The Responsiveness of Breast Cancer Cells to Varied Levels of Vitamin B12, Cisplatin, and G-CSF
Abstract
1. Introduction
2. Results
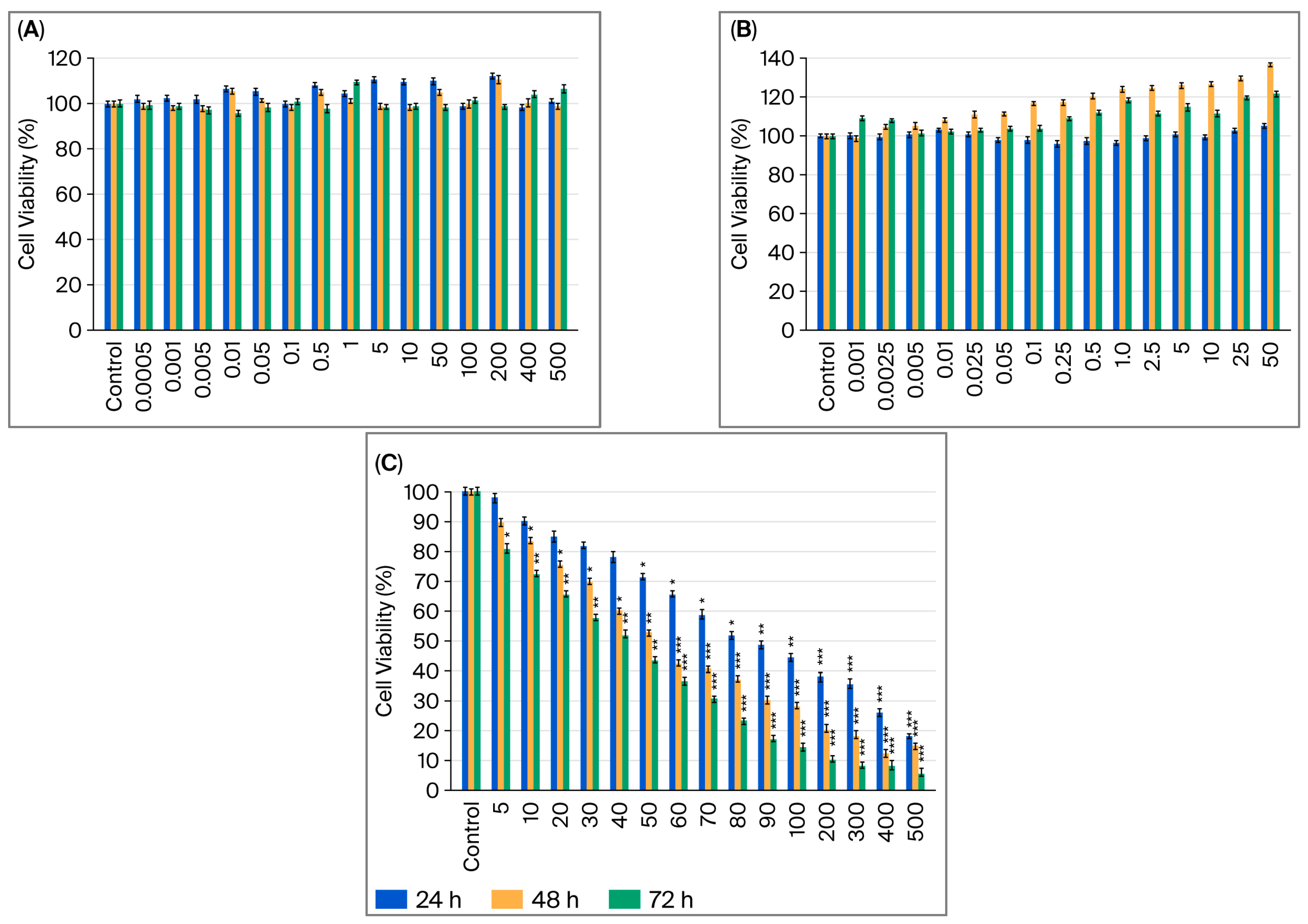
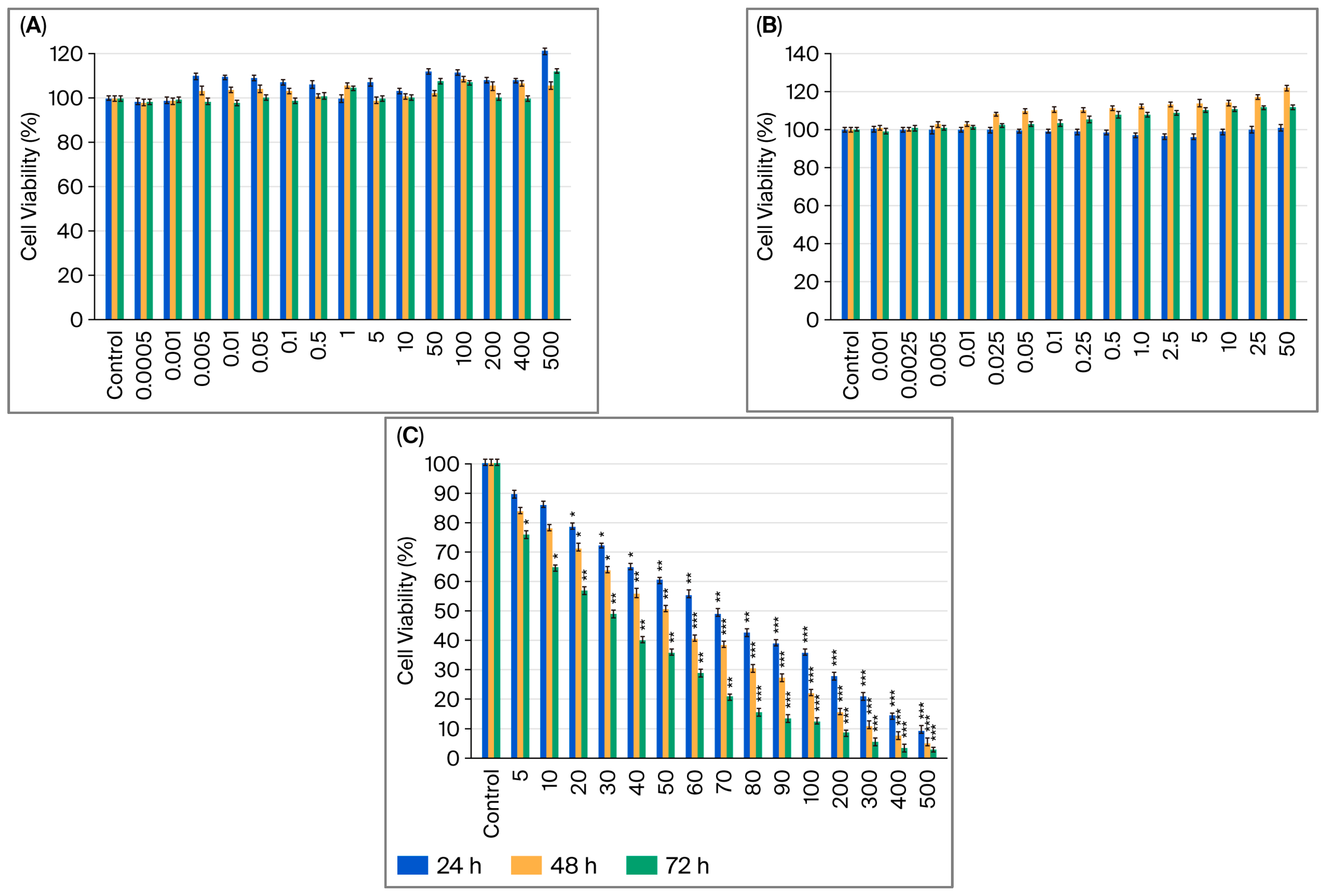
2.1. Assessment of How Single Doses of Vitamin B12, G-CSF, and Cisplatin Affect Cell Viability
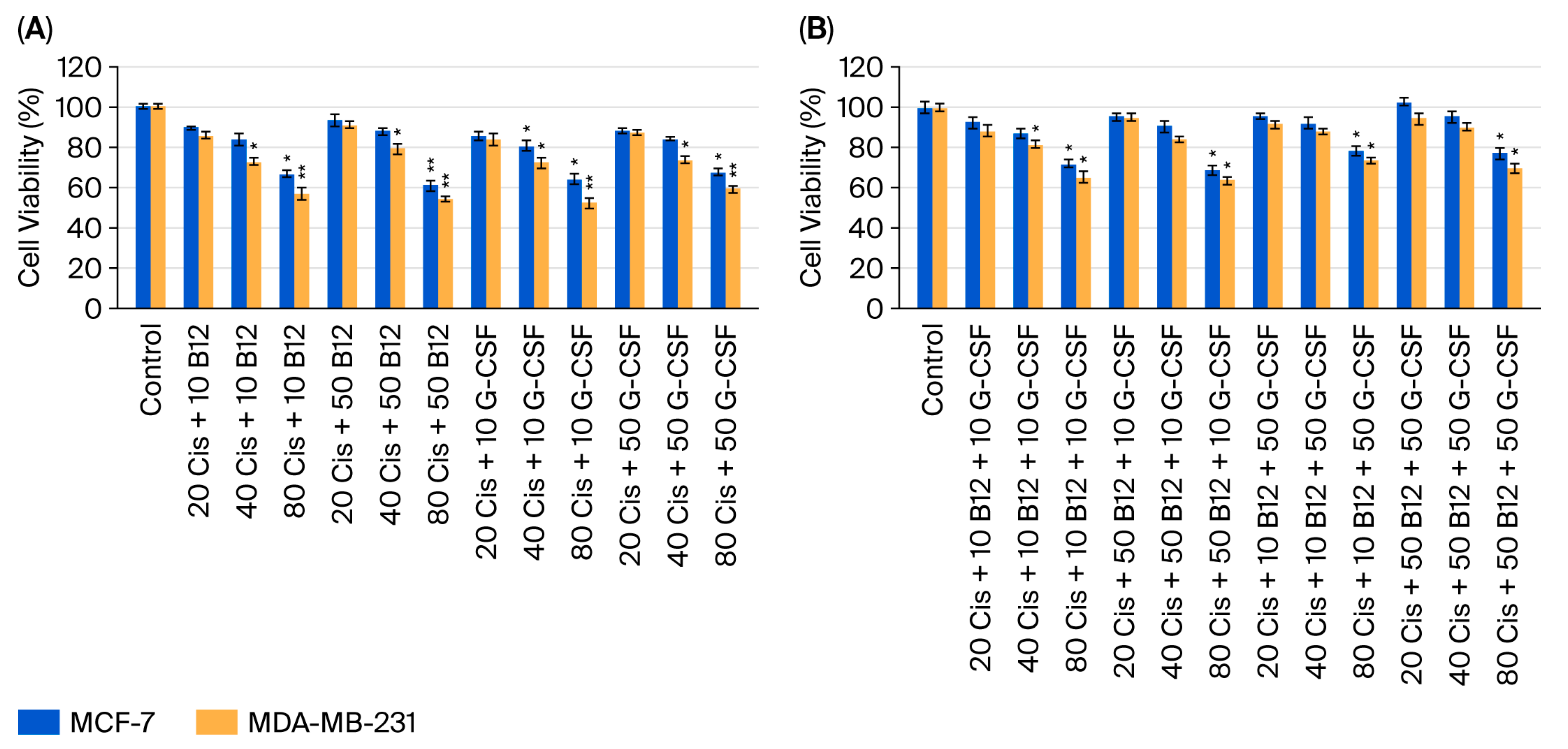
2.2. Effect of Dual and Triple Combinations of Cisplatin with Vitamin B12 and G-CSF on Cell Viability
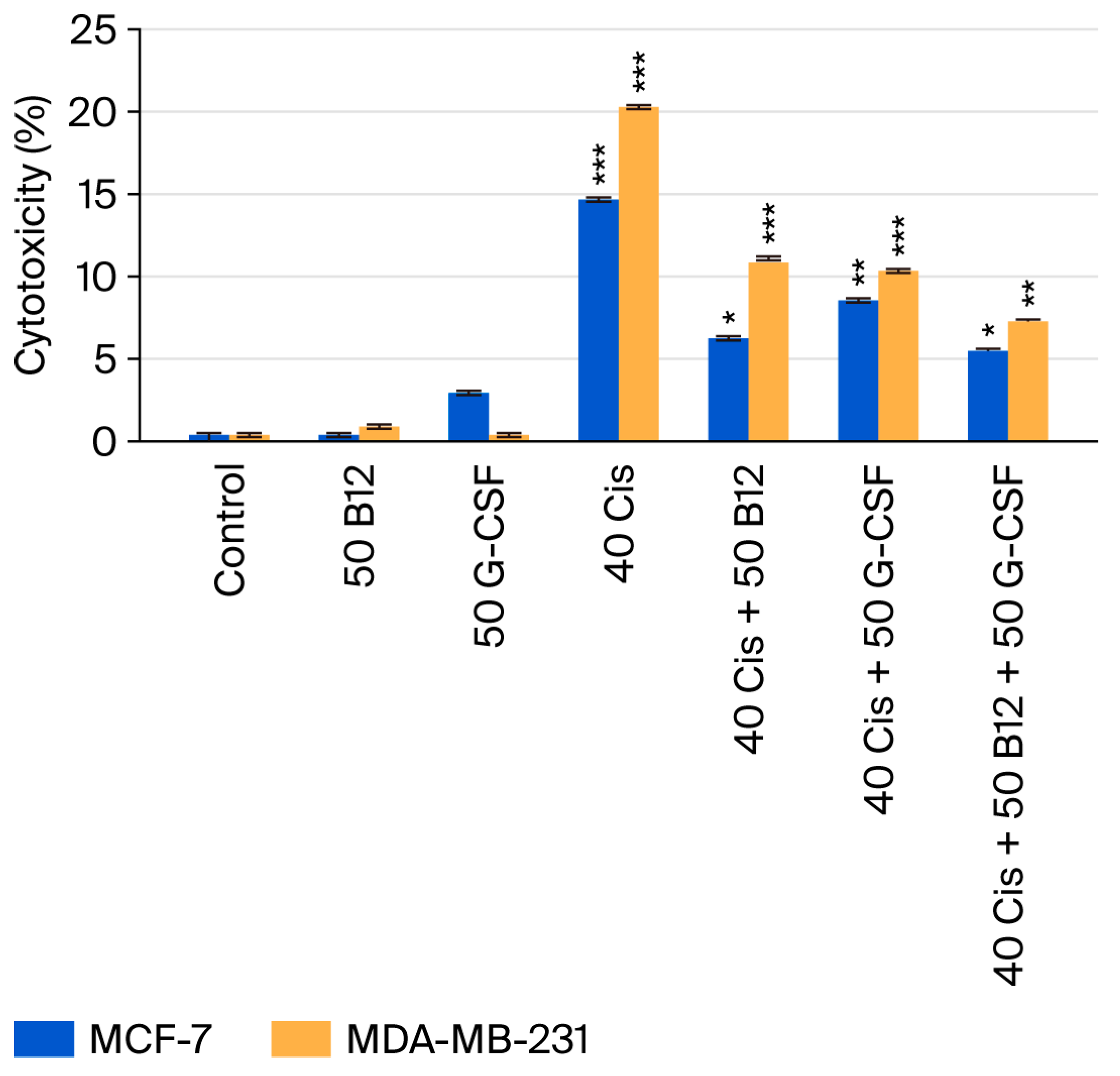
2.3. Cytotoxic Effects of Cisplatin and Its Combinations with Vitamin B12 and G-CSF
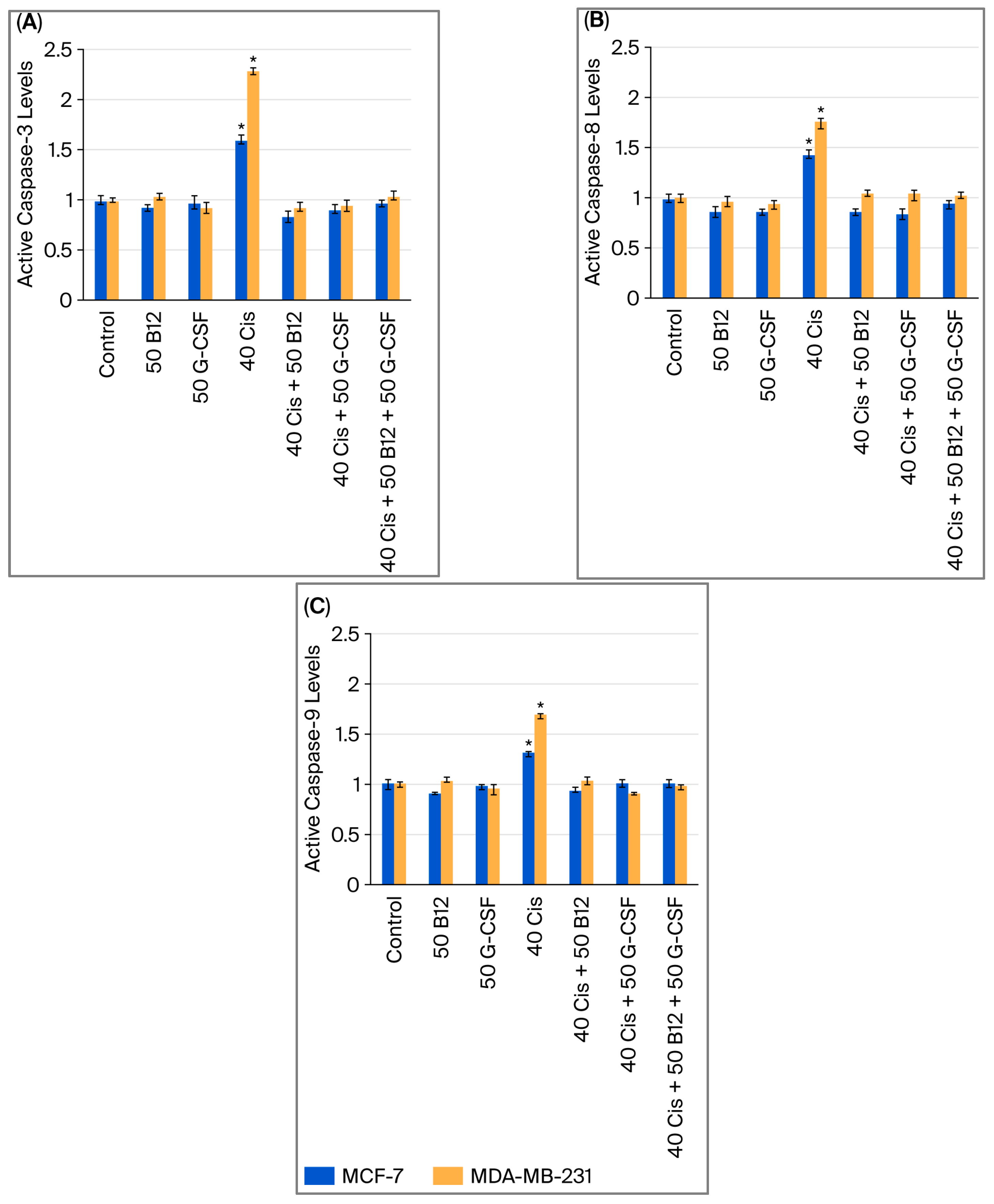
2.4. Effects of Vitamin B12 and G-CSF on Cisplatin-Induced Caspase Activation
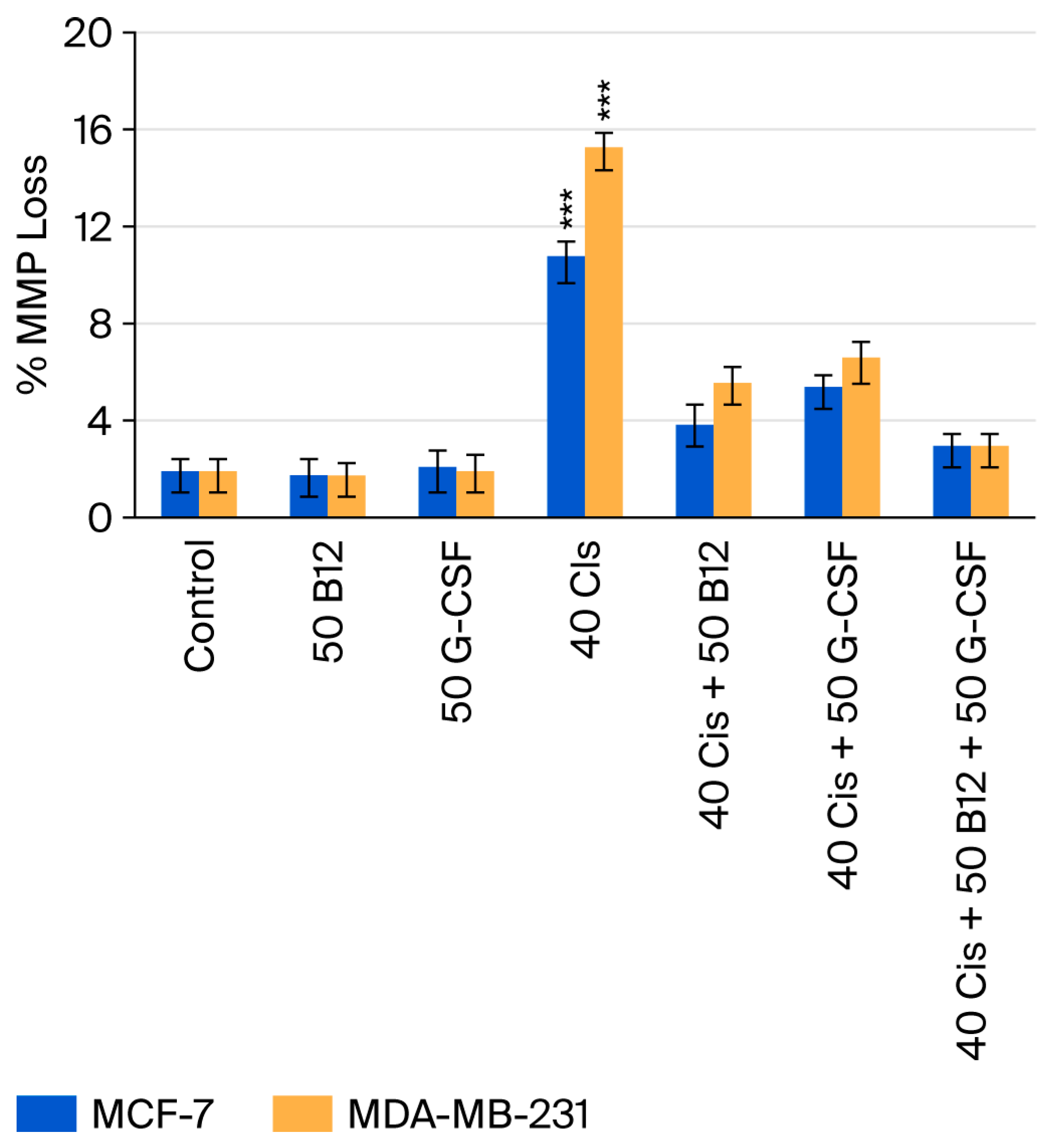
2.5. Effects of Vitamin B12 and G-CSF on Cisplatin-Induced Loss of Mitochondrial Membrane Potential
2.6. Effects of Vitamin B12 and G-CSF on Cisplatin-Induced Perforin and Granzyme Expression in MCF-7 and MDA-MB-231 Cells
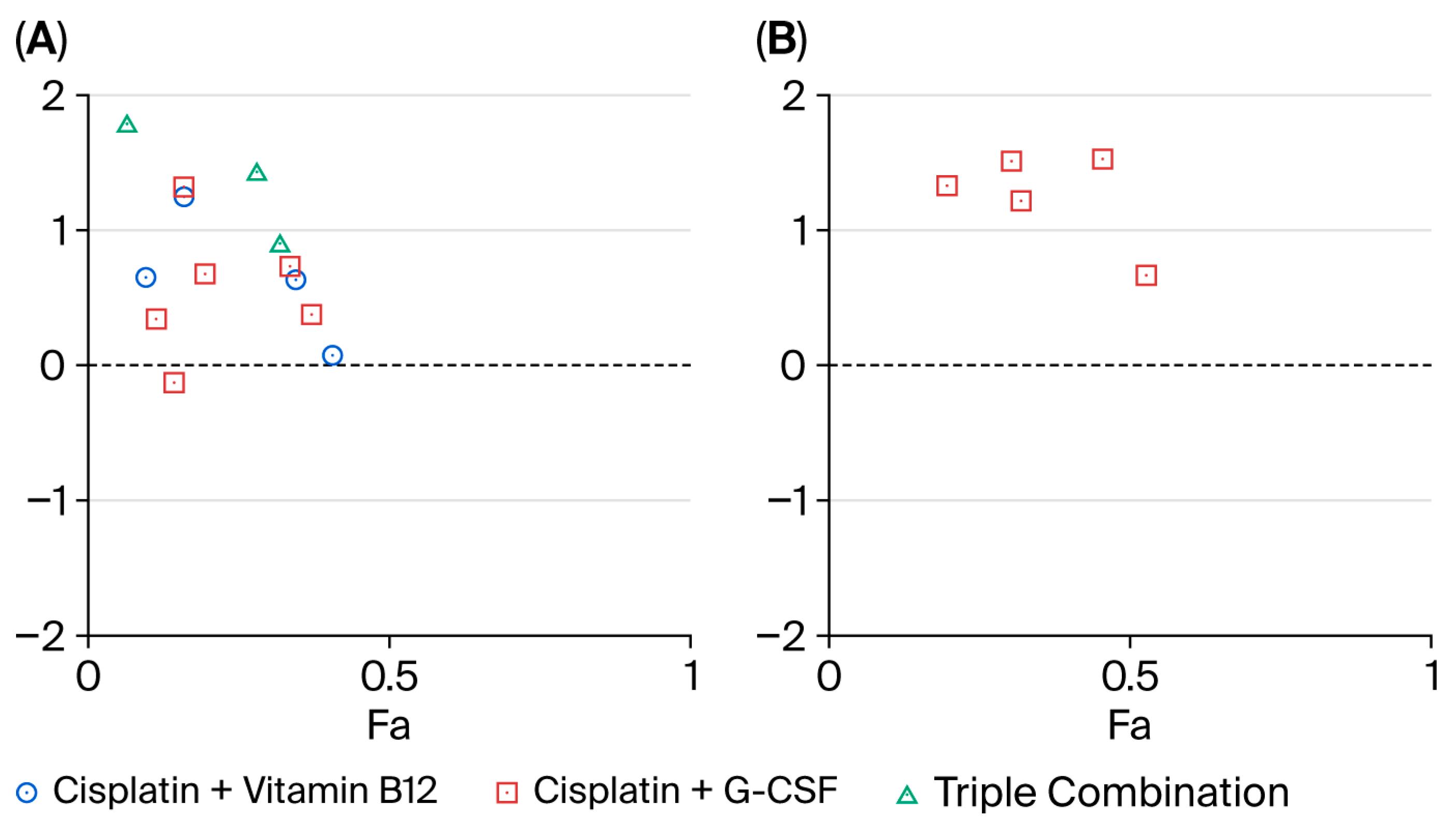
2.7. Combination Index Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Cell Viability Assessment
4.4. Cytotoxicity Assay
4.5. Caspase Activity Assays
4.6. Mitochondrial Membrane Potential Measurement
4.7. Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| B12 | Vitamin B12 (cobalamin, B12) |
| G-CSF | Granulocyte colony-stimulating factor |
| TNBC | Triple-negative breast cancer |
| HR+ | Hormone receptor-positive breast cancer |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | Fetal bovine serum |
| DMSO | Dimethyl sulfoxide |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| LDH | Lactate dehydrogenase |
| Cis | Cisplatin |
| MMP | Mitochondrial membrane potential |
| Cmax | Maximum plasma concentration |
| SD | Standard deviation |
| ANOVA | Analysis of variance |
| PBS | Phosphate-buffered saline |
| CD320 | Transcobalamin receptor |
| CSF3R | Colony-stimulating factor 3 receptor |
| STAT3 | Signal transducer and activator of transcription 3 |
