Relevance of STIM/Orai Calcium Entry System Hyperactivation in Human Prostate Contractility in Benign Prostate Hyperplasia
Abstract
1. Introduction
2. Results
2.1. Study Subjects
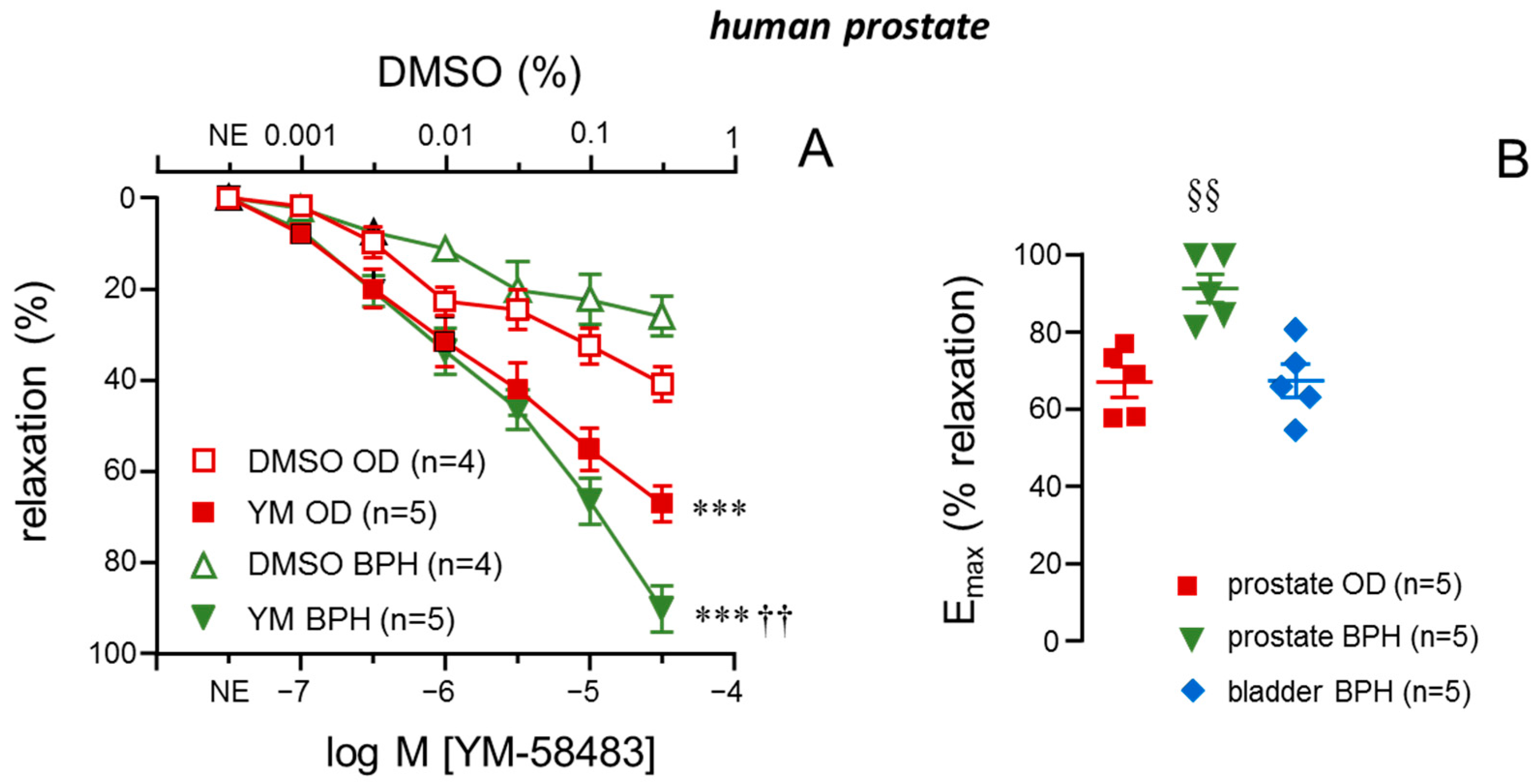
2.2. Inhibitor of STIM/Orai, YM-58483, Causes More Effective Relaxations in Prostate Strips from Patients with BPH
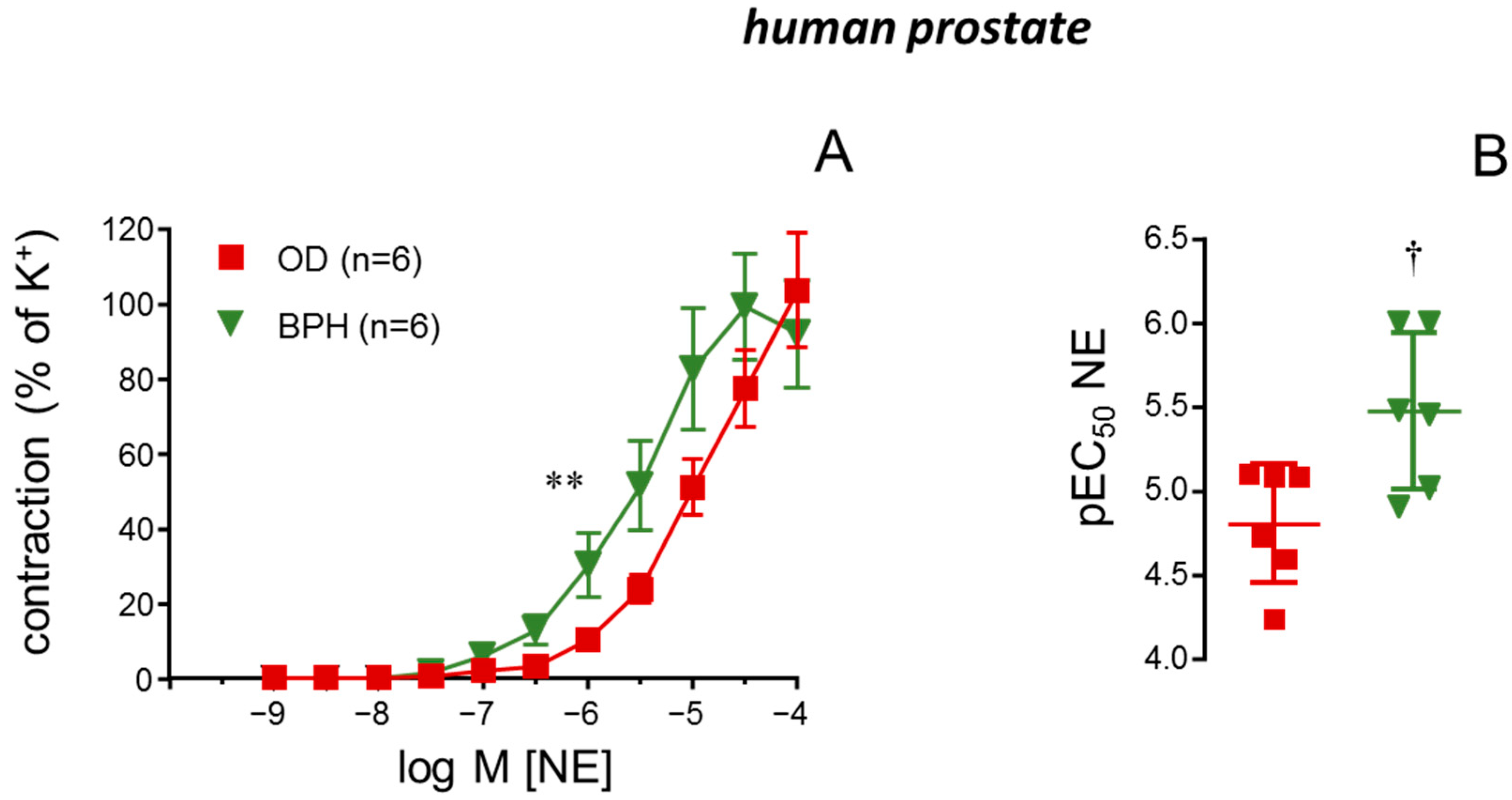
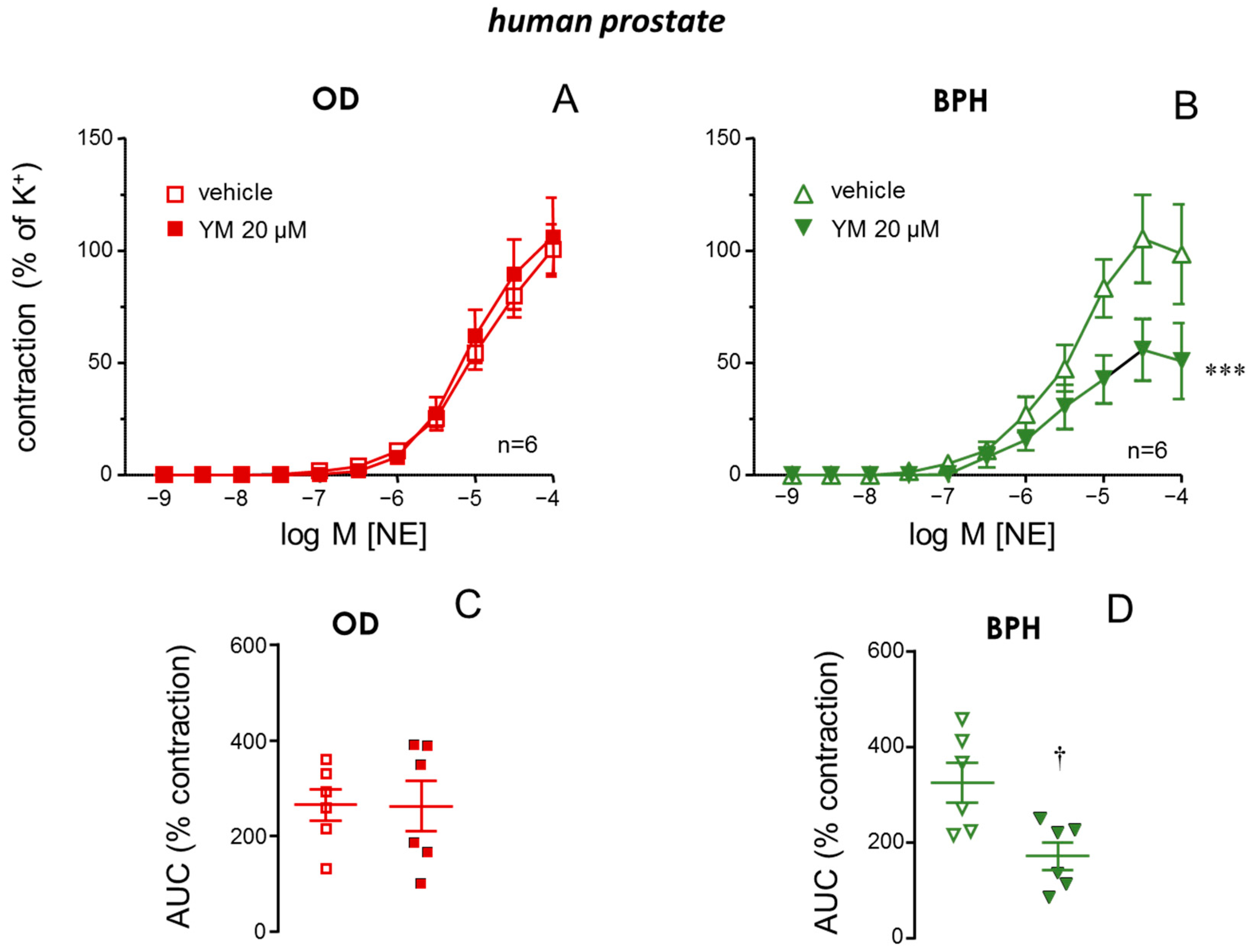
2.3. STIM/Orai Inhibition Reduced NE-Induced Contractions in Prostate from BPH Patients
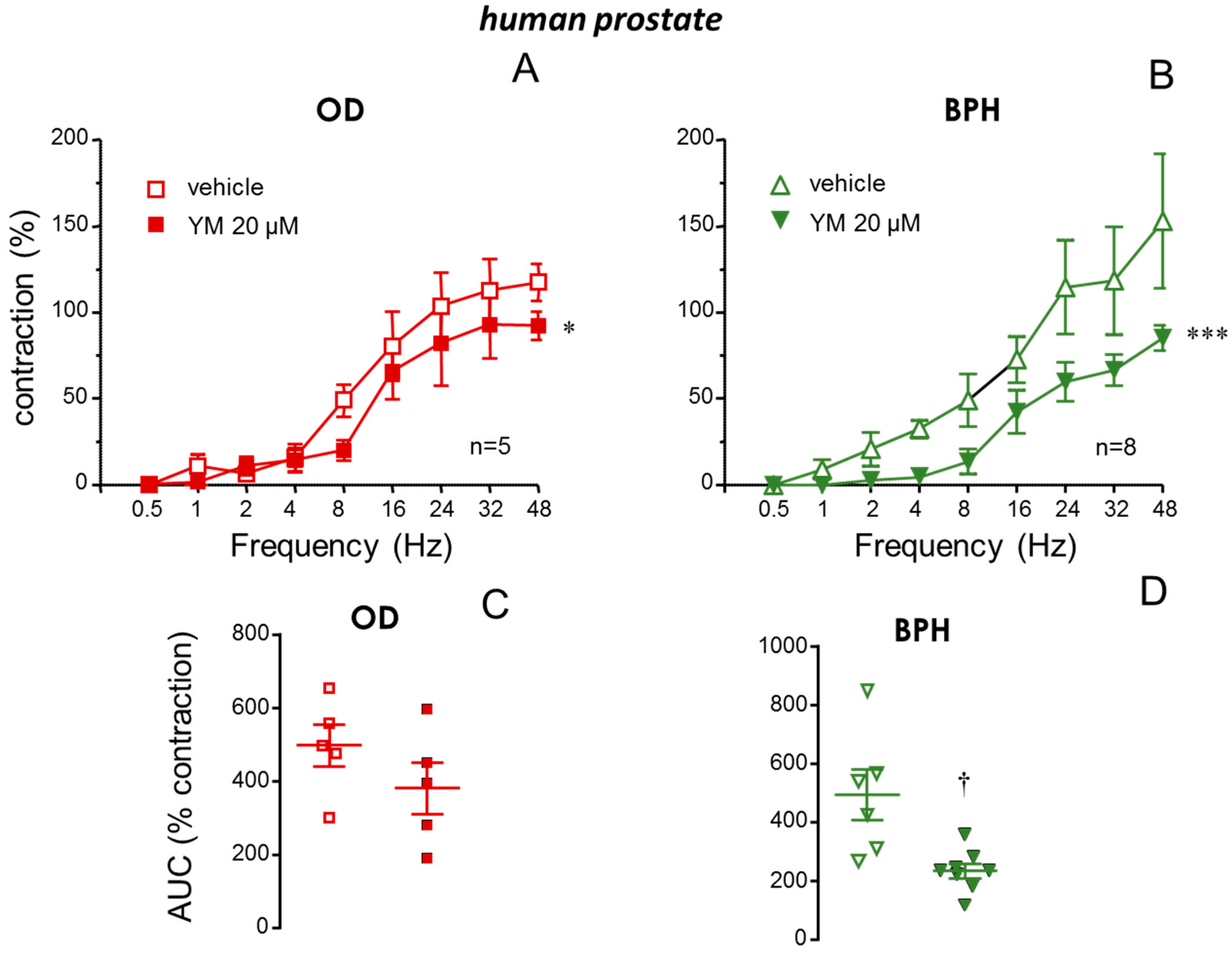
2.4. Neurogenic Contractions of the Prostate from BPH Patients Were Also Diminished by STIM/Orai Inhibition
2.5. STIM/Orai Inhibition Did Not Modify Adrenergic nor Neurogenic Contractile Responses in Bladder Neck from BPH Patients
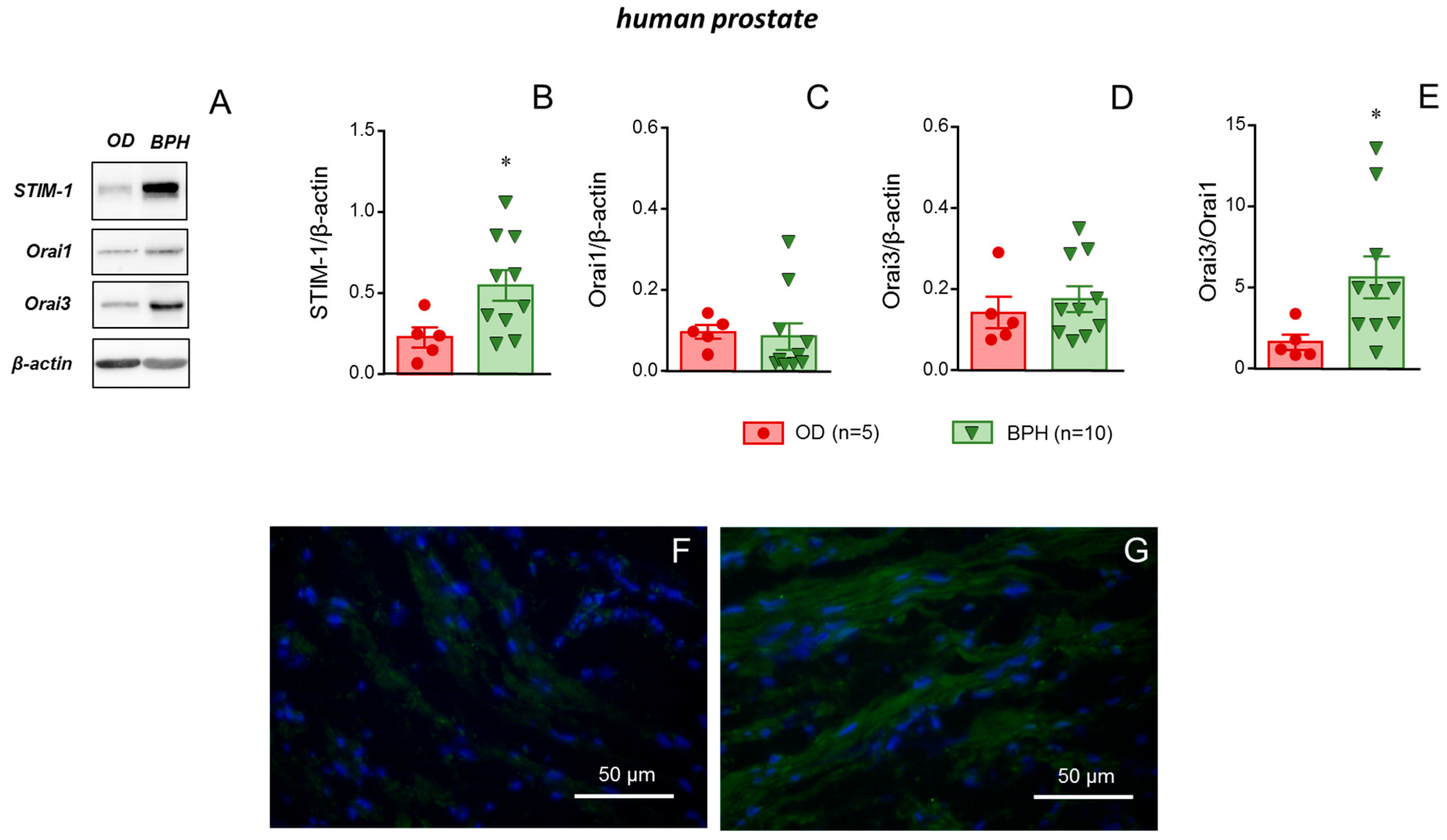
2.6. STIM-1 Was Upregulated in Prostate Tissue from Patients with BPH
3. Discussion
4. Materials and Methods
4.1. Human Tissues
4.2. Functional Evaluation of Tissues in Organ Bath
4.3. Western Blot Experiments
4.4. Immunofluorescence Assays
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| BPH | Benign prostate hyperplasia |
| DMSO | Dimethyl sulfoxide |
| Emax | Maximum response |
| HB | Human bladder |
| HP | Human prostate |
| LUTS | Lower urinary tract symptoms |
| NE | Norepinephrine |
| OD | Organ donor |
| PBS | Phosphate-buffered saline |
| pEC50 | −log M of the concentration required for 50% of contraction |
| SOCE | Store-operated calcium entry |
| STIM-1 | Stromal interaction protein-1 |
| YM | YM-58483 |
References
- Soler, R.; Andersson, K.E.; Chancellor, M.B.; Chapple, C.R.; de Groat, W.C.; Drake, M.J.; Gratzke, C.; Lee, R.; Cruz, F. Future direction in pharmacotherapy for non-neurogenic male lower urinary tract symptoms. Eur. Urol. 2013, 64, 610–621. [Google Scholar] [CrossRef]
- Madersbacher, S.; Sampson, N.; Culig, Z. Pathophysiology of Benign Prostatic Hyperplasia and Benign Prostatic Enlargement: A Mini-Review. Gerontology 2019, 65, 458–464. [Google Scholar] [CrossRef]
