Osteoporosis-Improving Effects of Extracellular Vesicles from Human Amniotic Membrane Stem Cells in Ovariectomized Rats
Abstract
1. Introduction
2. Results
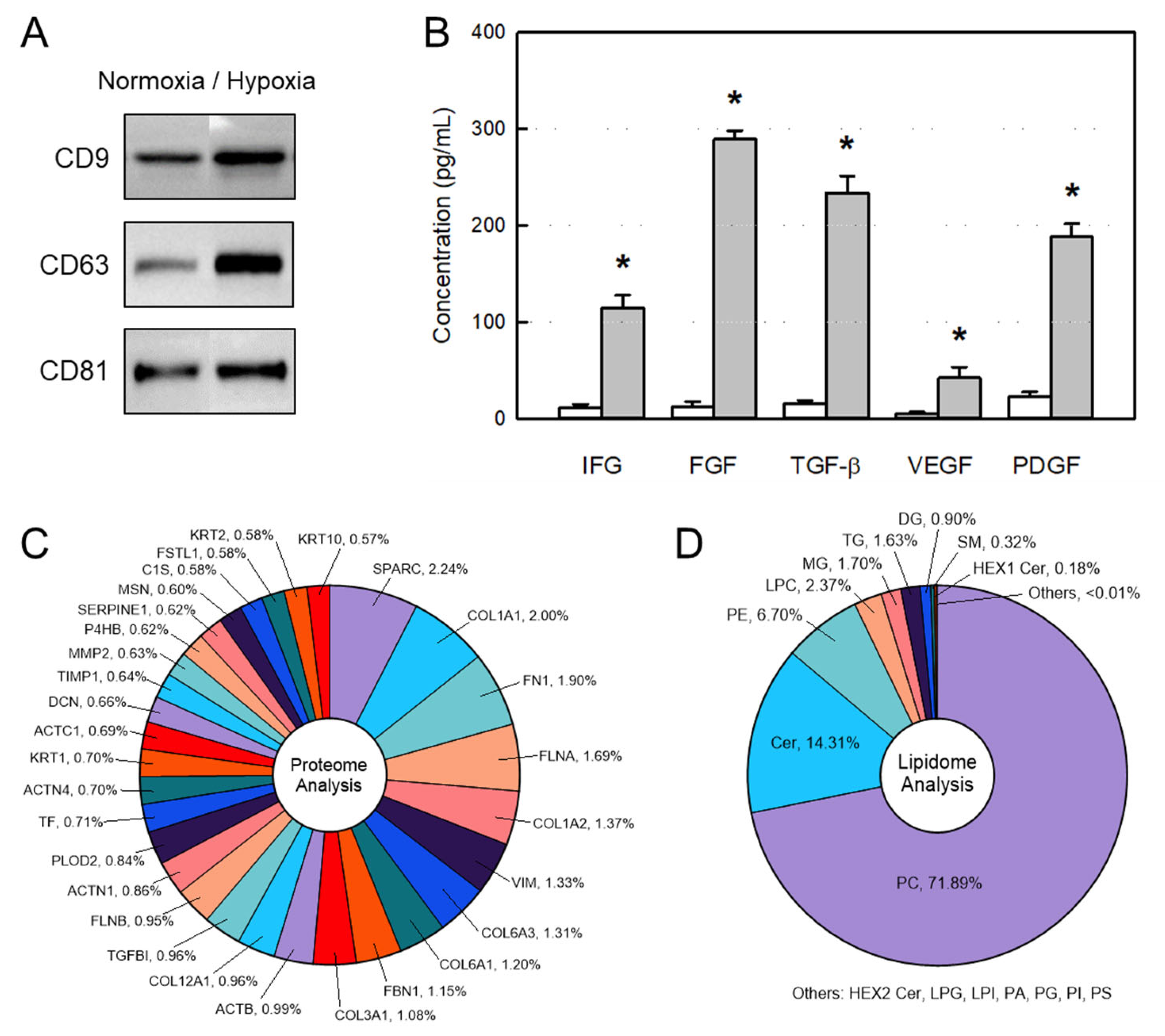
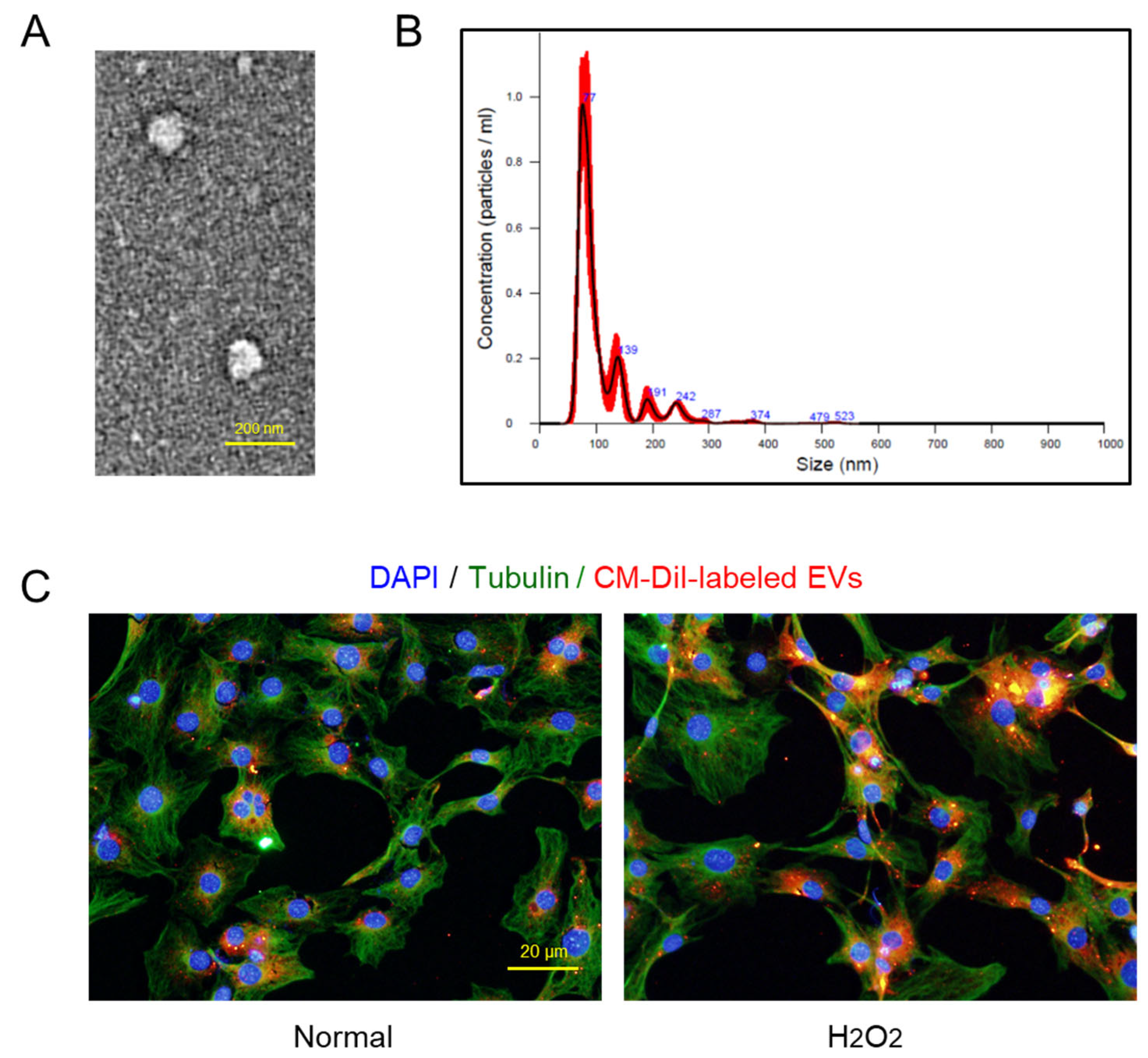
2.1. Characteristics of AMSC-Derived EVs
2.2. Osteoblast-Proliferative and -Protective Activities of EVs
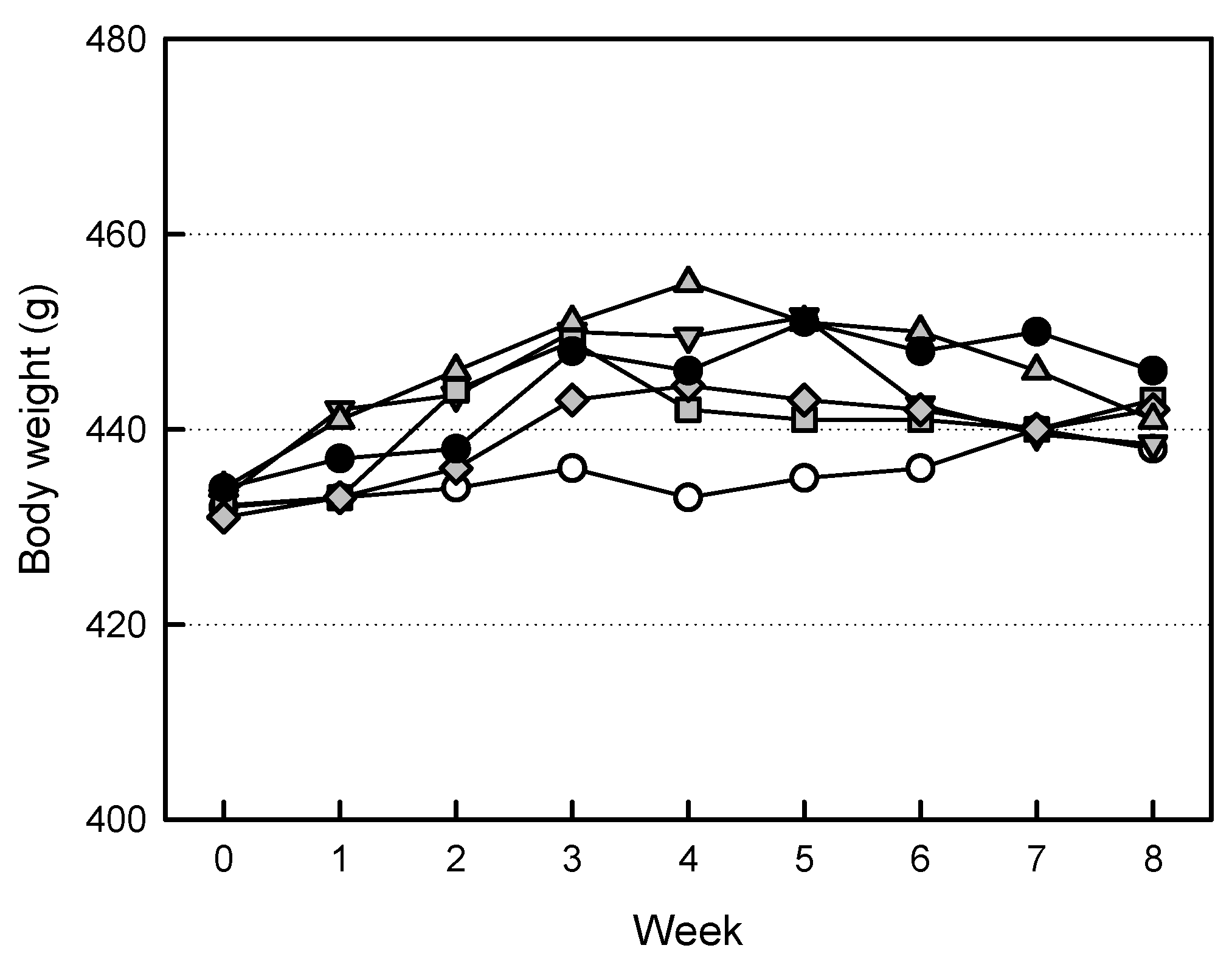
2.3. Effects of EVs on Body Weights
2.4. Effects of EVs on Organ Weights
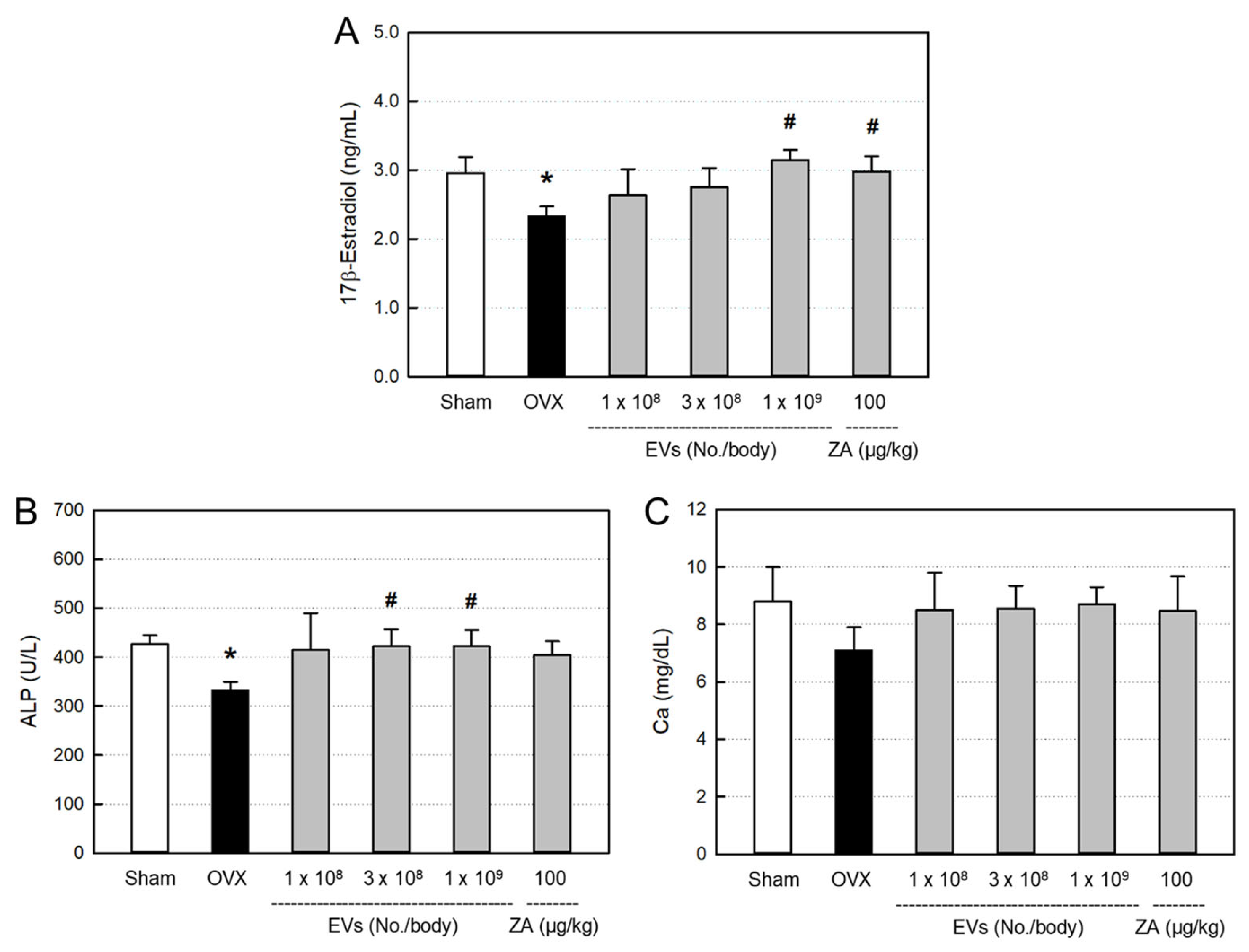
2.5. Effects of EVs on Serum Markers
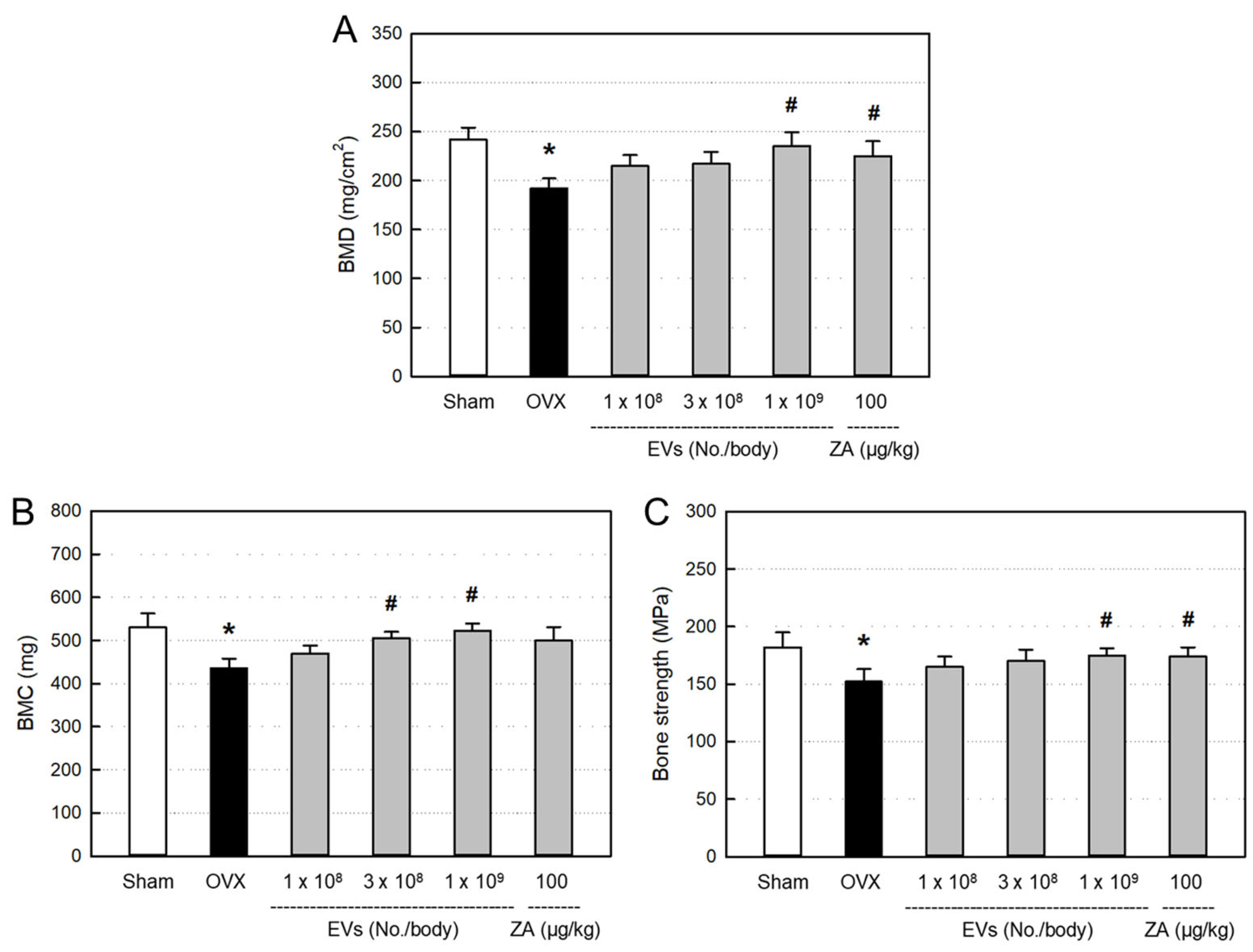
2.6. Effects of EVs on Bone Minerals and Strength
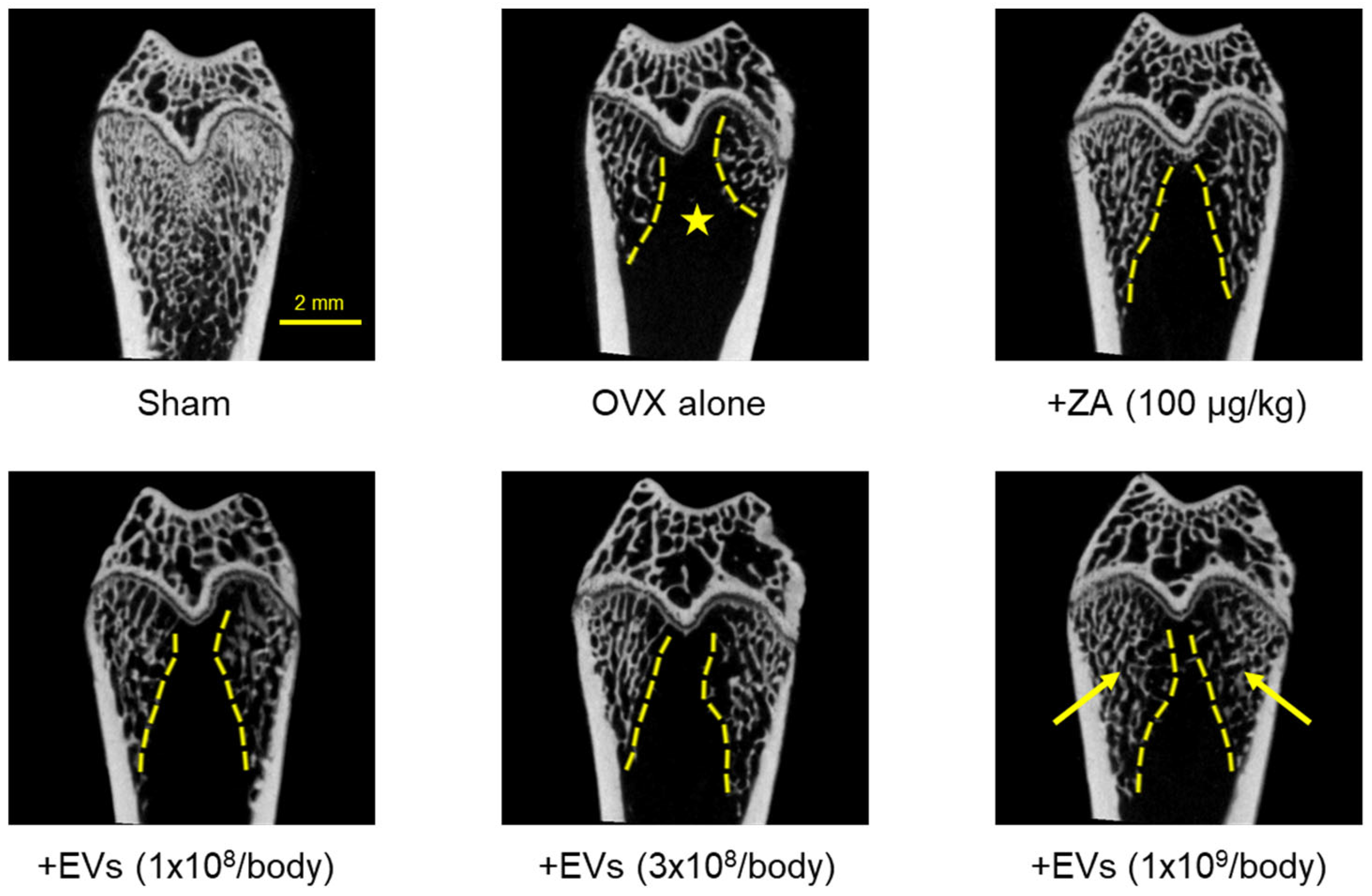
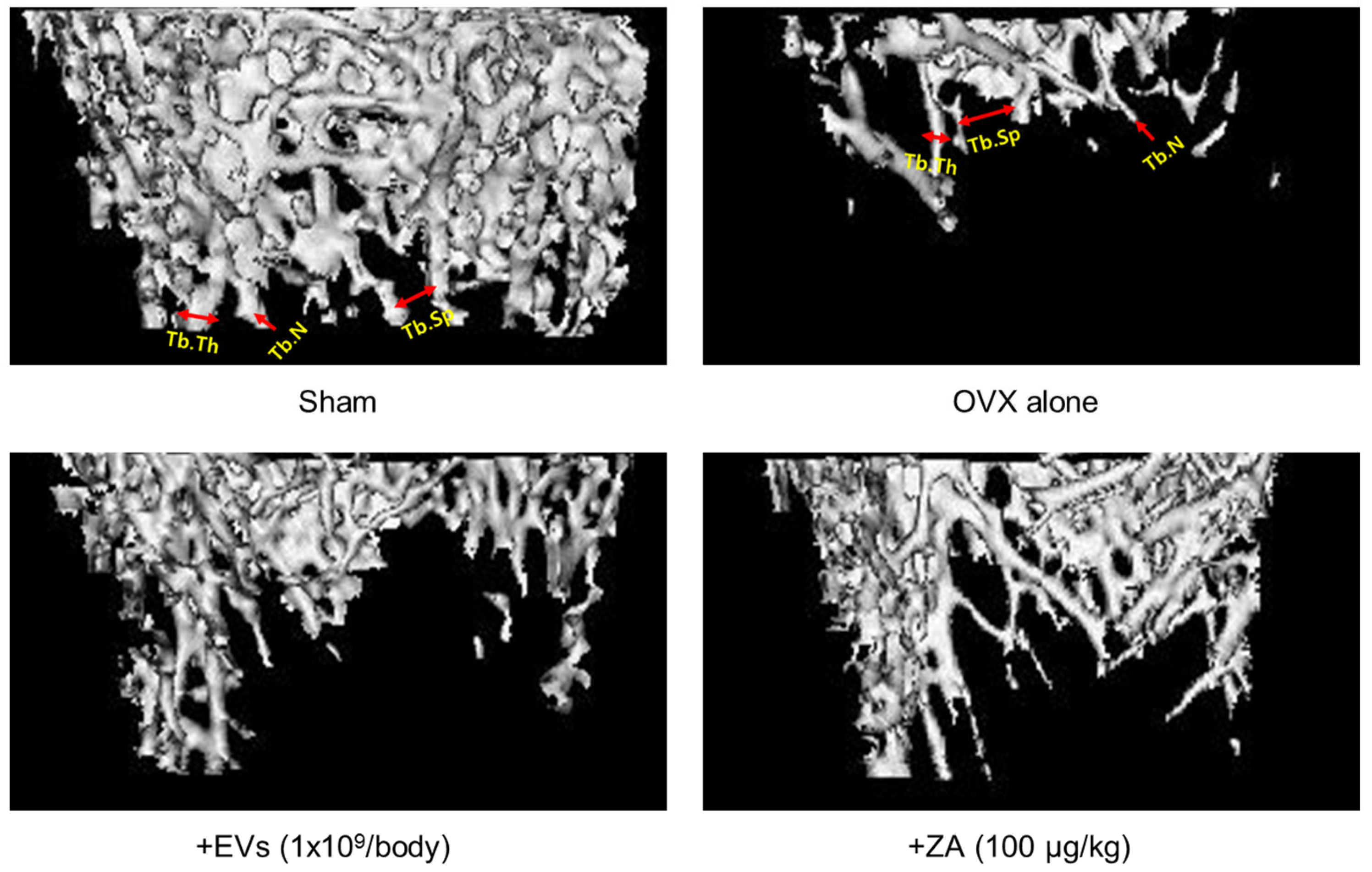
2.7. Effect of EVs on Bone Structure
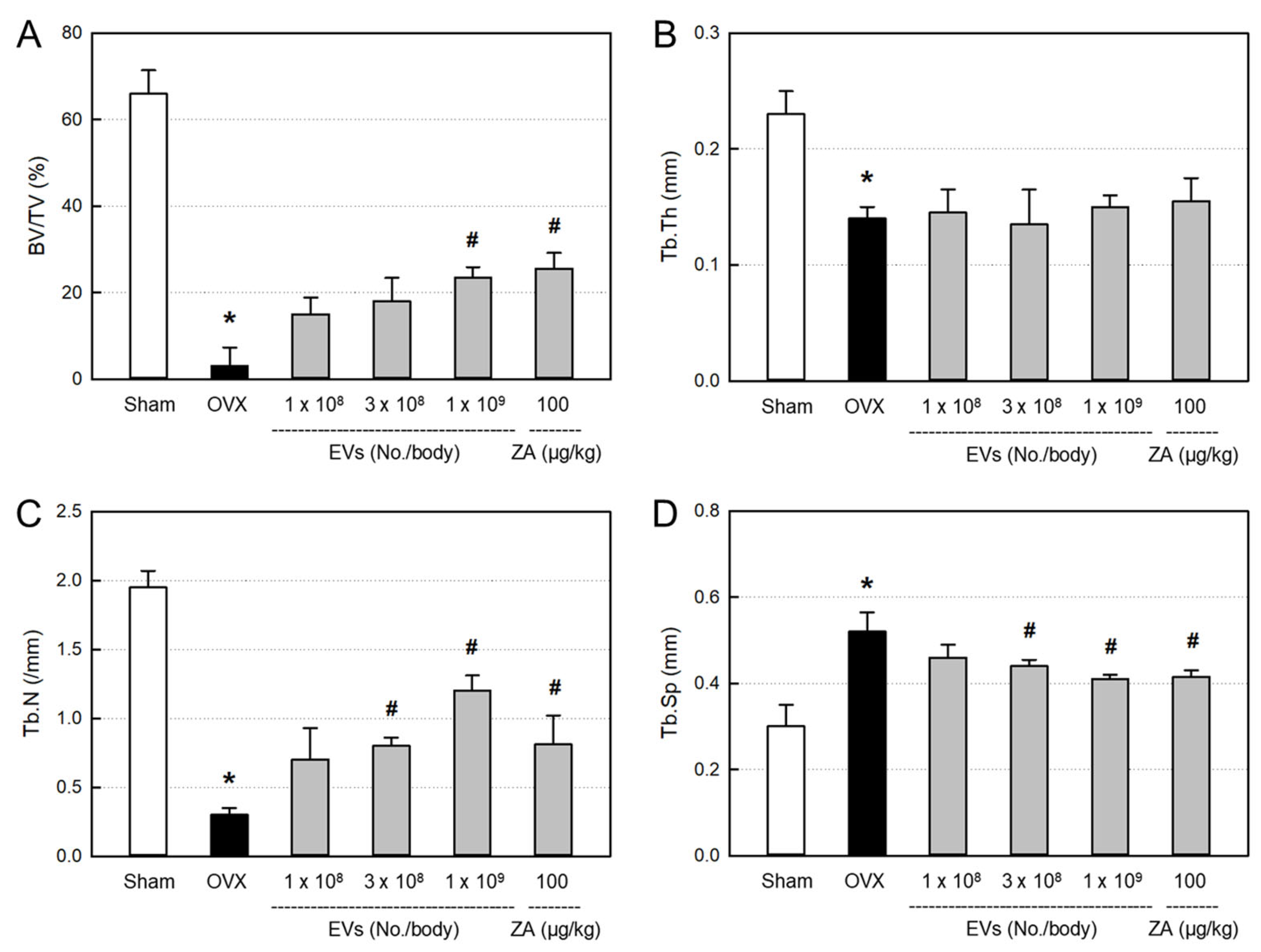
2.8. Effects of EVs on Bone Structural Parameters
3. Discussion
4. Materials and Methods
4.1. Preparation of AMSCs
4.2. Preparation of EVs
4.3. Western Blot Analysis of EV Markers
4.4. ELISA of GFs and NFs
4.5. Proteome Analysis of EVs
4.6. Lipidome Analysis of EVs
4.7. Transmission Electron Microscopy (TEM) of EVs
4.8. Nanoparticle-Tracking Analysis (NTA) of EVs
4.9. Osteoblast Culture
4.10. EV Uptake Assay
4.11. Osteoblast-Proliferative Activity
4.12. Osteoblast-Protective Activity
4.13. Design of Animal Experiment
4.14. Analysis of Serum Markers
4.14.1. ELISA of 17β-Estradiol
4.14.2. Biochemistry Analysis of ALP and Calcium
4.15. Analysis of Bone Parameters
4.15.1. X-Ray Analysis of Bone Minerals
4.15.2. Biomechanical Measurement of Three-Point Bending Strength
4.15.3. Micro-CT Analysis of Bone Structure
4.16. Statistical Analysis
5. Conclusions
Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- LeBoff, M.S.; Greenspan, S.L.; Insogna, K.L.; Lewiecki, E.M.; Saag, K.G.; Singer, A.J.; Siris, E.S. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos. Int. 2022, 33, 2049–2102. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Kekatpure, L. Postmenopausal osteoporosis: A literature review. Cureus 2022, 14, e29367. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Chen, L.R.; Chen, K.H. Osteoporosis due to hormone imbalance: An overview of the effects of estrogen deficiency and glucocorticoid overuse on bone turnover. Int. J. Mol. Sci. 2022, 23, 1376. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.X.; Yu, Q. Primary osteoporosis in postmenopausal women. Chronic Dis. Transl. Med. 2015, 1, 9–13. [Google Scholar] [CrossRef]
- Khalid, A.B.; Krum, S.A. Estrogen receptors alpha and beta in bone. Bone 2016, 87, 130–135. [Google Scholar] [CrossRef]
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.H. Osteoblast–osteoclast communication and bone homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Khan, A.A.; Sandor, G.K.B.; Dore, E.; Morrison, A.D.; Alsahli, M.; Amin, F.; Peters, E.; Hanley, D.A.; Chaudry, S.R.; Lentle, B.; et al. Bisphosphonate associated osteonecrosis of the jaw. J. Rheumatol. 2009, 36, 478–490. [Google Scholar] [CrossRef]
- Lo, J.C.; O’Ryan, F.S.; Gordon, N.P.; Yang, J.; Hui, R.L.; Martin, D.; Hutchinson, M.; Lathon, P.V.; Sanchez, G.; Silver, P.; et al. Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. J. Oral Maxillofac. Surg. 2013, 68, 243–253. [Google Scholar] [CrossRef]
- Saleh, A.; Hegde, V.V.; Potty, A.G.; Lane, J.M. Bisphosphonate therapy and atypical fractures. Orthop. Clin. N. Am. 2013, 44, 137–151. [Google Scholar] [CrossRef]
- Li, G.W.; Zheng, X.; Chang, S.X.; Zhou, L.; Wang, X.Y.; Nian, H.; Shi, X. Influence of early zoledronic acid administration on bone marrow fat in ovariectomized rats. Endocrinology 2014, 155, 4731–4738. [Google Scholar] [CrossRef]
- Undale, A.H.; Westendorf, J.J.; Yaszemski, M.J.; Khosla, S. Mesenchymal stem cells for bone repair and metabolic bone diseases. Mayo Clin. Proc. 2009, 84, 893–902. [Google Scholar] [CrossRef]
