Modulation of Mesangial Cells by Tamsulosin and Pioglitazone Under Hyperglycemic Conditions: An In Vitro and In Vivo Study
Abstract
1. Introduction
2. Results
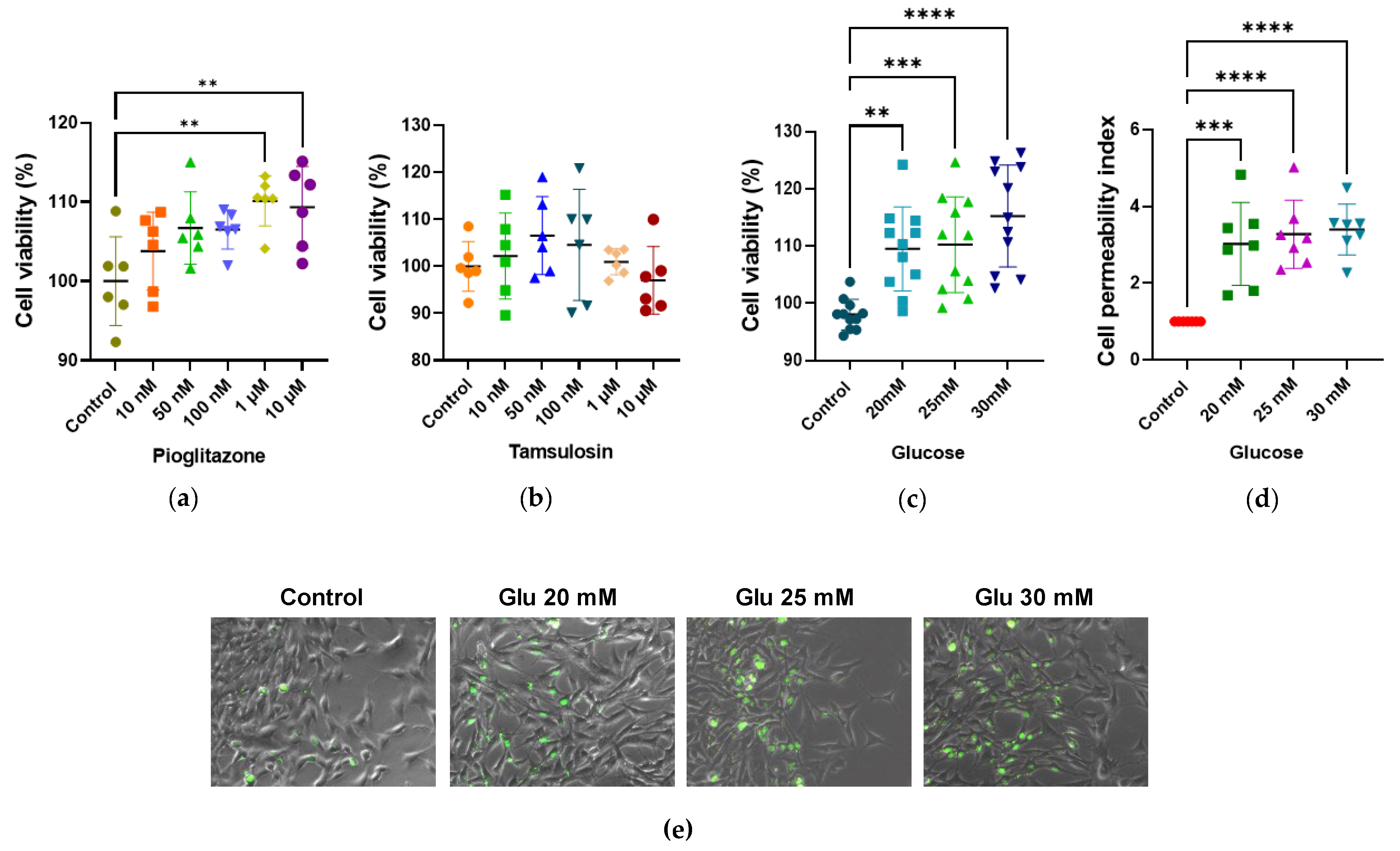
2.1. Impacts of HG, Tamsulosin, and Pioglitazone on Viability and Cellular Integrity of SV40 MES 13 Cells
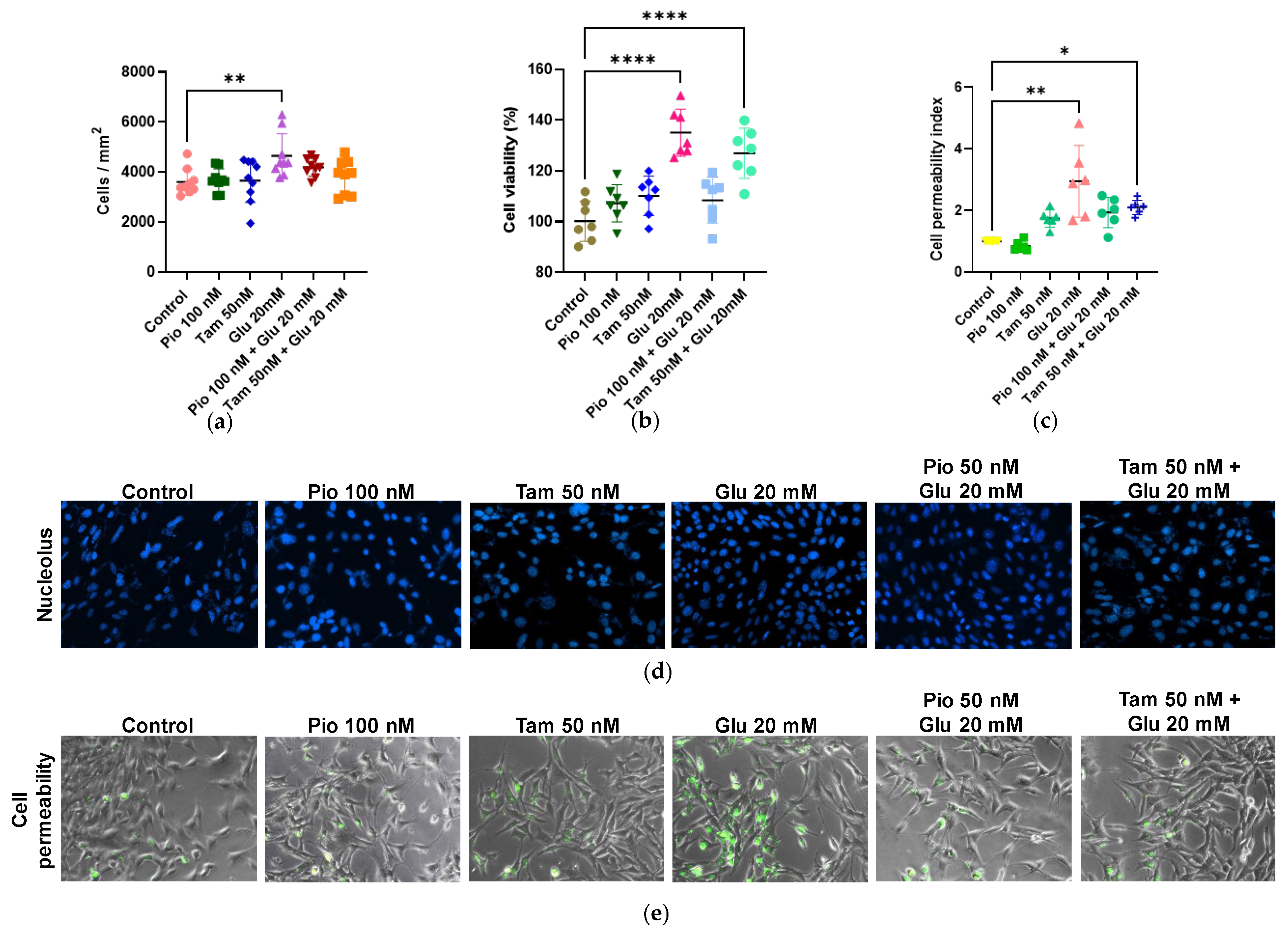
2.2. Effects of Tamsulosin and Pioglitazone on Proliferation and Viability in SV40 MES 13 Cells Activated by HG
2.3. Tamsulosin and Pioglitazone Modulate α1- and β2-AR Expression Under HG Conditions
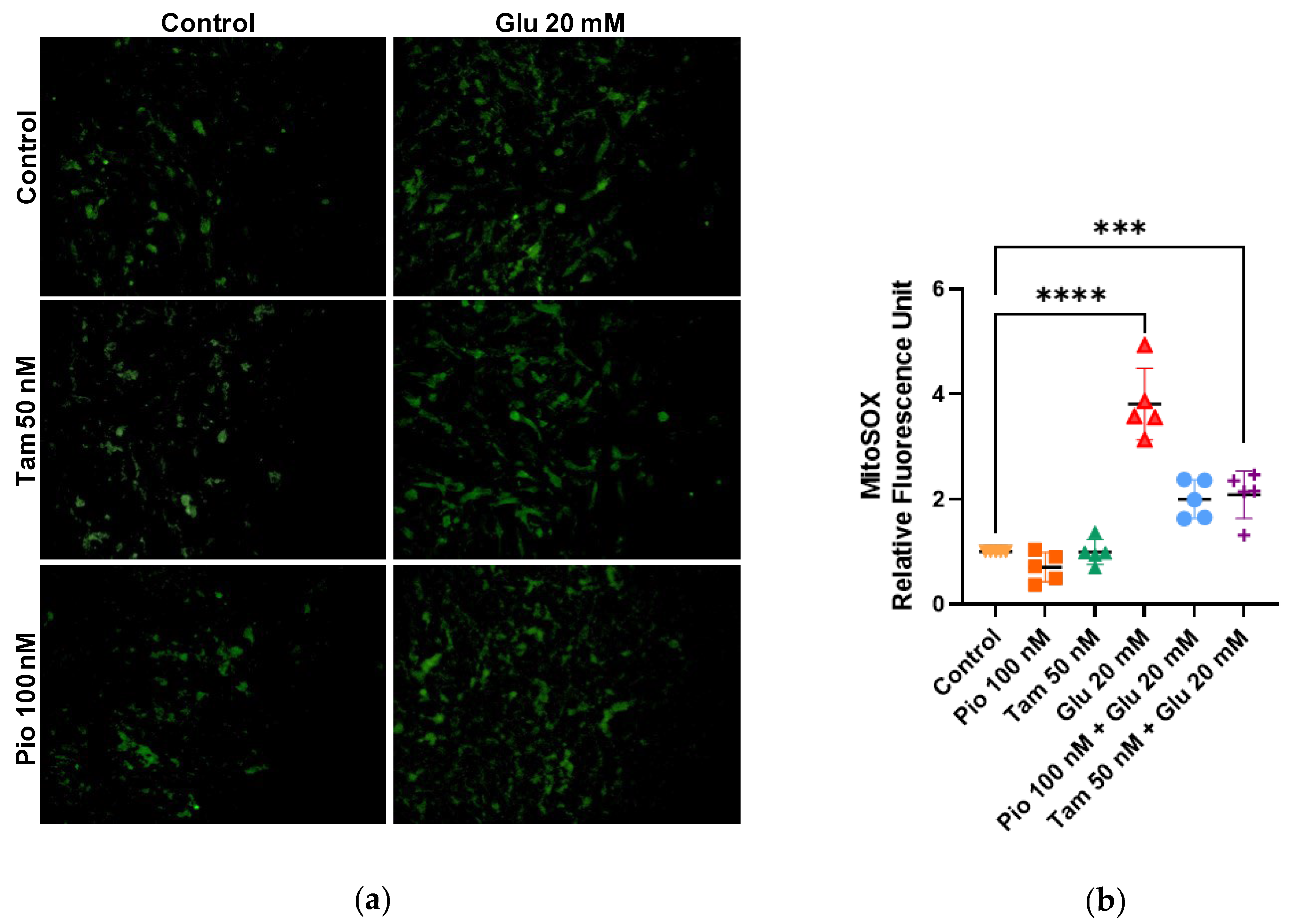
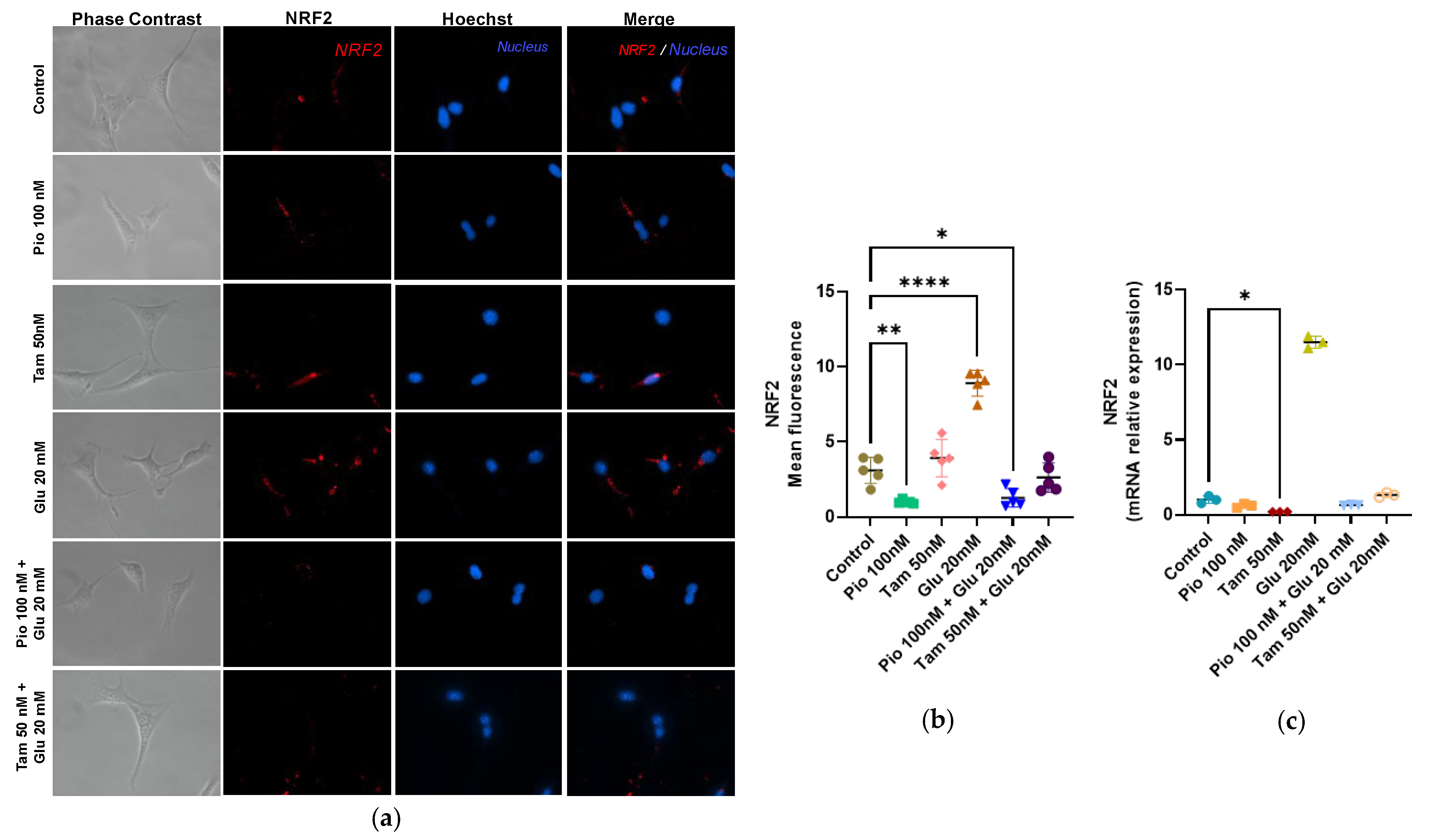
2.4. Tamsulosin and Pioglitazone Attenuate HG-Induced Mitochondrial Superoxide Production and Regulate NRF2 Expression in SV40 MES 13 Cells
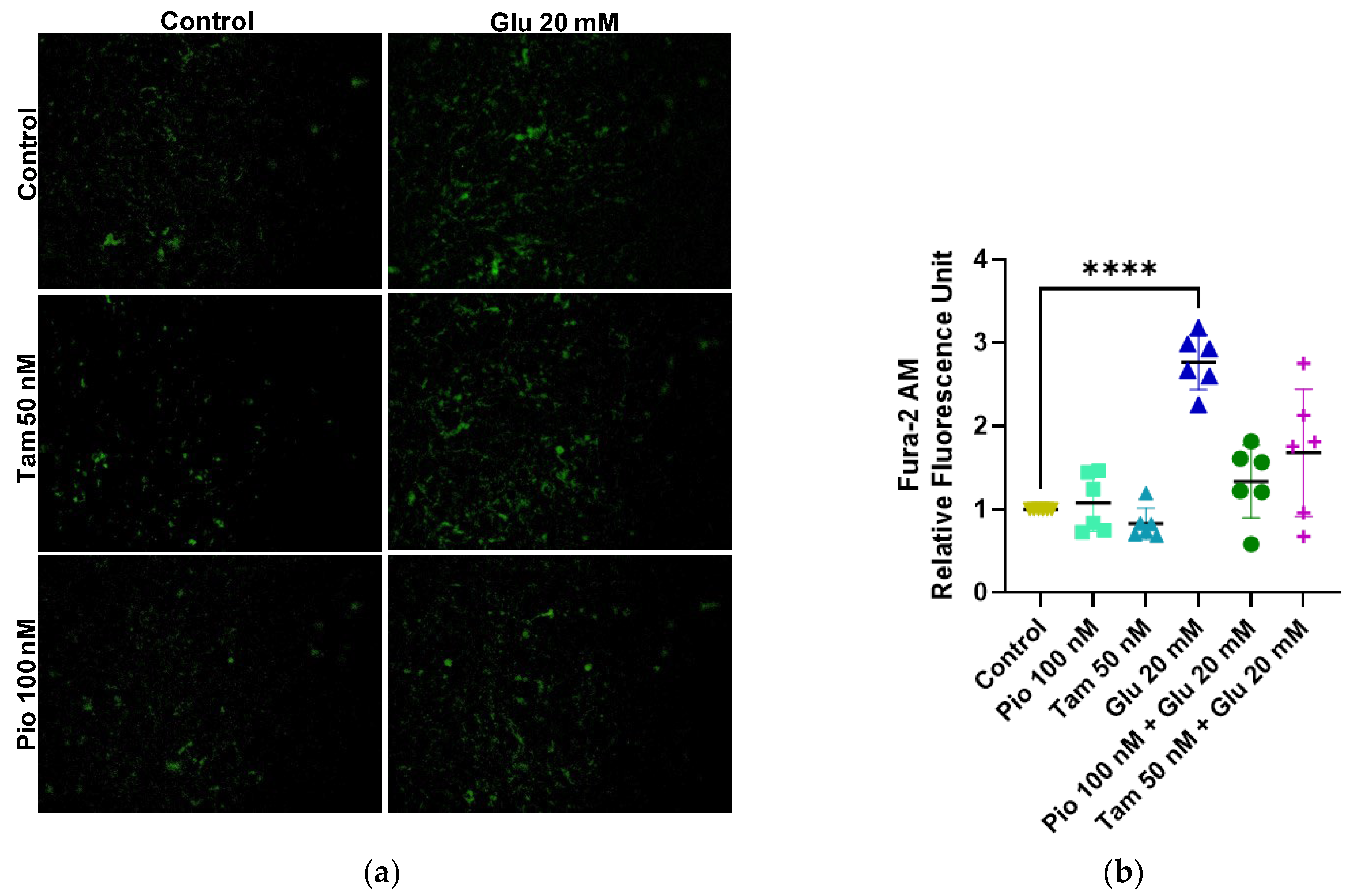
2.5. Tamsulosin and Pioglitazone Reduced Cytoplasmic Calcium Caused by HG
2.6. Tamsulosin and Pioglitazone Significantly Attenuated the Inflammatory Profile in HG-Induced SV40 MES 13 MCs
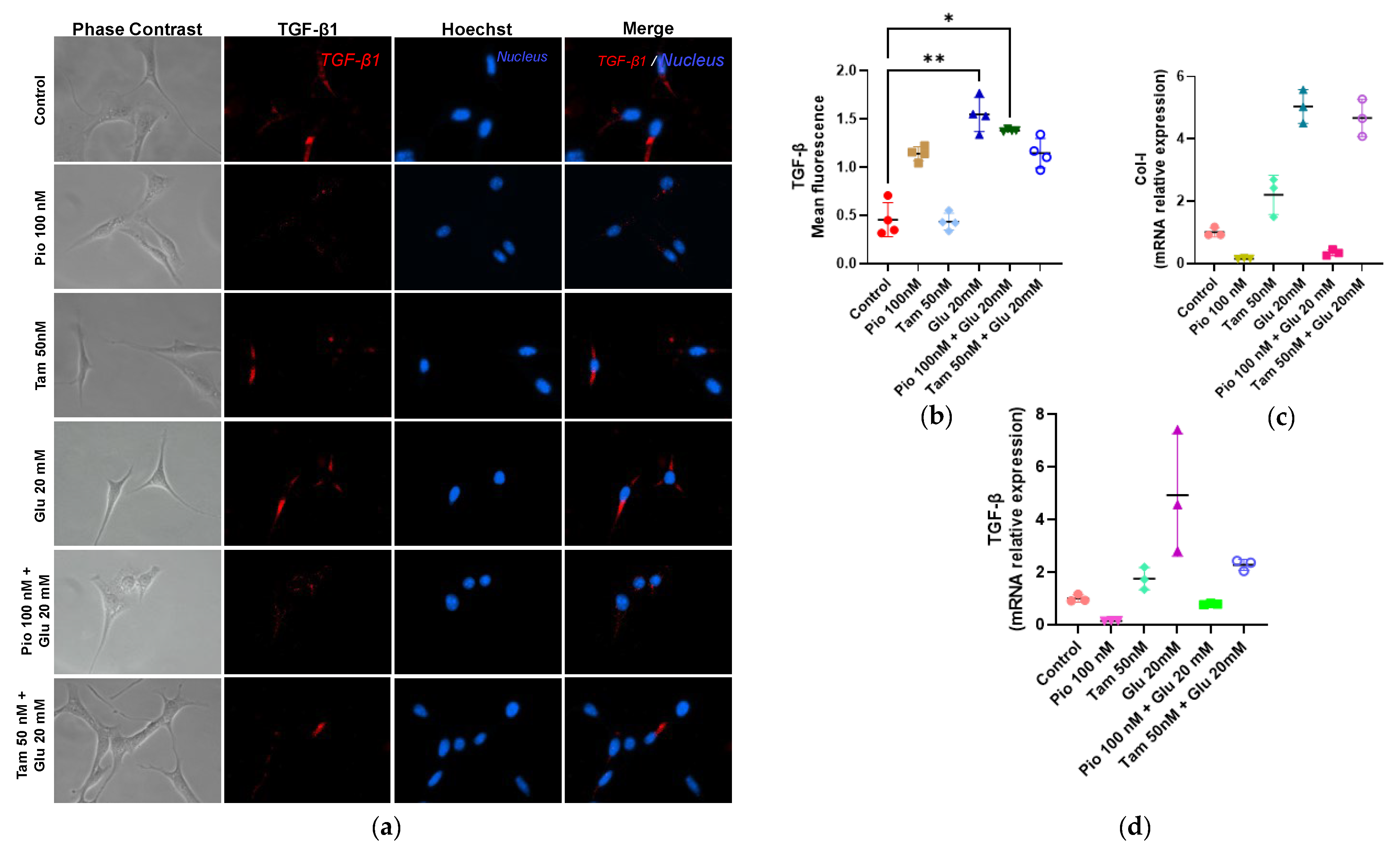
2.7. Tamsulosin and Pioglitazone Significantly Attenuated the Fibrogenic Profile in HG-Induced SV40 MES 13 MCs
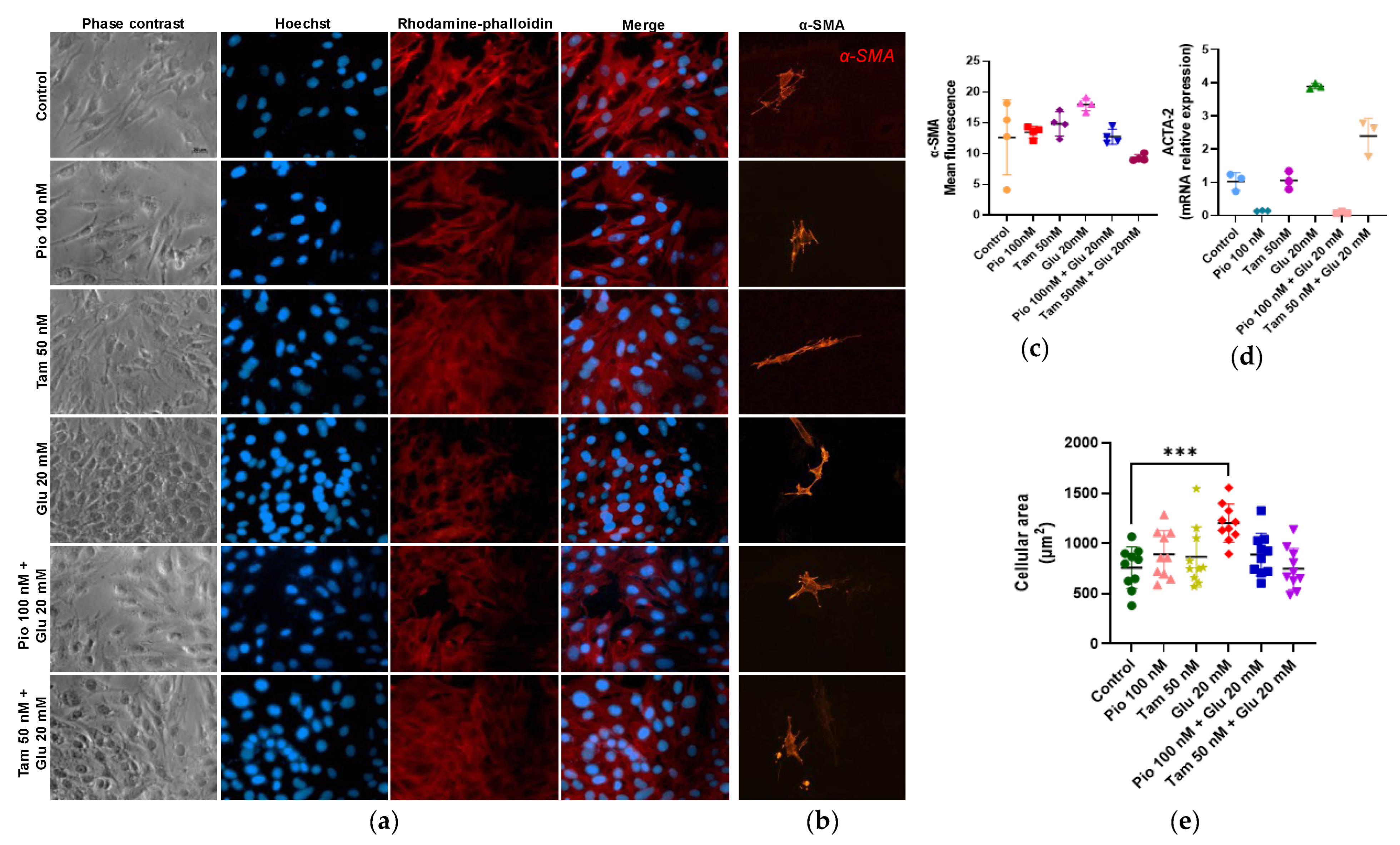
2.8. Regulation of Actin Polymerization and Cellular Hypertrophy Under HG Conditions by Tamsulosin and Pioglitazone
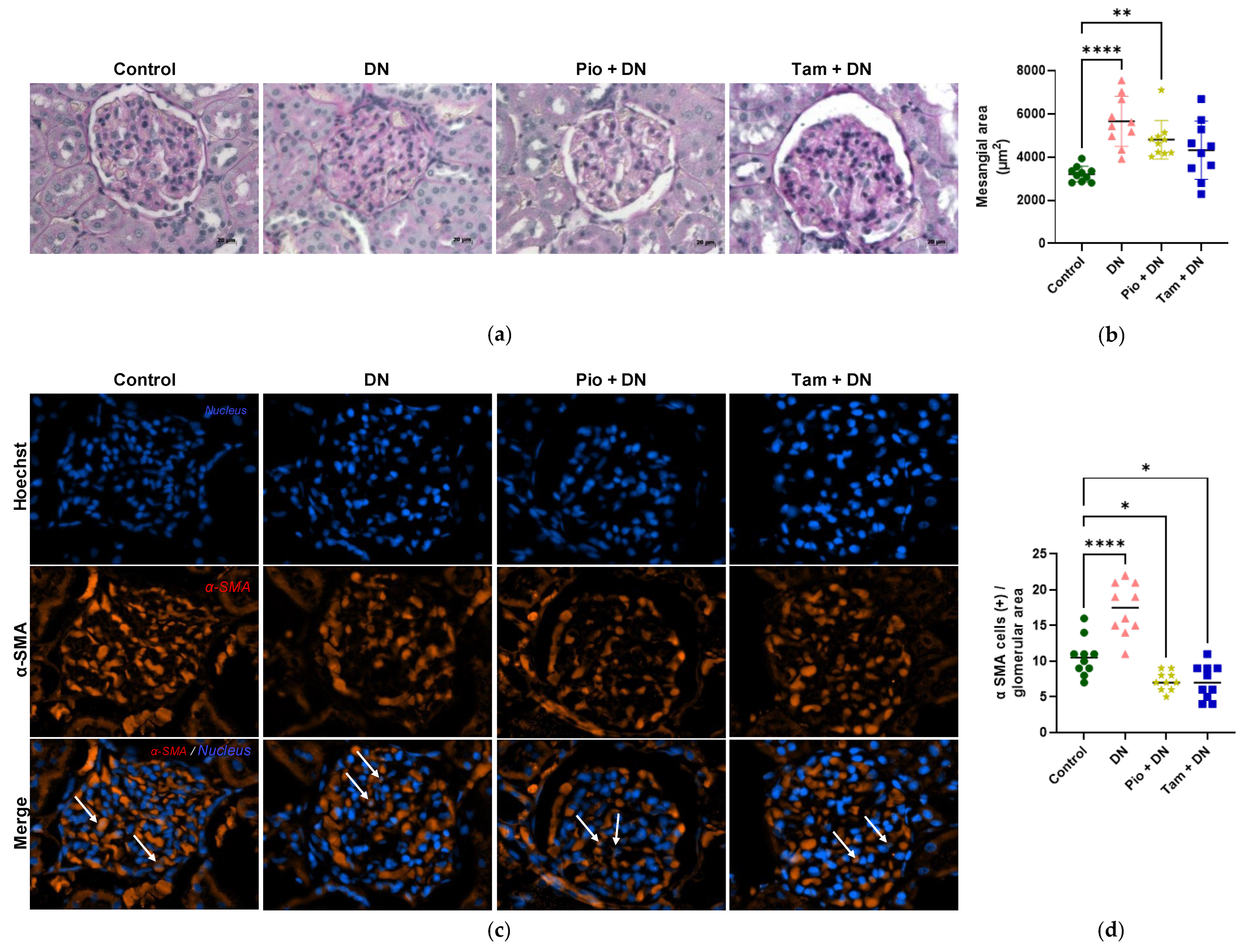
2.9. Tamsulosin and Pioglitazone Diminished Mesangial Expansion and Positive Cells for α-SMA in an Experimental DN Model
3. Discussion
4. Materials and Methods
4.1. Animals
4.1.1. Mesangial Expansion Measurement
4.1.2. Mesangial α-SMA by Immunofluorescence
4.2. Cell Culture
4.2.1. Colorimetric MTT (Tetrazolium) Viability Assay
4.2.2. SYTOX Green Membrane Cell Permeability Determination by Using High Glucose Medium
4.2.3. Detection of Mitochondrial Superoxide Formation by MitoSOX Assay
4.2.4. Intracytoplasmic Ca2+ Measurement
4.2.5. Polymerized Actin Detection Assay by Identification of Transformation from G-Actin to F-Actin
4.2.6. Immunofluorescence
4.2.7. Mesangial Cell Area Measurement
4.2.8. Analyze Gene Expression by RT-qPCR
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dwivedi, S.; Sikarwar, M.S. Diabetic Nephropathy: Pathogenesis, Mechanisms, and Therapeutic Strategies. Horm. Metab. Res. 2025, 57, 7–17. [Google Scholar] [CrossRef]
- Anders, H.-J.; Huber, T.B.; Isermann, B.; Schiffer, M. CKD in Diabetes: Diabetic Kidney Disease versus Nondiabetic Kidney Disease. Nat. Rev. Nephrol. 2018, 14, 361–377. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Xing, X.; Li, M.; Liu, Y.; Xu, A.; Zhang, J. Podocyte Injury of Diabetic Nephropathy: Novel Mechanism Discovery and Therapeutic Prospects. Biomed. Pharmacother. 2023, 168, 115670. [Google Scholar] [CrossRef]
