Exploring the Bioactive Potential and Biocompatibility of Extracts from Agro-Industrial Residues for Cosmetic Applications
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Samples and Reagents
3.2. Phenolic Compounds Extraction
3.3. Extracts Characterisation
3.3.1. Total Phenolic Content
3.3.2. Antioxidant Activity
3.4. Extracts Biocompatibility
3.4.1. Cell Culture
3.4.2. Resazurin Assay
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development. A/RES/70/1. 2015. Available online: https://sdgs.un.org/sites/default/files/publications/21252030%20Agenda%20for%20Sustainable%20Development%20web.pdf (accessed on 5 March 2025).
- Food and Agriculture Organization. State of Food and Agriculture 2019. Moving Forward on Food Loss and Waste Reduction. Rome. 2019. Available online: http://www.fao.org/3/ca6030en/ca6030en.pdf (accessed on 5 March 2025).
- Singh, R.; Das, R.; Sangwan, S.; Rohatgi, B.; Khanam, R.; Peera, S.K.P.G.; Das, S.; Lyngdoh, Y.A.; Langyan, S.; Shukla, A.; et al. Utilisation of agro-industrial waste for sustainable green production: A review. Environ. Sustain. 2021, 4, 619–636. [Google Scholar] [CrossRef]
- Reguengo, L.M.; Salgaço, M.K.; Sivieri, K.; Maróstica Júnior, M.R. Agro-industrial by-products: Valuable sources of bioactive compounds. Food Res. Int. 2022, 152, 110871. [Google Scholar] [CrossRef]
- Zuñiga-Martínez, B.S.; Domínguez-Avila, J.A.; Robles-Sánchez, R.M.; Ayala-Zavala, J.F.; Villegas-Ochoa, M.A.; González-Aguilar, G.A. Agro-Industrial Fruit Byproducts as Health-Promoting Ingredients Used to Supplement Baked Food Products. Foods 2022, 11, 3181. [Google Scholar] [CrossRef]
- Simitzis, P.E. Agro-Industrial by-Products and Their Bioactive Compounds—An Ally against Oxidative Stress and Skin Aging. Cosmetics 2018, 5, 58. [Google Scholar] [CrossRef]
- Tapia-Blácido, D.R.; Garcia, A.L.; Beitum, L.R.; Zitei-Baptista, L.F.; Aguilar, P.F. Chapter 6—Use of biobased materials from agro-industrial residues in food packaging. In Advanced Applications of Biobased Materials; Ahmed, S., Annu, Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 173–229. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Ito, J.; Komuro, M.; Parida, I.S.; Shimizu, N.; Kato, S.; Meguro, Y.; Ogura, Y.; Kuwahara, S.; Miyazawa, T.; Nakagawa, K. Evaluation of lipid oxidation mechanisms in beverages and cosmetics via analysis of lipid hydroperoxide isomers. Sci. Rep. 2019, 9, 7387. [Google Scholar] [CrossRef]
- Pinto, D.; Lameirão, F.; Delerue-Matos, C.; Rodrigues, F.; Costa, P. Characterization and Stability of a Formulation Containing Antioxidants-Enriched Castanea sativa Shells Extract. Cosmetics 2021, 8, 49. [Google Scholar] [CrossRef]
- Silva, A.M.; Costa, P.C.; Delerue-Matos, C.; Rodrigues, F. Assessment of a Formulation Containing a Castanea sativa Shells Extract on Skin Face Parameters: In Vivo Evaluation. Processes 2022, 10, 2230. [Google Scholar] [CrossRef]
- Salem, Y.; Rajha, H.N.; Sunoqrot, S.; Hammad, A.M.; Castangia, I.; Manconi, M.; Manca, M.L.; Al Lababidi, D.; Touma, J.A.; Maroun, R.G.; et al. Exhausted Grape Seed Residues as a Valuable Source of Antioxidant Molecules for the Formulation of Biocompatible Cosmetic Scrubs. Molecules 2023, 28, 5049. [Google Scholar] [CrossRef]
