.; Giambelluca, L.A.; Dahinten, S.; Oyhenart, E.E. Estudio transversal de las parasitosis intestinales en poblaciones infantiles de Argentina. Rev. Panam. Salud Publica 2017, 41, e24. Available online: https://iris.paho.org/handle/10665.2/33879 (accessed on 11 August 2025). [CrossRef]
- Brito Núñez, J.D.; Landaeta Mejías, J.A.; Chávez Contreras, A.N.; Gastiaburú Castillo, P.K.; Blanco Martínez, Y.Y. Prevalencia de parasitosis intestinales en la comunidad rural apostadero, municipio sotillo, estado monagas, venezuela. Rev. Científica Cienc. Médica 2017, 20, 7–14. [Google Scholar] [CrossRef]
- Parrales-Toala, J.A.; Pilco-Romero, T.J.; Pin-Guerra, A.I.; Durán-Pincay, Y. Estudio de la prevalencia de la parasitosis intestinal a nivel de Latinoamérica. MQRInvestigar 2022, 6, 1373–1395. [Google Scholar] [CrossRef]
- Montes Franceschini, S. Contaminación ambiental e infecciones respiratorias en niños. Neumol. Pediátrica 2021, 16, 161–163. Available online: https://www.savalnet.ec/revistas/neumo_ped_diciembre_2021/30/ (accessed on 11 August 2025). [CrossRef]
- Gómez-Oliván, L.M. Pollution of water bodies in latin America: Impact of contaminants on species of ecological interest. In Pollution of Water Bodies in Latin America: Impact of Contaminants on Species of Ecological Interest; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–349. [Google Scholar]
- Jelenkovic, A.; Sund, R.; Yokoyama, Y.; Latvala, A.; Sugawara, M.; Tanaka, M.; Matsumoto, S.; Freitas, D.L.; Maia, J.A.; Knafo-Noam, A.; et al. Genetic and environmental influences on human height from infancy through adulthood at different levels of parental education. Sci. Rep. 2020, 10, 7974. [Google Scholar] [CrossRef] [PubMed]
- Lui, J.C.; Palmer, A.C.; Christian, P. Nutrition, Other Environmental Influences, and Genetics in the Determination of Human Stature. Annu. Rev. Nutr. 2024, 44, 205–229. [Google Scholar] [CrossRef]
- Sharma, V.; Varshney, R.; Sethy, N.K. Human adaptation to high altitude: A review of convergence between genomic and proteomic signatures. Hum. Genom. 2022, 16, 21. [Google Scholar] [CrossRef]
- Julian, C.G.; Moore, L.G. Human Genetic Adaptation to High Altitude: Evidence from the Andes. Genes 2019, 10, 150. [Google Scholar] [CrossRef]
- Ma, Q.; Xiong, F.; Zhang, L. Gestational hypoxia and epigenetic programming of brain development disorders. Drug Discov. Today 2014, 19, 1883. [Google Scholar] [CrossRef]
- Wang, B.; Zeng, H.; Liu, J.; Sun, M. Effects of Prenatal Hypoxia on Nervous System Development and Related Diseases. Front. Neurosci. 2021, 15, 755554. [Google Scholar] [CrossRef]
- Medina Jasso, J.J.; Pilo, J.; de Vera, T.D.; Rego, A.; Boughanem, H.; Macías González, M.; García Flores, L.A. Factores epigenéticos promotores de la obesidad en Latinoamérica. Revista de la Sociedad Española de Cirugía de Obesidad y Metabólica y de la Sociedad Española para el Estudio de la Obesidad. BMI J. 2025, 15, 4991–5002. [Google Scholar] [CrossRef]
- Cabana, J.; Sabatelli, D.; Tonietti, M.; Flores, A.; Conti, R.; Pasqualini, D.; Gaete, L.; Gil, S.M. Concepto de Developmental Origins of Health and Disease: El ambiente en los primeros mil días de vida y su asociación con las enfermedades no transmisibles. Arch. Argent. Pediatr. 2020, 118, 118–129. [Google Scholar]
- Wade, P.; López-Beltrán, C.; Restrepo, E.; Ventura Santos, R. Genomic research, publics and experts in Latin America: Nation, race and body. Soc. Stud. Sci. 2015, 45, 775. [Google Scholar] [CrossRef]
- Ortega-García, J.A.; Tellerías, L.; Ferrís-Tortajada, J.; Boldo, E.; Campillo-López, F.; Van den Hazel, P.; Cortes-Arancibia, S.; Ramis, R.; Gaioli, M.; Monroy-Torres, R.; et al. Amenazas, desafíos y oportunidades para la salud medioambiental pediátrica en Europa, América Latina y el Caribe. An. Pediatr. 2019, 90, e1–e124. [Google Scholar] [CrossRef]
- Cassorla, F.; Calzada León, R. Manual de diagnóstico y tratamiento de los trastornos del crecimiento en Latinoamérica. Available online: https://slep-endocrino.com/wp-content/uploads/2022/12/libro-merck.pdf (accessed on 7 August 2025).