- Qi, C.; Mao, X.; Zhang, Z.; Wu, H. Classification and Differential Diagnosis of Diabetic Nephropathy. J. Diabetes Res. 2017, 2017, 8637138. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Morgado-Pascual, J.L.; Opazo-Ríos, L.; Guerrero-Hue, M.; García-Caballero, C.; Vázquez-Carballo, C.; Mas, S.; Sanz, A.B.; Herencia, C.; Mezzano, S.; et al. Pathogenic Pathways and Therapeutic Approaches Targeting Inflammation in Diabetic Nephropathy. Int. J. Mol. Sci. 2020, 21, 3798. [Google Scholar] [CrossRef]
- Dagar, N.; Das, P.; Bisht, P.; Taraphdar, A.K.; Velayutham, R.; Arumugam, S. Diabetic Nephropathy: A Twisted Thread to Unravel. Life Sci. 2021, 278, 119635. [Google Scholar] [CrossRef] [PubMed]
- Hoogeveen, E.K. The Epidemiology of Diabetic Kidney Disease. Kidney Dial. 2022, 2, 433–442. [Google Scholar] [CrossRef]
- Wada, J.; Makino, H. Inflammation and the Pathogenesis of Diabetic Nephropathy. Clin. Sci. 2013, 124, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.K.; Nicholas, S.B. Pathomechanisms of Diabetic Kidney Disease. J. Clin. Med. 2023, 12, 7349. [Google Scholar] [CrossRef]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef]
- Brownlee, M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Haneda, M.; Koya, D.; Isono, M.; Kikkawa, R. Overview of Glucose Signaling in Mesangial Cells in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2003, 14, 1374–1382. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and Molecular Cell Biology of Diabetic Complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Du, X.-L.; Edelstein, D.; Rossetti, L.; Fantus, I.G.; Goldberg, H.; Ziyadeh, F.; Wu, J.; Brownlee, M. Hyperglycemia-Induced Mitochondrial Superoxide Overproduction Activates the Hexosamine Pathway and Induces Plasminogen Activator Inhibitor-1 Expression by Increasing Sp1 Glycosylation. Proc. Natl. Acad. Sci. USA 2000, 97, 12222–12226. [Google Scholar] [CrossRef]
- Ebefors, K.; Bergwall, L.; Nyström, J. The Glomerulus According to the Mesangium. Front. Med. 2021, 8, 740527. [Google Scholar] [CrossRef]
- Wakisaka, M.; He, Q.; Spiro, M.J.; Spiro, R.G. Glucose Entry into Rat Mesangial Cells Is Mediated by Both Na+-Coupled and Facilitative Transporters. Diabetologia 1995, 38, 291–297. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heilig, C.W.; Concepcion, L.A.; Riser, B.L.; Freytag, S.O.; Zhu, M.; Cortes, P. Overexpression of Glucose Transporters in Rat Mesangial Cells Cultured in a Normal Glucose Milieu Mimics the Diabetic Phenotype. J. Clin. Investig. 1995, 96, 1802–1814. [Google Scholar] [CrossRef]
