Stem Cells and Cell-Free Therapies for Olfactory Epithelium Regeneration: Insights from Experimental Models
Abstract
1. Introduction
2. Olfactory Nervous System
2.1. Peripheral Components: The Olfactory Epithelium
2.2. Central Components: The Olfactory Bulb and Higher Brain Regions
2.3. Distinctive Characteristics of the Olfactory Nervous System
3. Experimental Models of Anosmia
3.1. Chemical Ablation
3.2. Surgical and Mechanical Injury
3.3. Genetic Models
3.4. Infection and Inflammation-Induced Models
4. Effect of Stem Cells on Olfactory Epithelium Regeneration
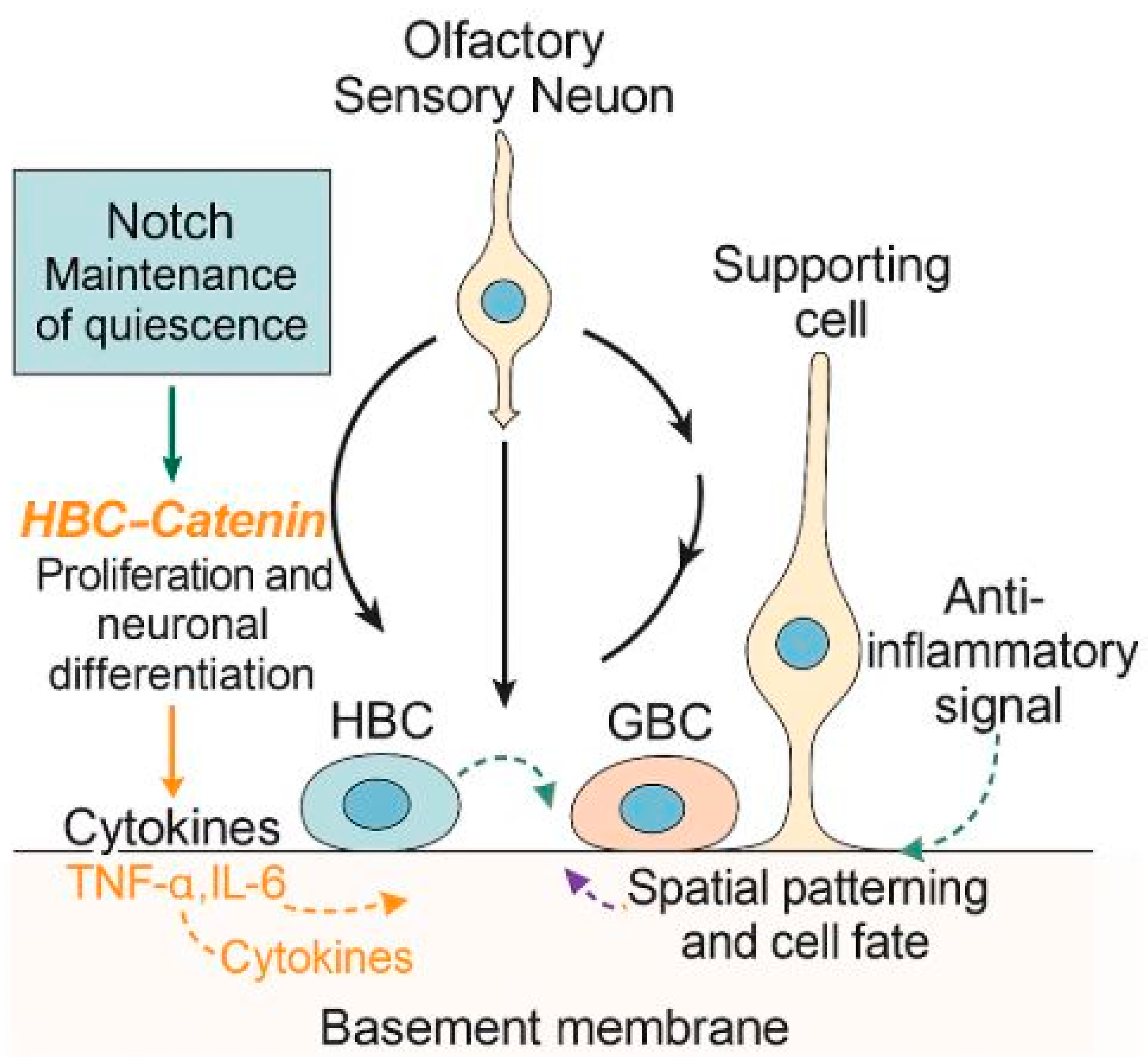
4.1. Endogenous Stem Cell-Mediated Regeneration
4.2. Molecular Signaling in Stem Cell-Mediated Regeneration
4.3. Exogenous Stem Cell Therapy
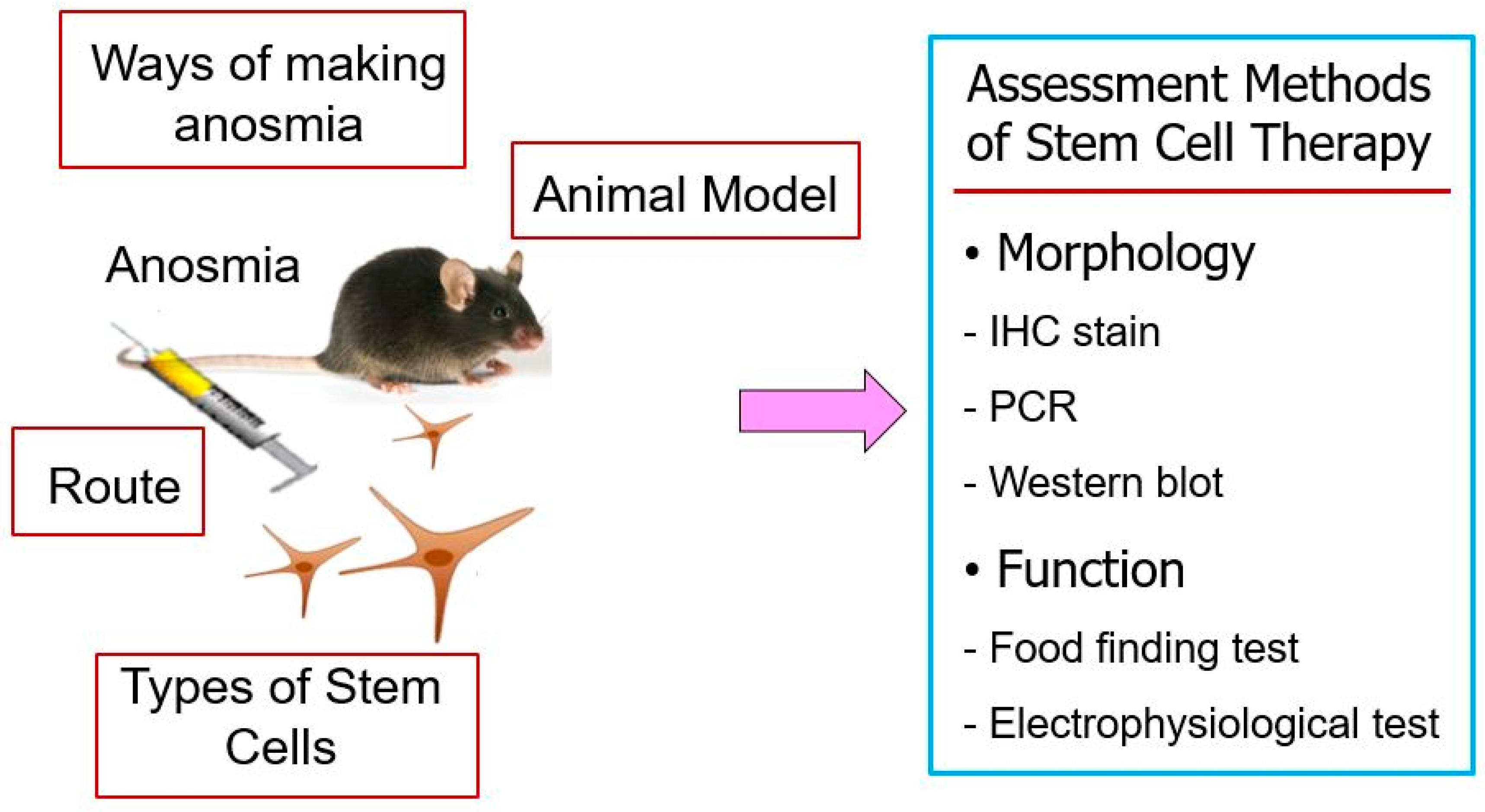
4.3.1. Animal Model of Olfactory Dysfunction
4.3.2. Stem Cell Sources and Characteristics
4.3.3. Administration Routes of Stem Cells and Timing
4.3.4. Functional and Histological Outcomes of Stem Cells
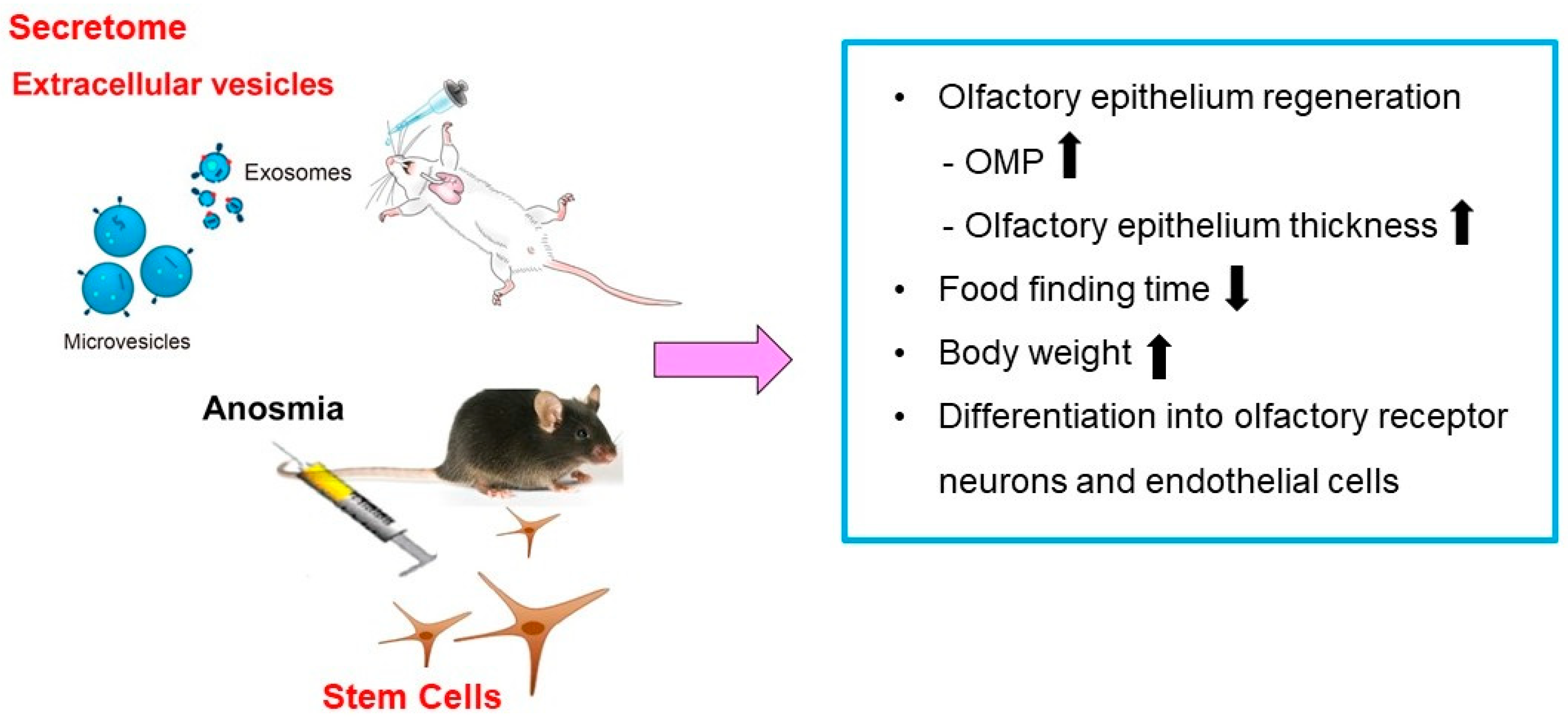
5. MSCs-Derived Secretome and Extracellular Vesicles as Cell-Free Therapeutic Strategy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, A.; Kumar, R.; Aier, I.; Semwal, R.; Tyagi, P.; Varadwaj, P. Sense of smell: Structural, functional, mechanistic advancements and challenges in human olfactory research. Curr. Neuropharmacol. 2019, 17, 891–911. [Google Scholar] [CrossRef]
- Boesveldt, S.; Parma, V. The importance of olfactory system in human well-being, through nutrition and social behavior. Cell Tissue Res. 2021, 383, 559–567. [Google Scholar] [CrossRef]
- Desai, M.; Oppenheimer, J. The importance of considering olfactory dysfunction during the COVID-19 pandemic and in clinical practice. J. Allergy Clin. Immunol. Pract. 2021, 9, 7–12. [Google Scholar] [CrossRef]
- Schäfer, L.; Schriever, V.A.; Croy, I. Human olfactory dysfunction: Causes and consequences. Cell Tissue Res. 2021, 383, 569–579. [Google Scholar] [CrossRef]
- Kondo, K.; Kikuta, S.; Ueha, R.; Suzukawa, K.; Yamasoba, T. Age-related olfactory dysfunction: Epidemiology, pathophysiology, and clinical management. Front. Aging Neurosci. 2020, 12, 208. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Holbrook, E.H. Therapies for olfactory dysfunction—An update. Curr. Allergy Asthma Rep. 2022, 22, 21–28. [Google Scholar] [CrossRef]
