The DNA Minor Groove Binders Trabectedin and Lurbinectedin Are Potent Antitumor Agents in Human Intrahepatic Cholangiocarcinoma
Abstract
1. Introduction
2. Results
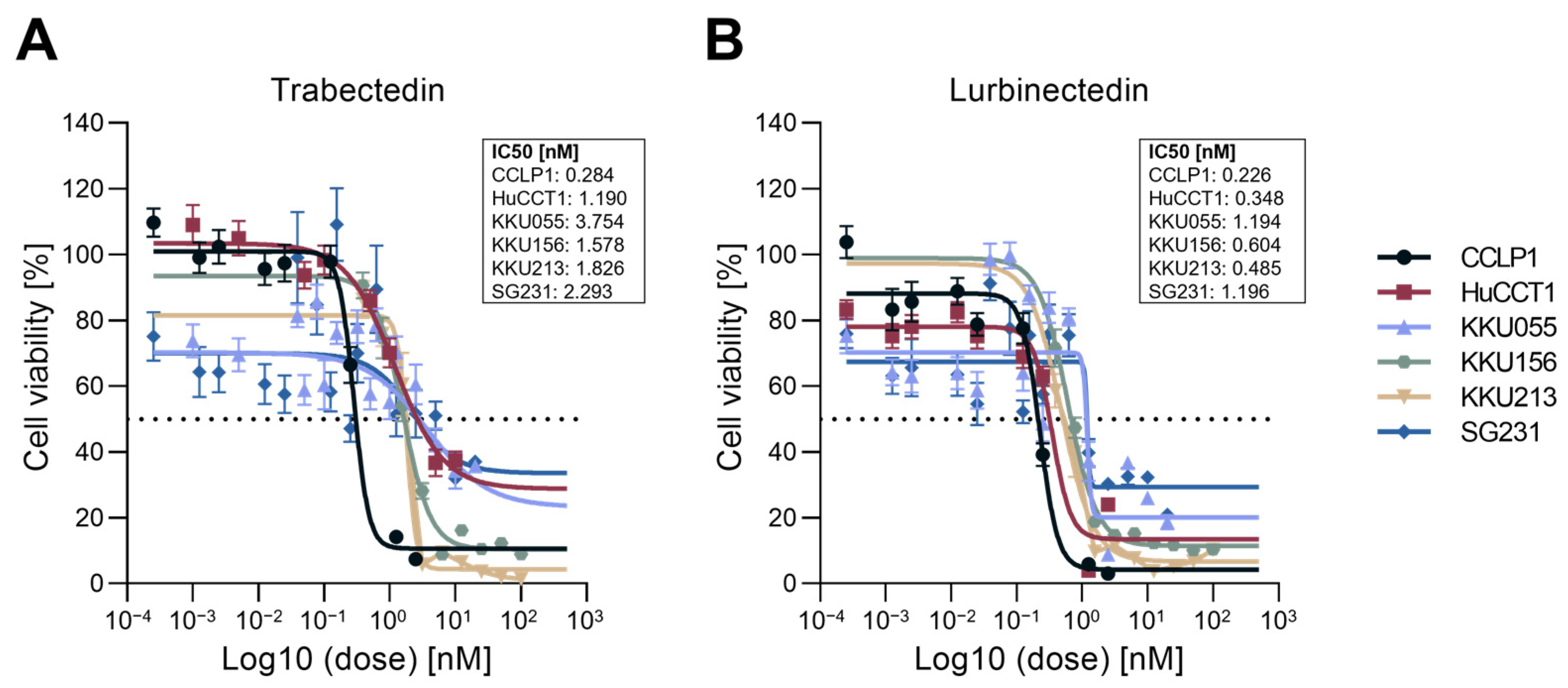
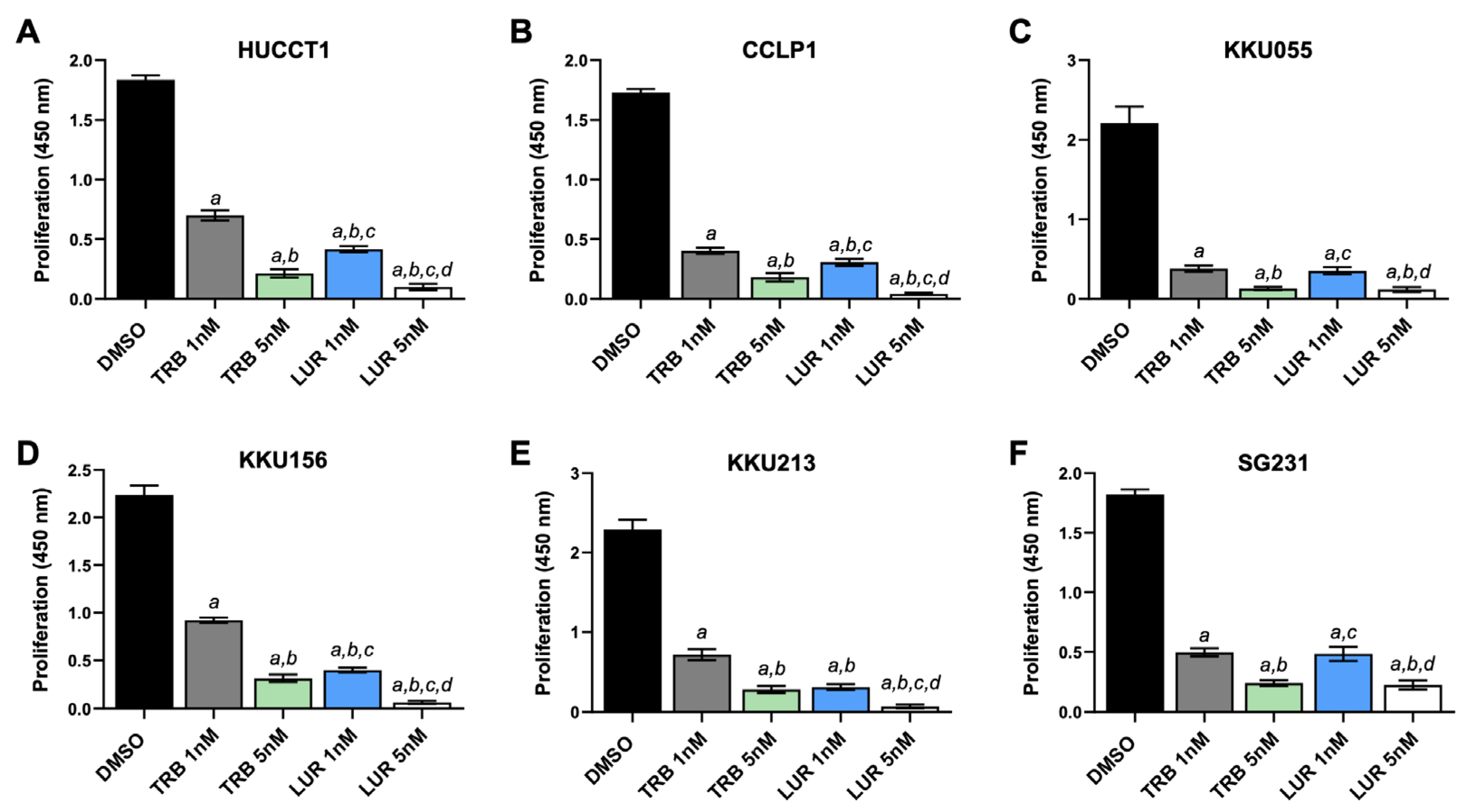
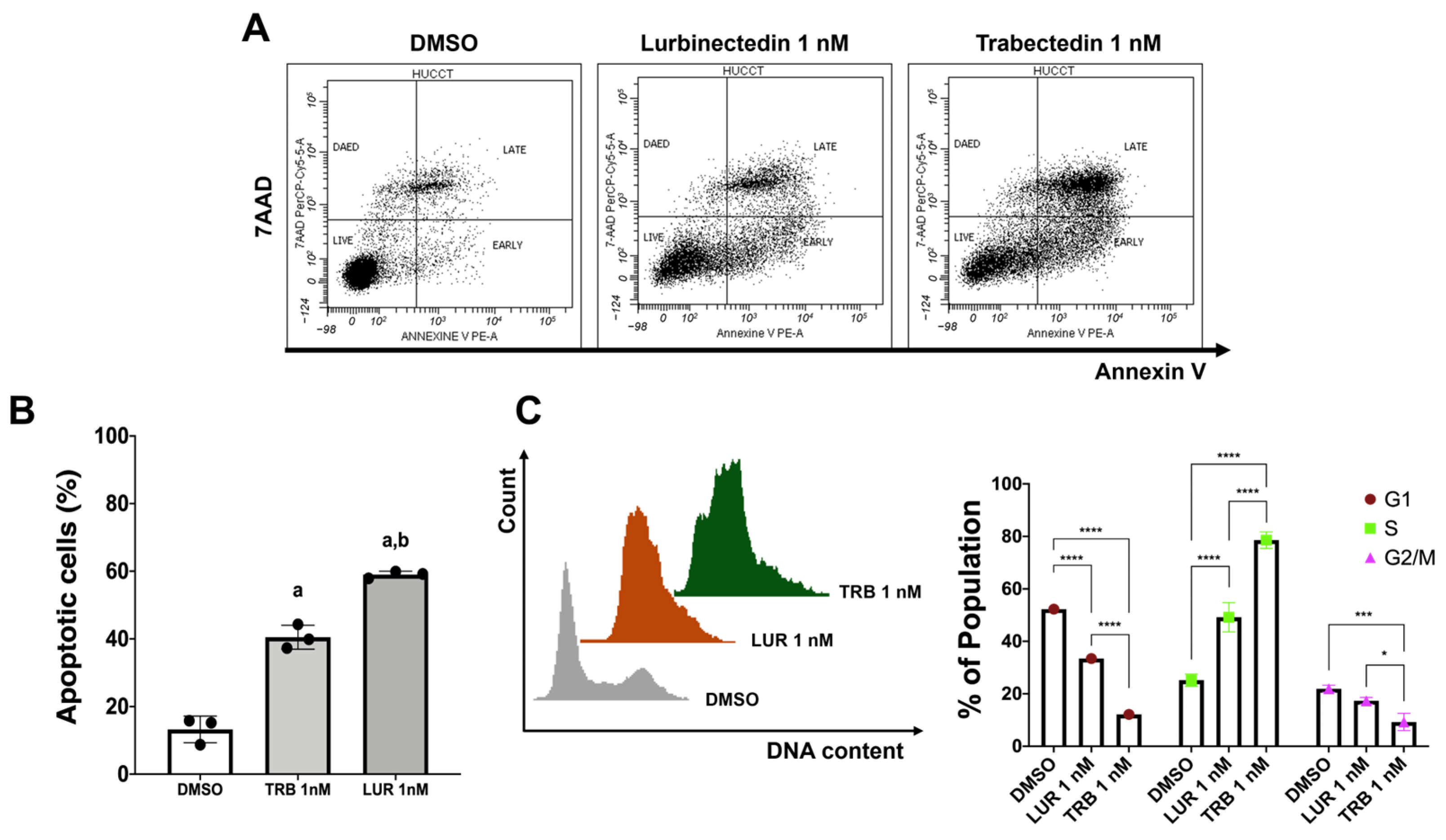
2.1. Trabectedin and Lurbinectedin Restrain Cell Growth and Trigger DNA Damage in iCCA Cell Lines
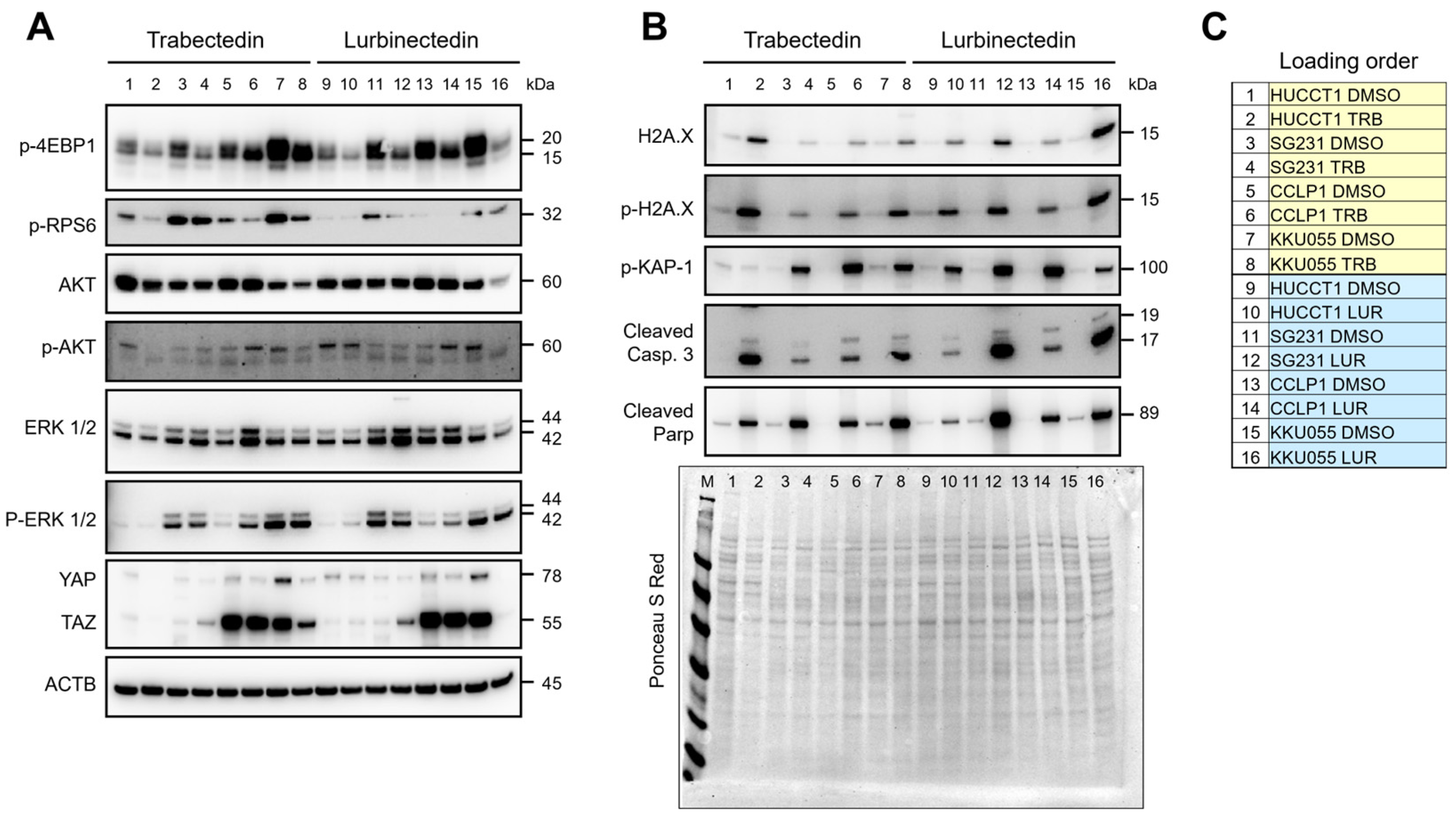
2.2. Signaling Pathways Affected by Trabectedin and Lurbinectedin in Intrahepatic Cholangiocarcinoma Cell Lines
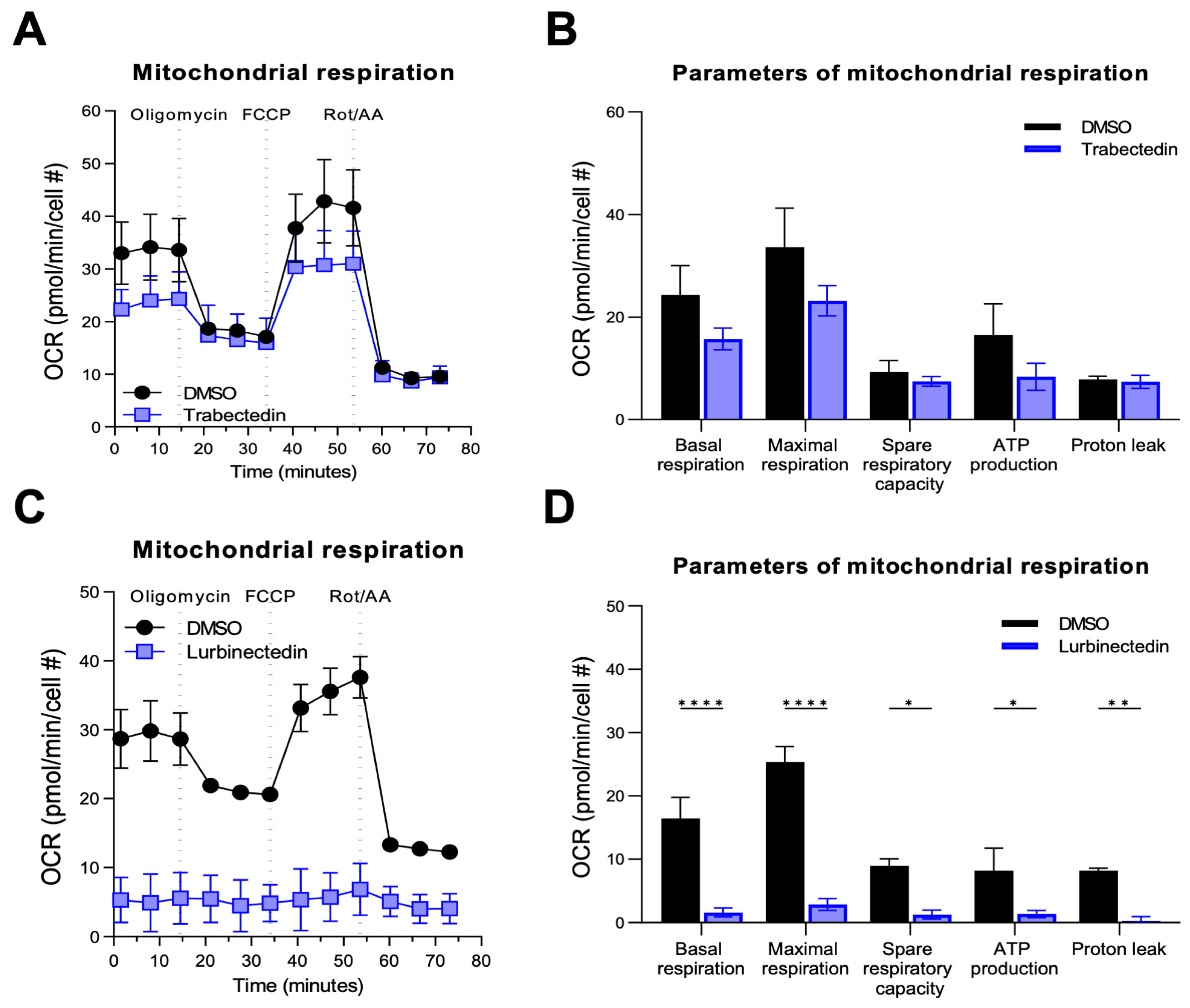
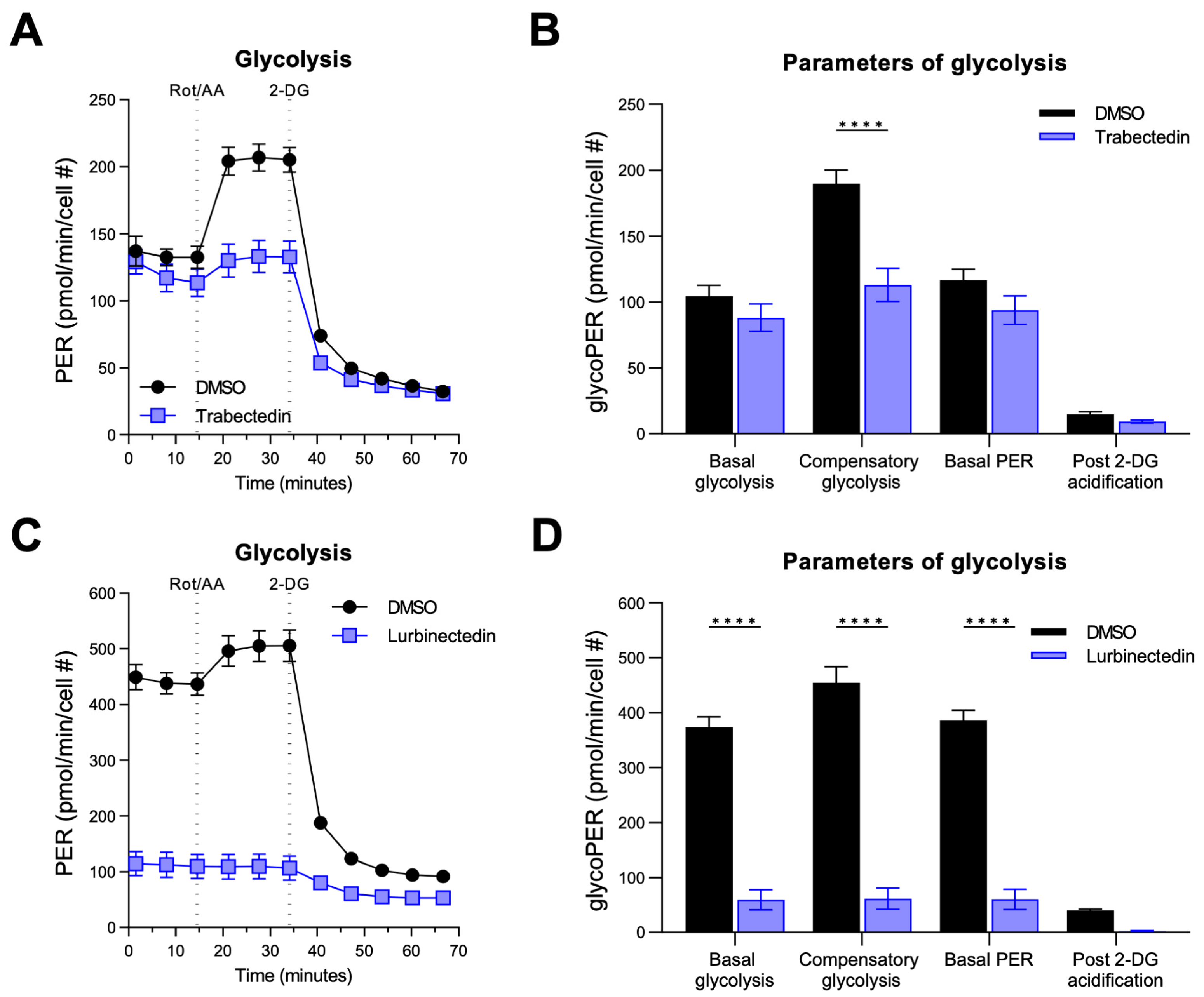
2.3. Trabectedin and Lurbinectedin Decrease Mitochondrial Respiration and Glycolysis in iCCA Cells
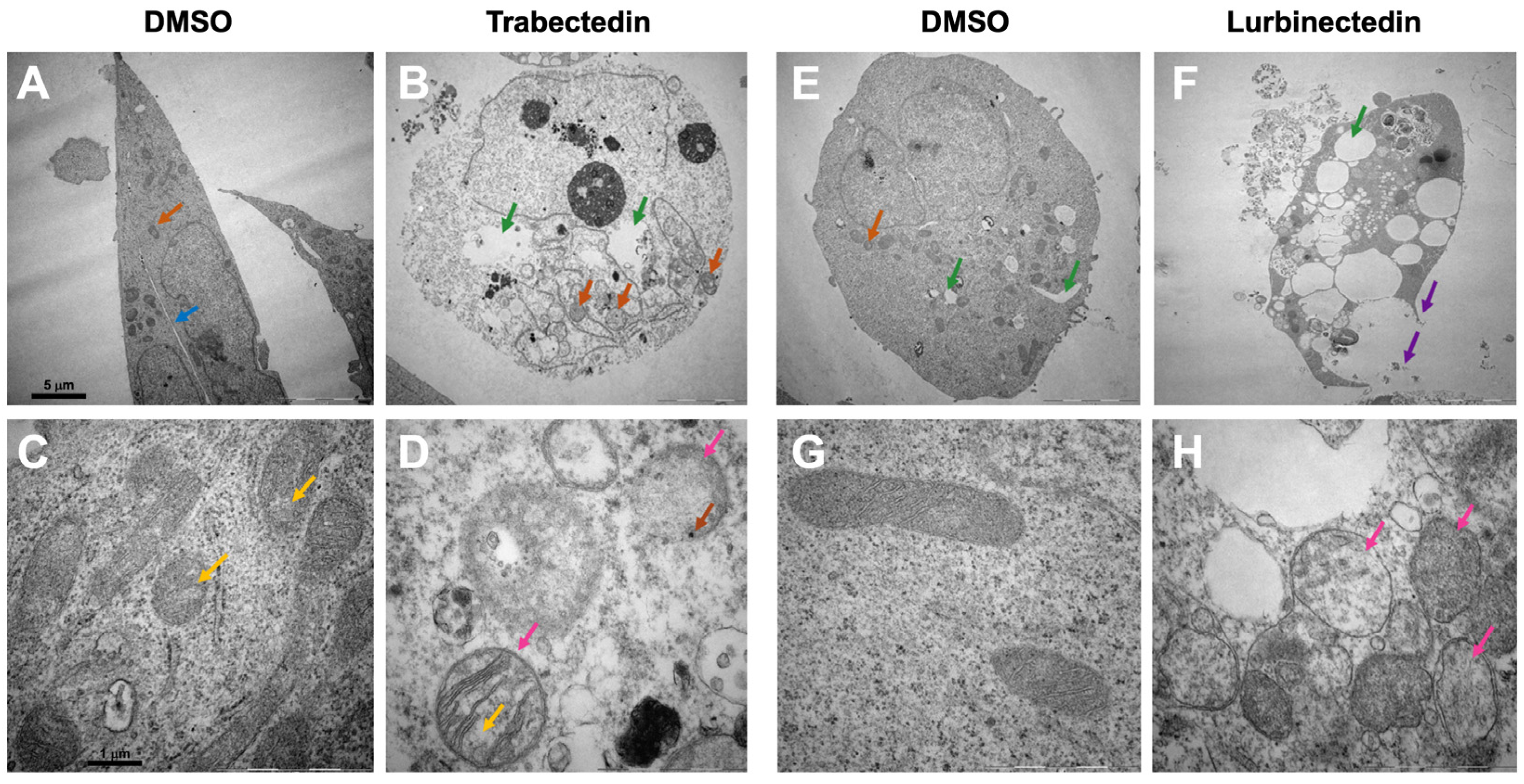
2.4. Effects of Trabectedin and Lurbinectedin on the Subcellular Structure of Intrahepatic Cholangiocarcinoma Cells
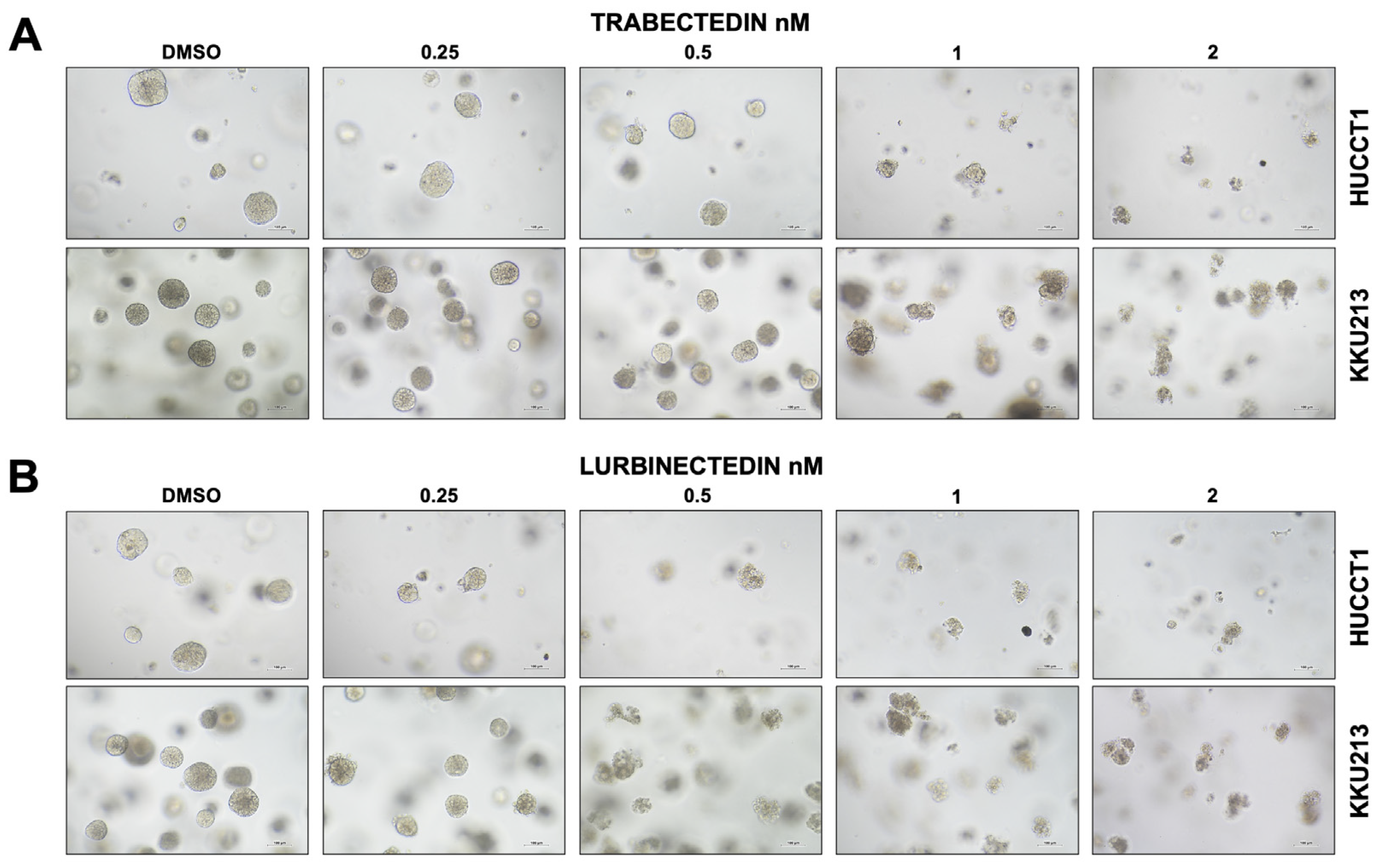
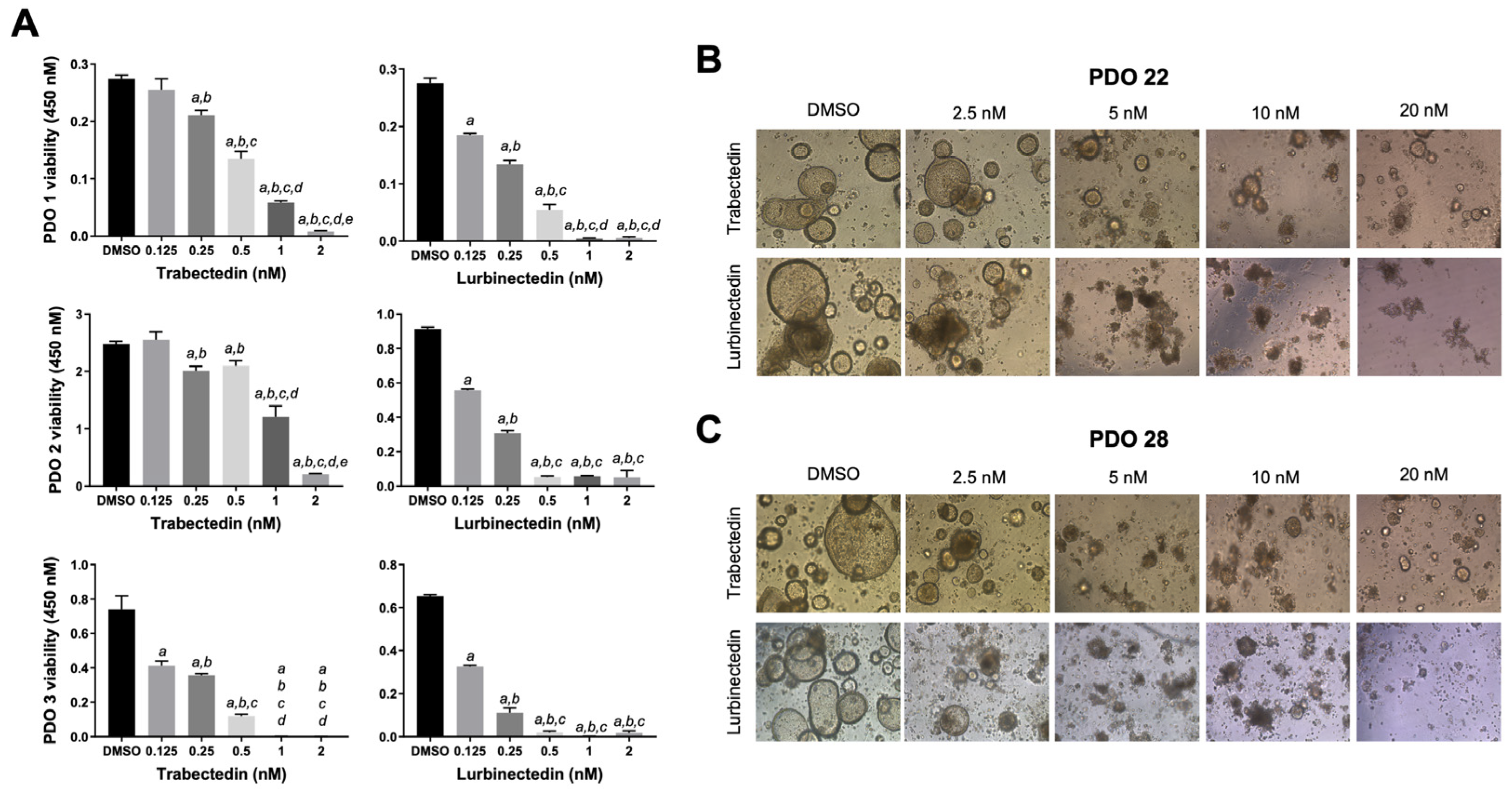
2.5. Trabectedin and Lurbinectedin Hamper Intrahepatic Cholangiocarcinoma Organoid Growth
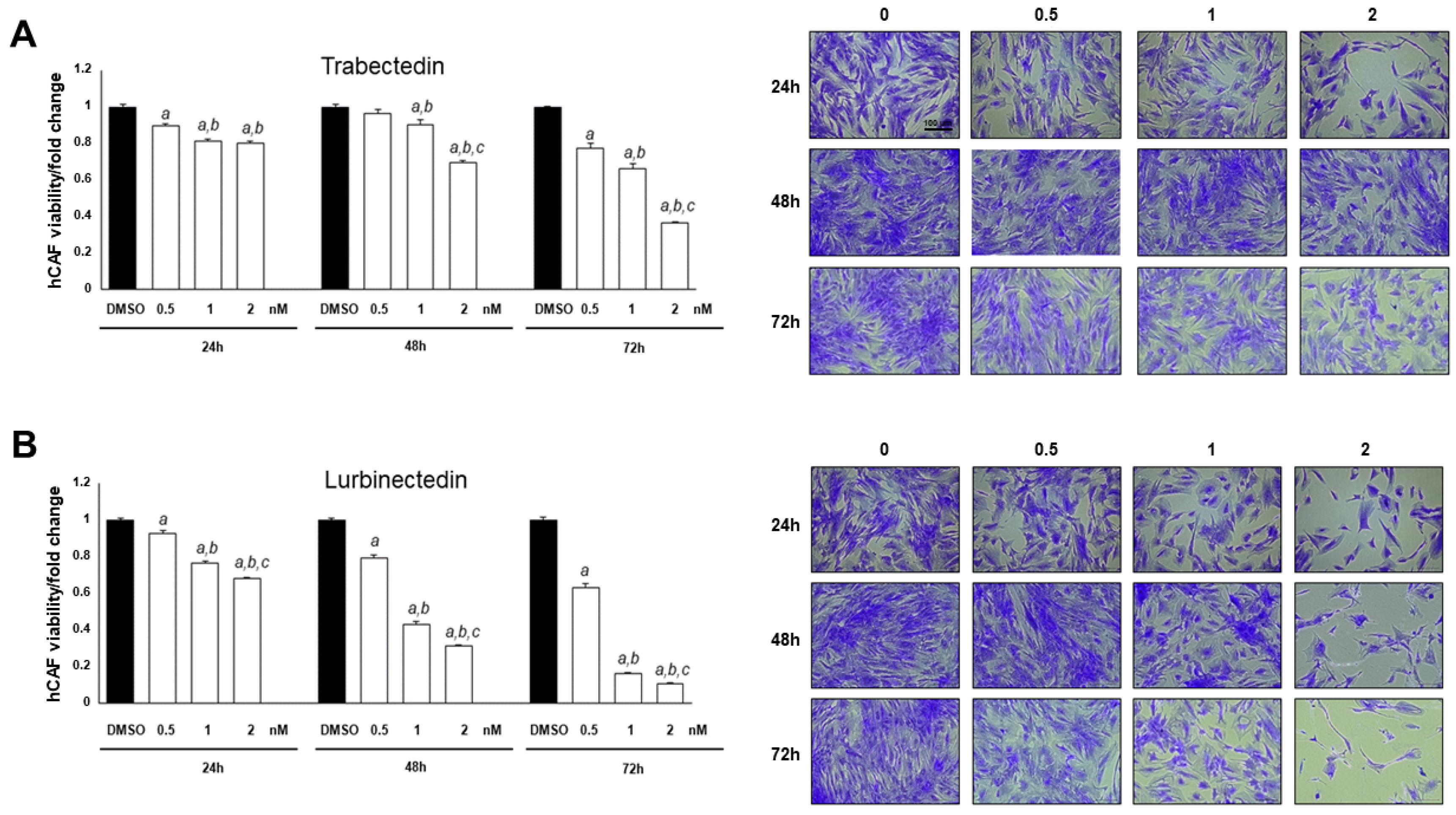
2.6. Trabectedin and Lurbinectedin Hinder Cancer-Associated Fibroblast Growth In Vitro
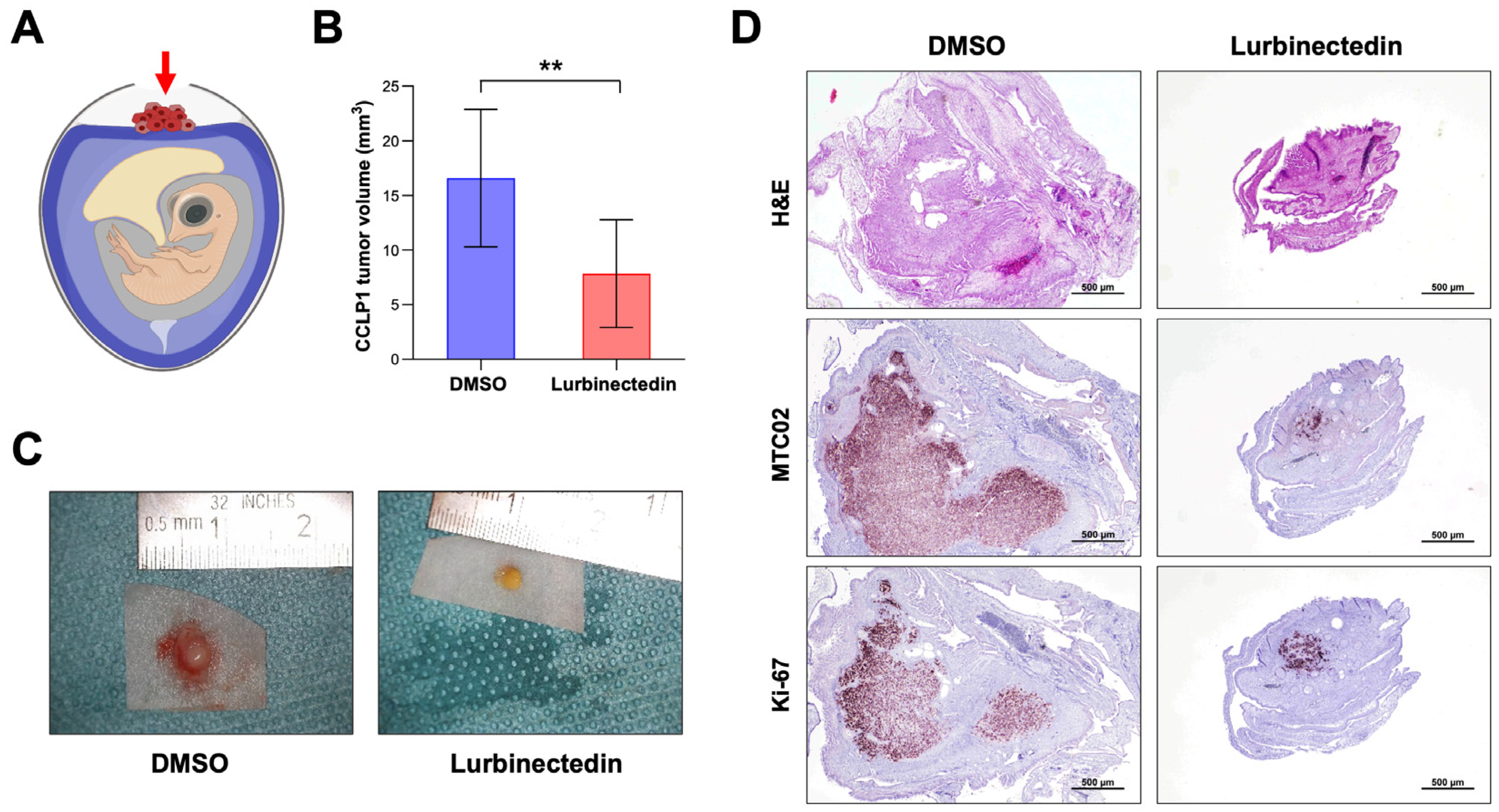
2.7. Lurbinectedin Reduces iCCA Growth In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Reagents
4.2. Viability, Proliferation, Apoptosis, and DNA Damage Assays
4.3. Flow Cytometry Assay
4.4. Spheroid Generation and Assessment of Cell Viability
4.5. Patient-Derived Tumor Organoids (PDOs)
4.6. Isolation and Treatment of Human Cancer-Associated Fibroblasts (hCAFs)
4.7. RNA Extraction and Quantitative Real-Time Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR)
4.8. Protein Extraction and Western Blot Analysis
4.9. Seahorse Mitochondrial Respiration and Glycolysis Analyses
4.10. Transmission Electron Microscopy Analysis
4.11. Chicken Chorioallantoic Membrane (CAM) Assay and Immunohistochemical Staining
