Hepatocyte Growth Factor-Mediated Chondrocyte Proliferation Induced by Adipose-Derived MSCs from Osteoarthritis Patients and Its Synergistic Enhancement by Hyaluronic Acid
Abstract
1. Introduction
2. Results
2.1. Phenotypic Characterization of ASCs and Spheroid Viability
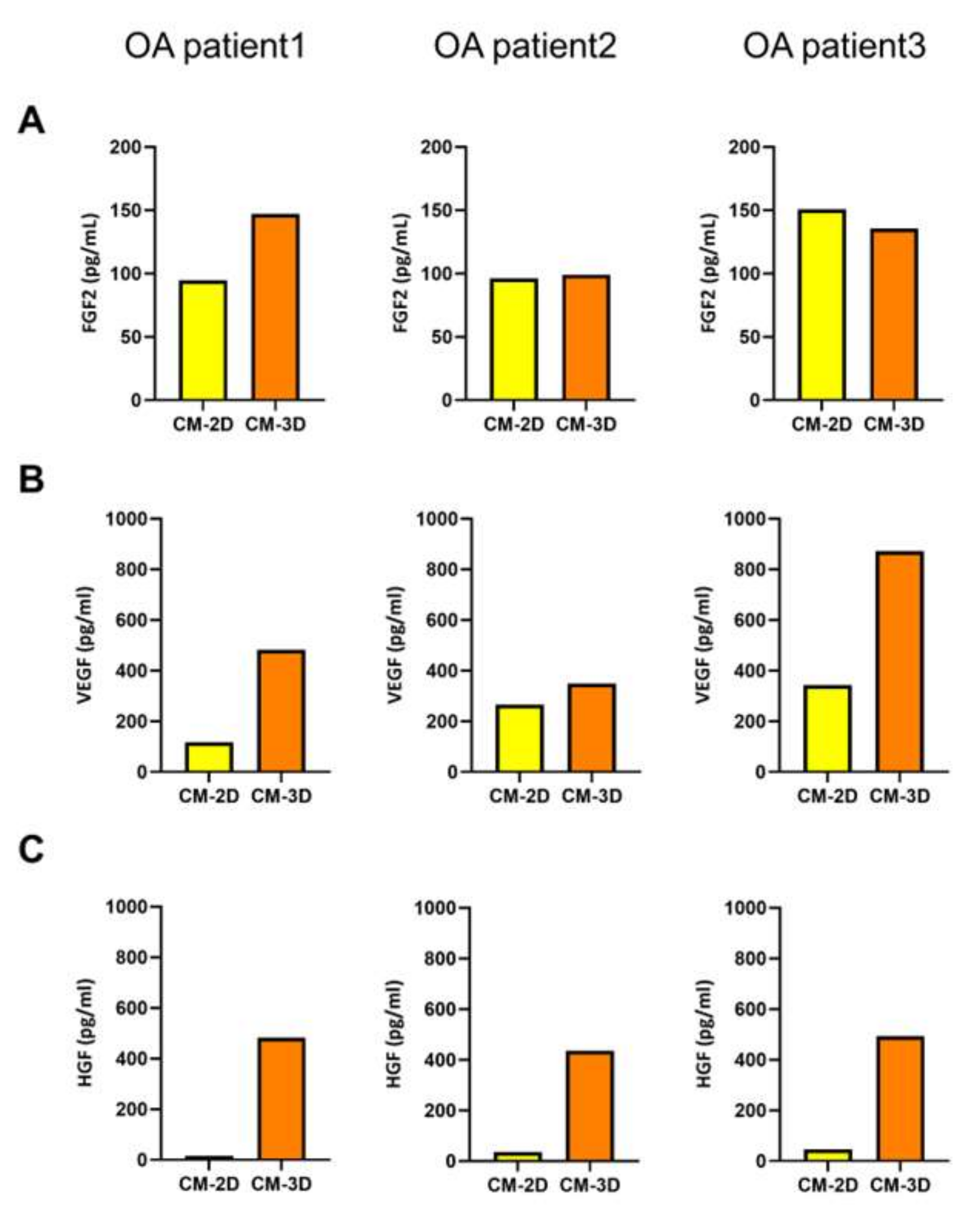
2.2. Growth-Factor Profile of Spheroid-Conditioned Medium
2.3. Three-Dimensional-Conditioned Medium Promotes Chondrocyte Proliferation More Effectively than 2D-Conditioned Medium
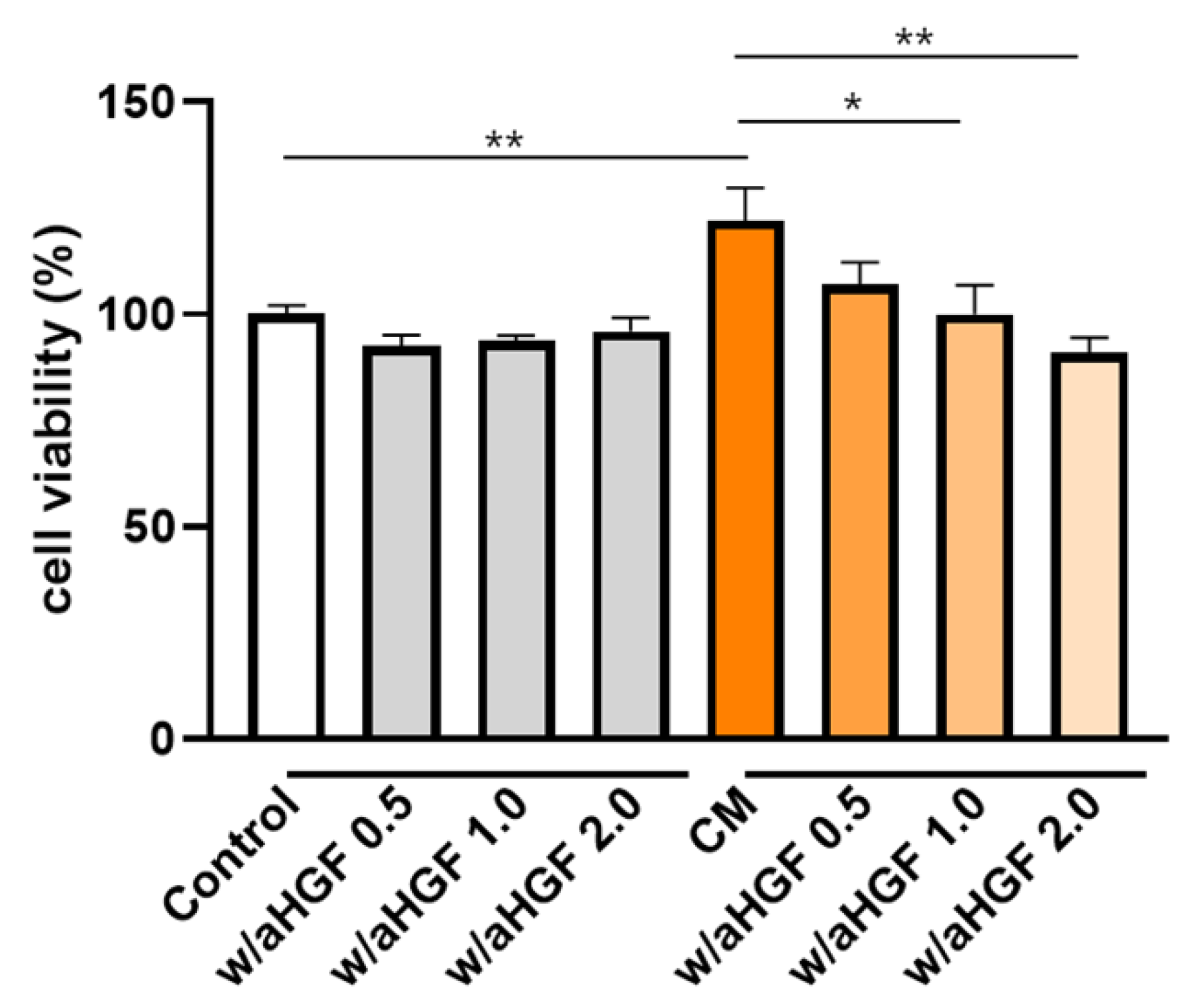
2.4. HGF Neutralization Reduces the Chondrocyte-Proliferative Effect of 3D ASC-Conditioned Medium
2.5. HA Modulates Growth-Factor Secretion in 30 Patient-Derived ASCs
2.6. HA Enhances ASC Proliferation in a Dose-Dependent Manner
3. Discussion
4. Materials and Methods
4.1. Patient Recruitment and Ethics
4.2. Isolation and Expansion of Osteoarthritis-Adipose-Derived Mesenchymal Stem Cells
4.3. Flow Cytometric Phenotyping
4.4. Spheroid Formation and Viability Assay
4.5. Collection of Conditioned Medium (CM) and Growth-Factor Quantification
4.6. Evaluation of Chondrocyte Proliferation by Conditioned Media Derived from 2D- and 3D-Cultured ASCs
4.7. Neutralization Assay to Evaluate the Role of HGF in Chondrocyte Proliferation
4.8. Hyaluronic Acid Dose–Response Study
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Pan, Z.; Zou, Y.; He, Y.; Yang, P.; Tang, Q.Q.; Yin, F. A comparative assessment of adipose-derived stem cells from subcutaneous and visceral fat as a potential cell source for knee osteoarthritis treatment. J. Cell. Mol. Med. 2017, 21, 2153–2162. [Google Scholar] [CrossRef]
- Han, J.H.; Jung, M.; Chung, K.; Jung, S.-H.; Choi, C.-H.; Kim, S.-H. Effects of Concurrent Cartilage Procedures on Cartilage Regeneration in High Tibial Osteotomy: A Systematic Review. Knee Surg. Relat. Res. 2024, 36, 13. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Ball, L.M.; Locatelli, F.; Fibbe, W.E. Immunomodulatory properties of MSCs. In Mesenchymal Stromal Cells; Springer: New York, NY, USA, 2013; pp. 179–202. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, D.-Y.; Noh, H.J.; Lee, S.Y.; Yoo, J.A.; Won, S.J.; Jeon, Y.S.; Baek, J.H.; Ryu, D.J. Elevated IL-6 Expression in Autologous Adipose-Derived Stem Cells Regulates RANKL Mediated Inflammation in Osteoarthritis. Cells 2024, 13, 2046. [Google Scholar] [CrossRef]
