Abstract
Variants in COL4A3 and COL4A4 cause autosomal dominant and recessive Alport syndrome, yet data on their distribution and clinical expression in different populations remain limited. This study investigated genotype–phenotype correlations and the distribution of COL4A3/COL4A4 variants in a Lithuanian Alport syndrome cohort. A total of 221 individuals from Lithuania were analyzed for COL4A3 and COL4A4 variants using either next-generation sequencing or Sanger sequencing in order to assess variant distribution and associated clinical features. Only individuals with pathogenic, likely pathogenic, or uncertain significance variants were included. Fifty-two individuals (38 index cases) with pathogenic, likely pathogenic, or variants of uncertain significance were identified, as follows: forty-eight were heterozygous, four had autosomal recessive, and four had digenic Alport syndrome. COL4A3 variants were found in 9.5% (21/221) and COL4A4 in 17.6% (39/221). Among the 28 identified variants, 18 were novel. Glycine substitutions (n = 8) were the most frequent and associated with worse kidney outcomes and increased hearing abnormalities. Hematuria was diagnosed significantly earlier than proteinuria (p = 0.05). Most individuals with autosomal dominant Alport syndrome had normal kidney function (eGFR > 90 mL/min/1.73 m2), while those with autosomal recessive Alport syndrome had more severe disease. Kidney failure occurred in 2/4 (50%) autosomal recessive Alport syndrome and 2/48 (4%) autosomal dominant Alport syndrome cases. A significant inverse correlation was found between eGFR and age in proteinuric individuals (r = –0.737; p = 0.013). This study expands knowledge of Alport syndrome in the Lithuanian population and contributes novel variant data to the global Alport syndrome genetic database.
1. Introduction
Alport syndrome (AS) is an inherited kidney disorder characterized by microhematuria, progressive kidney dysfunction, and often sensorineural hearing loss and ocular abnormalities [1]. It is the second-most common genetic cause of kidney failure (KF), responsible for up to 2% of all KF cases [2,3]. AS is caused by pathogenic variants in COL4A3, COL4A4, or COL4A5 (COL4A3–A5) genes, which encode the α3, α4, and α5 chains of type IV collagen—a key component of the glomerular, cochlear, and ocular basement membranes [4,5,6]. Disruption in these chains affects collagen synthesis, assembly, and function, leading to organ-specific damage [7].
AS may follow X-linked (COL4A5), autosomal recessive, or autosomal dominant (COL4A3/4) inheritance patterns [5,7,8]. Digenic inheritance involving two of the COL4A3–A5 genes has also been described [9]. While AS prevalence is estimated at 1 in 5000 to 1 in 53,000 individuals [10,11], newer studies suggest that X-linked AS (XLAS) may occur in at least 1 in 2000 and autosomal dominant AS (ADAS) in up to 1 in 100 individuals [12]. These findings highlight the likelihood of underdiagnosed or asymptomatic carriers of pathogenic variants [13].
Clinical severity varies depending on the gene, sex, and type of variant [7,14,15,16,17,18,19]. Comprehensive clinical assessment, including family history, persistent hematuria, extrarenal signs, and molecular analysis of COL4A3–A5, is essential for diagnosis [13]. In this study, we focused on core clinical features typically associated with COL4A3- and COL4A4-related AS to assess genotype–phenotype correlations. These included hematuria, proteinuria, estimated glomerular filtration rate (eGFR), hearing loss, and ocular abnormalities. These phenotypic characteristics were selected based on established diagnostic criteria and their relevance to disease progression and severity, as documented in previous literature.
Global data on AS remain limited, particularly regarding COL4A3 and COL4A4. This study aims to describe the genotype–phenotype relationship and the distribution of COL4A3 and COL4A4 variants in a Lithuanian cohort—marking the first such analysis in this population.
2. Results
2.1. General Genetic Findings
NGS and Sanger sequencing of the COL4A3, COL4A4, and COL4A5 genes in 221 individuals from 120 families identified 28 distinct COL4A3–A4 variants in 52 individuals from 38 families. Although COL4A5 variants were also detected, the analysis focused on COL4A3 and COL4A4. COL4A3 variants were identified in 32% (12/38) of unrelated families while COL4A4 variants in 50% (19/38). Among the 28 variants, 12 (9 novel and 3 known) were in COL4A3 and 16 (9 novel and 7 known) in COL4A4. A summary of the COL4A3 and COL4A4 genetic changes found in the Lithuanian cohort is shown in Table 1, Table 2 and Table S1. Novel variants have been described in detail [20].

Table 1.
Classification of 12 different COL4A3 genetic variants.

Table 2.
Classification of 16 different COL4A4 genetic variants.