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Kelley, R.K.; Bridgewater, J.; Gores, G.J.; Zhu, A.X. Systemic therapies for intrahepatic cholangiocarcinoma. J. Hepatol. 2020, 72, 353–363. [Google Scholar] [CrossRef]
- Saha, S.K.; Zhu, A.X.; Fuchs, C.S.; Brooks, G.A. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016, 21, 594–599. [Google Scholar] [CrossRef]
- Clements, O.; Eliahoo, J.; Kim, J.U.; Taylor-Robinson, S.D.; Khan, S.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J. Hepatol. 2020, 72, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Sirica, A.E.; Gores, G.J.; Groopman, J.D.; Selaru, F.M.; Strazzabosco, M.; Wei Wang, X.; Zhu, A.X. Intrahepatic Cholangiocarcinoma: Continuing Challenges and Translational Advances. Hepatology 2019, 69, 1803–1815. [Google Scholar] [CrossRef]
- Lamarca, A.; Edeline, J.; Goyal, L. How I treat biliary tract cancer. ESMO Open 2022, 7, 100378. [Google Scholar] [CrossRef]
- Ali, I.; Lone, M.N.; Alothman, Z.A.; Alwarthan, A. Insights into the pharmacology of new heterocycles embedded with oxopyrrolidine rings: DNA binding, molecular docking, and anticancer studies. J. Mol. Liq. 2017, 234, 391–402. [Google Scholar] [CrossRef]
- Høgdall, D.; Lewinska, M.; Andersen, J.B. Desmoplastic Tumor Microenvironment and Immunotherapy in Cholangiocarcinoma. Trends Cancer 2018, 4, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Moris, D.; Palta, M.; Kim, C.; Allen, P.J.; Morse, M.A.; Lidsky, M.E. Advances in the treatment of intrahepatic cholangiocarcinoma: An overview of the current and future therapeutic landscape for clinicians. CA Cancer J. Clin. 2023, 73, 198–222. [Google Scholar] [CrossRef]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.-J.; Chan, S.L.; Ozaka, M.; et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1853–1865, Erratum in Lancet 2024, 403, 1140. [Google Scholar] [CrossRef]
