A Sensitive SPE-LC-MS/MS Method for Determination of Selected Veterinary Drugs and Other Organic Contaminants in Human Urine: Development, Validation, and Application Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Hydroxy-Fipronil Investigation
2.2. Method Development
2.2.1. Filtration Loss Experiment
2.2.2. Extraction Cartridge Selection
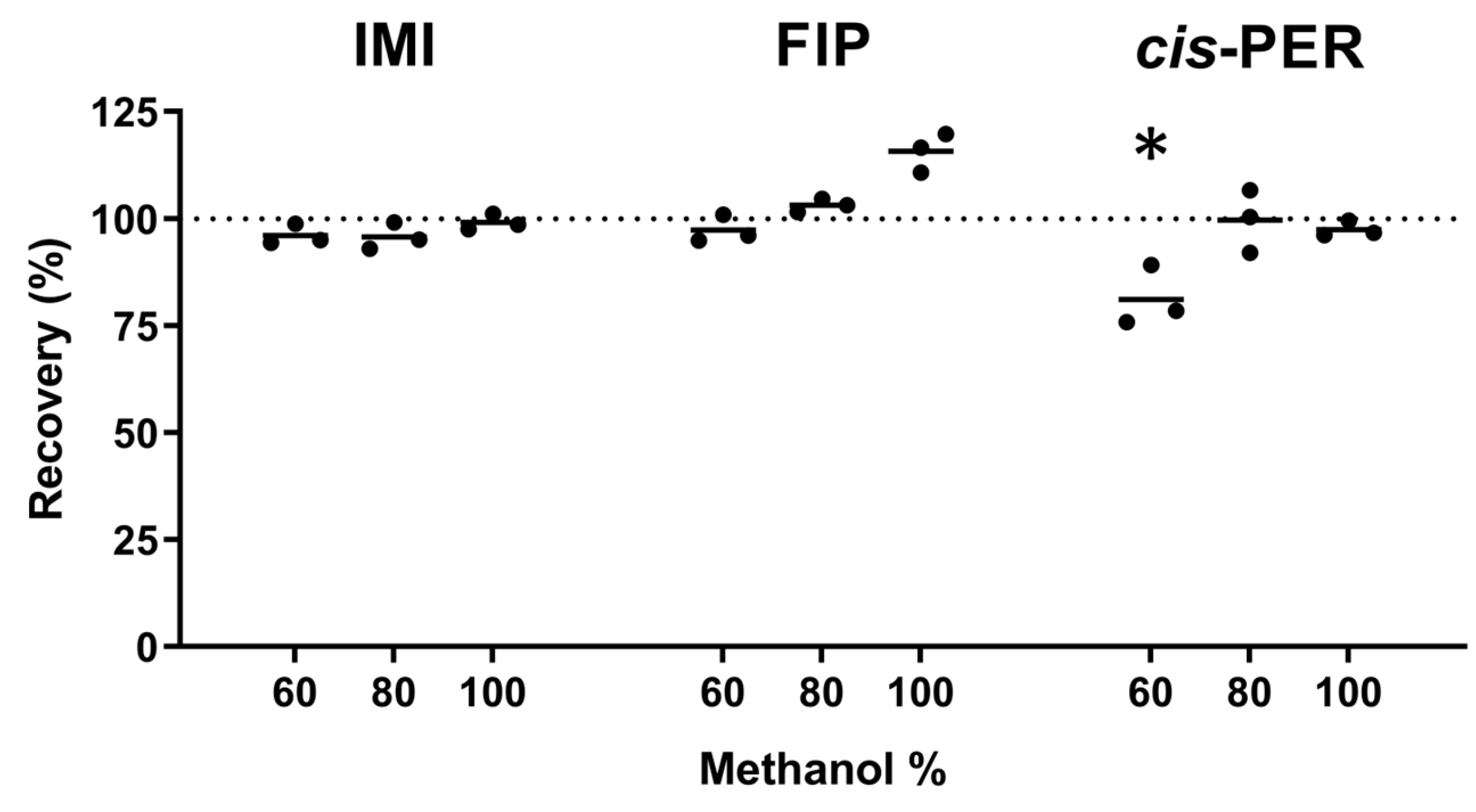
2.2.3. Washing Step Optimization
2.3. Method Validation
2.3.1. Selectivity
2.3.2. Internal Standard Selection and Matrix Effect
2.3.3. Linearity and Lower Limit of Quantification
2.3.4. Accuracy and Precision
2.3.5. Carry-Over
2.3.6. Dilution Integrity
2.3.7. Stability
2.4. Method Application
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Instrumental Analysis
3.3. Fipronil-Hydroxy
3.4. Final Protocol of Sample Preparation
3.5. Method Development
3.5.1. Filtration Loss Experiment
3.5.2. Extraction Cartridge Selection
- 20 pg/mL: FIP-desulfinyl, FIP, FIP-sulfide, FIP-sulfone;
- 100 pg/mL: DEET, FIP-amide, PFOA;
- 500 pg/mL: BPS, DPhP, FIP-dtfms, TEB-OH;
- 1000 pg/mL: 4F3PBA;
- 2000 pg/mL: IMI;
- 5000 pg/mL: IMZ-OH, BOS-OH;
- 10,000 pg/mL: IMI-OH, 4OH3PBA, CPhCA.
3.5.3. Washing Step Optimization
3.6. Method Validation
3.6.1. Selectivity
3.6.2. Internal Standard Selection and Matrix Effect
3.6.3. Linearity and Lower Limit of Quantification
3.6.4. Accuracy and Precision
3.6.5. Carry-Over
3.6.6. Dilution Integrity
3.6.7. Stability
3.7. Method Application
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HBM | Human biomonitoring |
| PFAS | Per-/polyfluoroalkyl substance |
| FIP | Fipronil |
| IMI | Imidacloprid |
| EU | European Union |
| FIP-sulfone | Fipronil-sulfone |
| FIP-hydroxy | Fipronil-hydroxy |
| FIP-dtfms | Fipronil-detrifluoromethylsulfinyl |
| FIP-amide | Fipronil-amide |
| FIP-desulfinyl | Fipronil-desulfinyl |
| FIP-sulfide | Fipronil-sulfide |
| FIPs | Fiproles |
| IMI-OH | Imidacloprid-5-hydroxy |
| 4OH3PBA | 4′-hydroxy-3-phenoxybenzoic acid |
| CPhCA | 3-(2-chloro-2-(4-chlorophenyl)vinyl)-2,2-dimethylcyclopropanecarboxylic acid |
| 4F3PBA | 4-fluoro-3-phenoxybenzoic acid |
| IMZ-OH | Imazalil-despropenyl |
| BOS-OH | Boscalid-5-hydroxy |
| TEB-OH | Tebuconazole-tert-butylhydroxy |
| DEET | N,N-Diethyl-meta-toluamide |
| BPS | Bisphenol S |
| OPFRs | Organophosphate flame retardants |
| DPhP | Diphenyl phosphate |
| PFOA | Perfluorooctanoic acid |
| LC-MS/MS | Liquid chromatography-tandem mass spectrometry |
| SPE | Solid phase extraction |
| cis-PER | cis-Permethrin |
| SG | Specific gravity |
| IS | Internal standard |
| CV | Coefficient of variation |
| LLOQ | Lower limit of quantification |
| R2 | Coefficient of determination |
| LOQ | Limit of quantification |
| LOD | Limit of detection |
| ULOQ | Upper limit of quantification |
| EMA | European Medicines Agency |
| QC | Quality control |
| LQC | Quality control sample at low concentration |
| HQC | Quality control sample at high concentration |
| GM | Geometric mean |
References
- Løkke, H.; Ragas, A.M.J.; Holmstrup, M. Tools and Perspectives for Assessing Chemical Mixtures and Multiple Stressors. Toxicology 2013, 313, 73–82. [Google Scholar] [CrossRef]
- Angerer, J.; Ewers, U.; Wilhelm, M. Human Biomonitoring: State of the Art. Int. J. Hyg. Environ. Health 2007, 210, 201–228. [Google Scholar] [CrossRef] [PubMed]
- Willey, J.B.; Pollock, T.; Thomson, E.M.; Liang, C.L.; Maquiling, A.; Walker, M.; St-Amand, A. Exposure Load: Using Biomonitoring Data to Quantify Multi-Chemical Exposure Burden in a Population. Int. J. Hyg. Environ. Health 2021, 234, 113704. [Google Scholar] [CrossRef] [PubMed]
- Needham, L.L.; Calafat, A.M.; Barr, D.B. Uses and Issues of Biomonitoring. Int. J. Hyg. Environ. Health 2007, 210, 229–238. [Google Scholar] [CrossRef]
- Ougier, E.; Ganzleben, C.; Lecoq, P.; Bessems, J.; David, M.; Schoeters, G.; Lange, R.; Meslin, M.; Uhl, M.; Kolossa-Gehring, M.; et al. Chemical Prioritisation Strategy in the European Human Biomonitoring Initiative (HBM4EU)—Development and Results. Int. J. Hyg. Environ. Health 2021, 236, 113778. [Google Scholar] [CrossRef] [PubMed]
- Vorkamp, K.; Castaño, A.; Antignac, J.P.; Boada, L.D.; Cequier, E.; Covaci, A.; Esteban López, M.; Haug, L.S.; Kasper-Sonnenberg, M.; Koch, H.M.; et al. Biomarkers, Matrices and Analytical Methods Targeting Human Exposure to Chemicals Selected for a European Human Biomonitoring Initiative. Environ. Int. 2021, 146, 106082. [Google Scholar] [CrossRef]
- Bolognesi, C.; Merlo, F.D. Pesticides: Human Health Effects. In Encyclopedia of Environmental Health; Nriagu, J.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 118–132. [Google Scholar] [CrossRef]
- Tingle, C.C.D.; Rother, J.A.; Dewhurst, C.F.; Lauer, S.; King, W.J. Health and Environmental Effects of Fipronil; Pesticide Action Network UK: Brighton and Hove, UK, 2000; pp. 1–30. [Google Scholar]
- Rust, M.K. Advances in the Control of Ctenocephalides Felis (Cat Flea) on Cats and Dogs. Trends Parasitol. 2005, 21, 232–236. [Google Scholar] [CrossRef]
- Matsuo, N. Discovery and Development of Pyrethroid Insecticides. Proc. Japan Acad. Ser. B Phys. Biol. Sci. 2019, 95, 378–400. [Google Scholar] [CrossRef]
- Nakagawa, L.E.; do Nascimento, C.M.; Costa, A.R.; Polatto, R.; Papini, S. Persistence of Indoor Permethrin and Estimation of Dermal and Non-Dietary Exposure. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 547–553. [Google Scholar] [CrossRef]
- FAO/WHO. Pesticide Residues in Food—1997 Evaluations, Part II—Toxicological; FAO/WHO: Geneva, Switzerland, 1998; Available online: https://www.inchem.org/documents/jmpr/jmpmono/v097pr01.htm (accessed on 5 August 2025).