| PI3K/AKT | Phosphoinositide 3-kinase/Protein kinase B pathway |
| EMT | Epithelial–mesenchymal transition |
| MDSCs | Myeloid-derived suppressor cells |
| GSH | Glutathione |
| KLF4 | Krüppel-like factor 4 |
| DNMT | DNA methyltransferase |
References
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.-L.; Brito, A.; Guéant, J.-L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.-H.; et al. Vitamin B12 deficiency. Nat. Rev. Dis. Primers 2017, 3, 17040. [Google Scholar] [CrossRef]
- Zelder, F.; Sonnay, M.; Prieto, L. Antivitamins for medicinal applications. ChemBioChem 2015, 16, 1264–1278. [Google Scholar] [CrossRef]
- Sysel, A.M.; Valli, V.E.; Nagle, R.B.; Bauer, J.A. Immunohistochemical quantification of the vitamin B12 transport protein (TCII), cell surface receptor (TCII-R) and Ki-67 in human tumor xenografts. Anticancer Res. 2013, 33, 4203–4212. [Google Scholar]
- Gick, G.G.; Arora, K.; Sequeira, J.M.; Nakayama, Y.; Lai, S.-C.; Quadros, E.V. Cellular uptake of vitamin B12: Role and fate of TCblR/CD320, the transcobalamin receptor. Exp. Cell Res. 2020, 396, 112256. [Google Scholar]
- Arendt, J.F.B.; Pedersen, L.; Nexo, E.; Sørensen, H.T. Elevated plasma vitamin B12 levels as a marker for cancer: A population-based cohort study. J. Natl. Cancer Inst. 2013, 105, 1799–1805. [Google Scholar] [PubMed]
- Aleksic, D.; Djokic, D.; Golubicic, I.; Jakovljevic, V.; Djuric, D. The importance of the blood levels of homocysteine, folic acid and vitamin B12 in children with malignant diseases. J. BUON 2013, 18, 1019–1025. [Google Scholar] [PubMed]
- Obeid, R. High Plasma Vitamin B12 and Cancer in Human Studies: A Scoping Review to Judge Causality and Alternative Explanations. Nutrients 2022, 14, 4476. [Google Scholar] [CrossRef]
- Lo-Bisgaard, T.; Espelund, U.; Frystyk, J.; Rasmussen, T.R.; Nexo, E.; Arendt, J.F.H. Vitamin B12 and its binding proteins in patients with non-small cell lung cancer referred to fast-track diagnostic work-up for lung cancer. Scand. J. Clin. Lab. Investig. 2020, 80, 14–19. [Google Scholar] [CrossRef]