- Burris, H.A.; Okusaka, T.; Vogel, A.; Lee, M.A.; Takahashi, H.; Breder, V.; Blanc, J.-F.; Li, J.; Bachini, M.; Żotkiewicz, M.; et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer (TOPAZ-1): Patient-reported outcomes from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2024, 25, 626–635. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807, Erratum in Lancet Oncol. 2024, 25, e61. [Google Scholar] [CrossRef]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; Kelley, R.K.; Cassier, P.A.; et al. Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma. N. Engl. J. Med. 2023, 388, 228–239. [Google Scholar] [CrossRef]
- Makawita, S.; K Abou-Alfa, G.; Roychowdhury, S.; Sadeghi, S.; Borbath, I.; Goyal, L.; Cohn, A.; Lamarca, A.; Oh, D.-Y.; Macarulla, T.; et al. Infigratinib in patients with advanced cholangiocarcinoma with FGFR2 gene fusions/translocations: The PROOF 301 trial. Future Oncol. 2020, 16, 2375–2384. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684, Erratum in Lancet Oncol. 2024, 25, e3. [Google Scholar] [CrossRef]
- D’Incalci, M.; Zambelli, A. Trabectedin for the treatment of breast cancer. Expert Opin. Investig. Drugs 2016, 25, 105–115. [Google Scholar] [CrossRef]
- Trigo, J.; Subbiah, V.; Besse, B.; Moreno, V.; López, R.; Sala, M.A.; Peters, S.; Ponce, S.; Fernández, C.; Alfaro, V.; et al. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: A single-arm, open-label, phase 2 basket trial. Lancet Oncol. 2020, 21, 645–654, Erratum in Lancet Oncol. 2020, 21, e553. [Google Scholar] [CrossRef]
- Erba, E.; Bergamaschi, D.; Bassano, L.; Damia, G.; Ronzoni, S.; Faircloth, G.T.; D’Incalci, M. Ecteinascidin-743 (ET-743), a natural marine compound, with a unique mechanism of action. Eur. J. Cancer 2001, 37, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.F.M.; Martínez-Díez, M.; García-Hernández, V.; Moneo, V.; Domingo, A.; Bueren-Calabuig, J.A.; Negri, A.; Gago, F.; Guillén-Navarro, M.J.; Avilés, P.; et al. PM01183, a new DNA minor groove covalent binder with potent in vitro and in vivo anti-tumour activity. Br. J. Pharmacol. 