CD163/CD63+ Monocyte-Derived DC Profiled in Tissue by Multi-Antigen Analysis (MAA) Discriminate Chronic Eczema and Psoriasis
Abstract
1. Introduction
2. Results
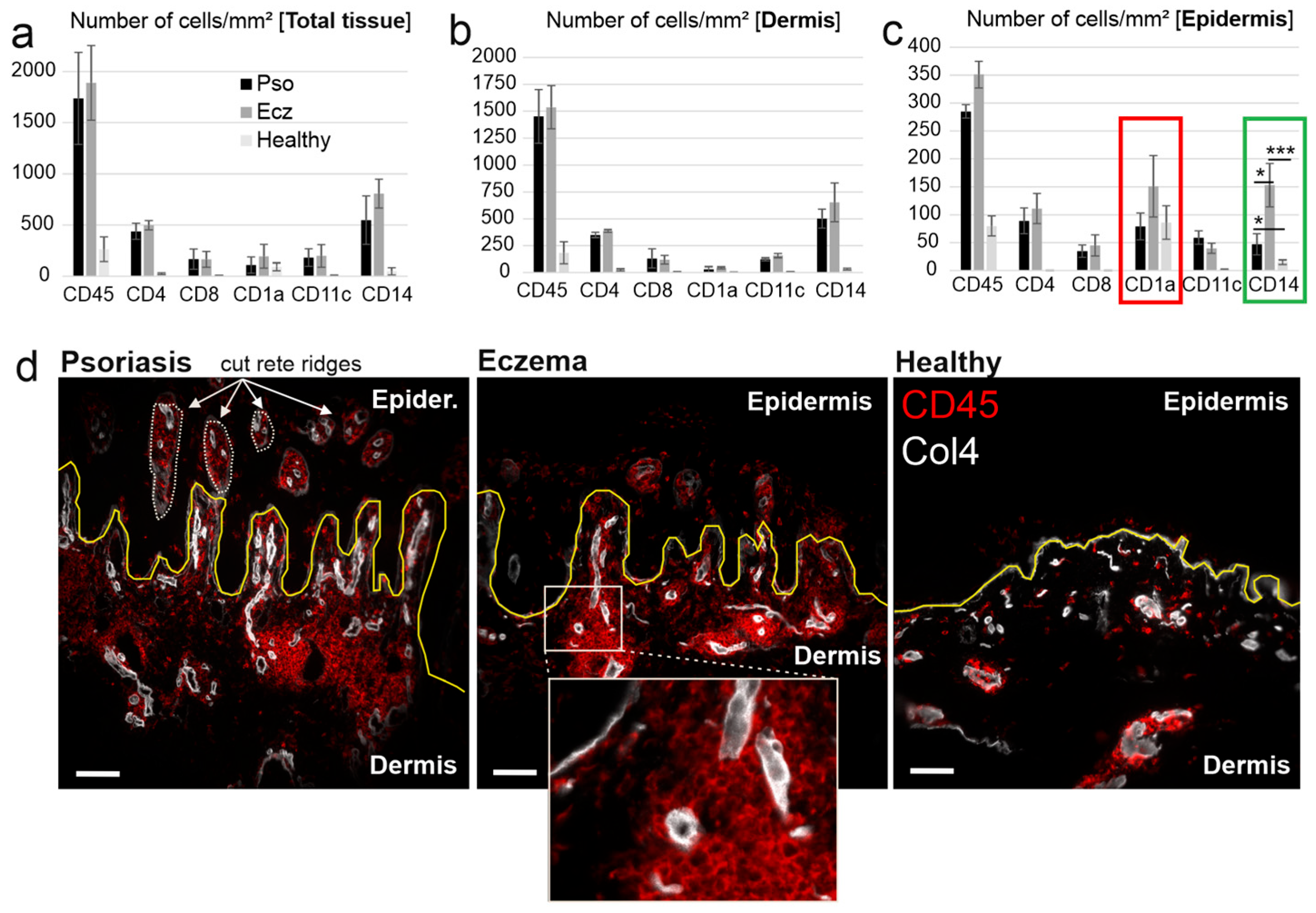
2.1. CD14+ and CD1a+ Cells Dominate in the Epidermis of Chronic Eczema
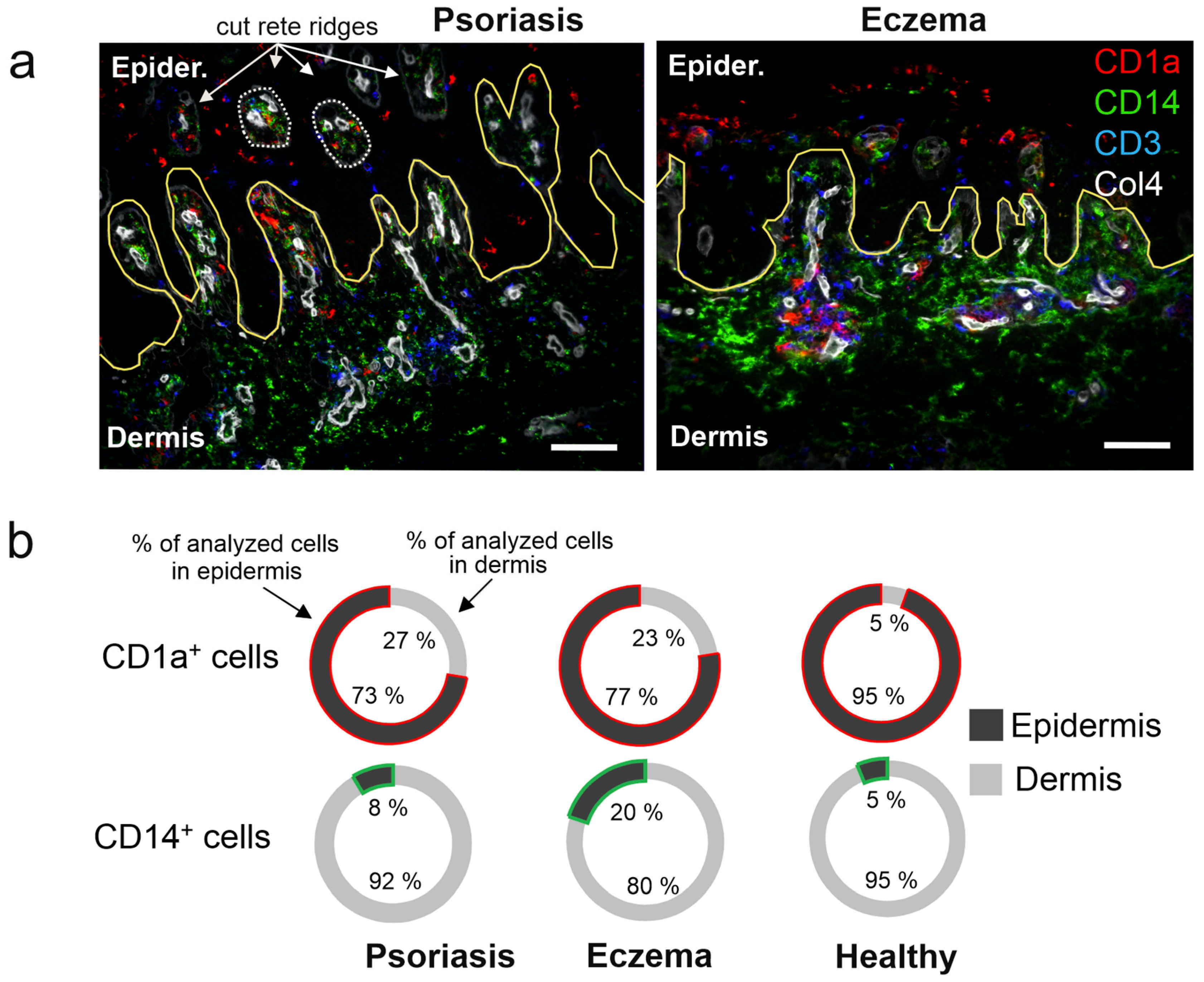
2.2. High Recruitment of CD14+ Cells to the Eczema Epidermis
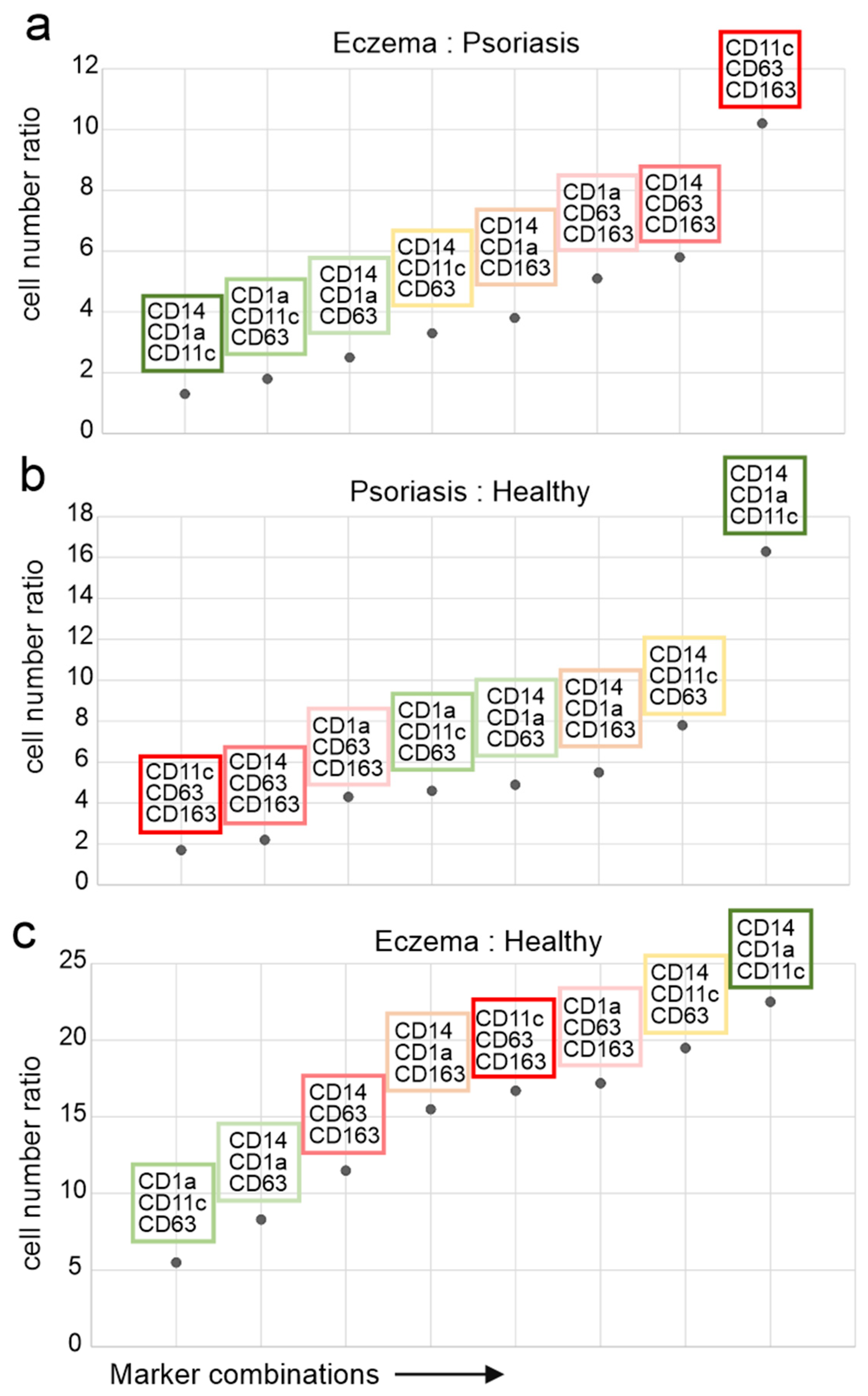
2.3. DC Expressing CD63 and CD163 Are Predominant in Eczema
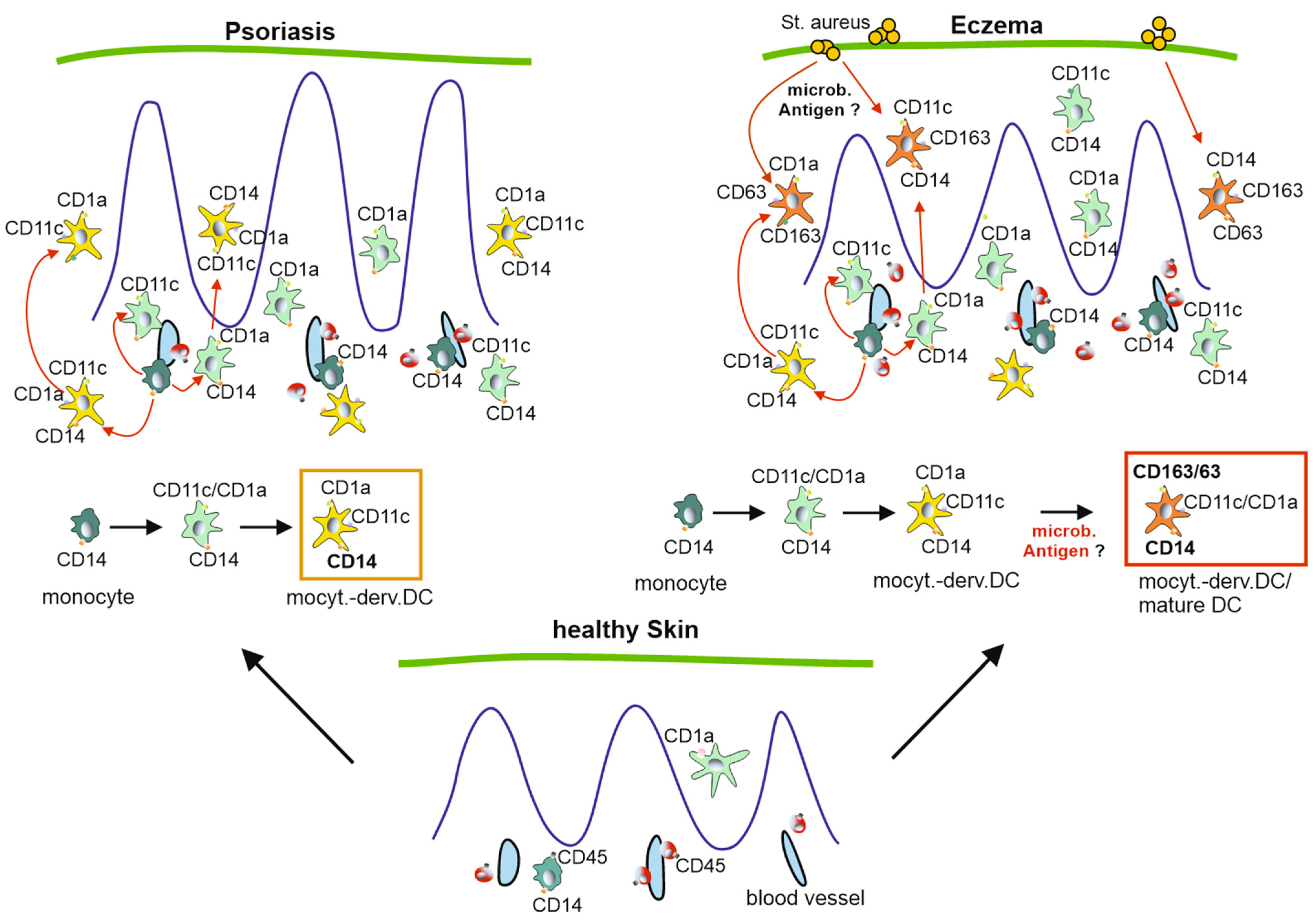
2.4. CD11c+/CD63+/CD163+ Cells Discriminate Pso from Eczema
3. Discussion
4. Materials and Methods
4.1. Tissue Samples
4.2. MAA Sample Preparation
4.3. MAA Data Generation
4.4. MAA Antibody Library
4.5. MAA Analysis
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayala-Fontánez, N.; Soler, D.C.; McCormick, T.S. Current knowledge on psoriasis and autoimmune diseases. Psoriasis 2016, 6, 7–32. [Google Scholar] [CrossRef]
- Boehncke, W.-H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Burfield, L.; Burden, A. Psoriasis. J. R. Coll. Physicians Edinb. 2013, 43, 334–338, quiz 339. [Google Scholar] [CrossRef] [PubMed]
- Navarini, A.A.; Trüeb, R.M. Psoriasis. Ther. Umsch. Rev. Ther. 2010, 67, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Sticherling, M. Psoriasis and autoimmunity. Autoimmun. Rev. 2016, 15, 1167–1170. [Google Scholar] [CrossRef] [PubMed]