- Kwon, D.G.; Kim, M.K.; Jeon, Y.S.; Nam, Y.C.; Park, J.S.; Ryu, D.J. State of the Art: The Immunomodulatory Role of MSCs for Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 1618. [Google Scholar] [CrossRef]
- Lavelle, W.F.; Lavelle, E.D.; Lavelle, L.A. Intra-articular injections. Med. Clin. N. Am. 2007, 91, 241–250. [Google Scholar] [CrossRef]
- Kang, J.S.; Suh, Y.J.; Moon, K.H.; Park, J.S.; Roh, T.H.; Park, M.H.; Ryu, D.J. Clinical Efficiency of Bone Marrow Mesenchymal Stem Cell Implantation for Osteonecrosis of the Femoral Head: A Matched Pair Control Study with Simple Core Decompression. Stem Cell Res. Ther. 2018, 9, 274. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.-I.; Yoon, W.-K.; Song, S.J.; Jin, W. Intra-Articular Injection of Mesenchymal Stem Cells After High Tibial Osteotomy in Osteoarthritic Knee: Two-Year Follow-up of Randomized Control Trial. Stem Cells Transl. Med. 2022, 11, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Freitag, J.; Bates, D.; Wickham, J.; Shah, K.; Huguenin, L.; Tenen, A.; Paterson, K.L.; Boyd, R.L. Adipose-Derived Mesenchymal Stem Cell Therapy in the Treatment of Knee Osteoarthritis: A Randomized Controlled Trial. Regen. Med. 2019, 14, 213–230. [Google Scholar] [CrossRef]
- Kim, K.-I.; Lee, M.C.; Lee, J.-H.; Moon, Y.-W.; Lee, W.-S.; Lee, H.-J.; Hwang, S.-C.; In, Y.; Shon, O.-J.; Bae, K.-C.; et al. Clinical Efficacy and Safety of the Intra-Articular Injection of Autologous Adipose-Derived Mesenchymal Stem Cells for Knee Osteoarthritis: A Phase III, Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Sports Med. 2023, 51, 2243–2253. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Sun, H.; Chen, J.; Liu, X.; Pan, B.; He, L.; Jiang, H. A single dose of local injection of adipose stem cells promotes ectopic cartilage regeneration in vivo by modulating inflammatory response and enhancing cartilage extracellular matrix synthesis in a porcine model. Curr. Stem Cell Res. Ther. 2022, 17, 787–798. [Google Scholar] [CrossRef]
- Toupet, K.; Maumus, M.; Peyrafitte, J.-A.; Bourin, P.; van Lent, P.L.E.M.; Ferreira, R.; Orsetti, B.; Pirot, N.; Casteilla, L.; Jorgensen, C.; et al. Long-term detection of human adipose-derived mesenchymal stem cells after intraarticular injection in SCID mice. Arthritis Rheum. 2013, 65, 1786–1794. [Google Scholar] [CrossRef]
- Rodríguez-Merchán, E.C. Intra-articular injections of mesenchymal stem cells (MSCs) as a treatment for hemophilic arthropathy. Expert Rev. Hematol. 2016, 9, 511–516. [Google Scholar] [CrossRef]
