OxyVita®C Hemoglobin-Based Oxygen Carrier Improves Viability and Reduces Tubular Necrosis in Ex Vivo Preserved Rabbit Kidneys
Abstract
1. Introduction
2. Results
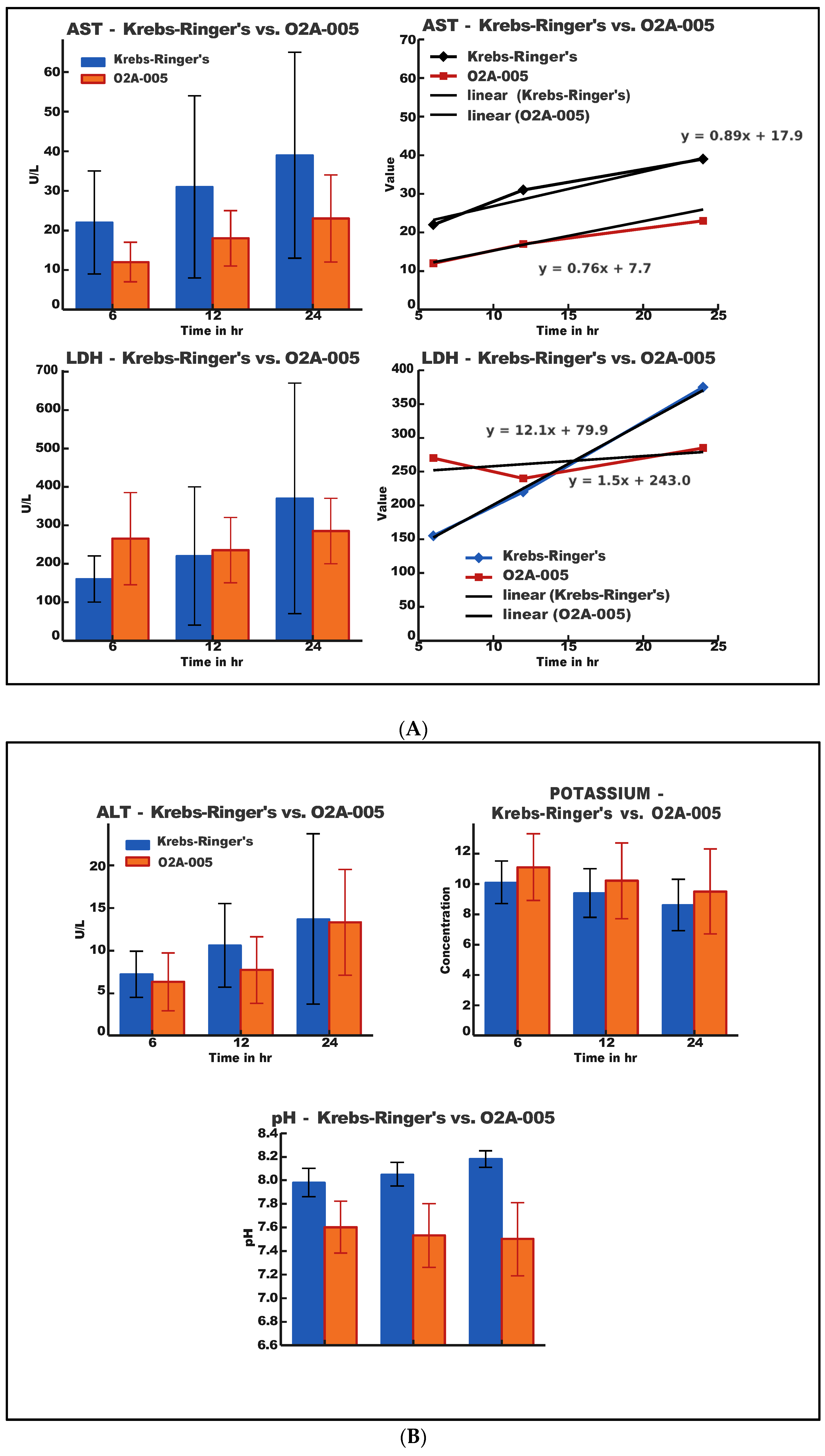
2.1. Biochemical Analysis
2.2. Histopathology
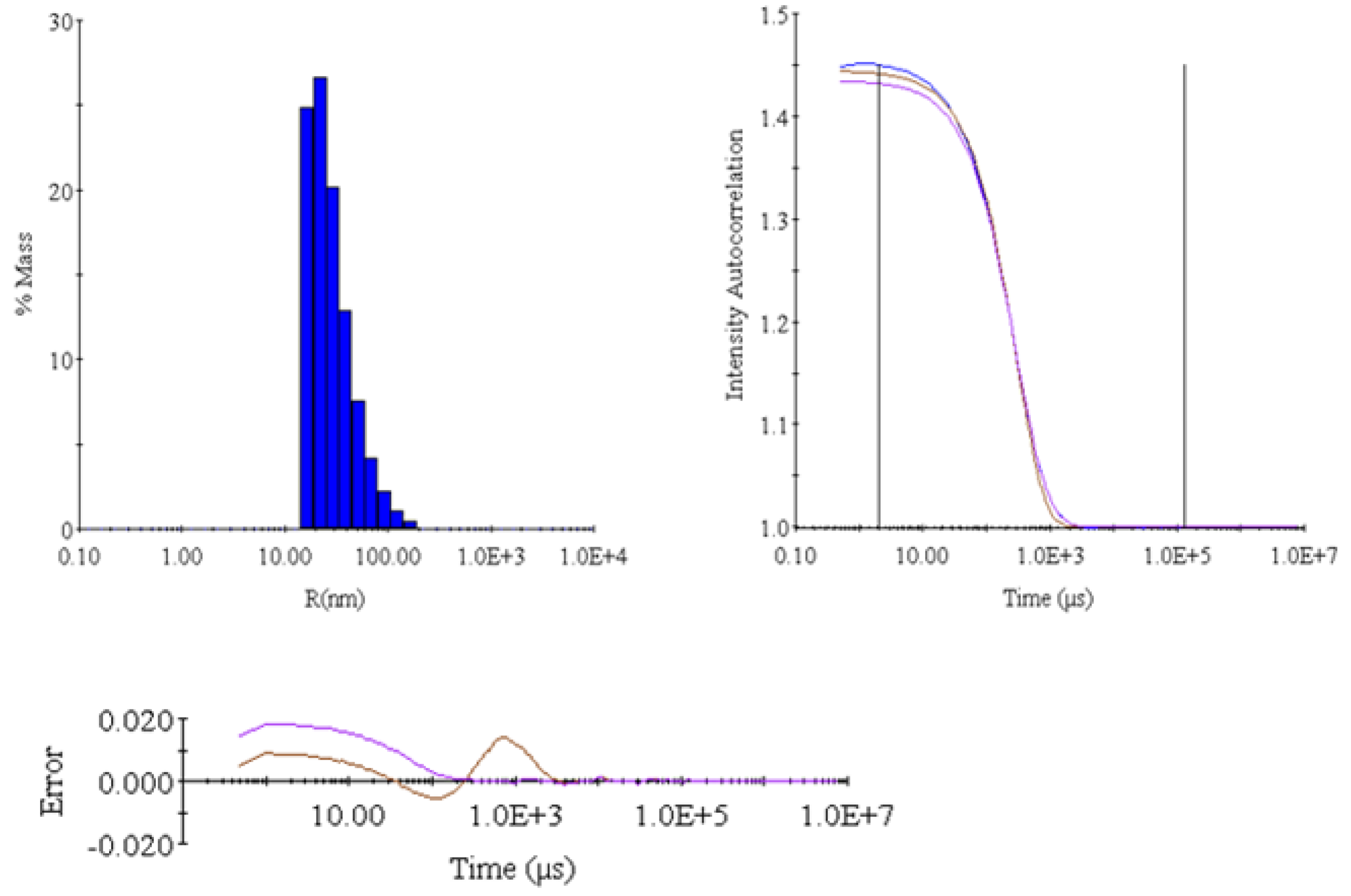
2.3. Dynamic Light Scattering (DLS) Analysis
| Item | Intensity (Cnt/s) | R (nm) | %Pd | MW (kDa) |
|---|---|---|---|---|
| Mean | 2,498,440 | 39.3658 | 99.5 | 17,569 |
| S | 31,056 | 0.596 | 0.687 | 312.5 |
| %S | 1.24 | 1.514 | 0.6905 | 1.7787 |
| S2 | 964,475,136 | 0.3552 | 0.4729 | 97,656 |
2.4. Summary of Key Findings
- O2A-005 solution slowed the accumulation of AST and LDH compared to Krebs-Ringer’s solution.
