Effects of Rice Bran Supplementation on Metabolic Syndrome-Related Parameters: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection and Eligibility Criteria
2.3. Data Extraction
2.4. Risk of Bias Assessment
2.5. Quality of Evidence Assessment
2.6. Data Analysis
3. Results
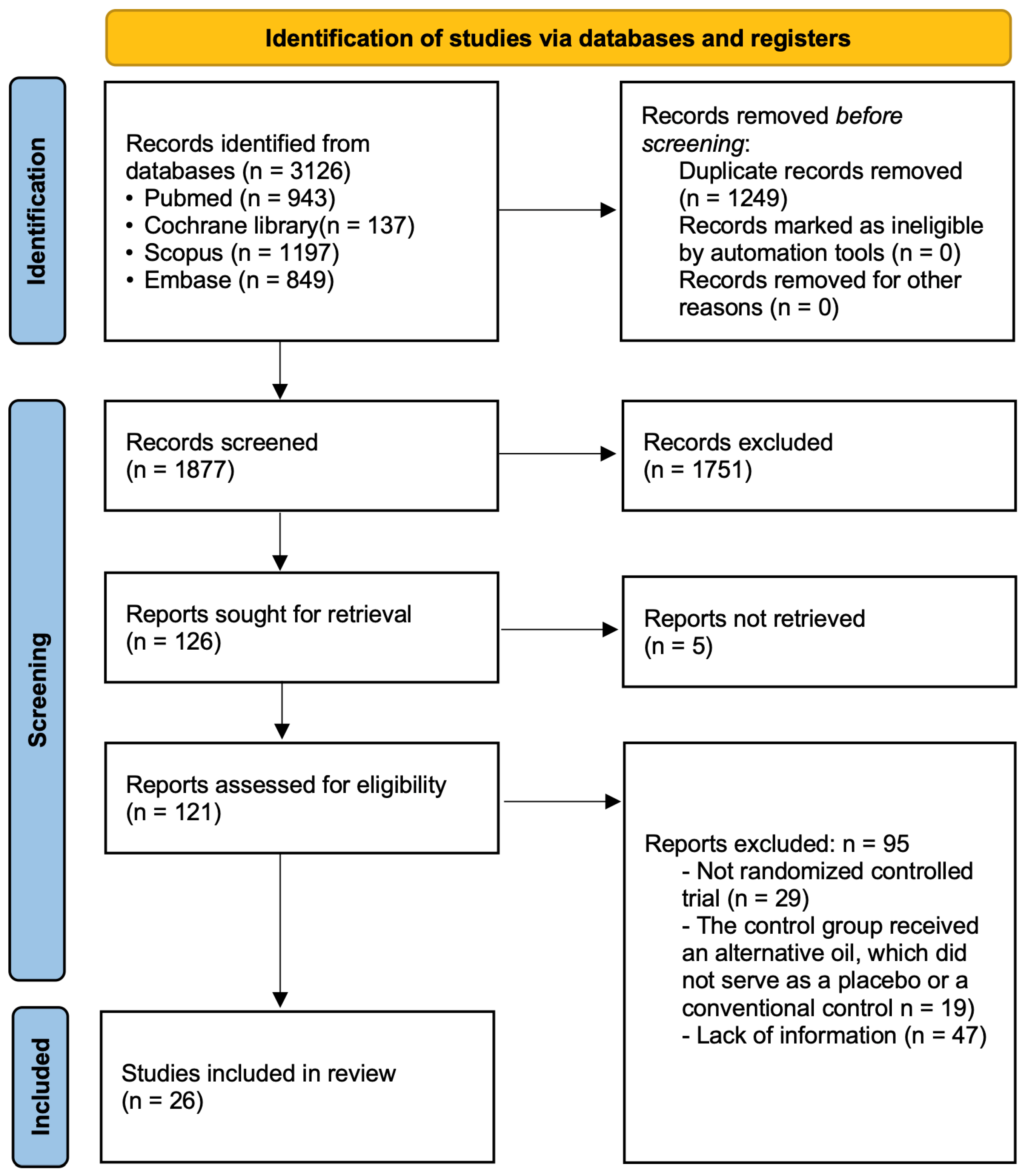
3.1. Search Results
3.2. Study Characteristics and Risk of Bias Assessment
3.3. Effects of Rice Bran on MetS-Related Parameters
3.3.1. Effects of Rice Bran on Anthropometric Parameters
Effect of Rice Bran on Body Mass Index Levels
Effect of Rice Bran on Waist Circumference
3.3.2. Effects of Rice Bran on Blood Pressure Parameters
Effect of Rice Bran on Systolic Blood Pressure
Effect of Rice Bran on Diastolic Blood Pressure
3.3.3. Effects of Rice Bran on Glycemic Parameters
Effect of Rice Bran on Fasting Blood Glucose
Effect of Rice Bran on HbA1c
Effect of Rice Bran on Insulin Levels
3.3.4. Effects of Rice Bran on Lipid Profiles
Effect of Rice Bran on Triglyceride Levels
Effects of Rice Bran on Total Cholesterol Levels
Effect of Rice Bran on LDL-C Levels
Effect of Rice Bran on HDL-C Levels
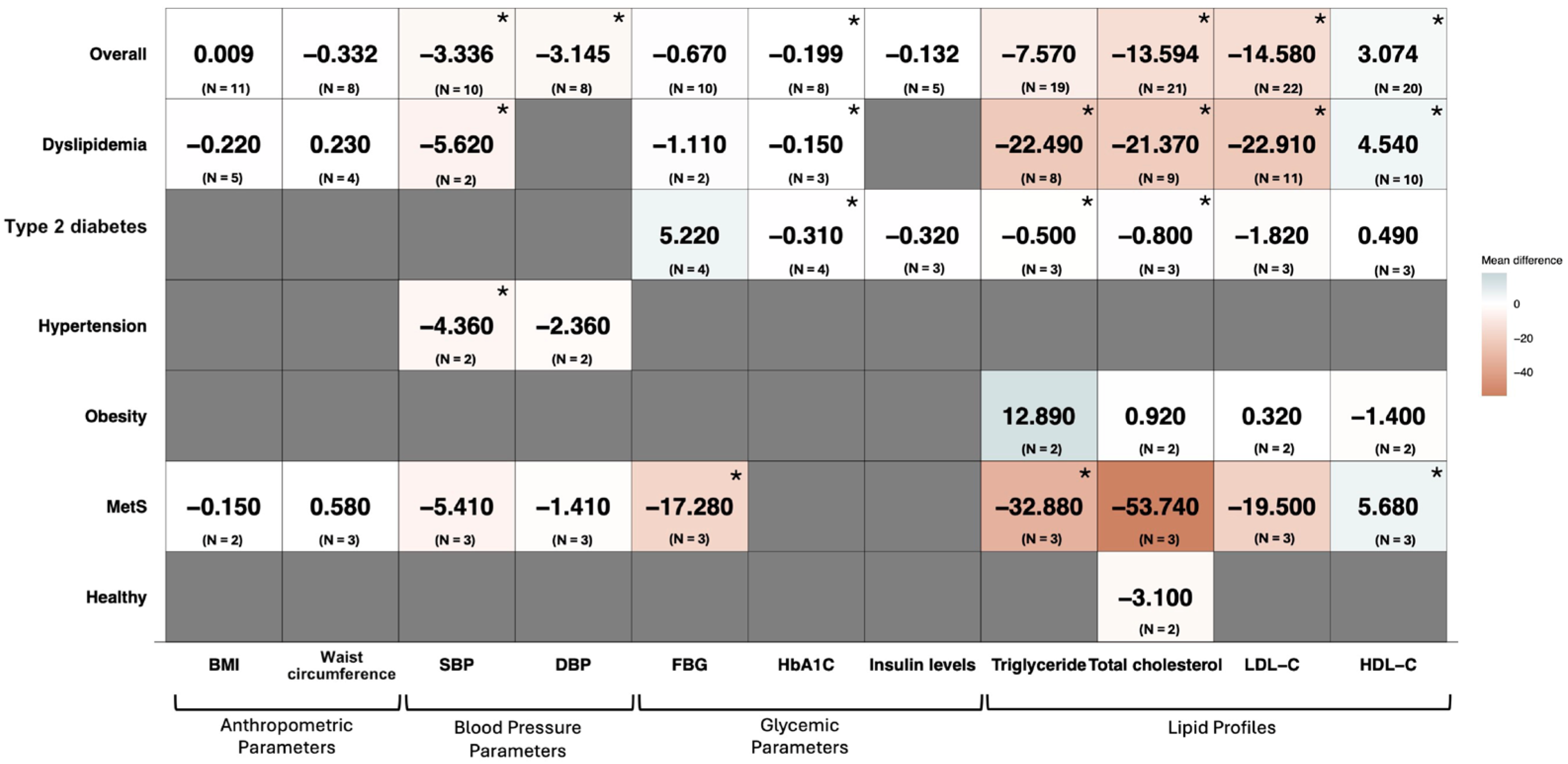
3.4. Subgroup Analysis
3.4.1. Participants’ Health Conditions
3.4.2. Rice Bran Dosage (≥20 g/d, <20 g/d, or Oryzanol)
3.4.3. Duration of the Intervention (≥90 Days or <90 Days)
3.5. Meta-Regression Analysis
3.6. Results of Quality of Evidence Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| BP | Blood pressure |
| CI | Confidence interval |
| FBG | Fasting blood glucose |
| GRADE | Grading of Recommendations Assessment, Development, and Evaluation |
| HbA1c | Hemoglobin A1C |
| HDL-C | High-density lipoprotein cholesterol |
| HMG-CoA | β-hydroxy-β-methylglutaryl-CoA |
| I2 | I-squared statistic |
| LDL-C | Low-density lipoprotein cholesterol |
| MetS | Metabolic syndrome |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCTs | Randomized controlled trials |
| ROB2 | Risk of Bias 2 Tool |
| SBP | Systolic blood pressure |
| SD | Standard deviation |
| SE | Standard error |
| VLDL | Very-low-density lipoprotein |
| WMD | Weighted mean difference |
References
