Disorders of Redox Homeostasis and Its Importance in Acrolein Toxicity
Abstract
1. Introduction
2. Results
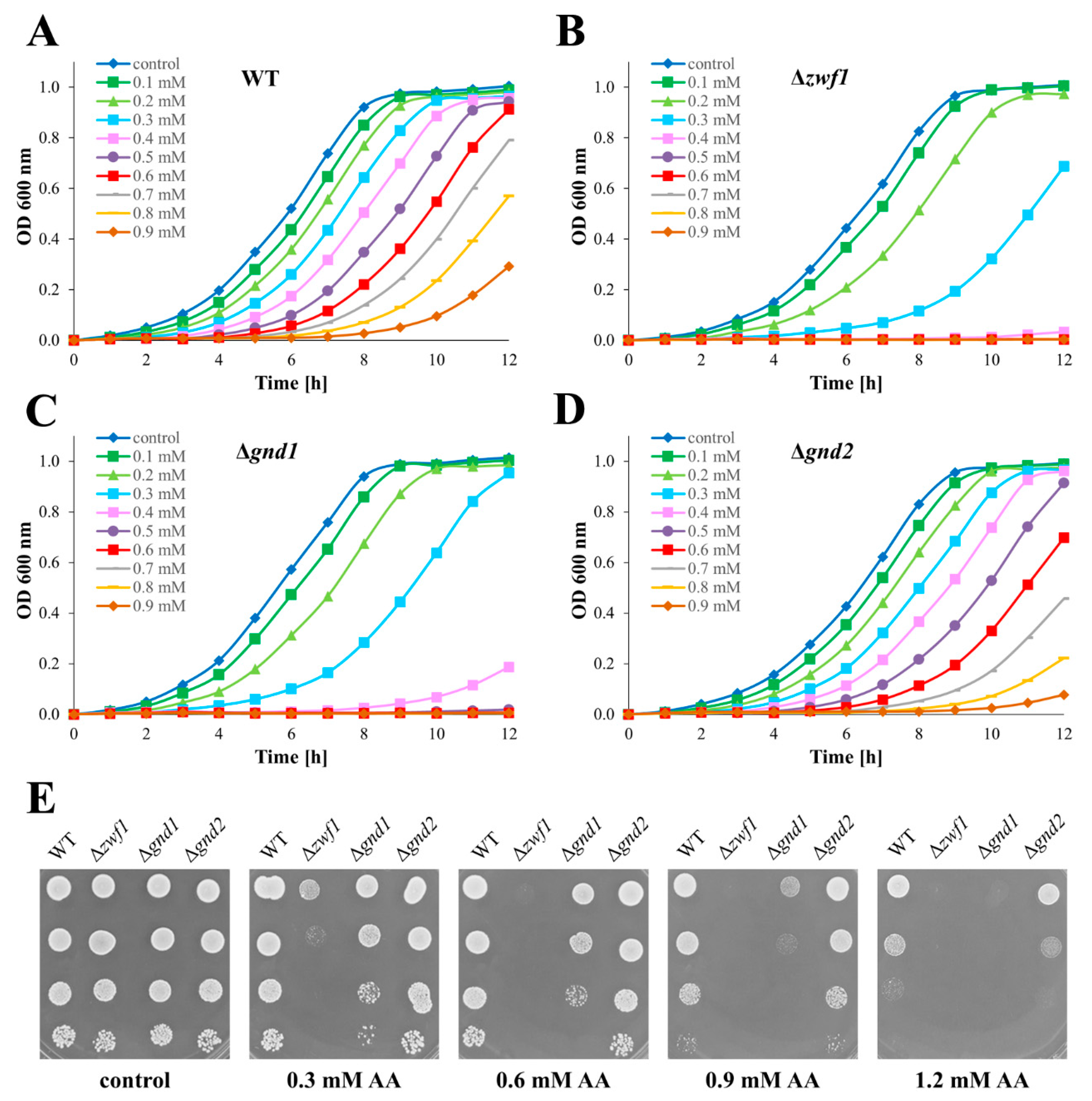
2.1. Yeast Mutants Defective in PPP-Dependent NADPH Generation Are Sensitive to Acrolein
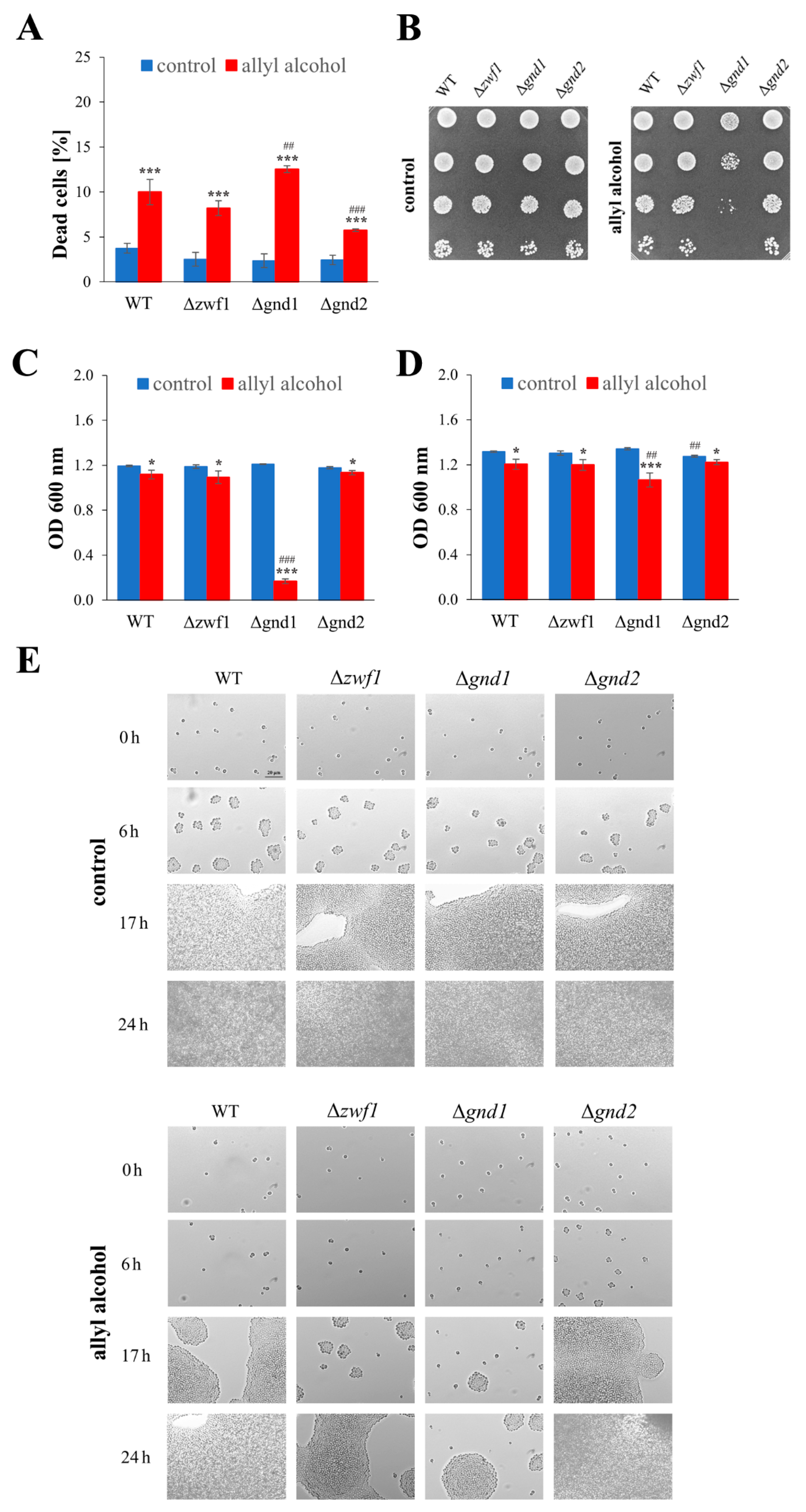
2.2. Deletion of the GND1 Gene Has More Severe Growth Repercussions than Deletion of the ZWF1 Gene in Conditions of Short-Term Allyl Alcohol Exposure
2.3. Short-Term Allyl Alcohol Exposure Disrupts Mitochondria Activity and Modifies Cellular Metabolism, Especially in the Case of Deletion of the GND1 Gene
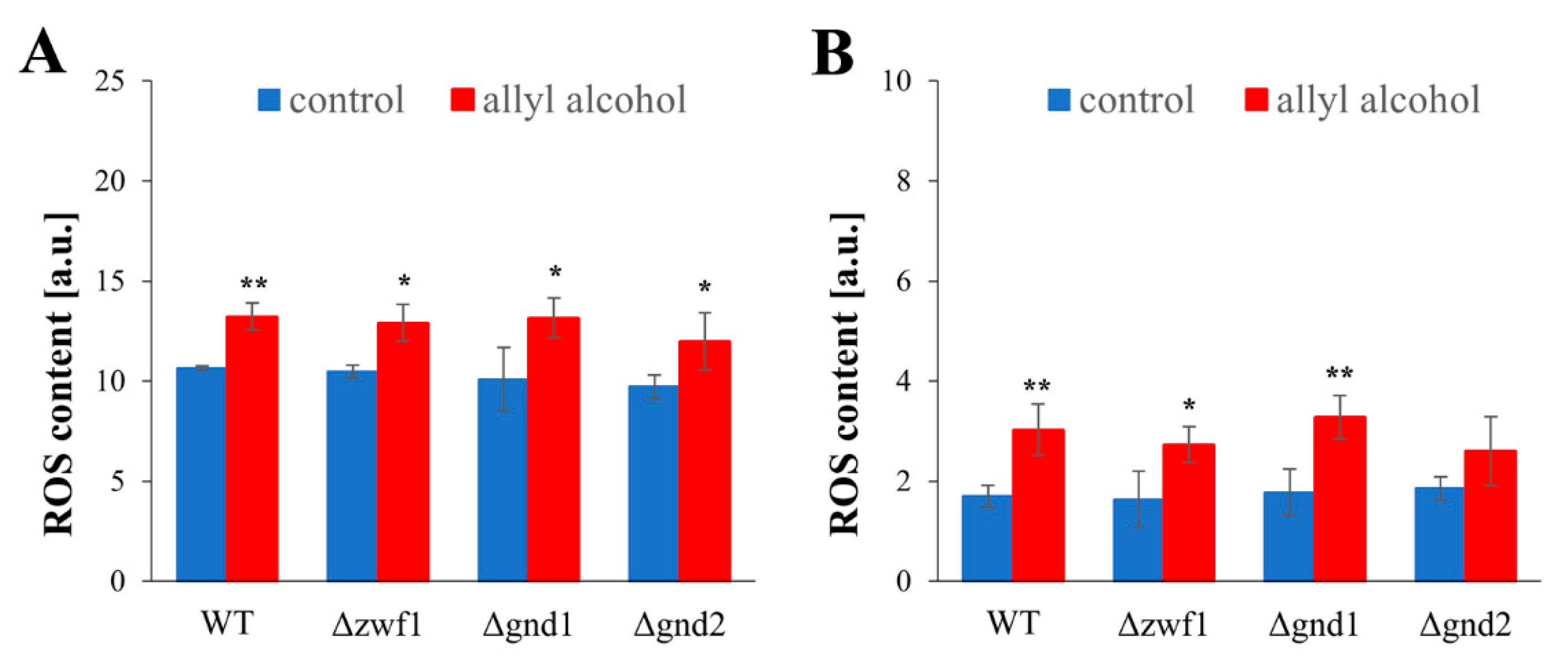
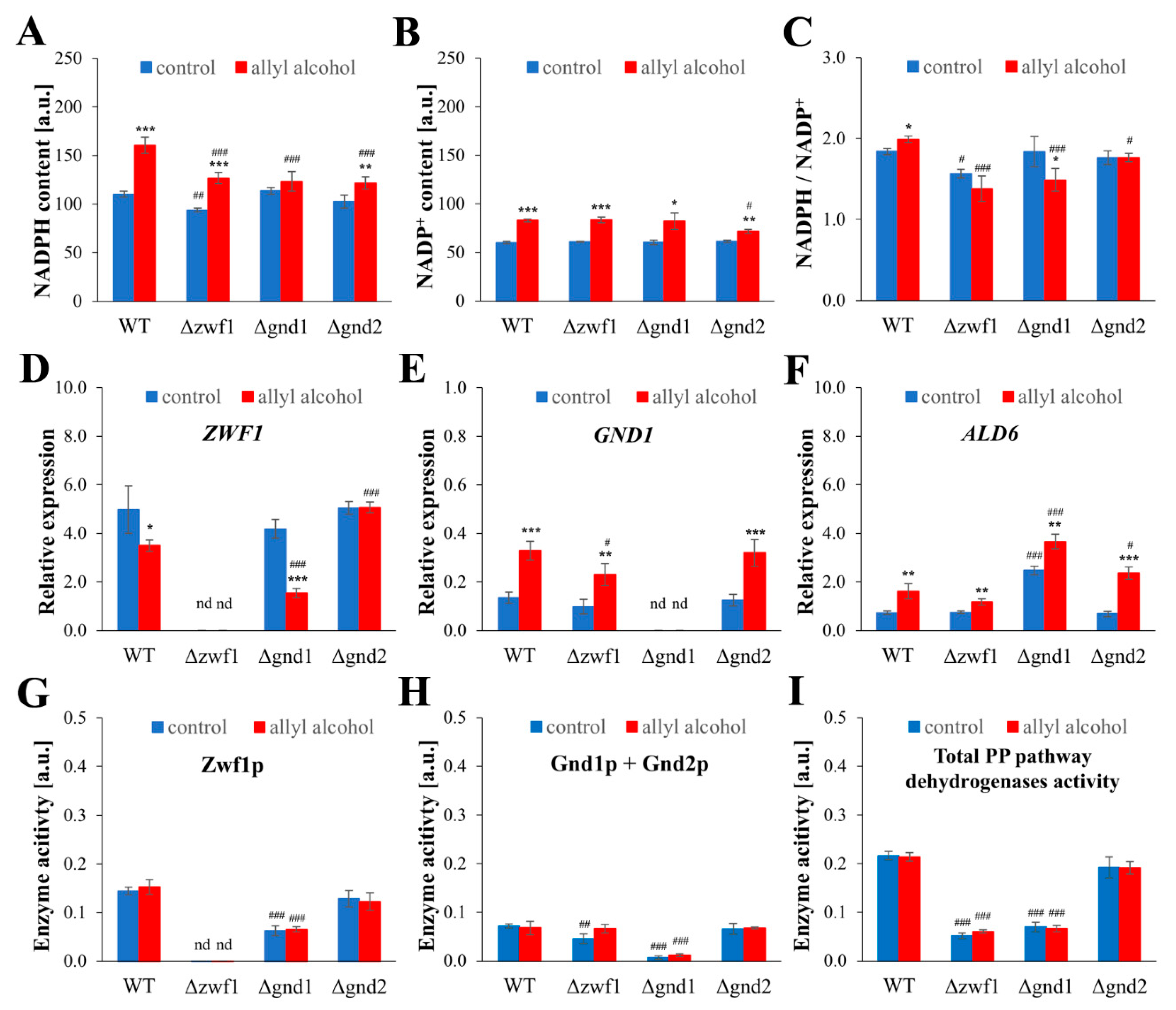
2.4. Acrolein-Induced Oxidative Stress Is Caused by Glutathione Depletion and Inadequate Redox Compensation in PPP Mutants
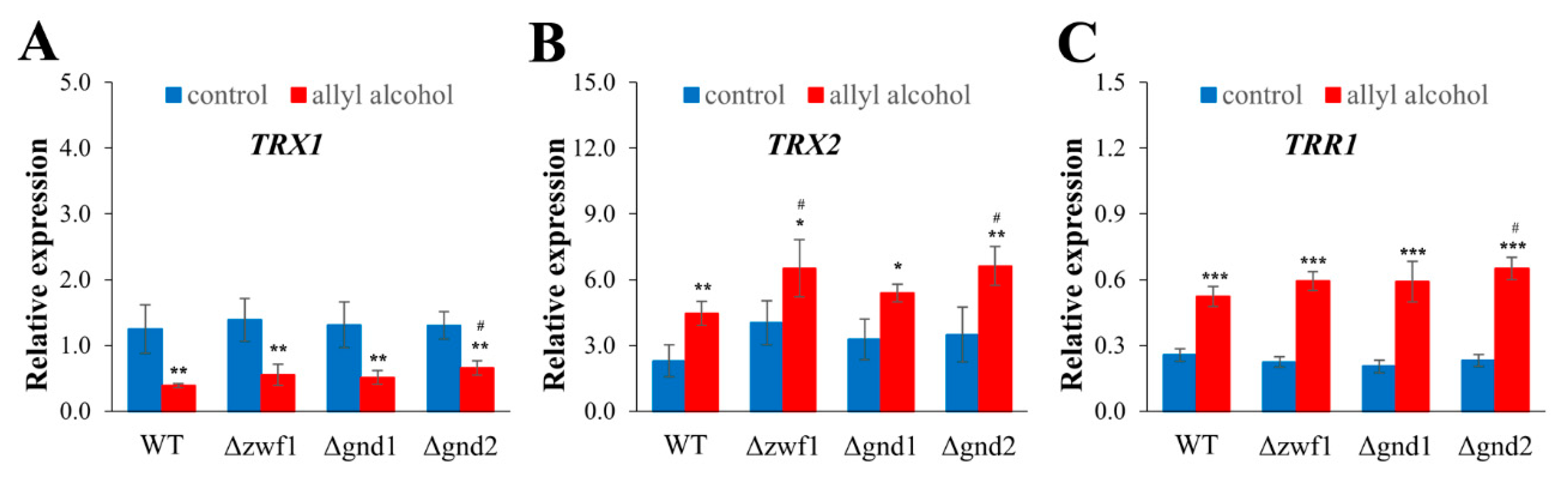
2.5. NADPH-Mediated Redox Compensation and the Thioredoxin System Are Key Determinants of Stress Response to Acrolein in Yeast Cells
3. Discussion
3.1. Disorders of Redox Homeostasis Under Exposure to Acrolein
3.2. Response Mechanisms and Possible Adaptations Under Acrolein Exposure Conditions
3.3. The Strategy of Supplying NADPH in Strains Defective in the PP Pathway Does Not Work When Cells Are Exposed to Allyl Alcohol
4. Materials and Methods
4.1. Yeast Strains, Growth and Incubation Conditions
4.2. Cell Growth Assays
4.3. Cell Viability and Vitality Assays
4.4. Assessment of Cellular ATP Content
4.5. Estimation of Mitochondrial Activity and Morphology of the Mitochondrial Network
4.6. Determination of Glutathione and Thiol Content
4.7. Determination of ROS Content
4.8. Determination of NAD(H) and NADP(H) Content
4.9. RNA Samples
4.10. Real-Time PCR