- ALT release was minimal in both groups but slightly lower in O2A-005.
- pH and potassium remained within physiological ranges; O2A-005 maintained slightly more stable values.
- Histopathology showed minimal tissue damage in O2A-005-stored kidneys, with RBC-derived capillary casts only in O2A-005.
- DLS confirmed polymer stability and uniformity in O2A-005.
- The presence of OxyVita®C in the storage solution created a more aerobic environment, preserving cellular integrity and reducing biochemical and histological markers of damage during ex vivo kidney storage.
3. Discussion
4. Materials and Methods
4.1. The O2A-005 Storage Solution
4.2. Methodology
4.3. Animal Husbandry
4.4. Surgical Procedure and Protocol Design
4.5. Experimental Design/Protocol Summary
4.6. Analytical Data
4.7. Analytical Methods
4.7.1. UV/Vis Method
4.7.2. Size Exclusion Chromatography (SEC)
4.7.3. Dynamic Light Scattering (DLS)
4.7.4. Osmotic Pressure
4.7.5. pH Determination
4.7.6. p50 Determination
4.7.7. LDH, AST, ALT Determination
4.7.8. Potassium Determination
4.8. Pathology
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| ATN | acute tubular necrosis |
| DLS | dynamic light scattering |
| HBOC | hemoglobin-based oxygen carrier |
| HC | histochemistry |
| IHC | immunohistochemistry |
| LDH | lactate dehydrogenase |
| RBC | red blood cell |
References
- Thongprayoon, C.; Kaewput, W.; Pattharanitima, P.; Cheungpasitporn, W. Progress and Recent Advances in Solid Organ Transplantation. J. Clin. Med. 2022, 11, 2112. [Google Scholar] [CrossRef]
- Beyar, R. Challenges in Organ Transplantation. Rambam Maimonides Med. J. 2011, 2, e0020. [Google Scholar] [CrossRef]
- Dugbartey, G.J. Cellular and Molecular Mechanisms of Cell Damage and Cell Death in Ischemia–Reperfusion Injury in Organ Transplantation. Mol. Biol. Rep. 2024, 51, 473–489. [Google Scholar] [CrossRef]
- Gourdin, M.; Dubois, P. Impact of Ischemia on Cellular Metabolism. Artery Bypass 2013, 54, 3–18. [Google Scholar]
- Patel, K.J.; Atkinson, C.; Broome, A.-M.; Nadig, S.N. Organ Preservation, Ischemia Reperfusion Injury, and Nanotherapeutics in Transplantation. In Technological Advances in Organ Transplantation; Nadig, S.N., Wertheim, J.A., Eds.; Springer International Publishing: Cham, Germany, 2017; pp. 151–179. ISBN 978-3-319-62141-8. [Google Scholar]
- Sicim, H.; Tam, W.S.V.; Tang, P.C. Primary Graft Dysfunction in Heart Transplantation: The Challenge to Survival. J. Cardiothorac. Surg. 2024, 19, 313. [Google Scholar] [CrossRef]
- Jing, L.; Yao, L.; Zhao, M.; Peng, L.; Liu, M. Organ Preservation: From the Past to the Future. Acta Pharmacol. Sin. 2018, 39, 845–857. [Google Scholar] [CrossRef]
- Ferng, A.S.; Schipper, D.; Connell, A.M.; Marsh, K.M.; Knapp, S.; Khalpey, Z. Novel vs. Clinical Organ Preservation Solutions: Improved Cardiac Mitochondrial Protection. J. Cardiothorac. Surg. 2017, 12, 7. [Google Scholar] [CrossRef]
