IL-20 Subfamily Biological Effects: Mechanistic Insights and Therapeutic Perspectives in Cancer
Abstract
1. Introduction
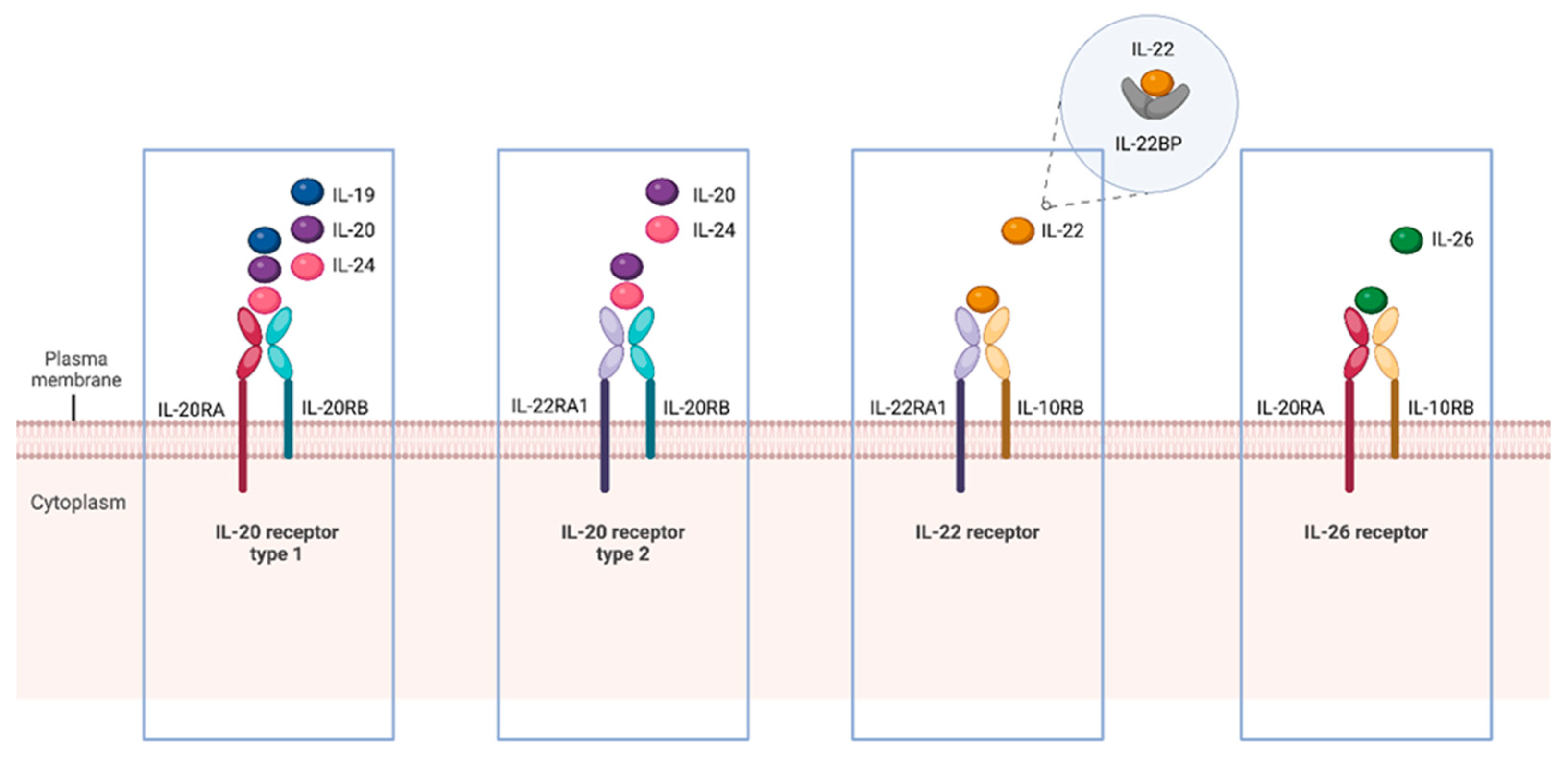
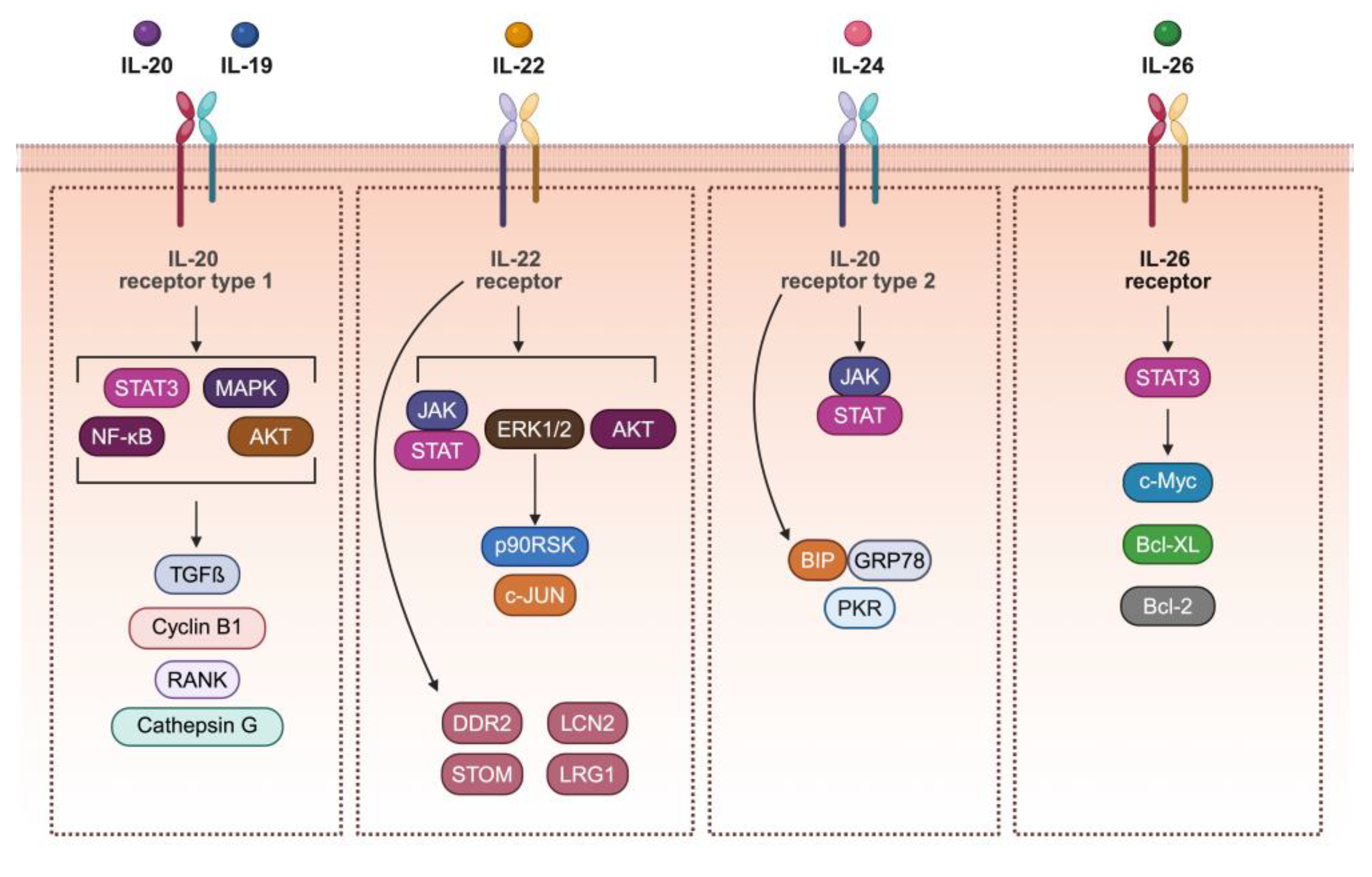
2. The IL-20 Subfamily Members, Receptors, and Signaling
3. Expression of IL-20 Subfamily Members and Their Receptors in Association with Specific Cancers
3.1. IL-19
3.2. IL-20
3.3. IL-22
3.4. IL-24
3.5. IL-26
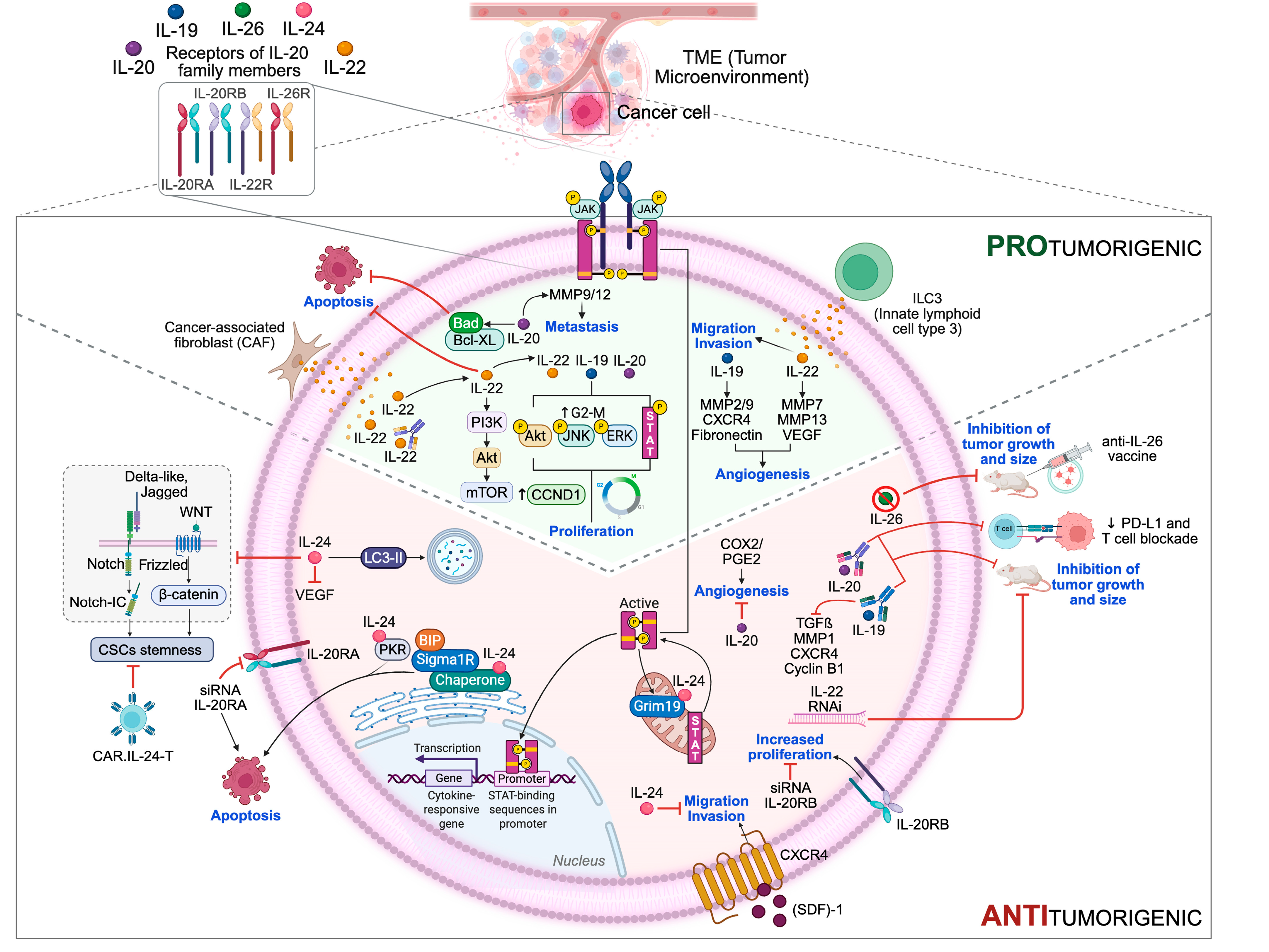
4. Impact of IL-20 Subfamily Members on Cancer Initiation and Progression: Pro- and Anti-Tumor Effects
4.1. IL-20 Family Members and Proliferation
4.1.1. IL-19
4.1.2. IL-20
4.1.3. IL-22
4.1.4. IL-24
4.1.5. IL-26
4.1.6. Receptor Complexes of IL-20 Subfamily Members
4.2. IL-20 Family Members and Resistance to Cell Death
4.2.1. IL-20
4.2.2. IL-22
4.2.3. IL-24
4.2.4. Receptor Complexes of IL-20 Subfamily Members
4.3. IL-20 Family Members and Angiogenesis
4.3.1. IL-19, IL-20, IL-22
4.3.2. IL-24
4.4. IL-20 Family Members and Invasiveness and Metastases
4.4.1. IL-19
4.4.2. IL-20
4.4.3. IL-22
4.4.4. IL-24
4.4.5. Receptor Complexes of Il-20 Subfamily Members
5. IL-20 Family/IL-20RA-Targeted Therapy: Mechanisms and Therapeutic Implications
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Mascaux, C.; Angelova, M.; Vasaturo, A.; Beane, J.; Hijazi, K.; Anthoine, G.; Buttard, B.; Rothe, F.; Willard-Gallo, K.; Haller, A.; et al. Immune evasion before tumour invasion in early lung squamous carcinogenesis. Nature 2019, 571, 570–575. [Google Scholar] [CrossRef]
- Hibino, S.; Kawazoe, T.; Kasahara, H.; Itoh, S.; Ishimoto, T.; Sakata-Yanagimoto, M.; Taniguchi, K. Inflammation-Induced Tumorigenesis and Metastasis. Int. J. Mol. Sci. 2021, 22, 5421. [Google Scholar] [CrossRef] [PubMed]
- Naser, R.; Fakhoury, I.; El-Fouani, A.; Abi-Habib, R.; El-Sibai, M. Role of the tumor microenvironment in cancer hallmarks and targeted therapy (Review). Int. J. Oncol. 2023, 62, 23. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Huynh, J.; Chand, A.; Gough, D.; Ernst, M. Therapeutically exploiting STAT3 activity in cancer—Using tissue repair as a road map. Nat. Rev. Cancer 2019, 19, 82–96. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Q.; Tan, L.; Wu, X.; Huang, R.; Zuo, Y.; Chen, L.; Yang, J.; Zhang, Z.X.; Ruan, W.; et al. Neutralizing IL-8 potentiates immune checkpoint blockade efficacy for glioma. Cancer Cell 2023, 41, 693–710.e8. [Google Scholar] [CrossRef] [PubMed]
- Rutz, S.; Wang, X.; Ouyang, W. The IL-20 subfamily of cytokines--from host defence to tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wen, H.; Liang, L.; Dong, X.; Du, R.; Zhou, W.; Zhang, X.; Zhang, C.; Xiang, R.; Li, N. IL20RA signaling enhances stemness and promotes the formation of an immunosuppressive microenvironment in breast cancer. Theranostics 2021, 11, 2564–2580. [Google Scholar] [CrossRef]
- Logsdon, N.J.; Jones, B.C.; Allman, J.C.; Izotova, L.; Schwartz, B.; Pestka, S.; Walter, M.R. The IL-10R2 binding hot spot on IL-22 is located on the N-terminal helix and is dependent on N-linked glycosylation. J. Mol. Biol. 2004, 342, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Zhou, Q. Th17 cells and their related cytokines: Vital players in progression of malignant pleural effusion. Cell. Mol. Life Sci. 2022, 79, 194. [Google Scholar] [CrossRef]
- Wu, T.; Cui, L.; Liang, Z.; Liu, C.; Liu, Y.; Li, J. Elevated serum IL-22 levels correlate with chemoresistant condition of colorectal cancer. Clin. Immunol. 2013, 147, 38–39. [Google Scholar] [CrossRef]
- Protopsaltis, N.J.; Liang, W.; Nudleman, E.; Ferrara, N. Interleukin-22 promotes tumor angiogenesis. Angiogenesis 2019, 22, 311–323. [Google Scholar] [CrossRef]
