First Look at Chemopreventive Properties of Chlorella pyrenoidosa Water Extract in Human Endometrial Adenocarcinoma Cells—Preliminary In Vitro Study
Abstract
1. Introduction
2. Results
2.1. Main Components of Water Extract of Chlorella pyrenoidosa
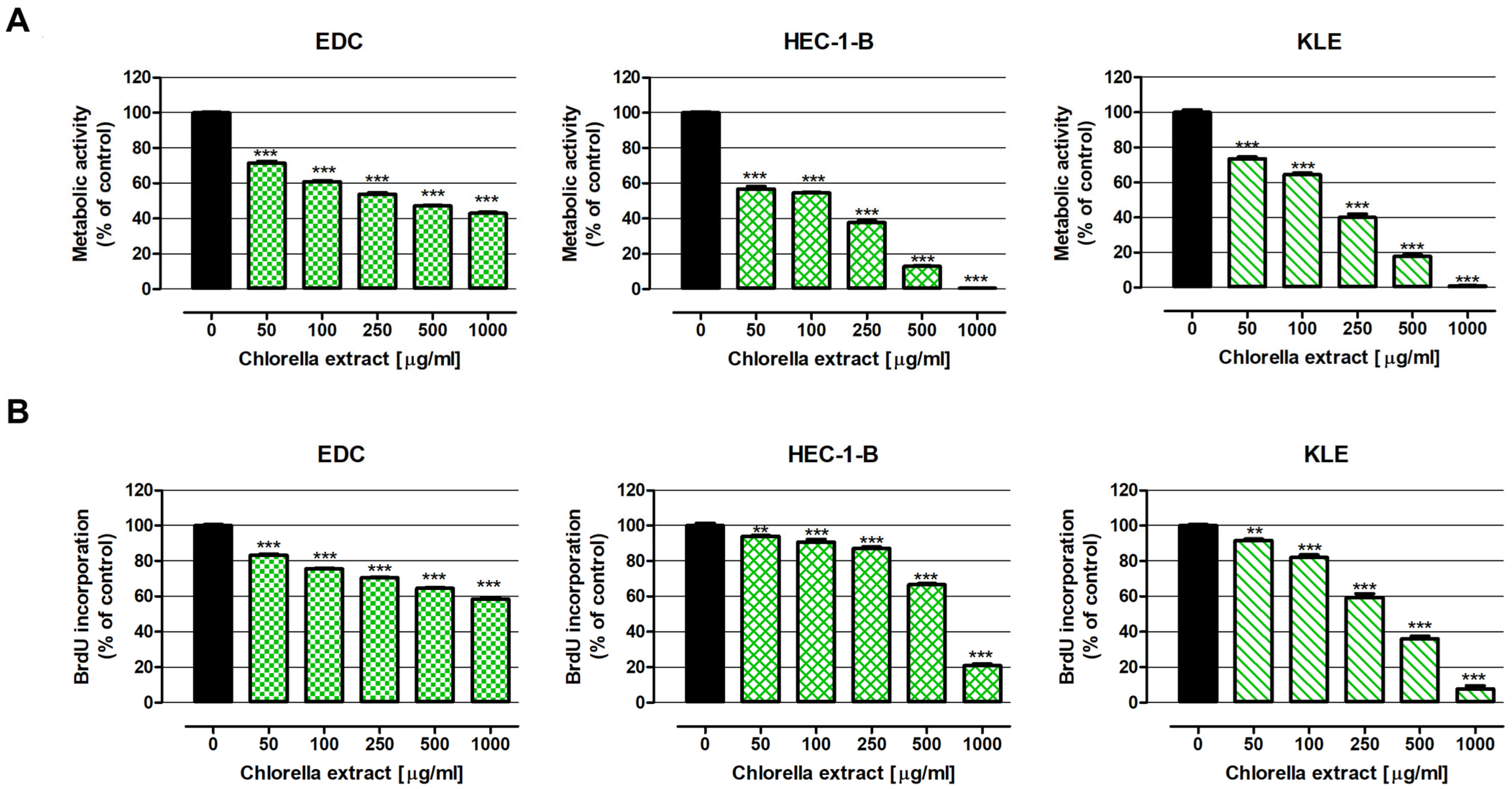
2.2. Antiproliferative Effect of Water Extract of Chlorella pyrenoidosa
2.3. Antimigratory Effect of Water Extract of Chlorella pyrenoidosa
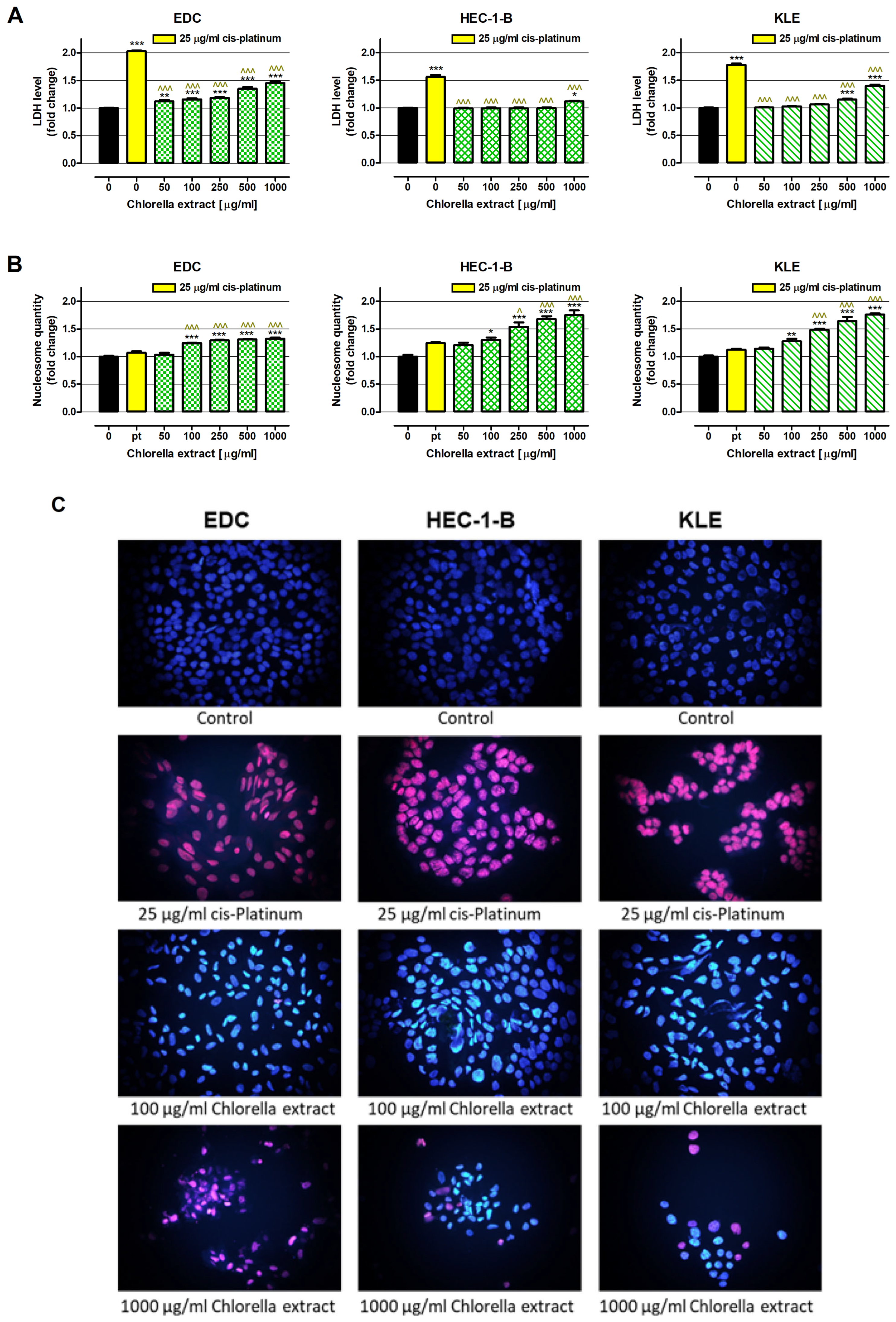
2.4. Cancer Cell Death Induction by Water Extract of Chlorella pyrenoidosa
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Chlorella Extracts
4.3. Evaluation of Sugar, Protein and Nucleic Acid Amounts in Chlorella Extract
4.4. Commercial Cell Lines
4.5. Non-Commercial Cell Line
4.6. Evaluation of Chemopreventive Abilities of Chlorella Extract
4.6.1. Examination of Cell Metabolic Activity—MTT Assay
4.6.2. Evaluation of Cell Proliferation—BrdU Assay
4.6.3. Assessment of Cell Membrane Integrity—LDH Assay
4.6.4. Examination of Apoptosis—ELISA
4.6.5. Visualization of Cell Death—Nuclear Double Staining
4.6.6. Examination of Cell Migratory Capacity—Wound Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brooks, R.A.; Fleming, G.F.; Lastra, R.R.; Lee, N.K.; Moroney, J.W.; Son, C.H.; Tatebe, K.; Veneris, J.L. Current recommendations and recent progress in endometrial cancer. CA Cancer J. Clin. 2019, 69, 258–279. [Google Scholar] [CrossRef]
- Mazidimoradi, A.; Momenimovahed, Z.; Khalajinia, Z.; Allahqoli, L.; Salehiniya, H.; Alkatout, I. The global incidence, mortality, and burden of uterine cancer in 2019 and correlation with SDI, tobacco, dietary risks, and metabolic risk factors: An ecological study. Health Sci. Rep. 2024, 7, e1835. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Available online: https://www.cancer.gov/types/uterine/hp/endometrial-treatment-pdq (accessed on 4 June 2025).
- Baker-Rand, H.; Kitson, S.J. Recent advances in endometrial cancer prevention, early diagnosis and treatment. Cancers 2024, 16, 1028. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Dowdy, S.C.; Cliby, W.A.; Ghezzi, F.; Rossetti, D.; Frigerio, L.; Mariani, A. Management of endometrial cancer: Issues and controversies. Eur. J. Gynaecol. Oncol. 2016, 37, 6–12. [Google Scholar]