- Fontes, P.A.; Light, W.R.; Van Der Plaats, A.; Cornett, E.M.; Kaye, A.D. Hemoglobin-Based Oxygen Carrier Solutions for Organ and Tissue Preservation and Transplantation. In Blood Substitutes and Oxygen Biotherapeutics; Liu, H., Kaye, A.D., Jahr, J.S., Eds.; Springer International Publishing: Cham, Germany, 2022; pp. 385–408. ISBN 978-3-030-95974-6. [Google Scholar]
- Brezonik, P.L.; Arnold, W.A. Dissolved Oxygen. In Water Chemistry: The Chemical Processes and Composition of Natural and Engineered Aquatic Systems; Brezonik, P.L., Arnold, W.A., Eds.; Oxford University Press: Oxford, UK, 2022; pp. 772–804. ISBN 978-0-19-760470-0. [Google Scholar]
- Cao, M.; Wang, G.; He, H.; Yue, R.; Zhao, Y.; Pan, L.; Huang, W.; Guo, Y.; Yin, T.; Ma, L. Hemoglobin-Based Oxygen Carriers: Potential Applications in Solid Organ Preservation. Front. Pharmacol. 2021, 12, 760215. [Google Scholar] [CrossRef]
- Riehmann, M.; Rhodes, P.R.; Ametani, M.S.; Sall, M.; Bruskewitz, R.C. Preservation of Bladder Tissue in Different Solutions for Varying Times. Urology 1997, 50, 142–149. [Google Scholar] [CrossRef]
- Kozlova, I.; Khalid, Y.; Roomans, G.M. Preservation of Mouse Liver Tissue during Cold Storage in Experimental Solutions Assessed by X-Ray Microanalysis. Liver Transplant. 2003, 9, 268–278. [Google Scholar] [CrossRef]
- Harrington, J.P.; Wollocko, J.; Kostecki, E.; Wollocko, H. Physicochemical Characteristics of OxyVita Hemoglobin, a Zero-Linked Polymer: Liquid and Powder Preparations. Artif. Cells Blood Substit. Biotechnol. 2011, 39, 12–18. [Google Scholar] [CrossRef]
- Wollocko, H.; Anvery, S.; Wollocko, J.; Harrington, J.M.; Harrington, J.P. Zero-Link Polymerized Hemoglobin (OxyVita® Hb) Stabilizes the Heme Environment: Potential for Lowering Vascular Oxidative Stress. Artif. Cells Nanomed. Biotechnol. 2017, 45, 701–709. [Google Scholar] [CrossRef]
- Forkasiewicz, A.; Dorociak, M.; Stach, K.; Szelachowski, P.; Tabola, R.; Augoff, K. The Usefulness of Lactate Dehydrogenase Measurements in Current Oncological Practice. Cell. Mol. Biol. Lett. 2020, 25, 35. [Google Scholar] [CrossRef]
- Harrington, J.P.; Wollocko, H. Zero-Link Hemoglobin (OxyVita®): Impact of Molecular Design Characteristics on Pre-Clinical Studies. In Hemoglobin-Based Oxygen Carriers as Red Cell Substitutes and Oxygen Therapeutics; Kim, H.W., Greenburg, A.G., Eds.; Springer: Berlin, Germany, 2013; pp. 283–297. ISBN 978-3-642-40716-1. [Google Scholar]
- Wollocko, H.; Harrington, J.; Jahr, J.; Steier, K.; Wollocko, J. OxyVita® Hb: A Step Forward in Delivering Oxygen Carrying Capacity for Therapeutic Applications. In Regenerative Medicine, Artificial Cells and Nanomedicine; World Scientific: Singapore, 2022; Volume 6, pp. 903–933. ISBN 9789811228681. [Google Scholar]