- Singh, P.; Kaushik, U.; Mir, S.R.; Kukreti, N.; Visht, S. Dietary and Nutritional Aspects of Metabolic Syndrome Management: An Overview. Endocr. Metab. Immune. Disord. Drug Targets 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.-L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Geographic distribution of metabolic syndrome and its components in the general adult population: A meta-analysis of global data from 28 million individuals. Diabetes Res. Clin. Pract. 2022, 188, 109924. [Google Scholar] [CrossRef] [PubMed]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Milić, M.; Kazensky, L.; Matovinović, M. The Impact of the Metabolic Syndrome Severity on the Appearance of Primary and Permanent DNA Damage. Medicina 2025, 61, 21. [Google Scholar] [CrossRef]
- Calle, E.E.; Kaaks, R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 2004, 4, 579–591. [Google Scholar] [CrossRef]
- Dalamaga, M. Obesity, insulin resistance, adipocytokines and breast cancer: New biomarkers and attractive therapeutic targets. World J. Exp. Med. 2013, 3, 34–42. [Google Scholar] [CrossRef]
- Grundy, S.M. Drug therapy of the metabolic syndrome: Minimizing the emerging crisis in polypharmacy. Nat. Rev. Drug Discov. 2006, 5, 295–309. [Google Scholar] [CrossRef]
- Rohatgi, K.W.; Humble, S.; McQueen, A.; Hunleth, J.M.; Chang, S.-H.; Herrick, C.J.; James, A.S. Medication Adherence and Characteristics of Patients Who Spend Less on Basic Needs to Afford Medications. J. Am. Board Fam. Med. 2021, 34, 561–570. [Google Scholar] [CrossRef]
- Kvarnström, K.; Westerholm, A.; Airaksinen, M.; Liira, H. Factors Contributing to Medication Adherence in Patients with a Chronic Condition: A Scoping Review of Qualitative Research. Pharmaceutics 2021, 13, 1100. [Google Scholar] [CrossRef]
- Chen, G.-C.; Tong, X.; Xu, J.-Y.; Han, S.-F.; Wan, Z.-X.; Qin, J.-B.; Qin, L.-Q. Whole-grain intake and total, cardiovascular, and cancer mortality: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2016, 104, 164–172. [Google Scholar] [CrossRef]
- Sharif, M.K.; Butt, M.S.; Anjum, F.M.; Khan, S.H. Rice bran: A novel functional ingredient. Crit. Rev. Food Sci. Nutr. 2014, 54, 807–816. [Google Scholar] [CrossRef]
- Filippou, C.; Thomopoulos, C.; Konstantinidis, D.; Dimitriadis, K.; Tatakis, F.; Siafi, E.; Manta, E.; Drogkaris, S.; Stathoulopoulou, M.; Polyzos, D.; et al. Effect of dash vs. Mediterranean diet on metabolic syndrome prevalence in adults with high normal blood pressure or grade 1 hypertension: A randomized controlled trial. J. Hypertens. 2024, 42, e36–e37. [Google Scholar] [CrossRef]
- Radić, J.; Vučković, M.; Belančić, A.; Đogaš, H.; Radić, M. Mediterranean Diet and Metabolic Syndrome. Diabetology 2025, 6, 4. [Google Scholar] [CrossRef]