- Gunasekara, A.S.; Truong, T.; Goh, K.S.; Spurlock, F.; Tjeerdema, R.S. Environmental Fate and Toxicology of Fipronil. J. Pestic. Sci. 2007, 32, 189–199. [Google Scholar] [CrossRef]
- European Comission. Commission Implementing Regulation (EU) No 2016/2035 of 21 November 2016 Amending Implementing Regulation (EU) No 540/2011 as Regards the Approval Periods of the Active Substances Fipronil and Maneb. Off. J. Eur. Union 2016, L 314, 7–8. [Google Scholar]
- EMA. Reflection Paper on the Environmental Risk Assessment of Ectoparasiticidal Veterinary Medicinal Products Used in Cats and Dogs; EMA/CVMP/ERA/31905/2021; EMA: Amsterdam, The Netherlands, 2023. [Google Scholar]
- FAO/WHO. Pesticide Residues in Food 2021: Joint FAO/WHO Meeting on Pesticide Residues. Evaluation Part II—Toxicological; FAO/WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Chen, D.; Li, J.; Zhao, Y.; Wu, Y. Human Exposure of Fipronil Insecticide and the Associated Health Risk. J. Agric. Food Chem. 2022, 70, 63–71. [Google Scholar] [CrossRef]
- Cravedi, J.P.; Delous, G.; Zalko, D.; Viguié, C.; Debrauwer, L. Disposition of Fipronil in Rats. Chemosphere 2013, 93, 2276–2283. [Google Scholar] [CrossRef] [PubMed]
- Verner, M.A.; Salame, H.; Housand, C.; Birnbaum, L.S.; Bouchard, M.F.; Chevrier, J.; Aylward, L.L.; Naiman, D.Q.; Lakind, J.S. How Many Urine Samples Are Needed to Accurately Assess Exposure to Non-Persistent Chemicals? The Biomarker Reliability Assessment Tool (BRAT) for Scientists, Research Sponsors, and Risk Managers. Int. J. Environ. Res. Public Health 2020, 17, 9102. [Google Scholar] [CrossRef] [PubMed]
- Vasylieva, N.; Barnych, B.; Wan, D.; El-Sheikh, E.S.A.; Nguyen, H.M.; Wulff, H.; McMahen, R.; Strynar, M.; Gee, S.J.; Hammock, B.D. Hydroxy-Fipronil Is a New Urinary Biomarker of Exposure to Fipronil. Environ. Int. 2017, 103, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Kasiotis, K.M.; Machera, K. Neonicotinoids and Their Metabolites in Human Biomonitoring: A Review. Hell. Plant Prot. J. 2015, 8, 33–45. [Google Scholar] [CrossRef]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic Insecticides (Neonicotinoids and Fipronil): Trends, Uses, Mode of Action and Metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2020/1643 of 5 November 2020 Amending Implementing Regulation (EU) No 540/2011 as Regards the Approval Periods of the Active Substances Calcium Phosphide, Denathonium Benzoate, Haloxyfop-P, Imidacloprid, Pencycuron. Off. J. Eur. Union 2020, L 370, 18–20. [Google Scholar]
- Han, W.; Tian, Y.; Shen, X. Human Exposure to Neonicotinoid Insecticides and the Evaluation of Their Potential Toxicity: An Overview. Chemosphere 2018, 192, 59–65. [Google Scholar] [CrossRef]
- Wrobel, S.A.; Bury, D.; Koslitz, S.; Hayen, H.; Koch, H.M.; Brüning, T.; Käfferlein, H.U. Quantitative Metabolism and Urinary Elimination Kinetics of Seven Neonicotinoids and Neonicotinoid-Like Compounds in Humans. Environ. Sci. Technol. 2023, 57, 19285–19294. [Google Scholar] [CrossRef]
- Soderlund, D.M.; Clark, J.M.; Sheets, L.P.; Mullin, L.S.; Piccirillo, V.J.; Sargent, D.; Stevens, J.T.; Weiner, M.L. Mechanisms of Pyrethroid Neurotoxicity: Implications for Cumulative Risk Assessment. Toxicology 2002, 171, 3–59. [Google Scholar] [CrossRef]
- Rodzaj, W.; Wileńska, M.; Klimowska, A.; Dziewirska, E.; Jurewicz, J.; Walczak-Jędrzejowska, R.; Słowikowska-Hilczer, J.; Hanke, W.; Wielgomas, B. Concentrations of Urinary Biomarkers and Predictors of Exposure to Pyrethroid Insecticides in Young, Polish, Urban-Dwelling Men. Sci. Total Environ. 2021, 773, 145666. [Google Scholar] [CrossRef] [PubMed]
- Sudakin, D.L. Pyrethroid Insecticides: Advances and Challenges in Biomonitoring. Clin. Toxicol. 2006, 44, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H. Pyrethroid Chemistry and Metabolism. In Hayes’ Handbook of Pesticide Toxicology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 1, pp. 1635–1663. [Google Scholar] [CrossRef]
- Toda, M.; Beer, K.D.; Kuivila, K.M.; Chiller, T.M.; Jackson, B.R. Trends in Agricultural Triazole Fungicide Use in the United States, 1992–2016 and Possible Implications for Antifungal-Resistant Fungi in Human Disease. Environ. Health Perspect. 2021, 129, 055001. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P.; Witschel, M.; Kramer, W.; Schirmer, U. Modern Crop Protection Compounds: Volume 2: Fungicides, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2019. [Google Scholar]
- FAO/WHO. Pesticide Residues in Food—2018 Evaluations, Part II—Toxicological; FAO/WHO: Geneva, Switzerland, 2018. [Google Scholar]
- FAO/WHO. Pesticide Residues in Food—2006 Evaluations, Part II—Toxicological; FAO/WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Mercadante, R.; Polledri, E.; Scurati, S.; Moretto, A.; Fustinoni, S. Identification and Quantification of Metabolites of the Fungicide Tebuconazole in Human Urine. Chem. Res. Toxicol. 2014, 27, 1943–1949. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, R.; Fu, Z.; Jin, Y. Maternal Exposure to Imazalil Disrupts the Endocrine System in F 1 Generation Mice. Mol. Cell. Endocrinol. 2019, 486, 105–112. [Google Scholar] [CrossRef]
- Taxvig, C.; Hass, U.; Axelstad, M.; Dalgaard, M.; Boberg, J.; Andeasen, H.R.; Vinggaard, A.M. Endocrine-Disrupting Activities In Vivo of the Fungicides Tebuconazole and Epoxiconazole. Toxicol. Sci. 2007, 100, 464–473. [Google Scholar] [CrossRef]
- Wang, H.; Meng, Z.; Liu, F.; Zhou, L.; Su, M.; Meng, Y.; Zhang, S.; Liao, X.; Cao, Z.; Lu, H. Characterization of Boscalid-Induced Oxidative Stress and Neurodevelopmental Toxicity in Zebrafish Embryos. Chemosphere 2020, 238, 124753. [Google Scholar] [CrossRef]