- Brann, J.H.; Firestein, S.J. A lifetime of neurogenesis in the olfactory system. Front. Neurosci. 2014, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Liang, F. Sustentacular cell enwrapment of olfactory receptor neuronal dendrites: An update. Genes 2020, 11, 493. [Google Scholar] [CrossRef]
- Kikuta, S.; Nagayama, S.; Hasegawa-Ishii, S. Structures and functions of the normal and injured human olfactory epithelium. Front. Neural Circuits 2024, 18, 1406218. [Google Scholar] [CrossRef]
- Kang, Y.J.; Jang, D.W.; Kim, D.H. Stem cell niches for olfactory regeneration and their therapeutic applications. J. Rhinol. 2025, 32, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Louie, J.D.; Bromberg, B.H.; Zunitch, M.J.; Schwob, J.E. Horizontal basal cells self-govern their neurogenic potential during injury-induced regeneration of the olfactory epithelium. Development 2023, 150, dev201552. [Google Scholar] [CrossRef]
- Li, X.; Tong, M.; Wang, L.; Qin, Y.; Yu, H.; Yu, Y. Age-dependent activation and neuronal differentiation of Lgr5+ basal cells in injured olfactory epithelium via notch signaling pathway. Front. Aging Neurosci. 2020, 12, 602688. [Google Scholar] [CrossRef]
- Packard, A.I.; Lin, B.; Schwob, J.E. Sox2 and Pax6 play counteracting roles in regulating neurogenesis within the murine olfactory epithelium. PLoS ONE 2016, 11, e0155167. [Google Scholar] [CrossRef]
- Maresh, A.; Rodriguez Gil, D.; Whitman, M.C.; Greer, C.A. Principles of glomerular organization in the human olfactory bulb—implications for odor processing. PLoS ONE 2008, 3, e2640. [Google Scholar] [CrossRef] [PubMed]
- Noto, T.; Zhou, G.; Yang, Q.; Lane, G.; Zelano, C. Human primary olfactory amygdala subregions form distinct functional networks, suggesting distinct olfactory functions. Front. Syst. Neurosci. 2021, 15, 752320. [Google Scholar] [CrossRef] [PubMed]
- Merrick, C.; Godwin, C.A.; Geisler, M.W.; Morsella, E. The olfactory system as the gateway to the neural correlates of consciousness. Front. Psychol. 2014, 4, 1011. [Google Scholar] [CrossRef]
- Bergström, U.; Giovanetti, A.; Piras, E.; Brittebo, E.B. Methimazole-induced damage in the olfactory mucosa: Effects on ultrastructure and glutathione levels. Toxicol. Pathol. 2003, 31, 379–387. [Google Scholar] [CrossRef]