- Ichiseki, T.; Shimazaki, M.; Ueda, Y.; Ueda, S.; Tsuchiya, M.; Souma, D.; Kaneuji, A.; Kawahara, N. Intraarticularly-injected mesenchymal stem cells stimulate anti-inflammatory molecules and inhibit pain related protein and chondrolytic enzymes in a monoiodoacetate-induced rat arthritis model. Int. J. Mol. Sci. 2018, 19, 203. [Google Scholar] [CrossRef]
- Lee, B.-W.; Kwok, S.-K. Mesenchymal stem/stromal cell-based therapies in systemic rheumatic disease: From challenges to new approaches for overcoming restrictions. Int. J. Mol. Sci. 2023, 24, 10161. [Google Scholar] [CrossRef] [PubMed]
- Kavianpour, M.; da Silva Meirelles, L.; Ahmadbeigi, N. Challenges in Mesenchymal Stromal Cell-based Therapies. Curr. Stem Cell Res. Ther. 2023, 18, 937–946. [Google Scholar] [CrossRef]
- Li, M.; Luo, X.; Lv, X.; Liu, V.; Zhao, G.; Zhang, X.; Cao, W.; Wang, R.; Wang, W. In vivo human adipose-derived mesenchymal stem cell tracking after intra-articular delivery in a rat osteoarthritis model. Stem Cell Res. Ther. 2016, 7, 160. [Google Scholar] [CrossRef]
- Satué, M.; Schüler, C.; Ginner, N.; Erben, R.G.; Proell, V.; Godoy, J.R.; Odörfer, K.I.; Flicker, M.; Hoffmann, S.C.; Zwolanek, D.; et al. Intra-articularly injected mesenchymal stem cells promote cartilage regeneration, but do not permanently engraft in distant organs. Sci. Rep. 2019, 9, 10153. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.; Shamdani, S.; Zarei, M.; Soudi, S.; Hashemi, S.M. Co-aggregation of MSC/chondrocyte in a dynamic 3D culture elevates the therapeutic effect of secreted extracellular vesicles on osteoarthritis in a rat model. Sci. Rep. 2022, 12, 19487. [Google Scholar] [CrossRef] [PubMed]
- Freeman, F.E.; Brennan, M.Á.; Browe, D.C.; Schiavi, J.; Critchley, S.E.; Layrolle, P.; Kelly, D.J. Effects of in vitro endochondral priming and pre-vascularisation of human MSC cellular aggregates in vivo. Stem Cell Res. Ther. 2015, 6, 218. [Google Scholar] [CrossRef]
- Suzuki, S.; Muneta, T.; Tsuji, K.; Ichinose, S.; Nakagawa, T.; Sekiya, I. Properties and usefulness of aggregates of synovial mesenchymal stem cells as a source for cartilage regeneration. Arthritis Res. Ther. 2012, 14, R136. [Google Scholar] [CrossRef]
- Peng, Y.N.; Peng, Y.H.; Chen, J.L.; Chen, C.P.C. Intraarticular leukocyte-poor platelet-rich plasma injection is more effective than intraarticular hyaluronic acid injection in the treatment of knee osteoarthritis: A systematic review and meta-analysis of 12 randomized controlled trials. Knee Surg. Relat. Res. 2025, 37, 15. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic acid: Molecular mechanisms and therapeutic trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef]