4.11. Protein Extraction
4.12. Enzyme Assays
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tretter, V.; Hochreiter, B.; Zach, M.L.; Krenn, K.; Klein, K.U. Understanding Cellular Redox Homeostasis: A Challenge for Precision Medicine. Int. J. Mol. Sci. 2022, 23, 106. [Google Scholar] [CrossRef] [PubMed]
- Zadrąg-Tęcza, R.; Maślanka, R.; Bednarska, S.; Kwolek-Mirek, M. Response Mechanisms to Oxidative Stress in Yeast and Filamentous Fungi. In Stress Response Mechanisms in Fungi: Theoretical and Practical Aspects; Skoneczny, M., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–34. [Google Scholar]
- Kwolek-Mirek, M.; Maslanka, R.; Bednarska, S.; Przywara, M.; Kwolek, K.; Zadrag-Tecza, R. Strategies to Maintain Redox Homeostasis in Yeast Cells with Impaired Fermentation-Dependent NADPH Generation. Int. J. Mol. Sci. 2024, 25, 9296. [Google Scholar] [CrossRef] [PubMed]
- Ayer, A.; Sanwald, J.; Pillay, B.A.; Meyer, A.J.; Perrone, G.G.; Dawes, I.W.; Rutherford, J. Distinct redox regulation in sub-cellular compartments in response to various stress conditions in Saccharomyces cerevisiae. PLoS ONE 2013, 8, e65240. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Maier, C.S. Acrolein: Sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol. Nutr. Food Res. 2008, 52, 7–25. [Google Scholar] [CrossRef]
- Cai, J.; Bhatnagar, A.; Pierce, W.M., Jr. Protein modification by acrolein: Formation and stability of cysteine adducts. Chem. Res. Toxicol. 2009, 22, 708–716. [Google Scholar] [CrossRef]
- Uchida, K.; Kanematsu, M.; Sakai, K.; Matsuda, T.; Hattori, N.; Mizuno, Y.; Suzuki, D.; Miyata, T.; Noguchi, N.; Niki, E.; et al. Protein-bound acrolein: Potential markers for oxidative stress. Proc. Natl. Acad. Sci. USA 1998, 95, 4882–4887. [Google Scholar] [CrossRef] [PubMed]