In this study, three different types of genetic variants including missense 85% (24/28), frameshift 3.5% (1/28), and splice site 10.7% (3/28) variants in COL4A3–A4 genes were identified. Different missense variants including Gly substitutions (n = 16) and non-Gly substitutions (other amino acid change, n = 8) were the most frequent types of genetic changes in all COLA4A3-A4 genes and accounted for 90.3% of all individuals with AS. The amino acid changes in different Gly substitutions distributed as follows 50% arginine (Arg, R), 25% valine (Val, V), 18.75% glutamic acid (Glu, E), 12.5% serine (Ser, S). Some individuals carried multiple variants within or across these genes and were included due to their potential clinical significance.
2.2. Autosomal Dominant Alport Syndrome
In total, 48 (82.8%) individuals with one heterozygous COL4A3 or COL4A4 variant, thus ADAS, were identified and included 24 males. In ADAS group, the probands represented 54.2% (n = 26) of individuals, with the remaining 45.8% (n = 22) identified during family screening. Two males were incidentally diagnosed with AS through genetic testing during screening for autosomal dominant polycystic kidney disease, with no pathogenic variants found in the PKD1 or PKD2 genes. Among ADAS patients, 33.3% (n = 16) were found to be heterozygous for COL4A3 genetic variants, while 66.7% (n = 32) were heterozygous for COL4A4 genetic variants (Table 3 and Table S2).

Table 3.
General characteristics of 48 individuals with ADAS depending on affected gene.
The average age at diagnosis was 24 ± 18.7 for males and 37.7 ± 20.3 for females. In this research, no notable distinctions in kidney function, hearing abnormalities, ocular lesions, or other characteristics were identified across genders (p = 0.13) or different COL4A3 or COL4A4 genes (p = 0.56). Therefore, genotype–phenotype correlations of females and males with ADAS and different COL4A3 and COL4A4 genes were considered together in further analysis. KF was linked to one non-Gly substitution (c.4421T>C, p.Leu1474Pro) in the COL4A3 gene and one splice site variant (c.594+1G>A) in COL4A4 (Table 4 and Table S3).

Table 4.
Genotype–phenotype correlations in individuals with ADAS.
All 48 ADAS heterozygotes experienced hematuria, whereas proteinuria was detected in 26 individuals. However, only one patient developed nephrotic-range proteinuria and chronic kidney disease (CKD) stage 2. Among the individuals with ADAS and proteinuria, more than half maintained normal kidney function. In contrast, only two individuals developed KF at the ages of 55 and 44. A significant association was identified between lower eGFR levels and older age in individuals with proteinuria (n = 25; r = −0.737; p = 0.013), while no significant correlation was observed in individuals without proteinuria (n = 23; r = −0.321, p = 0.135) (Figure 1).
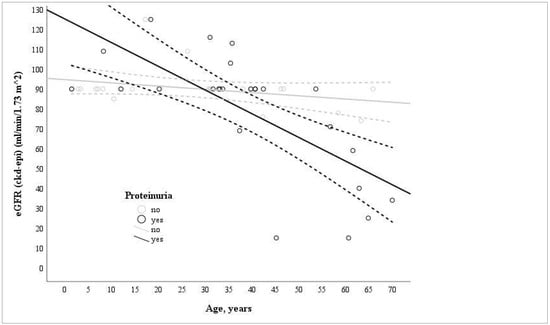
Figure 1.
Correlation of age and eGFR levels in proteinuric (n = 25; r = −0.737; p = 0.013) and non-proteinuric (n = 23; r = −0.321, p = 0.135) groups of individuals with ADAS at single time points.
Similar eGFR rate levels were observed among individuals with different types of genetic variants in COL4A3–A4 genes, and there were no significant differences detected between the groups.
Kidney biopsy was performed in 21 individuals with ADAS, with the mean age being 43.0 ± 15.1. Thin basement membrane (n = 19) and foot process effacement (n = 19) were the most common characteristics described in electron microscopy (EM). Lamellation or thickening of the glomerular basement membrane (GBM) was observed in 28.6% (6/21) of individuals at a mean age of 49 ± 17.4 years and was associated with higher eGFR values compared to those with focal segmental glomerulosclerosis (FSGS) (eGFR 85.5 ± 38.8 mL/min/1.73 m2, n = 6 vs. 58.6 ± 31.4 mL/min/1.73 m2, n = 8). The general findings from kidney biopsy in individuals with ADAS are shown in Table S4.