- Sakamoto, T.; Kondo, K.; Kashio, A.; Suzukawa, K.; Yamasoba, T. Methimazole-induced cell death in rat olfactory receptor neurons occurs via apoptosis triggered through mitochondrial cytochrome c-mediated caspase-3 activation pathway. J. Neurosci. Res. 2007, 85, 548–557. [Google Scholar] [CrossRef]
- McBride, K.; Slotnick, B.; Margolis, F.L. Does intranasal application of zinc sulfate produce anosmia in the mouse? An olfactometric and anatomical study. Chem. Senses 2003, 28, 659–670. [Google Scholar] [CrossRef]
- Xie, F.; Fang, C.; Schnittke, N.; Schwob, J.E.; Ding, X. Mechanisms of permanent loss of olfactory receptor neurons induced by the herbicide 2,6-dichlorobenzonitrile: Effects on stem cells and noninvolvement of acute induction of the inflammatory cytokine IL-6. Toxicol. Appl. Pharmacol. 2013, 272, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, J.H.; Dhong, H.-J.; Kim, K.R.; Chung, S.-K.; Chung, S.-C.; Kang, J.M.; Jung, Y.G.; Jang, S.Y.; Hong, S.D. Effects of statins on the recovery of olfactory function in a 3-methylindole-induced anosmia mouse model. Am. J. Rhinol. Allergy 2012, 26, e81-4. [Google Scholar] [CrossRef]
- Coppola, D.M.; Parrish Waters, R. The olfactory bulbectomy disease model: A re-evaluation. Physiol. Behav. 2021, 240, 113548. [Google Scholar] [CrossRef] [PubMed]
- Anders, J.J.; Johnson, J.A. Transection of the rat olfactory nerve increases glial fibrillary acidic protein immunoreactivity from the olfactory bulb to the piriform cortex. Glia 1990, 3, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.D.; Margolis, F.L. Cell suspensions from rat olfactory neuroepithelium: Biochemical and histochemical characterization. Brain Res. 1979, 161, 277–291. [Google Scholar] [CrossRef]
- Lane, A.P.; Zhao, H.; Reed, R.R. Development of transgenic mouse models for the study of human olfactory dysfunction. Am. J. Rhinol. 2005, 19, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, M.; Im, S.K.; Fang, S. Mouse Cre-LoxP system: General principles to determine tissue-specific roles of target genes. Lab. Anim. Res. 2018, 34, 147–159. [Google Scholar] [CrossRef]
- Ahn, J.M.; Lee, C.H.; Kim, D.Y.; Rhee, C.S.; Min, Y.G.; Kim, J.W. Maintenance of regional difference in cellular composition of neurospheres derived from adult mouse olfactory bulb. Eur. Arch. Otorhinolaryngol. 2008, 265, 429–434. [Google Scholar] [CrossRef]