- Lemieszek, M.K.; Rzeski, W. Synergism of antiproliferative effects of young green barley and chlorella water extracts against human breast cancer cells. Ann. Agric. Environ. Med. 2023, 20, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Sawasdee, N.; Jantakee, K.; Wathikthinnakon, M.; Panwong, S.; Pekkoh, J.; Duangjan, K.; Yenchitsomanus, P.T.; Panya, A. Microalga Chlorella sp. extract induced apoptotic cell death of cholangiocarcinoma via AKT/mTOR signaling pathway. Biomed. Pharmacother. 2023, 160, 114306. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Abd El Latif, A.; Elkaw, E.M.; Alotaibi, A.S.; Alenzi, A.M.; Hamza, H.A. Assessment of antioxidant and anticancer activities of microgreen alga Chlorella vulgaris and its blend with different vitamins. Molecules 2022, 27, 1602. [Google Scholar] [CrossRef]
- Ishiguro, S.; Robben, N.; Burghart, R.; Cote, P.; Greenway, S.; Thakkar, R.; Upreti, D.; Nakashima, A.; Suzuki, K.; Comer, J.; et al. Cell wall membrane fraction of Chlorella sorokiniana enhances host antitumor immunity and inhibits colon carcinoma growth in mice. Integr. Cancer Ther. 2020, 19, 1534735419900555. [Google Scholar] [CrossRef]
- Lemieszek, M.K.; Rzeski, W. The enhancement of chemopreventive properties of young green barley and chlorella extracts used together against colon cancer cells. Ann. Agric. Environ. Med. 2020, 27, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Karakaş, C.Y.; Tekarslan Şahin, H.; İnan, B.; Özçimen, D.; Erginer, Y.Ö. In vitro cytotoxic activity of microalgal extracts loaded nano-micro particles produced via electrospraying and microemulsion methods. Biotechnol. Prog. 2019, 35, e2876. [Google Scholar] [CrossRef]
- Lee, C.; Lim, K.; Kim, S.S.; Thien, L.X.; Lee, E.S.; Oh, K.T.; Choi, H.G.; Youn, Y.S. Chlorella-gold nanorods hydrogels generating photosynthesis-derived oxygen and mild heat for the treatment of hypoxic breast cancer. J. Control. Release 2019, 294, 77–90. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Chen, F. Production and characterization of exopolysaccharides from Chlorella zofingiensis and Chlorella vulgaris with anti-colorectal cancer activity. Int. J. Biol. Macromol. 2019, 134, 976–983. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Ren, Y.; Chen, F. Characterization of exopolysaccharides produced by microalgae with antitumor activity on human colon cancer cells. Int. J. Biol. Macromol. 2019, 128, 761–767. [Google Scholar] [CrossRef]
- Khalilnezhad, A.; Mahmoudian, E.; Mosaffa, N.; Anissian, A.; Rashidi, M.; Amani, D. Effects of Chlorella vulgaris on tumor growth in mammary tumor-bearing Balb/c mice: Discussing association of an immune-suppressed protumor microenvironment with serum IFNγ and IgG decrease and spleen IgG potentiation. Eur. J. Nutr. 2018, 57, 1025–1044. [Google Scholar] [CrossRef]
