1. Introduction
Parkinson’s disease (PD) is a complex, progressive, and incurable neurodegenerative condition, posing a significant health burden [
1,
2]. It is clinically characterized by the presence of core motor symptoms, with bradykinesia being a hallmark, accompanied by either resting tremor or muscle rigidity [
3]. The condition arises due to the gradual loss of dopamine-producing neurons, especially in the substantia nigra pars compacta (SNpc). In addition to neuronal loss, the accumulation of Lewy bodies—abnormal clumps of the protein alpha-synuclein—is a key pathological feature. The underlying mechanisms thought to drive PD progression include the toxic buildup of proteins, disruption of mitochondrial function, oxidative stress, and damage to mitochondrial DNA, leading to impaired cellular energy production and abnormal cell structure. These factors together contribute to the steady decline in motor and non-motor functions seen in the disease [
4,
5].
As the global population ages, the number of PD cases is rapidly increasing, making it one of the fastest-growing neurological disorders in terms of disability and mortality. In 2020, an estimated 9.4 million people worldwide were affected by PD [
6].
In Sweden, early studies revealed widespread use of antiparkinsonian drugs, highlighting PD as a significant public health issue [
7] with reported prevalence rates between 115 and 136 per 100,000 individuals, confirming the disease’s impact [
8,
9]. A large twin study estimated a higher adjusted prevalence of 496 per 100,000, with men being 1.45 times more likely than women to develop PD [
10]. These findings illustrate the growing burden of Parkinson’s disease in Sweden.
While most PD cases are idiopathic (iPD), it remains unclear why many patients exhibit familial clustering [
11]. A growing list of studies have identified over 40 genetic loci associated with an increased risk of developing the disease [
12,
13,
14,
15]. These findings highlight the role genetic factors may play, even in cases previously considered sporadic.
Studies in Swedish PD patients have revealed strong links between specific alterations in the glucocerebrosidase (GBA1) gene and PD [
16]. Additionally, although ~22% of Swedish PD patients exhibit signs of familial PD, common variants, such as
LRRK2 p.(Gly2019Ser) and
SNCA duplication, have been found at unexpectedly low frequencies in more than 2000 PD patients [
17]. This suggests that other genetic factors may play a larger role in the Swedish PD population.
Unlike single-variant alterations, Nucleotide Repeat Expansions (NREs) exhibit intergenerational instability, leading to either expansion or contraction across generations [
18,
19]. This instability may contribute to the phenomenon known as “missing heritability,” where genetic risk factors for diseases like Parkinson’s remain undetected. These genetic alterations were difficult to detect in the early days of whole-genome association studies, which likely explains the earlier lack of positive associations with PD. Unless candidate gene studies specifically targeted their role in PD, these alterations are only identified through a combination of short-read sequencing and bioinformatics. However, with recent advances in long-read sequencing (LRS), these alterations are becoming more readily detectable [
20].
Abnormal NREs in certain genes typically lead to distinct disorders, such as ataxic conditions affecting the cerebellum or neuromuscular diseases like
C9ORF72-ALS (C9-ALS) that target motor neurons. However, some individuals display atypical phenotypes, including parkinsonian features [
21,
22,
23,
24,
25], suggesting a broader overlap in neurodegenerative pathways. This blurs the lines between conditions once thought to be entirely separate and hints at shared pathological mechanisms across these seemingly distinct disorders. A clear example of this overlap occurs with Spinocerebellar ataxia Type-2 (SCA2) repeat expansions in
ATXN2. The pathological range in SCA2 extends from 32 to 200 CAG repeats. A cerebellar phenotype is most often observed in fully penetrant alleles with ≥35 uninterrupted CAG repeats, whereas alleles in the lower range (32–34 repeats) containing CAA interruptions consistently manifest with features indistinguishable from idiopathic Parkinson’s disease [
26]. Broadening this view, individuals with NREs in
ATXN3,
TBP,
C9ORF72,
PRNP, and
POLGA have also been reported to display a spectrum of parkinsonian phenotypes, ranging from atypical to classical forms [
27,
28,
29,
30]. This phenotypic variability raises important questions about the role of NREs in basal ganglia degeneration, a key pathological feature of PD. However, the impact of NREs varies significantly across different parkinsonian populations, adding complexity to understanding their role in neurodegeneration [
19]. Yet, many Swedish PD cases remain underrepresented in such studies.
Sweden, like other Scandinavian countries, has experienced extended periods of genetic isolation, resulting in a distinct genetic landscape that may differ from other European populations in both the prevalence and expression of certain genetic diseases [
31,
32,
33]. This isolation has likely shaped the genetic factors contributing to PD, with NREs potentially presenting a unique genetic signature that influences both the risk and manifestation of the disorder in Sweden.
As a result, PD in the Swedish population may be driven by a different combination of genetic factors, setting it apart from patterns seen elsewhere in Europe. Despite these genetic nuances, the role of NREs in Swedish PD patients remains largely unexplored. Furthermore, most studies investigating repeat expansions in PD fail to integrate clinical phenotypes and biomarker profiles of affected carriers, limiting translational insights. This study investigates the contribution of key NRE-associated genes—ATXN2, ATXN3, CACNA1A, TBP, C9ORF72, PRNP, POLGA, and TOMM40—to PD in a Swedish cohort, examining their influence on clinical phenotype and associated molecular signatures to better characterize the overt stages of the disease. By focusing on these genes, we hope to shed light on the unique genetic underpinnings of PD in Sweden, contributing to a more nuanced understanding of its pathogenesis and potential avenues for targeted interventions.
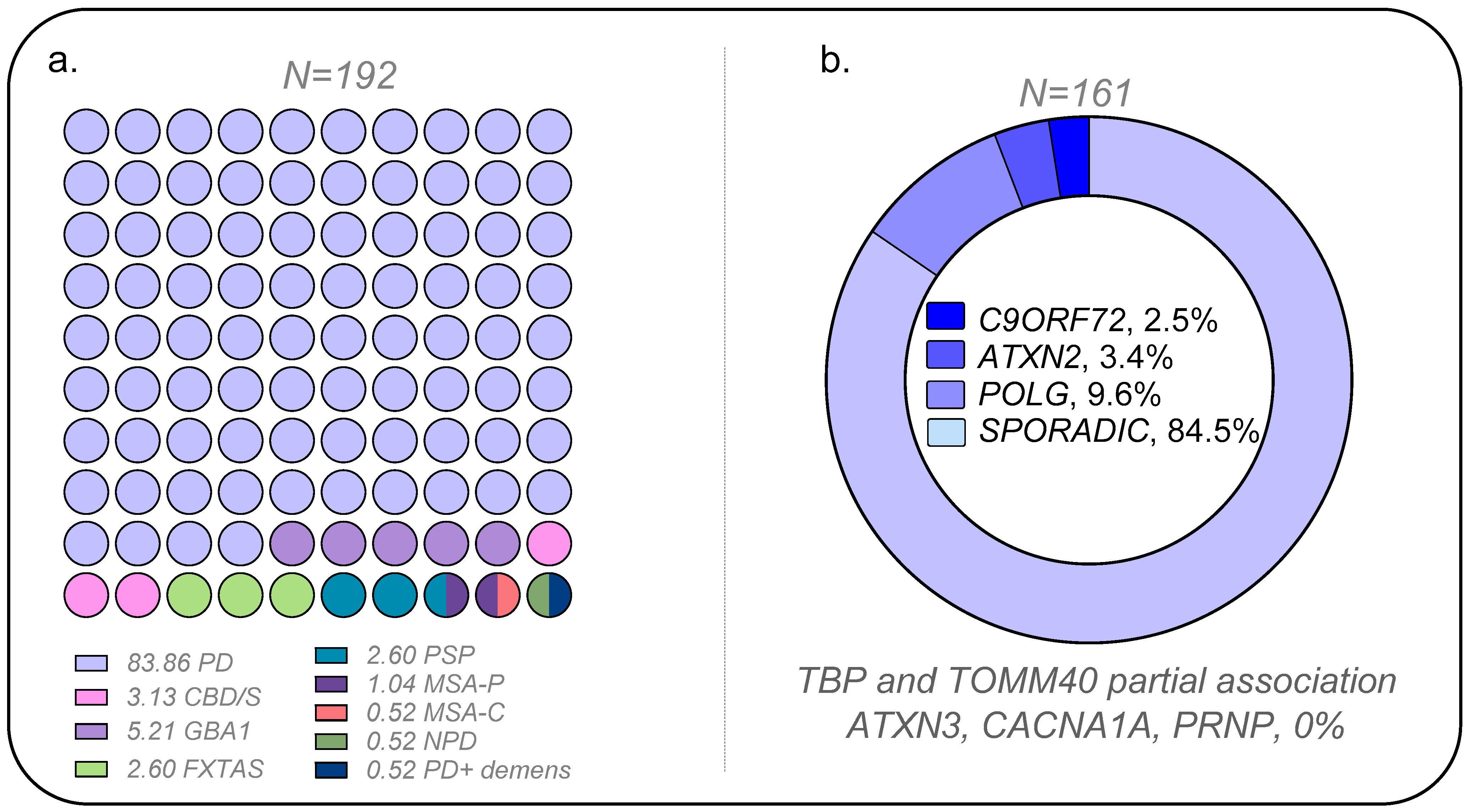
2. Results
Figure 1 presents the overall results of the association analysis conducted in the PD and control cohorts (demography and clinical data in
Table S1–S3). Positive associations were observed for
POLG,
ATXN2, and
C9ORF72. The positive association for CAG repeats in
TBP was marginal. Likewise,
TOMM40 was only significant for specific alleles or genotype groups (refer to details below). Conversely, neither the CAG repeats at
loci,
ATXN3, and
CACNA1A nor octapeptide repeat insertions (OPRI) in the Prion gene (
PRNP) were found to be positively associated with PD. Two cases with one OPRI deletion, considered a polymorphism, were found, but the frequency was like controls (sizing traces in
Figure S1).