- Kuklenyik, P.; Baker, S.E.; Bishop, A.M.; Morales-A, P.; Calafat, A.M. On-Line Solid Phase Extraction-High Performance Liquid Chromatography-Isotope Dilution-Tandem Mass Spectrometry Approach to Quantify N,N-Diethyl-m-Toluamide and Oxidative Metabolites in Urine. Anal. Chim. Acta 2013, 787, 267–273. [Google Scholar] [CrossRef]
- del-Rahman, A.; Dechkovskaia, A.M.; Goldstein, L.B.; Bullman, S.H.; Khan, W.; El-Masry, E.M.; Abou-Donia, M.B. Neurological Deficits Induced by Malathion, Deet, and Permethrin, Alone or in Combination in Adult Rats. J. Toxicol. Environ. Health Part A 2004, 67, 331–356. [Google Scholar] [CrossRef]
- Eladak, S.; Grisin, T.; Moison, D.; Guerquin, M.J.; N’Tumba-Byn, T.; Pozzi-Gaudin, S.; Benachi, A.; Livera, G.; Rouiller-Fabre, V.; Habert, R. A New Chapter in the Bisphenol a Story: Bisphenol S and Bisphenol F Are Not Safe Alternatives to This Compound. Fertil. Steril. 2015, 103, 11–21. [Google Scholar] [CrossRef]
- Silva, J.P.A.; Ramos, J.G.; Campos, M.S.; da Silva Lima, D.; de Azevedo Brito, P.V.; Mendes, E.P.; Taboga, S.R.; Biancardi, M.F.; Ghedini, P.C.; Santos, F.C.A. Bisphenol-S Promotes Endocrine-Disrupting Effects Similar to Those Promoted by Bisphenol-A in the Prostate of Adult Gerbils. Reprod. Toxicol. 2019, 85, 83–92. [Google Scholar] [CrossRef]
- Frenzilli, G.; Martorell-Ribera, J.; Bernardeschi, M.; Scarcelli, V.; Jönsson, E.; Diano, N.; Moggio, M.; Guidi, P.; Sturve, J.; Asker, N. Bisphenol A and Bisphenol S Induce Endocrine and Chromosomal Alterations in Brown Trout. Front. Endocrinol. 2021, 12, 645519. [Google Scholar] [CrossRef]
- Oh, J.; Choi, J.W.; Ahn, Y.A.; Kim, S. Pharmacokinetics of Bisphenol S in Humans after Single Oral Administration. Environ. Int. 2018, 112, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Marklund, A.; Andersson, B.; Haglund, P. Screening of Organophosphorus Compounds and Their Distribution in Various Indoor Environments. Chemosphere 2003, 53, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Reemtsma, T.; García-López, M.; Rodríguez, I.; Quintana, J.B.; Rodil, R. Organophosphorus Flame Retardants and Plasticizers in Water and Air I. Occurrence and Fate. TrAC—Trends Anal. Chem. 2008, 27, 727–737. [Google Scholar] [CrossRef]
- Saillenfait, A.M.; Ndaw, S.; Robert, A.; Sabaté, J.P. Recent Biomonitoring Reports on Phosphate Ester Flame Retardants: A Short Review; Springer: Berlin/Heidelberg, Germany, 2018; Volume 92. [Google Scholar] [CrossRef]
- Hu, W.; Gao, P.; Wang, L.; Hu, J. Endocrine Disrupting Toxicity of Aryl Organophosphate Esters and Mode of Action. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1–18. [Google Scholar] [CrossRef]
- Steenland, K.; Fletcher, T.; Savitz, D.A. Epidemiologic Evidence on the Health Effects of Perfluorooctanoic Acid (PFOA). Environ. Health Perspect. 2010, 118, 1100–1108. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, L.; Yu, Y.; Yang, G.; Xu, Z.; Wang, Q.; Cai, L. Single and Joint Toxic Effects of Five Selected Pesticides on the Early Life Stages of Zebrafish (Denio Rerio). Chemosphere 2017, 170, 61–67. [Google Scholar] [CrossRef]
- Wei, F.; Wang, D.; Li, H.; You, J. Joint Toxicity of Imidacloprid and Azoxystrobin to Chironomus Dilutus at Organism, Cell, and Gene Levels. Aquat. Toxicol. 2021, 233, 105783. [Google Scholar] [CrossRef]
- Martin, O.; Scholze, M.; Ermler, S.; McPhie, J.; Bopp, S.K.; Kienzler, A.; Parissis, N.; Kortenkamp, A. Ten Years of Research on Synergisms and Antagonisms in Chemical Mixtures: A Systematic Review and Quantitative Reappraisal of Mixture Studies. Environ. Int. 2021, 146, 106206. [Google Scholar] [CrossRef]
- Kertesz, V.; Van Berkel, G.J. Minimizing Analyte Electrolysis in an Electrospray Emitter. J. Mass Spectrom. 2001, 36, 204–210. [Google Scholar] [CrossRef]
- Hassan, I.; Pavlov, J.; Errabelli, R.; Attygalle, A.B. Oxidative Ionization Under Certain Negative-Ion Mass Spectrometric Conditions. J. Am. Soc. Mass Spectrom. 2017, 28, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, G.W.; Masucci, J.A.; Yan, Z.; Hageman, W. Allometric Scaling of Pharmacokinetic Parameters in Drug Discovery: Can Human CL, Vss and T1/2 Be Predicted from in-Vivo Rat Data? Eur. J. Drug Metab. Pharmacokinet. 2004, 29, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Gibbs, S.T.; Fang, L.; Miller, H.A.; Landowski, C.P.; Shin, H.C.; Lennernas, H.; Zhong, Y.; Amidon, G.L.; Yu, L.X.; et al. Why Is It Challenging to Predict Intestinal Drug Absorption and Oral Bioavailability in Human Using Rat Model. Pharm. Res. 2006, 23, 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Indorf, P.; Patzak, A.; Lichtenberger, F.B. Drug Metabolism in Animal Models and Humans: Translational Aspects and Chances for Individual Therapy. Acta Physiol. 2021, 233, e13734. [Google Scholar] [CrossRef]
- Martignoni, M.; Groothuis, G.M.M.; de Kanter, R. Species Differences between Mouse, Rat, Dog, Monkey and Human CYP-Mediated Drug Metabolism, Inhibition and Induction. Expert Opin. Drug Metab. Toxicol. 2006, 2, 875–894. [Google Scholar] [CrossRef]
- Tang, J.; Usmani, K.A.; Hodgson, E.; Rose, R.L. In Vitro Metabolism of Fipronil by Human and Rat Cytochrome P450 and Its Interactions with Testosterone and Diazepam. Chem. Biol. Interact. 2004, 147, 319–329. [Google Scholar] [CrossRef]
- McMahen, R.L.; Strynar, M.J.; Dagnino, S.; Herr, D.W.; Moser, V.C.; Garantziotis, S.; Andersen, E.M.; Freeborn, D.L.; McMillan, L.; Lindstrom, A.B. Identification of Fipronil Metabolites by Time-of-Flight Mass Spectrometry for Application in a Human Exposure Study. Environ. Int. 2015, 78, 16–23. [Google Scholar] [CrossRef]