- Averill-Bates, D.A.; Tanel, A. Activation of cellular signalling pathways and apoptosis by the aldehyde acrolein—A major environmental hazard. Redox Biochem. Chem. 2024, 7, 100019. [Google Scholar] [CrossRef]
- Golla, U.; Bandi, G.; Tomar, R.S. Molecular Cytotoxicity Mechanisms of Allyl Alcohol (Acrolein) in Budding Yeast. Chem. Res. Toxicol. 2015, 28, 1246–1264. [Google Scholar] [CrossRef]
- Janeczko, A.; Przywara, M.; Maslanka, R.; Ras, B.; Ziaja, K.; Kwolek-Mirek, M.; Zadrag-Tecza, R.; Bednarska, S. Redox perturbations in yeast cells lacking glutathione reductase. Fungal Genet. Biol. 2023, 167, 103810. [Google Scholar] [CrossRef]
- Kwolek-Mirek, M.; Bednarska, S.; Bartosz, G.; Bilinski, T. Acrolein toxicity involves oxidative stress caused by glutathione depletion in the yeast Saccharomyces cerevisiae. Cell Biol. Toxicol. 2009, 25, 363–378. [Google Scholar] [CrossRef]
- Kwolek-Mirek, M.; Bednarska, S.; Dubicka-Lisowska, A.; Maslanka, R.; Zadrag-Tecza, R.; Kaszycki, P. Unbalance between Pyridine Nucleotide Cofactors in The SOD1 Deficient Yeast Saccharomyces cerevisiae Causes Hypersensitivity to Alcohols and Aldehydes. Int. J. Mol. Sci. 2022, 24, 659. [Google Scholar] [CrossRef]
- Kwolek-Mirek, M.; Zadrag-Tecza, R. Effects of antioxidants on acrolein-induced oxidative protein damage in the yeast Saccharomyces cerevisiae. Anim. Welf. Ethol. Hous. Syst. 2013, 9, 545–550. [Google Scholar]
- Kwolek-Mirek, M.; Zadrag-Tecza, R.; Bednarska, S.; Bartosz, G. Acrolein-Induced Oxidative Stress and Cell Death Exhibiting Features of Apoptosis in the Yeast Saccharomyces cerevisiae Deficient in SOD1. Cell Biochem. Biophys. 2015, 71, 1525–1536. [Google Scholar] [CrossRef][Green Version]