- Wolf, G.; Sharma, K.; Chen, Y.; Ericksen, M.; Ziyadeh, F.N. High Glucose-Induced Proliferation in Mesangial Cells Is Reversed by Autocrine TGF-Beta. Kidney Int. 1992, 42, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.Y.; Ford Versypt, A.N. Pathophysiology of Mesangial Expansion in Diabetic Nephropathy: Mesangial Structure, Glomerular Biomechanics, and Biochemical Signaling and Regulation. J. Biol. Eng. 2022, 16, 19. [Google Scholar] [CrossRef]
- Hu, S.; Hang, X.; Wei, Y.; Wang, H.; Zhang, L.; Zhao, L. Crosstalk among Podocytes, Glomerular Endothelial Cells and Mesangial Cells in Diabetic Kidney Disease: An Updated Review. Cell Commun. Signal 2024, 22, 136. [Google Scholar] [CrossRef]
- Gruden, G.; Perin, P.C.; Camussi, G. Insight on the Pathogenesis of Diabetic Nephropathy from the Study of Podocyte and Mesangial Cell Biology. Curr. Diabetes Rev. 2005, 1, 27–40. [Google Scholar] [CrossRef]
- Abrass, C.K. Diabetic Nephropathy. Mechanisms of Mesangial Matrix Expansion. West. J. Med. 1995, 162, 318–321. [Google Scholar] [PubMed]
- Bülow, R.D.; Boor, P. Extracellular Matrix in Kidney Fibrosis: More Than Just a Scaffold. J. Histochem. Cytochem. 2019, 67, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Herrera, G.A. Plasticity of Mesangial Cells: A Basis for Understanding Pathological Alterations. Ultrastruct. Pathol. 2006, 30, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guerrero, C.; Hernández-Vargas, P.; López-Franco, O.; Ortiz-Muñóz, G.; Egido, J. Mesangial Cells and Glomerular Inflammation: From the Pathogenesis to Novel Therapeutic Approaches. Curr. Drug Targets-Inflamm. Allergy 2005, 4, 341–351. [Google Scholar] [CrossRef]
- Nastase, M.V.; Zeng-Brouwers, J.; Wygrecka, M.; Schaefer, L. Targeting Renal Fibrosis: Mechanisms and Drug Delivery Systems. Adv. Drug Deliv. Rev. 2018, 129, 295–307. [Google Scholar] [CrossRef]
- Zhao, J.-H. Mesangial Cells and Renal Fibrosis. In Renal Fibrosis: Mechanisms and Therapies; Liu, B.-C., Lan, H.-Y., Lv, L.-L., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; Volume 1165, pp. 165–194. ISBN 978-981-13-8870-5. [Google Scholar]
- Elendu, C.; John Okah, M.; Fiemotongha, K.D.J.; Adeyemo, B.I.; Bassey, B.N.; Omeludike, E.K.; Obidigbo, B. Comprehensive Advancements in the Prevention and Treatment of Diabetic Nephropathy: A Narrative Review. Medicine 2023, 102, e35397. [Google Scholar] [CrossRef]