- Glezer, I.; Bruni-Cardoso, A.; Schechtman, D.; Malnic, B. Viral infection and smell loss: The case of COVID-19. J. Neurochem. 2021, 157, 930–943. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, J.; He, Q.; Li, R.-T.; Yang, G.; Zhang, Y.; Wu, S.-J.; Chen, Q.; Shi, J.-H.; Zhang, R.-R.; et al. SARS-CoV-2 infection in the mouse olfactory system. Cell Discov. 2021, 7, 49. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, H.; Xi, Z.; Huang, J.; Nie, J.; Zhou, B.; Deng, Y.; Tao, Z. Establishment of a mouse model of lipopolysaccharide-induced neutrophilic nasal polyps. Exp. Ther. Med. 2017, 14, 5275–5282. [Google Scholar] [CrossRef]
- Gan, S.; Qu, S.; Zhu, H.; Gong, M.; Xiang, Y.; Ye, D. Role and mechanism of olfactory stem cells in the treatment of olfactory disorders. Stem Cells Int. 2025, 2025, 6631857. [Google Scholar] [CrossRef]
- Chen, M.; Tian, S.; Yang, X.; Lane, A.P.; Reed, R.R.; Liu, H. Wnt-responsive Lgr5+ globose basal cells function as multipotent olfactory epithelium progenitor cells. J. Neurosci. 2014, 34, 8268–8276. [Google Scholar] [CrossRef] [PubMed]
- Schnittke, N.; Herrick, D.B.; Lin, B.; Peterson, J.; Coleman, J.H.; Packard, A.I.; Jang, W.; Schwob, J.E. Transcription factor p63 controls the reserve status but not the stemness of horizontal basal cells in the olfactory epithelium. Proc. Natl. Acad. Sci. USA 2015, 112, E5068-77. [Google Scholar] [CrossRef]
- Schwob, J.E.; Jang, W.; Holbrook, E.H.; Lin, B.; Herrick, D.B.; Peterson, J.N.; Coleman, J.H. Stem and progenitor cells of the mammalian olfactory epithelium: Taking poietic license. J. Comp. Neurol. 2017, 525, 1034–1054. [Google Scholar] [CrossRef]
- Gadye, L.; Das, D.; Sanchez, M.A.; Street, K.; Baudhuin, A.; Wagner, A.; Cole, M.B.; Choi, Y.G.; Yosef, N.; Purdom, E.; et al. Injury activates transient olfactory stem cell states with diverse lineage capacities. Cell Stem Cell 2017, 21, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Duan, C.; Ren, W.; Li, F.; Zheng, Q.; Wang, L.; Li, W.; Lu, X.; Ni, W.; Zhang, Y.; et al. Notch signaling regulates Lgr5+ olfactory epithelium progenitor/stem cell turnover and mediates recovery of lesioned olfactory epithelium in mouse model. Stem Cells 2018, 36, 1259–1272. [Google Scholar] [CrossRef]