- Li, C.; Wu, X.; Zhang, H.; Zhang, Y.; Wu, W.; Wang, Z.; Wang, M. A review on the wide range applications of hyaluronic acid as a promising rejuvenating biomacromolecule in the treatments of bone related diseases. Int. J. Biol. Macromol. 2020, 165, 1264–1279. [Google Scholar] [CrossRef]
- Tian, Y.B.; Wang, N.X.; Xu, Y.; Yu, C.Y.; Liu, R.M.; Luo, Y.; Xiao, J.H. Hyaluronic Acid Ameliorates the Proliferative Ability of Human Amniotic Epithelial Cells through Activation of TGF-β/BMP Signaling. PeerJ 2020, 8, e10104. [Google Scholar] [CrossRef]
- Liu, R.M.; Sun, R.G.; Zhang, L.T.; Zhang, Q.F.; Chen, D.X.; Zhong, J.J.; Xiao, J.H. Hyaluronic Acid Enhances Proliferation of Human Amniotic Mesenchymal Stem Cells through Activation of Wnt/β-Catenin Signaling Pathway. Exp. Cell Res. 2016, 345, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lin, C.; Jia, X.; Fang, D.; Gao, Q.; Han, C. HGF/c-Met Signaling Promotes the Migration and Proliferation of Deer Antler MSCs. Sci. Rep. 2023, 13, 11121. [Google Scholar] [CrossRef]
- Della Sala, F.; Longobardo, G.; Lista, G.; Messina, F.; Borzacchiello, A. Effect of Hyaluronic Acid and Mesenchymal Stem Cells Secretome Combination in Promoting Alveolar Regeneration. Int. J. Mol. Sci. 2023, 24, 3642. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Duan, X.; Fan, Z.; Chen, L.; Xing, F.; Xu, Z.; Chen, Q.; Xiang, Z. Mesenchymal Stem Cells in Combination with Hyaluronic Acid for Articular Cartilage Defects. Sci. Rep. 2018, 8, 9900. [Google Scholar] [CrossRef]
- Ryu, D.J.; Jeon, Y.S.; Park, J.S.; Bae, G.C.; Kim, J.-S.; Kim, M.K. Comparison of bone marrow aspirate concentrate and allogenic human umbilical cord blood derived mesenchymal stem cell implantation on chondral defect of knee: Assessment of Clinical and Magnetic Resonance Imaging Outcomes at 2-Year Follow-Up. Cell Transplant. 2020, 29, 963689720943581. [Google Scholar] [CrossRef]
- Czerwiec, K.; Zawrzykraj, M.; Deptuła, M.; Skoniecka, A.; Tymińska, A.; Zieliński, J.; Kosiński, A.; Pikuła, M. Adipose-Derived Mesenchymal Stromal Cells in Basic Research and Clinical Applications. Int. J. Mol. Sci. 2023, 24, 3888. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.L.; He, F.; Zhu, X. Intra-articular injection choice for osteoarthritis: Making sense of cell source—An updated systematic review and dual network meta-analysis. Arthritis Res. Ther. 2022, 24, 151. [Google Scholar] [CrossRef]