- Moorman, C.D.; Sohn, S.J.; Phee, H. Emerging Therapeutics for Immune Tolerance: Tolerogenic Vaccines, T cell Therapy, and IL-2 Therapy. Front. Immunol. 2021, 12, 657768. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Krueger, J.G. Atopic dermatitis and psoriasis: Two different immune diseases or one spectrum? Curr. Opin. Immunol. 2017, 48, 68–73. [Google Scholar] [CrossRef]
- McGregor, S.; Farhangian, M.E.; Feldman, S.R. Dupilumab for the treatment of atopic dermatitis: A clinical trial review. Expert Opin. Biol. Ther. 2015, 15, 1657–1660. [Google Scholar] [CrossRef]
- Antal, D.; Alimohammadi, S.; Bai, P.; Szöllősi, A.G.; Szántó, M. Antigen-Presenting Cells in Psoriasis. Life 2022, 12, 234. [Google Scholar] [CrossRef]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A.; Grading, C. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef]
- Furue, M.; Kadono, T. “Inflammatory skin march” in atopic dermatitis and psoriasis. Inflamm. Res. 2017, 66, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Krueger, J.G. The immunopathogenesis of psoriasis. Dermatol. Clin. 2015, 33, 13–23. [Google Scholar] [CrossRef]
- Wang, A.; Bai, Y. Dendritic cells: The driver of psoriasis. J. Dermatol. 2020, 47, 104–113. [Google Scholar] [CrossRef]