- Roehrborn, C.G. Benign prostatic hyperplasia: An overview. Rev. Urol. 2005, 7 (Suppl. 9), S3–S14. [Google Scholar]
- Eckhardt, M.D.; van Venrooij, G.E.; Boon, T.A. Symptoms and quality of life versus age, prostate volume, and urodynamic parameters in 565 strictly selected men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Urology 2001, 57, 695–700. [Google Scholar] [CrossRef]
- Eckhardt, M.D.; van Venrooij, G.E.; Boon, T.A. Symptoms, prostate volume, and urodynamic findings in elderly male volunteers without and with LUTS and in patients with LUTS suggestive of benign prostatic hyperplasia. Urology 2001, 58, 966–971. [Google Scholar] [CrossRef]
- Lepor, H. Alpha blockers for the treatment of benign prostatic hyperplasia. Rev. Urol. 2007, 9, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, E.; Hartanto, V.; Lepor, H. The response to alpha blockade in benign prostatic hyperplasia is related to the percent area density of prostate smooth muscle. Prostate 1992, 21, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Rigatti, P.; Brausi, M.; Scarpa, R.M.; Porru, D.; Schumacher, H.; Rizzi, C.A.; MICTUS Study Group. A comparison of the efficacy and tolerability of tamsulosin and finasteride in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2003, 6, 315–323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hasan, M.; Parveen, F.; Shamsuzzaman, A.K.; Kibria, M.D. Comparison of efficacy between Tamsulosin and Finasteride on symptomatic Benign Prostatic Hyperplasia. Mymensingh Med. J. 2007, 16, 154–159. [Google Scholar][Green Version]
- Ventura, S.; Oliver, V.I.; White, C.W.; Xie, J.H.; Haynes, J.M.; Exintaris, B. Novel drug targets for the pharmacotherapy of benign prostatic hyperplasia (BPH). Br. J. Pharmacol. 2011, 163, 891–907. [Google Scholar] [CrossRef]