- Pintore, A.; Notarfrancesco, D.; Zara, A.; Oliviero, A.; Migliorini, F.; Oliva, F.; Maffulli, N. Intra-articular injection of bone marrow aspirate concentrate (BMAC) or adipose-derived stem cells (ADSCs) for knee osteoarthritis: A prospective comparative clinical trial. J. Orthop. Surg. Res. 2023, 18, 315. [Google Scholar] [CrossRef]
- Emadedin, M.; Aghdami, N.; Taghiyar, L.; Fazeli, R.; Moghadasali, R.; Jahangir, S.; Farjad, R.; Baghaban Eslaminejad, M. Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch. Iran. Med. 2012, 15, 422–428. [Google Scholar] [PubMed]
- Ahrens, C.C.; Dong, Z.; Li, W. Engineering cell aggregates through incorporated polymeric microparticles. Acta Biomater. 2017, 59, 74–84. [Google Scholar] [CrossRef]
- Amimoto, I.; Watanabe, R.; Hirano, Y. Cell Behavior on Peptide-Immobilized Substrate with Cell Aggregation Inducing Property. Processes 2022, 10, 1779. [Google Scholar] [CrossRef]
- Bijonowski, B.M.; Fu, Q.; Yuan, X.; Irianto, J.; Li, Y.; Grant, S.C.; Ma, T. Aggregation-induced integrated stress response rejuvenates culture-expanded human mesenchymal stem cells. Biotechnol. Bioeng. 2020, 117, 2363–2375. [Google Scholar] [CrossRef]
- Egger, D.; Tripisciano, C.; Weber, V.; Dominici, M.; Kasper, C. Dynamic Cultivation of Mesenchymal Stem Cell Aggregates. Bioengineering 2018, 5, 48. [Google Scholar] [CrossRef]
- Mélou, C.; Pellen-Mussi, P.; Novello, S.; Brezulier, D.; Novella, A.; Tricot, S.; Bellaud, P.; Chauvel-Lebret, D. Spheroid Culture System, a Promising Method for Chondrogenic Differentiation of Dental Mesenchymal Stem Cells. Biomedicines 2023, 11, 1314. [Google Scholar] [CrossRef]
- Tu, V.T.-K.; Le, H.T.-N.; To, X.H.-V.; Nguyen, P.D.-N.; Huynh, P.D.; Le, T.M.; Vu, N.B. Method for in vitro production of cartilage microtissues from scaffold-free spheroids composed of human adipose-derived stem cells. Biomed. Res. Ther. 2020, 7, 3647–3657. [Google Scholar] [CrossRef]
- Fürsatz, M.; Gerges, P.; Wolbank, S.; Nürnberger, S. Autonomous spheroid formation by culture plate compartmentation. Biofabrication 2021, 13, 035010. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, S.M.; Tatsumi, R.; Temm-Grove, C.J.; Allen, R.E. HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro. Muscle Nerve 2000, 23, 239–245. [Google Scholar] [CrossRef]