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in Metabolic Diseases. Molecules 2022, 27, 6280. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, E.; Giacco, A. Diet, Exercise, and the Metabolic Syndrome: Enrollment of the Mitochondrial Machinery. Nutrients 2022, 14, 4519. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Garcia, C.; Gavino, G.; Baragaño-Mosqueda, M.; Hevia, P.; Gavino, V.C. Correlation of tocopherol, tocotrienol, γ-oryzanol and total polyphenol content in rice bran with different antioxidant capacity assays. Food Chem. 2007, 102, 1228–1232. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kim, Y.; Park, M.J.; Kim, J.Y. Rice Bran Consumption Improves Lipid Profiles: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2025, 17, 114. [Google Scholar] [CrossRef]
- Devarajan, S.; Singh, R.; Chatterjee, B.; Zhang, B.; Ali, A. A blend of sesame oil and rice bran oil lowers blood pressure and improves the lipid profile in mild-to-moderate hypertensive patients. J. Clin. Lipidol. 2016, 10, 339–349. [Google Scholar] [CrossRef]
- Lin, J.H.; Lin, Y.H.; Chao, H.C.; Chang, D.M.; Hong, D.W. A clinical empirical study on the role of refined rice bran in the prevention and improvement of metabolic syndrome. J. Food Biochem. 2020, 44, e13492. [Google Scholar] [CrossRef]
- Yang, S.C.; Huang, W.C.; Ng, X.E.; Lee, M.C.; Hsu, Y.J.; Huang, C.C.; Wu, H.H.; Yeh, C.L.; Shirakawa, H.; Budijanto, S.; et al. Rice Bran Reduces Weight Gain and Modulates Lipid Metabolism in Rats with High-Energy-Diet-Induced Obesity. Nutrients 2019, 11, 2033. [Google Scholar] [CrossRef]
- Hariri, Z.; Afzalzade, F.; Sohrab, G.; Saadati, S.; Yari, Z. The effects of rice bran supplementation for management of blood lipids: A GRADE-assessed systematic review, dose–response meta-analysis, and meta-regression of randomized controlled trials. Syst. Rev. 2023, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 6.4; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar]
- Walters, S.J.; Jacques, R.M.; Henriques-Cadby, I.B.d.A.; Candlish, J.; Totton, N.; Xian, M.T.S. Sample size estimation for randomised controlled trials with repeated assessment of patient-reported outcomes: What correlation between baseline and follow-up outcomes should we assume? Trials 2019, 20, 566, Erratum in Trials 2019, 20, 611. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A. Web Based Tool to Extract Data from Plots, Images, and Maps, Version 4.6; Ankit Rohatgi: Pacifica, CA, USA, 2022.