- Yu, Z.J.; Yan, H.L.; Xu, F.H.; Chao, H.C.; Deng, L.H.; Xu, X.D.; Huang, J.B.; Zeng, T. Efficacy and Side Effects of Drugs Commonly Used for the Treatment of Lower Urinary Tract Symptoms Associated with Benign Prostatic Hyperplasia. Front. Pharmacol. 2020, 11, 658. [Google Scholar] [CrossRef] [PubMed]
- Müderrisoglu, A.E.; de la Rosette, J.J.M.C.H.; Michel, M.C. Potential side effects of currently available pharmacotherapies in male lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Expert. Opin. Drug Saf. 2023, 22, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Collins, H.E.; Zhang, D.; Chatham, J.C. STIM and Orai Mediated Regulation of Calcium Signaling in Age-Related Diseases. Front. Aging 2022, 3, 876785. [Google Scholar] [CrossRef] [PubMed]
- Kodakandla, G.; Akimzhanov, A.M.; Boehning, D. Regulatory mechanisms controlling store-operated calcium entry. Front. Physiol. 2023, 14, 1330259. [Google Scholar] [CrossRef]
- El Assar, M.; García-Rojo, E.; Sevilleja-Ortiz, A.; Sánchez-Ferrer, A.; Fernández, A.; García-Gómez, B.; Romero-Otero, J.; Rodríguez-Mañas, L.; Angulo, J. Functional Role of STIM-1 and Orai1 in Human Microvascular Aging. Cells 2022, 11, 3675. [Google Scholar] [CrossRef]
- Sevilleja-Ortiz, A.; El Assar, M.; García-Rojo, E.; García-Gómez, B.; Fernández, A.; Sánchez-Ferrer, A.; La Fuente, J.M.; Romero-Otero, J.; Rodríguez-Mañas, L.; Angulo, J. Ageing-induced hypercontractility is related to functional enhancement of STIM/Orai and upregulation of Orai 3 in rat and human penile tissue. Mech. Ageing Dev. 2021, 200, 111590. [Google Scholar] [CrossRef]
- Sevilleja-Ortiz, A.; El Assar, M.; García-Gómez, B.; La Fuente, J.M.; Alonso-Isa, M.; Romero-Otero, J.; Martínez-Salamanca, J.I.; Fernández, A.; Rodríguez-Mañas, L.; Angulo, J. STIM/Orai Inhibition as a Strategy for Alleviating Diabetic Erectile Dysfunction Through Modulation of Rat and Human Penile Tissue Contractility and in vivo Potentiation of Erectile Responses. J. Sex. Med. 2022, 19, 1733–1749. [Google Scholar] [CrossRef]