- Liu, G.-j.; Wang, Y.-j.; Yue, M.; Zhao, L.-m.; Guo, Y.-D.; Liu, Y.-p.; Yang, H.-c.; Liu, F.; Zhang, X.; Zhi, L.-h.; et al. High expression of TCN1 is a negative prognostic biomarker and can predict neoadjuvant chemosensitivity of colon cancer. Sci. Rep. 2020, 10, 11951. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, K.; Bradbury, D.; Davies, J.; Ryrie, D. Cobalamin and folate binding proteins in human tumour tissue. J. Clin. Pathol. 1984, 37, 1336–1338. [Google Scholar] [CrossRef]
- Lacombe, V.; Chabrun, F.; Lacout, C.; Ghali, A.; Capitain, O.; Patsouris, A.; Lavigne, C.; Urbanski, G. Persistent elevation of plasma vitamin B12 is strongly associated with solid cancer. Sci. Rep. 2021, 11, 13361. [Google Scholar] [CrossRef]
- Piyathilake, C.J.; Macaluso, M.; Alvarez, R.D.; Bell, W.C.; Heimburger, D.C.; Partridge, E.E. Lower risk of cervical intraepithelial neoplasia in women with high plasma folate and sufficient vitamin B12 in the post-folic acid fortification era. Cancer Prev. Res. 2009, 2, 658–664. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Hollmén, M.; Karaman, S.; Schwager, S.; Lisibach, A.; Christiansen, A.J.; Maksimow, M.; Varga, Z.; Jalkanen, S.; Detmar, M. G-CSF regulates macrophage phenotype and associates with poor overall survival in human triple-negative breast cancer. Oncoimmunology 2016, 5, e1115177. [Google Scholar]
- Mei, X.; Ouyang, H.; Zhang, H.; Jia, W.; Lu, B.; Zhang, J.; Ji, L. Scutellarin suppresses the metastasis of triple-negative breast cancer via targeting TNFα/TNFR2-RUNX1-triggered G-CSF expression in endothelial cells. Biochem. Pharmacol. 2023, 217, 115808. [Google Scholar]
- Ali, S.; Coombes, R.C. Estrogen receptor alpha in human breast cancer: Occurrence and significance. J. Mammary Gland Biol. Neoplasia 2000, 5, 271–281. [Google Scholar]