- Miranda, J.; Maestre, N.; Paternina-Caicedo, Á.; Parra-Saavedra, M.; Caradeux, J.; Sepulveda-Martinez, Á.; Pelaez-Chomba, M.; Torres, A.; Parra-Cordero, M.; Diaz-Corvillón, P.; et al. Performance of the INTERGROWTH-21st and World Health Organization fetal growth charts for the detection of small-for-gestational age neonates in Latin America Synopsis Funding information Universidad de Cartagena. Int. J. Gynecol. Obs. 2023, 161, 1083–1091. [Google Scholar] [CrossRef]
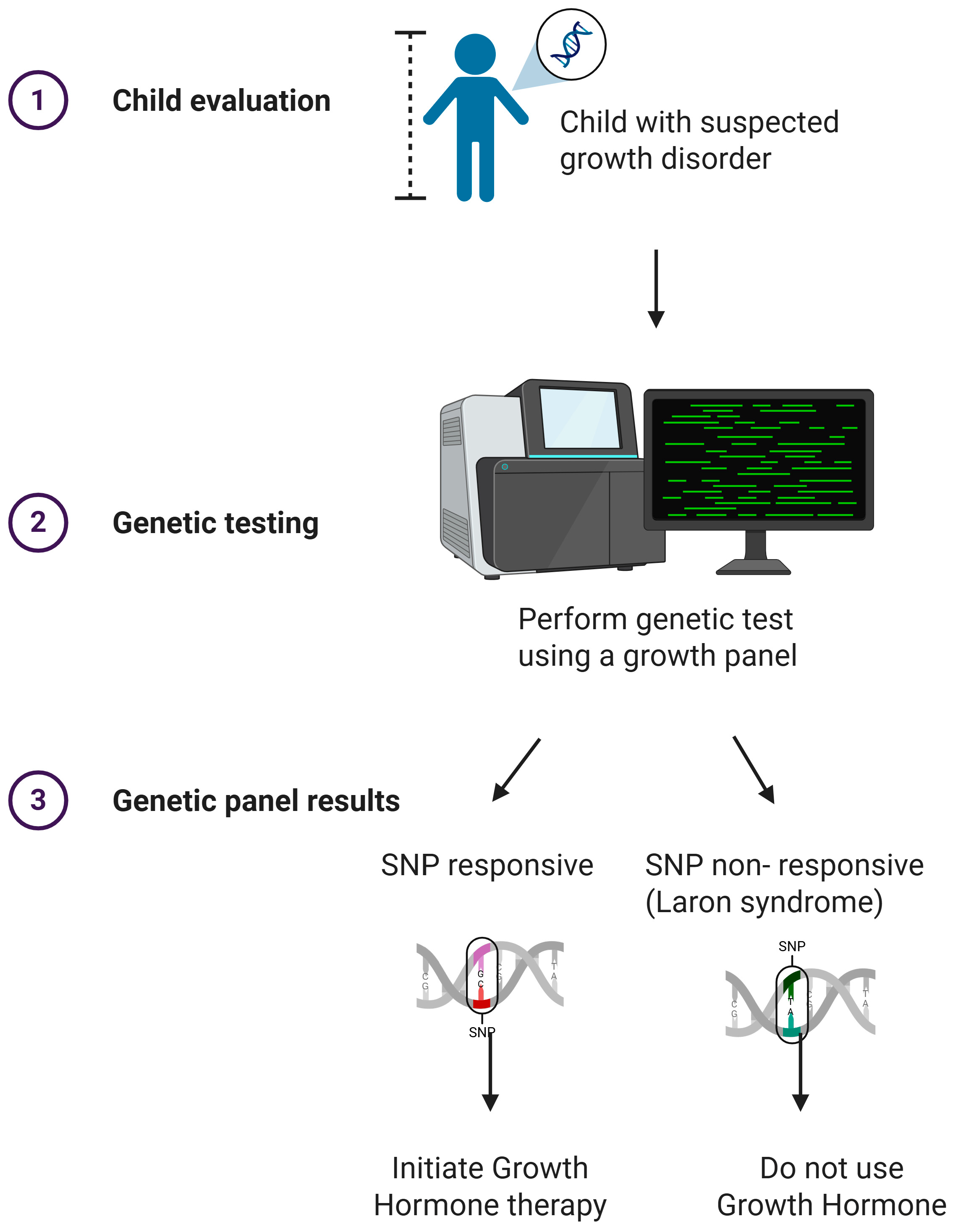
| Gene/Variant | Global Evidence | Latin American Evidence |
|---|---|---|
| GHR exon 3 deletion | Study: Wassenaar et al., 2009 (meta-analysis of 15 studies) [112]. N: meta-analysis of 15 studies Duration: 1 year Countries: Germany, Spain, The Netherlands, Taiwan, others Association: Carriers of d3 allele (GHRd3) showed higher baseline height SDS (+0.16), greater growth velocity (+0.52 cm/year), and modest height SDS gain (+0.075) vs. fl/fl. Effect stronger at lower GH doses and older age. | Study: Jorge et al., 2005 (retrospective cohort) [113]. N: 75 GHD children Duration: mean 7.5 ± 3.0 years Country: Brazil Association: d3 carriers had significantly higher growth velocity (12.3 ± 2.6 vs. 10.6 ± 2.3 cm/year) and taller final height (SDS −0.8 vs. −1.7; p < 0.05) than fl/fl homozygotes. |
| SHOX | Study: Sodero et al., 2024 (scoping review of 22 studies) [114]. N: 22 studies Duration: variable Countries: Germany, Italy, others Association: GH therapy improves growth velocity and is generally safe, though prospective studies are still required. | Study: Griffero González et al., 2024 (retrospective cohort) [115]. N: 9 SHOX patients (within 73 treated) Duration: ≥12 months Country: Chile (Santiago) Association: Significant height SDS gain (+0.8 ± 0.7; p = 0.007). Treatment well tolerated; adverse events mainly mild (headache, limb pain). |
| NF1 | Study: Howell et al., 1998 (KIGS database, retrospective cohort) [117]. N: 102 GH-deficient NF1 children Treatment duration: up to 3 years Countries: International (Europe, USA) Association: Median height velocity increased from 4.2 cm/year (pre-treatment) to 7.1 cm/year in the first year and remained >5.7 cm/year at years 2–3. Median height SDS improved from −2.4 to −1.8 after 3 years. No excess malignant risk was detected compared with NF1 background incidence. | Not reported. |
| Study: Haas-Lude et al., 2000 (retrospective, Germany) [128]. N: 10 NF1 patients Duration: variable, retrospective follow-up Countries: Germany Association: Overall, GH therapy was beneficial; one case showed tumor progression, another resolution. No second tumors or cutaneous neurofibromas were detected. | ||
