Folcisteine Safeguards Maize Against Copper–Cadmium Stress by Boosting the Activity of Photosynthesis-Related Enzymes and Antioxidant Defense Systems, Mediating Ascorbate–Glutathione Pathways and Hormonal Regulation
Abstract
1. Introduction
2. Results
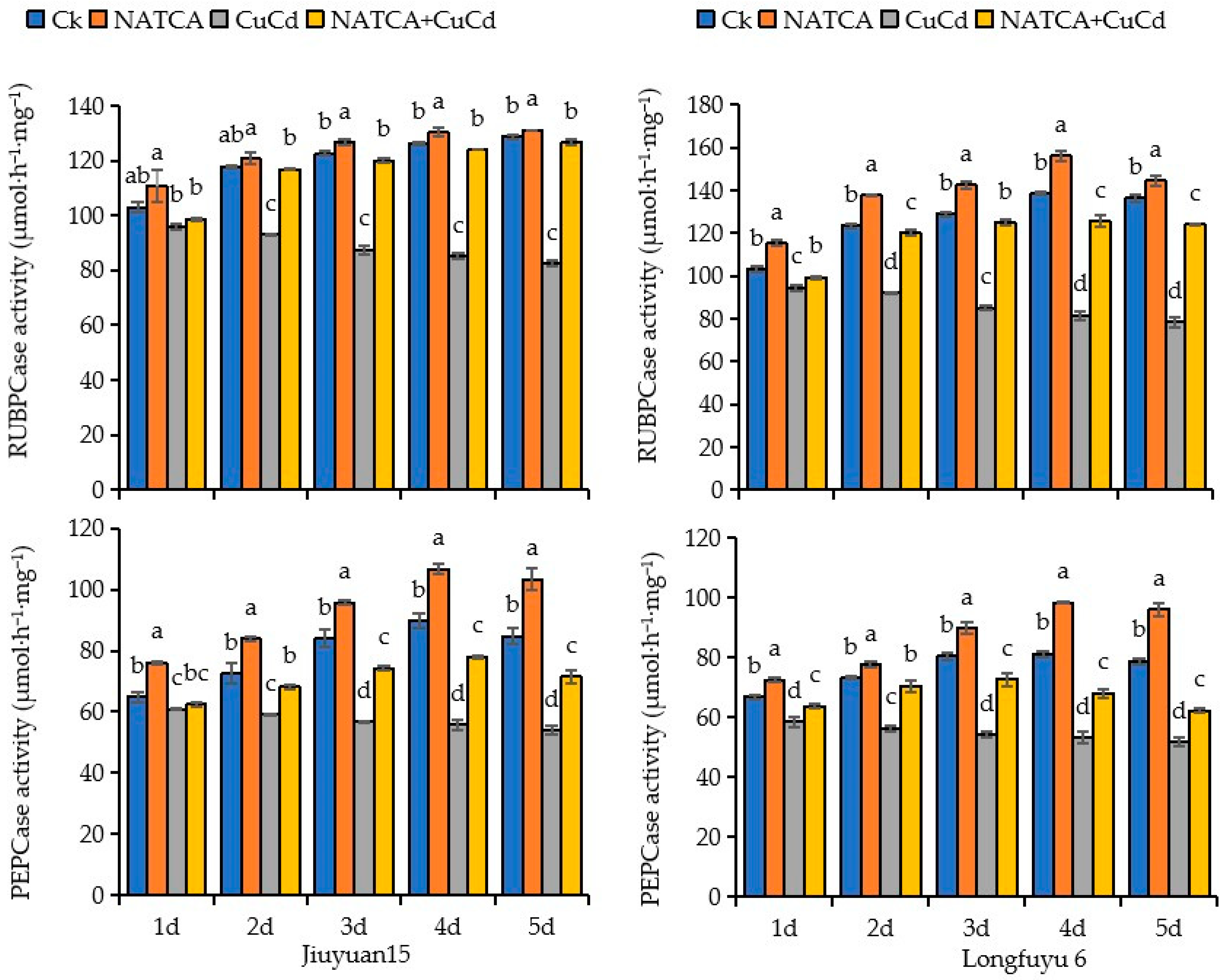