- Reyna-Martinez, R.; Gomez-Flores, R.; López-Chuken, U.; Quintanilla-Licea, R.; Caballero-Hernandez, D.; Rodríguez-Padilla, C.; Beltrán-Rocha, J.C.; Tamez-Guerra, P. Antitumor activity of Chlorella sorokiniana and Scenedesmus sp. microalgae native of Nuevo León State, México. Peer J. 2018, 6, e4358. [Google Scholar] [CrossRef] [PubMed]
- Tajul Arifin, K.; Sulaiman, S.; Md Saad, S.; Ahmad Damanhuri, H.; Wan Ngah, W.Z.; Mohd Yusof, Y.A. Elevation of tumour markers TGF-β, M2-PK, OV-6 and AFP in hepatocellular carcinoma (HCC)-induced rats and their suppression by microalgae Chlorella vulgaris. BMC Cancer 2017, 17, 879. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.Y.; Tsai, C.T.; Chuang, W.L.; Chao, Y.H.; Pan, I.H.; Chen, Y.K.; Lin, C.C.; Wang, B.Y. Chlorella sorokiniana induces mitochondrial-mediated apoptosis in human non-small cell lung cancer cells and inhibits xenograft tumor growth in vivo. BMC Complement. Altern. Med. 2017, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Kubatka, P.; Kapinová, A.; Kružliak, P.; Kello, M.; Výbohová, D.; Kajo, K.; Novák, M.; Chripková, M.; Adamkov, M.; Péč, M.; et al. Antineoplastic effects of Chlorella pyrenoidosa in the breast cancer model. Nutrition 2015, 31, 560–569. [Google Scholar] [CrossRef]
- Renju, G.L.; Muraleedhara Kurup, G.; Bandugula, V.R. Effect of lycopene isolated from Chlorella marina on proliferation and apoptosis in human prostate cancer cell line PC-3. Tumour. Biol. 2014, 35, 10747–10758. [Google Scholar] [CrossRef]
- Kyadari, M.; Fatma, T.; Azad, R.; Velpandian, T. Evaluation of antiangiogenic and antiproliferative potential of the organis extract of green algae Chlorella pyrenoidosa. Indian J. Pharmaco. 2013, 45, 569. [Google Scholar]
- Wang, X.; Zhang, X. Separation, antitumor activities, and encapsulation of polypeptide from Chlorella pyrenoidosa. Biotechnol. Prog. 2013, 29, 681–687. [Google Scholar] [CrossRef]
- Cha, K.H.; Koo, S.Y.; Lee, D.U. Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J. Agric. Food Chem. 2008, 56, 10521–10526. [Google Scholar] [CrossRef]
- Saad, S.M.; Yusof, Y.A.M.; Ngah, W.Z.W. Comparison between locally produced Chlorella vulgaris and Chlorella vulgaris from Japan on proliferation and apoptosis of liver cancer cell line HepG2. Malays. J. Bchem. Mol. Biol. 2006, 13, 32–36. [Google Scholar]
- Sulaiman, S.; Shamaan, N.A.; Ngah, W.Z.W.; Yusof, Y.A.M. Chemopreventive effect of Chlorella vulgaris in choline deficient diet and ethionine induced liver carcinogenesis in rats. Int. J. Cancer Res. 2006, 2, 234–241. [Google Scholar] [CrossRef][Green Version]