2.1. ATXN2
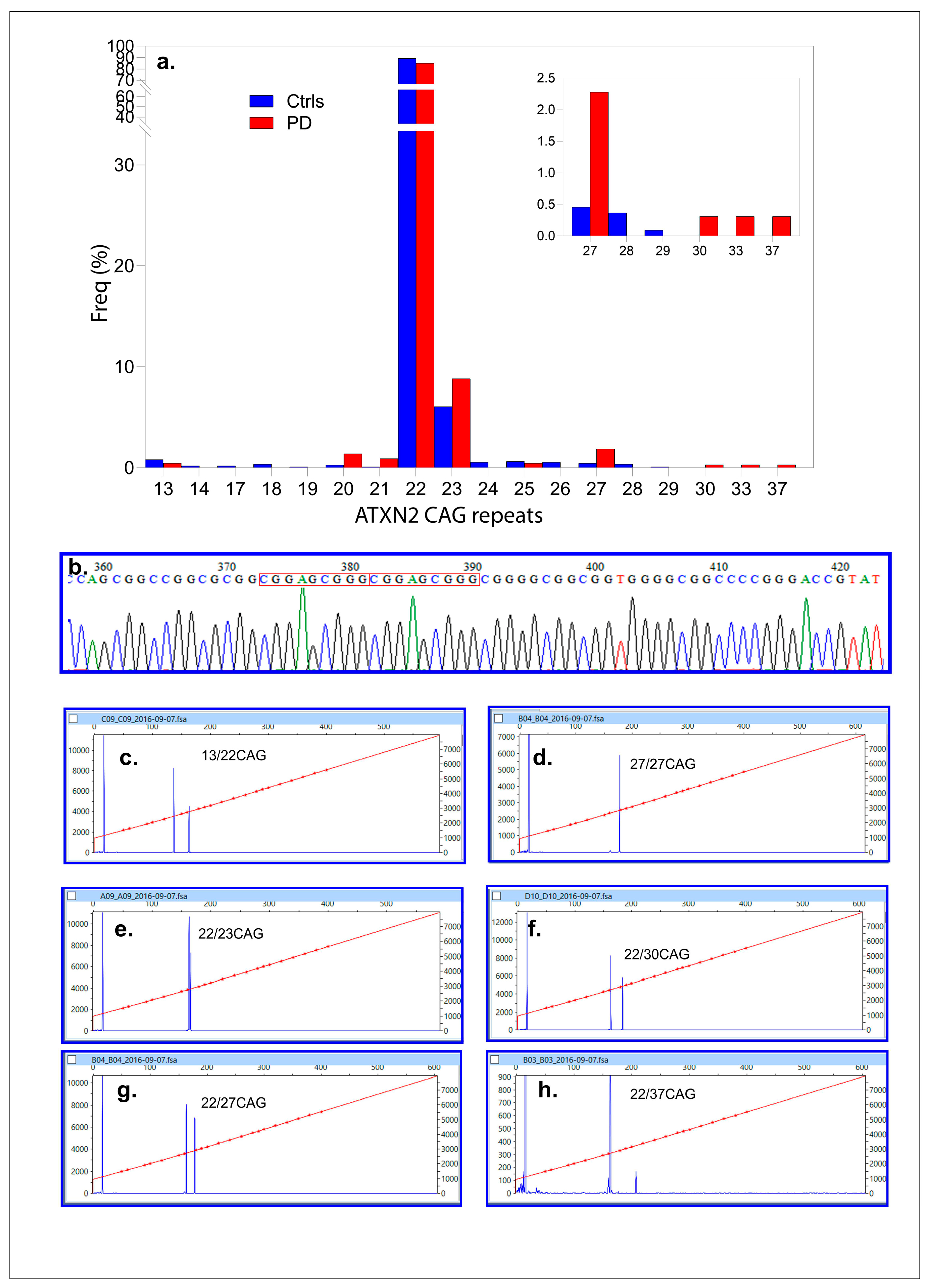
One hundred sixty-one PD patients (161, 100%, 2N = 322) and 547 (100%, 2N = 1094) control DNAs were successfully genotyped (control/case ratio = 3.39). CAG repeats varied from 13–37 in the PD population, while for the controls, from 13–29 CAG repeats. The most frequent allele was 22 CAG repeats for both populations (85.11 and 89.27%). Alleles, sized with 23 CAG repeats, were the second most frequently found at 8.83% and 6.04% (
Figure 2a,b). Among the PD cases, one had a CAG repeat length of 37, and another was homozygous for an intermediate allele (27/27 CAG repeats). In contrast, no individuals in the control population had CAG repeats of ≥30. Intermediate CAG repeats expansions in
ATXN2 (27–37 CAG repeats) were significantly associated with PD, occurring in 11 out of 322 alleles (3.40%) compared to 10 out of 1094 controls (0.91%). This association was statistically significant (Fisher’s exact test:
p = 0.0027; FDR-adjusted α
c = 0.005 for ten tests or allelic classes) with an odds ratio (OR) of 3.83 (95% CI: 1.61–9.1;
p = 0.0023). A post hoc power analysis confirmed the study’s ability to detect significant differences between cases (2N = 322) and controls (2N = 1094), with allele frequencies of 0.0376 and 0.00091, respectively. The study achieved 99.97% power at α = 0.05, confirming that the sample size was sufficient to detect significant differences in CAG repeat distributions between PD patients and controls.
2.1.1. ATXN2 Methylation
Methylation analysis of ATXN2, in both sense and antisense directions, revealed that it was generally unmethylated. Gene expression analysis was not performed due to the unavailability of sufficient material.
2.1.2. Novel Variants in ATXN2
Four PD cases were found to carry a 9-bp duplication (g271_279delinsCGGAGCGGG) (
Figure 2b). Among these, three had 22/22 CAG repeats, while one was a compound heterozygote with an intermediate allele genotype (22/27 CAG repeats). The age at diagnosis (ADx) and other clinical parameters were unremarkable and have been detailed elsewhere [
34].
2.1.3. Clinical Characteristics of ATXN2 Intermediate PD Expansion Carriers
Table S1 illustrates the phenotypic manifestations associated with abnormal
ATXN2 CAG repeats within our studied population. Among these cases, 3 out of 10 were male (30%), and 86% were Swedish. All cases demonstrated favorable responses to dopamine treatment. Additionally, clinical parameters such as age at diagnosis (ADx) and Disease Duration (DD) were comparable between carriers and individuals with normal CAG repeat expansions. Specifically, for ADx, carriers had a mean of M = 70.20 (SD = 9.09) compared to M = 62.76 (SD = 10.43) for non-carriers,
t (151) = −0.92,
p = 0.18, ns. Likewise, for DD, carriers had a mean of M = 5.70 years (SD = 5.14) compared to M = 6.20 years (SD = 5.49) for non-carriers,
t (151) = −0.92,
p = 0.18, ns.
No noticeable differences were found in other clinical measures, including MDS-UPDRS (M: 47.86 (SD = 11.64)) and Hoehn and Yahr staging (M = 2.65 (SD = 0.78)), concerning genetic statuses. Notably, two subjects exhibited elevated levels of CSF tau and
p-tau, indicating the presence of tau pathology, as illustrated in
Table S1.
Dopamine Transporter scan (DaTscan) data were available for one ATXN2 anomalous carrier: ATX-01 (22/27). DaTscan showed reduced binding with the typical rostro-caudal gradient observed in PD. This reduced DAT binding was more severe in the striatum and less in the putamen than in the caudate, as indicated by the right and left caudate binding ratio (2.07 (z-score = −4.08 **); 1.90 (z-score − 4.24 **)); this reduction was more prominent in the left than in the right putamen (binding ratio of right and left putamen (1.69 (z-score − 4.39 **); 1.52 (z-score − 6.40 **))).
The homozygous case, ATX-05, with 27/27 CAG repeats in the ATXN2, consisted of patients that were 67 years old on average and presented with significant cognitive decline, reflected by a MoCA score of 8.5, and was also homozygous E3/E3 for APOE.
2.1.4. ATXN2 in Atypical Parkinsonism
Four patients with MSA-P (22/27 CAG repeats), CBS (22/27 CAG repeats), PSP (22/30 CAG repeats), and CBD (22/35 CAG repeats) phenotypes were identified as carriers of
ATXN2 alleles ≥27 repeats. These findings may highlight the pleiotropic role of
ATXN2 in atypical parkinsonism, extending its involvement beyond classic neurodegenerative conditions. The PSP case exhibited low but detectable methylation levels for
ATXN2-AS (1%), as shown in the representative 2D ddPCR plot (
Figure S2). The CBD case demonstrated a significant reduction in striatal DAT binding, with symmetric losses in the caudate (right: 2.60; left: 2.53; z-score for both: −2.87 *) and putamen (right: 2.27, z-score: −3.03 **; left: 2.28, z-score: −4.19 **). Our findings underscore the pleiotropic role of
ATXN2 in the parkinsonian spectrum, with intermediate CAG repeat expansions (≥27 repeats) significantly associated with PD and observed across various neurodegenerative phenotypes, including PSP, CBD, and MSA-P. These atypical cases were not included in the above association analysis for typical PD.
2.2. C9ORF72
All individuals in the PD cohort (100%, 161) and 518 controls (94.87%, 2n = 1036) were successfully genotyped using both the two-primer method or Triple-Primed PCR, with a control-to-case ratio of 3.21.