2010, 161, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Gedminas, J.M.; Kaufman, R.; Boguslawski, E.A.; Gross, A.C.; Adams, M.; Beddows, I.; Kitchen-Goosen, S.M.; Roberts, R.D.; Grohar, P.J. Lurbinectedin Inhibits the EWS-WT1 Transcription Factor in Desmoplastic Small Round Cell Tumor. Mol. Cancer Ther. 2022, 21, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, E.; Mabuchi, S.; Shimura, K.; Komura, N.; Kozasa, K.; Kuroda, H.; Takahashi, R.; Sasano, T.; Kawano, M.; Matsumoto, Y.; et al. Lurbinectedin (PM01183), a selective inhibitor of active transcription, effectively eliminates both cancer cells and cancer stem cells in preclinical models of uterine cervical cancer. Investig. New Drugs 2019, 37, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Santamaría Nuñez, G.; Robles, C.M.G.; Giraudon, C.; Martínez-Leal, J.F.; Compe, E.; Coin, F.; Aviles, P.; Galmarini, C.M.; Egly, J.-M. Lurbinectedin Specifically Triggers the Degradation of Phosphorylated RNA Polymerase II and the Formation of DNA Breaks in Cancer Cells. Mol. Cancer Ther. 2016, 15, 2399–2412. [Google Scholar] [CrossRef] [PubMed]
- Povo-Retana, A.; Mojena, M.; Stremtan, A.B.; Fernández-García, V.B.; Gómez-Sáez, A.; Nuevo-Tapioles, C.; Molina-Guijarro, J.M.; Avendaño-Ortiz, J.; Cuezva, J.M.; López-Collazo, E.; et al. Specific Effects of Trabectedin and Lurbinectedin on Human Macrophage Function and Fate-Novel Insights. Cancers 2020, 12, 3060. [Google Scholar] [CrossRef] [PubMed]
- D’Incalci, M.; Galmarini, C.M. A review of trabectedin (ET-743): A unique mechanism of action. Mol. Cancer Ther. 2010, 9, 2157–2163. [Google Scholar] [CrossRef]
- Minuzzo, M.; Marchini, S.; Broggini, M.; Faircloth, G.; D’Incalci, M.; Mantovani, R. Interference of transcriptional activation by the antineoplastic drug ecteinascidin-743. Proc. Natl. Acad. Sci. USA 2000, 97, 6780–6784. [Google Scholar] [CrossRef]