2.1. Photosynthetic Key Enzymes Activity
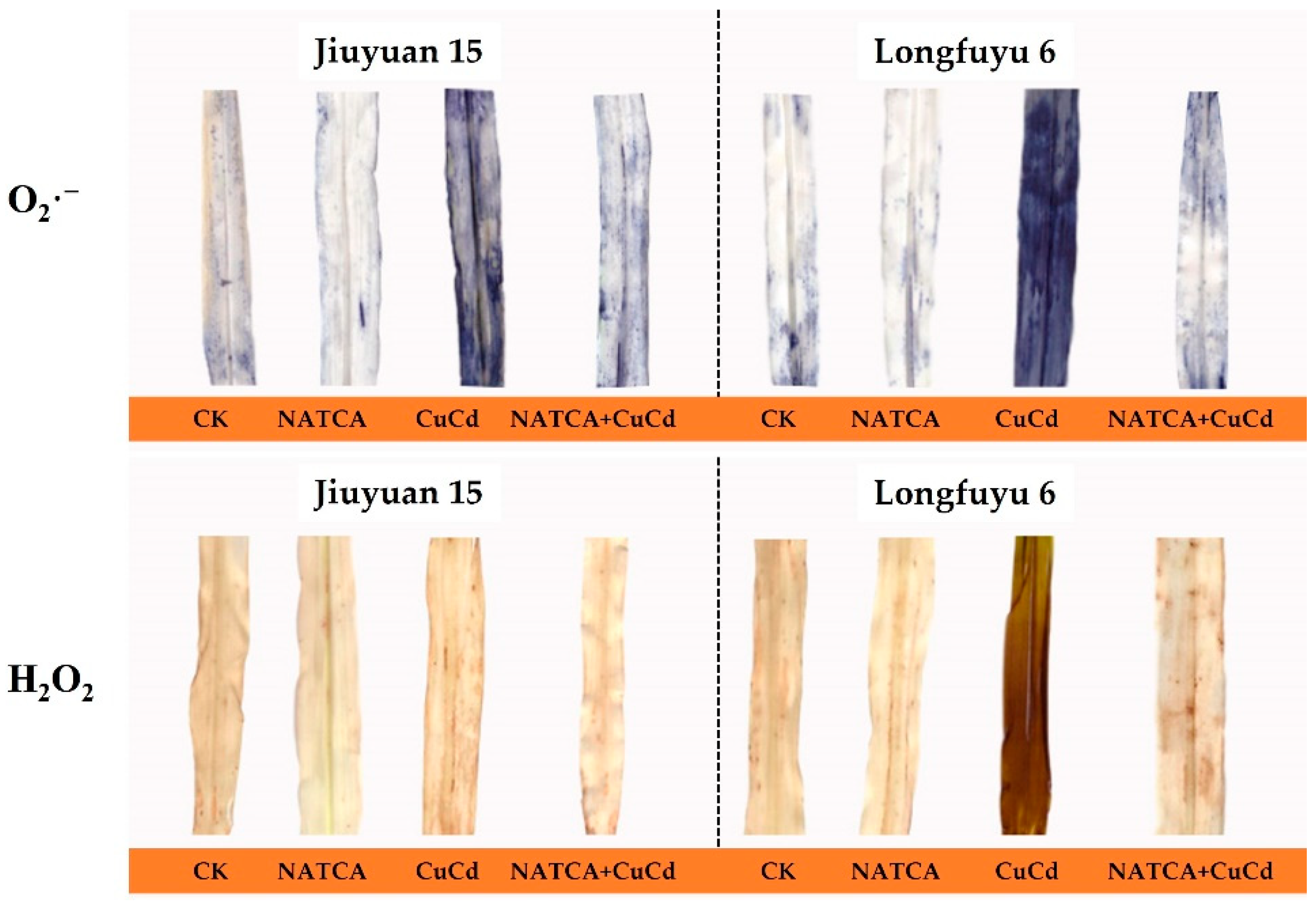
2.2. Superoxide Anion (O2·−)and Hydrogen Peroxide (H2O2)