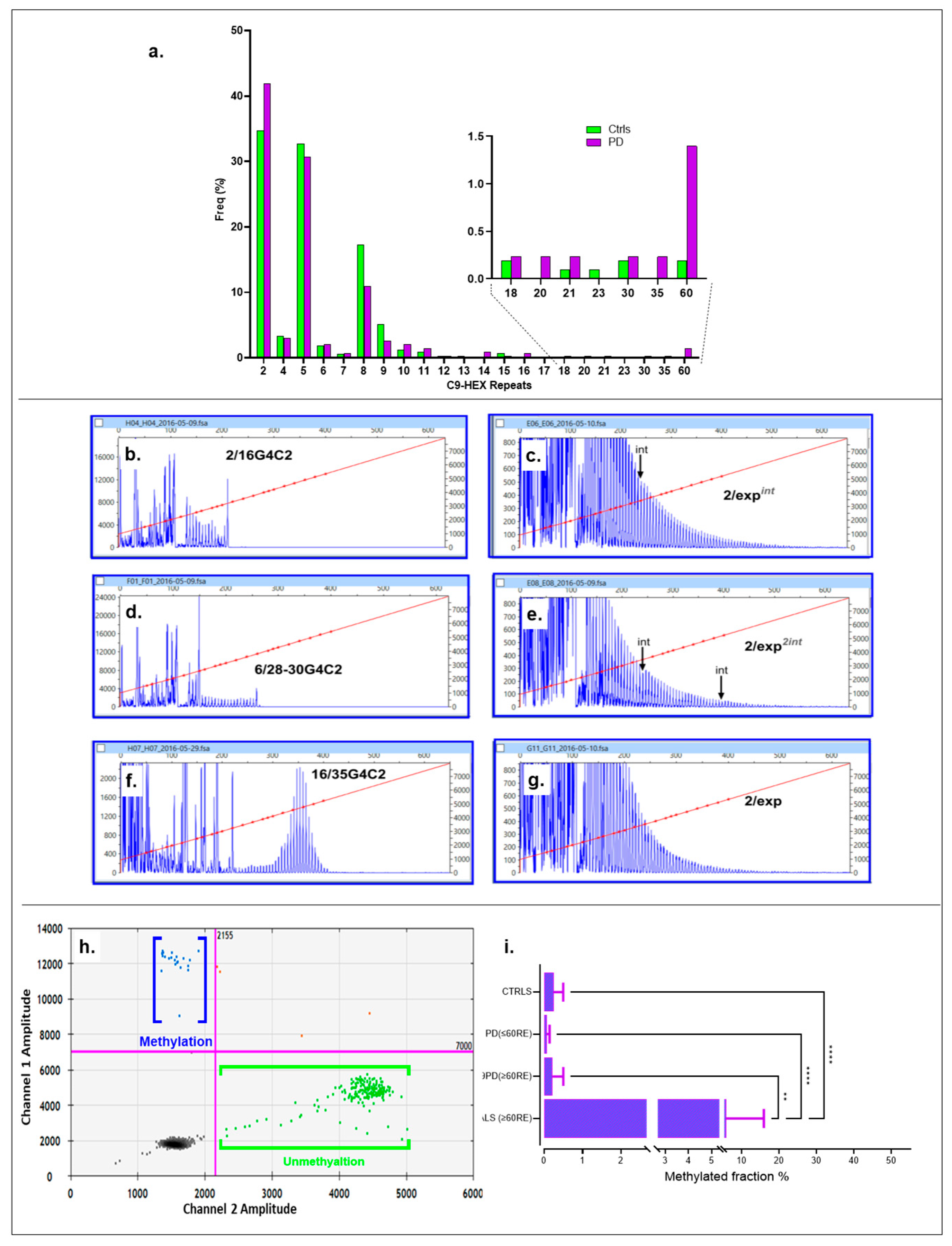
Figure 3a displays the frequency for both cohorts, with the inset highlighting the association range for PD. The hexanucleotide repeat ranged from 2 to ≥60C9RE in PD subjects. Specifically, three intermediate alleles were identified, one containing 21 30 repeats and another with 35 repeats. Six alleles exhibited moderate expansion. Although the traces displayed the characteristic saw-tooth-like pattern, the tails were short, indicating the absence of fully expanded alleles (
Figure 3b–g and
Figure S3). Given the limitations of routine methods (fluorescent PCR and RP-PCR) in accurately quantifying allele size, along with the ongoing debate surrounding this issue [
35], we estimated the alleles to range from intermediate (20–32 repeats) to moderately long (35–60 repeats). Regarding the controls (with a normal range of 2–30 repeats), they showed two alleles with 30 repeats, alongside two moderate long/moderate/fully expanded alleles, all in a heterozygous state for both cohorts. All ≥30C9RE alleles in the control group were identified in the DNA samples from the blood bank collection, with none found in the PD spouse control group.
Using ≥30 repeats as the threshold, abnormal C9REs were significantly associated with PD, with a prevalence of 8 out of 322 (2.48%) in the case group compared to 4 out of 1036 (0.38%) in the control group. The Fisher’s exact test yielded p = 0.0018, α = 0.05, and OR = 6.6 (95% CI: 2.00–22.00, p = 0.0022). Using different cutoffs (≥35 repeats representing moderate repeat expansions), the association with anomalous alleles linked to PD was still highly significant. For repeats ≥35, the frequency was seven out of 322 (2.17%) in the case group compared to two out of 1036 (0.19%) in the control group. The Fisher exact test yielded p = 0.0053 and OR = 5.73 (95% CI: 1.70–19.71), with a significant result (p = 0.0056) that remained significant after adjusting for multiple comparisons using FDR (αc = 0.025 for two tests). Significance persisted even after excluding cases with intermediate expansions and including them in the normal allele group.
Ataxin-2 and ApoE genotypes for this cohort were unremarkable. However, the digenic inheritance of TBP and C9ORF72 repeats were observed in one sporadic PD case, which exhibited a reduced penetrance allele with 42 CAG repeats in TBP and C9ORF72 repeats (≥60 repeats). Age at diagnosis was 69 year, and the course of disease was severe (MDS-UPDRS = 75, H & Y = 4) in a follow-up two years after diagnosis. This patient also suffered from moderate levels of anxiety and depression (HADS =12, MADRS = 14).
2.2.1. C9ORF72 Promoter Methylation
We investigated the blood methylation levels in a CpG island within the
C9ORF72 gene. Our analysis included 69 amyotrophic lateral sclerosis (ALS) DNA samples with full expansions of C9RE from Coriell cell repositories, five PD cases with intermediate to moderate expansions (range = 35–≥60 C9RE), 25 individuals with PD but possessing normal repeat expansions in
C9ORF72 (range = 2–18 repeats), and control DNA comprising a panel of 30 individuals with diverse ethnic backgrounds with an EDP-1 plate (repeats: HAM 006 and ECACC 94082275; repeat range = 2–15). Using ddPCR, we observed methylation patterns in PD patients carrying anomalous C9RE, as depicted by positive droplets in the 2D ddPCR plot for a representative case (C9-03, genotype 2/≥35–60RE) (
Figure 3h). However, further one-way ANOVA with a Kruskal–Wallis test (K-W statistic = 93.32,
p = 0.0001), followed by Dunn’s test and FDR control using the Benjamini–Hochberg method, revealed that methylation levels in PD are not comparable to C9ALS, as this group has significantly higher methylation levels compared to CTRLS (q = 0.0001), PD (≤60RE) (q = 0.0001), and C9PD (≥60RE) (q = 0.0021) (
Figure 3i).
2.2.2. Clinical Characteristics of PD Patients with RE in C9ORF72
Table S1 depicts the clinical phenotype for all
C9ORF72 carriers, alongside DaTscan data, available for 4 out of 8 subjects (50%). All subjects were diagnosed with PD, with a predominant representation of individuals of Caucasian descent and a male-to-female ratio of 6 to 8 (75%). The average age at diagnosis was 66.00 years (SD = 6.52), and disease duration was 6.88 years (SD = 7.97), which did not differ significantly from the C9RE non-carriers (
t (145) = −0.92,
p = 0.19;
t (145) = −0.39,
p = 0.35). They were all responsive to L-DOPA therapy, consistent with the typical late onset of PD. DaTscan results available for four patients consistently reveal dopamine transporter loss in the striatum (
Table S1). Additionally, among the subjects with ≥60C9RE, two exhibited severe cerebellar degeneration. Lastly, CSF markers, including β-amyloid, tau, and phospho-tau, were normal for all subjects with available data (50%).
Our findings highlight a significant association between anomalous hexanucleotide repeat expansions (≥30 repeats) in C9ORF72 and PD, with increased prevalence in the PD cases compared to controls. Moderate long expansions (35–≥60 repeats) were more frequent and clinically impactful in PD, correlating with severe dopamine transporter loss in the striatum, and, in some cases, the cerebellum showed degeneration. Promoter methylation levels were significantly higher in the ALS cases with full expansions compared to the PD and controls, underscoring the distinct molecular characteristics of C9ORF72-related pathology.