- Olivier, M.; Langerød, A.; Carrieri, P.; Bergh, J.; Klaar, S.; Eyfjord, J.; Theillet, C.; Rodriguez, C.; Lidereau, R.; Bièche, I.; et al. The clinical value of somatic TP53 gene mutations in 1794 patients with breast cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Flores-Guerrero, J.L.; Minović, I.; Groothof, D.; Gruppen, E.G.; Riphagen, I.J.; Kootstra-Ros, J.; Kobold, A.M.; Hak, E.; Navis, G.; Gansevoort, R.T.; et al. Association of plasma concentration of vitamin B12 with all-cause mortality in the general population in the Netherlands. JAMA Netw. Open 2020, 3, e1919274. [Google Scholar] [PubMed]
- Karagiannidis, I.; Salataj, E.; Egal, E.S.A.; Beswick, E.J. G-CSF in tumors: Aggressiveness, tumor microenvironment and immune cell regulation. Cytokine 2021, 142, 155479. [Google Scholar] [CrossRef] [PubMed]
- Tsukazaki, Y.; Ogino, H.; Okano, Y.; Kakiuchi, S.; Harada, S.; Toyoda, Y.; Matsumura, Y.; Ichihara, S.; Imakura, T.; Matsumoto, R.; et al. Granulocyte colony-stimulating factor has the potential to attenuate the therapeutic efficacy of chemo-immunotherapy for extensive-stage small-cell lung cancer. Int. J. Clin. Oncol. 2024, 29, 1451–1460. [Google Scholar] [CrossRef]
- Ilhan, Y.; Ucar, G.; Baser, M.N.; Guzel, H.G.; Efil, S.C.; Demir, B.; Ercan Uzundal, D.; Karacelik, T.; Sever, N.; Balcik, O.Y.; et al. Efficacy and safety of G-CSF prophylaxis in patients with extensive-stage small cell lung cancer receiving chemoimmunotherapy. Expert Opin. Pharmacother. 2024, 25, 1555–1563. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Yan, X.; Zhou, C.; Xiong, X. The role of granulocyte colony-stimulating factor in breast cancer development: A review. Mol. Med. Rep. 2020, 21, 2019–2029. [Google Scholar] [CrossRef]