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Dettori, J.R.; Norvell, D.C.; Chapman, J.R. Seeing the Forest by Looking at the Trees: How to Interpret a Meta-Analysis Forest Plot. Glob. Spine J. 2021, 11, 614–616. [Google Scholar] [CrossRef]
- Accinni, R.; Rosina, M.; Bamonti, F.; Della Noce, C.; Tonini, A.; Bernacchi, F.; Campolo, J.; Caruso, R.; Novembrino, C.; Ghersi, L.; et al. Effects of combined dietary supplementation on oxidative and inflammatory status in dyslipidemic subjects. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 121–127. [Google Scholar] [CrossRef]
- Balters, S.K.; Kies, C.; Fox, H.M. Lipid utilization of human adults as affected by dietary bran supplementation. Nutr. Res. 1981, 1, 339–347. [Google Scholar] [CrossRef]
- Borresen, E.C.; Brown, D.G.; Harbison, G.; Taylor, L.; Fairbanks, A.; O’Malia, J.; Bazan, M.; Rao, S.; Bailey, S.M.; Wdowik, M.; et al. A Randomized Controlled Trial to Increase Navy Bean or Rice Bran Consumption in Colorectal Cancer Survivors. Nutr. Cancer 2016, 68, 1269–1280. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Chongsuwat, R.; Phosat, C.; Butacnum, A. Rice Bran Oil Containing Gamma-Oryzanol Improves Lipid Profiles and Antioxidant Status in Hyperlipidemic Subjects: A Randomized Double-Blind Controlled Trial. J. Altern. Complement Med. 2019, 25, 353–358. [Google Scholar] [CrossRef]
- Cheng, H.H.; Huang, H.Y.; Chen, Y.Y.; Huang, C.L.; Chang, C.J.; Chen, H.L.; Lai, M.H. Ameliorative effects of stabilized rice bran on type 2 diabetes patients. Ann. Nutr. Metab. 2010, 56, 45–51. [Google Scholar] [CrossRef]
- Choi, J.Y.; Paik, D.J.; Kwon, D.Y.; Park, Y. Dietary supplementation with rice bran fermented with Lentinus edodes increases interferon-γ activity without causing adverse effects: A randomized, double-blind, placebo-controlled, parallel-group study. Nutr. J. 2014, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- De Lellis, L.F.; Morone, M.V.; Buccato, D.G.; Cordara, M.; Larsen, D.S.; Ullah, H.; Piccinocchi, R.; Piccinocchi, G.; Balaji, P.; Baldi, A.; et al. Efficacy of Food Supplement Based on Monacolins, γ-Oryzanol, and γ-Aminobutyric Acid in Mild Dyslipidemia: A Randomized, Double-Blind, Parallel-Armed, Placebo-Controlled Clinical Trial. Nutrients 2024, 16, 2983. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, A.L.; Gallo, N.B. Full-Fat rice bran and oat bran similarly reduce hypercholesterolemia in humans. J. Nutr. 1998, 128, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, Z.; Shoaibinobarian, N.; Zamani, E.; Salari, A.; Mahdavi-Roshan, M.; Porteghali, P.; Ahmadnia, Z. Supplementing the standard diet with brown rice bran powder might effectively improve the metabolic syndrome characteristics and antioxidant status: An open label randomized controlled trial. Food Funct. 2025, 16, 750–762. [Google Scholar] [CrossRef]
- Ito, Y.; Nakashima, Y.; Matsuoka, S. Rice bran extract containing acylated steryl glucoside fraction decreases elevated blood LDL cholesterol level in obese Japanese men. J. Med. Investig. 2015, 62, 80–84. [Google Scholar] [CrossRef][Green Version]
- Kim, J.H.; Kim, O.H.; Ha, Y.L.; Kim, J.O. Supplementation of conjugated linoleic acid with γ-Oryzanol for 12 weeks effectively reduces body fat in healthy overweight Korean women. J. Food Sci. Nutr. 2008, 13, 146–156. [Google Scholar] [CrossRef]
- Lai, M.H.; Chen, Y.T.; Chen, Y.Y.; Chang, J.H.; Cheng, H.H. Effects of rice bran oil on the blood lipids profiles and insulin resistance in type 2 diabetes patients. J. Clin. Biochem. Nutr. 2012, 51, 15–18. [Google Scholar] [CrossRef]
- Mahdavi-Roshan, M.; Shoaibinobarian, N.; Evazalipour, M.; Salari, A.; Ghorbani, Z.; Savarrakhsh, A.; Ahmadnia, Z. An open label randomized controlled trial of the effects of rice bran oil on cardiometabolic risk factors, lipid peroxidation and antioxidant status in overweight/obese adults with metabolic syndrome. Lipids Heal. Dis. 2024, 23, 273. [Google Scholar] [CrossRef]