- Yarovaya, L.; Waranuch, N.; Wisuitiprot, W.; Khunkitti, W. Clinical study of Asian skin changes after application of a sunscreen formulation containing grape seed extract. J. Cosmet. Dermatol. 2022, 21, 4523–4535. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.M.; Miranda, R.; Santos, L. Sustainable Cosmetics: Valorisation of Kiwi (Actinidia deliciosa) by-Products by Their Incorporation into a Moisturising Cream. Sustainability 2023, 15, 14059. [Google Scholar] [CrossRef]
- Messias, M.A.; Ferreira, S.M.; Tavares, L.; Santos, L. A Comparative Study between Onion Peel Extracts, Free and Complexed with β-Cyclodextrin, as a Natural UV Filter to Cosmetic Formulations. Int. J. Mol. Sci. 2023, 24, 15854. [Google Scholar] [CrossRef]
- Ferreira, D.F.; da Silva, T.M.; de Melo, R.C.G.; Bastos, K.A.; Ucella-Filho, J.G.M.; Severi, J.A.; Villanova, J.C.O.; Resende, J.A. Development of a gel formulation with pomegranate peel extract (Punica granatum L.) for antimicrobial and wound healing action. S. Afr. J. Bot. 2024, 173, 284–294. [Google Scholar] [CrossRef]
- Regulation (EC) No. 1333/2008 of the European Parliament and of the Council on Food Additives. 2008. Available online: https://eur-lex.europa.eu/eli/reg/2008/1333/oj (accessed on 26 March 2025).
- Lameirão, F.; Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Sut, S.; Dall’acqua, S.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Green-Sustainable Recovery of Phenolic and Antioxidant Compounds from Industrial Chestnut Shells Using Ultrasound-Assisted Extraction: Optimization and Evaluation of Biological Activities In Vitro. Antioxidants 2020, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- Salem, Y.; Sunoqrot, S.; Rajha, H.N.; Abusulieh, S.; Afif, C.; Francis, H.; Touma, J.A.; Louka, N.; Maroun, R.G. Grape seed phenolic extracts encapsulation in polymeric nanoparticles: Characterization and in vitro evaluation against skin melanoma. J. Drug Deliv. Sci. Technol. 2024, 100, 106094. [Google Scholar] [CrossRef]
- George, D.; Maheswari, P.U.; Begum, K.M.M.S. Synergic formulation of onion peel quercetin loaded chitosan-cellulose hydrogel with green zinc oxide nanoparticles towards controlled release, biocompatibility, antimicrobial and anticancer activity. Int. J. Biol. Macromol. 2019, 132, 784–794. [Google Scholar] [CrossRef]
- Monika, P.; Chandraprabha, M.N.; Murthy, K.N.C. Catechin, epicatechin, curcumin, garlic, pomegranate peel and neem extracts of Indian origin showed enhanced anti-inflammatory potential in human primary acute and chronic wound derived fibroblasts by decreasing TGF-β and TNF-α expression. BMC Complement. Med. Ther. 2023, 23, 181. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Cádiz-Gurrea, M.d.l.L.; Garcia, J.; Saavedra, M.J.; Freitas, V.; Costa, P.; Sarmento, B.; Delerue-Matos, C.; Rodrigues, F. From soil to cosmetic industry: Validation of a new cosmetic ingredient extracted from chestnut shells. Sustain. Mater. Technol. 2021, 29, e00309. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Gomes, S.M.; Santos, L. The Chemistry Behind Biological Properties of Agro-Industrial Portuguese By-Products. Waste Biomass Valor. 2024, 15, 2721–2733. [Google Scholar] [CrossRef]
- Kocer, S.; Utku Copur, O.; Ece Tamer, C.; Suna, S.; Kayahan, S.; Uysal, E.; Cavus, S.; Akman, O. Optimization and characterization of chestnut shell pigment extract obtained microwave assisted extraction by response surface methodology. Food Chem. 2024, 443, 138424. [Google Scholar] [CrossRef]