- Zhou, W.; Yang, Y.; Tang, L.; Cheng, K.; Li, C.; Wang, H.; Liu, M.; Wang, W. Acrolein-stressed threshold adaptation alters the molecular and metabolic bases of an engineered Saccharomyces cerevisiae to improve glutathione production. Sci. Rep. 2018, 8, 4506. [Google Scholar] [CrossRef] [PubMed]
- Lobo, Z.; Maitra, P.K. Pentose phosphate pathway mutants of yeast. Mol. Gen. Genet. 1982, 185, 367–368. [Google Scholar] [CrossRef]
- Nogae, I.; Johnston, M. Isolation and characterization of the ZWF1 gene of Saccharomyces cerevisiae, encoding glucose-6-phosphate dehydrogenase. Gene 1990, 96, 161–169. [Google Scholar] [CrossRef]
- Sinha, A.; Maitra, P.K. Induction of specific enzymes of the oxidative pentose phosphate pathway by glucono-delta-lactone in Saccharomyces cerevisiae. J. Gen. Microbiol. 1992, 138, 1865–1873. [Google Scholar] [CrossRef]
- Grabowska, D.; Chelstowska, A. The ALD6 gene product is indispensable for providing NADPH in yeast cells lacking glucose-6-phosphate dehydrogenase activity. J. Biol. Chem. 2003, 278, 13984–13988. [Google Scholar] [CrossRef]
- Plapp, B.V.; Lee, A.T.; Khanna, A.; Pryor, J.M. Bradykinetic alcohol dehydrogenases make yeast fitter for growth in the presence of allyl alcohol. Chem. Biol. Interact. 2013, 202, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Trivić, S.; Leskovac, V. Novel substrates of yeast alcohol dehydrogenase--4. Allyl alcohol and ethylene glycol. Biochem. Mol. Biol. Int. 1999, 47, 1–8. [Google Scholar] [CrossRef]
- Wills, C.; Hom, D. An efficient selection producing structural gene mutants of yeast alcohol dehydrogenase resistant to pyrazole. Genetics 1988, 119, 791–795. [Google Scholar] [CrossRef]
