Effect of Sunlight-Induced Isomerisation on the Biotransformation of 4′-Hydroxychalcones by Yarrowia lipolytica KCh 71
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Analysis
3.2. Chemical Synthesis
- trans-4′-hydroxychalcone (trans-1)
- cis-4′-hydroxychalcone (cis-1)
- 4′-hydroxydihydrochalcone (1a)
- trans-4′-hydroxy-2-methoxychalcone (trans-2)
- cis-4′-hydroxy-2-methoxychalcone (cis-2)
- 4′-hydroxy-2-methoxydihydrochalcone (2a)
- trans-4′-hydroxy-3-methoxychalkone (trans-3)
- cis-4′-hydroxy-3-methoxychalcone (cis-3)
- 4′-hydroxy-3-methoxydihydrochalcone (3a)
- trans-4′-hydroxy-4-methoxychalcone (trans-4)
- cis-4′-hydroxy-4-methoxychalcone (cis-4)
- 4′-hydroxy-4-methoxydihydrochalcone (4a)
- trans-4′-hydroxy-2,4-dimethoxychalcone (trans-5)
- cis-4′-hydroxy-2,4-dimethoxychalcone (cis-5)
- 4′-hydroxy-2,4-dimethoxydihydrochalcone (5a)
- trans-4′-hydroxy-2,5-dimethoxychalcone (trans-6)
- cis-4′-hydroxy-2,5-dimethoxychalcone (cis-6)
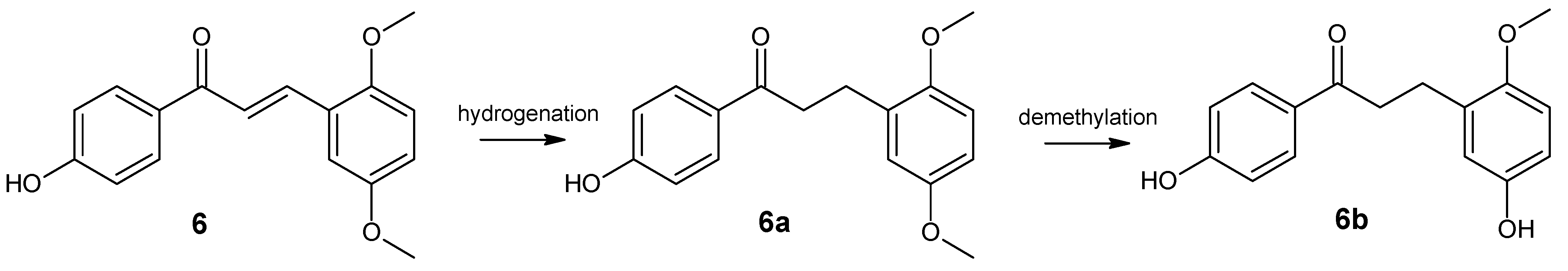
- 4′-hydroxy-2,5-dimethoxydihydrochalcone (6a)
- 4′,5-dihydroxy-2-methoxydihydrochalcone (6b)
- trans-4′-hydroxy-3,5-dimethoxychalcone (trans-7)
- cis-4′-hydroxy-3,5-dimethoxychalcone (cis-7)
- 4′-hydroxy-3,5-dimethoxydihydrochalcone (7a)
3.3. Microorganisms and Evaluation of Light Influence on the Biotransformation Process
3.4. Preparative Biotransformations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, D.; Gupta, R.K. Bioprotective properties of Dragon’s blood resin: In vitro evaluation of antioxidant activity and antimicrobial activity. BMC Complement. Altern. Med. 2011, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Al-Awthan, Y.S.; Bahattab, O.S. Phytochemistry and Pharmacological Activities of Dracaena cinnabari Resin. BioMed Res. Int. 2021, 2021, 8561696. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Nath, P.; Deb, V.K.; Das, N.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Pharmacological potential of natural chalcones: A recent studies and future perspective. Front. Pharmacol. 2025, 16, 1570385. [Google Scholar] [CrossRef]
- Wang, S.; Shi, Y.; Ma, J.; Ye, Z.; Yao, M.; Shang, J.; Liu, J. Enhanced intradermal delivery of Dragon’s blood in biocompatible nanosuspensions hydrogel patch for skin photoprotective effect. J. Cosmet. Dermatol. 2022, 22, 1046–1062. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.Y.; Yi, T.; Sze-To, C.M.; Zhu, L.; Peng, W.L.; Zhang, Y.Z.; Zhao, Z.Z.; Chen, H.B. A systematic review of the botanical, phytochemical and pharmacological profile of dracaena cochinchinensis, a plant source of the ethnomedicine dragon’s blood. Molecules 2014, 19, 10650–10669. [Google Scholar] [CrossRef] [PubMed]
- Chlipała, P.; Janeczko, T.; Mazur, M. Bioreduction of 4′-Hydroxychalcone in Deep Eutectic Solvents: Optimization and Efficacy with Various Yeast Strains. Int. J. Mol. Sci. 2024, 25, 7152. [Google Scholar] [CrossRef] [PubMed]
