First Successful Treatment of Advanced Intrahepatic Cholangiocarcinoma with Tasurgratinib Following Regulatory Approval: A Case Report from Clinical Practice
Abstract
1. Introduction
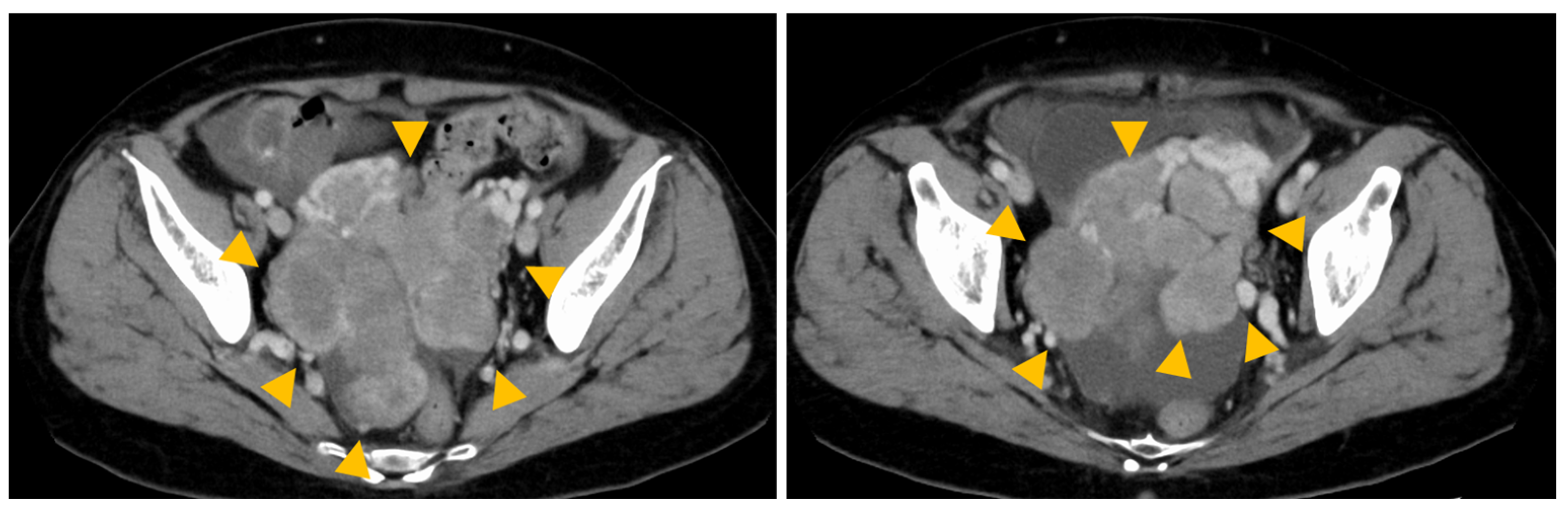
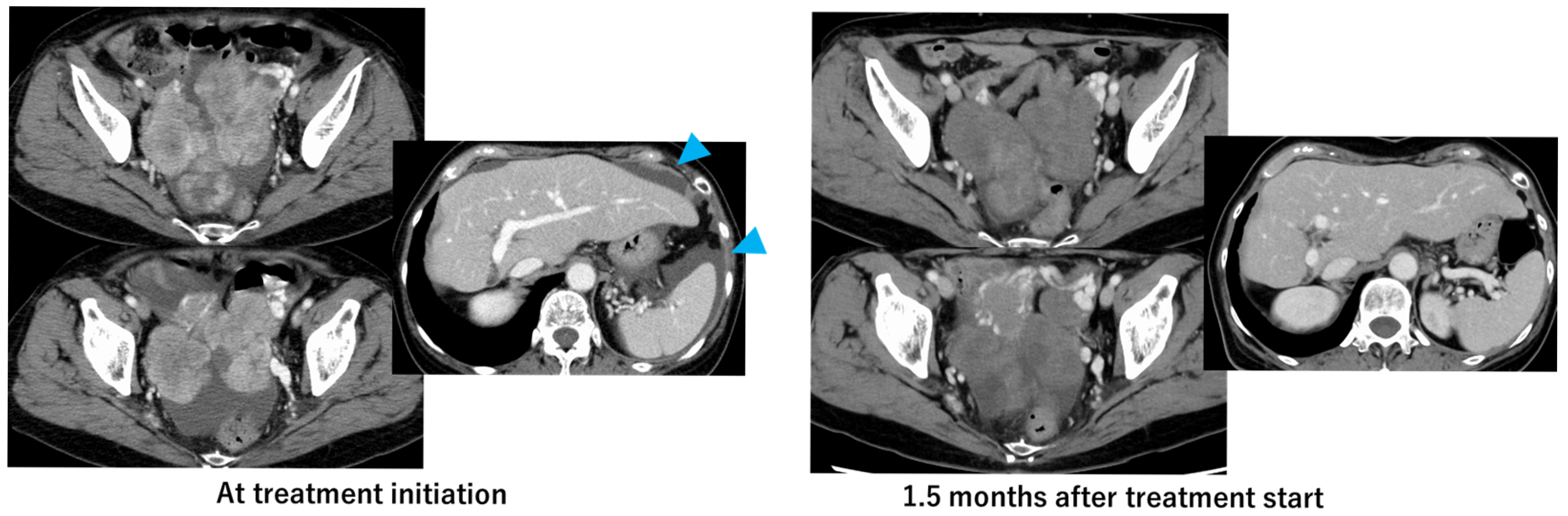
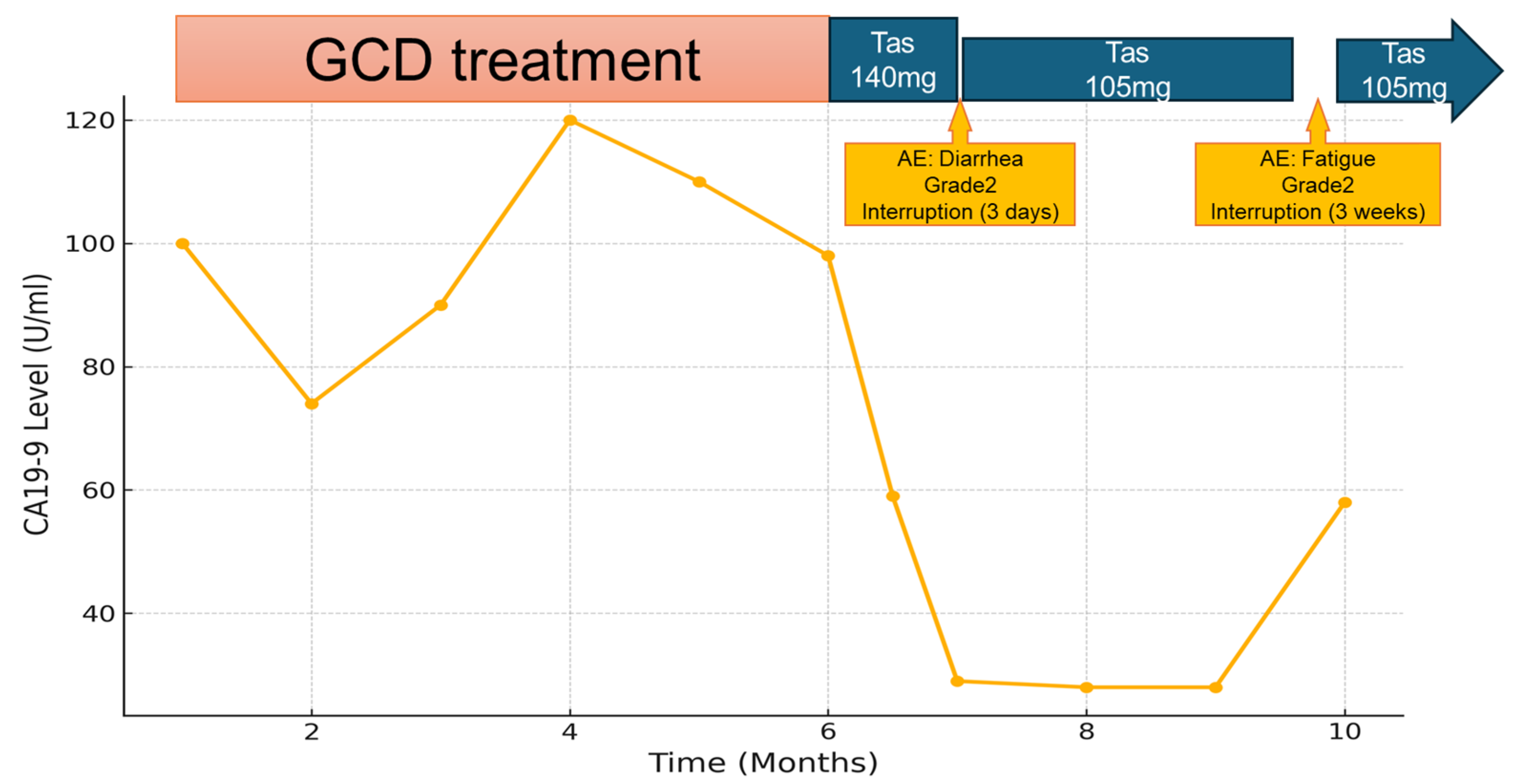
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| iCCA | Intrahepatic cholangiocarcinoma |
| FGFR2 | fibroblast growth factor receptor 2 |
| GCD | gemcitabine, cisplatin, and durvalumab |
| BTC | biliary tract cancer |
| GC | Gemcitabine plus cisplatin |
| ICIs | immune checkpoint inhibitors |
| IDH1 | Isocitrate dehydrogenase 1 |
| CT | computed tomography |
| EUS | TA Endoscopic ultrasound-guided tissue acquisition |
| CGP | Comprehensive genomic profiling |
| PR | partial response |
| FISH | fluorescence in situ hybridization |
| NGS | next-generation sequencing |
| IHC | immunohistochemistry |
References
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Woods, E.; Le, D.; Jakka, B.K.; Manne, A. Changing landscape of systemic therapy in biliary tract cancer. Cancers 2022, 14, 2137. [Google Scholar] [CrossRef] [PubMed]
- Ioka, T.; Kanai, M.; Kobayashi, S.; Sakai, D.; Eguchi, H.; Baba, H.; Seo, S.; Taketomi, A.; Takayama, T.; Yamaue, H.; et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer (KHBO1401- Mitsuba). J. Hepato-Bil. Pancreat. Sci. 2023, 30, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; Ruth He, A.