- Biliński, T.; Kwolek, M.; Sas, E.; Krynicka, M.; Koziol, S.; Owsiak-Teleon, A.; Krzepilko, A.; Bartosz, G. A novel test for identifying genes involved in aldehyde detoxification in the yeast. Increased sensitivity of superoxide-deficient yeast to aldehydes and their metabolic precursors. Biofactors 2005, 24, 59–65. [Google Scholar] [CrossRef]
- Kwolek-Mirek, M.; Maslanka, R.; Molon, M. Disorders in NADPH generation via pentose phosphate pathway influence the reproductive potential of the Saccharomyces cerevisiae yeast due to changes in redox status. J. Cell Biochem. 2019, 120, 8521–8533. [Google Scholar] [CrossRef]
- Ouyang, X.; Tran, Q.T.; Goodwin, S.; Wible, R.S.; Sutter, C.H.; Sutter, T.R. Yap1 activation by H2O2 or thiol-reactive chemicals elicits distinct adaptive gene responses. Free Radic. Biol. Med. 2011, 50, 1–13. [Google Scholar] [CrossRef]
- Azevedo, D.; Tacnet, F.; Delaunay, A.; Rodrigues-Pousada, C.; Toledano, M.B. Two redox centers within Yap1 for H2O2 and thiol-reactive chemicals signaling. Free Radic. Biol. Med. 2003, 35, 889–900. [Google Scholar] [CrossRef]
- Rodrigues-Pousada, C.; Menezes, R.A.; Pimentel, C. The Yap family and its role in stress response. Yeast 2010, 27, 245–258. [Google Scholar] [CrossRef]
- Kuge, S.; Jones, N.; Nomoto, A. Regulation of yAP-1 nuclear localization in response to oxidative stress. EMBO J. 1997, 16, 1710–1720. [Google Scholar] [CrossRef]
- Wu, A.L.; Moye-Rowley, W.S. GSH1, which encodes γ-glutamylcysteine synthetase, is a target gene for yAP-1 transcriptional regulation. Mol. Cell Biol. 1994, 14, 5832–5839. [Google Scholar] [CrossRef] [PubMed]