- Malve, H.; Kerkar, P.; Mishra, N.; Loke, S.; Rege, N.N.; Marwaha-Jaspal, A.; Jainani, K.J. LDL-cholesterol lowering activity of a blend of rice bran oil and safflower oil (8:2) in patients with hyperlipidaemia: A proof of concept, double blind, controlled, randomised parallel group study. J. Indian Med. Assoc. 2010, 108, 785–788. [Google Scholar]
- Most, M.M.; Tulley, R.; Morales, S.; Lefevre, M. Rice bran oil, not fiber, lowers cholesterol in humans. Am. J. Clin. Nutr. 2005, 81, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Nhung, B.T.; Tuyen, L.D.; Linh, V.A.; Van Anh, N.D.; Nga, T.T.; Thuc, V.T.M.; Yui, K.; Ito, Y.; Nakashima, Y.; Yamamoto, S. Rice bran extract reduces the risk of atherosclerosis in post-menopausal vietnamese women. J. Nutr. Sci. Vitaminol. 2016, 62, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Nikooyeh, B.; Zargaraan, A.; Ebrahimof, S.; Kalayi, A.; Zahedirad, M.; Yazdani, H.; Rismanchi, M.; Karami, T.; Khazraei, M.; Jafarpour, A.; et al. Daily consumption of γ-oryzanol-fortified canola oil, compared with unfortified canola and sunflower oils, resulted in a better improvement of certain cardiometabolic biomarkers of adult subjects with type 2 diabetes: A randomized controlled clinical trial. Eur. J. Med. Res. 2023, 28, 416. [Google Scholar] [CrossRef]
- Ogawa, Y.; Shobako, N.; Fukuhara, I.; Satoh, H.; Kobayashi, E.; Kusakari, T.; Suwa, M.; Matsumoto, M.; Ishikado, A. Rice Bran Supplement Containing a Functional Substance, the Novel Peptide Leu-Arg-Ala, Has Anti-Hypertensive Effects: A Double-Blind, Randomized, Placebo-Controlled Study. Nutrients 2019, 11, 726. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Shobako, N.; Kobayashi, E.; Suwa, M.; Matsumoto, M.; Ishikado, A. Anti-hypertensive effect of thermolysin-digested rice bran on high normal blood pressure and mild hypertension. J. Hypertens. 2018, 36, e136–e137. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Sami, S.A.; Salser, W.A.; Khan, A.F. Synergistic effect of tocotrienol-rich fraction (TRF25) of rice bran and lovastatin on lipid parameters in hypercholesterolemic humans. J. Nutr. Biochem. 2001, 12, 318–329. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Sami, S.A.; Salser, W.A.; Khan, F.A. Dose-dependent suppression of serum cholesterol by tocotrienol-rich fraction (TRF25) of rice bran in hypercholesterolemic humans. Atherosclerosis 2002, 161, 199–207. [Google Scholar] [CrossRef]
- Saphyakhajorn, W.; Sirirat, R.; Sapwarobol, S. Effect of defatted rice bran supplementation on metabolic parameters and inflammatory status in overweight/obese adults with hypercholesterolemia: A randomized, placebo-controlled intervention. BMC Nutr. 2022, 8, 94. [Google Scholar] [CrossRef]
- Yonei, Y.; Yagi, M.; Hamada, U.; Imamura, I.; Okamura, S.; Yamamoto, K.; Matsumoto, M. A Placebo-Controlled, Randomized, Single-Blind, Parallel-Group Comparative Study to Evaluate the Anti-Glycation Effect of a Functional Soymilk Beverage Supplemented with Rice Bran/Rice Bran Oil. Glycative Stress Res. 2015, 2, 80–100. [Google Scholar]
- Upadya, H.; Devaraju, C.J.; Joshi, S.R. Anti-inflammatory properties of blended edible oil with synergistic antioxidants. Indian J. Endocrinol. Metab. 2015, 19, 511–519. [Google Scholar] [CrossRef]
- Zavoshy, R.; Noroozi, M.; Jahanihashemi, H. Effect of low calorie diet with rice bran oil on cardiovascular risk factors in hyperlipidemic patients. J. Res. Med. Sci. 2012, 17, 626–631. [Google Scholar]