- Miao, L.; Qi, J.; Zhao, Q.; Wu, Q.N.; Wei, D.L.; Wei, X.L.; Liu, J.; Chen, J.; Zeng, Z.L.; Ju, H.Q.; et al. Targeting the STING pathway in tumor-associated macrophages regulates innate immune sensing of gastric cancer cells. Theranostics 2020, 10, 498–515. [Google Scholar] [CrossRef]
- Huber, S.; Gagliani, N.; Zenewicz, L.A.; Huber, F.J.; Bosurgi, L.; Hu, B.; Hedl, M.; Zhang, W.; O’Connor, W., Jr.; Murphy, A.J.; et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 2012, 491, 259–263. [Google Scholar] [CrossRef]
- Oberstein, P.E.; Dias Costa, A.; Kawaler, E.A.; Cardot-Ruffino, V.; Rahma, O.E.; Beri, N.; Singh, H.; Abrams, T.A.; Biller, L.H.; Cleary, J.M.; et al. Blockade of IL1β and PD1 with Combination Chemotherapy Reduces Systemic Myeloid Suppression in Metastatic Pancreatic Cancer with Heterogeneous Effects in the Tumor. Cancer Immunol. Res. 2024, 12, 1221–1235. [Google Scholar] [CrossRef]
- Briukhovetska, D.; Dörr, J.; Endres, S.; Libby, P.; Dinarello, C.A.; Kobold, S. Interleukins in cancer: From biology to therapy. Nat. Rev. Cancer 2021, 21, 481–499. [Google Scholar] [CrossRef] [PubMed]
- Jacenik, D.; Karagiannidis, I.; Beswick, E.J. Th2 cells inhibit growth of colon and pancreas cancers by promoting anti-tumorigenic responses from macrophages and eosinophils. Br. J. Cancer 2023, 128, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Dudakov, J.A.; Hanash, A.M.; van den Brink, M.R. Interleukin-22: Immunobiology and pathology. Annu. Rev. Immunol. 2015, 33, 747–785. [Google Scholar] [CrossRef] [PubMed]
- Stephen-Victor, E.; Fickenscher, H.; Bayry, J. IL-26: An Emerging Proinflammatory Member of the IL-10 Cytokine Family with Multifaceted Actions in Antiviral, Antimicrobial, and Autoimmune Responses. PLoS Pathog. 2016, 12, e1005624. [Google Scholar] [CrossRef]
- Jiang, Y.; Tsoi, L.C.; Billi, A.C.; Ward, N.L.; Harms, P.W.; Zeng, C.; Maverakis, E.; Kahlenberg, J.M.; Gudjonsson, J.E. Cytokinocytes: The diverse contribution of keratinocytes to immune responses in skin. JCI Insight 2020, 5, e142067. [Google Scholar] [CrossRef]
- Hiemstra, P.S.; McCray, P.B., Jr.; Bals, R. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur. Respir. J. 2015, 45, 1150–1162. [Google Scholar] [CrossRef]
- Giannou, A.D.; Kempski, J.; Shiri, A.M.; Lücke, J.; Zhang, T.; Zhao, L.; Zazara, D.E.; Cortesi, F.; Riecken, K.; Amezcua Vesely, M.C.; et al. Tissue resident iNKT17 cells facilitate cancer cell extravasation in liver metastasis via interleukin-22. Immunity 2023, 56, 125–142.e12. [Google Scholar] [CrossRef]
- Zenewicz, L.A. IL-22 Binding Protein (IL-22BP) in the Regulation of IL-22 Biology. Front. Immunol. 2021, 12, 766586. [Google Scholar] [CrossRef]
- Kotenko, S.V.; Krause, C.D.; Izotova, L.S.; Pollack, B.P.; Wu, W.; Pestka, S. Identification and functional characterization of a second chain of the interleukin-10 receptor complex. EMBO J. 1997, 16, 5894–5903. [Google Scholar] [CrossRef]
- Yu, D.; Yang, X.; Lin, J.; Cao, Z.; Lu, C.; Yang, Z.; Zheng, M.; Pan, R.; Cai, W. Super-Enhancer Induced IL-20RA Promotes Proliferation/Metastasis and Immune Evasion in Colorectal Cancer. Front. Oncol. 2021, 11, 724655. [Google Scholar] [CrossRef]
- He, W.; Wu, J.; Shi, J.; Huo, Y.M.; Dai, W.; Geng, J.; Lu, P.; Yang, M.W.; Fang, Y.; Wang, W.; et al. IL22RA1/STAT3 Signaling Promotes Stemness and Tumorigenicity in Pancreatic Cancer. Cancer Res. 2018, 78, 3293–3305. [Google Scholar] [CrossRef]
- Baird, A.M.; Gray, S.G.; O’Byrne, K.J. IL-20 is epigenetically regulated in NSCLC and down regulates the expression of VEGF. Eur. J. Cancer 2011, 47, 1908–1918. [Google Scholar] [CrossRef] [PubMed]