- Baraniak, P.R.; McDevitt, T.C. Stem cell paracrine actions and tissue regeneration. Regen. Med. 2010, 5, 121–143. [Google Scholar] [CrossRef]
- Linero, I.; Chaparro, O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS ONE 2014, 9, e107001, Erratum in PLoS ONE 2015, 10, e0119262. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.P.; Chio, C.C.; Cheong, C.U.; Chao, C.M.; Cheng, B.C.; Lin, M.T. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin. Sci. 2013, 124, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yoon, Y.M.; Lee, S.H. Hypoxic preconditioning promotes the bioactivities of mesenchymal stem cells via the HIF-1α-GRP78-Akt axis. Int. J. Mol. Sci. 2017, 18, 1320. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Thery, C. Communication by extracellular vesicles: Where we are and where we need to go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020, 22, 6917–6934. [Google Scholar] [CrossRef]
- Seong, H.R.; Noh, C.H.; Park, S.; Cho, S.; Hong, S.J.; Lee, A.y.; Geum, D.; Hong, S.C.; Park, D.; Kim, T.M.; et al. Intraocular pressure-lowering and retina-protective effects of exosome-rich conditioned media from human amniotic membrane stem cells in a rat model of glaucoma. Int. J. Mol. Sci. 2023, 24, 8073. [Google Scholar] [CrossRef]
- Noh, C.H.; Park, S.; Seong, H.R.; Lee, A.-y.; Tsolmon, K.E.; Geum, D.; Hong, S.C.; Kim, T.M.; Choi, E.K.; Kim, Y.B. An exosome-rich conditioned medium from human amniotic membrane stem cells facilitates wound healing via increased reepithelization, collagen synthesis, and angiogenesis. Cells 2023, 12, 2698. [Google Scholar] [CrossRef]
- Batoon, L.; Millard, S.M.; Raggatt, L.J.; Wu, A.C.; Kaur, S.; Sun, L.W.H.; Williams, K.; Sandrock, C.; Ng, P.Y.; Irvine, K.M.; et al. Osteal macrophages support osteoclast-mediated resorption and contribute to bone pathology in a postmenopausal osteoporosis mouse model. J. Bone Miner. Res. 2021, 36, 2214–2228. [Google Scholar] [CrossRef]
