The Impacts of Dengue Virus Infection on Mitochondrial Functions and Dynamics
Abstract
1. Introduction
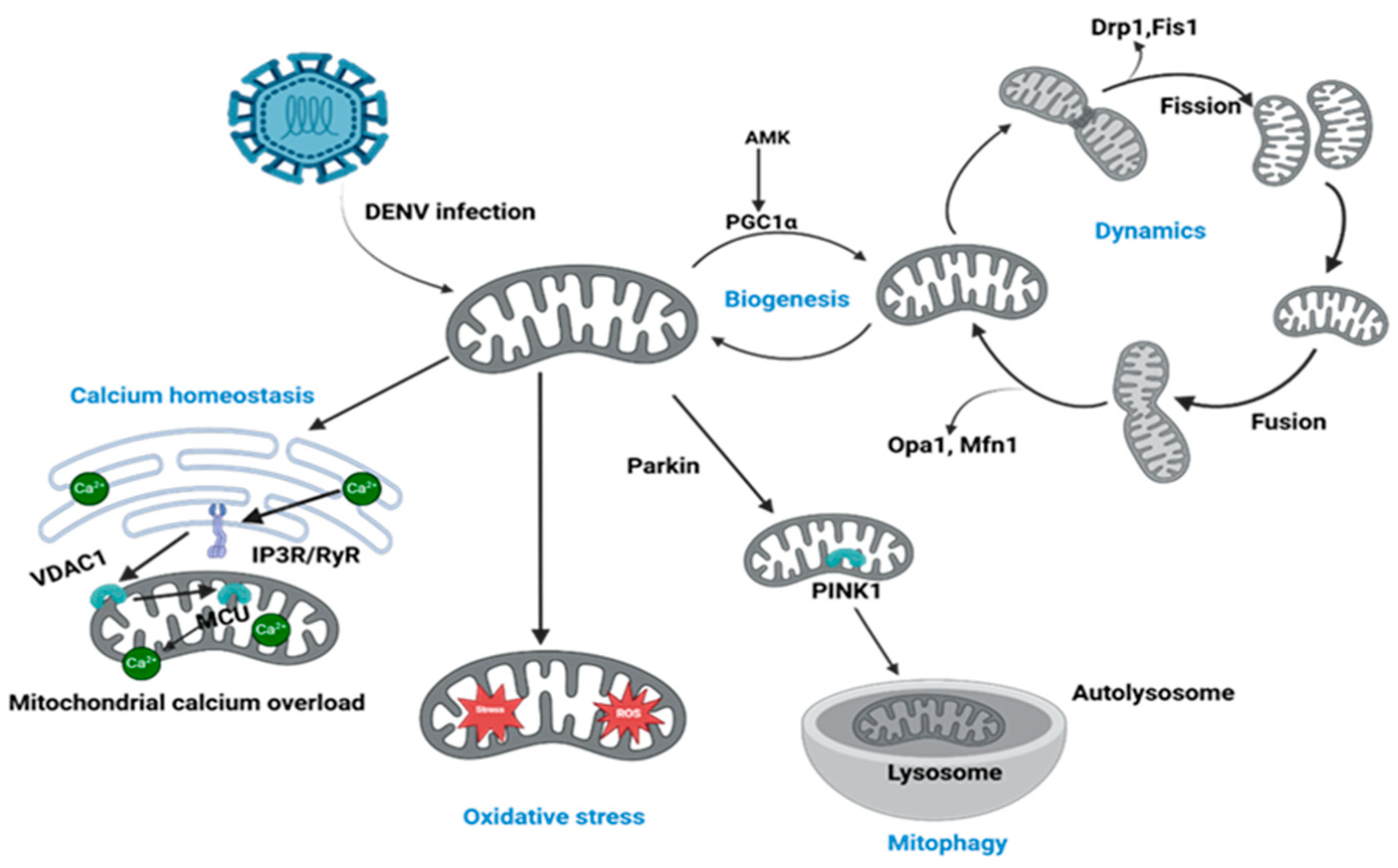
2. DENV-Induced Perturbation of Mitochondrial Dynamics
3. Compromised Mitochondrial Quality Control: The Blockade of Mitophagy and Biogenesis
4. DENV Hijacking of Host Cell Metabolism via Mitochondrial Modulation
5. DENV Evasion of Innate Immunity
6. Immune-Mediated Mitochondrial Alterations: The Hidden Cost of Antibody Dependent Enhancement (ADE) in Dengue
7. Mitochondrial Dysfunction, Oxidative Stress, and Apoptosis in DENV Infection
8. Mitochondria Targeted Therapeutic Implications for Dengue: Emerging Insights and Drug Candidates
9. Future Direction
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| DENV | Dengue virus |
| DHF | Dengue hemorrhagic fever |
| DF | Dengue fever |
| DHS | Dengue hemorrhagic syndrome |
| DSS | Dengue shock syndrome |
| ATP | Adenosine triphosphate |
| ROS | Reactive oxygen species |
| ETC | Electron transport chain |
| OXPHOS | Oxidative phosphorylation |
| mtDNA | Mitochondrial DNA |
| DRP1 | Dynamin related protein 1 |
| DNM2 | Dynamin 2 |
| OMM | Outer mitochondrial membrane |
| IMM | Inner mitochondrial membrane |
| MMP | Mitochondrial membrane potential |
| FcγR | Fragment crystallizable gamma receptors |
| MAVS | Mitochondrial antiviral signaling |
| AMPK | AMP-activated protein kinase |
| MASM7 | Mitochondrial activity stimulation molecule 7 |
| ADE | Antibody dependent enhancement |
| GSH | Glutathione |
| MFN1 | Mitofusin 1 |
| MFN2 | Mitofusin 2 |
| FIS1 | Fission 1 |
| OPA1 | Optic atrophy 1 |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| O2− | Superoxide |
| TNF-α | Tumor necrosis factor-alpha |
| IL-10 | Interleukin-10 |
| IFN1 | Type 1 interferon |
| NO | Nitric oxide |
| CI | Complex 1 |
| NS3pro | NS3 protease |
| LDs | Lipid droplets |
| NRF-2 | Nuclear factor erythroid 2-related factor 2 |
| PPAR-γ | Peroxisome proliferator-activated receptor gamma |
| RIG-1 | Retinoic acid-inducible gene I |
| IRF3 | Interferon regulatory factor 3 |
| TRAF 2/3/6 | TNF receptor-associated factors 2, 3, and 6 |
| STING | Stimulator of interferon genes. |
| UCP1 | Uncoupling protein 1 |
| PINK1 | PTEN-induced kinase 1 |
| Ser 616 | Serine 616 |
| TBK1 | TANK-binding kinase 1 |
| MAMs | Mitochondria-associated membranes |
| Sirtuin 1 | SIRT1 |
| Mdivi-1 | Mitochondrial division inhibitor-1 |
| NAC | N-acetylcysteine |
| FDA | Food and drug administration |
References
- Tsang, T.K.; Ghebremariam, S.L.; Gresh, L.; Gordon, A.; Halloran, M.E.; Katzelnick, L.C.; Rojas, D.P.; Kuan, G.; Balmaseda, A.; Sugimoto, J.; et al. Effects of Infection History on Dengue Virus Infection and Pathogenicity. Nat. Commun. 2019, 10, 1246. [Google Scholar] [CrossRef]
- Nisalak, A.; Clapham, H.E.; Kalayanarooj, S.; Klungthong, C.; Thaisomboonsuk, B.; Fernandez, S.; Reiser, J.; Srikiatkhachorn, A.; Macareo, L.R.; Lessler, J.T.; et al. Forty Years of Dengue Surveillance at a Tertiary Pediatric Hospital in Bangkok, Thailand, 1973–2012. Am. J. Trop. Med. Hyg. 2016, 94, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S. Recent Advances in Understanding Dengue. F1000Res 2019, 8, F1000. [Google Scholar] [CrossRef]
- Vasilakis, N.; Weaver, S.C. Chapter 1 The History and Evolution of Human Dengue Emergence. In Advances in Virus Research; Elsevier: Amsterdam, The Netherlands, 2008; Volume 72, pp. 1–76. ISBN 978-0-12-374322-0. [Google Scholar]
- Carey, D.E. Chikungunya and Dengue: A Case of Mistaken Identity? J. Hist. Med. Allied Sci. 1971, XXVI, 243–262. [Google Scholar] [CrossRef]
- Gubler, D.J. Dengue and Dengue Hemorrhagic Fever. Clin. Microbiol. Rev. 1998, 11, 480–496. [Google Scholar] [CrossRef]
- Halstead, S.B. Reappearance of Chikungunya, Formerly Called Dengue, in the Americas. Emerg. Infect. Dis. 2015, 21, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Zerfu, B.; Kassa, T.; Legesse, M. Epidemiology, Biology, Pathogenesis, Clinical Manifestations, and Diagnosis of Dengue Virus Infection, and Its Trend in Ethiopia: A Comprehensive Literature Review. Trop. Med. Health 2023, 51, 11. [Google Scholar] [CrossRef]