- Lande, R.; Gregorio, J.; Facchinetti, V.; Chatterjee, B.; Wang, Y.-H.; Homey, B.; Cao, W.; Wang, Y.-H.; Su, B.; Nestle, F.O.; et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimi-crobial peptide. Nature 2007, 449, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Di Meglio, P.; Qin, J.-Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef]
- Ha, H.-L.; Wang, H.; Pisitkun, P.; Kim, J.-C.; Tassi, I.; Tang, W.; Morasso, M.I.; Udey, M.C.; Siebenlist, U. IL-17 drives psoriatic inflammation via distinct, target cell-specific mechanisms. Proc. Natl. Acad. Sci. USA 2014, 111, E3422–E3431. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Lambrecht, B.N. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity 2015, 43, 29–40. [Google Scholar] [CrossRef]
- Ginhoux, F.; Tacke, F.; Angeli, V.; Bogunovic, M.; Loubeau, M.; Dai, X.-M.; Stanley, E.R.; Randolph, G.J.; Merad, M. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 2006, 7, 265–273. [Google Scholar] [CrossRef]
- Seré, K.; Baek, J.-H.; Ober-Blöbaum, J.; Müller-Newen, G.; Tacke, F.; Yokota, Y.; Zenke, M.; Hieronymus, T. Two Distinct Types of Langerhans Cells Populate the Skin during Steady State and Inflammation. Immunity 2012, 37, 905–916. [Google Scholar] [CrossRef]
- Wollenberg, A.; Kraft, S.; Hanau, D.; Bieber, T. Immunomorphological and ultrastructural characterization of Langerhans cells and a novel, inflammatory dendritic epidermal cell (IDEC) population in lesional skin of atopic eczema. J. Investig. Dermatol. 1996, 106, 446–453. [Google Scholar] [CrossRef]