- Arnér, E.S.J.; Holmgren, A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000, 267, 6102–6109. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Garrido, E.O.; Grant, C.M. Role of thioredoxins in the response of Saccharomyces cerevisiae to oxidative stress induced by hydroperoxides. Mol. Microbiol. 2002, 43, 993–1003. [Google Scholar] [CrossRef]
- Vignols, F.; Bréhélin, C.; Surdin-Kerjan, Y.; Thomas, D.; Meyer, Y. A yeast two-hybrid knockout strain to explore thioredoxin-interacting proteins in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 16729–16734. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.R.; Myers, J.M.; Kufahl, T.D.; Forbes, R.; Szadkowski, A. The effects of acrolein on the thioredoxin system: Implications for redox-sensitive signaling. Mol. Nutr. Food Res. 2011, 55, 1361–1374. [Google Scholar] [CrossRef]
- Szadkowski, A.; Myers, C.R. Acrolein oxidizes the cytosolic and mitochondrial thioredoxins in human endothelial cells. Toxicology 2008, 243, 164–176. [Google Scholar] [CrossRef][Green Version]
- Trotter, E.W.; Grant, C.M. Non-reciprocal regulation of the redox state of the glutathione-glutaredoxin and thioredoxin systems. EMBO Rep. 2003, 4, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Masanta, S.; Wiesyk, A.; Panja, C.; Pilch, S.; Ciesla, J.; Sipko, M.; De, A.; Enkhbaatar, T.; Maslanka, R.; Skoneczna, A.; et al. Fmp40 ampylase regulates cell survival upon oxidative stress by controlling Prx1 and Trx3 oxidation. Redox Biol. 2024, 73, 103201. [Google Scholar] [CrossRef]
- Gansemer, E.R.; McCommis, K.S.; Martino, M.; King-McAlpin, A.Q.; Potthoff, M.J.; Finck, B.N.; Taylor, E.B.; Rutkowski, D.T. NADPH and Glutathione Redox Link TCA Cycle Activity to Endoplasmic Reticulum Homeostasis. iScience 2020, 23, 101116. [Google Scholar] [CrossRef]