- Snow, G.E.; Haaland, B.; Ooi, E.E.; Gubler, D.J. Review Article: Research on Dengue during World War II Revisited. Am. J. Trop. Med. Hyg. 2014, 91, 1203–1217. [Google Scholar] [CrossRef]
- Halstead, S.B.; Cohen, S.N. Dengue Hemorrhagic Fever at 60 Years: Early Evolution of Concepts of Causation and Treatment. Microbiol. Mol. Biol. Rev. 2015, 79, 281–291. [Google Scholar] [CrossRef]
- Biswas, H.H.; Ortega, O.; Gordon, A.; Standish, K.; Balmaseda, A.; Kuan, G.; Harris, E. Early Clinical Features of Dengue Virus Infection in Nicaraguan Children: A Longitudinal Analysis. PLoS Negl. Trop. Dis. 2012, 6, e1562. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.S.; Rasotgi, V.; Jain, S.; Gupta, V. Discovery of Fifth Serotype of Dengue Virus (DENV-5): A New Public Health Dilemma in Dengue Control. Med. J. Armed. Forces India 2015, 71, 67–70. [Google Scholar] [CrossRef]
- Tariq, F.; Irfan, M.; Farooq, S.; Iqbal, H.; Atia-Tul-Wahab; Khan, I.A.; Iftner, T.; Choudhary, M.I. Dynamics and Genetic Variation of Dengue Virus Serotypes Circulating during the 2022 Outbreak in Karachi. Sci. Rep. 2025, 15, 22703. [Google Scholar] [CrossRef]
- Wang, W.-H.; Urbina, A.N.; Chang, M.R.; Assavalapsakul, W.; Lu, P.-L.; Chen, Y.-H.; Wang, S.-F. Dengue Hemorrhagic Fever —A Systemic Literature Review of Current Perspectives on Pathogenesis, Prevention and Control. J. Microbiol. Immunol. Infect. 2020, 53, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Shen, Y.J.; Chou, Y.J.; Tsai, T.F.; Lien, C.E. Advanced Age and Increased Risk for Severe Outcomes of Dengue Infection, Taiwan, 2014–2015. Emerg. Infect. Dis. 2023, 29, 1701–1702. [Google Scholar] [CrossRef] [PubMed]
- Hotta, S. Experimental Studies on Dengue: I.; Isolation, Identification and Modification of the Virus. J. Infect. Dis. 1952, 90, 1–9. [Google Scholar] [CrossRef]
- Lam, P.K.; Tam, D.T.H.; Diet, T.V.; Tam, C.T.; Tien, N.T.H.; Kieu, N.T.T.; Simmons, C.; Farrar, J.; Nga, N.T.N.; Qui, P.T.; et al. Clinical Characteristics of Dengue Shock Syndrome in Vietnamese Children: A 10-Year Prospective Study in a Single Hospital. Clin. Infect. Dis. 2013, 57, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Anders, K.L.; Nguyet, N.M.; Van Vinh Chau, N.; Hung, N.T.; Thuy, T.T.; Lien, L.B.; Farrar, J.; Wills, B.; Hien, T.T.; Simmons, C.P. Epidemiological Factors Associated with Dengue Shock Syndrome and Mortality in Hospitalized Dengue Patients in Ho Chi Minh City, Vietnam. Am. Soc. Trop. Med. Hyg. 2011, 84, 127–134. [Google Scholar] [CrossRef]
- Schmid, M.A.; Diamond, M.S.; Harris, E. Dendritic Cells in Dengue Virus Infection: Targets of Virus Replication and Mediators of Immunity. Front. Immunol. 2014, 5, 647. [Google Scholar] [CrossRef]
- de Arruda, T.B.; Bavia, L.; Mosimann, A.L.P.; Aoki, M.N.; Sarzi, M.L.; Conchon-Costa, I.; Wowk, P.F.; Duarte Dos Santos, C.N.; Pavanelli, W.R.; Silveira, G.F.; et al. Viremia and Inflammatory Cytokines in Dengue: Interleukin-2 as a Biomarker of Infection, and Interferon-α and -γ as Markers of Primary versus Secondary Infection. Pathogens 2023, 12, 1362. [Google Scholar] [CrossRef]
- Srikiatkhachorn, A.; Mathew, A.; Rothman, A.L. Immune-Mediated Cytokine Storm and Its Role in Severe Dengue. Semin. Immunopathol. 2017, 39, 563–574. [Google Scholar] [CrossRef]
- Vaughn, D.W.; Green, S.; Kalayanarooj, S.; Innis, B.L.; Nimmannitya, S.; Suntayakorn, S.; Rothman, A.L.; Ennis, F.A.; Nisalak, A. Dengue in the Early Febrile Phase: Viremia and Antibody Responses. J. Infect. Dis. 1997, 176, 322–330. [Google Scholar] [CrossRef]
- Chermahini, F.A.; Arvejeh, P.M.; Marincola, F.M.; Ahmad, S.; Naderian, R.; Pajand, O.; Eslami, M.; Hasannia, M.; Sanami, S. Investigating How Dengue Virus-Induced Metabolic Changes Affect the Host Immune Response and How to Develop Immunomodulatory Strategies. Virol. J. 2025, 22, 117. [Google Scholar] [CrossRef]