- Wegenka, U.M. IL-20: Biological functions mediated through two types of receptor complexes. Cytokine Growth Factor Rev. 2010, 21, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Parrish-Novak, J.; Xu, W.; Brender, T.; Yao, L.; Jones, C.; West, J.; Brandt, C.; Jelinek, L.; Madden, K.; McKernan, P.A.; et al. Interleukins 19, 20, and 24 signal through two distinct receptor complexes. Differences in receptor-ligand interactions mediate unique biological functions. J. Biol. Chem. 2002, 277, 47517–47523. [Google Scholar] [CrossRef]
- Ebersbach, C.; Beier, A.K.; Thomas, C.; Erb, H.H.H. Impact of STAT Proteins in Tumor Progress and Therapy Resistance in Advanced and Metastasized Prostate Cancer. Cancers 2021, 13, 4854. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef]
- Hsing, C.H.; Kwok, F.A.; Cheng, H.C.; Li, C.F.; Chang, M.S. Inhibiting interleukin-19 activity ameliorates esophageal squamous cell carcinoma progression. PLoS ONE 2013, 8, e75254. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Wu, C.Y.; Hsing, C.H.; Lai, W.T.; Wu, L.W.; Chang, M.S. Anti-IL-20 Monoclonal Antibody Suppresses Prostate Cancer Growth and Bone Osteolysis in Murine Models. PLoS ONE 2015, 10, e0139871. [Google Scholar] [CrossRef]
- Lejeune, D.; Dumoutier, L.; Constantinescu, S.; Kruijer, W.; Schuringa, J.J.; Renauld, J.C. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J. Biol. Chem. 2002, 277, 33676–33682. [Google Scholar] [CrossRef]
- Franz, J.; Jerome, J.; Lear, T.; Gong, Q.; Weathington, N.M. The Human IL-22 Receptor Is Regulated through the Action of the Novel E3 Ligase Subunit FBXW12, Which Functions as an Epithelial Growth Suppressor. J. Immunol. Res. 2015, 2015, 912713. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Wang, R.; Jeet, V.; McGuckin, M.A.; Hasnain, S.Z. Interleukin (IL)-22 from IL-20 Subfamily of Cytokines Induces Colonic Epithelial Cell Proliferation Predominantly through ERK1/2 Pathway. Int. J. Mol. Sci. 2019, 20, 3468. [Google Scholar] [CrossRef]
- Smith, S.; Lopez, S.; Kim, A.; Kasteri, J.; Olumuyide, E.; Punu, K.; de la Parra, C.; Sauane, M. Interleukin 24: Signal Transduction Pathways. Cancers 2023, 15, 3365. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Xu, R.; Ma, F.; Yang, N.; Li, Y.; Sun, X.; Jin, P.; Kang, W.; Jia, L.; Xiong, J.; et al. scRNA-seq of gastric tumor shows complex intercellular interaction with an alternative T cell exhaustion trajectory. Nat Commun. 2022, 13, 4943. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wigle, D.A.; Jurisica, I.; Radulovich, N.; Pintilie, M.; Rossant, J.; Liu, N.; Lu, C.; Woodgett, J.; Seiden, I.; Johnston, M.; et al. Molecular profiling of non-small cell lung cancer and correlation with disease-free survival. Cancer Res. 2002, 62, 3005–3008. [Google Scholar]
- Lee, G.A.; Hsu, J.B.; Chang, Y.W.; Hsieh, L.C.; Li, Y.T.; Wu, Y.C.; Chu, C.Y.; Chiang, Y.H.; Guo, W.Y.; Wu, C.C.; et al. IL-19 as a promising theranostic target to reprogram the glioblastoma immunosuppressive microenvironment. J. Biomed. Sci. 2025, 32, 34. [Google Scholar] [CrossRef]
- Small, S.H.; Tang, E.J.; Ragland, R.L.; Ruzankina, Y.; Schoppy, D.W.; Mandal, R.S.; Glineburg, M.R.; Ustelenca, Z.; Powell, D.J.; Simpkins, F.; et al. Induction of IL19 expression through JNK and cGAS-STING modulates DNA damage-induced cytokine production. Sci. Signal. 2021, 14, eaba2611. [Google Scholar] [CrossRef]
- Hsing, C.H.; Li, H.H.; Hsu, Y.H.; Ho, C.L.; Chuang, S.S.; Lan, K.M.; Chang, M.S. The distribution of interleukin-19 in healthy and neoplastic tissue. Cytokine 2008, 44, 221–228. [Google Scholar] [CrossRef]