- Huang, B.; Su, Y.; Shen, E.; Song, M.; Liu, D.; Qi, H. Extracellular vesicles from GPNMB-modified bone marrow mesenchymal stem cells attenuate bone loss in an ovariectomized rat model. Life Sci. 2021, 272, 119208. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Guo, Y.; Kong, L.; Shi, J.; Liu, P.; Li, R.; Geng, Y.; Gao, W.; Zhang, Z.; Fu, D. A bone-targeted engineered exosome platform delivering siRNA to treat osteoporosis. Bioact. Mater. 2022, 10, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Kang, M.J.; Kong, D.; Lee, J.E.; Lee, A.Y.; Geum, D.H.; Kim, B.S.; Kim, Y.S.; Hong, S.C. Effect of human amniotic epithelial stem cell transplantation on preterm premature rupture of fetal membrane using the amniotic pore culture technique in vitro. Gynecol. Obstet. Investig. 2022, 87, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, X.; Hao, H.; Xu, H.; Shu, J.; Hou, Q.; Wang, M. Exosomes derived from umbilical cord mesenchymal stem cells treat cutaneous nerve damage and promote wound healing. Front. Cell. Neurosci. 2022, 16, 913009. [Google Scholar] [CrossRef]
- Bradshaw, A.D.; Sage, E.H. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J. Clin. Investig. 2001, 107, 1049–1054. [Google Scholar] [CrossRef]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta 2010, 1803, 55–71. [Google Scholar] [CrossRef]
- Nakamura, F.; Stossel, T.P.; Hartwig, J.H. The filamins: Organizers of cell structure and function. Cell Adhes. Migr. 2011, 5, 160–169. [Google Scholar] [CrossRef]
- Vasta, J.D.; Raines, R.T. Collagen prolyl 4-hydroxylase as a therapeutic target. J. Med. Chem. 2018, 61, 10403–10411. [Google Scholar] [CrossRef]
- Hurme, T.; Kalimo, H.; Sandberg, M.; Lehto, M.; Vuorio, E. Localization of type I and III collagen and fibronectin production in injured gastrocnemius muscle. Lab. Investig. 1991, 64, 76–84. [Google Scholar]