Eight (16.7%) individuals with ADAS exhibited bilateral sensorineural hearing abnormalities, which were detected at a mean age of 46.5 ± 11.8 years. Eight (16.7%) individuals with ADAS exhibited ocular lesions specific to AS, which were detected at a mean age of 16.6 ± 17.3 years. The diagnosis of ocular abnormalities was higher in individuals with ADAS who were older than 6.6 years (p = 0.02, 95% CI [1.6–216.1]).
2.3. Autosomal Recessive Alport Syndrome
In total, four (3.5%) individuals from three different families with ARAS were identified in the study, including one male. ARAS was genetically confirmed in three probands, which accounted for 75% of the cases, and one of their relatives, constituting 25% of the cases. Three individuals were diagnosed based on clinical features alone or with a kidney biopsy, while one female was referred for genetic analysis due to her twin sister’s positive AS. The general characteristics and genotype–phenotype correlations of individuals with ARAS can be found in Tables S5 and S6. Three individuals were compound heterozygotes for genetic variants in COL4A3 (n = 2) or COL4A4 (n = 1), while one individual was homozygous for genetic variants in COL4A4. One variant was categorized as pathogenic, three as likely pathogenic, and four as VUS. Hematuria and proteinuria were observed in all four individuals, with two of them developing KF at the ages of 32 and 33, with homozygous variant p.Ala607Val in COL4A4 and compound heterozygous variants p.Pro1568Ser and p.Gly1083Arg in COL4A3, respectively. Three females with ARAS underwent kidney biopsy at a mean age of 30.0 ± 3.2. All three females exhibited proteinuria exceeding 1 g/L at the time of the KB, and two of them had abnormal kidney function (median eGFR was 50.0, with a minimum of 45 and a maximum of 55 mL/min/1.73 m2). The general findings of the KB are listed in Table S7. All four individuals exhibited sensorineural deafness, with the youngest being 7 years old. Hearing loss was detected in both patients with KF as well. Two individuals with pathogenic variants in the COL4A3 gene had ocular abnormalities at the ages of 7 and 32. One of these patients was diagnosed with KF at the age of 32, while the other maintained normal kidney function.
2.4. Digenic Alport Syndrome
Four (3.5%) unrelated individuals with suspected digenic AS were identified in the study, including three females. The general characteristics and genotype–phenotype correlations of individuals with digenic AS can be found in Tables S8 and S9. All three females had a gene combination of COL4A4 and COL4A5, while COL4A3 and COL4A4 genetic variants were identified in the male. All variants in COL4A3 and COL4A4 were classified as VUS, while changes in COL4A5 were classified as pathogenic (n = 1) or likely pathogenic (n = 2). Three missense alterations in COL4A4 were detected, and one of them had glycine substitutions. One splice site variant in COL4A4 and one missense variant in COL4A3 were found as well. Hematuria and proteinuria were detected in all four individuals, with three of them exhibiting normal kidney function (eGFR greater than 90 mL/min/1.73 m2). Only one female developed CKD stage 2 with her proteinuria reaching over 50 mg/mmol. Two females and one male underwent kidney biopsy at the mean ages of 25.5 ± 16.3 and 40, respectively. The general findings of kidney biopsy in individuals with digenic AS are shown in Table S10. Hearing loss and ocular abnormalities were detected only in one female, who also developed the 2nd stage of CKD.
3. Discussion
Identifying pathogenic variants in COL4A3 and COL4A4 is critical for diagnosing and managing AS. Although over 5000 variants have been reported [21], many remain undiscovered [22]. In our cohort of 221 individuals from 120 families, we identified 28 different COL4A3–A4 variants. Sixty-four percent of variants in COL4A3–A4 genes were novel [20], while others (36%) were previously described and found globally, originating from Central/East Europe around 750–900 years ago [23]. These findings broaden knowledge on AS in Lithuania and raise the estimated prevalence to at least 1 in 106 individuals, though actual rates are likely higher due to undetected variants [12].
It is important to emphasize that our analysis represented a cohort-specific interpretation and did not involve formal variant reclassification. In several cases, individuals carrying variants classified as VUS exhibited clinical features consistent with AS. This may suggest a potentially pathogenic role, possibly influenced by epigenetic factors or other genetic modifiers.
ADAS cases were mainly identified through family screening (71%), while ARAS diagnoses followed abnormal kidney biopsies (40.7%). This reflects ARAS’s more severe, early-onset phenotype, often requiring invasive diagnostics, while ADAS typically presents milder, later-onset symptoms. These results highlight the importance of family-based screening for early detection and renoprotective therapy initiation [24,25,26,27].