- Ramakrishna, K.; Nalla, L.V.; Naresh, D.; Venkateswarlu, K.; Viswanadh, M.K.; Nalluri, B.N.; Chakravarthy, G.; Duguluri, S.; Singh, P.; Rai, S.N.; et al. WNT-β catenin signaling as a potential therapeutic target for neurodegenerative diseases: Current status and future perspective. Diseases 2023, 11, 89. [Google Scholar] [CrossRef]
- Ito, A.; Miller, C.; Imamura, F. Suppression of BMP signaling restores mitral cell development impaired by FGF signaling deficits in mouse olfactory bulb. Mol. Cell Neurosci. 2024, 128, 103913. [Google Scholar] [CrossRef]
- Ziegler, A.N.; Levison, S.W.; Wood, T.L. Insulin and IGF receptor signaling in neural-stem cell homeostasis. Nat. Rev. Endocrinol. 2015, 11, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Torabi, A.; Mohammadbagheri, E.; Akbari Dilmaghani, N.; Bayat, A.-H.; Fathi, M.; Vakili, K.; Alizadeh, R.; Rezaeimirghaed, O.; Hajiesmaeili, M.; Ramezani, M.; et al. Proinflammatory cytokines in the olfactory mucosa result in COVID-19 induced anosmia. ACS Chem. Neurosci. 2022, 11, 1909–1913. [Google Scholar] [CrossRef]
- Novoseletskaya, E.S.; Evdokimov, P.V.; Efimenko, A.Y. Extracellular matrix-induced signaling pathways in mesenchymal stem/stromal cells. Cell Commun. Signal 2023, 21, 244. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.D.; Cho, K.S. Immunomodulatory effects of mesenchymal stem cell-derived extracellular vesicles in allergic airway disease. Life 2022, 12, 1994. [Google Scholar] [CrossRef]
- Tuan, R.S.; Boland, G.; Tuli, R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res. Ther. 2003, 5, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Kim, Y.M.; Choi, Y.S.; Choi, J.W.; Park, Y.H.; Koo, B.S.; Roh, H.; Rha, K. Effects of systemic transplantation of adipose tissue-derived stem cells on olfactory epithelium regeneration. Laryngoscope 2009, 119, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, V.; Bettini, S.; Pifferi, S.; Rosellini, A.; Menini, A.; Saccardi, R.; Ognio, E.; Jeffery, R.; Poulsom, R.; Revoltella, R.P. Human cord blood CD133+ stem cells transplanted to nod-scid mice provide conditions for regeneration of olfactory neuroepithelium after permanent damage induced by dichlobenil. Stem Cells 2009, 27, 825–835. [Google Scholar] [CrossRef]
- Lee, C.H.; Jeon, S.-W.; Seo, B.S.; Mo, J.-H.; Jeon, E.-H.; Choi, A.-R.; Kim, J.-W. Transplantation of neural stem cells in anosmic mice. Clin. Exp. Otorhinolaryngol. 2010, 3, 84–90. [Google Scholar] [CrossRef]