- Uraipong, C.; Zhao, J. In vitro digestion of rice bran proteins produces peptides with potent inhibitory effects on α-glucosidase and angiotensin I converting enzyme. J. Sci. Food Agric. 2018, 98, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Jan-On, G.; Sangartit, W.; Pakdeechote, P.; Kukongviriyapan, V.; Sattayasai, J.; Senaphan, K.; Kukongviriyapan, U. Virgin rice bran oil alleviates hypertension through the upregulation of eNOS and reduction of oxidative stress and inflammation in L-NAME–induced hypertensive rats. Nutrition 2020, 69, 110575. [Google Scholar] [CrossRef] [PubMed]
- Jan-on, G.; Sangartit, W.; Pakdeechote, P.; Kukongviriyapan, V.; Senaphan, K.; Boonla, O.; Thongraung, C.; Kukongviriyapan, U. Antihypertensive Effect and Safety Evaluation of Rice Bran Hydrolysates from Sang-Yod Rice. Plant Foods Hum. Nutr. 2020, 75, 89–95. [Google Scholar] [CrossRef]
- Shin, J.; Yang, S.J.; Lim, Y. Gamma-tocopherol supplementation ameliorated hyper-inflammatory response during the early cutaneous wound healing in alloxan-induced diabetic mice. Exp. Biol. Med. 2017, 242, 505–515. [Google Scholar] [CrossRef]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark. Insights 2016, 11, 95–104. [Google Scholar] [CrossRef]
- McPherson, R.A.; Pincus, M.R. Henry’s Clinical Diagnosis and Management by Laboratory Methods E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021. [Google Scholar]
- King, A.B. Misled by the Morning “Fasting” Plasma Glucose. J. Diabetes Sci. Technol. 2015, 9, 1342–1345. [Google Scholar] [CrossRef]
- Cara, L.; Dubois, C.; Borel, P.; Armand, M.; Senft, M.; Portugal, H.; Pauli, A.M.; Bernard, P.M.; Lairon, D. Effects of oat bran, rice bran, wheat fiber, and wheat germ on postprandial lipemia in healthy adults. Am. J. Clin. Nutr. 1992, 55, 81–88. [Google Scholar] [CrossRef]
- Mahdavi-Roshan, M.; Salari, A.; Ghorbani, Z.; Nikpey, Z.; Haghighatkhah, M.; Mousavi, A.F.; Gholipour, M.; Pourfarzad, A. The effects of rice bran oil on left ventricular systolic function, cardiometabolic risk factors and inflammatory mediators in men with coronary artery disease: A randomized clinical trial. Food Funct. 2021, 12, 4446–4457. [Google Scholar] [CrossRef]
- Sasaki, J.; Takada, Y.; Handa, K.; Kusuda, M.; Tanabe, Y.; Matsunaga, A.; Arakawa, K. Effects of gamma-oryzanol on serum-lipids and apolipoproteins in dyslipidemic schizophrenics receiving major tranquilizers. Clin. Ther. 1990, 12, 263–268. [Google Scholar]
- Seetharamaiah, G.S.; Chandrasekhara, N. Effect of oryzanol on cholesterol absorption & biliary & fecal bile acids in rats. Indian J. Med. Res. 1990, 92, 471–475. [Google Scholar]
- Zhao, G.; Zhang, R.; Huang, F.; Dong, L.; Liu, L.; Jia, X.; Chi, J.; Ma, Y.; Deng, M.; Chen, Y.; et al. Hydrolyzed Bound Phenolics from Rice Bran Alleviate Hyperlipidemia and Improve Gut Microbiota Dysbiosis in High-Fat-Diet Fed Mice. Nutrients 2022, 14, 1277. [Google Scholar] [CrossRef]
- Feng, L.; He, B.; Xia, J.; Wang, Z. Untargeted and Targeted Lipidomics Unveil Dynamic Lipid Metabolism Alterations in Type 2 Diabetes. Metabolites 2024, 14, 610. [Google Scholar] [CrossRef]
- Luciani, L.; Pedrelli, M.; Parini, P. Modification of lipoprotein metabolism and function driving atherogenesis in diabetes. Atherosclerosis 2024, 394, 117545. [Google Scholar] [CrossRef]
- García-Rodríguez, S.; Espinosa-Cabello, J.M.; García-González, A.; González-Jiménez, E.; Aguilar-Cordero, M.J.; Castellano, J.M.; Perona, J.S. Interplay of Postprandial Triglyceride-Rich Lipoprotein Composition and Adipokines in Obese Adolescents. Int. J. Mol. Sci. 2024, 25, 1112. [Google Scholar] [CrossRef]

| Author (Year) | Country | Participants | Intervention | Control | Duration (Days) | Risk of Bias Assessment | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D5 | Overall | ||||||
| Accinni (2006) [29] | Italy | Dyslipidemic individuals | Oryzanol (0.040 g/d) | Placebo (rice starch) | 120 |  |  |  |  |  |  |
| Balters (1981) [30] | United States of America | Healthy individuals | Basal diet with rice bran (20 g/d) | Placebo (Basal diet) | 34 |  |  |  |  |  |  |