- Yoshida, K.; Kubo, A.; Fujita, H.; Yokouchi, M.; Ishii, K.; Kawasaki, H.; Nomura, T.; Shimizu, H.; Kouyama, K.; Ebihara, T.; et al. Distinct behavior of human Langerhans cells and inflammatory dendritic epidermal cells at tight junctions in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 856–864. [Google Scholar] [CrossRef]
- Lee, J.-H.; Ostalecki, C.; Oberstein, T.; Schierer, S.; Zinser, E.; Eberhardt, M.; Blume, K.; Plosnita, B.; Stich, L.; Bruns, H.; et al. Alzheimer’s disease protease-containing plasma extracellular vesicles transfer to the hippocampus via the choroid plexus. eBioMedicine 2022, 77, 103903. [Google Scholar] [CrossRef]
- Schierer, S.; Ostalecki, C.; Zinser, E.; Lamprecht, R.; Plosnita, B.; Stich, L.; Dörrie, J.; Lutz, M.B.; Schuler, G.; Baur, A.S. Extracellular vesicles from mature dendritic cells (DC) differentiate monocytes into immature DC. Life Sci. Alliance 2018, 1, e201800093. [Google Scholar] [CrossRef]
- Bourdely, P.; Anselmi, G.; Vaivode, K.; Ramos, R.N.; Missolo-Koussou, Y.; Hidalgo, S.; Tosselo, J.; Nuñez, N.; Richer, W.; Vincent-Salomon, A.; et al. Transcriptional and Functional Analysis of CD1c+ Human Dendritic Cells Identifies a CD163+ Subset Priming CD8+CD103+ T Cells. Immunity 2020, 53, 335–352.e8. [Google Scholar] [CrossRef]