Clinical manifestations of AS are genotype-dependent [28]. Hematuria consistently appeared before proteinuria in all AS types. KF occurred in 50% of ARAS and 4% of ADAS cases, aligning with ARAS’s known severity. Proteinuria and male sex were risk factors for CKD progression, in line with findings in broader CKD populations [29,30,31,32]. Our data support using proteinuria as a key prognostic marker and rationale for early intervention [13,33]. In our cohort, the inverse correlation between estimated glomerular filtration rate and age among individuals with proteinuria suggests progressive kidney function decline, highlighting the prognostic value of proteinuria and the importance of initiating early therapeutic interventions to delay disease progression.
ADAS displays wide phenotypic variability. Only 2 of 48 individuals developed KF, both with additional risk factors (e.g., delayed diagnosis, FSGS, and severe proteinuria). One notable case involved a hypomorphic COL4A3 variant (p.Leu1474Pro) in a male who progressed to KF earlier than a female with a pathogenic COL4A4 splice-site variant, despite the literature suggesting non-missense variants typically result in more severe outcomes [34]. This case suggests that compound or undetected variants may modulate disease severity.
Extrarenal manifestations were observed in 17% of ADAS cases (ocular or auditory), consistent with previous studies [33,35]. Although less frequent than in ARAS, these findings support the heterogeneity of ADAS and the potential presence of additional undiagnosed variants or digenic inheritance in more severe cases.
Data on ARAS genotype–phenotype correlations remain limited [36,37,38]. In our study, two women with ARAS developed KF in their early 30s. One pair of dizygotic twins with identical compound heterozygous COL4A3 variants showed differing severity, suggesting environmental or epigenetic modifiers. One ARAS case with a homozygous COL4A4 variant arose from a consanguineous family, underscoring the impact of genetic background on disease risk. Another boy with compound heterozygous COL4A4 variants showed early-onset hematuria and proteinuria but preserved kidney function at age 12, further illustrating variability. All ARAS individuals had hearing loss, and half had ocular abnormalities, consistent with severe phenotypes similar to males with XLAS [21].
Our findings reinforce that ARAS and digenic AS are frequently underdiagnosed due to lack of family history and overlapping phenotypes. Both genders with ARAS show comparable risk of KF and extrarenal symptoms.
Limitations of this study include its single-center design, a small sample size for kidney failure (KF) cases, limited eGFR data, and potential bias due to missing data, inclusion of young children, and related individuals. Proteinuria and eGFR were measured at a single time point without longitudinal follow-up, limiting our ability to assess disease progression over time. Interpretations of genetic variants are specific to our study population and should not be generalized. Additionally, the absence of functional validation and the potential influence of epigenetic or other unidentified factors limit the broader applicability of our conclusions.
4. Materials and Methods
4.1. Study Design and Participants
A cross-sectional study between 2016 and 2022 was performed. Two hundred and twenty-one individuals (aged 2 months to 82 years) with suspected AS [13] or at-risk relatives were assessed by nephrologists at Vilnius University Hospital Santaros Klinikos (VUH SK), Vilnius, Lithuania, and provided blood samples for DNA analysis. Clinical, demographic, and family history data were collected at the initial visit. The study was approved by the Vilnius Regional Biomedical Research Ethics Committee (BioAlport, No. 158200-16-857-367).
4.2. Genetic Analysis
Genetic testing was conducted using next-generation sequencing (NGS) or Sanger sequencing. The genetic methodology is described in detail in our previous publication [20] and in the article available at https://www.mdpi.com/1648-9144/61/4/597 (accessed on 27 July 2025). Most samples (85%) were analyzed at Centogene (Rostock, Germany) and the rest at the Centre for Medical Genetics, VUH SK (Vilnius, Lithuania). We examined variants with a minor allele frequency <1% and known pathogenic mutations in COL4A3, COL4A4, and COL4A5. Individuals with pathogenic (P), likely pathogenic (LP), or VUS in COL4A3 or COL4A4 were included. While VUS do not confirm AS, they were retained due to clinical relevance and reassessed based on available clinical and genetic data.
4.3. Study-Based Interpretation of Pathogenicity of Genetic Variants
Variant classification followed ACMG guidelines, using databases (ClinVar, InterVar, Franklin, Centogene) and in silico tools (PPT, SIFT, MutationTaster, Align-GVGD). Genetic variant interpretation in this study was based solely on clinical evaluation, disease course, biopsy results, sex, age, and familial history observed in our patient cohort. We excluded other kidney pathologies and co-morbidities to support a more accurate phenotype–genotype correlation. VUS were upgraded to pathogenic or likely pathogenic if accompanied by features such as nephrotic-range proteinuria, reduced eGFR (<60 mL/min/1.73 m2), CKD stages III–V, extrarenal signs (hearing/ocular), or affected relatives with CKD/KF. The absence of Alport-specific features in multiple carriers and the presence of only isolated hematuria with mild albuminuria in a single individual without family history of KF or progression supported interpretation of the variant from VUS or pathogenic to likely benign or to remain a VUS.