- Di Giandomenico, S.; Frapolli, R.; Bello, E.; Uboldi, S.; Licandro, S.A.; Marchini, S.; Beltrame, L.; Brich, S.; Mauro, V.; Tamborini, E.; et al. Mode of action of trabectedin in myxoid liposarcomas. Oncogene 2014, 33, 5201–5210. [Google Scholar] [CrossRef]
- Andreeva-Gateva, P.; Chakar, S. The place of trabectedin in the treatment of soft tissue sarcoma: An umbrella review of the level one evidence. Expert Opin. Orphan Drugs 2019, 7, 105–115. [Google Scholar] [CrossRef]
- Grignani, G.; D’Ambrosio, L.; Pignochino, Y.; Palmerini, E.; Zucchetti, M.; Boccone, P.; Aliberti, S.; Stacchiotti, S.; Bertulli, R.; Piana, R.; et al. Trabectedin and olaparib in patients with advanced and non-resectable bone and soft-tissue sarcomas (TOMAS): An open-label, phase 1b study from the Italian Sarcoma Group. Lancet Oncol. 2018, 19, 1360–1371. [Google Scholar] [CrossRef]
- Colombo, N.; Hardy-Bessard, A.-C.; Ferrandina, G.; Marth, C.; Romero, I. Experience with trabectedin + pegylated liposomal doxorubicin for recurrent platinum-sensitive ovarian cancer unsuited to platinum rechallenge. Expert Rev. Anticancer Ther. 2016, 16, 11–19. [Google Scholar] [CrossRef]
- Grosso, F.; Jones, R.L.; Demetri, G.D.; Judson, I.R.; Blay, J.-Y.; Le Cesne, A.; Sanfilippo, R.; Casieri, P.; Collini, P.; Dileo, P.; et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: A retrospective study. Lancet Oncol. 2007, 8, 595–602. [Google Scholar] [CrossRef]
- Farago, A.F.; Drapkin, B.J.; Lopez-Vilarino de Ramos, J.A.; Galmarini, C.M.; Núñez, R.; Kahatt, C.; Paz-Ares, L. ATLANTIS: A Phase III study of lurbinectedin/doxorubicin versus topotecan or cyclophosphamide/doxorubicin/vincristine in patients with small-cell lung cancer who have failed one prior platinum-containing line. Future Oncol. 2019, 15, 231–239. [Google Scholar] [CrossRef]
- Chuk, M.K.; Balis, F.M.; Fox, E. Trabectedin. Oncologist 2009, 14, 794–799. [Google Scholar] [CrossRef]