- Jo, H.; Jung, M.; Seo, D.J.; Park, D.J. The effect of rat bone marrow derived mesenchymal stem cells transplantation for restoration of olfactory disorder. Biochem. Biophys. Res. Commun. 2015, 467, 395–399. [Google Scholar] [CrossRef]
- Hazir, B.; Ceylan, A.; Bagariack, E.Ü.; Dayanir, D.; Araz, M.; Ceylan, B.T.; Oruklu, N.; Sahin, M.M. Effect of intranasal neural stem cells transplantation on olfactory epithelium regeneration in an anosmia-induced mouse model. Sci. Rep. 2025, 15, 17015. [Google Scholar] [CrossRef]
- Basu, J.; Ludlow, J.W. Exosomes for repair, regeneration and rejuvenation. Expert. Opin. Biol. Ther. 2016, 16, 489–506. [Google Scholar] [CrossRef]
- Drago, D.; Cossetti, C.; Iraci, N.; Gaude, E.; Musco, G.; Bachi, A.; Pluchino, S. The stem cell secretome and its role in brain repair. Biochimie 2013, 95, 2271–2285. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Liu, Z.; Wang, X.; Shang, X.; Cui, Y.; Zhang, Z.G.; Chopp, M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells 2013, 31, 2737–2746. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.J.; Kang, S.A.; Park, H.K.; Yu, H.S.; Cho, K.S.; Roh, H.J. Intranasally administrated extracellular vesicles from adipose stem cells have immunomodulatory effects in a mouse model of asthma. Stem Cells Int. 2021, 2021, 6686625. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Kang, S.A.; Park, J.H.; Kim, S.D.; Yu, H.S.; Mun, S.J.; Cho, K.S. Immunomodulatory effect of adipose stem cell-derived extra-cellular vesicles on cytokine expression and regulatory T cells in patients with asthma. Int. J. Mol. Sci. 2024, 25, 10524. [Google Scholar] [CrossRef]
- Jung, J.H.; Kang, S.A.; Park, J.H.; Kim, S.D.; Yu, H.S.; Mun, S.J.; Cho, K.S. Paraoxonase-1 is a pivotal regulator responsible for suppressing allergic airway inflammation through adipose stem cell-derived extracellular vesicles. Int. J. Mol. Sci. 2024, 25, 12756. [Google Scholar] [CrossRef]
- Yavuz, B.; Mutlu, E.C.; Ahmed, Z.; Ben-Nissan, B.; Stamboulis, A. Applications of stem cell-derived extracellular vesicles in nerve regeneration. Int. J. Mol. Sci. 2024, 25, 5863. [Google Scholar] [CrossRef]