- Mantegazza, A.R.; Barrio, M.M.; Moutel, S.; Bover, L.; Weck, M.; Brossart, P.; Teillaud, J.-L.; Mordoh, J. CD63 tet-raspanin slows down cell migration and translocates to the endosomal-lysosomal-MIICs route after extracellular stimuli in human immature dendritic cells. Blood 2004, 104, 1183–1190. [Google Scholar] [CrossRef]
- Ostalecki, C.; Lee, J.-H.; Dindorf, J.; Collenburg, L.; Schierer, S.; Simon, B.; Schliep, S.; Kremmer, E.; Schuler, G.; Baur, A.S. Multiepitope tissue analysis reveals SPPL3-mediated ADAM10 activation as a key step in the transformation of melanocytes. Sci. Signal. 2017, 10, eaai8288. [Google Scholar] [CrossRef] [PubMed]
- Ronicke, M.; Baur, A.; Kirr, M.; Erdmann, M.; Erfurt-Berge, C.; Ostalecki, C. Epidermotropism of inflammatory cells differentiates pyoderma gangrenosum from venous leg ulcers. J. Dtsch. Dermatol. Ges. 2022, 20, 619–627. [Google Scholar] [CrossRef]
- Otsuka, M.; Egawa, G.; Kabashima, K. Uncovering the Mysteries of Langerhans Cells, Inflammatory Dendritic Ep-idermal Cells, and Monocyte-Derived Langerhans Cell-Like Cells in the Epidermis. Front. Immunol. 2018, 9, 1768. [Google Scholar] [CrossRef] [PubMed]
- Nakamizo, S.; Dutertre, C.-A.; Khalilnezhad, A.; Zhang, X.M.; Lim, S.; Lum, J.; Koh, G.; Foong, C.; Yong, P.J.A.; Tan, K.J.; et al. Single-cell analysis of human skin identifies CD14+ type 3 dendritic cells co-producing IL1B and IL23A in psoriasis. J. Exp. Med. 2021, 218, e20202345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Audiger, C.; Chopin, M.; Nutt, S.L. Transcriptional regulation of dendritic cell development and function. Front. Immunol. 2023, 14, 1182553. [Google Scholar] [CrossRef]