- Mirnaghi, F.S.; Goryński, K.; Rodriguez-Lafuente, A.; Boyaci, E.; Bojko, B.; Pawliszyn, J. Microextraction versus Exhaustive Extraction Approaches for Simultaneous Analysis of Compounds in Wide Range of Polarity. J. Chromatogr. A 2013, 1316, 37–43. [Google Scholar] [CrossRef]
- Klimowska, A.; Wielgomas, B. Off-Line Microextraction by Packed Sorbent Combined with on Solid Support Derivatization and GC-MS: Application for the Analysis of Five Pyrethroid Metabolites in Urine Samples. Talanta 2018, 176, 165–171. [Google Scholar] [CrossRef]
- Klimowska, A.; Wynendaele, E.; Wielgomas, B. Quantification and Stability Assessment of Urinary Phenolic and Acidic Biomarkers of Non-Persistent Chemicals Using the SPE-GC/MS/MS Method. Anal. Bioanal. Chem. 2023, 415, 2227–2238. [Google Scholar] [CrossRef]
- Michlig, N.; Lehotay, S.J.; Lightfield, A.R. Comparison of Filter Membranes in the Analysis of 183 Veterinary and Other Drugs by Liquid Chromatography-Tandem Mass Spectrometry. J. Sep. Sci. 2024, 47, 2300696. [Google Scholar] [CrossRef]
- Hebig, K.H.; Nödler, K.; Licha, T.; Scheytt, T.J. Impact of Materials Used in Lab and Field Experiments on the Recovery of Organic Micropollutants. Sci. Total Environ. 2014, 473–474, 125–131. [Google Scholar] [CrossRef] [PubMed]
- VanMiddlesworth, B.J.; Dorsey, J.G. Quantifying Injection Solvent Effects in Reversed-Phase Liquid Chromatography. J. Chromatogr. A 2012, 1236, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Waters. Beginner’s Guide to SPE: Solid Phase Extraction; Waters Corporation: Milford, MA, USA, 2014; pp. 1–212. [Google Scholar]
- Agilent. Bond Elut Plexa SPE Method Guide; 04034-0712; Agilent Technologies: Melbourne, VIC, Australia, 2012; pp. 1–2. [Google Scholar]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An International Database for Pesticide Risk Assessments and Management. Hum. Ecol. Risk Assess. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- EMA. ICH Guideline M10 on Bioanalytical Method Validation; EMA/CHMP/ICH/172948/2019; EMA: Amsterdam, The Netherlands, 2022. [Google Scholar]
- EMA. Guideline on Bioanalytical Method Validation; EMEA/CHMP/EWP/192217/2009 Rev. 1 Corr. 2; EMA: London, UK, 2011. [Google Scholar]
- Panuwet, P.; Hunter, R.E.; D’Souza, P.E.; Chen, X.; Radford, S.A.; Cohen, J.R.; Marder, M.E.; Kartavenka, K.; Ryan, P.B.; Barr, D.B. Biological Matrix Effects in Quantitative Tandem Mass Spectrometry-Based Analytical Methods: Advancing Biomonitoring. Crit. Rev. Anal. Chem. 2016, 46, 93–105. [Google Scholar] [CrossRef]
- Ferrer Amate, C.; Unterluggauer, H.; Fischer, R.J.; Fernández-Alba, A.R.; Masselter, S. Development and Validation of a LC-MS/MS Method for the Simultaneous Determination of Aflatoxins, Dyes and Pesticides in Spices. Anal. Bioanal. Chem. 2010, 397, 93–107. [Google Scholar] [CrossRef]
- Raposo, F.; Barceló, D. Challenges and Strategies of Matrix Effects Using Chromatography-Mass Spectrometry: An Overview from Research versus Regulatory Viewpoints. TrAC—Trends Anal. Chem. 2021, 134, 116068. [Google Scholar] [CrossRef]
- Gys, C.; Ait Bamai, Y.; Araki, A.; Bastiaensen, M.; Caballero-Casero, N.; Kishi, R.; Covaci, A. Biomonitoring and Temporal Trends of Bisphenols Exposure in Japanese School Children. Environ. Res. 2020, 191, 110172. [Google Scholar] [CrossRef]
- Rodríguez-Zamora, M.G.; Fuhrimann, S.; Winkler, M.S.; Rosa, M.J.; Reich, B.; Lindh, C.; Mora, A.M. Respiratory and Allergic Outcomes among Farmworkers Exposed to Pesticides in Costa Rica. Sci. Total Environ. 2024, 954, 176776. [Google Scholar] [CrossRef]
- Gao, B.; Poma, G.; Malarvannan, G.; Dumitrascu, C.; Bastiaensen, M.; Wang, M.; Covaci, A. Development of an Analytical Method Based on Solid-Phase Extraction and LC-MS/MS for the Monitoring of Current-Use Pesticides and Their Metabolites in Human Urine. J. Environ. Sci. 2022, 111, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Araujo, P. Key Aspects of Analytical Method Validation and Linearity Evaluation. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, S.A.; Bury, D.; Belov, V.N.; Klenk, J.M.; Hauer, B.; Hayen, H.; Martino-Andrade, A.J.; Koch, H.M.; Brüning, T.; Käfferlein, H.U. Rapid Quantification of Seven Major Neonicotinoids and Neonicotinoid-like Compounds and Their Key Metabolites in Human Urine. Anal. Chim. Acta 2023, 1239, 340680. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.E.; Serafim, A.B.; Morales-Agudelo, P.; Vidal, M.; Calafat, A.M.; Ospina, M. Quantification of DEET and Neonicotinoid Pesticide Biomarkers in Human Urine by Online Solid-Phase Extraction High-Performance Liquid Chromatography-Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2019, 411, 669–678. [Google Scholar] [CrossRef]
- Wrobel, S.A.; Koslitz, S.; Bury, D.; Hayen, H.; Koch, H.M.; Brüning, T.; Käfferlein, H.U. Human Biomonitoring of Neonicotinoid Exposures: Case Studies after the Use of a Spray-Agent to Ornamental Plants and a Topical Medication to Pets. Front. Public Health 2023, 11, 1321138. [Google Scholar] [CrossRef]
- Garí, M.; Bury, D.; Moos, R.K.; Wolniewicz, M.; Jankowska, A.; Brzozowska, A.; Jerzynska, J.; Bose-O’Reilly, S.; Koch, H.M.; Polanska, K. Urinary Concentrations of BPA and Analogous Bisphenols (BPF and BPS) among School Children from Poland: Exposure and Risk Assessment in the REPRO_PL Cohort. Expo. Health 2025, 17, 191–200. [Google Scholar] [CrossRef]
- Norén, E.; Lindh, C.; Bengtsson, M.; Rönnholm, A. Urin-Och Serumhalter Av Organiska Miljöföroreningar Hos Unga Vuxna i Skåne År 2000–2022. Resultat Från Den Sjätte Delstudien 2022; Naturvårdsverkets: Lund, Sweden, 2024; Available online: https://www.naturvardsverket.se/verktyg-och-tjanster/data-databaser-och-sokregister/naturvardsverkets-oppna-rapportarkiv---diva/ (accessed on 30 July 2025).