- Hsing, C.H.; Cheng, H.C.; Hsu, Y.H.; Chan, C.H.; Yeh, C.H.; Li, C.F.; Chang, M.S. Upregulated IL-19 in breast cancer promotes tumor progression and affects clinical outcome. Clin. Cancer Res. 2012, 18, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-W.; Pan, H.-C.; Hsu, Y.-H.; Chang, K.-C.; Wu, L.-W.; Chen, W.-Y.; Chang, M.-S. IL-20 antagonist suppresses PD-L1 expression and prolongs survival in pancreatic cancer models. Nat. Commun. 2020, 11, 4611. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.H.; Hsing, C.H.; Li, C.F.; Chan, C.H.; Chang, M.C.; Yan, J.J.; Chang, M.S. Anti-IL-20 monoclonal antibody suppresses breast cancer progression and bone osteolysis in murine models. J. Immunol. 2012, 188, 1981–1991. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, E.J.; Kim, S.K.; Jeong, P.; Cho, Y.H.; Yun, S.J.; Kim, S.; Kim, G.Y.; Choi, Y.H.; Cha, E.J.; et al. Identification of pro-inflammatory cytokines associated with muscle invasive bladder cancer; the roles of IL-5, IL-20, and IL-28A. PLoS ONE 2012, 7, e40267. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.S.; Hsing, C.H.; Li, C.F.; Lee, C.Y.; Hsu, Y.H.; Chang, M.S. Anti-IL-20 monoclonal antibody inhibited tumor growth in hepatocellular carcinoma. Sci. Rep. 2017, 7, 17609. [Google Scholar] [CrossRef]
- Guillon, A.; Gueugnon, F.; Mavridis, K.; Dalloneau, E.; Jouan, Y.; Diot, P.; Heuzé-Vourc’h, N.; Courty, Y.; Si-Tahar, M. Interleukin-22 receptor is overexpressed in nonsmall cell lung cancer and portends a poor prognosis. Eur. Respir. J. 2016, 47, 1277–1280. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Y.; Wei, H.; Zheng, C.; Sun, R.; Zhang, J.; Tian, Z. Antiapoptotic activity of autocrine interleukin-22 and therapeutic effects of interleukin-22-small interfering RNA on human lung cancer xenografts. Clin. Cancer Res. 2008, 14, 6432–6439. [Google Scholar] [CrossRef]
- Weinberg, F.D.; Ramnath, N. Targeting IL22: A potential therapeutic approach for Kras mutant lung cancer? Transl. Lung Cancer Res. 2018, 7 (Suppl. S3), S243–S247. [Google Scholar] [CrossRef]
- Tosti, N.; Cremonesi, E.; Governa, V.; Basso, C.; Kancherla, V.; Coto-Llerena, M.; Amicarella, F.; Weixler, B.; Däster, S.; Sconocchia, G.; et al. Infiltration by IL22-Producing T Cells Promotes Neutrophil Recruitment and Predicts Favorable Clinical Outcome in Human Colorectal Cancer. Cancer Immunol. Res. 2020, 8, 1452–1462. [Google Scholar] [CrossRef]
- Petanidis, S.; Anestakis, D.; Argyraki, M.; Hadzopoulou-Cladaras, M.; Salifoglou, A. Differential expression of IL-17, 22 and 23 in the progression of colorectal cancer in patients with K-ras mutation: Ras signal inhibition and crosstalk with GM-CSF and IFN-γ. PLoS ONE 2013, 8, e73616. [Google Scholar] [CrossRef]
- Kryczek, I.; Lin, Y.; Nagarsheth, N.; Peng, D.; Zhao, L.; Zhao, E.; Vatan, L.; Szeliga, W.; Dou, Y.; Owens, S.; et al. IL-22(+)CD4(+) T cells promote colorectal cancer stemness via STAT3 transcription factor activation and induction of the methyltransferase DOT1L. Immunity 2014, 40, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Peng, L.S.; Zhao, Y.L.; Shi, Y.; Mao, X.H.; Guo, G.; Chen, W.; Liu, X.F.; Zhang, J.Y.; Liu, T.; et al. Increased intratumoral IL-22-producing CD4(+) T cells and Th22 cells correlate with gastric cancer progression and predict poor patient survival. Cancer Immunol. Immunother. 2012, 61, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Peng, L.; Yu, P.; Zhao, Y.; Shi, Y.; Mao, X.; Chen, W.; Cheng, P.; Wang, T.; Chen, N.; et al. Increased circulating Th22 and Th17 cells are associated with tumor progression and patient survival in human gastric cancer. J. Clin. Immunol. 2012, 32, 1332–1339. [Google Scholar] [CrossRef]