- Trotter, E.W.; Collinson, E.J.; Dawes, I.W.; Grant, C.M. Old yellow enzymes protect against acrolein toxicity in the yeast Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2006, 72, 4885–4892. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.O.; Silva, P.G.P.; Lemos Junior, W.J.F.; de Oliveira, V.S.; Anschau, A. Glutathione production by Saccharomyces cerevisiae: Current state and perspectives. Appl. Microbiol. Biotechnol. 2022, 106, 1879–1894. [Google Scholar] [CrossRef]
- Plapp, B.V.; Charlier, H.A., Jr.; Ramaswamy, S. Mechanistic implications from structures of yeast alcohol dehydrogenase complexed with coenzyme and an alcohol. Arch. Biochem. Biophys. 2016, 591, 35–42. [Google Scholar] [CrossRef]
- Kwolek-Mirek, M.; Zadrag-Tecza, R. Comparison of methods used for assessing the viability and vitality of yeast cells. FEMS Yeast Res. 2014, 14, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Maslanka, R.; Zadrag-Tecza, R.; Kwolek-Mirek, M. Linkage between Carbon Metabolism, Redox Status and Cellular Physiology in the Yeast Saccharomyces cerevisiae Devoid of SOD1 or SOD2 Gene. Genes 2020, 11, 780. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B. Oxidative chemistry of fluorescent dyes: Implications in the detection of reactive oxygen and nitrogen species. Biochem. Soc. Trans. 2011, 39, 1221–1225. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Hardy, M.; Podsiadly, R.; Cheng, G.; Zielonka, J. Recent developments in detection of superoxide radical anion and hydrogen peroxide: Opportunities, challenges, and implications in redox signaling. Arch. Biochem. Biophys. 2017, 617, 38–47. [Google Scholar] [CrossRef]