- Van den Eede, N.; Neels, H.; Jorens, P.G.; Covaci, A. Analysis of Organophosphate Flame Retardant Diester Metabolites in Human Urine by Liquid Chromatography Electrospray Ionisation Tandem Mass Spectrometry. J. Chromatogr. A 2013, 1303, 48–53. [Google Scholar] [CrossRef]
- Fišerová, P.S.; Kohoutek, J.; Degrendele, C.; Dalvie, M.A.; Klánová, J. New Sample Preparation Method to Analyse 15 Specific and Non-Specific Pesticide Metabolites in Human Urine Using LC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1166, 122542. [Google Scholar] [CrossRef]
- Nishihara, N.; Isobe, T.; Takagi, M.; Tajima, T.; Kitahara, Y.; Hayashi, M.; Saito, I.; Watanabe, S.; Iwai-Shimada, M.; Ueyama, J. Determination of Skin-Insect Repellent Icaridin and DEET in Human Urine Using Solid-Phase Extraction and Liquid Chromatography with Tandem Mass Spectrometry and Its Application to a Sample of Japanese Adults. Environ. Health Prev. Med. 2025, 30, 18. [Google Scholar] [CrossRef]
- Šulc, L.; Janoš, T.; Figueiredo, D.; Ottenbros, I.; Šenk, P.; Mikeš, O.; Huss, A.; Čupr, P. Pesticide Exposure among Czech Adults and Children from the CELSPAC-SPECIMEn Cohort: Urinary Biomarker Levels and Associated Health Risks. Environ. Res. 2022, 214, 114002. [Google Scholar] [CrossRef]
- Davis, M.D.; Wade, E.L.; Restrepo, P.R.; Roman-Esteva, W.; Bravo, R.; Kuklenyik, P.; Calafat, A.M. Semi-Automated Solid Phase Extraction Method for the Mass Spectrometric Quantification of 12 Specific Metabolites of Organophosphorus Pesticides, Synthetic Pyrethroids, and Select Herbicides in Human Urine. J. Chromatogr. B 2013, 929, 18–26. [Google Scholar] [CrossRef]
- Le Grand, R.; Dulaurent, S.; Gaulier, J.M.; Saint-Marcoux, F.; Moesch, C.; Lachâtre, G. Simultaneous Determination of Five Synthetic Pyrethroid Metabolites in Urine by Liquid Chromatography-Tandem Mass Spectrometry: Application to 39 Persons without Known Exposure to Pyrethroids. Toxicol. Lett. 2012, 210, 248–253. [Google Scholar] [CrossRef]
- Shi, L.; Wan, Y.; Liu, J.; He, Z.; Xu, S.; Xia, W. Insecticide Fipronil and Its Transformation Products in Human Blood and Urine: Assessment of Human Exposure in General Population of China. Sci. Total Environ. 2021, 786, 147342. [Google Scholar] [CrossRef] [PubMed]
- Kruve, A.; Rebane, R.; Kipper, K.; Oldekop, M.L.; Evard, H.; Herodes, K.; Ravio, P.; Leito, I. Tutorial Review on Validation of Liquid Chromatography-Mass Spectrometry Methods: Part I. Anal. Chim. Acta 2015, 870, 29–44. [Google Scholar] [CrossRef]
- Evard, H.; Kruve, A.; Leito, I. Tutorial on Estimating the Limit of Detection Using LC-MS Analysis, Part I: Theoretical Review. Anal. Chim. Acta 2016, 942, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Bishop, A.M.; Reidy, J.A.; Needham, L.L.; Calafat, A.M. Temporal Stability of the Conjugated Species of Bisphenol A, Parabens, and Other Environmental Phenols in Human Urine. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Kruve, A.; Rebane, R.; Kipper, K.; Oldekop, M.L.; Evard, H.; Herodes, K.; Ravio, P.; Leito, I. Tutorial Review on Validation of Liquid Chromatography-Mass Spectrometry Methods: Part II. Anal. Chim. Acta 2015, 870, 8–28. [Google Scholar] [CrossRef]
- Li, W.; Zhang, J.; Tse, F.L.S. Strategies in Quantitative LC-MS/MS Analysis of Unstable Small Molecules in Biological Matrices. Biomed. Chromatogr. 2011, 25, 258–277. [Google Scholar] [CrossRef]
- ECHA. BPA Being Replaced by BPS in Thermal Paper, ECHA Survey Finds. Available online: https://echa.europa.eu/pl/-/bpa-being-replaced-by-bps-in-thermal-paper-echa-survey-finds (accessed on 25 August 2025).