- Malinowska, M.A.; Ferrier, M.; Sharafan, M.; Szopa, A.; Gémin, M.-P.; Miastkowska, M.; Bialik-Wąs, K.; Dziki, A.; Sikora, E.; Kiszka, A.; et al. The cosmetic potential evaluation of the fungus resistant grapevine extracts using metabolomic approaches. Sci. Rad. 2024, 3, 187–211. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Aladeselu, O.H.; Oboh, G.; Boligon, A.A. Drying alters the phenolic constituents, antioxidant properties, α-amylase, and α-glucosidase inhibitory properties of Moringa (Moringa oleifera) leaf. Food Sci. Nutr. 2018, 6, 2123–2133. [Google Scholar] [CrossRef]
- Guthrie, F.; Wang, Y.; Neeve, N.; Quek, S.Y.; Mohammadi, K.; Baroutian, S. Recovery of phenolic antioxidants from green kiwifruit peel using subcritical water extraction. Food Bioprod. Process 2020, 122, 136–144. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Liu, H.; Zhao, T.; Meng, C.; Liu, Z.; Liu, X. Bioactive compounds and in vitro antioxidant activities of peel, flesh and seed powder of kiwi fruit. Int. J. Food Sci. Technol. 2018, 53, 2239–2245. [Google Scholar] [CrossRef]
- Benito-Román, Ó.; Blanco, B.; Sanz, M.T.; Beltrán, S. Subcritical Water Extraction of Phenolic Compounds from Onion Skin Wastes (Allium cepa cv. Horcal): Effect of Temperature and Solvent Properties. Antioxidants 2020, 9, 1233. [Google Scholar] [CrossRef] [PubMed]
- Bordin Viera, V.; Piovesan, N.; Mello, R.D.O.; Barin, J.S.; Fogaça, A.D.O.; Bizzi, C.A.; Flores, É.M.D.M.; Costa, A.C.D.S.; Pereira, D.E.; Soares, J.K.B.; et al. Ultrasonic-assisted extraction of phenolic compounds with evaluation of red onion skin (Allium cepa L.) antioxidant capacity. J. Culin. Sci. Technol. 2023, 21, 156–172. [Google Scholar] [CrossRef]
- Pinto, D.; Silva, A.M.; Freitas, V.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Microwave-Assisted Extraction as a Green Technology Approach to Recover Polyphenols from Castanea sativa Shells. ACS Food Sci. Technol. 2021, 1, 229–241. [Google Scholar] [CrossRef]
- Pinto, D.; Cádiz-Gurrea, M.d.l.L.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Castanea sativa shells: A review on phytochemical composition, bioactivity and waste management approaches for industrial valorization. Food Res. Int. 2021, 144, 110364. [Google Scholar] [CrossRef]
- Fitrasyah, S.I.; Ariani, A.; Rahman, N.; Nurulfuadi, N.; Aiman, U.; Nadila, D.; Pradana, F.; Rakhman, A.; Hartini, D.A. Analysis of Chemical Properties and Antioxidant Activity of Sambiloto (Andrographis paniculata Nees.) Leaf Tea Formula as a Functional Drink in Preventing Coronavirus Diseases and Degenerative Diseases. Open Access Mac. J. Med. Sci. 2021, 9, 196–201. [Google Scholar] [CrossRef]
- Gomes, S.M.; Leitão, A.; Alves, A.; Santos, L. Incorporation of Moringa oleifera Leaf Extract in Yoghurts to Mitigate Children’s Malnutrition in Developing Countries. Molecules 2023, 28, 2526. [Google Scholar] [CrossRef]
- Ramôa, A.M.; Campos, F.; Moreira, L.; Teixeira, C.; Leiro, V.; Gomes, P.; das Neves, J.; Martins, M.C.L.; Monteiro, C. Antimicrobial peptide-grafted PLGA-PEG nanoparticles to fight bacterial wound infections. Biomater. Sci. 2023, 11, 499–508. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-05; Biological Evaluation of Medical Devices. International Organisation for Standardization: Geneva, Switzerland, 2009.