- Kamiar, A.; Yousefi, K.; Dunkley, J.C.; Webster, K.A.; Shehadeh, L.A. Β2-Adrenergic Receptor Agonism as a Therapeutic Strategy for Kidney Disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R575–R587. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New Data Content and Improved Web Interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Sun, L.; Sun, C.; Zhou, S.; Zhang, L.; Hu, W. Tamsulosin Attenuates High Glucose- Induced Injury in Glomerular Endothelial Cells. Bioengineered 2021, 12, 5184–5194. [Google Scholar] [CrossRef]
- Broeders, N.; Abramowicz, D. Peroxisome Proliferator-Activated Receptors (PPARs): Novel Therapeutic Targets in Renal Disease. Kidney Int. 2002, 61, 354–355. [Google Scholar] [CrossRef]
- Benkirane, K.; Viel, E.C.; Amiri, F.; Schiffrin, E.L. Peroxisome Proliferator-Activated Receptor Gamma Regulates Angiotensin II-Stimulated Phosphatidylinositol 3-Kinase and Mitogen-Activated Protein Kinase in Blood Vessels In Vivo. Hypertension 2006, 47, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Efrati, S.; Berman, S.; Ilgiyeav, E.; Averbukh, Z.; Weissgarten, J. PPAR-γ Activation Inhibits Angiotensin II Synthesis, Apoptosis, and Proliferation of Mesangial Cells from Spontaneously Hypertensive Rats. Nephron Exp. Nephrol. 2007, 106, e107–e112. [Google Scholar] [CrossRef] [PubMed]
- Weissgarten, J.; Berman, S.; Efrati, S.; Rapoport, M.; Averbukh, Z.; Feldman, L. Apoptosis and Proliferation of Cultured Mesangial Cells Isolated from Kidneys of Rosiglitazone-Treated Pregnant Diabetic Rats. Nephrol. Dial. Transpl. 2006, 21, 1198–1204. [Google Scholar] [CrossRef][Green Version]
- Ko, G.J.; Kang, Y.S.; Han, S.Y.; Lee, M.H.; Song, H.K.; Han, K.H.; Kim, H.K.; Han, J.Y.; Cha, D.R. Pioglitazone Attenuates Diabetic Nephropathy through an Anti-Inflammatory Mechanism in Type 2 Diabetic Rats. Nephrol. Dial. Transplant. 2008, 23, 2750–2760. [Google Scholar] [CrossRef]
- Maeda, A.; Horikoshi, S.; Gohda, T.; Tsuge, T.; Maeda, K.; Tomino, Y. Pioglitazone Attenuates TGF-β-Induction of Fibronectin Synthesis and Its Splicing Variant in Human Mesangial Cells via Activation of Peroxisome Proliferator-Activated Receptor (PPAR)γ. Cell Biol. Int. 2005, 29, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ye, S.; Sun, W.; Hu, Y. Pioglitazone Inhibits the Expressions of P22phox and P47phox in Rat Mesangial Cells In Vitro. Int. Sch. Res. Not. 2014, 2014, 601352. [Google Scholar] [CrossRef]
- Morones, J.; Pérez, M.; Muñoz, M.; Sánchez, E.; Ávila, M.; Topete, J.; Ventura, J.; Martínez, S. Evaluation of the Effect of an α-Adrenergic Blocker, a PPAR-γ Receptor Agonist, and a Glycemic Regulator on Chronic Kidney Disease in Diabetic Rats. Int. J. Mol. Sci. 2024, 25, 11372. [Google Scholar] [CrossRef]