- Frederiksen, H.; Nielsen, O.; Koch, H.M.; Skakkebaek, N.E.; Juul, A.; Jørgensen, N.; Andersson, A.M. Changes in Urinary Excretion of Phthalates, Phthalate Substitutes, Bisphenols and Other Polychlorinated and Phenolic Substances in Young Danish Men; 2009–2017. Int. J. Hyg. Environ. Health 2020, 223, 93–105. [Google Scholar] [CrossRef]
- Ospina, M.; Jayatilaka, N.K.; Wong, L.Y.; Restrepo, P.; Calafat, A.M. Exposure to Organophosphate Flame Retardant Chemicals in the U.S. General Population: Data from the 2013–2014 National Health and Nutrition Examination Survey. Environ. Int. 2018, 110, 32–41. [Google Scholar] [CrossRef]
- Jayatilaka, N.K.; Restrepo, P.; Williams, L.T.; Ospina, M.; Valentin-Blasini, L.; Calafat, A.M. Quantification of Three Chlorinated Dialkyl Phosphates, Diphenyl Phosphate, 2,3,4,5-Tetrabromobenzoic Acid, and Four Other Organophosphates in Human Urine by Solid Phase Extraction-High Performance Liquid Chromatography-Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2017, 409, 1323–1332. [Google Scholar] [CrossRef]
- Faÿs, F.; Palazzi, P.; Hardy, E.M.; Schaeffer, C.; Phillipat, C.; Zeimet, E.; Vaillant, M.; Beausoleil, C.; Rousselle, C.; Slama, R.; et al. Is There an Optimal Sampling Time and Number of Samples for Assessing Exposure to Fast Elimination Endocrine Disruptors with Urinary Biomarkers? Sci. Total Environ. 2020, 747, 141185. [Google Scholar] [CrossRef]
- Dyk, M.B.; Liu, Y.; Chen, Z.; Vega, H.; Krieger, R.I. Fate and Distribution of Fipronil on Companion Animals and in Their Indoor Residences Following Spot-on Flea Treatments. J. Environ. Sci. Health—Part B Pestic. Food Contam. Agric. Wastes 2012, 47, 913–924. [Google Scholar] [CrossRef]
- Cone, E.J.; Caplan, Y.H.; Moser, F.; Robert, T.; Shelby, M.K.; Black, D.L. Normalization of Urinary Drug Concentrations with Specific Gravity and Creatinine. J. Anal. Toxicol. 2009, 33, 1–7. [Google Scholar] [CrossRef]




| Analyte | Nominal Concentration (pg/mL) | Specific Gravity of Urine Lots | Mean | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1.006 | 1.010 | 1.011 | 1.017 | 1.026 | 1.028 | 1.031 | |||
| Matrix Factors (%) | |||||||||
| IMI-OH | 40,000 | 50 | 52 | 51 | 50 | 10 | 44 | 49 | 44 |
| IMI | 8000 | 43 | 32 | 40 | 40 | 21 | 37 | 51 | 38 |
| BPS | 2000 | 17 | 12 | 13 | 11 | 15 | 10 | 16 | 13 |
| BOS-OH | 20,000 | 5 | 3 | 2 | 2 | 1 | 1 | 3 | 2 |
| 4OH3PBA | 40,000 | 16 | 6 | 8 | 7 | 3 | 4 | 4 | 7 |
| DPhP | 2000 | 89 | 72 | 61 | 64 | 25 | 34 | 42 | 55 |
| DEET | 400 | 38 | 20 | 24 | 23 | 22 | 21 | 18 | 24 |
| FIP-dtfms | 2000 | 38 | 19 | 18 | 15 | 9 | 10 | 7 | 17 |
| FIP-amide | 400 | 19 | 11 | 14 | 10 | 5 | 6 | 5 | 10 |
| TEB-OH | 2000 | 81 | 58 | 56 | 48 | 42 | 45 | 34 | 52 |
| 4F3PBA | 4000 | 14 | 10 | 11 | 12 | 6 | 9 | 10 | 10 |
| FIP-desulfinyl | 80 | 67 | 40 | 34 | 36 | 30 | 32 | 21 | 37 |
| FIP | 80 | 71 | 48 | 52 | 48 | 34 | 39 | 26 | 46 |
| FIP-sulfide | 80 | 53 | 37 | 37 | 36 | 5 | 27 | 12 | 30 |
| FIP-sulfone | 80 | 61 | 31 | 41 | 35 | 28 | 28 | 20 | 35 |
| CPhCA | 40,000 | 55 | 31 | 39 | 33 | 28 | 29 | 27 | 35 |
| Analyte | Internal Standard | Specific Gravity of Urine Lots | Mean | CV | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1.006 | 1.010 | 1.011 | 1.017 | 1.026 | 1.028 | 1.031 | ||||
| IS-Corrected Matrix Factors (%) | ||||||||||
| IMI-OH | IMI-D4 | 110 | 146 | 114 | 117 | 38 | 106 | 85 | 102 | 30 |
| IMI | IMI-D4 | 93 | 91 | 90 | 95 | 78 | 88 | 90 | 89 | 6 |
| BPS | BPS-D8 | 85 | 74 | 74 | 60 | 79 | 61 | 74 | 72 | 12 |
| BOS-OH | BPS-D8 | 24 | 16 | 13 | 11 | 6 | 8 | 14 | 13 | 41 |
| 4OH3PBA | FIP-dtfms-13C215N2 | 48 | 36 | 44 | 47 | 42 | 44 | 60 | 46 | 15 |
| DPhP | FIP-13C4 | 118 | 140 | 106 | 125 | 69 | 80 | 150 | 112 | 25 |
| DEET | FIP-13C4 | 51 | 38 | 42 | 46 | 60 | 48 | 64 | 50 | 18 |
| FIP-dtfms | FIP-dtfms-13C215N2 | 114 | 110 | 105 | 110 | 111 | 106 | 116 | 110 | 3 |
| FIP-amide | FIP-dtfms-13C215N2 | 57 | 62 | 80 | 72 | 65 | 69 | 78 | 69 | 11 |
| TEB-OH | FIP-13C4 | 108 | 112 | 96 | 93 | 113 | 104 | 123 | 107 | 9 |
| 4F3PBA | 3PBA-13C6 | 52 | 79 | 65 | 71 | 74 | 66 | 89 | 71 | 15 |
| FIP-desulfinyl | FIP-13C4 | 89 | 78 | 58 | 70 | 82 | 73 | 76 | 75 | 12 |
| FIP | FIP-13C4 | 95 | 93 | 90 | 95 | 93 | 91 | 95 | 93 | 2 |
| FIP-sulfide | FIP-13C4 | 71 | 72 | 64 | 71 | 15 | 62 | 41 | 57 | 35 |
| FIP-sulfone | FIP-13C4 | 80 | 60 | 70 | 68 | 76 | 66 | 72 | 70 | 9 |
| CPhCA | FIP-13C4 | 73 | 60 | 68 | 65 | 76 | 68 | 96 | 72 | 15 |
| Analyte | IS | LLOQ (pg/mL) | Linear Range 1 (pg/mL) | Regression Equation | Curve Fit | Curve Weighting | Coefficient of Determination (R2) |
|---|---|---|---|---|---|---|---|
| IMI-OH | IMI-D4 | 1000 | 1000–200,000 | 0.0853x + 0.0065 | Linear | 1/x | 0.9972 |
| IMI | IMI-D4 | 100 | 100–20,000 (160,000) | 1.5555x + 0.0017 | Linear | 1/x | 0.9990 |
| BPS | BPS-D8 | 50 | 50–5000 (40,000) | 0.2526x + 0.0056 | Linear | 1/x | 0.9979 |