- Ambrosone, C.B.; Zirpoli, G.R.; Hutson, A.D.; McCann, W.E.; McCann, S.E.; Barlow, W.E.; Kelly, K.M.; Cannioto, R.; Sucheston-Campbell, L.E.; Hershman, D.L.; et al. Dietary supplement use during chemotherapy and survival outcomes of patients with breast cancer enrolled in a cooperative group clinical trial (SWOG S0221). J. Clin. Oncol. 2020, 38, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Kuo, C.-S.; Lu, C.-L.; Wu, M.-Y.; Huang, R.-F.S. Elevated serum vitamin B12 levels in association with tumor markers as the prognostic factors predictive for poor survival in patients with hepatocellular carcinoma. Nutr. Cancer 2010, 62, 190–197. [Google Scholar] [CrossRef]
- Arendt, J.F.H.; Farkas, D.K.; Pedersen, L.; Nexo, E.; Sørensen, H.T. Elevated plasma vitamin B12 levels and cancer prognosis: A population-based cohort study. Cancer Epidemiol. 2016, 40, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Rzepka, Z.; Rok, J.; Maszczyk, M.; Beberok, A.; Hermanowicz, J.M.; Pawlak, D.; Gryko, D.; Wrześniok, D. Response of human glioblastoma cells to vitamin B12 deficiency: A study using the non-toxic cobalamin antagonist. Biology 2021, 10, 69. [Google Scholar] [CrossRef]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef]
- Graeser, M.; McCarthy, A.; Lord, C.J.; Savage, K.; Hills, M.; Salter, J.; Orr, N.; Parton, M.; Smith, I.E.; Reis-Filho, J.S.; et al. A marker of homologous recombination predicts pathologic complete response to neoadjuvant chemotherapy in primary breast cancer. Clin. Cancer Res. 2010, 16, 6159–6168. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Yu, P.; Wen, J.; Li, N.; Huang, W.; Xie, X.; Ye, F. Survival benefit of platinum-based regimen in early stage triple negative breast cancer: A meta-analysis of randomized controlled trials. NPJ Breast Cancer 2021, 7, 157. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Kawaguchi, K.; Kawanishi, K.; Maeshima, Y.; Nakakura, A.; Kataoka, T.R.; Takahara, S.; Nakagawa, S.; Yonezawa, A.; Takada, M.; et al. Comparison of cisplatin-based versus standard preoperative chemotherapy in patients with operable triple-negative breast cancer: Propensity score matching and inverse probability of treatment weighting analysis. Breast Cancer Res. Treat. 2023, 204, 261–275. [Google Scholar] [CrossRef]