- Watanabe, K.; Murata, K.; Kimura, A. Purification and Characterization of γ-Glutamylcysteine Synthetase of Escherichia coli B. Agric. Biol. Chem. 1986, 50, 1925–1930. [Google Scholar] [CrossRef]
- Tian, W.N.; Braunstein, L.D.; Pang, J.; Stuhlmeier, K.M.; Xi, Q.C.; Tian, X.; Stanton, R.C. Importance of glucose-6-phosphate dehydrogenase activity for cell growth. J. Biol. Chem. 1998, 273, 10609–10617. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwolek-Mirek, M.; Maslanka, R.; Bednarska, S.; Szczypek, J.; Baran, J.; Przywara, M.; Janeczko, A.; Zadrag-Tecza, R. Disorders of Redox Homeostasis and Its Importance in Acrolein Toxicity. Int. J. Mol. Sci. 2025, 26, 9047. https://doi.org/10.3390/ijms26189047
Kwolek-Mirek M, Maslanka R, Bednarska S, Szczypek J, Baran J, Przywara M, Janeczko A, Zadrag-Tecza R. Disorders of Redox Homeostasis and Its Importance in Acrolein Toxicity. International Journal of Molecular Sciences. 2025; 26(18):9047. https://doi.org/10.3390/ijms26189047
Chicago/Turabian StyleKwolek-Mirek, Magdalena, Roman Maslanka, Sabina Bednarska, Joanna Szczypek, Justyna Baran, Michał Przywara, Agnieszka Janeczko, and Renata Zadrag-Tecza. 2025. "Disorders of Redox Homeostasis and Its Importance in Acrolein Toxicity" International Journal of Molecular Sciences 26, no. 18: 9047. https://doi.org/10.3390/ijms26189047
APA StyleKwolek-Mirek, M., Maslanka, R., Bednarska, S., Szczypek, J., Baran, J., Przywara, M., Janeczko, A., & Zadrag-Tecza, R. (2025). Disorders of Redox Homeostasis and Its Importance in Acrolein Toxicity. International Journal of Molecular Sciences, 26(18), 9047. https://doi.org/10.3390/ijms26189047