- Tsirakis, G.; Pappa, C.A.; Kolovou, A.; Kokonozaki, M.; Neonakis, I.; Alexandrakis, M.G. Clinical significance of interleukin-22 in multiple myeloma. Hematology 2015, 20, 143–147. [Google Scholar] [CrossRef]
- Lee, H.K.; Kim, S.Y.; Chung, S.H.; Choi, B.; Kim, J.E.; Yoon, D.; Jang, S.I.; Yeo, A.; Kang, H.G.; Lee, J.; et al. Tumour-associated myeloid cells expressing IL-10R2/IL-22R1 as a potential biomarker for diagnosis and recurrence of pancreatic ductal adenocarcinoma. Br. J. Cancer 2024, 130, 1979–1989. [Google Scholar] [CrossRef]
- Xu, X.; Tang, Y.; Guo, S.; Zhang, Y.; Tian, Y.; Ni, B.; Wang, H. Increased intratumoral interleukin 22 levels and frequencies of interleukin 22-producing CD4+ T cells correlate with pancreatic cancer progression. Pancreas 2014, 43, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.F.; Ren, Y.; Wu, C.J.; Zhao, X.H.; Xu, H.; Wu, D.Z.; Xu, J.; Zhang, X.L.; Ji, Y. Interleukin (IL)-24 transforms the tumor microenvironment and induces anticancer immunity in a murine model of colon cancer. Mol. Immunol. 2016, 75, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Menezes, M.E.; Bhatia, S.; Bhoopathi, P.; Das, S.K.; Emdad, L.; Dasgupta, S.; Dent, P.; Wang, X.Y.; Sarkar, D.; Fisher, P.B. MDA-7/IL-24: Multifunctional cancer killing cytokine. Adv. Exp. Med. Biol. 2014, 818, 127–153. [Google Scholar] [CrossRef]
- Ellerhorst, J.A.; Prieto, V.G.; Ekmekcioglu, S.; Broemeling, L.; Yekell, S.; Chada, S.; Grimm, E.A. Loss of MDA-7 expression with progression of melanoma. J. Clin. Oncol. 2002, 20, 1069–1074. [Google Scholar] [CrossRef]
- Patani, N.; Douglas-Jones, A.; Mansel, R.; Jiang, W.; Mokbel, K. Tumour suppressor function of MDA-7/IL-24 in human breast cancer. Cancer Cell Int. 2010, 10, 29. [Google Scholar] [CrossRef]
- Choi, Y.; Roh, M.S.; Hong, Y.S.; Lee, H.S.; Hur, W.J. Interleukin-24 is correlated with differentiation and lymph node numbers in rectal cancer. World J. Gastroenterol. 2011, 17, 1167–1173. [Google Scholar] [CrossRef]
- Fisher, P.B.; Gopalkrishnan, R.V.; Chada, S.; Ramesh, R.; Grimm, E.A.; Rosenfeld, M.R.; Curiel, D.T.; Dent, P. mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene: From the laboratory into the clinic. Cancer Biol. Ther. 2003, 2 (Suppl. 1), S23–S37. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liang, P. Interleukin-24 and its receptors. Immunology 2005, 114, 166–170. [Google Scholar] [CrossRef]
- Propper, D.J.; Balkwill, F.R. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 2022, 19, 237–253. [Google Scholar] [CrossRef]
- Xi, Z.F.; Jeong, S.; Wang, C.C.; Li, H.J.; Guo, H.; Cai, J.; Li, J.X.; Kong, X.N.; Tong, Y.; Xia, Q. Expression of IL-26 predicts prognosis of patients with hepatocellular carcinoma after surgical resection. Hepatobiliary Pancreat. Dis. Int. 2019, 18, 242–248. [Google Scholar] [CrossRef]
- Droeser, R.A.; Iezzi, G. IL-22-mediates Cross-talk between Tumor Cells and Immune Cells Associated with Favorable Prognosis in Human Colorectal Cancer. J. Cell. Immunol. 2021, 3, 118–121. [Google Scholar] [CrossRef]
- Xue, T.; Yang, J.; Song, P.; Zhou, G. Investigation on correlations of serum IL-26 with diagnosis and staging of gastric cancer. J. Buon. 2019, 24, 215–220. [Google Scholar]