- González, M.N.; de Mello, W.; Butler-Browne, G.; Silva-Barbosa, S.D.; Mouly, V.; Savino, W.; Riederer, I. HGF potentiates extracellular matrix-driven migration of human myoblasts: Involvement of matrix metalloproteinases and MAPK/ERK pathway. Skelet. Muscle 2017, 7, 18. [Google Scholar] [CrossRef]
- Tsou, H.-K.; Chen, H.-T.; Hung, Y.-H.; Chang, C.-H.; Li, T.-M.; Fong, Y.-C.; Tang, C.-H. HGF and c-Met Interaction Promotes Migration in Human Chondrosarcoma Cells. PLoS ONE 2013, 8, e53974. [Google Scholar] [CrossRef]
- Liang, Y.; Xie, X.; Ma, B.; Li, Z.; Zhang, M.; Guo, B.; Yin, Z.; Wei, X.; Wang, W. Human Umbilical Cord Mesenchymal Stem Cells in Combination with Hyaluronic Acid Ameliorate the Progression of Knee Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 1136. [Google Scholar] [CrossRef]
- Kota, D.J.; DiCarlo, B.; Hetz, R.A.; Smith, P.; Cox, C.S.; Olson, S.D. Differential MSC activation leads to distinct mononuclear leukocyte binding mechanisms. Sci. Rep. 2015, 5, 4512. [Google Scholar] [CrossRef]
- Bourin, P.; Gimble, J.M.; Casteilla, L.; Salgado, A. Editorial: MSC Communication in Physiological and Pathological Settings. Front. Cell Dev. Biol. 2022, 10, 909550. [Google Scholar] [CrossRef]
- Jia, P.; Guo, Z.; Li, Z. The Enhanced Therapeutic Potential of MSC with Biomaterials Application. In Biomaterials in Regenerative Medicine, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 17–41. [Google Scholar] [CrossRef]
- Zhubanova, G.; Mukhambetova, A.; Karzhauov, M.; Issabekova, A.S.; Raimagambetov, E.; Ogay, V. The effect of combinations of growth factors and hyaluronic acid on the proliferation and chondrogenic differentiation of human synovium derived mesenchymal stem cells. Eurasian J. Appl. Biotechnol. 2022, 3, 41–50. [Google Scholar] [CrossRef]
- Yu, S.; Cai, X.; Wu, C.; Wu, L.; Wang, Y.; Liu, Y.; Yu, Z.; Qin, S.; Ma, F.; Thiery, J.P.; et al. Adhesion glycoprotein CD44 functions as an upstream regulator of a network connecting ERK, AKT and Hippo-YAP pathways in cancer progression. Oncotarget 2015, 6, 2951–2965. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M.; Im, J.; Nho, R.S.; Han, Y.H.; Upadhyaya, P.; Kassie, F. Hyaluronan-CD44/RHAMM Interaction-Dependent Cell Proliferation and Survival in Lung Cancer Cells. Mol. Carcinog. 2019, 58, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.M.; Soares da Costa, D.; Paulo, P.M.R.; Reis, R.L.; Pashkuleva, I. Co-Localization and Crosstalk between CD44 and RHAMM Depend on Hyaluronan Presentation. Acta Biomater. 2021, 119, 114–124. [Google Scholar] [CrossRef]