- Takekoshi, H.; Mizoguchi, T.; Komasa, Y.; Chubachi, H.; Inoue, Y.; Imanishi, H.; Nakano, M. Suppression of glutathione S-transferase placental form-positive foci development in rat hepatocarcinogenesis by Chlorella pyrenoidosa. Oncol. Rep. 2005, 14, 409–414. [Google Scholar] [CrossRef]
- Ferdous, U.T.; Yusof, Z.N.B. Medicinal prospects of antioxidants from algal sources in cancer therapy. Front. Pharmacol. 2021, 12, 593116. [Google Scholar] [CrossRef]
- Bito, T.; Okumura, E.; Fujishima, M.; Watanabe, F. Potential of chlorella as a dietary supplement to promote human health. Nutrients 2020, 12, 2524. [Google Scholar] [CrossRef] [PubMed]
- Van der Paal, J.; Neyts, E.C.; Verlackt, C.C.; Bogaerts, A. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chem. Sci. 2016, 7, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Sheih, I.C.; Fang, T.J.; Wu, T.K.; Lin, P.H. Anticancer and antioxidant activities of the peptide fraction from algae protein waste. J. Agric. Food Chem. 2010, 58, 1202–1207. [Google Scholar] [CrossRef]
- Wang, H.M.; Pan, J.L.; Chen, C.Y.; Chiu, C.C.; Yang, M.H.; Chang, H.W.; Chang, J.S. Identification of anti-lung cancer extract from Chlorella vulgaris C-C by antioxidant property using supercritical carbon dioxide extraction. Process Biochem. 2010, 45, 1865–1872. [Google Scholar] [CrossRef]
- Wu, L.C.; Ho, J.A.A.; Shieh, M.C.; Lu, I.W. Antioxidant and antiproliferative activities of spirulina and chlorella water extracts. J. Agri. Food Chem. 2005, 53, 4207–4212. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Kim, S.M. Effects of the molecular weight and protein and sulfate content of Chlorella ellipsoidea polysaccharides on their immunomodulatory activity. Int. J. Biol. Macromol. 2018, 107, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Wan, Z.; Zhang, X.; Li, J.; Li, H.; Wang, C. Dietary Chlorella vulgaris ameliorates altered immunomodulatory functions in cyclophosphamide- induced immunosuppressive mice. Nutrients 2017, 9, 708, Erratum in Nutrients 2024, 16, 2867. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Kim, S.M. Characterization and immunomodulatory activities of polysaccharides extracted from green alga Chlorella ellipsoidea. Int. J. Biol. Macromol. 2017, 95, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Kim, K.Y.; Jeong, H.J.; Kim, H.M.; Hong, S.H.; Um, J.Y. Effects of hydrolyzed Chlorella vulgaris by malted barley on the immunomodulatory response in ICR mice and in Molt-4 cells. J. Sci. Food Agric. 2010, 90, 1551–1556. [Google Scholar] [CrossRef]