2.3. TBP
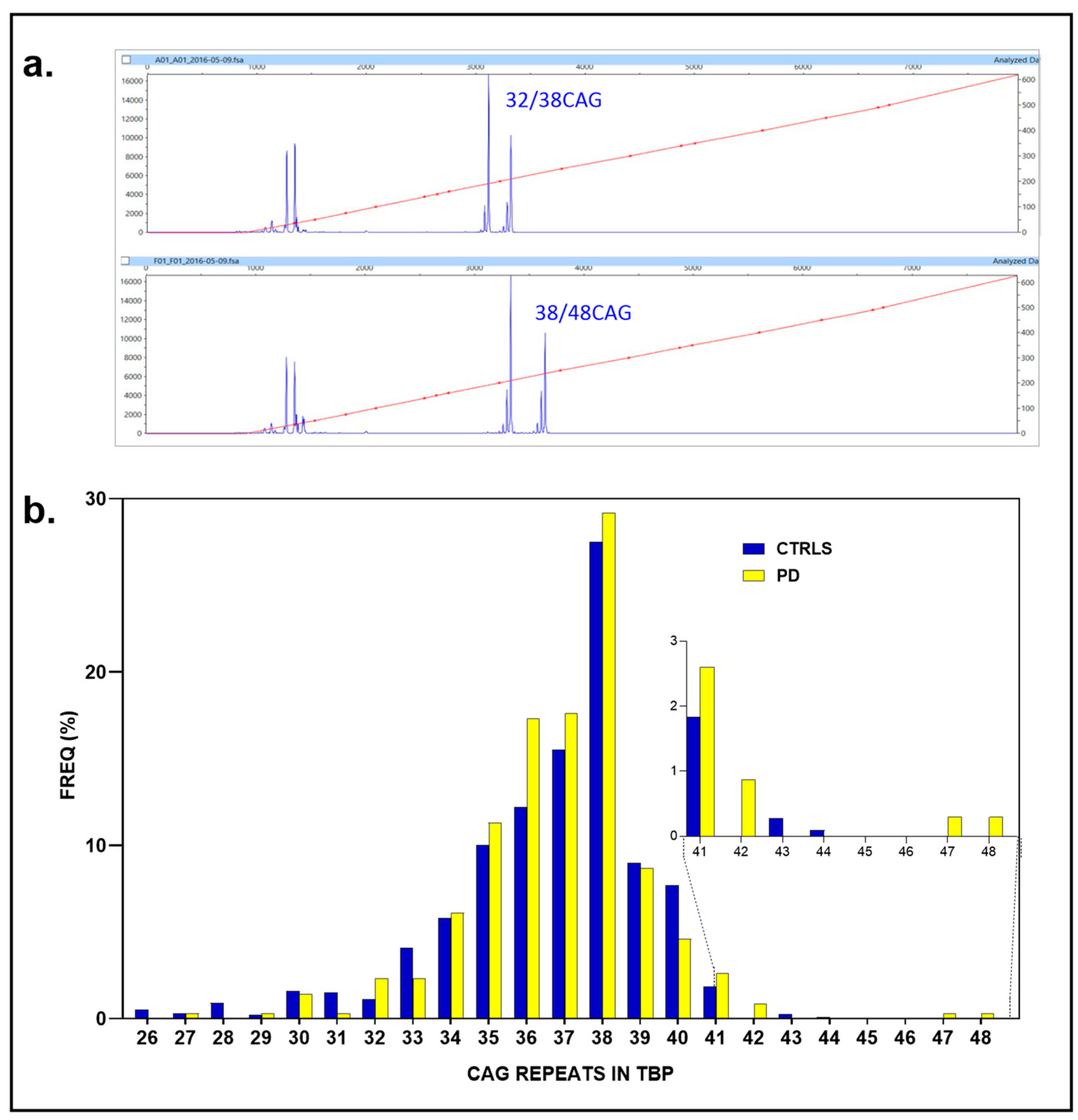
We successfully genotyped all PD subjects (161 individuals, 100%, 2N = 322) and all controls (547 individuals, 100%, 2N = 1094), with the control group outnumbering the PD cohort by a ratio of 3.39. Among the PD cases, 14 individuals exhibited anomalous CAG repeat expansions: nine alleles with 41 repeats (4.35%), three with 42 repeats (genotypes, two with 35/42 and 36/42 CAG repeats, 0.93%), one with 47 CAG repeats, and one with 48 repeats (37/47 and 38/48, 0.62%). In contrast, none of the controls had ≥48 CAG repeats; the largest repeat numbers found in the controls were 41 (20 individuals, 1.83%), 43 (3, 0.27%), and 44 (1, 0.09%) CAG repeats (
Figure 4a,b).
Only marginal associations were observed for ≥41–48 CAG repeats in 14 out of 322 vs. 4/1093 (4.35 vs. 0.36%) (p = 0.053; OR: 1.94, 95% CI: 1.00-to-3.80, p = 0.051). Similarly, the association for ≥42–48 CAG repeats reached nominal significance (5/322 and 4/1093 (1.5% vs. 0.36%, p = 0.031; OR: 4.29, 95% CI: 1.15-to-16.10, p = 0.030)) but did not meet the adjusted threshold (αc = 0.025) after FDR correction. Likewise, the association for ≥47 CAG repeats (0.62% vs. 0%, Fisher’s exact test, p = 0.052; OR: 17.07, 95% CI: 0.82 to 356.59, p = 0.067) did not achieve statistical significance at the conventional threshold of p = 0.05, nor after applying FDR adjustment (αc = 0.016).
To achieve sufficient power to detect a significant association with ≥42–48 CAG repeats in PD, the PD cohort size would need to increase to approximately 470–612 cases to ensure power levels of 80% and 90%, respectively. Similarly, the control cohort would need to be expanded to 944–1230 individuals. This increase in sample size, achievable for us, would ensure adequate statistical power to detect meaningful associations at the conventional
p = 0.05 significance level, while controlling for multiple comparisons using a Bonferroni-adjusted α threshold of 0.025 for two independent tests (
Figure S4).
Additionally, we identified potential digenic effects, where one allele with borderline repeat lengths (e.g., 42 CAG repeats) co-occurred with a C9ORF72 expansion. The frequency of this co-occurrence was 0.31% in the PD patients, compared to 0% in the controls (TBP × C9ORF72; Fisher’s exact test, p = 0.24), as previously discussed. Six additional C9ORF72 repeats (7–10 C9REs) were observed to be co-inherited with TBP expansions of ≥42–48 CAG repeats, though they did not reach the intermediate cutoff of 35 repeat units seen in the case with C9 ≥ 60 and ≥42 CAG repeats in TBP. Notably, five out of these seven subjects carried the C9ORF72 ‘Risk’ haplotype for the rs3849942 T-allele, whereas none of the repeats with the longest TBP expansions (CAG = 47 or 48) did.
2.4. Clinical Features
All subjects were diagnosed with PD and predominantly Caucasian, with a male-to-female ratio of 9:5 (64%). The mean age at diagnosis was 62.78 years (SD = 11.54), and disease duration averaged 5.75 years (SD = 5.4), comparable to non-carriers. All responded positively to DOPA therapy. MoCA scores (M= 23.1, SD = 3.64) showed no significant differences. DAT scans consistently revealed striatal DAT loss. CSF markers (β-amyloid, tau, and
p-tau) were available for only three subjects (
Table S1).
Our findings reveal nominal associations between anomalous TBP CAG repeat expansions (≥42CAG repeats) and PD, though statistical significance was not maintained after correction for multiple comparisons. Larger cohort sizes are needed to achieve sufficient power for robust detection of associations. Co-inheritance of borderline TBP repeats with full C9ORF72 expansions was observed in a subset of cases, which may suggest potential digenic interactions. Clinical characteristics, including ADx, DD, and treatment response, were like those of non-carriers, though DAT scans revealed consistent striatal dopamine transporter loss.
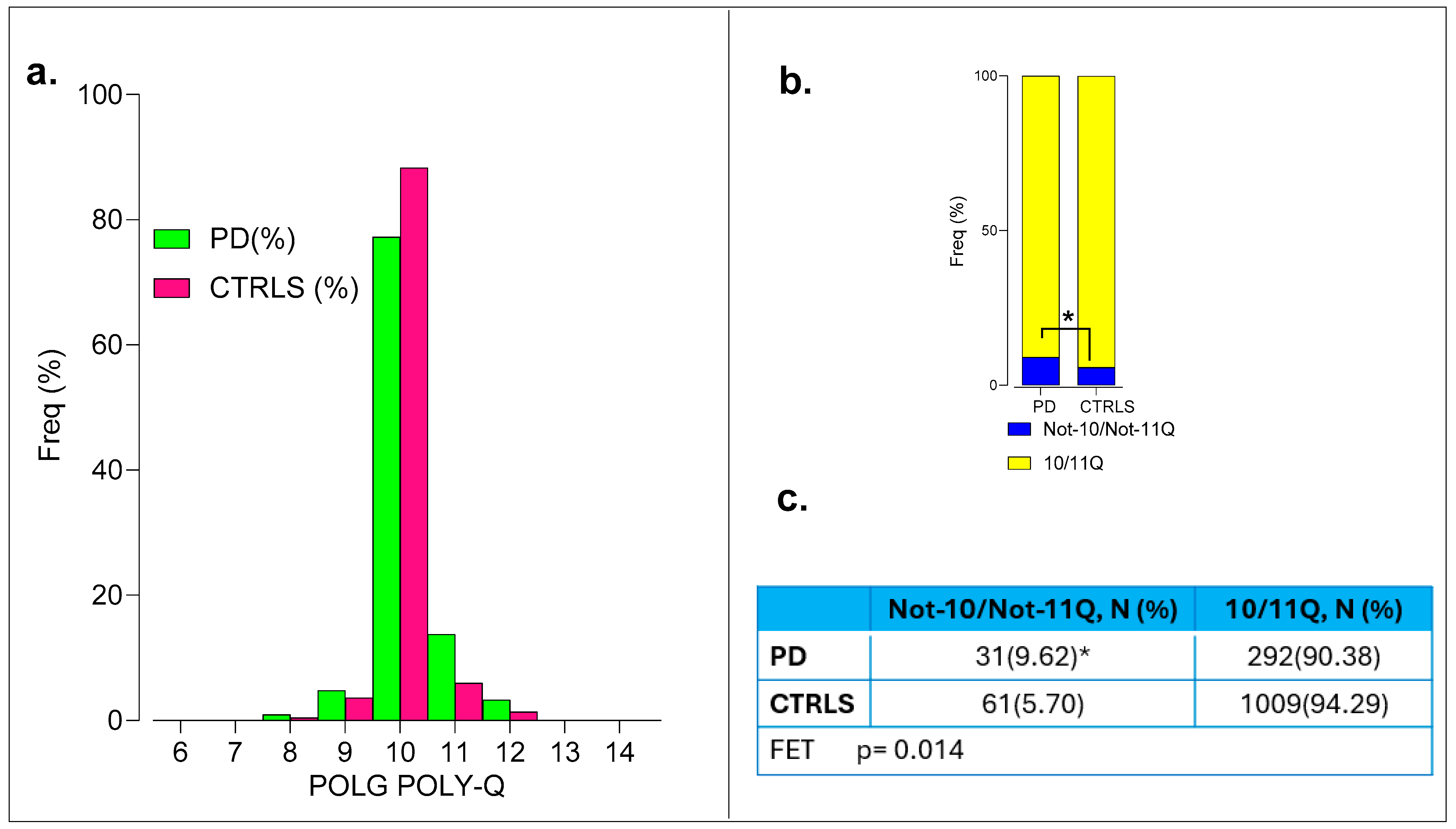
2.5. POLG
All PD individuals and controls had their DNA samples successfully genotyped. Positive association of the polyQ variant in
POLG was observed with the Not-10/Not-11Q group, showing a frequency of 9.62% (31 out of 322) vs. 5.57% (61 out of 1094) in the controls (Fisher’s exact test
p = 0.014 significant at
p = 0.05; α
c = 0.025, OR = 1.80, 95% CI 1.15-to-2.83,
p = 0.01) (
Figure 5a and
Figure S16).