- Singh, B.; Avula, K.; Sufi, S.A.; Parwin, N.; Das, S.; Alam, M.F.; Samantaray, S.; Bankapalli, L.; Rani, A.; Poornima, K.; et al. Defective Mitochondrial Quality Control during Dengue Infection Contributes to Disease Pathogenesis. J. Virol. 2022, 96, e0082822. [Google Scholar] [CrossRef]
- Kao, Y.-T.; Lai, M.M.C.; Yu, C.-Y. How Dengue Virus Circumvents Innate Immunity. Front. Immunol. 2018, 9, 2860. [Google Scholar] [CrossRef]
- Pan, Y.; Cheng, A.; Wang, M.; Yin, Z.; Jia, R. The Dual Regulation of Apoptosis by Flavivirus. Front. Microbiol. 2021, 12, 654494. [Google Scholar] [CrossRef]
- El-Bacha, T.; Da Poian, A.T. Virus-Induced Changes in Mitochondrial Bioenergetics as Potential Targets for Therapy. Int. J. Biochem. Cell Biol. 2013, 45, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Palma, F.R.; Gantner, B.N.; Sakiyama, M.J.; Kayzuka, C.; Shukla, S.; Lacchini, R.; Cunniff, B.; Bonini, M.G. ROS Production by Mitochondria: Function or Dysfunction? Oncogene 2024, 43, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative Stress, Mitochondrial Damage and Neurodegenerative Diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef]
- Lemeshko, V.V. Apparent “Mild Depolarization of the Inner Mitochondrial Membrane” as a Result of a Possible Generation of the Outer Membrane Potential. Biochim. Biophys. Acta Biomembr. 2022, 1864, 184032. [Google Scholar] [CrossRef]
- Ragonese, F.; Monarca, L.; De Luca, A.; Mancinelli, L.; Mariani, M.; Corbucci, C.; Gerli, S.; Iannitti, R.G.; Leonardi, L.; Fioretti, B. Resveratrol Depolarizes the Membrane Potential in Human Granulosa Cells and Promotes Mito-chondrial Biogenesis. Fertil. Steril. 2021, 115, 1063–1073. [Google Scholar] [CrossRef]
- Bravo-Sagua, R.; Parra, V.; López-Crisosto, C.; Díaz, P.; Quest, A.F.G.; Lavandero, S. Calcium Transport and Sig-naling in Mitochondria. Compr. Physiol. 2017, 7, 623–634. [Google Scholar] [CrossRef]
- Lai, J.-H.; Wu, D.-W.; Wu, C.-H.; Hung, L.-F.; Huang, C.-Y.; Ka, S.-M.; Chen, A.; Ho, L.-J. USP18 Enhances Dengue Virus Replication by Regulating Mitochondrial DNA Release. Sci. Rep. 2023, 13, 20126. [Google Scholar] [CrossRef]
- Kuczera, D.; Assolini, J.P.; Tomiotto-Pellissier, F.; Pavanelli, W.R.; Silveira, G.F. Highlights for Dengue Immuno-pathogenesis: Antibody-Dependent Enhancement, Cytokine Storm, and Beyond. J. Interferon Cytokine Res. 2018, 38, 69–80. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Zhao, L.; Long, J.; Feng, Z.; Su, J.; Gao, F.; Liu, J. Mitochondria as a Sensor, a Central Hub and a Biological Clock in Psychological Stress-Accelerated Aging. Ageing Res. Rev. 2024, 93, 102145. [Google Scholar] [CrossRef] [PubMed]
- Trajano, L.A.D.S.N.; Siqueira, P.B.; Rodrigues, M.M.D.S.; Pires, B.R.B.; Da Fonseca, A.D.S.; Mencalha, A.L. Does Photobiomodulation Alter Mitochondrial Dynamics? Photochem. Photobiol. 2025, 101, 21–37. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial Dysfunction and Oxidative Stress in Metabolic Disorders—A Step towards Mitochondria Based Therapeutic Strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hoppe, T. Role of Amino Acid Metabolism in Mitochondrial Homeostasis. Front. Cell Dev. Biol. 2023, 11, 1127618. [Google Scholar] [CrossRef]
- Stoolman, J.S.; Porcelli, A.M.; Martínez-Reyes, I. Editorial: Mitochondria as a Hub in Cellular Signaling. Front. Cell Dev. Biol. 2022, 10, 981464. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Baldassari, F.; Bononi, A.; Bonora, M.; De Marchi, E.; Marchi, S.; Missiroli, S.; Patergnani, S.; Rimessi, A.; Suski, J.M.; et al. Mitochondrial Ca2+ and Apoptosis. Cell Calcium 2012, 52, 36–43. [Google Scholar] [CrossRef]
- Arnoult, D.; Soares, F.; Tattoli, I.; Girardin, S.E. Mitochondria in Innate Immunity. EMBO Rep. 2011, 12, 901–910. [Google Scholar] [CrossRef]
- Gatti, P.; Ilamathi, H.S.; Todkar, K.; Germain, M. Mitochondria Targeted Viral Replication and Survival Strategies-Prospective on SARS-CoV-2. Front. Pharmacol. 2020, 11, 578599. [Google Scholar] [CrossRef]