- Romagnani, P.; Remuzzi, G.; Glassock, R.; Levin, A.; Jager, K.J.; Tonelli, M.; Massy, Z.; Wanner, C.; Anders, H.-J. Chronic Kidney Disease. Nat. Rev. Dis. Primers 2017, 3, 17088. [Google Scholar] [CrossRef]
- Esler, M.; Jennings, G.; Lambert, G. Noradrenaline Release and the Pathophysiology of Primary Human Hypertension. Am. J. Hypertens. 1989, 2, 140S–146S. [Google Scholar] [CrossRef]
- Kong, F.; Ma, L.; Zou, L.; Meng, K.; Ji, T.; Zhang, L.; Zhang, R.; Jiao, J. Alpha1-Adrenergic Receptor Activation Stimulates Calcium Entry and Proliferation via TRPC6 Channels in Cultured Human Mesangial Cells. Cell Physiol. Biochem. 2015, 36, 1928–1938. [Google Scholar] [CrossRef]
- Majumder, S.; Amin, M.; Pushpakumar, S.; Sen, U. Collagen Receptor- and Metalloproteinase-Dependent Hypertensive Stress Response in Mesangial and Glomerular Endothelial Cells. Mol. Cell Biochem. 2020, 466, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wei, G.; Xu, J.; Ma, X.; Wang, Q. Naringin Ameliorates the High Glucose-Induced Rat Mesangial Cell Inflammatory Reaction by Modulating the NLRP3 Inflammasome. BMC Complement. Altern. Med. 2018, 18, 192. [Google Scholar] [CrossRef]
- Jie, R.; Zhu, P.; Zhong, J.; Zhang, Y.; Wu, H. LncRNA KCNQ1OT1 Affects Cell Proliferation, Apoptosis and Fibrosis through Regulating miR-18b-5p/SORBS2 Axis and NF-ĸB Pathway in Diabetic Nephropathy. Diabetol. Metab. Syndr. 2020, 12, 77. [Google Scholar] [CrossRef]
- Ding, T.; Chen, W.; Li, J.; Ding, J.; Mei, X.; Hu, H. High Glucose Induces Mouse Mesangial Cell Overproliferation via Inhibition of Hydrogen Sulfide Synthesis in a TLR-4-Dependent Manner. Cell Physiol. Biochem. 2017, 41, 1035–1043. [Google Scholar] [CrossRef]
- Kang, B.P.S.; Frencher, S.; Reddy, V.; Kessler, A.; Malhotra, A.; Meggs, L.G. High Glucose Promotes Mesangial Cell Apoptosis by Oxidant-Dependent Mechanism. Am. J. Physiol. Ren. Physiol. 2003, 284, F455–F466. [Google Scholar] [CrossRef]
- Pawluczyk, I.Z.A.; Patel, S.R.; Harris, K.P.G. The Role of the Alpha-1 Adrenoceptor in Modulating Human Mesangial Cell Matrix Production. Nephrol. Dial. Transpl. 2006, 21, 2417–2424. [Google Scholar] [CrossRef][Green Version]