Clinical variables such as ADx and DD were not different between Not-10/Not-11Q compared to 10/11Q_10/11Q genotypes. No differences were found in the MDS-UPDRS, H & Y, and MoCA scores and the biochemical polypeptides (β-amyloid1-42, p-tau, and tau) in the CSF, as determined at disease diagnosis.
Thus, the POLG Not-10/Not-11Q variant was significantly associated with PD, but no differences were observed in clinical variables or CSF biomarkers between genotypes.
2.6. TOMM40
The
TOMM40 gene, located on chromosome 19q13.2, contains the
rs10524523 locus, known as the
TOMM40 poly-T repeat. Adjacent to
TOMM40 is the
APOE locus, crucial for cholesterol metabolism, particularly in the central nervous system. Roses et al. [
36] classified rs10524523 alleles at
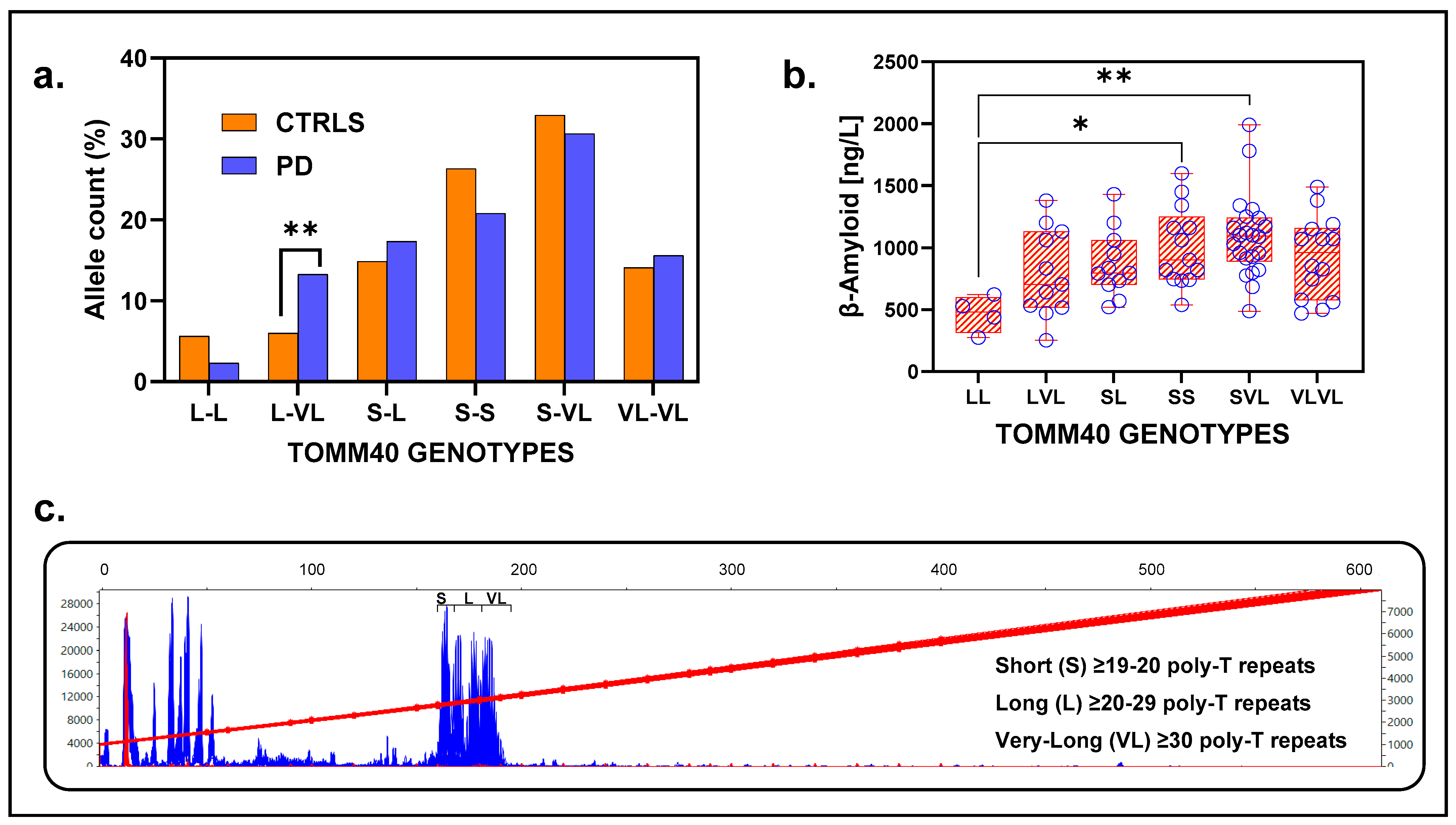
TOMM40 based on repeat sequence length, denoted as short (S), long (L), and very long (VL).
All 161 cases (100%) and only 531 controls (97.07%) were successfully genotyped, resulting in a control-to-case ratio of 3.29. The ApoE-E4 haplotype was strongly associated with the presence of long alleles (Fisher’s exact test,
p = 0.00001), confirming the observations of [
36]. However, no significant association was found between the
ApoE haplotypes with PD. Likewise, no association was found for TOMM40 haplotypes (short, long, and very long) and PD (
Figure S5).
Genotypes identified in the population were L-L, L-VL, S-L, S-S, S-VL, and VL-VL, with S-VL being the most frequent in both groups (PD = 30.63%, CTRL = 32.95%) (
Figure 6a). The genotype Long-poly-T/Very-Long-poly-T (L-VL) showed a strong association with PD, being significantly more prevalent in the patients (14.28%, 23/161) than in the controls (6.03%, 32/531) (Fisher’s exact test,
p = 0.0014). This association remained significant after FDR adjustment (α
c= 0.008, corrected for six tests), with an odds ratio (OR) of 2.60 (95% CI:1.50–4.60,
p = 0.0010) (
Figure 6a).
While PD genotypes at the
TOMM40 locus were in Hardy–Weinberg Equilibrium (PD: Chi2 = 1.26,
p = 0.74), the control population showed significant deviation (Chi2 = 36.36,
p = 0.0001), driven primarily by the genotypes L-L (Chi2 = 19.13,
p = 0.0001), L-VL (Chi2 = 11.29,
p = 0.0001), and VL-VL (Chi2 = 3.75 marginally significant,
p = 0.05), while others (S-L, S-S, and S-VL) were in HWE (
Figure S6). These results suggest potential population-specific effects or biases.
2.7. Clinical and Biochemical Features
ADx, DD, UPDRS, MoCA, and PD scores did not differ across genotypes (
Figure S7a–f). CSF analysis revealed that β-amyloid levels were significantly lower in the L_L genotype group compared to S_L and S_VL groups, with values for L_L (N = 2, M = 355, SD = 116.7 ng/L), S_S (N = 12, M = 1021, SD = 328.5 ng/L), and S_VL (N = 20, M = 1055, SD = 278.6 ng/L). A one-way ANOVA analysis indicated a significant effect of genotype on β-amyloid levels (F = 3.42,
p = 0.009), with post hoc Tukey’s test revealing significant differences between L_L vs. S_S (adjusted
p = 0.053) and L_L vs. S_VL (adjusted
p = 0.03) (
Figure 6b). Genotypes with long alleles (L-L, L-VL, and VL-VL) showed no differences for CSF β-amyloid levels compared to those with at least one short allele (S-S, S-L, and S-VL), designated as intermediate (t (62) 1.58,
p = 0.12) (
Figure S8a,b). To create the two groups, we combined the poly-T expansion lengths of alleles from the S-S, S-L, and S-VL genotypes for the Intermediates, and those from the L-L, L-VL, and VL-VL genotypes into Long. The following values were obtained for the Intermediate genotypes: N = 226, poly-T length M = 20.3, and SD = 8.8. The following values were obtained for the Long genotypes: N = 96, poly-T length M = 32.20, and SD = 4.01. No significant findings were observed for tau and phospho-tau levels.
In conclusion, while TOMM40 alleles alone are not directly associated with PD risk, specific genotypes involving long and very long alleles contribute significantly to risk in the Swedish population. This association may reflect selective assortment of these alleles within the population, potentially influenced by non-random mating or demographic factors. Moreover, the findings highlight the potential role of TOMM40 genotypes in influencing both PD risk and β-amyloid levels in CSF.
2.8. Cross-Sectional Insights into Gene Interactions and Their Impact on PD
2.8.1. APOE × POLG and TOMM40 × POLG
We conducted two independent two-way ANOVA analyses to examine the interactions between specific genetic variants and their effects on key biomarkers. The first analysis focused on the
APOE haplotypes (E3 vs. E4) and
POLG haplotypes (10/11Q_10/11Q vs. Non-10/11Q_10/11Q), while the other analysis assessed the
TOMM40 haplotypes (Intermediate vs. Long) in conjunction with the
POLG haplotypes (see description in the
Section 3). These analyses allowed us to evaluate both the main effects of the
POLG and
TOMM40 haplotypes, as well as their interactions, on important disease-related biomarkers.
