Combining CAR T-Cell Therapy and Nivolumab to Overcome Immune Resistance in THRLBCL: A Case Report
Abstract
1. Introduction
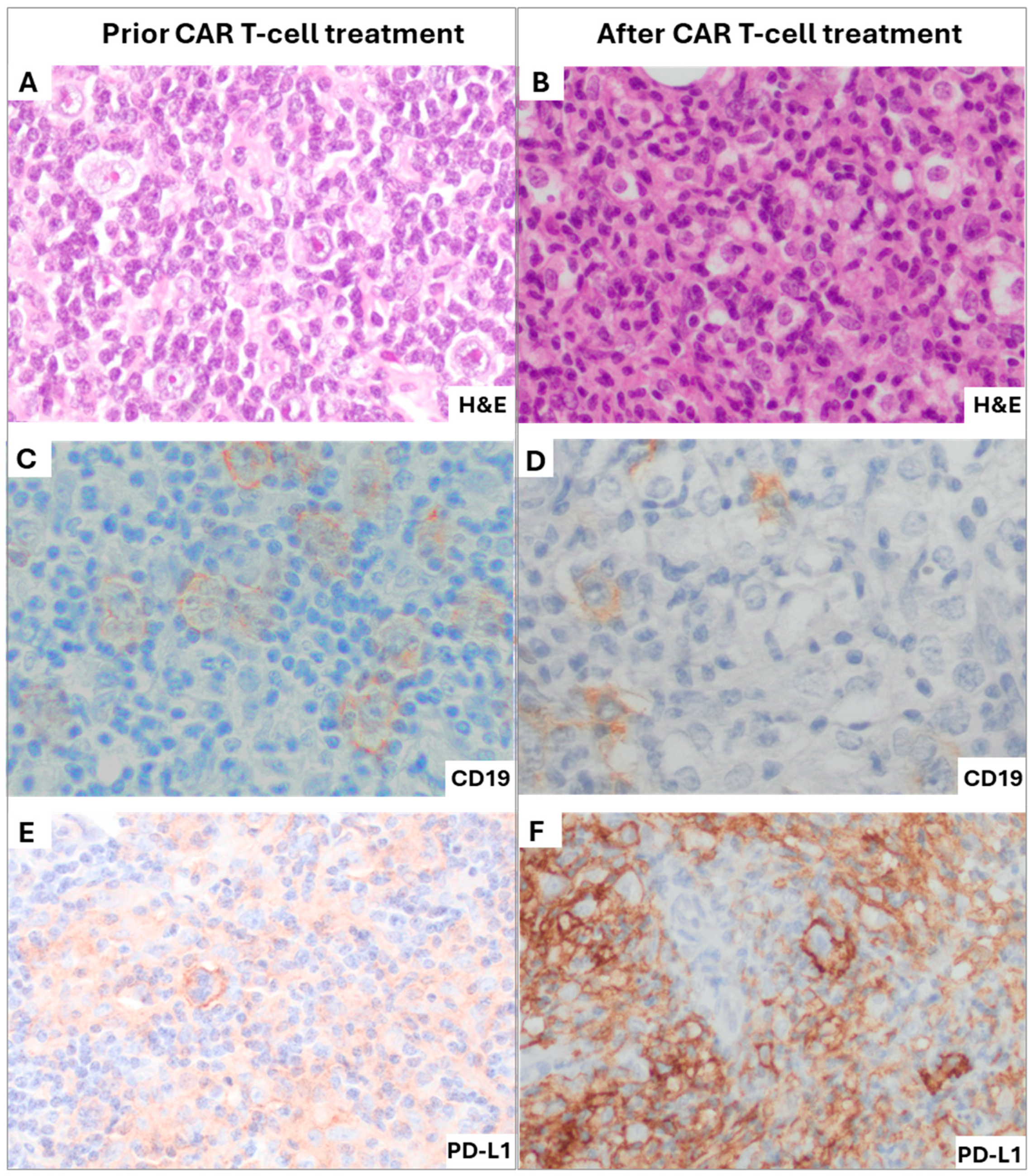
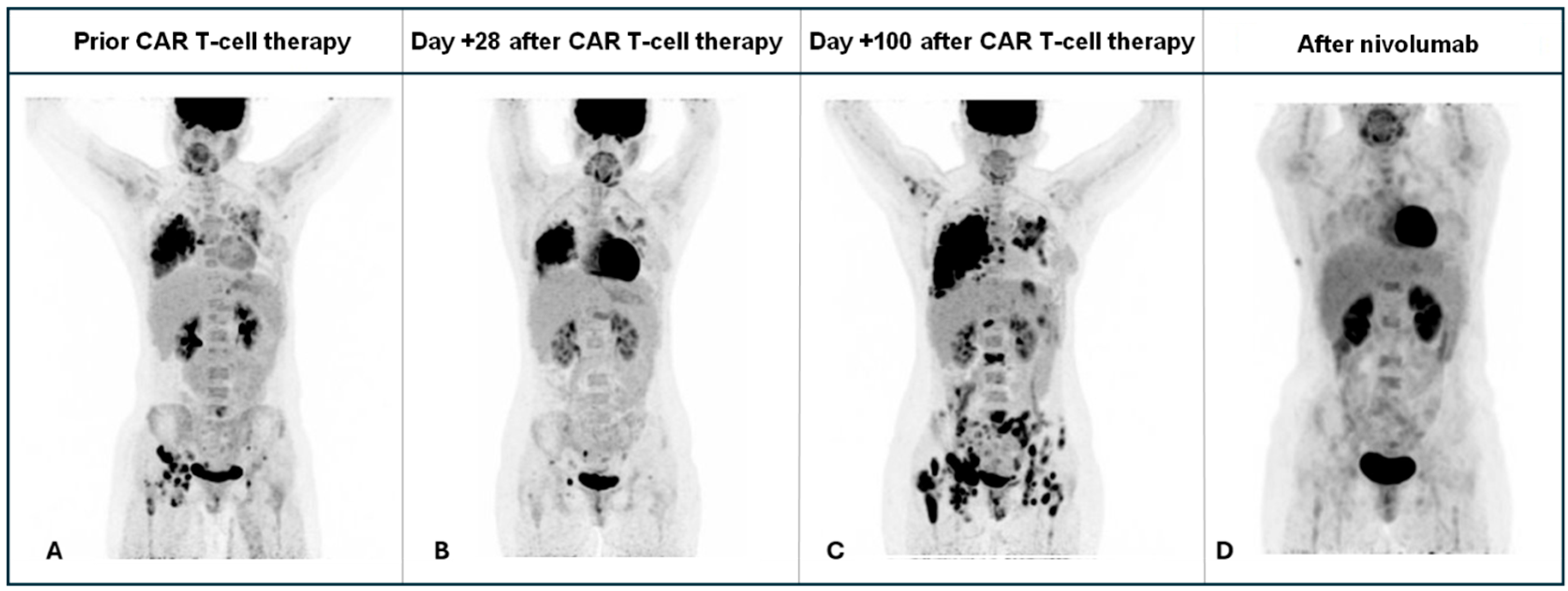
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| THRLBCL | T-cell/histiocyte-rich large B-cell lymphoma |
| DLBCL | Diffuse large B-cell lymphoma |
| PD-L1 | Programmed death-ligand 1 |
| cHL | Classical Hodgkin lymphoma |
References
- Griffin, G.K.; Weirather, J.L.; Roemer, M.G.M.; Lipschitz, M.; Kelley, A.; Chen, P.H.; Gusenleitner, D.; Jeter, E.; Pak, C.; Gjini, E.; et al. Spatial signatures identify immune escape via PD-1 as a defining feature of T-cell/histiocyte-rich large B-cell lymphoma. Blood 2021, 137, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Menter, T.; Bodmer-Haecki, A.; Dirnhofer, S.; Tzankov, A. Evaluation of the diagnostic and prognostic value of PDL1 expression in Hodgkin and B-cell lymphomas. Hum. Pathol. 2016, 54, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Armand, P.; Engert, A.; Younes, A.; Fanale, M.; Santoro, A.; Zinzani, P.L.; Timmerman, J.M.; Collins, G.P.; Ramchandren, R.; Cohen, J.B.; et al. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1428–1439, Erratum in J. Clin. Oncol. 2018, 36, 2748. [Google Scholar] [CrossRef] [PubMed]
- Sahin, T.K.; Akin, S. Immune checkpoint blockade and CAR T-cell therapy in T-cell/histiocyte-rich large B-cell lymphoma: Challenges and opportunities. Heliyon 2024, 10, e38023. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Zhang, J.; Gao, H.; Shen, H. Tumor microenvironment and CAR-T cell immunotherapy in B-cell lymphoma. Eur. J. Haematol. 2024, 112, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Li, H.; Shi, Y.; Wang, D.; Gong, J.; Xun, J.; Zhou, S.; Xiang, R.; Tan, X. M2 tumour-associated macrophages contribute to tumour progression via legumain remodelling the extracellular matrix in diffuse large B cell lymphoma. Sci. Rep. 2016, 6, 30347. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y. Tumor-associated macrophages: From basic research to clinical application. J. Hematol. Oncol. 