- Yang, M.-D.; Sun, Y.; Zhou, W.-J.; Xie, X.-Z.; Zhou, Q.-M.; Lu, Y.-Y.; Su, S.-B. Resveratrol enhances inhibition effects of cisplatin on cell migration and invasion and tumor growth in breast cancer MDA-MB-231 cell models in vivo and in vitro. Molecules 2021, 26, 2204. [Google Scholar] [CrossRef] [PubMed]
- Pascua, S.M.; McGahey, G.E.; Ma, N.; Wang, J.J.; Digman, M.A. Caffeine and cisplatin effectively targets the metabolism of a triple-negative breast cancer cell line assessed via phasor-FLIM. Int. J. Mol. Sci. 2020, 21, 2443. [Google Scholar] [CrossRef]
- Halczuk, K.; Kaźmierczak-Barańska, J.; Karwowski, B.T.; Karmańska, A.; Cieślak, M. Vitamin B12—Multifaceted in vivo functions and in vitro applications. Nutrients 2023, 15, 2734. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Dasari, S.; Noubissi, F.K.; Ray, P.; Kumar, S. Advances in our understanding of the molecular mechanisms of action of cisplatin in cancer therapy. J. Exp. Pharmacol. 2021, 2021, 303–328. [Google Scholar] [CrossRef]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B vitamins and one-carbon metabolism: Implications in human health and disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef]
- Bernasocchi, T.; Mostoslavsky, R. Subcellular one carbon metabolism in cancer, aging and epigenetics. Front. Epigenetics Epigenomics 2024, 2, 1451971. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Buque, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 2015, 28, 690–714. [Google Scholar] [CrossRef]
- Matos, I.; Barvalia, M.; Chehal, M.K.; Robertson, A.G.; Kulic, I.; Silva, J.A.; Ranganathan, A.; Short, A.; Huang, Y.-H.; Long, E.; et al. Tumor-derived GCSF alters tumor and systemic immune system cell subset composition and signaling. Cancer Res. Commun. 2023, 3, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; White, S.M.; Guha, S. Granulocyte colony-stimulating receptor promotes β1-integrin-mediated adhesion and invasion of bladder cancer cells. Urology 2006, 68, 208–213. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Chen, Y.; Xie, Y.; Liu, J.; Feng, Q.; Wang, Y.; Yuan, W.; Ma, J. G-CSF is a key modulator of MDSC and could be a potential therapeutic target in colitis-associated colorectal cancers. Protein Cell 2016, 7, 130–140. [Google Scholar] [CrossRef]
- Kortylewski, M.; Kujawski, M.; Wang, T.; Wei, S.; Zhang, S.; Pilon-Thomas, S.; Niu, G.; Kay, H.; Mulé, J.; Kerr, W.G.; et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat. Med. 2005, 11, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Miyake, M.; Onishi, S.; Morizawa, Y.; Nakai, Y.; Tatsumi, Y.; Onishi, K.; Iida, K.; Gotoh, D.; Itami, Y.; et al. Evaluation of pro-and anti-tumor effects induced by three colony-stimulating factors, G-CSF, GM-CSF and M-CSF, in bladder cancer cells: Is G-CSF a friend of bladder cancer cells? Int. J. Oncol. 2019, 54, 2237–2249. [Google Scholar] [CrossRef] [PubMed]
- Fukui, Y.; Kawashima, M.; Kawaguchi, K.; Takeuchi, M.; Hirata, M.; Kataoka, T.R.; Sakurai, T.; Kataoka, M.; Kanao, S.; Nakamoto, Y.; et al. Granulocyte-colony-stimulating factor-producing metaplastic carcinoma of the breast with significant elevation of serum interleukin-17 and vascular endothelial growth factor levels. Int. Cancer Conf. J. 2018, 7, 107–113. [Google Scholar] [CrossRef]
- Suzuki, K.; Ota, D.; Nishi, T.; Mori, M.; Kato, T.; Takeuchi, M.; Tsuji, M.; Teraoka, M.; Fukuuchi, A. A Case of Granulocyte-Colony Stimulating Factor–Producing Spindle Cell Carcinoma of the Breast. Clin. Breast Cancer 2015, 15, e213–e217. [Google Scholar] [CrossRef] [PubMed]
- Cavalloni, G.; Sarotto, I.; Pignochino, Y.; Gammaitoni, L.; Migliardi, G.; Sgro, L.; Piacibello, W.; Risio, M.; Aglietta, M.; Leone, F. Granulocyte-colony stimulating factor upregulates ErbB2 expression on breast cancer cell lines and converts primary resistance to trastuzumab. Anti-Cancer Drugs 2008, 19, 689–696. [Google Scholar] [CrossRef]