| Borresen (2016) [31] | United States of America | Colorectal cancer patients | Rice bran powder (30 g/d) | Placebo (rice starch) | 28 |  |  |  |  |  |  |
| Bumrungpert (2019) [32] | Thailand | Hyperlipidemic individuals | Rice bran oil (30 mL) | Oil without rice bran | 28 |  |  |  |  |  |  |
| Cheng (2010) [33] | Taiwan | Type 2 diabetes patients | Rice bran oil (3.9 g/d) | Placebo (milled rice flour) | 84 |  |  |  |  |  |  |
| Choi (2014) [34] | Korea | Healthy individuals | Rice bran fermented (3 g/d) | Placebo (rice starch) | 56 |  |  |  |  |  |  |
| De Lellis (2024) [35] | Italy | Dyslipidemic individuals | ROSSOPURO® Forte with γ-Oryzanol (0.062 g/d) | Placebo | 84 |  |  |  |  |  |  |
| Gerhardt (1998) [36] | United States of America | Hypercholesterolemic individuals | Medium-grain rice bran product (84 g/d) | Placebo (rice starch) | 42 |  |  |  |  |  |  |
| Ghorbani (2025) [37] | Iran | Metabolic syndrome individuals | Standard diet with rice bran powder (15 g/d) | Standard diet without rice bran powder | 56 |  |  |  |  |  |  |
| Ito (2015) [38] | Japan | Obese individuals with hypercholesterolemia | Rice bran acylated steryl glucosides (0.05 g/d) | Placebo (rice starch) | 84 |  |  |  |  |  |  |
| Kim (2008) [39] | Korea | Healthy overweight individuals | Conjugate linoleic acid with oryzanol (0.3 g/d) | Conjugate linoleic acid | 84 |  |  |  |  |  |  |
| Lai (2012) [40] | Taiwan | Type 2 diabetes patients | Rice bran oil-modified milk (18 g/d) | Placebo (rice starch) | 35 |  |  |  |  |  |  |
| Lin (2020) [19] | Taiwan | Metabolic syndrome and healthy individuals | Refined rice bran (20 g/d) | Refined oil without rice bran | 56 |  |  |  |  |  |  |
| Mahdavi-Roshan (2024) [41] | Iran | Metabolic syndrome individuals | Standard diet with rice bran oil (30 g/d) | Standard diet without rice bran oil | 56 |  |  |  |  |  |  |
| Malve (2010) [42] | India | Hyperlipidemic individuals | Rice bran oil (16.67 g/d) | Blend oil without rice bran oil | 90 |  |  |  |  |  |  |
| Most (2005) [43] | United States of America | Hypercholesterolemic individuals | Rice bran oil diet (56 g/d) | Control oil blend diet | 70 |  |  |  |  |  |  |
| Nhung (2016) [44] | Vietnam | Hypercholesterolemic individuals | Pre-germinated brown rice bran extract (50 g/d) | Placebo (rice starch) | 180 |  |  |  |  |  |  |
| Nikooyeh (2023) [45] | Iran | Type 2 diabetes patients | Oryzanol-fortified canola oil (30 g/d) | Unfortified canola oil (without oryzanol) | 84 |  |  |  |  |  |  |
| Ogawa (2019) [46] | Japan | High–normal-blood-pressure individuals | Processed rice bran (1 g/d) | Placebo (rice starch) | 84 |  |  |  |  |  |  |
| Ogawa (2018) [47] | Japan | High–normal-blood-pressure and mild hypertension individuals | Thermolysin digested rice bran (1 g/d) | Placebo | 84 |  |  |  |  |  |  |
| Qureshi (2001) [48] | United States of America | Hypercholesterolemic individuals | Tocotrienol-rich fraction (0.2 g/d) | AHA Step−1 diet | 35 |  |  |  |  |  |  |
| Qureshi (2002) [49] | United States of America | Hypercholesterolemic individuals | Tocotrienol-rich fraction (0.2 g/d) | AHA Step−1 diet | 35 |  |  |  |  |  |  |
| Saphyakhajorn (2022) [50] | Thailand | Overweight/obese individuals with hypercholesterolemia | Defatted rice bran (30 g/d) | Placebo | 84 |  |  |  |  |  |  |
| Umin (2015) [51] | Japan | Type 2 diabetes patients | Rice bran oil (8.2 g/d) | Placebo | 84 |  |  |  |  |  |  |
| Upadya (2015) [52] | India | Hyperlipidemic individuals | Blend oil with rice bran oil (1 L/person/ month) | Blend oil without rice bran oil | 90 |  |  |  |  |  |  |
| Zavoshy (2012) [53] | Iran | Hyperlipidemic individuals | Low-calorie diet with rice bran oil (30 g/d) | Low-calorie diet without rice bran oil | 70 |  |  |  |  |  |  |
 low risk of bias;
low risk of bias;  some concerns;
some concerns;  high risk of bias.