- Ishikawa, J.; Ohga, K.; Yoshino, T.; Takezawa, R.; Ichikawa, A.; Kubota, H.; Yamada, T. A pyrazole derivative, YM-58483, potently inhibits store-operated sustained Ca2+ influx and IL-2 production in T lymphocytes. J. Immunol. 2003, 170, 4441–4449. [Google Scholar] [CrossRef]
- Ohga, K.; Takezawa, R.; Yoshino, T.; Yamada, T.; Shimizu, Y.; Ishikawa, J. The suppressive effects of YM-58483/BTP-2, a store-operated Ca2+ entry blocker, on inflammatory mediator release in vitro and airway responses in vivo. Pulm. Pharmacol. Ther. 2008, 21, 360–369. [Google Scholar] [CrossRef]
- Masson, B.; Le Ribeuz, H.; Sabourin, J.; Laubry, L.; Woodhouse, E.; Foster, R.; Ruchon, Y.; Dutheil, M.; Boët, A.; Ghigna, M.R.; et al. Orai1 Inhibitors as Potential Treatments for Pulmonary Arterial Hypertension. Circ. Res. 2022, 131, e102–e119. [Google Scholar] [CrossRef]
- Zhang, W.; Qi, Z.; Wang, Y. BTP2, a Store-Operated Calcium Channel Inhibitor, Attenuates Lung Ischemia-Reperfusion Injury in Rats. Inflammation 2017, 40, 778–787. [Google Scholar] [CrossRef]
- Miyoshi, M.; Liu, S.; Morizane, A.; Takemasa, E.; Suzuki, Y.; Kiyoi, T.; Maeyama, K.; Mogi, M. Efficacy of constant long-term delivery of YM-58483 for the treatment of rheumatoid arthritis. Eur. J. Pharmacol. 2018, 824, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Nehme, A.; Ghahramanpouri, M.; Ahmed, I.; Golsorkhi, M.; Thomas, N.; Munoz, K.; Abdipour, A.; Tang, X.; Wilson, S.M.; Wasnik, S.; et al. Combination therapy of insulin-like growth factor I and BTP-2 markedly improves lipopolysaccharide-induced liver injury in mice. FASEB J. 2022, 36, e22444. [Google Scholar] [CrossRef] [PubMed]
- Ardura, J.A.; Álvarez-Carrión, L.; Gutiérrez-Rojas, I.; Alonso, V. Role of Calcium Signaling in Prostate Cancer Progression: Effects on Cancer Hallmarks and Bone Metastatic Mechanisms. Cancers 2020, 12, 1071. [Google Scholar] [CrossRef] [PubMed]