- Duan, X.; Liu, R.; Lan, W.; Liu, S. The Essential Role of Mitochondrial Dynamics in Viral Infections. Int. J. Mol. Sci. 2025, 26, 1955. [Google Scholar] [CrossRef]
- Chatel-Chaix, L.; Cortese, M.; Romero-Brey, I.; Bender, S.; Neufeldt, C.J.; Fischl, W.; Scaturro, P.; Schieber, N.; Schwab, Y.; Fischer, B.; et al. Dengue Virus Perturbs Mitochondrial Morphodynamics to Dampen Innate Immune Responses. Cell Host Microbe 2016, 20, 342–356. [Google Scholar] [CrossRef]
- Tseng, C.-K.; Lin, C.-K.; Wu, Y.-H.; Chen, Y.-H.; Chen, W.-C.; Young, K.-C.; Lee, J.-C. Human Heme Oxygenase 1 Is a Potential Host Cell Factor against Dengue Virus Replication. Sci. Rep. 2016, 6, 32176. [Google Scholar] [CrossRef] [PubMed]
- Ježek, J.; Cooper, K.F.; Strich, R. The Impact of Mitochondrial Fission-Stimulated ROS Production on Pro-Apoptotic Chemotherapy. Biology 2021, 10, 33. [Google Scholar] [CrossRef]
- Pickles, S.; Vigié, P.; Youle, R.J. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr. Biol. 2018, 28, R170–R185. [Google Scholar] [CrossRef]
- Wang, H.; Luo, W.; Chen, H.; Cai, Z.; Xu, G. Mitochondrial Dynamics and Mitochondrial Autophagy: Molecular Structure, Orchestrating Mechanism and Related Disorders. Mitochondrion 2024, 75, 101847. [Google Scholar] [CrossRef]
- Singh, B.; Kiran, A.; Sufi, S.A.; Syed, G.H. Fatal Attraction: Dengue Virus and the Mitochondrial Connection. Autophagy Rep. 2023, 2, 2167429. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Singh, K.; Ravi Kumar, Y.S.; Roy, R.; Phadnis, S.; Meena, V.; Bhattacharyya, S.; Verma, B. Dengue Virus Pathogenesis and Host Molecular Machineries. J. Biomed. Sci. 2024, 31, 43. [Google Scholar] [CrossRef] [PubMed]
- Santana-Román, M.E.; Maycotte, P.; Uribe-Carvajal, S.; Uribe-Alvarez, C.; Alvarado-Medina, N.; Khan, M.; Siddiqui, A.; Pando-Robles, V. Monitoring Mitochondrial Function in Aedes Albopictus C6/36 Cell Line during Dengue Virus Infection. Insects 2021, 12, 934. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Liang, J.-J.; Li, J.-K.; Lee, Y.-L.; Chang, B.-L.; Su, C.-I.; Huang, W.-J.; Lai, M.M.C.; Lin, Y.-L. Dengue Virus Impairs Mitochondrial Fusion by Cleaving Mitofusins. PLoS Pathog. 2015, 11, e1005350. [Google Scholar] [CrossRef]
- Barbier, V.; Lang, D.; Valois, S.; Rothman, A.L.; Medin, C.L. Dengue Virus Induces Mitochondrial Elongation through Impairment of Drp1-Triggered Mitochondrial Fission. Virology 2017, 500, 149–160. [Google Scholar] [CrossRef]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of Mitochondrial Biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar] [CrossRef]
- Kubli, D.A.; Gustafsson, Å.B. Mitochondria and Mitophagy: The Yin and Yang of Cell Death Control. Circ. Res. 2012, 111, 1208–1221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qin, Y.; Chen, M. Viral Strategies for Triggering and Manipulating Mitophagy. Autophagy 2018, 14, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Chen, G.; Li, W.; Kepp, O.; Zhu, Y.; Chen, Q. Mitophagy, Mitochondrial Homeostasis, and Cell Fate. Front. Cell Dev. Biol. 2020, 8, 467. [Google Scholar] [CrossRef]
- Wu, N.; Ji, J.; Gou, X.; Hu, P.; Cheng, Y.; Liu, Y.; Wang, Y.; Zhang, Q.; Zuo, L. DENV-2 NS1 Promotes AMPK-LKB1 Interaction to Activate AMPK/ERK/mTOR Signaling Pathway to Induce Autophagy. Virol. J. 2023, 20, 231. [Google Scholar] [CrossRef]
- Cloherty, A.P.M.; Rader, A.G.; Patel, K.S.; Eisden, T.-J.T.H.D.; van Piggelen, S.; Schreurs, R.R.C.E.; Ribeiro, C.M.S. Dengue Virus Exploits Autophagy Vesicles and Secretory Pathways to Promote Transmission by Human Dendritic Cells. Front. Immunol. 2024, 15, 1260439. [Google Scholar] [CrossRef] [PubMed]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Balancing Mitochondrial Biogenesis and Mitophagy to Maintain Energy Metabolism Homeostasis. Cell Death Differ. 2015, 22, 1399–1401. [Google Scholar] [CrossRef]