4.4. Clinical Evaluation
Participants with a genetic diagnosis of AS underwent follow-up evaluation by nephrologists, including assessment of hematuria (spot urine), proteinuria (spot urinary protein-to-creatinine ratio [uPr/uCr, PCR] and albumin-to-creatinine ratio [uA/Cr, ACR] in mg/mmol, per KDIGO 2020/2021 guidelines), and CKD staging using eGFR (CKD-EPI for adults, Schwartz 2009 for children). GFR and proteinuria values were evaluated at a single time point. Arterial hypertension was defined as blood pressure > 130/90 mmHg in adults (2017 ACC/AHA guideline) or ≥95th percentile for age, sex, and height in children (2016 European Society of Hypertension guidelines in children and adolescents). Individuals were referred for ophthalmologic evaluation [19]. AS-related ophthalmopathy was considered present if one or more of the specified criteria were met. Audiometric testing was performed in a soundproof booth (Interacoustics AC40), using air and bone conduction thresholds across frequencies of 125–8000 Hz. As early ocular and hearing abnormalities are often under-recognized, we recorded the age at which these abnormalities were first diagnosed.
4.5. Statistical Analysis
Data were analyzed using SPSS 27.0. Continuous variables were presented as the mean ± standard deviation or median with interquartile range, depending on the distribution. Categorical data are expressed as frequencies and percentages. Appropriate statistical tests, including ANOVA, Kruskal–Wallis, chi-square, Fisher’s exact test, and Mann–Whitney, were employed to compare variables. A significance level of p ≤ 0.05 was considered statistically significant. Due to the limited number of individuals with kidney failure, kidney survival analysis using Kaplan–Meier tests was not feasible. It is important to acknowledge that a direct comparison between ARAS and ADAS was not feasible due to the smaller cohort sizes of ARAS compared to ADAS.
4.6. Data Availability
The original findings presented in the study have been made publicly accessible and are located in the ClinVar Database, specifically under the accession SUB11112191 (SCV002098105–SCV002098129). However, it should be noted that the authors acknowledge a partial unavailability of raw data within this study. This is primarily due to the Centogene laboratory’s imposed time restriction, wherein variants and their corresponding raw data older than 5 years are not stored. The limited accessibility of data represents a potential significant constraint within the study.
5. Conclusions
In summary, 12 different COL4A3 and 16 different COL4A4 genetic variants were identified by NGS or Sanger method testing in 221 individuals from 120 families with suspected AS in a single-center Lithuanian cohort. The majority of the affected individuals had ADAS (82.8%) but ARAS (3.5%) and digenic disease (3.5%) were also identified. Despite the genetic variant, individuals with ADAS and digenic AS mostly retained normal kidney function, while individuals with ARAS developed more severe kidney disease. Ocular abnormalities and hearing loss were more severe and more frequent in individuals with ARAS compared to ADAS as well. However, Alport syndrome caused by COL4A3 and COL4A4 changes should always be regarded as potentially serious. Overall, it is the first such research of AS in Lithuania, and the results of this study expand the number of identified COL4A3 and COL4A4 variants. It increases our understanding of genotype–phenotype correlations in AS, which play a crucial role in diagnosing and prognosticating for individuals with AS.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26157639/s1.
Author Contributions
Conceptualization, A.C.-K. and R.C.; methodology, A.C.-K.; formal analysis, A.C.-K.; investigation, A.C.-K., R.C., D.B., V.V., R.S.-S., A.M., V.M., G.S., M.M. and A.L.; data curation, A.C.-K.; writing—original draft preparation, A.C.-K. and M.N.; writing—review and editing, R.C., J.S., K.A., G.M. and D.B.; supervision, R.C., J.S. and A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study received approval from the Vilnius Regional Biomedical Research Ethics Committee (protocol code no. 158200-16-857-367; date of approval: 12 July 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The online version of this article contains Supplementary Materials. Any additional information is available from the authors upon request.