| Case report: Vurallı et al., 2016 (NF1-Noonan syndrome) [129]. N: 1 girl (mutation in NF1) Duration: GH until final height Country: Turkey/France collaboration Association: Short stature due to GHD improved under GH treatment. | ||
| PTPN11 | Study: Jorge et al., 2022 [130]. N: 69 NS patients (71% PTPN11+) Treatment duration: 4 years of rhGH Countries: Multinational (Europe, USA, Japan) Association: Both PTPN11-positive and negative patients showed significant improvement in HSDS (+1.3 vs. +1.5 over 4 years, respectively; no statistical difference). Safety outcomes were consistent with prior GH studies. | Not reported. |
| Study: Wu et al., 2023 (case series, China) [116]. N: 8 children with NS (7 treated with rhGH, PTPN11-positive) Treatment duration: median follow-up ≈ 3 years Country: China Association: Growth velocity increased from 3.7 ± 0.5 cm/year to 8.0 ± 1.0 cm/year (p < 0.01). One patient developed osteochondroma during therapy, highlighting the need for bone monitoring in PTPN11 carriers. | ||
| IGF1/IGF1R | Study: Çelik et al., 2022 (case report) [122]. N: 1 boy with complete IGF1R deletion Duration: 5.7 years of rhGH (two courses) Country: Turkey/The Netherlands Association: Improved growth velocity and near final height. Partial hypogonadotropic hypogonadism and central hypothyroidism developed. rhGH had partial effect; early initiation may be more beneficial. | Not reported. |
| Study: Göpel & Pfäffle, 2021 (retrospective cohort) [131]. N: 23 IGF1R mutation carriers vs. 34 SGA controls Duration: ≥4 years rhGH Country: Germany Association: IGF1R carriers had lower growth response to rhGH (Δ height SDS 0.29 in year 1 vs. 0.65 in SGA, p < 0.01). Long-term NFH gain was modest (−2.59 SDS treated vs. −2.22 SDS in treated SGA). | ||
| Study: Zaitoon et al., 2024 (observational retrospective) [132]. N: 135 pediatric patients (64 GHD, 71 ISS) Duration: routine follow-up with BIA, cumulative rhGH dose assessed Country: Israel Association: GHD patients showed higher BMI z-scores, higher fat %, lower muscle-to-fat ratio compared to ISS. Higher IGF1 z-scores were positively associated with skeletal muscle mass but not with adiposity. Suggests rhGH therapy may mitigate muscle deficits by raising IGF1. | ||
| ACAN variants | Study: Stavber et al., 2025 (comparative cohort, Slovenia) [133]. N: 17 children with ACAN variants (vs. 16 with NPR2) Duration: mean 5.3 ± 2.2 years of rhGH Country: Slovenia Association: Prepubertal start produced greater gains (+1.35 SDS) vs. pubertal (+0.3 SDS). ACAN group showed stronger overall response compared with NPR2. | Not reported |