- Camponeschi, I.; Montanari, A.; Mazzoni, C.; Bianchi, M.M. Light Stress in Yeasts: Signaling and Responses in Creatures of the Night. Int. J. Mol. Sci. 2023, 24, 6929. [Google Scholar] [CrossRef]
- Chlipała, P.; Bienia, J.; Mazur, M.; Dymarska, M.; Janeczko, T. Efficient Production of 4′-Hydroxydihydrochalcones Using Non-Conventional Yeast Strains. Int. J. Mol. Sci. 2024, 25, 10735. [Google Scholar] [CrossRef]
- Eichenberger, M.; Lehka, B.J.; Folly, C.; Fischer, D.; Martens, S.; Simón, E.; Naesby, M. Metabolic engineering of Saccharomyces cerevisiae for de novo production of dihydrochalcones with known antioxidant, antidiabetic, and sweet tasting properties. Metab. Eng. 2017, 39, 80–89. [Google Scholar] [CrossRef]
- Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast Protein as an Easily Accessible Food Source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef]
- Stompor, M.; Broda, D.; Bajek-Bil, A. Dihydrochalcones: Methods of Acquisition and Pharmacological Properties—A First Systematic Review. Molecules 2019, 24, 4468. [Google Scholar] [CrossRef]
- Bekatorou, A.; Psarianos, C.; Koutinas, A.A. Production of food grade yeasts. Food Technol. Biotechnol. 2006, 44, 407–415. [Google Scholar]
- Tisch, D.; Schmoll, M. Light regulation of metabolic pathways in fungi. Appl. Microbiol. Biotechnol. 2010, 85, 1259–1277. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.B.; Davis, C.R.; Johnson, C.H. Visible light alters yeast metabolic rhythms by inhibiting respiration. Proc. Natl. Acad. Sci. USA 2013, 110, 21130–21135. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.H.; Huang, C.K.; Tsai, C.C. Effects of light wavelength and intensity on the production of ethanol by Saccharomyces cerevisiae in batch cultures. J. Chem. Technol. Biotechnol. 2009, 84, 1156–1162. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of Yarrowia lipolytica yeast biomass as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2019, 17, e05594. [Google Scholar] [CrossRef]
- Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of an extension of use of Yarrowia lipolytica yeast biomass as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2022, 20, 7450. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, M.; Rosazza, J.P.N. Microbial Transformations of Chalcones: Hydroxylation, O -Demethylation, and Cyclization to Flavanones. J. Nat. Prod. 2004, 67, 553–558. [Google Scholar] [CrossRef]
- Łebek, A.K.; Żarowska, B.; Dymarska, M.; Janeczko, T.; Susłow, E.K. Synthesis, fungal biotransformation, and evaluation of the antimicrobial potential of chalcones with a chlorine atom. Sci. Rep. 2024, 14, 15050. [Google Scholar] [CrossRef]