| Agro-Industrial Residue | Objectives | Main Results | Ref. |
|---|---|---|---|
| Chestnut Shell (CS) | Evaluate the organoleptic and technological properties, as well as the stability, of an oil-in-water semisolid formulation containing chestnut shell extracts. | The formulation presented pleasant organoleptic properties, a skin-compatible pH, and suitable viscosity for topical application, with no impact on general stability. | [10] |
| Assess the impact of a facial formulation containing chestnut shell extract on different human skin parameters. | The formulation improved the skin hydration, while slightly decreasing its roughness and wrinkles’ depth. The skin firmness increased after the application of the developed product. | [11] | |
| Grape Seed (GS) | Study the use of grape seed extracts, in different concentrations (0.5, 1, 1.5, and 2% w/w), as an ingredient for cosmetic scrubs. | All formulations presented suitable spreadability and excellent physical stability. Scrubs enriched with grape seed extracts up to 1.5% did not cause skin irritation in vivo. The addition of the extract increased the total phenolic content and antioxidant activity of the scrubs. | [12] |
| Evaluate the effectiveness of grape seed extract as a natural ingredient of sunscreen formulations for reducing skin age-related changes. | The application of the formulation containing the extract reduced melanin and erythema levels while improving skin tone, hydration and elasticity. | [13] | |
| Kiwi Peel (KP) | Investigate the impact of incorporating kiwi peel extract into a moisturising cream. | The incorporation of the extract increased the antioxidant activity without affecting the product’s microbial safety and stability for two weeks. | [14] |
| Onion Peel (OP) | Examine the efficacy of onion peel extracts (free or encapsulated) as natural UV filters. | Onion peel extract and its microparticles conferred antioxidant properties to the sunscreen without affecting its stability. These natural ingredients proved to be effective in protecting the skin from UV radiation. | [15] |