- Chapple, C.; Andersson, K.-E. Tamsulosin: An Overview. World J. Urol. 2002, 19, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Safi, S.Z.; Qvist, R.; Yan, G.O.S.; Ismail, I.S.B. Differential Expression and Role of Hyperglycemia Induced Oxidative Stress in Epigenetic Regulation of Β1, Β2 and Β3-Adrenergic Receptors in Retinal Endothelial Cells. BMC Med. Genomics 2014, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Papay, R.S.; Perez, D.M. A1-Adrenergic Receptors Increase Glucose Oxidation under Normal and Ischemic Conditions in Adult Mouse Cardiomyocytes. J. Recept. Signal Transduct. Res. 2021, 41, 138–144. [Google Scholar] [CrossRef]
- Samsu, N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. Bio. Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef] [PubMed]
- Jasielec, J.J.; Filipek, R.; Dołowy, K.; Lewenstam, A. Precipitation of Inorganic Salts in Mitochondrial Matrix. Membranes 2020, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 Signaling Pathway: Pivotal Roles in Inflammation. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- He, J.; Zhang, X.; Lian, C.; Wu, J.; Fang, Y.; Ye, X. KEAP1/NRF2 Axis Regulates H2O2-Induced Apoptosis of Pancreatic β-Cells. Gene 2019, 691, 8–17. [Google Scholar] [CrossRef]
- Dai, C.; Li, M.; Liu, Y.; Tran, D.H.; Jiang, H.; Tang, S.; Shen, J. Involvement of the Inhibition of Mitochondrial Apoptotic, P53, NF-κB Pathways and the Activation of Nrf2/HO-1 Pathway in the Protective Effects of Curcumin against Copper Sulfate-Induced Nephrotoxicity in Mice. Ecotoxicol. Environ. Saf. 2023, 249, 114480. [Google Scholar] [CrossRef]
- Sedor, J.R.; Nakazato, Y.; Konieczkowski, M. Interleukin-1 and the Mesangial Cell. Kidney Int. 1992, 41, 595–599. [Google Scholar] [CrossRef][Green Version]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A Key Role in Inflammatory Diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Ueta, M.; Wakisaka, M.; Ago, T.; Kitazono, T.; Nakamura, U.; Yoshinari, M.; Iwase, M.; Iida, M. PPARgamma Ligands Attenuate Mesangial Contractile Dysfunction in High Glucose. Kidney Int. 2004, 65, 961–971. [Google Scholar] [CrossRef]
- Moon, D.-O. Calcium’s Role in Orchestrating Cancer Apoptosis: Mitochondrial-Centric Perspective. Int. J. Mol. Sci. 2023, 24, 8982. [Google Scholar] [CrossRef] [PubMed]
- Stockand, J.D.; Sansom, S.C. Glomerular Mesangial Cells: Electrophysiology and Regulation of Contraction. Physiol. Rev. 1998, 78, 723–744. [Google Scholar] [CrossRef]
- Bonventre, J.V. Calcium and Calcium-Related Signalling Pathways in Glomerular Mesangial Cells. Clin. Exp. Pharmacol. Physiol. 1996, 23, 65–70. [Google Scholar] [CrossRef]
- Dong, Z.; Saikumar, P.; Weinberg, J.M.; Venkatachalam, M.A. Calcium in Cell Injury and Death. Annu. Rev. Pathol. 2006, 1, 405–434. [Google Scholar] [CrossRef]