- Popov, L.-D. Mitochondrial Biogenesis: An Update. J. Cell Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef]
- Valero, T. Mitochondrial Biogenesis: Pharmacological Approaches. Curr. Pharm. Des. 2014, 20, 5507–5509. [Google Scholar] [CrossRef]
- Yousefi, M.; Lee, W.S.; Yan, B.; Cui, L.; Yong, C.L.; Yap, X.; Tay, K.S.L.; Qiao, W.; Tan, D.; Nurazmi, N.I.; et al. TMEM41B and VMP1 Modulate Cellular Lipid and Energy Metabolism for Facilitating Dengue Virus Infection. PLoS Pathog. 2022, 18, e1010763. [Google Scholar] [CrossRef]
- Philipp, N.; Costa Navarro, G.S.; de Borba, L.; Gamarnik, A.V.; Estrada, L.C. Unraveling Viral Protein-Host Membrane Interaction for Dengue and Zika. Biophys. J. 2025, 124, 2523–2530. [Google Scholar] [CrossRef]
- Allonso, D.; Andrade, I.S.; Conde, J.N.; Coelho, D.R.; Rocha, D.C.P.; Da Silva, M.L.; Ventura, G.T.; Silva, E.M.; Mohana-Borges, R. Dengue Virus NS1 Protein Modulates Cellular Energy Metabolism by Increasing Glyceraldehyde-3-Phosphate Dehydrogenase Activity. J. Virol. 2015, 89, 11871–11883. [Google Scholar] [CrossRef]
- Alcalá, A.C.; Ludert, J.E. The Dengue Virus NS1 Protein; New Roles in Pathogenesis Due to Similarities with and Affinity for the High-Density Lipoprotein (HDL)? PLoS Pathog. 2023, 19, e1011587. [Google Scholar] [CrossRef]
- Sousa, B.G.; Mebus-Antunes, N.C.; Fernandes-Siqueira, L.O.; Caruso, M.B.; Saraiva, G.N.; Carvalho, C.F.; Neves-Martins, T.C.; Galina, A.; Zingali, R.B.; Zeidler, J.D.; et al. Dengue Virus Non-Structural Protein 3 Inhibits Mitochondrial Respiration by Impairing Complex I Function. mSphere 2024, 9, e0040624. [Google Scholar] [CrossRef] [PubMed]
- Heaton, N.S.; Perera, R.; Berger, K.L.; Khadka, S.; Lacount, D.J.; Kuhn, R.J.; Randall, G. Dengue Virus Nonstructural Protein 3 Redistributes Fatty Acid Synthase to Sites of Viral Replication and Increases Cellular Fatty Acid Synthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 17345–17350. [Google Scholar] [CrossRef]
- Gandhi, L.; Maisnam, D.; Rathore, D.; Chauhan, P.; Bonagiri, A.; Venkataramana, M. Differential Localization of Dengue Virus Protease Affects Cell Homeostasis and Triggers to Thrombocytopenia. iScience 2023, 26, 107024. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kang, C. Structures and Dynamics of Dengue Virus Nonstructural Membrane Proteins. Membranes 2022, 12, 231. [Google Scholar] [CrossRef]
- Nemésio, H.; Palomares-Jerez, F.; Villalaín, J. NS4A and NS4B Proteins from Dengue Virus: Membranotropic Regions. Biochim. Biophys. Acta 2012, 1818, 2818–2830. [Google Scholar] [CrossRef]
- Faustino, A.F.; Martins, I.C.; Carvalho, F.A.; Castanho, M.A.R.B.; Maurer-Stroh, S.; Santos, N.C. Understanding Dengue Virus Capsid Protein Interaction with Key Biological Targets. Sci. Rep. 2015, 5, 10592. [Google Scholar] [CrossRef] [PubMed]
- Byk, L.A.; Gamarnik, A.V. Properties and Functions of the Dengue Virus Capsid Protein. Annu. Rev. Virol. 2016, 3, 263–281. [Google Scholar] [CrossRef]
- He, Z.; Zhu, X.; Wen, W.; Yuan, J.; Hu, Y.; Chen, J.; An, S.; Dong, X.; Lin, C.; Yu, J.; et al. Dengue Virus Subverts Host Innate Immunity by Targeting Adaptor Protein MAVS. J. Virol. 2016, 90, 7219–7230. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, N.A.; Cimica, V.; Mackow, E.R. Dengue Virus NS Proteins Inhibit RIG-I/MAVS Signaling by Blocking TBK1/IRF3 Phosphorylation: Dengue Virus Serotype 1 NS4A Is a Unique Interferon-Regulating Virulence Determinant. mBio 2015, 6, e00553-15. [Google Scholar] [CrossRef]
- Tremblay, N.; Freppel, W.; Sow, A.A.; Chatel-Chaix, L. The Interplay between Dengue Virus and the Human Innate Immune System: A Game of Hide and Seek. Vaccines 2019, 7, 145. [Google Scholar] [CrossRef]
- Nie, Y.; Deng, D.; Mou, L.; Long, Q.; Chen, J.; Wu, J. Dengue Virus 2 NS2B Targets MAVS and IKKε to Evade the Antiviral Innate Immune Response. J. Microbiol. Biotechnol. 2023, 33, 600–606. [Google Scholar] [CrossRef]