| BOS-OH | BPS-D8 | 1000 | 1000–200,000 | 0.0138x + 0.0020 | Linear | 1/x | 0.9969 |
| 4OH3PBA | FIP-dtfms-13C215N2 | 2000 | 2000–200,000 | 0.0185x − 0.0018 | Linear | 1/x | 0.9907 |
| DPhP | FIP-13C4 | 100 | 100–5000 | 0.0668x + 0.0238 | Linear | 1/x | 0.9935 |
| DEET | FIP-13C4 | 10 | 10–2000 | 0.8049x + 0.0604 | Linear | 1/x | 0.9991 |
| FIP-dtfms | FIP-dtfms-13C215N2 | 200 | 200–40,000 (320,000) | 0.8586x − 0.0021 | Linear | 1/x | 0.9995 |
| FIP-amide | FIP-dtfms-13C215N2 | 50 | 50–5000 | 0.2650x − 0.0004 | Linear | 1/x | 0.9948 |
| TEB-OH | FIP-13C4 | 100 | 100–20,000 | 0.0499x + 0.0096 | Linear | 1/x | 0.9973 |
| 4F3PBA | 3PBA-13C6 | 100 | 100–10,000 | 3.8030x + 0.0050 | Linear | 1/x2 | 0.9921 |
| FIP-desulfinyl | FIP-13C4 | 5 | 5–1000 (8000) | 0.9155x + 0.0027 | Linear | 1/x | 0.9988 |
| FIP | FIP-13C4 | 1 | 1–200 (1600) | 1.2291x + 0.0013 | Linear | 1/x | 0.9996 |
| FIP-sulfide | FIP-13C4 | 1 | 1–200 (1600) | 0.8579x + 0.0007 | Linear | 1/x | 0.9992 |
| FIP-sulfone | FIP-13C4 | 0.5 | 0.5–100 (800) | 1.3612x + 0.0053 | Linear | 1/x | 0.9995 |
| CPhCA | FIP-13C4 | 500 | 500–100,000 (800,000) | 0.0012x + 0.0015 | Linear | 1/x | 0.9993 |
| Analyte | LQC | HQC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nominal Concentration (pg/mL) | Accuracy (%) | Precision (CV, %) | Nominal Concentration (pg/mL) | Accuracy (%) | Precision (CV, %) | |||||
| Intra-Run (n = 5) | Inter-Run (n = 15) | Intra-Run (n = 5) | Inter-Run (n = 15) | Intra-Run (n = 5) | Inter-Run (n = 15) | Intra-Run (n = 5) | Inter-Run (n = 15) | |||
| IMI-OH | 3000 | 102 | 86 | 13 | 20 | 50,000 | 96 | 95 | 8 | 8 |
| IMI | 300 | 99 | 100 | 6 | 8 | 5000 | 97 | 101 | 4 | 9 |
| BPS | 75 | 97 | 89 | 14 | 14 | 1250 | 110 | 102 | 7 | 10 |
| BOS-OH | 3000 | 100 | 100 | 9 | 13 | 50,000 | 101 | 96 | 7 | 12 |
| 4OH3PBA | 3000 | 101 | 97 | 12 | 14 | 50,000 | 88 | 86 | 5 | 7 |
| DPhP | 150 | 91 | 101 | 8 | 16 | 2500 | 100 | 86 | 7 | 14 |
| DEET | 30 | 105 | 113 | 15 | 14 | 500 | 97 | 104 | 3 | 4 |
| FIP-dtfms | 600 | 100 | 98 | 9 | 6 | 10,000 | 102 | 102 | 3 | 3 |
| FIP-amide | 150 | 95 | 88 | 7 | 13 | 2500 | 96 | 95 | 7 | 6 |
| TEB-OH | 300 | 104 | 100 | 8 | 10 | 5000 | 103 | 103 | 4 | 6 |
| 4F3PBA | 150 | 95 | 93 | 11 | 13 | 2500 | 97 | 99 | 4 | 6 |
| FIP-desulfinyl | 15 | 99 | 97 | 6 | 8 | 250 | 100 | 103 | 4 | 4 |
| FIP | 3 | 97 | 93 | 7 | 12 | 50 | 99 | 97 | 4 | 7 |
| FIP-sulfide | 3 | 102 | 96 | 10 | 14 | 50 | 95 | 99 | 7 | 11 |
| FIP-sulfone | 1.5 | 103 | 88 | 10 | 13 | 25 | 97 | 100 | 7 | 14 |
| CPhCA | 1500 | 93 | 99 | 10 | 12 | 25,000 | 99 | 101 | 3 | 4 |
| Analyte | Study Period | LQC | HQC | ||||
|---|---|---|---|---|---|---|---|
| Nominal Concentration (pg/mL) | Accuracy (n = 4, %) | Precision (n = 4, CV, %) | Nominal Concentration (pg/mL) | Accuracy (n = 4, %) | Precision (n = 4, CV, %) | ||
| 24 h | 88 | 13 | 96 | 8 | |||
| IMI-OH | 30-day | 3000 | 86 | 15 | 50,000 | 95 | 6 |
| 12-month | 92 | 12 | 90 | 4 | |||
| 24 h | 99 | 7 | 103 | 9 | |||
| IMI | 30-day | 300 | 99 | 14 | 5000 | 100 | 5 |
| 12-month | 108 | 14 | 103 | 3 | |||
| 24 h | 90 | 10 | 103 | 7 | |||
| BPS | 30-day | 75 | 115 | 4 | 1250 | 104 | 9 |
| 12-month | 113 | 14 | 108 | 6 | |||
| 24 h | 101 | 6 | 85 | 3 | |||
| BOS-OH | 30-day | 3000 | 80 | 8 | 50,000 | 89 | 1 |
| 12-month | 46 | 11 | 46 | 11 | |||
| 24 h | 88 | 7 | 89 | 4 | |||
| 4OH3PBA | 30-day | 3000 | 107 | 4 | 50,000 | 79 | 7 |
| 12-month | 77 | 10 | 58 | 2 | |||
| 24 h | 105 | 8 | 86 | 4 | |||
| DPhP | 30-day | 150 | 109 | 13 | 2500 | 87 | 9 |
| 12-month | 97 | 14 | 114 | 10 | |||
| 24 h | 113 | 5 | 110 | 2 | |||
| DEET | 30-day | 30 | 115 | 6 | 500 | 108 | 6 |
| 12-month | 97 | 6 | 98 | 5 | |||
| 24 h | 94 | 11 | 105 | 2 | |||
| FIP-dtfms | 30-day | 600 | 97 | 1 | 10,000 | 105 | 3 |
| 12-month | 98 | 1 | 99 | 2 | |||
| 24 h | 89 | 6 | 91 | 6 | |||
| FIP-amide | 30-day | 150 | 105 | 11 | 2500 | 93 | 6 |
| 12-month | 102 | 8 | 99 | 3 | |||
| 24 h | 96 | 11 | 100 | 2 | |||
| TEB-OH | 30-day | 300 | 97 | 6 | 5000 | 101 | 10 |
| 12-month | 102 | 10 | 99 | 2 | |||
| 24 h | 91 | 15 | 100 | 6 | |||
| 4F3PBA | 30-day | 150 | 99 | 14 | 2500 | 91 | 6 |
| 12-month | 115 | 13 | 92 | 4 | |||
| 24 h | 94 | 11 | 106 | 4 | |||
| FIP-desulfinyl | 30-day | 15 | 93 | 3 | 250 | 102 | 2 |
| 12-month | 98 | 14 | 90 | 3 | |||
| 24 h | 90 | 5 | 90 | 1 | |||
| FIP | 30-day | 3 | 89 | 1 | 50 | 90 | 4 |
| 12-month | 96 | 0 | 89 | 1 | |||
| 24 h | 91 | 13 | 92 | 0 | |||
| FIP-sulfide | 30-day | 3 | 90 | 10 | 50 | 92 | 4 |
| 12-month | 95 | 12 | 85 | 5 | |||
| 24 h | 89 | 12 | 89 | 4 | |||
| FIP-sulfone | 30-day | 1.5 | 86 | 9 | 25 | 89 | 5 |
| 12-month | 85 | 3 | 86 | 10 | |||
| 24 h | 97 | 2 | 105 | 1 | |||
| CPhCA | 30-day | 1500 | 95 | 3 | 25,000 | 99 | 4 |
| 12-month | 96 | 12 | 108 | 2 | |||
| Analyte | % ≥LLOQ 1 | Unadjusted (pg/mL) | Specific Gravity-Adjusted (pg/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GM | P25 | P50 | P75 | P95 | Max | GM | P25 | P50 | P75 | P95 | Max | ||