| Pomegranate Peel (PP) | Assess the potential for creating a gel for topical application using pomegranate peel extract for wound healing and antimicrobial purposes. | Gels incorporated with pomegranate peel extracts presented antimicrobial properties against different microorganisms (S. aureus, S. epidermidis, E. coli, C. albicans). The formulation’s in vitro antimicrobial activity remained effective over 30 days against all tested strains. | [16] |
| CS | GS | KP | OP | PP | ||
|---|---|---|---|---|---|---|
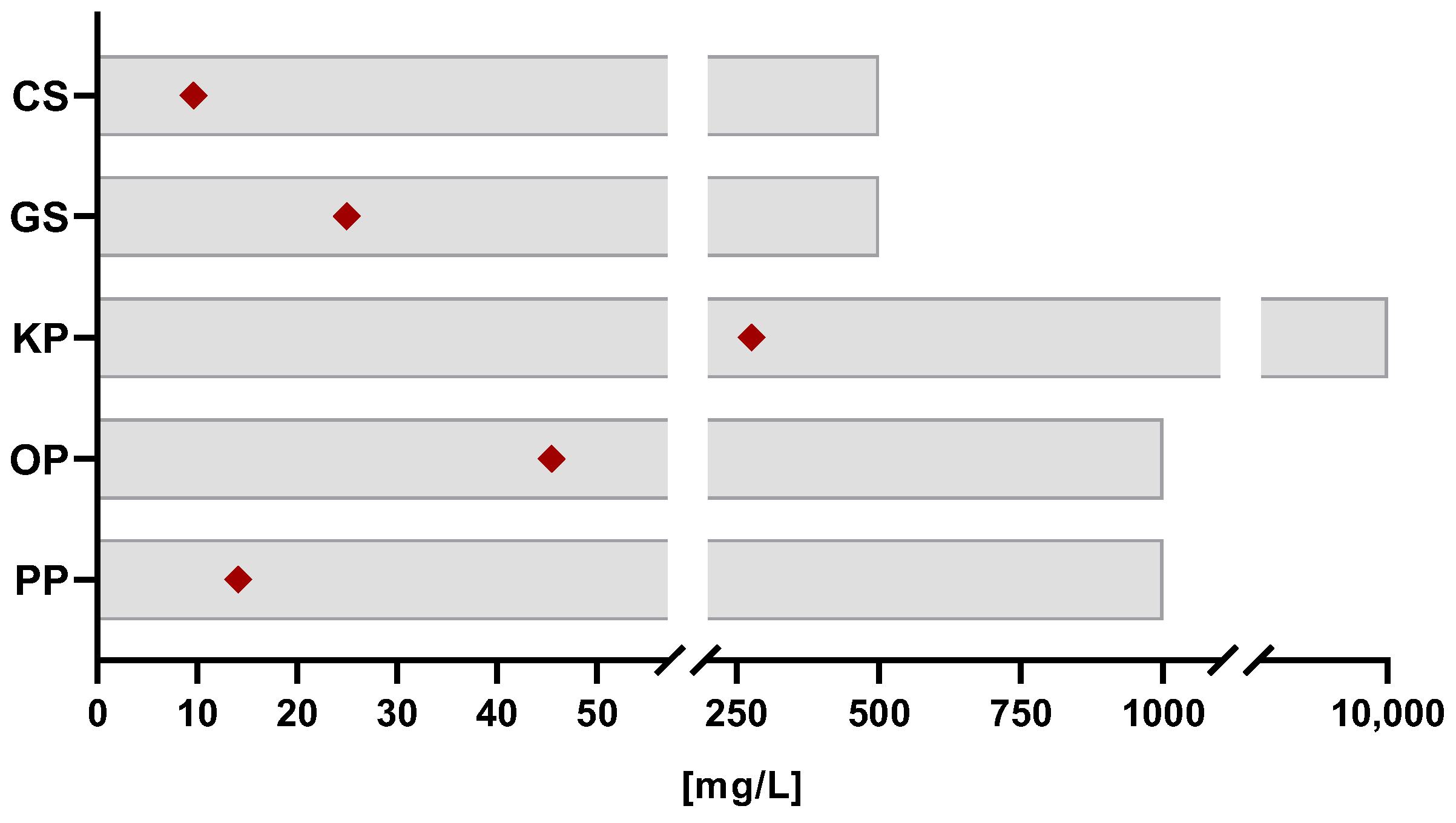
| TPC (mgGAE/gextract) | 343.9 ± 124.6 a | 208.5 ± 39.7 a | 37.6 ± 8.3 b | 243.8 ± 51.2 a | 260.3 ± 67.6 a | |
| DPPH | IC50 (mg/L) | 9.6 ± 1.2 a | 25.0 ± 12.7 a,c | 276.8 ± 169.7 b | 45.5 ± 21.4 b,c | 14.1 ± 4.3 a |
| mgTE/gextract | 413.5 ± 179.1 a | 169.0 ± 21.4 a,c | 16.2 ± 5.4 b | 93.9 ± 46.6 b,c | 270.6 ± 65.2 a | |
| ABTS | IC50 (mg/L) | 3.5 ± 1.7 a | 6.5 ± 1.9 a,c | 49.9 ± 3.6 b | 10.0 ± 0.4 b,c | 4.3 ± 1.1 a |
| mgTE/gextract | 657.8 ± 235.9 a | 324.8 ± 95.6 a,c | 39.1 ± 3.6 b | 194.6 ± 7.6 b,c | 478.3 ± 106.5 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, S.M.; Campos, F.; Martins, M.C.L.; Monteiro, C.; Santos, L. Exploring the Bioactive Potential and Biocompatibility of Extracts from Agro-Industrial Residues for Cosmetic Applications. Int. J. Mol. Sci. 2025, 26, 9169. https://doi.org/10.3390/ijms26189169
Gomes SM, Campos F, Martins MCL, Monteiro C, Santos L. Exploring the Bioactive Potential and Biocompatibility of Extracts from Agro-Industrial Residues for Cosmetic Applications. International Journal of Molecular Sciences. 2025; 26(18):9169. https://doi.org/10.3390/ijms26189169
Chicago/Turabian StyleGomes, Sandra M., Filipa Campos, M. Cristina L. Martins, Cláudia Monteiro, and Lúcia Santos. 2025. "Exploring the Bioactive Potential and Biocompatibility of Extracts from Agro-Industrial Residues for Cosmetic Applications" International Journal of Molecular Sciences 26, no. 18: 9169. https://doi.org/10.3390/ijms26189169
APA StyleGomes, S. M., Campos, F., Martins, M. C. L., Monteiro, C., & Santos, L. (2025). Exploring the Bioactive Potential and Biocompatibility of Extracts from Agro-Industrial Residues for Cosmetic Applications. International Journal of Molecular Sciences, 26(18), 9169. https://doi.org/10.3390/ijms26189169