- Shin, K.O.; Ha, D.H.; Kim, J.O.; Crumrine, D.A.; Meyer, J.M.; Wakefield, J.S.; Lee, Y.; Kim, B.; Kim, S.; Kim, H.K.; et al. Exosomes from human adipose tissue-derived mesenchymal stem cells promote epidermal barrier repair by inducing de novo synthesis of ceramides in atopic dermatitis. Cells 2020, 9, 680. [Google Scholar] [CrossRef] [PubMed]
- Negri, S.; Wang, Y.; Sono, T.; Lee, S.; Hsu, G.C.; Xu, J.; Meyers, C.A.; Qin, Q.; Broderick, K.; Witwer, K.W.; et al. Human perivascular stem cells prevent bone graft resorption in osteoporotic contexts by inhibiting osteoclast formation. Stem Cells Transl. Med. 2020, 9, 1617–1630. [Google Scholar] [CrossRef]
- Lizcano, F.; Guzman, G. Estrogen deficiency and the origin of obesity during menopause. Biomed. Res. Int. 2014, 2014, 757461. [Google Scholar] [CrossRef] [PubMed]
- Gaggini, M.; Morelli, M.; Buzzigoli, E.; DeFronzo, R.A.; Bugianesi, E.; Gastaldelli, A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients 2013, 5, 1544–1560. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef]
- Panneerselvam, S.; Packirisamy, R.M.; Bobby, Z.; Jacob, S.E.; Sridhar, M.G. Soy isoflavones (Glycine max) ameliorate hypertriglyceridemia and hepatic steatosis in high fat-fed ovariectomized Wistar rats (an experimental model of postmenopausal obesity). J. Nutr. Biochem. 2016, 38, 57–69. [Google Scholar] [CrossRef]
- Wang, R.; Lin, M.; Li, L.; Li, L.; Qi, G.; Rong, R.; Xu, M.; Zhu, T. Bone marrow mesenchymal stem cell-derived exosome protects kidney against ischemia reperfusion injury in rats. Zhonghua Yi Xue Za Zhi 2014, 94, 3298–3303. [Google Scholar]
- Narayanan, R.; Huang, C.C.; Ravindran, S. Hijacking the cellular mail: Exosome mediated differentiation of mesenchymal stem cells. Stem Cells Int. 2016, 2016, 3808674. [Google Scholar] [CrossRef]
- Tao, S.C.; Yuan, T.; Zhang, Y.L.; Yin, W.J.; Guo, S.C.; Zhang, C.Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 2017, 7, 180–195. [Google Scholar] [CrossRef]