- U.S. Food & Drug. Neupogen (Filgrastim) Prescribing Information; FDA: Silver Spring, MD, USA, 2013. [Google Scholar]
- Ocak, M.; Usta, D.D.; Arik Erol, G.N.; Kaplanoglu, G.T.; Konac, E.; Yar Saglam, A.S. Determination of in vitro and in vivo effects of taxifolin and epirubicin on epithelial–mesenchymal transition in mouse breast cancer cells. Technol. Cancer Res. Treat. 2024, 23, 15330338241241245. [Google Scholar] [CrossRef]
- Fossey, S.L.; Liao, A.T.; McCleese, J.K.; Bear, M.D.; Lin, J.; Li, P.-K.; Kisseberth, W.C.; London, C.A. Characterization of STAT3 activation and expression in canine and human osteosarcoma. BMC Cancer 2009, 9, 81. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslan, V.; Usta, D.D.; Yar Sağlam, A.S.; Özet, A.; Sütcüoglu, O.; Dikmen, K.; Özdemir, N. The Responsiveness of Breast Cancer Cells to Varied Levels of Vitamin B12, Cisplatin, and G-CSF. Int. J. Mol. Sci. 2025, 26, 9086. https://doi.org/10.3390/ijms26189086
Aslan V, Usta DD, Yar Sağlam AS, Özet A, Sütcüoglu O, Dikmen K, Özdemir N. The Responsiveness of Breast Cancer Cells to Varied Levels of Vitamin B12, Cisplatin, and G-CSF. International Journal of Molecular Sciences. 2025; 26(18):9086. https://doi.org/10.3390/ijms26189086
Chicago/Turabian StyleAslan, Volkan, Duygu Deniz Usta, Atiye Seda Yar Sağlam, Ahmet Özet, Osman Sütcüoglu, Kürşat Dikmen, and Nuriye Özdemir. 2025. "The Responsiveness of Breast Cancer Cells to Varied Levels of Vitamin B12, Cisplatin, and G-CSF" International Journal of Molecular Sciences 26, no. 18: 9086. https://doi.org/10.3390/ijms26189086
APA StyleAslan, V., Usta, D. D., Yar Sağlam, A. S., Özet, A., Sütcüoglu, O., Dikmen, K., & Özdemir, N. (2025). The Responsiveness of Breast Cancer Cells to Varied Levels of Vitamin B12, Cisplatin, and G-CSF. International Journal of Molecular Sciences, 26(18), 9086. https://doi.org/10.3390/ijms26189086