- Behr, N.J.; Pierre, S.; Ickelsheimer, T.; Ziegler, N.; Luckhardt, S.; Kannt, A.; Pinter, A.; Geisslinger, G.; Schäfer, S.M.G.; König, A.; et al. High content imaging shows distinct macrophage and dendritic cell phenotypes for psoriasis and atopic dermatitis. Sci. Rep. 2025, 15, 18904. [Google Scholar] [CrossRef]
- Singh, T.P.; Zhang, H.H.; Borek, I.; Wolf, P.; Hedrick, M.N.; Singh, S.P.; Kelsall, B.L.; Clausen, B.E.; Farber, J.M. Monocyte-derived inflammatory Langerhans cells and dermal dendritic cells mediate psoriasis-like inflammation. Nat. Commun. 2016, 7, 13581. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and chemokine receptors: Positioning cells for host defense and immunity. Annu. Rev. Immunol. 2014, 32, 659–702. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Conrad, C.; Tun-Kyi, A.; Homey, B.; Gombert, M.; Boyman, O.; Burg, G.; Liu, Y.-J.; Gilliet, M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 2005, 202, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Schubert, W.; Bonnekoh, B.; Pommer, A.J.; Philipsen, L.; Böckelmann, R.; Malykh, Y.; Gollnick, H.; Friedenberger, M.; Bode, M.; Dress, A.W.M. Analyzing proteome topology and function by automated multidimensional fluorescence microscopy. Nat. Biotechnol. 2006, 24, 1270–1278. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Windorfer, S.; Kirr, M.; Fröhlich, W.; Plosnita, B.; Ostalecki, C.; Berking, C.; Sticherling, M.; Baur, A.S. CD163/CD63+ Monocyte-Derived DC Profiled in Tissue by Multi-Antigen Analysis (MAA) Discriminate Chronic Eczema and Psoriasis. Int. J. Mol. Sci. 2025, 26, 9077. https://doi.org/10.3390/ijms26189077
Windorfer S, Kirr M, Fröhlich W, Plosnita B, Ostalecki C, Berking C, Sticherling M, Baur AS. CD163/CD63+ Monocyte-Derived DC Profiled in Tissue by Multi-Antigen Analysis (MAA) Discriminate Chronic Eczema and Psoriasis. International Journal of Molecular Sciences. 2025; 26(18):9077. https://doi.org/10.3390/ijms26189077
Chicago/Turabian StyleWindorfer, Sabrina, Michael Kirr, Waltraud Fröhlich, Bianca Plosnita, Christian Ostalecki, Carola Berking, Michael Sticherling, and Andreas S. Baur. 2025. "CD163/CD63+ Monocyte-Derived DC Profiled in Tissue by Multi-Antigen Analysis (MAA) Discriminate Chronic Eczema and Psoriasis" International Journal of Molecular Sciences 26, no. 18: 9077. https://doi.org/10.3390/ijms26189077
APA StyleWindorfer, S., Kirr, M., Fröhlich, W., Plosnita, B., Ostalecki, C., Berking, C., Sticherling, M., & Baur, A. S. (2025). CD163/CD63+ Monocyte-Derived DC Profiled in Tissue by Multi-Antigen Analysis (MAA) Discriminate Chronic Eczema and Psoriasis. International Journal of Molecular Sciences, 26(18), 9077. https://doi.org/10.3390/ijms26189077