Acknowledgments
The authors are very thankful to the study participants for their contribution.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACMG | The American College of Medical Genetics and Genomics |
| ADAS | autosomal dominant Alport syndrome |
| AMP | Association for Molecular Pathology |
| ARAS | autosomal recessive Alport syndrome |
| AS | Alport syndrome |
| CKD | chronic kidney disease |
| CKD-EPI | Chronic Kidney Disease Epidemiology Collaboration |
| eGFR | estimated glomerular filtration rate |
| FSGS | focal segmental glomerulosclerosis |
| KB | kidney biopsy |
| KF | kidney failure |
| NGS | next-generation sequencing |
| VUS | variant of uncertain significance |
| XLAS | X-linked Alport syndrome |
References
- Kashtan, C.E.; Michael, A.F. Alport Syndrome. Kidney Int. 1996, 50, 1445–1463. [Google Scholar] [CrossRef]
- Gretz, N.; Broyer, M.; Brunner, F.P.; Brynger, H.; Donckerwolcke, R.A.; Jacobs, C.; Kramer, P.; Selwood, N.H.; Wing, A.J. Alport’s syndrome as a cause of renal failure in Europe. Pediatr. Nephrol. 1987, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Wing, A.J.; Brunner, F.P. Twenty-Three Years of Dialysis and Transplantation in Europe: Experiences of the EDTA Registry. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 1989, 14, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Kashtan, C.E. Alport Syndrome. An Inherited Disorder of Renal, Ocular, and Cochlear Basement Membranes. Medicine 1999, 78, 338–360. [Google Scholar] [CrossRef]
- Rheault, M.N.; Kashtan, C.E. Inherited Glomerular Diseases. In Pediatric Nephrology, 7th ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 777–803. ISBN 978-3-662-43595-3. [Google Scholar]
- Suh, J.H.; Miner, J.H. The Glomerular Basement Membrane as a Barrier to Albumin. Nat. Rev. Nephrol. 2013, 9, 470–477. [Google Scholar] [CrossRef]
- Kashtan, C.E.; Ding, J.; Garosi, G.; Heidet, L.; Massella, L.; Nakanishi, K.; Nozu, K.; Renieri, A.; Rheault, M.; Wang, F.; et al. Alport Syndrome: A Unified Classification of Genetic Disorders of Collagen IV A345: A Position Paper of the Alport Syndrome Classification Working Group. Kidney Int. 2018, 93, 1045–1051. [Google Scholar] [CrossRef]
- Fallerini, C.; Dosa, L.; Tita, R.; Del Prete, D.; Feriozzi, S.; Gai, G.; Clementi, M.; La Manna, A.; Miglietti, N.; Mancini, R.; et al. Unbiased next Generation Sequencing Analysis Confirms the Existence of Autosomal Dominant Alport Syndrome in a Relevant Fraction of Cases. Clin. Genet. 2014, 86, 252–257. [Google Scholar] [CrossRef]
- Mencarelli, M.A.; Heidet, L.; Storey, H.; van Geel, M.; Knebelmann, B.; Fallerini, C.; Miglietti, N.; Antonucci, M.F.; Cetta, F.; Sayer, J.A.; et al. Evidence of digenic inheritance in Alport syndrome. J. Med. Genet. 2015, 52, 163–174. [Google Scholar] [CrossRef]
- Habib, R.; Gubler, M.C.; Hinglais, N.; Noël, L.H.; Droz, D.; Levy, M.; Mahieu, P.; Foidart, J.M.; Perrin, D.; Bois, E.; et al. Alport’s Syndrome: Experience at Hôpital Necker. Kidney Int. Suppl. 1982, 11, S20–S28. [Google Scholar]
- Gubler, M.; Levy, M.; Broyer, M.; Naizot, C.; Gonzales, G.; Perrin, D.; Habib, R. Alport’s Syndrome. A Report of 58 Cases and a Review of the Literature. Am. J. Med. 1981, 70, 493–505. [Google Scholar] [CrossRef]
- Gibson, J.; Fieldhouse, R.; Chan, M.M.Y.; Sadeghi-Alavijeh, O.; Burnett, L.; Izzi, V.; Persikov, A.V.; Gale, D.P.; Storey, H.; Savige, J.; et al. Prevalence Estimates of Predicted Pathogenic COL4A3-COL4A5 Variants in a Population Sequencing Database and Their Implications for Alport Syndrome. J. Am. Soc. Nephrol. 2021, 32, 2273–2290. [Google Scholar] [CrossRef] [PubMed]