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Ah Lee, M.; Kitano, M.; et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.J.; Chan, S.L.; Ozaka, M.; et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Arai, Y.; Totoki, Y.; Shirota, T.; Elzawahry, A.; Kato, M.; Hama, N.; Hosoda, F.; Urushidate, T.; Ohashi, S.; et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015, 47, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Barriuso, J.; McNamara, M.G.; Valle, J.W. Molecular targeted therapies: Ready for “prime time” in biliary tract cancer. J. Hepatol. 2020, 73, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; Kelley, R.K.; Cassier, P.A.; et al. Futibatinib for FGFR2-rearranged intrahepatic cholangiocarcinoma. N. Engl. J. Med. 2023, 388, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. Tasurgratinib Succinate: First Approval. Drugs 2025, 85, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Furuse, J.; Jiang, B.; Kuwahara, T.; Satoh, T.; Ma, X.; Yan, S.; Zhao, H.-T.; Ikeda, M.; Cui, T.; Sasaki, T.; et al. Pivotal Single-Arm, Phase 2 Trial of Tasurgratinib for Patients with Fibroblast Growth Factor Receptor (FGFR)-2 Gene Fusion-Positive Cholangiocarcinoma (CCA). J. Clin. Oncol. 2024, 45. [Google Scholar] [CrossRef]
- Inada, H.; Miyamoto, H.; Shinriki, S.; Oda, H.; Narahara, S.; Yoshinari, M.; Nagaoka, K.; Yoshii, D.; Fukubayashi, K.; Hayashi, H.; et al. Clinical utility of a comprehensive genomic profiling test for patient with advanced biliary tract cancer. Int. J. Clin. Oncol. 