- Strom, J.O.; Theodorsson, E.; Theodorsson, A. Order of magnitude differences between methods for maintaining physiological 17beta-oestradiol concentrations in ovariectomized rats. Scand. J. Clin. Lab. Investig. 2008, 68, 814–822. [Google Scholar] [CrossRef]
- El-Gendy, A.A.; Elsaed, W.M.; Abdallah, H.I. Potential role of estradiol in ovariectomy-induced derangement of renal endocrine functions. Ren. Fail. 2019, 41, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Jang, J.W.; Lee, J.K.; Park, C.K. Rutin Improves Bone histomorphometric values by reduction of osteoclastic activity in osteoporosis mouse model induced by bilateral ovariectomy. J. Korean Neurosurg. Soc. 2020, 63, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R. Sources of estrogen and their importance. J. Steroid Biochem. Mol. Biol. 2003, 86, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shen, Y.; Li, R. Estrogen synthesis and signaling pathways during ageing: From periphery to brain. Trends Mol. Med. 2013, 19, 197–209. [Google Scholar] [CrossRef]
- Hetemaki, N.; Mikkola, T.S.; Tikkanen, M.J.; Wang, F.; Hamalainen, E.; Turpeinen, U.; Haanpaa, M.; Vihma, V.; Peltonen, H.S. Adipose tissue estrogen production and metabolism in premenopausal women. J. Steroid Biochem. Mol. Biol. 2021, 209, 105849. [Google Scholar] [CrossRef]
- Bauss, F.; Dempster, D.W. Effects of ibandronate on bone quality: Preclinical studies. Bone 2007, 40, 265–273. [Google Scholar] [CrossRef]
- Lei, Z.; Xiaoying, Z.; Xingguo, L. Ovariectomy-associated changes in bone mineral density and bone marrow haematopoiesis in rats. Int. J. Exp. Pathol. 2009, 90, 512–519. [Google Scholar] [CrossRef]
- Huang, X.L.; Huang, L.Y.; Cheng, Y.T.; Li, F.; Zhou, Q.; Wu, C.; Shi, Q.H.; Guan, Z.Z.; Liao, J.; Hong, W. Zoledronic acid inhibits osteoclast differentiation and function through the regulation of NF-κB and JNK signalling pathways. Int. J. Mol. Med. 2019, 44, 582–592. [Google Scholar] [CrossRef]