- Saleh, H.; Schlatter, E.; Lang, D.; Pauels, H.-G.; Heidenreich, S. Regulation of Mesangial Cell Apoptosis and Proliferation by Intracellular Ca2+ Signals. Kidney Int. 2000, 58, 1876–1884. [Google Scholar] [CrossRef] [PubMed]
- Maruri, D.P.; Iyer, K.S.; Schmidtke, D.W.; Petroll, W.M.; Varner, V.D. Signaling Downstream of Focal Adhesions Regulates Stiffness-Dependent Differences in the TGF-Β1-Mediated Myofibroblast Differentiation of Corneal Keratocytes. Front. Cell Dev. Biol. 2022, 10, 886759. [Google Scholar] [CrossRef]
- Stephenson, L.A.; Haney, L.B.; Hussaini, I.M.; Karns, L.R.; Glass, W.F. Regulation of Smooth Muscle Alpha-Actin Expression and Hypertrophy in Cultured Mesangial Cells. Kidney Int. 1998, 54, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Medina-Pizaño, M.Y.; Llanos-Angulo, L.A.G.; Ventura-Juárez, J.; Quintanar-Stephano, A.; Saucedo-Cárdenas, O.; de Oca-Luna, R.M.; de Jesús Loera-Arias, M.; Muñoz-Ortega, M.H. Tamsulosin Regulates Antifibrotic Effects in Thioacetamide-Induced Liver Damage and Ameliorates Portal Hypertension. Life Sci. 2025, 379, 123859. [Google Scholar] [CrossRef] [PubMed]
- Alabdali, H.H.; Ibrahim, M.A. Study the Anti-Inflammatory Effects of Tamsulosin by the Evaluation of Inflammatory Cells and Lung Histopathology in an Airway Inflammation Model in Rats. Bull. Pharm. Sci. Assiut Univ. 2023, 46, 633–645. [Google Scholar] [CrossRef]
- Zhou, X.; Li, C.; Dlugosz, J.; Kapor-Drezgic, J.; Munk, S.; Whiteside, C. Mesangial Cell Actin Disassembly in High Glucose Mediated by Protein Kinase C and the Polyol Pathway. Kidney Int. 1997, 51, 1797–1808. [Google Scholar] [CrossRef]
- Dubus, I.; L’Azou, B.; Gordien, M.; Delmas, Y.; Labouyrie, J.-P.; Bonnet, J.; Combe, C. Cytoskeletal Reorganization by Mycophenolic Acid Alters Mesangial Cell Migration and Contractility. Hypertension 2003, 42, 956–961. [Google Scholar] [CrossRef][Green Version]
- Wang, X.X.; Shen, E.; Wang, Y.Z.; Jiang, Z.Z.; Gui, D.K.; Cheng, D.S.; Chen, T.F.; Wang, N.S. MiR-196a Regulates High Glucose-Induced Mesangial Cell Hypertrophy by Targeting P27kip1. J. Lab. Autom. 2015, 20, 491–499. [Google Scholar] [CrossRef]
- Oh, J.H.; Ha, H.; Yu, M.R.; Lee, H.B. Sequential Effects of High Glucose on Mesangial Cell Transforming Growth Factor-Beta 1 and Fibronectin Synthesis. Kidney Int. 1998, 54, 1872–1878. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, L.I.O. NORMA Oficial Mexicana NOM-062-ZOO-1999, Especificaciones Tecnicas Para la Produccion, Cuidado y Uso de los Animales de Laboratorio. 2001. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 29 July 2025).