- Daba, M.Y.; Fan, Z.; Li, Q.; Yuan, X.; Liu, B. The role of calcium channels in prostate cancer progression and potential as a druggable target for prostate cancer treatment. Crit. Rev. Oncol. Hematol. 2023, 186, 104014. [Google Scholar] [CrossRef]
- Shan, S.; Su, M. The role of RhoA-ROCK signaling in benign prostatic hyperplasia: A review. Hum. Cell 2025, 38, 48. [Google Scholar] [CrossRef]
- Angulo, J.; Cuevas, P.; Fernández, A.; La Fuente, J.M.; Allona, A.; Moncada, I.; Sáenz de Tejada, I. Tadalafil enhances the inhibitory effects of tamsulosin on neurogenic contractions of human prostate and bladder neck. J. Sex. Med. 2012, 9, 2293–2306. [Google Scholar] [CrossRef]
- Huang, R.; Liu, Y.; Hu, S.; Tamalunas, A.; Waidelich, R.; Strittmatter, F.; Stief, C.G.; Hennenberg, M. Inhibition of α1-Adrenergic, Non-Adrenergic and Neurogenic Human Prostate Smooth Muscle Contraction and of Stromal Cell Growth by the Isoflavones Genistein and Daidzein. Nutrients 2022, 14, 4943. [Google Scholar] [CrossRef]
- Wang, R.; Huang, R.; Liu, Y.; Tamalunas, A.; Stief, C.G.; Hennenberg, M. Silencing of CDC42 inhibits contraction and growth-related functions in prostate stromal cells, which is mimicked by ML141. Life Sci. 2023, 329, 121928. [Google Scholar] [CrossRef]
- Bester, B.; Koslowa, K.; Gronau, A.C.; Mietens, A.; Nowell, C.; Whittaker, M.R.; Pilatz, A.; Wagenlehner, F.; Exintaris, B.; Middendorff, R. The oxytocin antagonist cligosiban reduces human prostate contractility: Implications for the treatment of benign prostatic hyperplasia. Br. J. Pharmacol. 2024, 181, 2869–2885. [Google Scholar] [CrossRef]
- Hu, S.; Müderrisoglu, A.E.; Ciotkowska, A.; Kale, O.; Keller, P.; Schott, M.; Tamalunas, A.; Waidelich, R.; Stief, C.G.; Hennenberg, M. Effects of carvedilol on human prostate tissue contractility and stromal cell growth pointing to potential clinical implications. Pharmacol. Rep. 2024, 76, 807–822. [Google Scholar] [CrossRef]