- Trotter, T.N.; Shuptrine, C.W.; Tsao, L.C.; Marek, R.D.; Acharya, C.; Wei, J.P.; Yang, X.Y.; Lei, G.; Wang, T.; Lyerly, H.K.; et al. IL26, a Noncanonical Mediator of DNA Inflammatory Stimulation, Promotes TNBC Engraftment and Progression in Association with Neutrophils. Cancer Res. 2020, 80, 3088–3100. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Ye, L.; Peng, W.; Wang, Z.; Wei, X.; Wang, X.; Li, Y.; Zhang, S.; Xiang, X.; Zhou, Q. IL-26 promotes the pathogenesis of malignant pleural effusion by enhancing CD4(+) IL-22(+) T-cell differentiation and inhibiting CD8(+) T-cell cytotoxicity. J. Leukoc. Biol. 2021, 110, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Sofi, S.; Jan, N.; Qayoom, H.; Alkhanani, M.; Almilaibary, A.; Ahmad Mir, M. Elucidation of interleukin-19 as a therapeutic target for breast cancer by computational analysis and experimental validation. Saudi J. Biol. Sci. 2023, 30, 103774. [Google Scholar] [CrossRef]
- Shaji, V.; Dagamajalu, S.; Sanjeev, D.; George, M.; Kanekar, S.; Prasad, G.; Keshava Prasad, T.S.; Raju, R.; Devasahayam Arokia Balaya, R. Deciphering the Receptor-Mediated Signaling Pathways of Interleukin-19 and Interleukin-20. J. Interferon Cytokine Res. 2024, 44, 388–398. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Wei, C.C.; Shieh, D.B.; Chan, C.H.; Chang, M.S. Anti-IL-20 monoclonal antibody alleviates inflammation in oral cancer and suppresses tumor growth. Mol. Cancer Res. 2012, 10, 1430–1439. [Google Scholar] [CrossRef]
- Lee, S.J.; Cho, S.C.; Lee, E.J.; Kim, S.; Lee, S.B.; Lim, J.H.; Choi, Y.H.; Kim, W.J.; Moon, S.K. Interleukin-20 promotes migration of bladder cancer cells through extracellular signal-regulated kinase (ERK)-mediated MMP-9 protein expression leading to nuclear factor (NF-κB) activation by inducing the up-regulation of p21(WAF1) protein expression. J. Biol. Chem. 2013, 288, 5539–5552. [Google Scholar] [CrossRef]
- Su, C.H.; Lin, I.H.; Tzeng, T.Y.; Hsieh, W.T.; Hsu, M.T. Regulation of IL-20 Expression by Estradiol through KMT2B-Mediated Epigenetic Modification. PLoS ONE 2016, 11, e0166090. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Cao, J.; Jin, S.; Lv, L.; Qi, L.; Liu, F.; Geng, J.; Yu, Y. Interleukin-22 promotes lung cancer cell proliferation and migration via the IL-22R1/STAT3 and IL-22R1/AKT signaling pathways. Mol. Cell. Biochem. 2016, 415, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hunzeker, Z.E.; Zhao, L.; Kim, A.M.; Parker, J.M.; Zhu, Z.; Xiao, H.; Bai, Q.; Wakefield, M.R.; Fang, Y. The role of IL-22 in cancer. Med. Oncol. 2024, 41, 240. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Tan, Z.; Deng, L.; Chen, Y.; Xia, Y.; Gao, Y.; Wang, X.; Sun, B. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology 2011, 54, 900–909. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Wu, Q.; Cui, Y.; Zhu, H.; Fang, M.; Zhou, X.; Sun, Z.; Yu, J. Interleukin-22 secreted by cancer-associated fibroblasts regulates the proliferation and metastasis of lung cancer cells via the PI3K-Akt-mTOR signaling pathway. Am. J. Transl. Res. 2019, 11, 4077–4088. [Google Scholar]
- Xuan, X.; Zhou, J.; Tian, Z.; Lin, Y.; Song, J.; Ruan, Z.; Ni, B.; Zhao, H.; Yang, W. ILC3 cells promote the proliferation and invasion of pancreatic cancer cells through IL-22/AKT signaling. Clin. Transl. Oncol. 2020, 22, 563–575. [Google Scholar] [CrossRef]
- Emdad, L.; Bhoopathi, P.; Talukdar, S.; Pradhan, A.K.; Sarkar, D.; Wang, X.Y.; Das, S.K.; Fisher, P.B. Recent insights into apoptosis and toxic autophagy: The roles of MDA-7/IL-24, a multidimensional anti-cancer therapeutic. Semin. Cancer Biol. 2020, 66, 140–154. [Google Scholar] [CrossRef]
- Whitaker, E.L.; Filippov, V.A.; Duerksen-Hughes, P.J. Interleukin 24: Mechanisms and therapeutic potential of an anti-cancer gene. Cytokine Growth Factor Rev. 2012, 23, 323–331. [Google Scholar] [CrossRef]
- Zhuo, B.; Wang, X.; Shen, Y.; Li, J.; Li, S.; Li, Y.; Wang, R. Interleukin-24 inhibits the phenotype and tumorigenicity of cancer stem cell in osteosarcoma via downregulation Notch and Wnt/β-catenin signaling. J. Bone Oncol. 2021, 31, 100403. [Google Scholar] [CrossRef]