- Huang, X.L.; Liu, C.; Shi, X.M.; Cheng, Y.T.; Zhou, Q.; Li, J.P.; Liao, J. Zoledronic acid inhibits osteoclastogenesis and bone resorptive function by suppressing RANKL-mediated NF-κB and JNK and their downstream signalling pathways. Mol. Med. Rep. 2022, 25, 59. [Google Scholar] [CrossRef]
- Wang, B.; Zhan, Y.; Yan, L.; Hao, D. How zoledronic acid improves osteoporosis by acting on osteoclasts. Front. Pharmacol. 2022, 13, 961941. [Google Scholar] [CrossRef]
- Hu, K.; Olsen, B.R. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J. Clin. Investig. 2016, 126, 509–526. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Z.; Zhao, W.J.; Ye, T.W.; Chen, A. Resveratrol attenuates lipopolysaccharides (LPS)-induced inhibition of osteoblast differentiation in MC3T3-E1 cells. Med. Sci. Monit. 2018, 24, 2045–2052. [Google Scholar] [CrossRef]
- Komori, T. Animal models for osteoporosis. Eur. J. Pharmacol. 2015, 759, 287–294. [Google Scholar] [CrossRef]
- Souza, V.R.; Mendes, E.; Casaro, M.; Antiorio, A.T.F.B. Description of ovariectomy protocol in mice. Methods Mol. Biol. 2019, 1916, 303–309. [Google Scholar] [PubMed]
- Jiang, G.Z.; Matsumoto, H.; Hori, M.; Gunji, A.; Hakozaki, K.; Akimoto, Y.; Fujii, A. Correlation among geometric, densitometric, and mechanical properties in mandible and femur of osteoporotic rats. J. Bone Miner. Metab. 2008, 26, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Lee, J.H.; Han, S.S.; Kim, Y.H.; Choi, Y.J.; Jeon, K.J.; Jung, H.I. Site-specific and time-course changes of postmenopausal osteoporosis in rat mandible: Comparative study with femur. Sci. Rep. 2019, 9, 14155. [Google Scholar] [CrossRef]

| Weight | Sham Control | OVX Alone | +EVs (1 × 108/Body) | +EVs (3 × 108/Body) | +EVs (1 × 109/Body) | +ZA (100 μg/kg) | |
|---|---|---|---|---|---|---|---|
| Body weight (g) | 434.3 ±37.7 | 442.9 ±30.9 | 439.7 ±30.0 | 446.4 ±18.0 | 435.5 ±20.4 | 441.7 ±17.6 | |
| Liver | Absolute (g) | 8.99 ±0.21 | 9.68 ±0.94 | 9.63 ±0.27 | 9.62 ±1.01 | 9.48 ±0.80 | 9.36 ±1.25 |