- Lee, M.F.; Voon, G.Z.; Lim, H.X.; Chua, M.L.; Poh, C.L. Innate and Adaptive Immune Evasion by Dengue Virus. Front. Cell Infect. Microbiol. 2022, 12, 1004608. [Google Scholar] [CrossRef]
- Chen, S.; Wu, Z.; Wang, M.; Cheng, A. Innate Immune Evasion Mediated by Flaviviridae Non-Structural Proteins. Viruses 2017, 9, 291. [Google Scholar] [CrossRef]
- Kuan, G.; Gordon, A.; Aviles, W.; Ortega, O.; Hammond, S.N.; Elizondo, D.; Nunez, A.; Coloma, J.; Balmaseda, A.; Harris, E. The Nicaraguan Pediatric Dengue Cohort Study: Study Design, Methods, Use of Information Technology, and Extension to Other Infectious Diseases. Am. J. Epidemiol. 2009, 170, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Smatti, M.K.; Ouhtit, A.; Cyprian, F.S.; Almaslamani, M.A.; Thani, A.A.; Yassine, H.M. Antibody-Dependent Enhancement (ADE) and the Role of Complement System in Disease Pathogenesis. Mol. Immunol. 2022, 152, 172–182. [Google Scholar] [CrossRef]
- Narayan, R.; Tripathi, S. Intrinsic ADE: The Dark Side of Antibody Dependent Enhancement During Dengue Infection. Front. Cell Infect. Microbiol. 2020, 10, 580096. [Google Scholar] [CrossRef]
- Aynekulu Mersha, D.G.; van der Sterren, I.; van Leeuwen, L.P.M.; Langerak, T.; Hakim, M.S.; Martina, B.; van Lelyveld, S.F.L.; van Gorp, E.C.M. The Role of Antibody-Dependent Enhancement in Dengue Vaccination. Trop. Dis. Travel. Med. Vaccines 2024, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Katzelnick, L.C.; Gresh, L.; Halloran, M.E.; Mercado, J.C.; Kuan, G.; Gordon, A.; Balmaseda, A.; Harris, E. Antibody-Dependent Enhancement of Severe Dengue Disease in Humans. Science 2017, 358, 929–932. [Google Scholar] [CrossRef]
- Sasmal, S.K.; Takeuchi, Y.; Nakaoka, S. T-Cell Mediated Adaptive Immunity and Antibody-Dependent Enhancement in Secondary Dengue Infection. J. Theor. Biol. 2019, 470, 50–63. [Google Scholar] [CrossRef]
- Bhol, N.K.; Bhanjadeo, M.M.; Singh, A.K.; Dash, U.C.; Ojha, R.R.; Majhi, S.; Duttaroy, A.K.; Jena, A.B. The Interplay between Cytokines, Inflammation, and Antioxidants: Mechanistic Insights and Therapeutic Potentials of Various Antioxidants and Anti-Cytokine Compounds. Biomed. Pharmacother. 2024, 178, 117177. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial Oxidative Stress-A Causative Factor and Therapeutic Target in Many Diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef]
- León-Juárez, M.; Martínez-Castillo, M.; Shrivastava, G.; García-Cordero, J.; Villegas-Sepulveda, N.; Mondragón-Castelán, M.; Mondragón-Flores, R.; Cedillo-Barrón, L. Recombinant Dengue Virus Protein NS2B Alters Membrane Permeability in Different Membrane Models. Virol. J. 2016, 13, 1. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Meuren, L.M.; Prestes, E.B.; Papa, M.P.; de Carvalho, L.R.P.; Mustafá, Y.M.; da Costa, L.S.; Da Poian, A.T.; Bozza, M.T.; Arruda, L.B. Infection of Endothelial Cells by Dengue Virus Induces ROS Production by Different Sources Affecting Virus Replication, Cellular Activation, Death and Vascular Permeability. Front. Immunol. 2022, 13, 810376. [Google Scholar] [CrossRef]
- Li, B.; Li, B.; Qiao, X.; Meng, W.; Xie, Y.; Gong, J.; Fan, Y.; Zhao, Z.; Li, L. Targeting Mitochondrial Transfer as a Promising Therapeutic Strategy. Trends Mol. Med. 2025, 6, S1471-4914. [Google Scholar] [CrossRef]
- Bottani, E.; Brunetti, D. Advances in Mitochondria-Targeted Drug Delivery. Pharmaceutics 2023, 15, 2089. [Google Scholar] [CrossRef]
- Hottz, E.D.; Oliveira, M.F.; Nunes, P.C.G.; Nogueira, R.M.R.; Valls-de-Souza, R.; Da Poian, A.T.; Weyrich, A.S.; Zimmerman, G.A.; Bozza, P.T.; Bozza, F.A. Dengue Induces Platelet Activation, Mitochondrial Dysfunction and Cell Death through Mechanisms That Involve DC-SIGN and Caspases. J. Thromb. Haemost. 2013, 11, 951–962. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial Dynamics in Health and Disease: Mechanisms and Potential Targets. Signal Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef]
- Whitley, B.N.; Engelhart, E.A.; Hoppins, S. Mitochondrial Dynamics and Their Potential as a Therapeutic Target. Mitochondrion 2019, 49, 269–283. [Google Scholar] [CrossRef]