- Janeczko, T.; Gładkowski, W.; Kostrzewa-Susłow, E. Microbial transformations of chalcones to produce food sweetener derivatives. J. Mol. Catal. B Enzym. 2013, 98, 55–61. [Google Scholar] [CrossRef]
- Łużny, M.; Kaczanowska, D.; Gawdzik, B.; Wzorek, A.; Pawlak, A.; Obmińska-Mrukowicz, B.; Dymarska, M.; Kozłowska, E.; Kostrzewa-Susłow, E.; Janeczko, T. Regiospecific Hydrogenation of Bromochalcone by Unconventional Yeast Strains. Molecules 2022, 27, 3681. [Google Scholar] [CrossRef] [PubMed]
- Łużny, M.; Krzywda, M.; Kozłowska, E.; Kostrzewa-Susłow, E.; Janeczko, T. Effective Hydrogenation of 3-(2″-furyl)- and 3-(2″-thienyl)-1-(2′-hydroxyphenyl)-prop-2-en-1-one in Selected Yeast Cultures. Molecules 2019, 24, 3185. [Google Scholar] [CrossRef] [PubMed]
- Żyszka, B.; Anioł, M.; Lipok, J. Highly effective, regiospecific reduction of chalcone by cyanobacteria leads to the formation of dihydrochalcone: Two steps towards natural sweetness. Microb. Cell Factories 2017, 16, 136. [Google Scholar] [CrossRef]
- Kozłowska, J.; Potaniec, B.; Anioł, M. Biotransformation of Hydroxychalcones as a Method of Obtaining Novel and Unpredictable Products Using Whole Cells of Bacteria. Catalysts 2020, 10, 1167. [Google Scholar] [CrossRef]
- de Matos, I.L.; Nitschke, M.; Porto, A.L.M. Regioselective and chemoselective biotransformation of 2′-hydroxychalcone derivatives by marine-derived fungi. Biocatal. Biotransformation 2023, 41, 46–56. [Google Scholar] [CrossRef]
- Kozłowska, J.; Potaniec, B.; Zarowska, B.; Anioł, M. Microbial transformations of 4′-methylchalcones as an efficient method of obtaining novel alcohol and dihydrochalcone derivatives with antimicrobial activity. RSC Adv. 2018, 8, 30379–30386. [Google Scholar] [CrossRef] [PubMed]
- Łużny, M.; Kozłowska, E.; Kostrzewa-Susłow, E.; Janeczko, T. Highly effective, regiospecific hydrogenation of methoxychalcone by Yarrowia lipolytica enables production of food sweeteners. Catalysts 2020, 10, 1135. [Google Scholar] [CrossRef]
- Su, X.Q.; Song, Y.L.; Zhang, J.; Huo, H.X.; Huang, Z.; Zheng, J.; Zhang, Q.; Zhao, Y.F.; Xiao, W.; Li, J.; et al. Dihydrochalcones and homoisoflavanes from the red resin of Dracaena cochinchinensis (Chinese dragon’s blood). Fitoterapia 2014, 99, 64–71. [Google Scholar] [CrossRef]
- Helal, I.E.; Elsbaey, M.; Zaghloul, A.M.; Mansour, E.S.S. A unique C-linked chalcone-dihydrochalcone dimer from Dracaena cinnabari resin. Nat. Prod. Res. 2021, 35, 2558–2563. [Google Scholar] [CrossRef]
- Mothana, R.A.; Arbab, A.H.; Elgamal, A.A.; Parvez, M.K.; Al-Dosari, M.S. Isolation and Characterization of Two Chalcone Derivatives with Anti-Hepatitis B Virus Activity from the Endemic Socotraen Dracaena cinnabari (Dragon’s Blood Tree). Molecules 2022, 27, 952. [Google Scholar] [CrossRef]