| Relative (%) | 2.06 ±0.19 | 2.19 ±0.09 | 2.19 ±0.13 | 2.16 ±0.20 | 2.15 ±0.14 | 2.15 ±0.26 | |
| Kidneys | Absolute (g) | 1.82 ±0.12 | 2.04 ±0.22 | 1.86 ±0.08 | 1.86 ±0.20 | 1.90 ±0.26 | 1.82 ±0.64 |
| Relative (%) | 0.42 ±0.01 | 0.46 ±0.02 | 0.42 ±0.02 | 0.42 ±0.04 | 0.43 ±0.06 | 0.42 ±0.18 | |
| Spleen | Absolute (g) | 0.86 ±0.17 | 0.75 ±0.22 | 0.81 ±0.14 | 0.70 ±0.09 | 0.76 ±0.09 | 0.82 ±0.13 |
| Relative (%) | 0.20 ±0.03 | 0.17 ±0.05 | 0.18 ±0.03 | 0.16 ±0.02 | 0.17 ±0.02 | 0.19 ±0.03 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.Y.; Tsolmon, K.-E.; Bavuu, Z.; Noh, C.H.; Kim, H.-S.; Jeong, H.-S.; Park, D.; Hong, S.-C.; Kim, Y.-B. Osteoporosis-Improving Effects of Extracellular Vesicles from Human Amniotic Membrane Stem Cells in Ovariectomized Rats. Int. J. Mol. Sci. 2025, 26, 9503. https://doi.org/10.3390/ijms26199503
Kim KY, Tsolmon K-E, Bavuu Z, Noh CH, Kim H-S, Jeong H-S, Park D, Hong S-C, Kim Y-B. Osteoporosis-Improving Effects of Extracellular Vesicles from Human Amniotic Membrane Stem Cells in Ovariectomized Rats. International Journal of Molecular Sciences. 2025; 26(19):9503. https://doi.org/10.3390/ijms26199503
Chicago/Turabian StyleKim, Ka Young, Khan-Erdene Tsolmon, Zolzaya Bavuu, Chan Ho Noh, Hyun-Soo Kim, Heon-Sang Jeong, Dongsun Park, Soon-Cheol Hong, and Yun-Bae Kim. 2025. "Osteoporosis-Improving Effects of Extracellular Vesicles from Human Amniotic Membrane Stem Cells in Ovariectomized Rats" International Journal of Molecular Sciences 26, no. 19: 9503. https://doi.org/10.3390/ijms26199503
APA StyleKim, K. Y., Tsolmon, K.-E., Bavuu, Z., Noh, C. H., Kim, H.-S., Jeong, H.-S., Park, D., Hong, S.-C., & Kim, Y.-B. (2025). Osteoporosis-Improving Effects of Extracellular Vesicles from Human Amniotic Membrane Stem Cells in Ovariectomized Rats. International Journal of Molecular Sciences, 26(19), 9503. https://doi.org/10.3390/ijms26199503