2.8.2. APOE × POLG Analysis
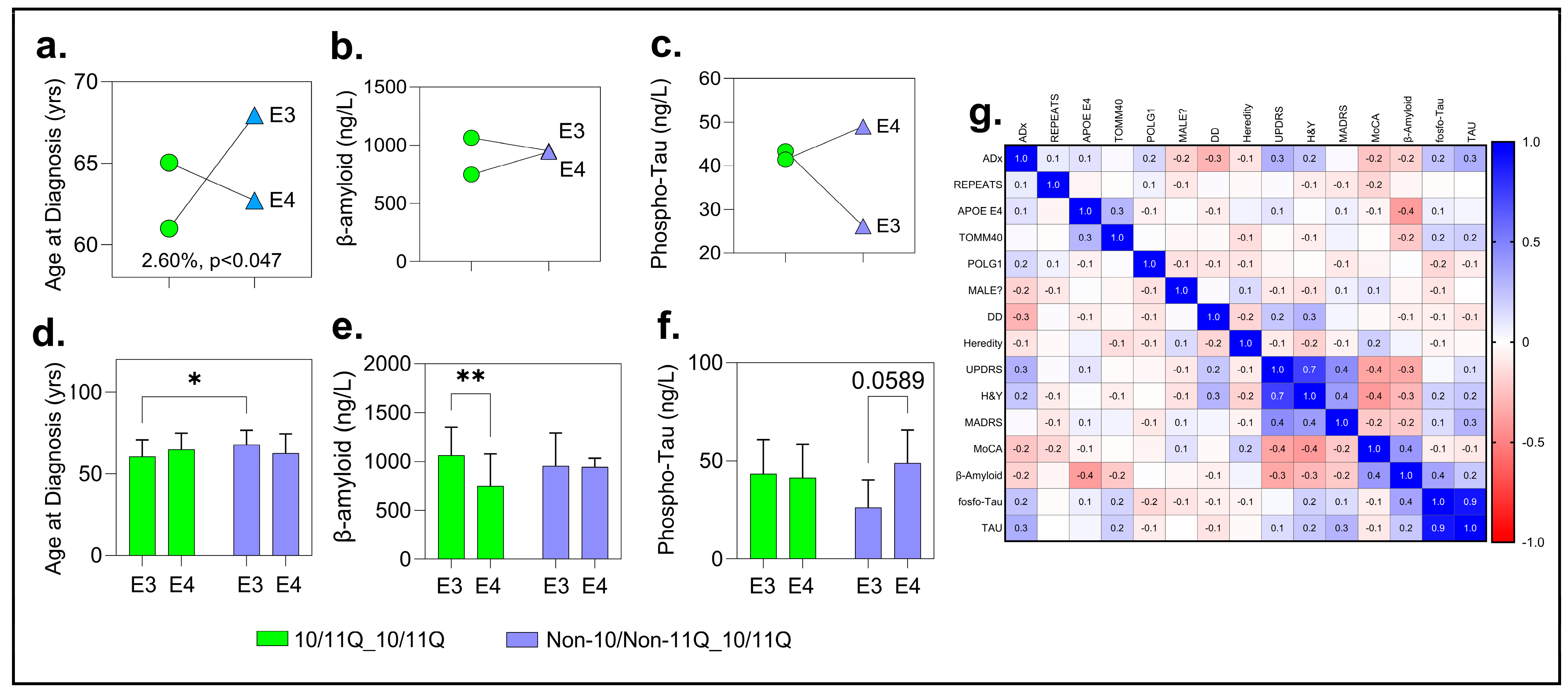
This analysis revealed significant positive associations for ADx, beta-amyloid, and tau levels in CSF for the PD subjects. Although trends were observed for MoCA, this did not reach statistical significance (F (1, 128) =3.42; p = 0.06). For ADx, the two-way ANOVA revealed a significant interaction effect between the APOE haplotypes (E3, E4) and POLG genotypes (10/11Q_10/11Q, Non-10/Non-11Q_10/11Q) on the age at diagnosis, accounting for 2.60% of the total variation (F (1, 146) = 4.02; p = 0.046)). This suggests that the combination of these genetic factors significantly influences the age at which individuals are diagnosed. However, the main effects of the APOE haplotypes (p = 0.85) and POLG genotypes (p = 0.30) were not significant, contributing only 0.02% and 0.67% of the total variation, respectively.
Post hoc analysis using Tukey’s multiple comparison test further examined these interactions, suggesting that the observed variation was primarily driven by a cross-interaction between the
APOE and
POLG groups. Specifically, a significant difference was identified between the E3:10/11Q_10/11Q group (N = 78) and the E3: Non-10/Non-11Q_10/11Q group (N = 19), with a mean difference of −7.32 years (95% CI: −14.02-to-0.62,
p = 0.025). Individuals with the E3/11Q_10/11Q genotype had a significantly earlier age at diagnosis (60.03 ± 10.17 years) compared to those with the E3: Non-10/Non-11Q_10/11Q genotype (67.95 ± 8.70 years) (see
Figure 7a,d and
Figures S9 and S10 for full comparisons of all parameters).
The age at diagnosis showed no significant differences between the
APOE E3 and E4 haplotypes or between the intermediate and long
POLG genotypes and the TOMM40 haplotypes (
p > 0.05,
Figure S11a,h,o), suggesting that the interaction effect is primarily driven by the specific comparison between these subgroups.
No interaction effects were observed for CSF beta-amyloid or phospho-tau levels. However, for beta-amyloid, a significant main effect was found with E3:10/11Q_10/11Q carriers (N = 33, M = 1064 ng/L, SD = 286) showing higher levels than E4:10/11Q_10/11Q carriers (N = 20, M = 748 ng/L, SD = 328; adjusted
p = 0.0024). For phospho-tau, a trend-level difference (adjusted
p = 0.06) suggested slightly higher levels in the E4:10/11Q_10/11Q carriers compared to their E3 counterparts, warranting further investigation (
Figure 7b,c,e,f).
2.8.3. TOMM40 × POLG Analysis
The
TOMM40 × POLG two-way ANOVA revealed no significant interaction or main effects for any biomarkers, including age at diagnosis, beta-amyloid, or phospho-tau levels in CSF. Post hoc analyses confirmed no subgroup differences (all
p > 0.05,
Figures S12 and S13), suggesting that
TOMM40 haplotypes alone or with
POLG genotypes do not significantly influence these biomarkers in this cohort.
Thus, APOE and POLG interactions significantly influenced age at diagnosis and beta-amyloid levels, while TOMM40 and POLG showed no impact on clinical outcomes or biomarkers in this cohort.
2.9. Regression Analysis and Correlation Analysis
We conducted a multiple linear regression analysis to assess the effects of genetic and clinical variables on the dependent variable, Age at Diagnosis (ADx). The model (ADx ~ Intercept + REPEATS + APOE E4 + TOMM40 + POLG + MALE + DD + Heredity + UPDRS + H & Y + MADRS + MoCA + β-Amyloid + p-Tau + Total Tau) was statistically significant (F (14, 32) = 2.07, p = 0.044)), with R2 = 0.4751 and adjusted R2 = 0.245.
Among the predictors,
TAU levels were significant contributors (F (1, 32) = 5.98,
p = 0.02). REPEATS and Disease Duration (DD) showed trends toward significance (
p = 0.064 and
p = 0.063, respectively). Other predictors, including
APOE E4,
TOMM40,
POLG, and clinical parameters (e.g., MoCA, MADRS, and UPDRS), were not significant (
p > 0.05) (
Table S4). Residual variance (SS = 2563) suggests additional unaccounted factors influencing ADx. These findings highlight the importance of tau as a key factor in the variation of ADx (
Table S5).
2.10. Correlation Analysis
To further explore the relationships among variables, we performed a Pearson correlation analysis. Tau showed a significant positive correlation with ADx (Pearson R = 0.324,
p = 0.009). MoCA exhibited a significant negative correlation with ADx (R = −0.238,
p = 0.045).
p-tau had a near-significant positive correlation with ADx (R = 0.237,
p = 0.057). Significant correlations were also observed between phospho-tau and tau (R = 0.752,
p = 0.001) and between MoCA and MADRS (R = −0.296,
p = 0.029). Variables such as REPEATS,
APOE E4, and
TOMM40 showed weak or non-significant correlations with ADx (
p > 0.05) (
Figure 7g; QC and parameters for the correlations and linear regression are detailed in
Figures S14 and S15).
Overall, our analysis explored the interplay between genetic, clinical, and biomarker variables to understand their influence on PD progression and ADx. Tau levels stood out as a significant predictor of age at diagnosis, while other factors, such as repeats and DD, showed trends toward significance. These findings underscore the importance of tau and its associated biomarkers in disease progression, while also highlighting the need for further research to uncover additional contributing factors.
2.11. Long-Term Effects of POLG, APOE, and TOMM40 on PD
We conducted a longitudinal study examining the association between genetic haplotypes in three genes—APOE, TOMM40, and POLG—and key clinical parameters: MoCA and UPDRS. These genes were selected due to their ample sample sizes and the availability of comprehensive data at two time points.
The analysis revealed significant cognitive decline (MoCA) in the
APOE gene, specifically within the E4 haplotype group (t (41) = 2.63,
p = 0.015, q = 0.0162 adjusted by the Benjamini–Yekutieli method (BKY); see
Figure 8a). This group showed a decline of four points per year in cognitive function (M = 24 SD = 3.83 at T1 to M = 20 SD = 5.63 at T2;
Figure 8i and
Figure S17a). However, no significant changes were observed for the E3 haplotype or in the genes
TOMM40 and
POLG when analyzed independently (
Figure 8b,c and
Figure S17b,c).
In terms of interactions, a significant decline in cognitive function was noted only in the
APOE–POLG combination for the E4-Q1 haplotype (
Figure 8k), which paralleled the decline observed in the APOE E4 group alone (t = 2.84, df = 18,
p = 0.01, q = 0.03 after FDR adjustment).
For the UPDRS scores, one-way ANOVA with Geisser correction revealed significant differences among the haplotypes. For
APOE, significant motor deficits were associated with the E4 haplotype, showing an increase in scores from T1 (39.89 ± 16.94) to T2 (52.33 ± 23.22), with t (18) = 3.49,
p = 0.002, and an FDR-adjusted q-value of 0.003. The E3 haplotype showed no discovery (q > 0.05), with a non-significant finding (
p = 0.096). Both haplotypes of
TOMM40 exhibited marked motor deficits, with the long haplotype showing an increase from INT-T1 to INT-T2 by 11 UPDRS points (±3.42), t (36) = 2.109, and
p = 0.042 and the intermediate haplotype increasing by 4.3 points (±2.03), t (18) = 3.214, and
p = 0.0051.
POLG showed scores rising from T1 (38.14 ± 17.68) to T2 (45.14 ± 20.23), t (41) = 3.418, and
p = 0.0014, with adjusted FDR (
Figure 8d–f,k and
Figure S18).