2017, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Van Loo, P.; Tousseyn, T.; Vanhentenrijk, V.; Dierickx, D.; Malecka, A.; Bempt, I.V.; Verhoef, G.; Delabie, J.; Marynen, P.; Matthys, P.; et al. T-cell/histiocyte-rich large B-cell lymphoma shows transcriptional features suggestive of a tolerogenic host immune response. Haematologica 2010, 95, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Abiko, K.; Matsumura, N.; Hamanishi, J.; Horikawa, N.; Murakami, R.; Yamaguchi, K.; Yoshioka, Y.; Baba, T.; Konishi, I.; Mandai, M. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br. J. Cancer 2015, 112, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Chiba, T.; Kondo, T.; Kanzaki, H.; Kanayama, K.; Ao, J.; Kojima, R.; Kusakabe, Y.; Nakamura, M.; Saito, T.; et al. Interferon-γ induced PD-L1 expression and soluble PD-L1 production in gastric cancer. Oncol. Lett. 2020, 20, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Robin, E.T.; Drill, E.; Batlevi, C.L.; Caron, P.; Falchi, L.; Hamilton, A.; Hamlin, P.A.; Horwitz, S.M.; Intlekofer, A.M.; Joffe, E.; et al. Favorable Outcomes Among Patients with T-Cell/Histiocyte-Rich Large B-Cell Lymphoma Treated with Higher-Intensity Therapy in the Rituximab Era. Blood 2020, 136 (Suppl. S1), 36–38. [Google Scholar] [CrossRef]
- Tabanelli, V.; Melle, F.; Motta, G.; Mazzara, S.; Fabbri, M.; Agostinelli, C.; Calleri, A.; Del Corvo, M.; Fiori, S.; Lorenzini, D.; et al. The identification of TCF1+ progenitor exhausted T cells in THRLBCL may predict a better response to PD-1/PD-L1 blockade. Blood Adv. 2022, 6, 4634. [Google Scholar] [CrossRef] [PubMed]
- Pophali, P.A.; Fein, J.A.; Ahn, K.W.; Allbee-Johnson, M.; Ahmed, N.; Awan, F.T.; Farhan, S.; Grover, N.S.; Hilal, T.; Iqbal, M.; et al. CD19-directed CART therapy for T-cell/histiocyte–rich large B-cell lymphoma. Blood Adv. 2024, 8, 5290–5296. [Google Scholar] [CrossRef] [PubMed]