- Papagiannakis, N.; Ragias, D.; Ntalarizou, N.; Laou, E.; Kyriakaki, A.; Mavridis, T.; Vahedian-Azimi, A.; Sakellakis, M.; Chalkias, A. Transitions from Aerobic to Anaerobic Metabolism and Oxygen Debt during Elective Major and Emergency Non-Cardiac Surgery. Biomedicines 2024, 12, 1754. [Google Scholar] [CrossRef]
- Biro, G.P. Oxygen and ATP: The Energy Economy of the Cell. In Blood Substitutes and Oxygen Biotherapeutics; Liu, H., Kaye, A.D., Jahr, J.S., Eds.; Springer International Publishing: Cham, Germany, 2022; pp. 21–32. ISBN 978-3-030-95974-6. [Google Scholar]
- Da Poian, A.T.; Castanho, M.A.R.B. Energy Conservation in Metabolism: The Mechanisms of ATP Synthesis. In Integrative Human Biochemistry; Springer International Publishing: Cham, Germany, 2021; pp. 301–362. ISBN 978-3-030-48739-3. [Google Scholar]
- Pirahanchi, Y.; Jessu, R.; Aeddula, N.R. Physiology, Sodium Potassium Pump. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Azad, N.; Iyer, A.K.V. Reactive Oxygen Species and Apoptosis. In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer: Berlin, Germany, 2014; pp. 113–135. ISBN 978-3-642-30018-9. [Google Scholar]
- Krumova, K.; Cosa, G. Overview of Reactive Oxygen Species. In Singlet Oxygen: Applications in Biosciences and Nanosciences; Springer: Berlin, Germany, 2016; pp. 1–20. [Google Scholar]
- Farhana, A.; Lappin, S.L. Biochemistry, Lactate Dehydrogenase. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Yang, Y.; Wu, Y.; Sun, X.-D.; Zhang, Y. Reactive Oxygen Species, Glucose Metabolism, and Lipid Metabolism. In Oxidative Stress: Human Diseases and Medicine; Huang, C., Zhang, Y., Eds.; Springer: Singapore, 2021; pp. 213–235. ISBN 9789811605222. [Google Scholar]
- Wollocko, H.; Wollocko, J.; Jahr, J.S.; Steier, K. OxyVita: History, Studies, and Future. In Blood Substitutes and Oxygen Biotherapeutics; Liu, H., Kaye, A.D., Jahr, J.S., Eds.; Springer International Publishing: Cham, Germany, 2022; pp. 267–276. ISBN 978-3-030-95974-6. [Google Scholar]
- Solà-Porta, E.; Redondo-Pachón, D.; Arias-Cabrales, C.; Navazo, D.; Buxeda, A.; Burballa, C.; Crespo, M.; García-Retortillo, M.; Pascual, J.; Pérez-Sáez, M.J. Early Hypertransaminasemia after Kidney Transplantation: Significance and Evolution According to Donor Type. J. Clin. Med. 2021, 10, 5168. [Google Scholar] [CrossRef]
- Ochiai, H.; Shirasawa, T.; Yoshimoto, T.; Nagahama, S.; Watanabe, A.; Sakamoto, K.; Kokaze, A. Elevated Alanine Aminotransferase and Low Aspartate Aminotransferase/Alanine Aminotransferase Ratio Are Associated with Chronic Kidney Disease among Middle-Aged Women: A Cross-Sectional Study. BMC Nephrol. 2020, 21, 471. [Google Scholar] [CrossRef]
- Storjord, E.; Wahlin, S.; Karlsen, B.O.; Hardersen, R.I.; Dickey, A.K.; Ludviksen, J.K.; Brekke, O.-L. Potential Biomarkers for the Earlier Diagnosis of Kidney and Liver Damage in Acute Intermittent Porphyria. Life 2023, 14, 19. [Google Scholar] [CrossRef]