- Michel, M.C.; Vrydag, W. Alpha1-, alpha2- and beta-adrenoceptors in the urinary bladder, urethra and prostate. Br. J. Pharmacol. 2006, 147 (Suppl. 2), S88–S119. [Google Scholar] [CrossRef]
- Luptak, J.; Kocmalova, M.; Franova, S.; Sutovsky, J.; Grendar, M.; Svihra, J.; Kliment, J., Sr.; Dusenka, R.; Sutovska, M. Involvement of calcium regulating ion channels in contractility of human isolated urinary bladder. Gen. Physiol. Biophys. 2018, 37, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.C.; Barendrecht, M.M. Physiological and pathological regulation of the autonomic control of urinary bladder contractility. Pharmacol. Ther. 2008, 117, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Souza Bomfim, G.H.; Mendez-Lopez, I.; Arranz-Tagarro, J.A.; Ferraz Carbonel, A.A.; Roman-Campos, D.; Padín, J.F.; Garcia, A.G.; Jurkiewicz, A.; Jurkiewicz, N.H. Functional Upregulation of STIM-1/Orai-1-Mediated Store-Operated Ca2+ Contributing to the Hypertension Development Elicited by Chronic EtOH Consumption. Curr. Vasc. Pharmacol. 2017, 15, 265–281. [Google Scholar] [CrossRef]
- Kim, J.H.; Hwang, K.H.; Oh, J.; Kim, S.E.; Lee, M.Y.; Lee, T.S.; Cha, S.K. Differential expression of ORAI channels and STIM proteins in renal cell carcinoma subtypes: Implications for metastasis and therapeutic targeting. Korean J. Physiol. Pharmacol. 2025, 29, 33–43. [Google Scholar] [CrossRef]
- Nguyen, A.; Sung, Y.; Lee, S.H.; Martin, C.E.; Srikanth, S.; Chen, W.; Kang, M.K.; Kim, R.H.; Park, N.H.; Gwack, Y.; et al. Orai3 Calcium Channel Contributes to Oral/Oropharyngeal Cancer Stemness through the Elevation of ID1 Expression. Cells 2023, 12, 2225. [Google Scholar] [CrossRef]
- Kouba, S.; Buscaglia, P.; Guéguinou, M.; Ibrahim, S.; Félix, R.; Guibon, R.; Fromont, G.; Pigat, N.; Capiod, T.; Vandier, C.; et al. Pivotal role of the ORAI3-STIM2 complex in the control of mitotic death and prostate cancer cell cycle progression. Cell. Calcium 2023, 115, 102794. [Google Scholar] [CrossRef]