- Torra, R.; Lipska-Zietkiewicz, B.; Acke, F.; Antignac, C.; Becker, J.U.; Cornec-Le Gall, E.; van Eerde, A.M.; Feltgen, N.; Ferrari, R.; Gale, D.P.; et al. Diagnosis, management and treatment of the Alport syndrome—2024 guideline on behalf of ERKNet, ERA and ESPN. Nephrol. Dial. Transplant. 2025, 40, 1091–1106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Storey, H.; Savige, J.; Sivakumar, V.; Abbs, S.; Flinter, F.A. COL4A3/COL4A4 Mutations and Features in Individuals with Autosomal Recessive Alport Syndrome. J. Am. Soc. Nephrol. 2013, 24, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Jais, J.P.; Knebelmann, B.; Giatras, I.; De Marchi, M.; Rizzoni, G.; Renieri, A.; Weber, M.; Gross, O.; Netzer, K.-O.; Flinter, F.; et al. X-Linked Alport Syndrome: Natural History and Genotype-Phenotype Correlations in Girls and Women Belonging to 195 Families: A “European Community Alport Syndrome Concerted Action” Study. J. Am. Soc. Nephrol. 2003, 14, 2603–2610. [Google Scholar] [CrossRef]
- Jais, J.P.; Knebelmann, B.; Giatras, I.; Marchi, M.D.; Rizzoni, G.; Renieri, A.; Weber, M.; Gross, O.; Netzer, K.-O.; Flinter, F.; et al. X-Linked Alport Syndrome: Natural History in 195 Families and Genotype- Phenotype Correlations in Males. J. Am. Soc. Nephrol. 2000, 11, 649–657. [Google Scholar] [CrossRef]
- Gross, O.; Netzer, K.-O.; Lambrecht, R.; Seibold, S.; Weber, M. Meta-Analysis of Genotype-Phenotype Correlation in X-Linked Alport Syndrome: Impact on Clinical Counselling. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2002, 17, 1218–1227. [Google Scholar] [CrossRef]
- Sabljar-Matovinoviæ, M.; Radiæ-Krišto, D.; Putarek, K.; Šèukanec-Špoljar, M.; Moroviæ-Verglas, J.; Galešiæ, K. Alport’s Syndrome in Adult Age. Lijeè Vjesn 1996, 118, 11–13. [Google Scholar]
- Savige, J.; Sheth, S.; Leys, A.; Nicholson, A.; Mack, H.G.; Colville, D. Ocular Features in Alport Syndrome: Pathogenesis and Clinical Significance. Clin. J. Am. Soc. Nephrol. 2015, 10, 703–709. [Google Scholar] [CrossRef]
- Cerkauskaite, A.; Savige, J.; Janonyte, K.; Jeremiciute, I.; Miglinas, M.; Kazenaite, E.; Laurinavicius, A.; Strupaite-Sileikiene, R.; Vainutiene, V.; Burnyte, B.; et al. Identification of 27 Novel Variants in Genes COL4A3, COL4A4, and COL4A5 in Lithuanian Families with Alport Syndrome. Front. Med. 2022, 9, 859521. [Google Scholar] [CrossRef]
- Savige, J.; Huang, M.; Croos Dabrera, M.S.; Shukla, K.; Gibson, J. Genotype-Phenotype Correlations for Pathogenic COL4A3-COL4A5 Variants in X-Linked, Autosomal Recessive, and Autosomal Dominant Alport Syndrome. Front. Med. 2022, 9, 865034. [Google Scholar] [CrossRef]
- Weber, S.; Strasser, K.; Rath, S.; Kittke, A.; Beicht, S.; Alberer, M.; Lange-Sperandio, B.; Hoyer, P.F.; Benz, M.R.; Ponsel, S.; et al. Identification of 47 Novel Mutations in Patients with Alport Syndrome and Thin Basement Membrane Nephropathy. Pediatr. Nephrol. Berl. Ger. 2016, 31, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Żurowska, A.M.; Bielska, O.; Daca-Roszak, P.; Jankowski, M.; Szczepańska, M.; Roszkowska-Bjanid, D.; Kuźma-Mroczkowska, E.; Pańczyk-Tomaszewska, M.; Moczulska, A.; Drożdż, D.; et al. Mild X-Linked Alport Syndrome Due to the COL4A5 G624D Variant Originating in the Middle Ages Is Predominant in Central/East Europe and Causes Kidney Failure in Midlife. Kidney Int. 2021, 99, 1451–1458. [Google Scholar] [CrossRef]
- Kashtan, C.E.; Segal, Y.; Flinter, F.; Makanjuola, D.; Gan, J.-S.; Watnick, T. Aortic Abnormalities in Males with Alport Syndrome. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2010, 25, 3554–3560. [Google Scholar] [CrossRef]
- Savige, J.; Gregory, M.; Gross, O.; Kashtan, C.; Ding, J.; Flinter, F. Expert Guidelines for the Management of Alport Syndrome and Thin Basement Membrane Nephropathy. J. Am. Soc. Nephrol. 2013, 24, 364–375. [Google Scholar] [CrossRef]