- Alvites, R.D.; Branquinho, M.V.; Sousa, A.C.; Lopes, B.; Sousa, P.; Prada, J.; Pires, I.; Ronchi, G.; Raimondo, S.; Luís, A.L.; et al. Effects of olfactory mucosa stem/stromal cell and olfactory ensheating cells secretome on peripheral nerve regeneration. Biomolecules 2022, 12, 818. [Google Scholar] [CrossRef]
| Agent | Route | Target Cells | Mechanism of Injury | Regeneration Potential | Notable Features |
|---|---|---|---|---|---|
| Methimazole | IP | OSNs, basal cells | Apoptosis via oxidative stress | High | Consistent, reversible, OE-specific |
| Zinc sulfate | IN | Epithelial cells | Necrosis via oxidative damage | Variable | Inconsistent damage, affects other nasal tissues |
| Dichlobenil | IN | Sustentacular cells | Disruption of cell structure | Moderate | Targets non-neuronal cells, less widely used |
| 3-Methylindole | IP | OSNs, basal cells, Clara cells | Reactive metabolites, inflammation | Moderate to high | Severe acute epithelial damage |
| Study (Year) | Animal Model | Stem Cell | Delivery Route | Outcome Measures | Key Findings |
|---|---|---|---|---|---|
| Kim et al. (2009) [45] | Rats, Nerve transection | ASCs | IV | Histology (OMP, PCNA) | Partial structural recovery, differentiation into OE neurons and endothelial cells |
| Franceschini et al. (2009) [46] | Mice, Dichlobenil | HSCs | IV | Histology, FISH chimerism analysis, electro-olfactogram | Partial structural and functional recovery |
| Lee at al. (2010) [47] | Mice, 3-methylindole | NSCs | Transnasal | Food-finding test, histology (OMP) | Improved survival and faster olfactory recovery |
| Jo et al. (2015) [48] | Rats, Triton X-100 | BMSCs | Transnasal | Food-finding test, histology (OMP) | Increased NGF and BDNF expression, improved OE thickness |
| Hazir et al. (2025) [49] | Mice, 3-methylindole | NSCs | Intranasal | Food-finding test, Histology (OE thickness, OMP+ neurons) | Behavioral improvement and epithelial regeneration |
| Potential Study Model | Secretome/EVs | Delivery Route | Expected Outcome Measures | Rationale/Precedent |
|---|---|---|---|---|
| 3-MI anosmia | Olfactory MSC-CM | Intranasal | Restore OMP+ neuron density, reduce epithelial thinning, improved FFT | Based on Alvites et al. (2022) [58] olfactory MSC CM in peripheral nerve repair |
| 3-MI anosmia | NSC-derived exosomes | Intranasal | Modulate inflammation, promote basal cell proliferation, accelerate OSN restoration | Builds on Yavuz et al. (2024) [57] nerve EV regenerative mechanisms |