- Zacharioudakis, E.; Gavathiotis, E. Mitochondrial Dynamics Proteins as Emerging Drug Targets. Trends Pharmacol. Sci. 2023, 44, 112–127. [Google Scholar] [CrossRef]
- Zacharioudakis, E.; Agianian, B.; Kumar Mv, V.; Biris, N.; Garner, T.P.; Rabinovich-Nikitin, I.; Ouchida, A.T.; Margulets, V.; Nordstrøm, L.U.; Riley, J.S.; et al. Modulating Mitofusins to Control Mitochondrial Function and Signaling. Nat. Commun. 2022, 13, 3775. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.-L.; Wang, T.-F.; Wang, C.-L.; Zhang, Z.-P.; Zhao, J.-W.; Heng, W.; Tang, Z.; Du, M.-R.; Yan, X.-D.; Li, X.-X.; et al. Cytoplasmic Escape of Mitochondrial DNA Mediated by Mfn2 Downregulation Promotes Microglial Activation via cGas-Sting Axis in Spinal Cord Injury. Adv. Sci. 2024, 11, e2305442. [Google Scholar] [CrossRef] [PubMed]
- Zacharioudakis, E.; Biris, N.; Garner, T.P.; Chen, Y.; Pekson, R.; Dhingra, R.; Santulli, G.; Kirshenbaum, L.A.; Kitsis, R.N.; Gavathiotis, E. Direct Small Molecule Activation of Mitofusins. bioRxiv 2018. [Google Scholar] [CrossRef]
- Cassidy-Stone, A.; Chipuk, J.E.; Ingerman, E.; Song, C.; Yoo, C.; Kuwana, T.; Kurth, M.J.; Shaw, J.T.; Hinshaw, J.E.; Green, D.R.; et al. Chemical Inhibition of the Mitochondrial Division Dynamin Reveals Its Role in Bax/Bak-Dependent Mitochondrial Outer Membrane Permeabilization. Dev. Cell 2008, 14, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.V.; Javadov, S.; Sommer, N. Cellular ROS and Antioxidants: Physiological and Pathological Role. Antioxidants 2024, 13, 602. [Google Scholar] [CrossRef]
- Antonenko, Y.N.; Roginsky, V.A.; Pashkovskaya, A.A.; Rokitskaya, T.I.; Kotova, E.A.; Zaspa, A.A.; Chernyak, B.V.; Skulachev, V.P. Protective Effects of Mitochondria-Targeted Antioxidant SkQ in Aqueous and Lipid Membrane Environments. J. Membr. Biol. 2008, 222, 141–149. [Google Scholar] [CrossRef]
- Punetha, M.; Saini, S.; Chaudhary, S.; Bala, R.; Sharma, M.; Kumar, P.; Kumar, D.; Yadav, P.S. Mitochondria-Targeted Antioxidant MitoQ Ameliorates ROS Production and Improves Cell Viability in Cryopreserved Buffalo Fibroblasts. Tissue and Cell 2023, 82, 102067. [Google Scholar] [CrossRef] [PubMed]
- Morchang, A.; Malakar, S.; Poonudom, K.; Noisakran, S.; Yenchitsomanus, P.-T.; Limjindaporn, T. Melatonin Inhibits Dengue Virus Infection via the Sirtuin 1-Mediated Interferon Pathway. Viruses 2021, 13, 659. [Google Scholar] [CrossRef]
- Melhuish Beaupre, L.M.; Brown, G.M.; Gonçalves, V.F.; Kennedy, J.L. Melatonin’s Neuroprotective Role in Mitochondria and Its Potential as a Biomarker in Aging, Cognition and Psychiatric Disorders. Transl. Psychiatry 2021, 11, 339. [Google Scholar] [CrossRef]
- Tafere, G.G.; Wondafrash, D.Z.; Demoz, F.B. Repurposing of N-Acetylcysteine for the Treatment of Dengue Virus-Induced Acute Liver Failure. Hepatic Med. Evid. Res. 2020, 12, 173–178. [Google Scholar] [CrossRef]
- Dissanayake, D.M.D.I.B.; Gunaratne, W.M.S.N.; Kumarihamy, K.W.M.P.P.; Kularatne, S.A.M.; Kumarasiri, P.V.R. Use of Intravenous N-Acetylcysteine in Acute Severe Hepatitis Due to Severe Dengue Infection: A Case Series. BMC Infect. Dis. 2021, 21, 978. [Google Scholar] [CrossRef] [PubMed]
- Tardiolo, G.; Bramanti, P.; Mazzon, E. Overview on the Effects of N-Acetylcysteine in Neurodegenerative Diseases. Molecules 2018, 23, 3305. [Google Scholar] [CrossRef]
- Ooi, S.L.; Green, R.; Pak, S.C. N-Acetylcysteine for the Treatment of Psychiatric Disorders: A Review of Current Evidence. BioMed Res. Int. 2018, 2018, 2469486. [Google Scholar] [CrossRef]
- Niewoehner, D.; Johnson, K.; McEvoy, C.; Naqvi, S.; Wendt, C.; Reilkoff, R.; Kunisaki, K.; Wetherbee, E.; Nelson, D.; Tirouvanziam, R. High-Dose Oral N-Acetylcysteine Fails to Improve Respiratory Health Status in Patients with Chronic Obstructive Pulmonary Disease and Chronic Bronchitis: A Randomized, Placebo-Controlled Trial. Int. J. Chronic Obstr. Pulm. Dis. 2016, 799, 807. [Google Scholar] [CrossRef] [PubMed]