- Prestwich, G.D. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J. Control. Release 2011, 155, 193–199. [Google Scholar] [CrossRef]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019, 286, 2883–2908. [Google Scholar] [CrossRef]
- Rampratap, P.; Lasorsa, A.; Arunachalam, A.; Kamperman, M.; Walvoort, M.T.C.; van der Wel, P.C.A. Resolving Atomic-Level Dynamics and Interactions of High-Molecular-Weight Hyaluronic Acid by Multidimensional Solid-State NMR. ACS Appl. Mater. Interfaces 2024, 16, 43317–43328. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Goldring, S.R. Osteoarthritis. J. Cell. Physiol. 2007, 213, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Jaraba-Álvarez, W.V.; Uscanga-Palomeque, A.C.; Sanchez-Giraldo, V.; Madrid, C.; Ortega-Arellano, H.; Halpert, K.; Quintero-Gil, C. Hypoxia-induced metabolic reprogramming in mesenchymal stem cells: Unlocking the regenerative potential of secreted factors. Front. Cell Dev. Biol. 2025, 13, 1609082. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, J.; Liu, B.; Shao, C.; Shi, Y. Reciprocal regulation of mesenchymal stem cells and immune responses. Cell Stem Cell 2022, 29, 1515–1530. [Google Scholar] [CrossRef]
- Česnik, A.B.; Švajger, U. The issue of heterogeneity of MSC-based advanced therapy medicinal products—A review. Front. Cell Dev. Biol. 2024, 12, 1400347. [Google Scholar] [CrossRef]
- Ambrosi, T.H.; Taheri, S.; Chen, K.; Sinha, R.; Wang, Y.; Hunt, E.J.; Goodnough, L.H.; Murphy, M.P.; Steininger, H.M.; Hoover, M.Y.; et al. Human skeletal development and regeneration are shaped by functional diversity of stem cells across skeletal sites. Cell Stem Cell 2025, 32, 811–823.e11. [Google Scholar] [CrossRef]
- Chan, C.K.F.; Gulati, G.S.; Sinha, R.; Tompkins, J.V.; Lopez, M.; Carter, A.C.; Ransom, R.C.; Reinisch, A.; Wearda, T.; Murphy, M.; et al. Identification of the Human Skeletal Stem Cell. Cell 2018, 175, 43–56.e21. [Google Scholar] [CrossRef] [PubMed]
- Mebarki, M.; Abadie, C.; Larghero, J.; Cras, A. Human umbilical cord-derived mesenchymal stem/stromal cells: A promising candidate for the development of advanced therapy medicinal products. Stem Cell Res. Ther. 2021, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- De Windt, T.S.; Vonk, L.A.; Slaper-Cortenbach, I.C.M.; van den Broek, M.P.; van Rijen, M.H.P.; Dhert, W.J.A.; Saris, D.B.F. Allogeneic mesenchymal stem cells from the synovial membrane to treat knee osteoarthritis: A randomized, double-blind, placebo-controlled clinical trial. Stem Cells Transl. Med. 2017, 6, 2062–2071. [Google Scholar] [CrossRef]