- Allavena, P.; Belgiovine, C.; Digifico, E.; Frapolli, R.; D’Incalci, M. Effects of the Anti-Tumor Agents Trabectedin and Lurbinectedin on Immune Cells of the Tumor Microenvironment. Front. Oncol. 2022, 12, 851790. [Google Scholar] [CrossRef]
- Povo-Retana, A.; Fariñas, M.; Landauro-Vera, R.; Mojena, M.; Alvarez-Lucena, C.; Fernández-Moreno, M.A.; Castrillo, A.; de La Rosa Medina, J.V.; Sánchez-García, S.; Foguet, C.; et al. Immunometabolic actions of trabectedin and lurbinectedin on human macrophages: Relevance for their anti-tumor activity. Front. Immunol. 2023, 14, 1211068. [Google Scholar] [CrossRef]
- Raggi, C.; Taddei, M.L.; Sacco, E.; Navari, N.; Correnti, M.; Piombanti, B.; Pastore, M.; Campani, C.; Pranzini, E.; Iorio, J.; et al. Mitochondrial oxidative metabolism contributes to a cancer stem cell phenotype in cholangiocarcinoma. J. Hepatol. 2021, 74, 1373–1385. [Google Scholar] [CrossRef]
- Raggi, C.; Correnti, M.; Sica, A.; Andersen, J.B.; Cardinale, V.; Alvaro, D.; Chiorino, G.; Forti, E.; Glaser, S.; Alpini, G.; et al. Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J. Hepatol. 2017, 66, 102–115. [Google Scholar] [CrossRef]
- Cigrang, M.; Obid, J.; Nogaret, M.; Seno, L.; Ye, T.; Davidson, G.; Catez, P.; Berico, P.; Capelli, C.; Marechal, C.; et al. Pan-inhibition of super-enhancer-driven oncogenic transcription by next-generation synthetic ecteinascidins yields potent anti-cancer activity. Nat. Commun. 2025, 16, 512. [Google Scholar] [CrossRef]
- Verstegen, M.M.A.; Coppes, R.P.; Beghin, A.; de Coppi, P.; Gerli, M.F.M.; de Graeff, N.; Pan, Q.; Saito, Y.; Shi, S.; Zadpoor, A.A.; et al. Clinical applications of human organoids. Nat. Med. 2025, 31, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Grabinger, T.; Luks, L.; Kostadinova, F.; Zimberlin, C.; Medema, J.P.; Leist, M.; Brunner, T. Ex vivo culture of intestinal crypt organoids as a model system for assessing cell death induction in intestinal epithelial cells and enteropathy. Cell Death Dis. 2014, 5, e1228. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, F.; Mellone, S.; D’Incalci, M.; Stornaiuolo, M.; Leonardi, A.; Crescenzi, E. Trabectedin suppresses escape from therapy-induced senescence in tumor cells by interfering with glutamine metabolism. Biochem. Pharmacol. 2022, 202, 115159. [Google Scholar] [CrossRef] [PubMed]
- Grupp, K.; Jedrzejewska, K.; Tsourlakis, M.C.; Koop, C.; Wilczak, W.; Adam, M.; Quaas, A.; Sauter, G.; Simon, R.; Izbicki, J.R.; et al. High mitochondria content is associated with prostate cancer disease progression. Mol. Cancer 2013, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Peraldo Neia, C.; Cavalloni, G.; Chiorino, G.; Ostano, P.; Aglietta, M.; Leone, F. Gene and microRNA modulation upon trabectedin treatment in a human intrahepatic cholangiocarcinoma paired patient derived xenograft and cell line. Oncotarget 2016, 7, 86766–86780. [Google Scholar] [CrossRef][Green Version]
- Zhang, S.; Song, X.; Cao, D.; Xu, Z.; Fan, B.; Che, L.; Hu, J.; Chen, B.; Dong, M.; Pilo, M.G.; et al. Pan-mTOR inhibitor MLN0128 is effective against intrahepatic cholangiocarcinoma in mice. J. Hepatol. 2017, 67, 1194–1203. [Google Scholar] [CrossRef]
- Cai, S.; Ding, Z.; Liu, X.; Zeng, J. Trabectedin induces ferroptosis via regulation of HIF-1α/IRP1/TFR1 and Keap1/Nrf2/GPX4 axis in non-small cell lung cancer cells. Chem. Biol. Interact. 2023, 369, 110262. [Google Scholar] [CrossRef]
- Kepp, O.; Zitvogel, L.; Kroemer, G. Lurbinectedin: An FDA-approved inducer of immunogenic cell death for the treatment of small-cell lung cancer. Oncoimmunology 2020, 9, 1795995. [Google Scholar] [CrossRef]