- Kashtan, C. Alport Syndrome: Facts and Opinions. F1000Research 2017, 6, 50. [Google Scholar] [CrossRef]
- Temme, J.; Peters, F.; Lange, K.; Pirson, Y.; Heidet, L.; Torra, R.; Grunfeld, J.-P.; Weber, M.; Licht, C.; Müller, G.-A.; et al. Incidence of Renal Failure and Nephroprotection by RAAS Inhibition in Heterozygous Carriers of X-Chromosomal and Autosomal Recessive Alport Mutations. Kidney Int. 2012, 81, 779–783. [Google Scholar] [CrossRef]
- Groopman, E.E.; Marasa, M.; Cameron-Christie, S.; Petrovski, S.; Aggarwal, V.S.; Milo-Rasouly, H.; Li, Y.; Zhang, J.; Nestor, J.; Krithivasan, P.; et al. Diagnostic Utility of Exome Sequencing for Kidney Disease. N. Engl. J. Med. 2019, 380, 142–151. [Google Scholar] [CrossRef]
- Pierides, A.; Voskarides, K.; Athanasiou, I.; Ioannou, K.; Damianou, L.; Arsali, M.; Zavros, M.; Pierides, M.; Vargemezis, V.; Patsias, C.; et al. Clinico-Pathological Correlations in 127 Patients in 11 Large Pedigrees, Segregating One of Three Heterozygous Mutations in the COL4A3/COL4A4 Genes Associated with Familial Haematuria and Significant Late Progression to Proteinuria and Chronic Kidney Disease from Focal Segmental Glomerulosclerosis. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2009, 24, 2721–2729. [Google Scholar] [CrossRef]
- Voskarides, K.; Damianou, L.; Neocleous, V.; Zouvani, I.; Christodoulidou, S.; Hadjiconstantinou, V.; Ioannou, K.; Athanasiou, Y.; Patsias, C.; Alexopoulos, E.; et al. COL4A3/COL4A4 Mutations Producing Focal Segmental Glomerulosclerosis and Renal Failure in Thin Basement Membrane Nephropathy. J. Am. Soc. Nephrol. 2007, 18, 3004–3016. [Google Scholar] [CrossRef]
- Torra, R.; Furlano, M. New Therapeutic Options for Alport Syndrome. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2019, 34, 1272–1279. [Google Scholar] [CrossRef]
- Nozu, K.; Nakanishi, K.; Abe, Y.; Udagawa, T.; Okada, S.; Okamoto, T.; Kaito, H.; Kanemoto, K.; Kobayashi, A.; Tanaka, E.; et al. A Review of Clinical Characteristics and Genetic Backgrounds in Alport Syndrome. Clin. Exp. Nephrol. 2019, 23, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Furlano, M.; Martínez, V.; Pybus, M.; Arce, Y.; Crespí, J.; Venegas, M.D.P.; Bullich, G.; Domingo, A.; Ayasreh, N.; Benito, S.; et al. Clinical and Genetic Features of Autosomal Dominant Alport Syndrome: A Cohort Study. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2021, 78, 560–570.e1. [Google Scholar] [CrossRef] [PubMed]
- Matthaiou, A.; Poulli, T.; Deltas, C. Prevalence of Clinical, Pathological and Molecular Features of Glomerular Basement Membrane Nephropathy Caused by COL4A3 or COL4A4 Mutations: A Systematic Review. Clin. Kidney J. 2020, 13, 1025–1036. [Google Scholar] [CrossRef]
- Zhang, Y.; Böckhaus, J.; Wang, F.; Wang, S.; Rubel, D.; Gross, O.; Ding, J. Genotype-Phenotype Correlations and Nephroprotective Effects of RAAS Inhibition in Patients with Autosomal Recessive Alport Syndrome. Pediatr. Nephrol. Berl. Ger. 2021, 36, 2719–2730. [Google Scholar] [CrossRef]
- Oka, M.; Nozu, K.; Kaito, H.; Fu, X.J.; Nakanishi, K.; Hashimura, Y.; Morisada, N.; Yan, K.; Matsuo, M.; Yoshikawa, N.; et al. Natural History of Genetically Proven Autosomal Recessive Alport Syndrome. Pediatr. Nephrol. Berl. Ger. 2014, 29, 1535–1544. [Google Scholar] [CrossRef]
- Savige, J.; Rana, K.; Tonna, S.; Buzza, M.; Dagher, H.; Wang, Y.Y. Thin Basement Membrane Nephropathy. Kidney Int. 2003, 64, 1169–1178. [Google Scholar] [CrossRef]
- Mochizuki, T.; Lemmink, H.H.; Mariyama, M.; Antignac, C.; Gubler, M.C.; Pirson, Y.; Verellen-Dumoulin, C.; Chan, B.; Schröder, C.H.; Smeets, H.J. Identification of Mutations in the Alpha 3(IV) and Alpha 4(IV) Collagen Genes in Autosomal Recessive Alport Syndrome. Nat. Genet. 1994, 8, 77–81. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).