- Ozgur, O.S.; Namsrai, B.-E.; Pruett, T.L.; Bischof, J.C.; Toner, M.; Finger, E.B.; Uygun, K. Current Practice and Novel Approaches in Organ Preservation. Front. Transplant. 2023, 2, 1156845. [Google Scholar] [CrossRef]
- Kniepeiss, D.; Houben, P.; Stiegler, P.; Berghold, A.; Riedl, R.; Kahn, J.; Schemmer, P. A Prospective, Randomized, Single-Blind, Multicentre, Phase III Study on Organ Preservation with Custodiol-N Solution Compared with Custodiol® Solution in Organ Transplantation (Kidney, Liver and Pancreas). Trials 2020, 21, 62. [Google Scholar] [CrossRef]
- Hunter, R.W.; Bailey, M.A. Hyperkalemia: Pathophysiology, Risk Factors and Consequences. Nephrol. Dial. Transplant. 2019, 34, iii2–iii11. [Google Scholar] [CrossRef]
- Viera, A.J.; Wouk, N. Potassium Disorders: Hypokalemia and Hyperkalemia. Am. Fam. Physician 2015, 92, 487–495. [Google Scholar]
- Serova, O.V.; Gantsova, E.A.; Deyev, I.E.; Petrenko, A.G. The Value of pH Sensors in Maintaining Homeostasis of the Nervous System. Russ. J. Bioorganic Chem. 2020, 46, 506–519. [Google Scholar] [CrossRef]
- DuBose, T.D., Jr. Hyperkalemic Hyperchloremic Metabolic Acidosis: Pathophysiologic Insights. Kidney Int. 1997, 51, 591–602. [Google Scholar] [CrossRef]


| (A) | |||||
| Krebs-Ringer’s Composition | Molecular Formula | MW | Krebs-Ringer’s Solution Composition (1000 mL) | ||
| g/mol | g | mM | |||
| 1 | Sodium Chloride, | NaCl | 58.4 | 7.02 | 120.2 |
| 2 | Sodium Bicarbonate, | NaHCO3 | 84.0 | 1.302 | 15.5 |
| 3 | Magnesium Chloride Hexahydrate, | MgCl2·6H2O | 203.3 | 0.244 | 1.2 |
| 4 | Sodium Phosphate, Monobasic | NaH2PO4 | 119.98 | 0.188 | 1.3 |
| 5 | Potassium Chloride, | KCl | 74.6 | 0.44 | 5.8 |
| 6 | Calcium Chloride, | CaCl2 | 110.98 | 0.28 | 1.9 |
| 7 | D-glucose (dextrose), | C6H12O6 | 180.16 | 2.08 | 11.6 |
| (B) | |||||
| O2A-005 Composition | Molecular Formula | MW | Krebs-Ringer’s Solution composition (1000 mL) | ||
| g/mol | g | mM | |||
| 1 | Krebs-Ringer’s | See Table 2 | |||
| 2 | Hb | Polymer | 17MDa | 15 | 15 |
| ALT | Krebs-Ringer’s | O2A-005 | ||
| Time (h) | Mean | SD | Mean | SD |
| 6 | 7.25 | 2.605 | 6.44 | 3.47 |
| 12 | 10.63 | 4.809 | 7.72 | 3.98 |
| 24 | 13.63 | 10.084 | 13.21 | 6.20 |
| AST | Krebs-Ringer’s | O2A-005 | ||
| Time (h) | Mean | SD | Mean | SD |
| 6 | 22.00 | 4.683 | 11.78 | 6.44 |
| 12 | 30.63 | 6.842 | 17.52 | 7.72 |
| 24 | 38.75 | 11.463 | 25.74 | 13.21 |
| LDH | Krebs-Ringer’s | O2A-005 | ||
| Time (h) | Mean | SD | Mean | SD |
| 6 | 157.75 | 61.46 | 265.32 | 130.16 |
| 12 | 217.75 | 179.75 | 238.49 | 83.22 |
| 24 | 373.43 | 270.64 | 286.57 | 81.49 |
| POTASSIUM | Krebs-Ringer’s | O2A-005 | ||
| Time (h) | Mean | SD | Mean | SD |
| 6 | 10.18 | 1.454 | 11.11 | 2.125 |
| 12 | 9.44 | 1.595 | 10.26 | 2.495 |
| 24 | 8.51 | 1.852 | 9.48 | 2.844 |
| pH | Krebs-Ringer’s | O2A-005 | ||
| Time (h) | Mean | SD | Mean | SD |
| 6 | 7.98 | 0.099 | 7.61 | 0.248 |
| 12 | 8.05 | 0.108 | 7.54 | 0.275 |