Significant results were also observed in interactions between
TOMM40-POLG and specific items of UPDRS. These differences varied subtly among the groups across UPDRS Items I, II, and III (
Figure 8i and
Figure S19).
Overall, our analysis identified the APOE E4 haplotype as a reliable marker for cognitive decline. We also found that interactions between APOE and POLG genes affect cognition in a similar way. However, the study showed that motor deficits are more commonly associated with TOMM40 haplotypes.
3. Discussion
Briefly, through a comprehensive analysis, we identified significant associations between PD and intermediate repeat expansions in POLG, ATXN2, and C9ORF72, as well as novel structural variations in the ATXN2 promoter region. Additionally, distinct methylation patterns emerged for C9ORF72 expansions, reinforcing the complex genetic landscape underlying PD in this population. Our findings also highlight the contribution of POLG alleles to PD risk, while TOMM40 and TBP showed partial associations. Conversely, we found no significant links between PD and repeat expansions in ATXN3, CACNA1A, or PRNP. We also found significant contributions of the genes APOE, POLG, and TOMM40 in key phenotype aspects of PD, specifically, age at diagnosis, cognitive function (MoCA), clinical scales (UPDRS), and biochemical endophenotypes (β-amyloid and tau).
Previously, a collection of thirty studies (search strategy in
Table S7) surfaced in the genetic of Parkinson disease in Sweden, with alterations in the following genes:
GBA,
SNCA,
POLG,
PLPP4,
HFE,
LRRK2,
S100B,
PARK16,
SLC45A3,
NUCKS1,
RAB7L1,
SLC41A1,
PM20D1,
NFE2L2,
GRIN2A,
HLA-DRA,
MAPT,
GPNMB,
CCDC62/HIP1R,
SYT11,
GAK,
STX1B,
MCCC1/LAMP3,
ACMSD,
FGF20,
COMT,
C9ORF72,
POLG,
GSTM1,
NAT2,
GSTP1,
PSEN2,
CYP2E1,
UCH-L1,
ERβ,
CALCA,
BDNF, and
PON1. Except for two studies that independently analyzed
POLG and
C9ORF72 (see below), most of the existing research does not explore NREs/STRs in PD, nor do they assess clinical correlates. This oversight adds both novelty and translational value to our study, potentially bridging the gap between genetic findings and clinical applications.
Among the studied genes, GBA1 (variants: E326K, N370S, and L444P), LRRK2 (G2019S), NFE2L2, GRIN2A, POLG, and UCH-L1 (S18Y) have shown a positive association or protective function against PD. Conversely, GBA (T369M), HLA-DRA (rs3129882), C9ORF72, and COMT (Val158Met) have demonstrated negative associations. Meanwhile, the roles of SNCA, PARK16, BMP6, and CALCA remain inconclusive.
Alterations in
GBA1 (E326K, N370S, and L444P) are the most frequently observed and are strongly associated with PD, particularly the L444P variant, which exhibits a significant effect size and confers an eight-fold increased risk of developing the disease compared to controls [
16]. In contrast, another study reported a low frequency of the most common mutations in a well-powered (99.9%) PD sample, with
LRRK2 p.(Gly2019Ser) present in only 0.11% of cases and
SNCA duplications in 0.045% [
17]. The scarcity of monogenic causative genes in PD (
SNCA,
PRKN,
PINK1, and
DJ-1) is striking and suggests that much of the genetic architecture may be attributed to susceptibility genes, unless novel monogenic variants are uncovered in the future. In contrast to the low frequency of PD-associated genes, the predominance of L444P mutation carriers in northern Sweden [
16] highlights a distinct and region-specific genetic landscape for PD. This regional disparity may be due to founder effects, which is the case for Gaucher’s disease in northern Sweden. Our study contributes to the list of PD susceptibility genes in Sweden, including
POLG (9.6%),
ATXN2 (3.1%), and
C9ORF72 (2.5%). Although these genes are not monogenic causes of PD, they can be considered risk factors and modifiers of the disease phenotype. This adds translational value by enhancing cohort stratification for clinical trials and improving diagnostic approaches.
Anvret et al. [
37] reported similar frequencies of
POLG variants, and while the role of this gene in PD remains under debate [
38,
39,
40], our study validates the previous association. Both studies indicate that
POLG is relevant as a PD gene, with odds ratios (ORs) and 95% confidence intervals consistently >1 and suggest that this association may be population-specific, with a predilection for Scandinavia. A potential caveat is the possible overrepresentation of PD subjects in our cohort and that of Anvret et al. [
37], as both were drawn from the Stockholm area. However, several factors suggest distinct subject pools. Our cohort included individuals of mixed ethnicity (see
Table S2); however, ethnicity was not specified in the study by Anvret. Additionally, the mean ADx in our cohort was 62.95 ± 10.55 years, which coincided with the age of recruitment, ensuring a temporal alignment. In contrast, Anvret et al. [
37] reported a mean age of diagnosis of 59.4 years and a mean collection age of 67.3 years, reflecting a disease duration of approximately 8 years at recruitment, compared to ~5 years in our cohort. These demographic and temporal differences, combined with the inclusion of subjects from diverse ethnic backgrounds in our study, further differentiates the two cohorts, despite their geographic proximity.
Beyond the observed genetic contributions, our study provides new insights into the
APOE–
POLG interaction, particularly its influence on age at diagnosis and CSF endophenotypes. E3 carriers with rare
POLG variants show a protective effect, leading to a later diagnosis of PD. A striking discovery is the substantial increase in beta-amyloid levels in CSF among
APOE E3 carriers with the most common
POLG haplotype, compared to their E4 counterparts. Therefore,
APOE–
POLG interaction not only influences the age of diagnosis of PD but also impacts cerebrospinal fluid (CSF) biomarkers, offering potential avenues for early diagnosis and targeted therapies.
APOE E4 has been modestly associated with lower cognitive scores, though not without discrepancies [
38,
39,
40,
41,
42]. It has also been linked to rapid motor progression in PD [
43], and genome-wide analyses have identified it as a determinant of mortality [
44]. However, its interaction with
POLG remains unexplored.
Our screening identified
ATXN2 as the second most frequent gene of interest, with allele sizes ranging from 27 to 37 CAG repeats, and the most frequent were those sized with 27 repeats. Alleles with 27 CAG repeats are generally considered low or no risk for ALS [
45].
However, the risk for ALS diminishes as the CAG repeat length approaches the SCA2 threshold of 33 repeats, a range where the repeat length is conclusively pathogenic for the Central Nervous System, leading to SCA2 [
46], and where genetic overlap with FTD has been identified [
47]. While this relative risk estimate may suggest a potential pan-neuronal toxicity, the association appears to be ALS-specific and not necessarily relevant to PD. We presented a case of PD with homozygous
ATXN2 intermediate alleles (27/27 CAG repeats). A prior case by [
48] reported homozygous 31/31 CAG repeats as non-pathogenic but conferring ALS risk, prompting to propose varied mechanisms by which these variants may contribute to
ATXN2-spectrum diseases. The frequency of these co-occurrences in gnomAD v4.10 is 27/27 repeats = 4 × 10
−4 vs. 3 × 10
−3 in our population. The summation for each genotype is 54, corresponding to
ATXN2 risk genotypes, such as 22/32 CAG repeats. In this patient, homozygosity could exacerbate neurodegeneration via a gene dosage effect, a pseudo-recessive inheritance pattern where bi-allelic intermediate alleles amplify disease risk, or through RNA gain-of-function effects and disrupted stress granule dynamics. These mechanisms may synergize in homozygous states, driving severe motor and cognitive phenotypes. This case highlights the need to investigate diverse inheritance patterns and the molecular mechanisms by which intermediate alleles contribute to disease heterogeneity in
ATXN2-related disorders.
Notably, inconsistencies have been reported regarding the association between
ATXN2 intermediate alleles and PD. Gispert et al. [
49] analyzed a large cohort of ~1500 PD subjects and found a significant enrichment in 27–28 CAG alleles in the Düsseldorf subgroup. However, the overall risk of sporadic PD remained unchanged, as no such enrichment was observed in the Frankfurt or Tübingen subgroups from the same study. In contrast, Yamashita et al. [
50] concluded that
ATXN2 polyQ expansion is a specific predisposing factor for PD, reporting that alleles with ≥24 repeats were significantly enriched in PD patients with typical L-DOPA-responsive phenotypes. However, Wang et al. [
19] refuted this finding using a large dataset of >12K PD cases from the Genetic Epidemiology of PD Consortium (GEOPD) and applying the same cutoff (≥24 repeats). The unbalanced control/subject ratio (0.66) in the Wang et al. study may have limited the precision of their estimations. Nevertheless, their findings were ultimately taken as evidence to dismiss the routine screening of SCA2 in PD patients [
51]. Ataxin-2 remains a conundrum, with emerging studies reinforcing its role in PD through diverse mechanisms. These include novel variants, full expansion, and recessive inheritance, such as the 9-bp duplication, all highlighted here;
ATXN2 double dosage [
52]; and intermediate or fully expanded CAG repeats (35–39 CAG repeats), which have recently been linked to the parkinsonian spectrum [
26,
53,
54].
C9ORF72 is rarely, if ever, associated with PD [
55,
56,
57,
58,
59,
60], making the presence of six alleles displaying the characteristic saw-tooth-like pattern particularly striking. We assumed the alleles range from intermediate to moderately long to address the contentious dilemma between evidence and consensus, supported by the empirical observation that long tails were absent in the fragment analysis compared to the long alleles typically observed in ALS (
Figure S20). Additionally, two other alleles, with repeat lengths of 30 and 35, completed the C9 cohort. Notably, 66% of expanded alleles shared the risk-associated (T) haplotype at rs3849942 and exhibited detectable methylation at the CpG island. All C9 carriers showed pure PD, and the clinical and imaging data were typical for PD with DAT striatal loss and also cerebellar degeneration. While previous studies in Sweden have excluded
C9ORF72 as a PD gene based on the saw-tooth pattern [
61], further investigations using orthogonal techniques, such as Asuragen or Long-Read Sequencing, are necessary to accurately estimate the size and classification of these likely intermediate/moderately long, mutatis mutandis, alleles in our PD cohorts. Our cases challenge the binary all-or-nothing criteria by demonstrating the presence of a saw-tooth pattern.