| Methimazole anosmia | miRNA-enriched EVs (miR-124, miR-133b) | Intranasal | Increase neurogenic miRNA expression, new synaptic markers, behavioral recovery | Based on Xin et al. (2013) [53] miRNA transfer in neural cells |
| Approach | Efficacy | Safety | Scalability | Translational Challenges |
|---|---|---|---|---|
| Endogenous regeneration (HBCs, GBCs) | Effective in mild to moderate injury; limited under aging or chronic inflammation | High (native cell source) | Intrinsic but declines with age/disease | Insufficient in severe or irreversible damage |
| Exogenous stem cell transplantation | Promotes OE thickening, OSN recovery, functional improvement in animal models | Risk of immune rejection, poor engraftment, tumorigenesis | Complex cell isolation, expansion, and quality control | Long-term neuronal integration uncertain; regulatory and ethical hurdles |
| Secretome/EV-based therapy | Comparable regenerative effects via paracrine cargo; enhances neurogenesis and reduces inflammation | High (cell-free, low immune risk) | Amenable to standardization, storage, and off-the-shelf use | Identification of key functional cargo; optimization of intranasal delivery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, K.-I.; Park, J.-H.; Kim, S.-D.; Mun, S.J.; Cho, K.-S. Stem Cells and Cell-Free Therapies for Olfactory Epithelium Regeneration: Insights from Experimental Models. Int. J. Mol. Sci. 2025, 26, 9024. https://doi.org/10.3390/ijms26189024
Yi K-I, Park J-H, Kim S-D, Mun SJ, Cho K-S. Stem Cells and Cell-Free Therapies for Olfactory Epithelium Regeneration: Insights from Experimental Models. International Journal of Molecular Sciences. 2025; 26(18):9024. https://doi.org/10.3390/ijms26189024
Chicago/Turabian StyleYi, Keun-Ik, Ji-Hwan Park, Sung-Dong Kim, Sue Jean Mun, and Kyu-Sup Cho. 2025. "Stem Cells and Cell-Free Therapies for Olfactory Epithelium Regeneration: Insights from Experimental Models" International Journal of Molecular Sciences 26, no. 18: 9024. https://doi.org/10.3390/ijms26189024
APA StyleYi, K.-I., Park, J.-H., Kim, S.-D., Mun, S. J., & Cho, K.-S. (2025). Stem Cells and Cell-Free Therapies for Olfactory Epithelium Regeneration: Insights from Experimental Models. International Journal of Molecular Sciences, 26(18), 9024. https://doi.org/10.3390/ijms26189024