- Ramos, A.L.; Torello, C.O.; Queiroz, M.L. Chlorella vulgaris modulates immunomyelopoietic activity and enhances the resistance of tumor-bearing mice. Nutr. Cancer 2010, 6, 1170–1180. [Google Scholar] [CrossRef]
- Morris, H.J.; Carrillo, O.; Almarales, A.; Bermúdez, R.C.; Lebequee, Y.; Fontaine, R.; Llauradó Maury, G.; Beltrán, Y. Immunostimulant activity of an enzymatic protein hydrolysate from green microalga Chlorella vulgaris on undernourished mice. Enzym. Microb. Tech. 2007, 40, 456–460. [Google Scholar] [CrossRef]
- Merchant, R.E.; Andre, C.A. A review of recent clinical trials of the nutritional supplement Chlorella pyrenoidosa in the treatment of fibromyalgia, hypertension, and ulcerative colitis. Altern. Ther. Health Med. 2001, 7, 79–91. [Google Scholar]
- Hu, R.; Feng, H.; Chang, H.; Wei, Z.; Zhang, C.; Zhong, N.; Zhang, Y.; Zhang, S.; Ho, S.H. Improving reverse osmosis concentrate treatment and nutrients conversion to Chlorella vulgaris bioenergy assisted with granular activated carbon. Sci. Total Environ. 2022, 815, 152663. [Google Scholar] [CrossRef]
- Lv, K.; Yuan, Q.; Li, H.; Li, T.; Ma, H.; Gao, C.; Zhang, S.; Liu, Y.; Zhao, L. Chlorella pyrenoidosa polysaccharides as a prebiotic to modulate gut microbiota: Physicochemical properties and fermentation characteristics in vitro. Foods 2022, 11, 725. [Google Scholar] [CrossRef]
- Andrade, L.M.; Andrade, C.J.; Dias, M.; Nascimento, C.A.O.; Mendes, M.A. Chlorella and spirulina microalgae as sources of functional foods, nutraceuticals, and food supplements; an overview. MOJ Food Process Technol. 2018, 6, 45–58. [Google Scholar] [CrossRef]
- Panahi, Y.; Darvishi, B.; Jowzi, N.; Beiraghdar, F.; Sahebkar, A. Chlorella vulgaris: A multifunctional dietary supplement with diverse medicinal properties. Curr. Pharm. Des. 2016, 22, 164–173. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Takehara, I.; Masuzawa, T.; Saito, T.; Naoki, Y. Nutrigenomic studies of effects of Chlorella on subjects with high-risk factors for lifestyle-related disease. J. Med. Food 2008, 11, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B.; Dunlop, N.M.; Newton, D.L.; Smith, J.M. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids). Fed. Proc. 1976, 35, 1332–1338. [Google Scholar]
- Available online: https://www.atcc.org/ (accessed on 10 July 2025).