high risk of bias.| Patient or Population: Participants with Metabolic Syndrome-Related Parameters Intervention: Rice Bran and Its Bioactive Compound Comparison: Control Group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Design | No. of Studies | Certainty Assessment | No. of Participants | Effect | |||||||
| Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Rice Bran and Its Bioactive Compound | Control | Estimation of Absolute Effects | Certainty | |||
| Relative (95% CI) | Absolute (95% CI) | ||||||||||
| Body Mass Index | |||||||||||
| RCT | 11 | Serious a | Not serious | Not serious | Serious b | None | 278 | 273 | - | MD 0.009 higher (0.413 lower to 0.432 higher) | ⨁⨁◯◯ Low |
| Waist Circumference | |||||||||||
| RCT | 8 | Serious a | Not serious | Not serious | Serious d | None | 217 | 206 | - | MD 0.332 lower (1.975 lower to 1.424 lower) | ⨁⨁◯◯ Low |
| Systolic Blood Pressure | |||||||||||
| RCT | 10 | Serious e | Not serious | Not serious | Not serious | None | 304 | 285 | - | MD 3.336 lower (5.248 lower to 1.311 higher) | ⨁⨁⨁◯ Moderate |
| Diastolic Blood Pressure | |||||||||||
| RCT | 8 | Serious f | Very serious g | Not serious | Not serious | None | 230 | 212 | - | MD 3.145 lower (5.690 lower to 0.600 lower) | ⨁◯◯◯ Very low |
| Fasting Blood Glucose | |||||||||||
| RCT | 10 | Not serious | Not serious | Not serious | Very serious h | None | 268 | 246 | - | MD 0.670 lower (4.844 lower to 3.505 higher) | ⨁⨁◯◯ Low |
| Hemoglobin A1c | |||||||||||
| RCT | 8 | Serious a | Very serious i | Not serious | Not serious | None | 195 | 188 | - | MD 0.199 lower (0.332 lower to 0.067 lower) | ⨁◯◯◯ Very low |
| Insulin Level | |||||||||||
| RCT | 5 | Not serious | Not serious | Not serious | Very serious j | None | 104 | 103 | - | MD 0.132 lower (1.098 lower to 0.834 higher) | ⨁⨁◯◯ Low |
| Triglycerides | |||||||||||
| RCT | 19 | Serious a | Very serious g | Not serious | Very serious k | None | 459 | 440 | - | MD 7.570 lower (16.714 lower to 1.573 higher) | ⨁◯◯◯ Very low |
| Total Cholesterol | |||||||||||
| RCT | 21 | Serious a | Very serious g | Not serious | Not serious | Publication bias strongly suspected c | 517 | 495 | - | MD 13.594 lower (20.289 lower to 6.900 lower) | ⨁◯◯◯ Very low |
| Low-Density Lipoprotein | |||||||||||
| RCT | 22 | Serious e | Very serious g | Not serious | Not serious | none | 536 | 512 | - | MD 14.580 lower (21.124 lower to 8.036 lower) | ⨁◯◯◯ Very low |
| High-Density Lipoprotein | |||||||||||
| RCT | 20 | Serious a | Very serious g | Not serious | Not serious | none | 487 | 467 | - | MD 3.074 lower (0.829 higher to 5.319 higher) | ⨁◯◯◯ Very low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tantayakhom, S.; Inpan, R.; Yaja, K.; Koonrungsesomboon, N.; Teekachunhatean, S.; Na Takuathung, M. Effects of Rice Bran Supplementation on Metabolic Syndrome-Related Parameters: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 9051. https://doi.org/10.3390/ijms26189051
Tantayakhom S, Inpan R, Yaja K, Koonrungsesomboon N, Teekachunhatean S, Na Takuathung M. Effects of Rice Bran Supplementation on Metabolic Syndrome-Related Parameters: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2025; 26(18):9051. https://doi.org/10.3390/ijms26189051
Chicago/Turabian StyleTantayakhom, Sirapatsorn, Ratchanon Inpan, Kantirat Yaja, Nut Koonrungsesomboon, Supanimit Teekachunhatean, and Mingkwan Na Takuathung. 2025. "Effects of Rice Bran Supplementation on Metabolic Syndrome-Related Parameters: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 26, no. 18: 9051. https://doi.org/10.3390/ijms26189051
APA StyleTantayakhom, S., Inpan, R., Yaja, K., Koonrungsesomboon, N., Teekachunhatean, S., & Na Takuathung, M. (2025). Effects of Rice Bran Supplementation on Metabolic Syndrome-Related Parameters: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 26(18), 9051. https://doi.org/10.3390/ijms26189051