- Chang, T.S.; Wu, J.Y.; Ding, H.Y.; Wang, T.Y.; Liu, G.C.; Wang, M.L.; Ting, H.J. Enzymatic synthesis of a new and bioactive dihydrochalcone: 3,4-dihydroxy-2′,6′-dimethoxy dihydrochalcone. Nat. Prod. Res. 2025, 1–12. [Google Scholar] [CrossRef]
- Chlipała, P.; Matera, A.; Sordon, S.; Popłoński, J.; Mazur, M.; Janeczko, T. Enzymatic Glycosylation of 4′-Hydroxychalcones: Expanding the Scope of Nature’s Catalytic Potential. Int. J. Mol. Sci. 2024, 25, 11482. [Google Scholar] [CrossRef] [PubMed]
- Dimmock, J.R.; Murthi Kandepu, N.; Hetherington, M.; Wilson Quail, J.; Pugazhenthi, U.; Sudom, A.M.; Chamankhah, M.; Rose, P.; Pass, E.; Allen, T.M.; et al. Cytotoxic activities of Mannich bases of chalcones and related compounds. J. Med. Chem. 1998, 41, 1014–1026. [Google Scholar] [CrossRef]
- Vitali, A.; Giardina, B.; Delle Monache, G.; Rocca, F.; Silvestrini, A.; Tafi, A.; Botta, B. Chalcone dimethylallyltransferase from Morus nigra cell cultures. Substrate specificity studies. FEBS Lett. 2004, 557, 33–38. [Google Scholar] [CrossRef]
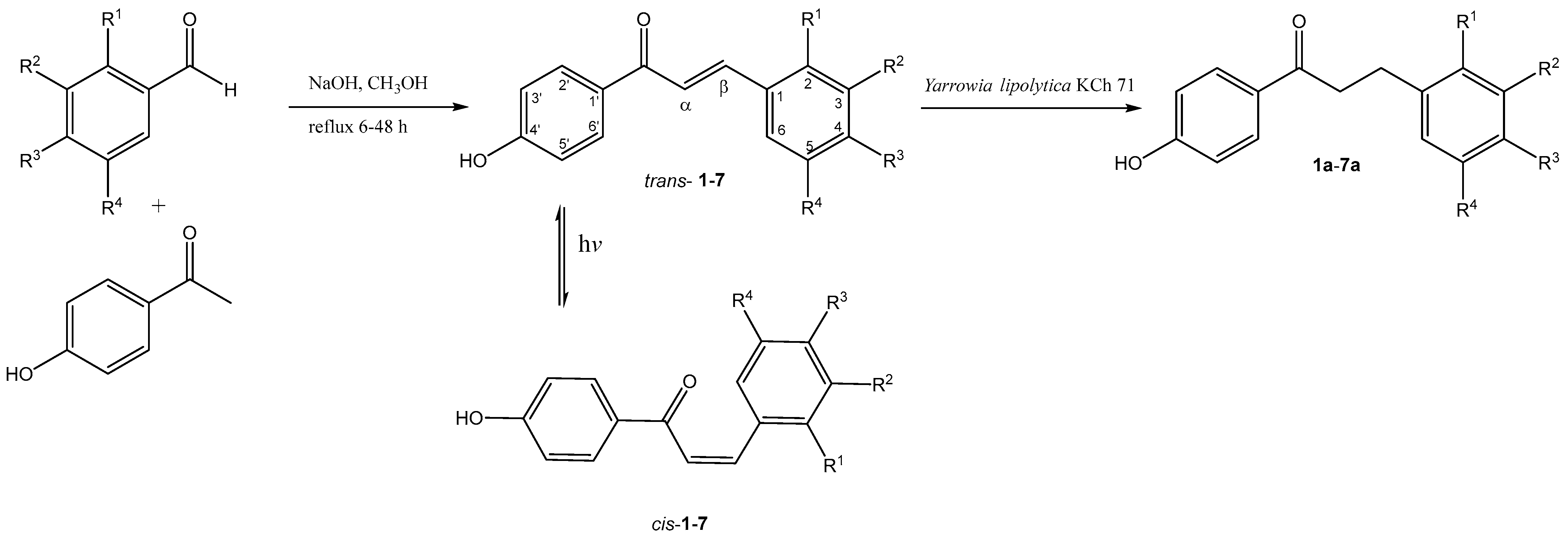

| trans-chalcone cis-chalcone | dihydrochalcones | R1 | R2 | R3 | R4 |
|---|---|---|---|---|---|
| trans-1, cis-1 | 1a | -H | -H | -H | -H |
| trans-2, cis-2 | 2a | -OCH3 | -H | -H | -H |
| trans-3, cis-3 | 3a | -H | -OCH3 | -H | -H |
| trans-4, cis-4 | 4a | -H | -H | -OCH3 | -H |
| trans-5, cis-5 | 5a | -OCH3 | -H | -OCH3 | -H |
| trans-6, cis-6 | 6a | -OCH3 | -H | -H | -OCH3 |
| trans-7, cis-7 | 7a | -H | -OCH3 | -H | -OCH3 |
| chalcone | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| cis | 65.3 | 36.9 | 69.1 | 15.0 | 11.6 | 29.3 | 47.3 |
| trans | 34.7 | 63.1 | 30.9 | 85.0 | 88.4 | 70.7 | 52.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chlipała, P.; Janeczko, T.; Mazur, M. Effect of Sunlight-Induced Isomerisation on the Biotransformation of 4′-Hydroxychalcones by Yarrowia lipolytica KCh 71. Int. J. Mol. Sci. 2025, 26, 9027. https://doi.org/10.3390/ijms26189027
Chlipała P, Janeczko T, Mazur M. Effect of Sunlight-Induced Isomerisation on the Biotransformation of 4′-Hydroxychalcones by Yarrowia lipolytica KCh 71. International Journal of Molecular Sciences. 2025; 26(18):9027. https://doi.org/10.3390/ijms26189027
Chicago/Turabian StyleChlipała, Paweł, Tomasz Janeczko, and Marcelina Mazur. 2025. "Effect of Sunlight-Induced Isomerisation on the Biotransformation of 4′-Hydroxychalcones by Yarrowia lipolytica KCh 71" International Journal of Molecular Sciences 26, no. 18: 9027. https://doi.org/10.3390/ijms26189027
APA StyleChlipała, P., Janeczko, T., & Mazur, M. (2025). Effect of Sunlight-Induced Isomerisation on the Biotransformation of 4′-Hydroxychalcones by Yarrowia lipolytica KCh 71. International Journal of Molecular Sciences, 26(18), 9027. https://doi.org/10.3390/ijms26189027