- Setiawan, V.W.; Yang, H.P.; Pike, M.C.; McCann, S.E.; Yu, H.; Xiang, Y.B.; Wolk, A.; Wentzensen, N.; Weiss, N.S.; Webb, P.M.; et al. Type I and II endometrial cancers: Have they different risk factors? J. Clin. Oncol. 2013, 31, 2607–2618. [Google Scholar] [CrossRef] [PubMed]
- Lelle, R.J.; Talavera, F.; Gretz, H.; Roberts, J.A.; Menon, K.M.J. Epidermal growth factor receptor expression in three different human endometrial cancer cell lines. Cancer 1993, 72, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Kozak, J.; Wdowiak, P.; Maciejewski, R.; Torres, A. A guide for endometrial cancer cell lines functional assays using the measurements of electronic impedance. Cytotechnology 2018, 70, 339–350. [Google Scholar] [CrossRef]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Pozzoli, G.; Morrione, A.; Giordano, A.; Cenciarelli, C. p53 signaling in cancer progression and therapy. Cancer Cell Int. 2021, 21, 703. [Google Scholar] [CrossRef]
- Meng, X.; Dang, T.; Chai, J. From apoptosis to necroptosis: The death wishes to cancer. Cancer Control 2021, 28, 10732748211066311. [Google Scholar] [CrossRef]
- Kanduc, D.; Mittelman, A.; Serpico, R.; Sinigaglia, E.; Sinha, A.A.; Natale, C.; Santacroce, R.; Di Corcia, M.G.; Lucchese, A.; Dini, L.; et al. Cell death: Apoptosis versus necrosis (review). Int. J. Oncol. 2002, 21, 165–170. [Google Scholar] [CrossRef]
- Miyazawa, Y.; Murayama, T.; Ooya, N.; Wang, L.F.; Tung, Y.C.; Yamaguchi, N. Immunomodulation by a unicellular green algae (Chlorella pyrenoidosa) in tumor-bearing mice. J. Ethnopharmacol. 1988, 24, 135–146. [Google Scholar] [CrossRef]
- Yusof, Y.A.M.; Saad, S.M.; Makpol, S.; Shamaan, N.A.; Ngah, W.Z.W. Hot water extract of Chlorella vulgaris induced DNA damage and apoptosis. Clinics 2010, 65, 1371–1377. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, L.; Yin, L.; Rao, H.; Zheng, H.; Xun, C.; Hao, J. Application and prospect of microbial food Chlorella. Heliyon. 2024, 10, e37025. [Google Scholar] [CrossRef]
- Hemalatha, M.; Mohan, S.V. Amino acids rich biomass cultivation: Trophic mode influence on Chlorella Growth Factor (CGF) production. Algal Res. 2024, 80, 103449. [Google Scholar] [CrossRef]
- Talib, W.H.; Awajan, D.; Hamed, R.A.; Azzam, A.O.; Mahmod, A.I.; Al-Yasari, I.H. Combination anti-cancer therapies using selected phytochemicals. Molecules 2022, 27, 5452. [Google Scholar] [CrossRef] [PubMed]
- Pezzani, R.; Salehi, B.; Vitalini, S.; Iriti, M.; Zuñiga, F.A.; Sharifi-Rad, J.; Martorell, M.; Martins, N. Synergistic effects of plant derivatives and conventional chemotherapeutic agents: An update on the cancer perspective. Medicina 2019, 55, 110. [Google Scholar] [CrossRef] [PubMed]