- National Research Council, Division on Earth; Institute for Laboratory Animal Research; Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011; ISBN 978-0-309-15400-0.
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef]
- Song, Y.; Liu, W.; Tang, K.; Zang, J.; Li, D.; Gao, H. Mangiferin Alleviates Renal Interstitial Fibrosis in Streptozotocin-Induced Diabetic Mice through Regulating the PTEN/PI3K/Akt Signaling Pathway. J. Diabetes Res. 2020, 2020, 9481720. [Google Scholar] [CrossRef]
- Korstanje, C.; Krauwinkel, W.; van Doesum-Wolters, F.L.C. Tamsulosin Shows a Higher Unbound Drug Fraction in Human Prostate than in Plasma: A Basis for Uroselectivity? Br. J. Clin. Pharmacol. 2011, 72, 218–225. [Google Scholar] [CrossRef]
- Medina-Pizaño, M.Y.; Medina-Rosales, M.N.; Martínez-Hernández, S.L.; Aldaba-Muruato, L.R.; Macías-Pérez, J.R.; Sánchez-Alemán, E.; Ventura-Juárez, J.; Muñoz-Ortega, M.H. Protective Effect of Curcumin against Doxazosin- and Carvedilol-Induced Oxidative Stress in HepG2 Cells. Oxid. Med. Cell Longev. 2022, 2022, 6085515. [Google Scholar] [CrossRef]
- Rivera-Navarro, J.C.; Pizaño, M.Y.M.; Martínez-Hernández, S.L.; Ventura-Juárez, J.; Ibarra-Martínez, D.; Muñoz-Ortega, M.H. Effects of the Adrenergic Blockers Tamsulosin and Carvedilol on the Homeostasis of the Endoplasmic Reticulum of Liver Cells. WJPSR 2024, 3, 38–53. [Google Scholar]
- Zhao, Y.; Lucht, K.; Zuhayra, M.; Cascorbi, I.; Lützen, U.; Culman, J. P0180 Effect of Pioglitazone on Proliferation and Apoptosis in Human Uterine Leiomyosarcoma Cells. Eur. J. Cancer 2015, 51, e34. [Google Scholar] [CrossRef]
- van der Veen, J.N.; Lingrell, S.; Gao, X.; Quiroga, A.D.; Takawale, A.; Armstrong, E.A.; Yager, J.Y.; Kassiri, Z.; Lehner, R.; Vance, D.E.; et al. Pioglitazone Attenuates Hepatic Inflammation and Fibrosis in Phosphatidylethanolamine N-Methyltransferase-Deficient Mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2016, 310, G526–G538. [Google Scholar] [CrossRef] [PubMed]


| Genes | Sequences |
|---|---|
| IL-1β | Fw: 5′-TGCCACCTTTTGACAGTGATG-3′ |
| Rv: 5′-AAGGTCCACGGGAAAGACAC-3′ | |
| IL-17A | Fw: 5′-CCCCTTCACTTTCAGGGTCG-3′ |
| Rv: 5′-GGGGGTTTCTTAGGGGTCAG-3′ | |
| NRF2 | Fw: 5′-CTAAAGCACAGCCAGCACAT-3′ |
| Rv: 5′-TGTCTTTTGTGAATGGGGCTTT-3′ | |
| NF-κB | Fw: 5′-ACTGGAGTTGTACGGCAGTG-3′ |
| Rv: 5′-TGTAAAATGCATAAAACGGGGAAA-3′ | |
| Acta-2 | Fw: 5′-GTACCCAGGCATTGCTGACA-3′ |
| Rv: 5′-GAGGCGCTGATCCACAAAAC-3′ | |
| Col I | Fw: 5′-GGGGCAAGACAGTCATCGAA-3′ |
| Rv: 5′-GAGGGAACCAGATTGGGGTG-3′ | |
| TGF-β1 | Fw: 5′-ACTGGAGTTGTACGGCAGTG-3′ |
| Rv: 5′-GGGGCTGATCCCGTTGATTT-3′ | |
| ADRA1A | Fw: 5′-GGTCTGCTAGAGCCATCCTG-3′ |
| Rv: 5′-TGATCTTGGTGTCTGCAAGC-3′ | |
| ADRA1B | Fw: 5′-CGCCCACCAACTACTTCATT-3′ |
| Rv: 5′-AATGGAGATGGCACATAGGC-3′ | |
| ADRB1 | Fw: 5′-CGGCCTTTCGTGTGTTTAAT-3′ |
| Rv: 5′-CACACCAAACCTGAGCTGAA-3′ | |
| ADRB2 | Fw: 5′-TGGTTGGGCTACGTCAACTC-3′ |
| Rv: 5′-TCCGTTCTGCCGTTGCTATT-3′ | |
| ADRB3 | Fw: 5′-GACAGCCTCAAATGCATCCT-3′ |
| Rv: 5′-CCCAGTCCACACACCTTTCT-3′ | |
| GAPDH | Fw: 5′-AGTCTACTGGCGTCTTCACC-3′ |
| Rv: 5′-CCACGATGCCAAAGTTGTCA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilera-Martínez, S.L.; Muñoz-Ortega, M.H.; Martínez-Hernández, S.L.; Morones-Gamboa, J.C.; Ventura-Juárez, J. Modulation of Mesangial Cells by Tamsulosin and Pioglitazone Under Hyperglycemic Conditions: An In Vitro and In Vivo Study. Int. J. Mol. Sci. 2025, 26, 9277. https://doi.org/10.3390/ijms26199277
Aguilera-Martínez SL, Muñoz-Ortega MH, Martínez-Hernández SL, Morones-Gamboa JC, Ventura-Juárez J. Modulation of Mesangial Cells by Tamsulosin and Pioglitazone Under Hyperglycemic Conditions: An In Vitro and In Vivo Study. International Journal of Molecular Sciences. 2025; 26(19):9277. https://doi.org/10.3390/ijms26199277
Chicago/Turabian StyleAguilera-Martínez, Sandra Lizbeth, Martín Humberto Muñoz-Ortega, Sandra Luz Martínez-Hernández, Jorge Christopher Morones-Gamboa, and Javier Ventura-Juárez. 2025. "Modulation of Mesangial Cells by Tamsulosin and Pioglitazone Under Hyperglycemic Conditions: An In Vitro and In Vivo Study" International Journal of Molecular Sciences 26, no. 19: 9277. https://doi.org/10.3390/ijms26199277
APA StyleAguilera-Martínez, S. L., Muñoz-Ortega, M. H., Martínez-Hernández, S. L., Morones-Gamboa, J. C., & Ventura-Juárez, J. (2025). Modulation of Mesangial Cells by Tamsulosin and Pioglitazone Under Hyperglycemic Conditions: An In Vitro and In Vivo Study. International Journal of Molecular Sciences, 26(19), 9277. https://doi.org/10.3390/ijms26199277