- Wu, Q.; Fang, Y.; Huang, X.; Zheng, F.; Ma, S.; Zhang, X.; Han, T.; Gao, H.; Shen, B. Role of Orai3-Mediated Store-Operated Calcium Entry in Radiation-Induced Brain Microvascular Endothelial Cell Injury. Int. J. Mol. Sci. 2023, 24, 6818. [Google Scholar] [CrossRef]
- Yu, C.; Zhou, Z.; Huang, W.; Li, X.; Zou, F.; Meng, X.; Cai, S. Orai3 mediates Orai channel remodelling to activate fibroblast in pulmonary fibrosis. J. Cell. Mol. Med. 2022, 26, 4974–4985. [Google Scholar] [CrossRef]
- Zhang, X.; Xin, P.; Yoast, R.E.; Emrich, S.M.; Johnson, M.T.; Pathak, T.; Benson, J.C.; Azimi, I.; Gill, D.L.; Monteith, G.R.; et al. Distinct pharmacological profiles of ORAI1, ORAI2, and ORAI3 channels. Cell. Calcium 2020, 91, 102281. [Google Scholar] [CrossRef]
- Huang, Q.; Li, B.H.; Wang, Y.B.; Zi, H.; Zhang, Y.Y.; Li, F.; Fang, C.; Tang, S.D.; Jin, Y.H.; Huang, J.; et al. Clinical biomarker-based biological aging and risk of benign prostatic hyperplasia: A large prospective cohort study. Aging Med. (Milton) 2024, 7, 393–405. [Google Scholar] [CrossRef]
- Mancarella, S.; Potireddy, S.; Wang, Y.; Gao, H.; Gandhirajan, R.K.; Autieri, M.; Scalia, R.; Cheng, Z.; Wang, H.; Madesh, M.; et al. Targeted STIM deletion impairs calcium homeostasis, NFAT activation, and growth of smooth muscle. FASEB J. 2013, 27, 893–906. [Google Scholar] [CrossRef]
- La Fuente, J.M.; Fernández, A.; Cuevas, P.; González-Corrochano, R.; Chen, M.X.; Angulo, J. Stimulation of large-conductance calcium-activated potassium channels inhibits neurogenic contraction of human bladder from patients with urinary symptoms and reverses acetic acid-induced bladder hyperactivity in rats. Eur. J. Pharmacol. 2014, 735, 68–76. [Google Scholar] [CrossRef]