| IMI-OH | 0 | - | - | - | - | - | - | - | - | - | - | - | - |
| IMI | 4 | - | - | - | - | - | 101 | - | - | - | - | - | 122 |
| BPS | 89 | 412 | 277 | 663 | 791 | 2888 | 3947 | 484 | 257 | 537 | 840 | 2437 | 2978 |
| BOS-OH | 50 | 1238 | - | 538 2 | 2732 | 11,950 | 14,190 | 1454 | - | 561 | 3726 | 13,374 | 13,384 |
| 4OH3PBA | 18 | - | - | - | - | 9813 | 14,008 | - | - | - | - | 8811 | 13,350 |
| DPhP | 93 | 1541 | 952 | 1712 | 3531 | >ULOQ 3 | >ULOQ | 1810 | 930 | 1656 | 3692 | >ULOQ | >ULOQ |
| DEET | 68 | 20.2 | - | 15.4 | 78.5 | 212 | 214 | 23.7 | - | 17.7 | 101 | 190 | 216 |
| FIP-dtfms | 0 | - | - | - | - | - | - | - | - | - | - | - | - |
| FIP-amide | 0 | - | - | - | - | - | - | - | - | - | - | - | - |
| TEB-OH | 86 | 281 | 134 | 259 | 741 | 4565 | 6905 | 330 | 108 | 254 | 580 | 3012 | 4465 |
| 4F3PBA | 4 | - | - | - | - | - | 416 | - | - | - | - | - | 290 |
| FIP-desulfinyl | 0 | - | - | - | - | - | - | - | - | - | - | - | - |
| FIP | 71 | 3.94 | - | 3.32 | 9.98 | 129 | 150 | 4.63 | - | 3.39 | 24.9 | 233 | 306 |
| FIP-sulfide | 0 | - | - | - | - | - | - | - | - | - | - | - | - |
| FIP-sulfone | 39 | - | - | - | 0.858 | 16.7 | 17.7 | - | - | - | 1.04 | 14.5 | 16.9 |
| CPhCA | 0 | - | - | - | - | - | - | - | - | - | - | - | - |
| Compound | Status | Retention Time (min) | Precursor Ion | Precursor m/z | Capillary Voltage (V) | Product Ions m/z 1 | Collision Energy (V) 2 |
|---|---|---|---|---|---|---|---|
| IMI-OH | analyte | 4.90 | [M+H]+ [M−H]− | 272.0 270.0 | 70 −70 | 225.0, 228.0 46.1 | 14, 10 10 |
| IMZ-OH | analyte | 5.18 | [M+H]+ | 257.0 | 90 | 69.0, 125.0, 136.0 | 19, 33, 42 |
| IMI | analyte | 5.33 | [M+H]+ | 256.0 | 70 | 209.0, 175.2, 212.2 | 12, 14, 11 |
| BPS | analyte | 6.11 | [M−H]− | 249.1 | −110 | 155.4, 91.9, 107.9 | 21, 33, 27 |
| BOS-OH | analyte | 8.31 | [M−H]− [M+H]+ | 357.0 359.0 | −100 100 | 243.7 140.0, 323.0 | 18 21, 20 |
| 4OH3PBA | analyte | 8.51 | [M−H]− | 229.3 | −70 | 108.9, 109.8, 185.6 | 21, 20.5, 12 |
| DPhP | analyte | 8.52 | [M−H]− | 249.0 | −92 | 92.7, 154.6 | 26, 19.5 |
| DEET | analyte | 9.09 | [M+H]+ | 192.0 | 88 | 119.0, 91.0 | 14, 26 |
| FIP-dtfms | analyte | 9.14 | [M−H]− | 319.0 | −70 | 282.9, 262.8 | 8, 20 |
| FIP-amide | analyte | 9.26 | [M−H]− | 453.0 | −70 | 347.8, 271.9, 303.8 | 15, 41, 25 |
| TEB-OH | analyte | 10.04 | [M+H]+ | 324.0 | 90 | 70.0, 125.0, 179.1 | 20, 37, 19 |
| 4F3PBA | analyte | 10.39 | [M−H]− | 230.9 | −75 | 92.7, 186.6 | 25, 10 |
| FIP-desulfinyl | analyte | 10.77 | [M−H]− | 387.0 | −50 | 350.9, 281.9, 330.8 | 12, 30, 28 |
| FIP | analyte | 10.96 | [M−H]− | 435.0 | −70 | 329.7, 249.6, 277.6 | 15, 26, 27 |
| FIP-sulfide | analyte | 11.12 | [M−H]− | 419.0 | −70 | 261.8, 313.9, 382.9 | 26, 18, 11 |
| FIP-sulfone | analyte | 11.34 | [M−H]− | 451.0 | −70 | 281.9, 243.8, 414.9 | 25, 44, 17 |
| PFOA | analyte | 11.79 | [M−H]− [M−CO2−H]− | 413.0 369.0 | −30 −72 | 369.0, 169.0 219.0 | 7, 18.5 10.5 |
| CPhCA | analyte | 12.11 | [M−H]− | 283.0 | −90 | 246.6, 35.2 | 7, 9 |
| IMI-D4 | internal standard | 5.30 | [M+H]+ | 260.0 | 70 | 213.0, 214.0, 216.0 | 12, 8, 10 |
| BPS-D8 | internal standard | 6.05 | [M−H]− | 257.0 | −110 | 112.0, 96.0, 160.0 | 27, 33, 21 |
| FIP-dtfms-13C215N2 | internal standard | 9.13 | [M−H]− | 323.0 | −70 | 287.0, 184.9 | 8, 28 |
| 3PBA-13C4 | internal standard | 10.40 | [M−H]− | 219.0 | −90 | 99.0, 175.0 | 19, 11 |
| FIP-13C4 | internal standard | 10.97 | [M−H]− | 439.0 | −70 | 334.0, 250.9, 321.9 | 15, 26, 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodzaj, W.; Wacławik, M.; Jurewicz, J.; Wielgomas, B. A Sensitive SPE-LC-MS/MS Method for Determination of Selected Veterinary Drugs and Other Organic Contaminants in Human Urine: Development, Validation, and Application Study. Int. J. Mol. Sci. 2025, 26, 9025. https://doi.org/10.3390/ijms26189025
Rodzaj W, Wacławik M, Jurewicz J, Wielgomas B. A Sensitive SPE-LC-MS/MS Method for Determination of Selected Veterinary Drugs and Other Organic Contaminants in Human Urine: Development, Validation, and Application Study. International Journal of Molecular Sciences. 2025; 26(18):9025. https://doi.org/10.3390/ijms26189025
Chicago/Turabian StyleRodzaj, Wojciech, Małgorzata Wacławik, Joanna Jurewicz, and Bartosz Wielgomas. 2025. "A Sensitive SPE-LC-MS/MS Method for Determination of Selected Veterinary Drugs and Other Organic Contaminants in Human Urine: Development, Validation, and Application Study" International Journal of Molecular Sciences 26, no. 18: 9025. https://doi.org/10.3390/ijms26189025
APA StyleRodzaj, W., Wacławik, M., Jurewicz, J., & Wielgomas, B. (2025). A Sensitive SPE-LC-MS/MS Method for Determination of Selected Veterinary Drugs and Other Organic Contaminants in Human Urine: Development, Validation, and Application Study. International Journal of Molecular Sciences, 26(18), 9025. https://doi.org/10.3390/ijms26189025