Notably, one carrier of ≥60 C9RE repeats exhibited co-occurring digenic inheritance with an intermediate
TBP allele, further underscoring the complexity and significance of this case series. Intermediate or incomplete penetrant
TBP41–46 alleles have also been identified in cases of digenism associated with
STUB1 mutations. These findings are now recognized as defining two distinct spinocerebellar autosomal recessive type 16 (SCAR16 or SCA17-DI) entities separated from SCA48 [
62].
In our study, although TBP was not statistically significant, it remains clinically relevant, as two patients were identified with full penetrant expansions. Precisely assigning risk helps determine the proportion of affected individuals, but even a single case with a pathogenic variant carries significant translational value for advancing precision medicine. While statistical significance aids in assigning risk within a population, it often overlooks the importance of low-prevalence variants. Non-significance is frequently misinterpreted as no effect; however, when a variant is known to be pathogenic, its clinical relevance is undeniable, regardless of statistical outcomes. This underscores the crucial need to integrate statistical analysis with clinical evidence to accurately assess its impact on population-level risk and individual patient care. In a clinical context, we treat patients, not p-values.
The association of intermediate expansions in
TBP with PD has been a topic of significant debate. It is important to highlight that the inconsistency in defining thresholds for TBP intermediate and incomplete penetrance is not merely a matter of debate but often reflects a superficial approach to the subject. For instance, while one publication after another cites thresholds of 41–46 repeats as the range for intermediate penetrance [
62], other studies arbitrarily extend this range to 49 repeats [
63,
64]. Such ad hoc adjustments not only lack rigorous justification but also complicate consistent data interpretation and cross-study comparisons. However, expert consensus established the ranges as 43–48 repeats for reduced penetrance and 49–66 repeats for full penetrance [
65]. Two meta-analyses, conducted by the GEO-PD consortium [
19] and Rossi et al. (MDSGene Task Force) [
66], further addressed this question. The GEO-PD study used a threshold of 42–47 repeats (based on SCA17 criteria) and found no evidence of an association with PD. However, Rossi et al. [
66], redefining the threshold, observed that pure parkinsonism was more prevalent in ATX-TBP patients with 41–45 repeats, while those with ≥46 repeats more frequently presented with a complex phenotype characterized by mixed movement disorders. An updated genotype–phenotype assessment for ATX-TBP is presented, proposing new repeat expansion cutoffs: reduced penetrance (41–45 repeats) and full penetrance (46–66 repeats). These revisions carry diagnostic and counseling implications and may inform future clinical trial protocols [
66].
In our study, the mutation range spanned from reduced to full penetrance (41–48 CAG repeats), including one case with dual inheritance. The estimated sample size is promising for detecting small effects in our new cohorts, laying a strong foundation for impactful future studies.
The association of the
ApoE-E4 haplotype with the presence of long
TOMM40 alleles has been well-documented, with previous studies, such as Roses et al. [
36], highlighting this linkage and relevance in Alzheimer’s Disease. Our findings confirm this strong association with long and very long alleles being overrepresented in the
ApoE-E4 haplotype. However, the association of specific haplotypes with PD is inconsistent in our and other studies [
67,
68,
69]. Surprisingly, long genotypes (L-VL) were significantly associated with PD, which may initially be attributed to other effects, such as the enrichment of
APOE-ε4 alleles and the effects of LD with E4. However, the S-L genotype, despite similar ε4 enrichment, showed no significant association. The most plausible explanation for this finding is the observed HWE disequilibrium in controls for the same genotype, which contributed to deviations. Notably, when the expected frequency is adjusted, this association disappears. Therefore, the deviation in the control group raises questions about whether the association is either artifact-driven (the result of comparing a biased control population to PD cases) or disease-driven (a reflection of a genuine biological predisposition in PD patients), which would suggest that the association is spurious and that
TOMM40 is irrelevant to PD. Beyond any speculation, these results should be interpreted with caution and require further confirmation in a more specifically designed control cohort.
Independent of genetic association, we found that
TOMM40 in our cohort significantly influences severity and long-term progression of PD, outperforming the more uneven influences of
APOE and
POLG. Its effects span nearly all UPDRS subdomains, making it a stronger predictor of disease progression and severity. In follow-up studies, short and long alleles have been shown to predict cognitive performance over time [
69]; however, this was not supported by our observations but still validate
TOMM40’s role in PD.
Gene expression studies, using cloned LD regions encompassing the
TOMM40-
APOE-APOC genomic signature, have revealed that enhancers within
TOMM40 regulate the promoter activity of both
TOMM40 and
APOE in a haplotype- and cell-type-specific manner. This regulatory activity is further influenced by the length of the poly-T repeat, with enhanced expression notably observed in neurons [
70].
Our analysis reveals a disequilibrium in some genotypes when using the unified long haplotype (L), contrasting with studies that divide it into subgroups (La and Lb) [
71,
72]. This division creates artificial haplotypes and genotypes, diluting potential signals of disequilibrium by generating lower expected frequencies that favor the Hardy–Weinberg Equilibrium (HWE). When calculating HWE without separating the haplotypes, we observed expected frequencies of zero for some genotypes, resulting in infinitum chi-square values and complicated interpretations. Maruszak et al. [
71] state that
La and
Lb have similar effects. Unifying haplotypes better reflects the genetic structure, as demonstrated in Australian cohorts [
68], where no HWE disequilibrium was seen (calculations were made by us). Despite methodological challenges, we recommend harmonized approaches in future studies to avoid artifacts and improve comparability.
Repeat length variations in ATXN3, PRNP, and CACNA1A were irrelevant for PD in our cohort.
Mutations in
ATXN3,
PRNP, and
CACNA1A have been implicated in PD and parkinsonism through diverse mechanisms and clinical presentations in isolated cases.
ATXN3, associated with Machado–Joseph disease (SCA3), has been reported in cases of atypical parkinsonism with L-dopa responsiveness, especially in patients with relatively low repeat numbers [
21,
72,
73,
74].
CACNA1A, linked to SCA6, has been described in cases of parkinsonism combined with cerebellar ataxia, with both L-dopa-responsive and non-responsive presentations, suggesting variability in dopaminergic dysfunction [
75]. In contrast, polymorphisms in
PRNP, including codon 129 variations, show no association with PD genetics across diverse populations [
76].
We present observations that underscore the meaningful contribution of these genes rather than mere associations. Age at diagnosis was correlated with POLG, male sex, UPDRS, H & Y, MoCA, and tau levels. Among these, tau levels were validated through regression analysis, while repeat expansions exhibited a trend toward association. Additionally, ADx correlated with DD. While many of these correlations (sex, UPDRS, H & Y, MoCA, and tau) have been confirmed with age at onset in other cohorts [
77,
78,
79], the positive correlation between
POLG variants and age at diagnosis is particularly intriguing.
Our analysis reveals that carriers of more frequent POLG variants experience faster disease progression, as measured by the UPDRS, with significant differences across all UPDRS subdomains. This highlights a potential role for POLG in influencing motor symptom severity and progression in PD, warranting further investigation into its pathogenic contribution. Furthermore, TOMM40 demonstrates superior predictive value over APOE and POLG when grouping PD individuals for progression analyses. Importantly, the TOMM40 effect is not a simple surrogate for APOE-related mechanisms, as the PD profiles of individuals differ significantly, underscoring its distinct role in disease progression.
Limitations
Our study was technically robust: all samples were tested in a single facility with rigorous quality control, internal standards consistently applied, and a control/patient design offering substantial predictive value. However, several limitations should be acknowledged. Increasing the sample size of the PD subjects, given the wide range of odds ratios, would enhance the power of our study and improve the precision of the associations. The low or null marginal frequencies of some variants may affect the robustness of these associations. Using ADx instead of age at onset may introduce inaccuracies in estimating the true onset of the disease. Likewise, missing values affect the stability of ANOVA, regression analyses, and correlations, posing a significant challenge. This is a common issue in tertiary centers and clinics managing large datasets, where incomplete data can undermine the robustness and reliability of statistical analyses. Addressing this limitation is critical to ensure accurate and meaningful interpretations of the findings. The lack of nuclear imaging analyses limits our ability to closely examine neurodegeneration and correlate it with genetic or clinical findings. Additionally, the absence of sequencing for some samples prevented us from confirming both repeat length and specific genetic variants or detecting triplet interruptions, which are known to modify disease risk and progression. For
C9ORF72, while three-primer G4C2-Repeat Primed (RP)–PCR coupled to capillary electrophoresis is reliable for sizing alleles with ~24–45 repeats, it is less accurate for larger expansions, which could be better resolved using methods like Asuragen (Limit Of Detection ~145 rep) or long-read sequencing. However, we followed the routine practices commonly conducted in most research labs, given the prohibitive costs of both techniques (USD 50 and USD 3500 per individual, respectively) compared to USD 10 for conventional (RP)-PCR. Furthermore, our study was conducted at a time when these techniques were not yet available, and some of our DNA samples were of suboptimal quality. The confirmation of low-risk alleles (
ATXN2: 27 repeats,
TBP: 41–42 repeats,
C9ORF72: 21–30 repeats) through functional studies is essential, along with observational analyses at the neuronal level, to establish their correlation or potential causal role. We also did not evaluate newly discovered non-repeat expansions, such as GGC repeats in
ZFHX3 [
80], which may explain additional PD cases or interact with other mutations to influence phenotypes. The longitudinal analysis, with only two time points, may have failed to capture stable or nuanced changes over time, leaving the data vulnerable to experimenter or clinician biases. Moreover, the use of blood bank controls instead of a population-representative control group may have introduced a recruitment bias, as blood bank participants often include undiagnosed cases. Finally, while our study identified significant associations, these do not establish causality. Functional research is essential to validate these findings and explore the underlying mechanisms, especially for the genetic variants and repeat expansions highlighted in this study. However, while these limitations are acknowledged, they do not compromise our conclusions given the screening nature of our study, as our findings align with and are validated by previous studies that have established the role of these genes.