- Babazadeh, S.M.; Zolfaghari, M.R.; Zargar, M.; Baesi, K.; Hosseini, S.Y.; Ghaemi, A. Interleukin-24-mediated antitumor effects against human glioblastoma via upregulation of P38 MAPK and endogenous TRAIL-induced apoptosis and LC3-II activation-dependent autophagy. BMC Cancer 2023, 23, 519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Hu, W.; Li, F.; Wen, C.; Zhou, L.; Zhang, L.; Lian, J.; Liu, S.; Wang, S.; Zhang, Y. IL-24 improves efficacy of CAR-T cell therapy by targeting stemness of tumor cells. Br. J. Cancer 2024, 130, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.F.; Cui, X.G.; Leng, N. Overexpression of interleukin-20 receptor subunit beta (IL20RB) correlates with cell proliferation, invasion and migration enhancement and poor prognosis in papillary renal cell carcinoma. J. Toxicol. Pathol. 2019, 32, 245–251. [Google Scholar] [CrossRef]
- Li, X.H.; Huang, G.Z.; Xu, Z.L.; Zhao, C.Y.; Dong, X.Y.; Cui, B.K.; Lin, X.J. IL20RB signaling enhances stemness and chemotherapy resistance in pancreatic cancer. J. Transl. Med. 2023, 21, 911. [Google Scholar] [CrossRef] [PubMed]
- Curd, L.M.; Favors, S.E.; Gregg, R.K. Pro-tumour activity of interleukin-22 in HPAFII human pancreatic cancer cells. Clin. Exp. Immunol. 2012, 168, 192–199. [Google Scholar] [CrossRef]
- Sauane, M.; Gopalkrishnan, R.V.; Lebedeva, I.; Mei, M.X.; Sarkar, D.; Su, Z.Z.; Kang, D.C.; Dent, P.; Pestka, S.; Fisher, P.B. Mda-7/IL-24 induces apoptosis of diverse cancer cell lines through JAK/STAT-independent pathways. J. Cell Physiol. 2003, 196, 334–345. [Google Scholar] [CrossRef]
- Lebedeva, I.V.; Su, Z.Z.; Sarkar, D.; Kitada, S.; Dent, P.; Waxman, S.; Reed, J.C.; Fisher, P.B. Melanoma differentiation associated gene-7, mda-7/interleukin-24, induces apoptosis in prostate cancer cells by promoting mitochondrial dysfunction and inducing reactive oxygen species. Cancer Res. 2003, 63, 8138–8144. [Google Scholar]
- Sauane, M.; Lebedeva, I.V.; Su, Z.Z.; Choo, H.T.; Randolph, A.; Valerie, K.; Dent, P.; Gopalkrishnan, R.V.; Fisher, P.B. Melanoma differentiation associated gene-7/interleukin-24 promotes tumor cell-specific apoptosis through both secretory and nonsecretory pathways. Cancer Res. 2004, 64, 2988–2993. [Google Scholar] [CrossRef]
- Sauane, M.; Gupta, P.; Lebedeva, I.V.; Su, Z.Z.; Sarkar, D.; Randolph, A.; Valerie, K.; Gopalkrishnan, R.V.; Fisher, P.B. N-glycosylation of MDA-7/IL-24 is dispensable for tumor cell-specific apoptosis and “bystander” antitumor activity. Cancer Res. 2006, 66, 11869–11877. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Liu, M.; Liu, Y.; Zeng, Y.; Zhang, W.; Wu, S.; Hu, L.; Ruan, X.; Zheng, X.; et al. Programmed cell death-related gene IL20RA facilitates tumor progression and remodels tumor microenvironment in thyroid cancer. Sci. Rep. 2025, 15, 3520. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Heuzé-Vourc’h, N.; Liu, M.; Dalwadi, H.; Baratelli, F.E.; Zhu, L.; Goodglick, L.; Põld, M.; Sharma, S.; Ramirez, R.D.; Shay, J.W.; et al. IL-20, an anti-angiogenic cytokine that inhibits COX-2 expression. Biochem. Biophys. Res. Commun. 2005, 333, 470–475. [Google Scholar] [CrossRef]
- Ramesh, R.; Mhashilkar, A.M.; Tanaka, F.; Saito, Y.; Branch, C.D.; Sieger, K.; Mumm, J.B.; Stewart, A.L.; Boquoi, A.; Dumoutier, L.; et al. Melanoma differentiation-associated gene 7/interleukin (IL)-24 is a novel ligand that regulates angiogenesis via the IL-22 receptor. Cancer Res. 2003, 63, 5105–5113. [Google Scholar]
- Inoue, S.; Branch, C.D.; Gallick, G.E.; Chada, S.; Ramesh, R. Inhibition of Src kinase activity by Ad-mda7 suppresses vascular endothelial growth factor expression in prostate carcinoma cells. Mol. Ther. 2005, 12, 707–715. [Google Scholar] [CrossRef]