2024, 29, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Goyal, L.; Kongpetch, S.; Crolley, V.E.; Bridgewater, J. Targeting FGFR inhibition in cholangiocarcinoma. Cancer Treat. Rev. 2021, 95, 102170. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M.; Loriot, Y.; Brandi, G.; Tavolari, S.; Wainberg, Z.A.; Katoh, M. FGFR-targeted therapeutics: Clinical activity, mechanisms of resistance and new directions. Nat. Rev. Clin. Oncol. 2024, 21, 312–329. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, G.; Du, L.; Zhao, J.; Pan, L.; Zhang, G.; Wang, F.; Liu, R. Case Report: Persistent response to combination therapy of pemigatinib, chemotherapy, and immune checkpoint inhibitor in a patient with advanced intrahepatic cholangiocarcinoma. Front. Immunol. 2023, 14, 1124482. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Angerilli, V.; Fornaro, L.; Pepe, F.; Rossi, S.M.; Perrone, G.; Malapelle, U.; Fassan, M. FGFR2 testing in cholangiocarcinoma: Translating molecular studies into clinical practice. Pathologica 2023, 115, 71. [Google Scholar] [CrossRef] [PubMed]
- Tsujie, M.; Iwai, T.; Kubo, S.; Ura, T.; Hatano, E.; Sakai, D.; Takeda, Y.; Kaibori, M.; Kobayashi, T.; Katanuma, A.; et al. Fibroblast growth factor receptor 2 (FGFR2) fusions in Japanese patients with intrahepatic cholangiocarcinoma. Jpn. J. Clin. Oncol. 2021, 51, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Yang, Y.; Liu, S.; Sun, L.; Liu, Y.; Luo, Y.; Wang, J.; Sun, Y. FGFR2 fusions assessed by NGS, FISH, and immunohistochemistry in intrahepatic cholangiocarcinoma. J. Gastroenterol. 