- Cantu-Gonzalez, G. 50 Years Ago in The Journal of Pediatrics: The Use of N-Acetylcysteine in the Treatment of Cystic Fibrosis. J. Pediatr. 2014, 165, 721. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, L.; Liu, Y.; Chen, M.; Guo, X.; Wang, J. N-Acetylcysteine, a Small Molecule Scavenger of Reactive Oxygen Species, Alleviates Cardiomyocyte Damage by Regulating OPA1-Mediated Mitochondrial Quality Control and Apoptosis in Response to Oxidative Stress. J. Thorac. Dis. 2024, 16, 5323–5336. [Google Scholar] [CrossRef]
- Pang, M.; Wang, S.; Shi, T.; Chen, J. Overview of MitoQ on Prevention and Management of Cardiometabolic Diseases: A Scoping Review. Front. Cardiovasc. Med. 2025, 12, 1506460. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.Y.; Chupyrkina, A.A.; Jankauskas, S.S.; Pevzner, I.B.; Silachev, D.N.; Skulachev, V.P.; Zorov, D.B. Mechanisms of Nephroprotective Effect of Mitochondria-Targeted Antioxidants under Rhabdomyolysis and Ischemia/Reperfusion. Biochim. Biophys. Acta-Mol. Basis Dis. 2011, 1812, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.; Lee, J.H. N-Acetylcysteine in Children with Dengue-Associated Liver Failure: A Case Report. J. Trop. Pediatr. 2012, 58, 409–413. [Google Scholar] [CrossRef]
- Srivastava, A.; Johnson, M.; Renna, H.A.; Sheehan, K.M.; Ahmed, S.; Palaia, T.; Pinkhasov, A.; Gomolin, I.H.; De Leon, J.; Reiss, A.B. Therapeutic Potential of P110 Peptide: New Insights into Treatment of Alzheimer’s Disease. Life 2023, 13, 2156. [Google Scholar] [CrossRef]
- Singh, A.; Faccenda, D.; Campanella, M. Pharmacological Advances in Mitochondrial Therapy. EBioMedicine 2021, 65, 103244. [Google Scholar] [CrossRef] [PubMed]

| Aspect | Dengue Viral Infection | Mitochondrial Dysfunctions | Ref. |
|---|---|---|---|
| Mitophagy | Modulates mitophagy to promote viral replication | Impaired mitophagy fails to clear damaged mitochondria | [24] |
| Innate Immune Response | Inhibits mitochondrial antiviral signaling (MAVS) protein to evade immune detection | MAVS dysfunction leads to impaired antiviral responses | [25] |
| Apoptosis Pathways | Activates intrinsic apoptosis pathways | Leads to cell death and tissue damage | [26] |
| Energy Production | Impairs mitochondrial oxidative phosphorylation | Leads to reduced Adenosine triphosphate (ATP) production | [27] |
| Oxidative stress | Increases reactive oxygen species (ROS) levels due to viral-induced stress | Excessive ROS damages mitochondrial DNA (mt-DNA) and proteins | [28,29] |
| Mitochondrial membrane potential (MMP) | Reduces MMP (Δψm) | Loss of MMP (Δψm) disrupts mitochondrial integrity | [30,31] |
| Calcium Homeostasis | Causes mitochondrial calcium overload | Impairs calcium signaling and buffering capacity | [32] |
| mt-DNA | Causes mt-DNA damage through oxidative stress | mt-DNA mutations disrupt mitochondrial function | [33] |
| Inflammatory Response | Triggers cytokine production (e.g., IL-6, TNF-α) | Inflammation is both a cause and consequence of dysfunction | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, S.; Varga, R.D.; Yang, J. The Impacts of Dengue Virus Infection on Mitochondrial Functions and Dynamics. Int. J. Mol. Sci. 2025, 26, 8968. https://doi.org/10.3390/ijms26188968
Ahmed S, Varga RD, Yang J. The Impacts of Dengue Virus Infection on Mitochondrial Functions and Dynamics. International Journal of Molecular Sciences. 2025; 26(18):8968. https://doi.org/10.3390/ijms26188968
Chicago/Turabian StyleAhmed, Showkot, Réka Dorottya Varga, and Jinsung Yang. 2025. "The Impacts of Dengue Virus Infection on Mitochondrial Functions and Dynamics" International Journal of Molecular Sciences 26, no. 18: 8968. https://doi.org/10.3390/ijms26188968
APA StyleAhmed, S., Varga, R. D., & Yang, J. (2025). The Impacts of Dengue Virus Infection on Mitochondrial Functions and Dynamics. International Journal of Molecular Sciences, 26(18), 8968. https://doi.org/10.3390/ijms26188968