- Nishikawa, T.; Ramesh, R.; Munshi, A.; Chada, S.; Meyn, R.E. Adenovirus-mediated mda-7 (IL24) gene therapy suppresses angiogenesis and sensitizes NSCLC xenograft tumors to radiation. Mol. Ther. 2004, 9, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Hartman, A.; Branch, C.D.; Bucana, C.D.; Bekele, B.N.; Stephens, L.C.; Chada, S.; Ramesh, R. mda-7 In combination with bevacizumab treatment produces a synergistic and complete inhibitory effect on lung tumor xenograft. Mol. Ther. 2007, 15, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H.; Zhang, X.; Sun, C.; Hara, K.; Kikuchi, S.; Yamasaki, T.; Kondo, T.; Tomita, T.; Oshima, T.; Watari, J.; et al. IL-22 produced by cancer-associated fibroblasts promotes gastric cancer cell invasion via STAT3 and ERK signaling. Br. J. Cancer 2014, 111, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Katara, G.K.; Kulshrestha, A.; Schneiderman, S.; Riehl, V.; Ibrahim, S.; Beaman, K.D. Interleukin-22 promotes development of malignant lesions in a mouse model of spontaneous breast cancer. Mol. Oncol. 2020, 14, 211–224. [Google Scholar] [CrossRef]
- Ramesh, R.; Ito, I.; Gopalan, B.; Saito, Y.; Mhashilkar, A.M.; Chada, S. Ectopic production of MDA-7/IL-24 inhibits invasion and migration of human lung cancer cells. Mol. Ther. 2004, 9, 510–518. [Google Scholar] [CrossRef]
- Panneerselvam, J.; Jin, J.; Shanker, M.; Lauderdale, J.; Bates, J.; Wang, Q.; Zhao, Y.D.; Archibald, S.J.; Hubin, T.J.; Ramesh, R. IL-24 inhibits lung cancer cell migration and invasion by disrupting the SDF-1/CXCR4 signaling axis. PLoS ONE 2015, 10, e0122439. [Google Scholar] [CrossRef]
- Maggisano, V.; Gargano, A.; Maiuolo, J.; Ortuso, F.; De Amicis, F.; Alcaro, S.; Bulotta, S. Rational Identification of Ritonavir as IL-20 Receptor A Ligand Endowed with Antiproliferative Properties in Breast Cancer Cells. Int. J. Mol. Sci. 2025, 26, 1285. [Google Scholar] [CrossRef] [PubMed]
- Lyssiotis, C.A.; Vander-Heiden, M.G.; Muñoz-Pinedo, C.; Emerling, B.M. Emerging concepts: Linking hypoxic signaling and cancer metabolism. Cell Death Dis. 2012, 3, e303. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggisano, V.; D’Amico, M.; Aquila, S.; Giordano, F.; Battaglia, A.M.; Chimento, A.; Biamonte, F.; Russo, D.; Pezzi, V.; Bulotta, S.; et al. IL-20 Subfamily Biological Effects: Mechanistic Insights and Therapeutic Perspectives in Cancer. Int. J. Mol. Sci. 2025, 26, 7320. https://doi.org/10.3390/ijms26157320
Maggisano V, D’Amico M, Aquila S, Giordano F, Battaglia AM, Chimento A, Biamonte F, Russo D, Pezzi V, Bulotta S, et al. IL-20 Subfamily Biological Effects: Mechanistic Insights and Therapeutic Perspectives in Cancer. International Journal of Molecular Sciences. 2025; 26(15):7320. https://doi.org/10.3390/ijms26157320
Chicago/Turabian StyleMaggisano, Valentina, Maria D’Amico, Saveria Aquila, Francesca Giordano, Anna Martina Battaglia, Adele Chimento, Flavia Biamonte, Diego Russo, Vincenzo Pezzi, Stefania Bulotta, and et al. 2025. "IL-20 Subfamily Biological Effects: Mechanistic Insights and Therapeutic Perspectives in Cancer" International Journal of Molecular Sciences 26, no. 15: 7320. https://doi.org/10.3390/ijms26157320
APA StyleMaggisano, V., D’Amico, M., Aquila, S., Giordano, F., Battaglia, A. M., Chimento, A., Biamonte, F., Russo, D., Pezzi, V., Bulotta, S., & De Amicis, F. (2025). IL-20 Subfamily Biological Effects: Mechanistic Insights and Therapeutic Perspectives in Cancer. International Journal of Molecular Sciences, 26(15), 7320. https://doi.org/10.3390/ijms26157320