- Gazeau, N.; Mitra, S.; Nudel, M.; Tilmont, R.; Chauvet, P.; Srour, M.; Moreau, A.; Varlet, P.; Alidjinou, E.K.; Manier, S.; et al. Safety and efficacy of nivolumab in patients who failed to achieve a complete remission after CD19-directed CAR T-cell therapy in diffuse large B cell lymphoma. Br. J. Haematol. 2023, 202, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, J.A.; Godfrey, J.; Hu, Y.; Huang, J.; Smith, S.M.; Frigault, M.J.; DeFilipp, Z.; Appelbaum, D.; Pu, Y.; Feinberg, N.; et al. Primary resistance to CD19-directed chimeric antigen receptor T-cell therapy in T-cell/histiocyte-rich large B-cell lymphoma. Blood 2021, 137, 3454–3459. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munarriz, D.; López-Godino, O.; Martinez-Cibrian, N.; Albiol, N.; Brillembourg, H.; Navarro-Velázquez, S.; Español-Rego, M.; Casanueva, S.; García-Tomás, L.; Muñoz-Sanchez, G.; et al. Combining CAR T-Cell Therapy and Nivolumab to Overcome Immune Resistance in THRLBCL: A Case Report. Int. J. Mol. Sci. 2025, 26, 9265. https://doi.org/10.3390/ijms26199265
Munarriz D, López-Godino O, Martinez-Cibrian N, Albiol N, Brillembourg H, Navarro-Velázquez S, Español-Rego M, Casanueva S, García-Tomás L, Muñoz-Sanchez G, et al. Combining CAR T-Cell Therapy and Nivolumab to Overcome Immune Resistance in THRLBCL: A Case Report. International Journal of Molecular Sciences. 2025; 26(19):9265. https://doi.org/10.3390/ijms26199265
Chicago/Turabian StyleMunarriz, Daniel, Oriana López-Godino, Nuria Martinez-Cibrian, Nil Albiol, Helena Brillembourg, Sergio Navarro-Velázquez, Marta Español-Rego, Sebastián Casanueva, Lucía García-Tomás, Guillermo Muñoz-Sanchez, and et al. 2025. "Combining CAR T-Cell Therapy and Nivolumab to Overcome Immune Resistance in THRLBCL: A Case Report" International Journal of Molecular Sciences 26, no. 19: 9265. https://doi.org/10.3390/ijms26199265
APA StyleMunarriz, D., López-Godino, O., Martinez-Cibrian, N., Albiol, N., Brillembourg, H., Navarro-Velázquez, S., Español-Rego, M., Casanueva, S., García-Tomás, L., Muñoz-Sanchez, G., Alserawan, L., Benitez-Ribas, D., Magnano, L., Correa, J. G., Rivero, A., Mozas, P., Gine, E., Rodríguez-Lobato, L. G., Martínez-Roca, A., ... Ortiz-Maldonado, V. (2025). Combining CAR T-Cell Therapy and Nivolumab to Overcome Immune Resistance in THRLBCL: A Case Report. International Journal of Molecular Sciences, 26(19), 9265. https://doi.org/10.3390/ijms26199265