2025, 60, 235–246. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maruki, Y.; Morizane, C.; Okada, M.; Harai, S.; Nagashio, Y.; Hijioka, S.; Ueno, H.; Okusaka, T. First Successful Treatment of Advanced Intrahepatic Cholangiocarcinoma with Tasurgratinib Following Regulatory Approval: A Case Report from Clinical Practice. Int. J. Mol. Sci. 2025, 26, 5586. https://doi.org/10.3390/ijms26125586
Maruki Y, Morizane C, Okada M, Harai S, Nagashio Y, Hijioka S, Ueno H, Okusaka T. First Successful Treatment of Advanced Intrahepatic Cholangiocarcinoma with Tasurgratinib Following Regulatory Approval: A Case Report from Clinical Practice. International Journal of Molecular Sciences. 2025; 26(12):5586. https://doi.org/10.3390/ijms26125586
Chicago/Turabian StyleMaruki, Yuta, Chigusa Morizane, Mao Okada, Shota Harai, Yoshikuni Nagashio, Susumu Hijioka, Hideki Ueno, and Takuji Okusaka. 2025. "First Successful Treatment of Advanced Intrahepatic Cholangiocarcinoma with Tasurgratinib Following Regulatory Approval: A Case Report from Clinical Practice" International Journal of Molecular Sciences 26, no. 12: 5586. https://doi.org/10.3390/ijms26125586
APA StyleMaruki, Y., Morizane, C., Okada, M., Harai, S., Nagashio, Y., Hijioka, S., Ueno, H., & Okusaka, T. (2025). First Successful Treatment of Advanced Intrahepatic Cholangiocarcinoma with Tasurgratinib Following Regulatory Approval: A Case Report from Clinical Practice. International Journal of Molecular Sciences, 26(12), 5586. https://doi.org/10.3390/ijms26125586