| 24 | 8.16 | 0.090 | 7.52 | 0.316 |
| Parameter | Factor/Test | Df | Sum Sq/F Value | Mean Sq | F Value | Pr (> F) | Significance |
|---|---|---|---|---|---|---|---|
| ALT | group | 1 | 16.7 | 16.70 | 0.521 | 0.47350 | |
| time point | 2 | 442.2 | 221.09 | 6.898 | 0.00215 | ** | |
| group: time point | 2 | 6.5 | 3.27 | 0.102 | 0.90320 | ||
| Residuals | 54 | 1730.7 | 32.05 | ||||
| AST | group | 1 | 2084 | 2084 | 9.923 | 0.00266 | ** |
| time point | 2 | 2775 | 1388 | 6.608 | 0.00271 | ** | |
| group: time point | 2 | 86 | 43 | 0.205 | 0.81534 | ||
| Residuals | 54 | 11338 | 210 | ||||
| LDH | group | 1 | 11454 | 11454 | 0.529 | 0.470 | |
| time point | 2 | 92498 | 46249 | 2.136 | 0.128 | ||
| group: time point | 2 | 65015 | 32507 | 1.501 | 0.232 | ||
| Residuals | 54 | 1169442 | 21656 | ||||
| Potassium | group | 1 | 14.40 | 14.40 | 2.945 | 0.0920 | (trend) |
| time point | 2 | 33.89 | 16.95 | 3.466 | 0.0385 | * | |
| group: time point | 2 | 0.59 | 0.30 | 0.061 | 0.9414 | ||
| Residuals | 53 | 259.07 | 4.89 |
| Comparison | Difference (diff) | Lower Bound (lwr) | Upper Bound (upr) | p Value adj | Significance |
|---|---|---|---|---|---|
| 24–12 | 3.95 | −0.36 | 8.26 | 0.079 | |
| 6–12 | −2.66 | −6.97 | 1.66 | 0.306 | |
| 6–24 | −6.61 | −10.92 | −2.29 | 0.0015 | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzegorzewski, W.; Smyk, Ł.; Puchała, Ł.; Adadynski, L.; Szadurska-Noga, M.; Wojtkiewicz, J.; Derkaczew, M.; Wollocko, J.; Wollocko, B.; Wollocko, H. OxyVita®C Hemoglobin-Based Oxygen Carrier Improves Viability and Reduces Tubular Necrosis in Ex Vivo Preserved Rabbit Kidneys. Int. J. Mol. Sci. 2025, 26, 9266. https://doi.org/10.3390/ijms26199266
Grzegorzewski W, Smyk Ł, Puchała Ł, Adadynski L, Szadurska-Noga M, Wojtkiewicz J, Derkaczew M, Wollocko J, Wollocko B, Wollocko H. OxyVita®C Hemoglobin-Based Oxygen Carrier Improves Viability and Reduces Tubular Necrosis in Ex Vivo Preserved Rabbit Kidneys. International Journal of Molecular Sciences. 2025; 26(19):9266. https://doi.org/10.3390/ijms26199266
Chicago/Turabian StyleGrzegorzewski, Waldemar, Łukasz Smyk, Łukasz Puchała, Leszek Adadynski, Marta Szadurska-Noga, Joanna Wojtkiewicz, Maria Derkaczew, Jacek Wollocko, Brian Wollocko, and Hanna Wollocko. 2025. "OxyVita®C Hemoglobin-Based Oxygen Carrier Improves Viability and Reduces Tubular Necrosis in Ex Vivo Preserved Rabbit Kidneys" International Journal of Molecular Sciences 26, no. 19: 9266. https://doi.org/10.3390/ijms26199266
APA StyleGrzegorzewski, W., Smyk, Ł., Puchała, Ł., Adadynski, L., Szadurska-Noga, M., Wojtkiewicz, J., Derkaczew, M., Wollocko, J., Wollocko, B., & Wollocko, H. (2025). OxyVita®C Hemoglobin-Based Oxygen Carrier Improves Viability and Reduces Tubular Necrosis in Ex Vivo Preserved Rabbit Kidneys. International Journal of Molecular Sciences, 26(19), 9266. https://doi.org/10.3390/ijms26199266