| Study: Sun et al., 2022 (familial short stature, China) [121]. N: 7 families (32 screened; 7 novel ACAN variants identified; 6 patients followed) Duration: mean 1.85 ± 1.91 years of rhGH Country: China Association: Height SDS improved from −2.89 ± 0.68 to −1.91 ± 0.93 after treatment. All showed good therapeutic response, expanding the pathogenic variant spectrum. | ||
| NPR2 variants | Study: Stavber et al., 2025 (comparative cohort) [133]. N: 16 children with NPR2 variants (vs. 17 ACAN) Duration: mean 3.2 ± 1.7 years of rhGH Country: Slovenia Association: Prepubertal start yielded greater benefit (+1.01 SDS) than pubertal (+0.37 SDS). Effect positive but smaller than ACAN group. | Not reported |
| Study: Chen et al., 2023 (case series) [134]. N: 3 unrelated Chinese patients with novel NPR2 variants Duration: 2 years of rhGH Country: China Association: Height gain of +1.59 ± 0.1 SDS after 2 years. Functional studies confirmed severe loss of cGMP signaling in pathogenic variants. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zambrano, A.K.; Guevara-Ramírez, P.; Cadena-Ullauri, S.; Basantes, C.; Nicola, S.; Hidalgo, S.; Felix, M.L. Genetic Variability in Child Growth Among South American Populations: A Perspective Integrating Population Genetics, Growth Standards, and Precision Growth Medicine. Int. J. Mol. Sci. 2025, 26, 9300. https://doi.org/10.3390/ijms26199300
Zambrano AK, Guevara-Ramírez P, Cadena-Ullauri S, Basantes C, Nicola S, Hidalgo S, Felix ML. Genetic Variability in Child Growth Among South American Populations: A Perspective Integrating Population Genetics, Growth Standards, and Precision Growth Medicine. International Journal of Molecular Sciences. 2025; 26(19):9300. https://doi.org/10.3390/ijms26199300
Chicago/Turabian StyleZambrano, Ana Karina, Patricia Guevara-Ramírez, Santiago Cadena-Ullauri, Carmen Basantes, Susana Nicola, Susana Hidalgo, and Maria L. Felix. 2025. "Genetic Variability in Child Growth Among South American Populations: A Perspective Integrating Population Genetics, Growth Standards, and Precision Growth Medicine" International Journal of Molecular Sciences 26, no. 19: 9300. https://doi.org/10.3390/ijms26199300
APA StyleZambrano, A. K., Guevara-Ramírez, P., Cadena-Ullauri, S., Basantes, C., Nicola, S., Hidalgo, S., & Felix, M. L. (2025). Genetic Variability in Child Growth Among South American Populations: A Perspective Integrating Population Genetics, Growth Standards, and Precision Growth Medicine. International Journal of Molecular Sciences, 26(19), 9300. https://doi.org/10.3390/ijms26199300