- Broutier, L.; Mastrogiovanni, G.; Verstegen, M.M.; Francies, H.E.; Gavarró, L.M.; Bradshaw, C.R.; Allen, G.E.; Arnes-Benito, R.; Sidorova, O.; Gaspersz, M.P.; et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 2017, 23, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Mancarella, S.; Gigante, I.; Pizzuto, E.; Serino, G.; Terzi, A.; Dituri, F.; Maiorano, E.; Vincenti, L.; de Bellis, M.; Ardito, F.; et al. Targeting cancer-associated fibroblasts/tumor cells cross-talk inhibits intrahepatic cholangiocarcinoma progression via cell-cycle arrest. J. Exp. Clin. Cancer Res. 2024, 43, 286. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gäbele, E.; Gigante, I.; Pastore, M.; Cigliano, A.; Galleri, G.; Bauer, T.; Pizzuto, E.; Mancarella, S.; Müller, M.; Marra, F.; et al. The DNA Minor Groove Binders Trabectedin and Lurbinectedin Are Potent Antitumor Agents in Human Intrahepatic Cholangiocarcinoma. Int. J. Mol. Sci. 2025, 26, 9085. https://doi.org/10.3390/ijms26189085
Gäbele E, Gigante I, Pastore M, Cigliano A, Galleri G, Bauer T, Pizzuto E, Mancarella S, Müller M, Marra F, et al. The DNA Minor Groove Binders Trabectedin and Lurbinectedin Are Potent Antitumor Agents in Human Intrahepatic Cholangiocarcinoma. International Journal of Molecular Sciences. 2025; 26(18):9085. https://doi.org/10.3390/ijms26189085
Chicago/Turabian StyleGäbele, Erwin, Isabella Gigante, Mirella Pastore, Antonio Cigliano, Grazia Galleri, Thea Bauer, Elena Pizzuto, Serena Mancarella, Martina Müller, Fabio Marra, and et al. 2025. "The DNA Minor Groove Binders Trabectedin and Lurbinectedin Are Potent Antitumor Agents in Human Intrahepatic Cholangiocarcinoma" International Journal of Molecular Sciences 26, no. 18: 9085. https://doi.org/10.3390/ijms26189085
APA StyleGäbele, E., Gigante, I., Pastore, M., Cigliano, A., Galleri, G., Bauer, T., Pizzuto, E., Mancarella, S., Müller, M., Marra, F., Siegmund, H., Giannelli, G., Evert, M., Raggi, C., Calvisi, D. F., & Steinmann, S. M. (2025). The DNA Minor Groove Binders Trabectedin and Lurbinectedin Are Potent Antitumor Agents in Human Intrahepatic Cholangiocarcinoma. International Journal of Molecular Sciences, 26(18), 9085. https://doi.org/10.3390/ijms26189085