- Temple, N.J.; Gladwin, K.K. Fruits, vegetables, and the prevention of cancer: Research challenges. Nutrition 2003, 19, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.F.; Sun, J.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of vegetables. J. Agric. Food Chem. 2002, 50, 6910–6916. [Google Scholar] [CrossRef]
- Sun, J.; Chu, Y.F.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Heptinstall, J.; Rapley, R. Spectrophotometric Analysis of Nucleic Acids. In The Nucleic Acid Protocols Handbook; Springer Protocols Handbooks; Rapley, R., Ed.; Humana Press: Totowa, NJ, USA, 2000. [Google Scholar]

| Total Proteins (Mean ± SD) | Total Sugar (Mean ± SD) | Total Nucleic Acids (Mean ± SD) | |
|---|---|---|---|
| Amount in water extract of Chlorella pyrenoidosa | 26.86 ± 3.49% | 42.32 ± 2.01% | 24.43 ± 0.99% |
| Commercial Product of Chlorella pyrenoidosa | |
|---|---|
| Energy | 1634 kJ, 389 kcal |
| Proteins | 54.13 ± 0.85 g |
| Fat | 11.60 ± 0.41 g |
| Carbohydrates | 11.63 ± 1.55 g |
| Total Dietary Fiber | 10.84 ± 0.73 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rzeska, W.; Chojnacki, M.; Adamiak-Godlewska, A.; Semczuk, A.; Lemieszek, M.K. First Look at Chemopreventive Properties of Chlorella pyrenoidosa Water Extract in Human Endometrial Adenocarcinoma Cells—Preliminary In Vitro Study. Int. J. Mol. Sci. 2025, 26, 9142. https://doi.org/10.3390/ijms26189142
Rzeska W, Chojnacki M, Adamiak-Godlewska A, Semczuk A, Lemieszek MK. First Look at Chemopreventive Properties of Chlorella pyrenoidosa Water Extract in Human Endometrial Adenocarcinoma Cells—Preliminary In Vitro Study. International Journal of Molecular Sciences. 2025; 26(18):9142. https://doi.org/10.3390/ijms26189142
Chicago/Turabian StyleRzeska, Weronika, Michał Chojnacki, Aneta Adamiak-Godlewska, Andrzej Semczuk, and Marta Kinga Lemieszek. 2025. "First Look at Chemopreventive Properties of Chlorella pyrenoidosa Water Extract in Human Endometrial Adenocarcinoma Cells—Preliminary In Vitro Study" International Journal of Molecular Sciences 26, no. 18: 9142. https://doi.org/10.3390/ijms26189142
APA StyleRzeska, W., Chojnacki, M., Adamiak-Godlewska, A., Semczuk, A., & Lemieszek, M. K. (2025). First Look at Chemopreventive Properties of Chlorella pyrenoidosa Water Extract in Human Endometrial Adenocarcinoma Cells—Preliminary In Vitro Study. International Journal of Molecular Sciences, 26(18), 9142. https://doi.org/10.3390/ijms26189142