- Angulo, J.; El Assar, M.; Sevilleja-Ortiz, A.; Fernández, A.; Sánchez-Ferrer, A.; Romero-Otero, J.; Martínez-Salamanca, J.I.; La Fuente, J.M.; Rodríguez-Mañas, L. Short-term pharmacological activation of Nrf2 ameliorates vascular dysfunction in aged rats and in pathological human vasculature. A potential target for therapeutic intervention. Redox Biol. 2019, 26, 101271. [Google Scholar] [CrossRef]

| OD | BPH | p Value | |
|---|---|---|---|
| n | 6 | 20 | |
| Age (years) | 54.5 ± 18.7 | 70.2 ± 5.5 | 0.0436 |
| Diabetes (%) | 0 (0.0) | 5 (25.0) | 0.2981 |
| Dyslipidemia (%) | 0 (0.0) | 4 (20.0) | 0.5425 |
| Hypertension (%) | 1 (12.5) | 8 (40.0) | 0.3798 |
| Obesity (%) | 0 (0.0) | 0 (0.0) | 1.0000 |
| Smoking habit (%) | 0 (0.0) | 4 (20.0) | 0.3214 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Fuente, J.M.; El Assar, M.; Fernández, A.; Rodríguez-Mañas, L.; Angulo, J. Relevance of STIM/Orai Calcium Entry System Hyperactivation in Human Prostate Contractility in Benign Prostate Hyperplasia. Int. J. Mol. Sci. 2025, 26, 8985. https://doi.org/10.3390/ijms26188985
La Fuente JM, El Assar M, Fernández A, Rodríguez-Mañas L, Angulo J. Relevance of STIM/Orai Calcium Entry System Hyperactivation in Human Prostate Contractility in Benign Prostate Hyperplasia. International Journal of Molecular Sciences. 2025; 26(18):8985. https://doi.org/10.3390/ijms26188985
Chicago/Turabian StyleLa Fuente, José M., Mariam El Assar, Argentina Fernández, Leocadio Rodríguez-Mañas, and Javier Angulo. 2025. "Relevance of STIM/Orai Calcium Entry System Hyperactivation in Human Prostate Contractility in Benign Prostate Hyperplasia" International Journal of Molecular Sciences 26, no. 18: 8985. https://doi.org/10.3390/ijms26188985
APA StyleLa Fuente, J. M., El Assar, M., Fernández, A., Rodríguez-Mañas, L., & Angulo, J. (2025). Relevance of STIM/Orai Calcium Entry System Hyperactivation in Human Prostate Contractility in Benign Prostate Hyperplasia. International Journal of Molecular Sciences, 26(18), 8985. https://doi.org/10.3390/ijms26188985