- Carneiro, D.C.; de Araújo, L.T.; Santos, G.C.; Damasceno, P.K.F.; Vieira, J.L.; dos Santos, R.R.; Soares, M.B.P. Clinical trials with mesenchymal stem cell therapies for osteoarthritis: Challenges in the regeneration of articular cartilage. Int. J. Mol. Sci. 2023, 24, 9939. [Google Scholar] [CrossRef] [PubMed]
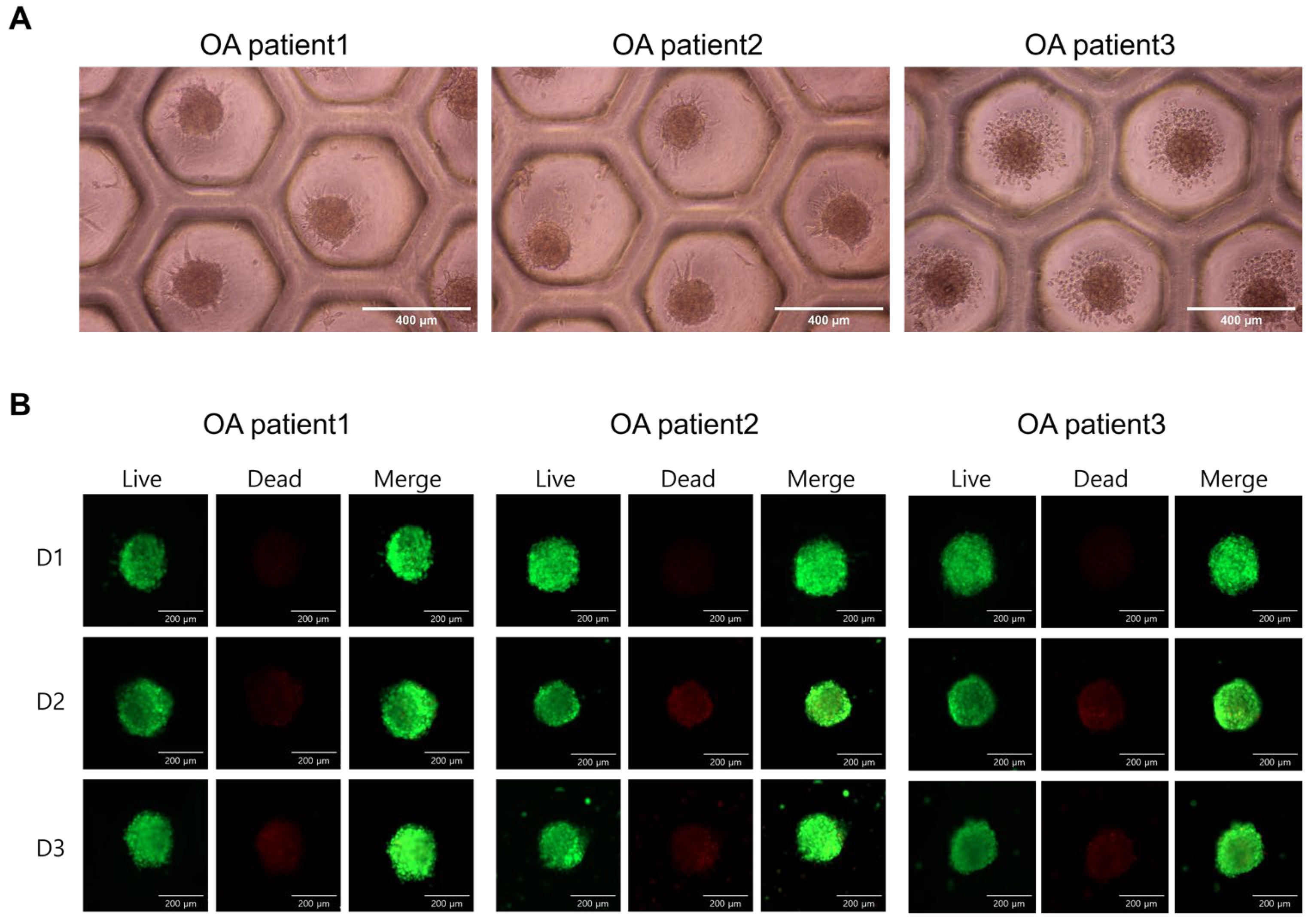



| OA Patient 1 | OA Patient 2 | OA Patient 3 | |
|---|---|---|---|
| Age (years) | 62 | 61 | 54 |
| Sex | Female | Female | Male |
| BMI (kg/m2) | 25.4 | 23.6 | 27.1 |
| Smoking | - | - | 1 pack/day |
| Alcohol | - | - | 1 bottle/week |
| K-L grade | II | IV | IV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, S.J.; Lee, H.-J.; Kim, D.-Y.; Noh, H.; Lee, S.y.; Yoo, J.A.; Jeon, Y.S.; Shin, H.; Ryu, D.J. Hepatocyte Growth Factor-Mediated Chondrocyte Proliferation Induced by Adipose-Derived MSCs from Osteoarthritis Patients and Its Synergistic Enhancement by Hyaluronic Acid. Int. J. Mol. Sci. 2025, 26, 9296. https://doi.org/10.3390/ijms26199296
Won SJ, Lee H-J, Kim D-Y, Noh H, Lee Sy, Yoo JA, Jeon YS, Shin H, Ryu DJ. Hepatocyte Growth Factor-Mediated Chondrocyte Proliferation Induced by Adipose-Derived MSCs from Osteoarthritis Patients and Its Synergistic Enhancement by Hyaluronic Acid. International Journal of Molecular Sciences. 2025; 26(19):9296. https://doi.org/10.3390/ijms26199296
Chicago/Turabian StyleWon, Samuel Jaeyoon, Hyun-Joo Lee, Dae-Yong Kim, Hyeonjeong Noh, Song yi Lee, Ji Ae Yoo, Yoon Sang Jeon, Heebeom Shin, and Dong Jin Ryu. 2025. "Hepatocyte Growth Factor-Mediated Chondrocyte Proliferation Induced by Adipose-Derived MSCs from Osteoarthritis Patients and Its Synergistic Enhancement by Hyaluronic Acid" International Journal of Molecular Sciences 26, no. 19: 9296. https://doi.org/10.3390/ijms26199296
APA StyleWon, S. J., Lee, H.-J., Kim, D.-Y., Noh, H., Lee, S. y., Yoo, J. A., Jeon, Y. S., Shin, H., & Ryu, D. J. (2025). Hepatocyte Growth Factor-Mediated Chondrocyte Proliferation Induced by Adipose-Derived MSCs from Osteoarthritis Patients and Its Synergistic Enhancement by Hyaluronic Acid. International Journal of Molecular Sciences, 26(19), 9296. https://doi.org/10.3390/ijms26199296