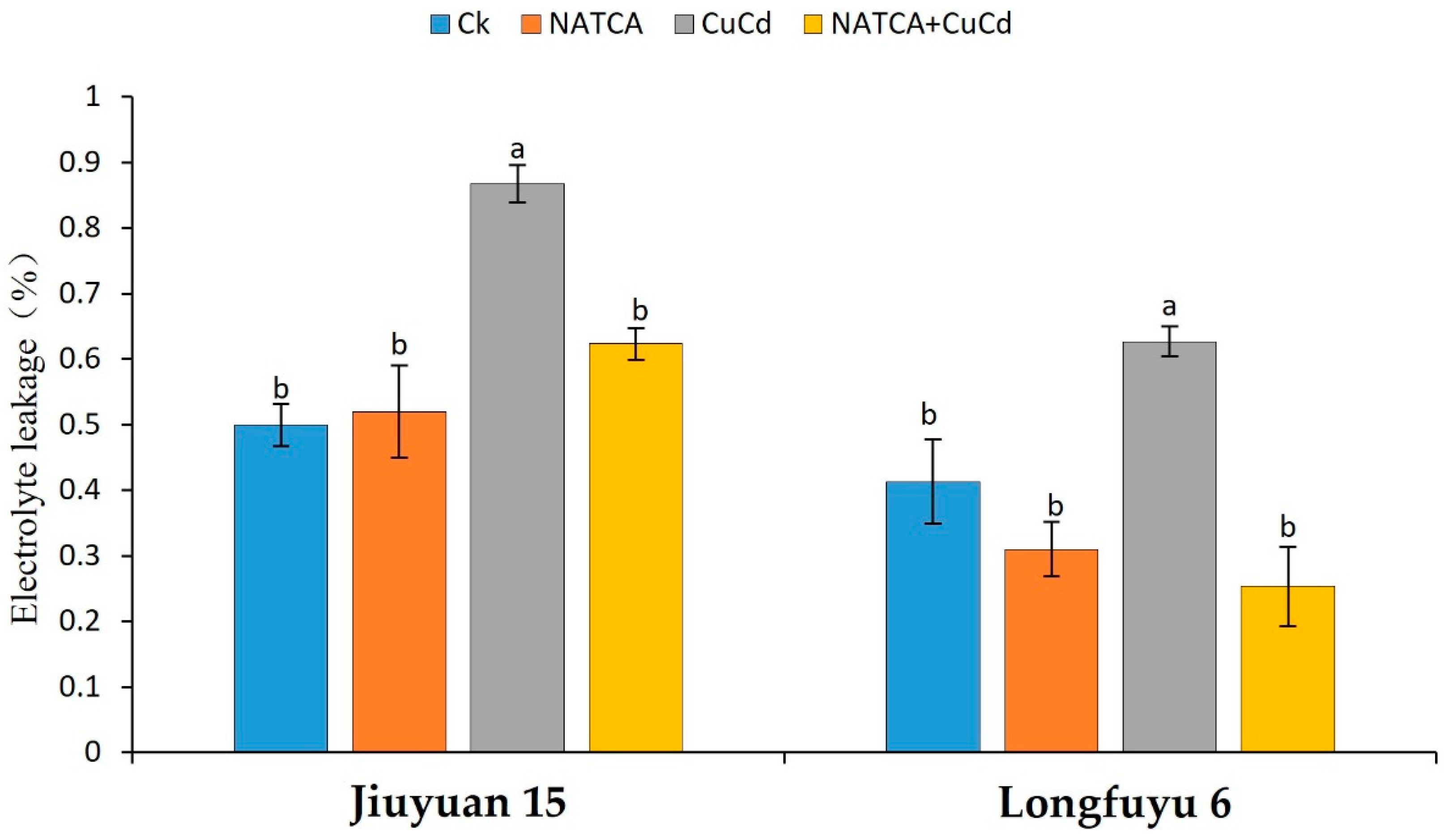
2.3. Electrolyte Leakage (EL)
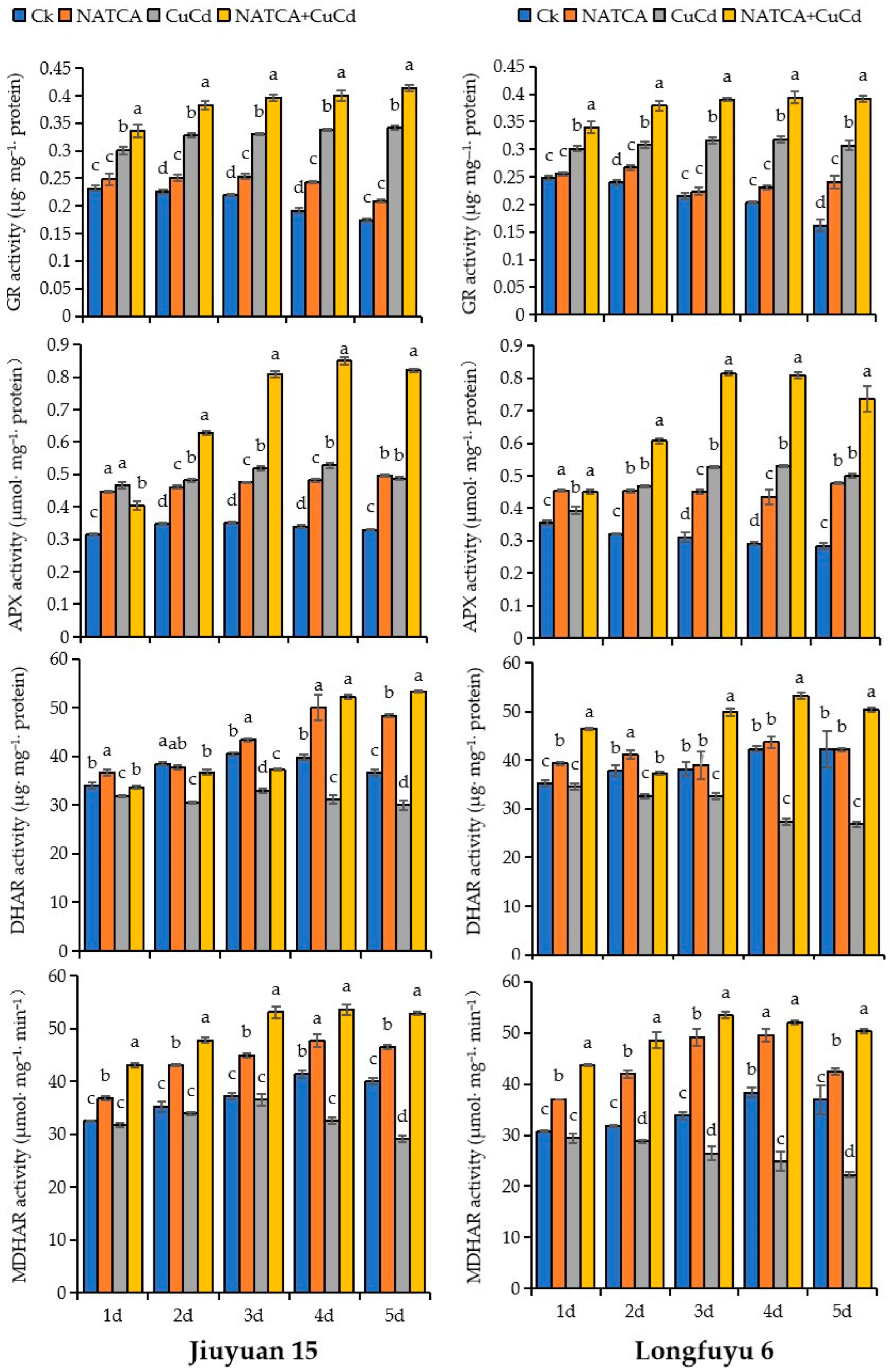
2.4. Activity of AsA–GSH Cycle Enzyme
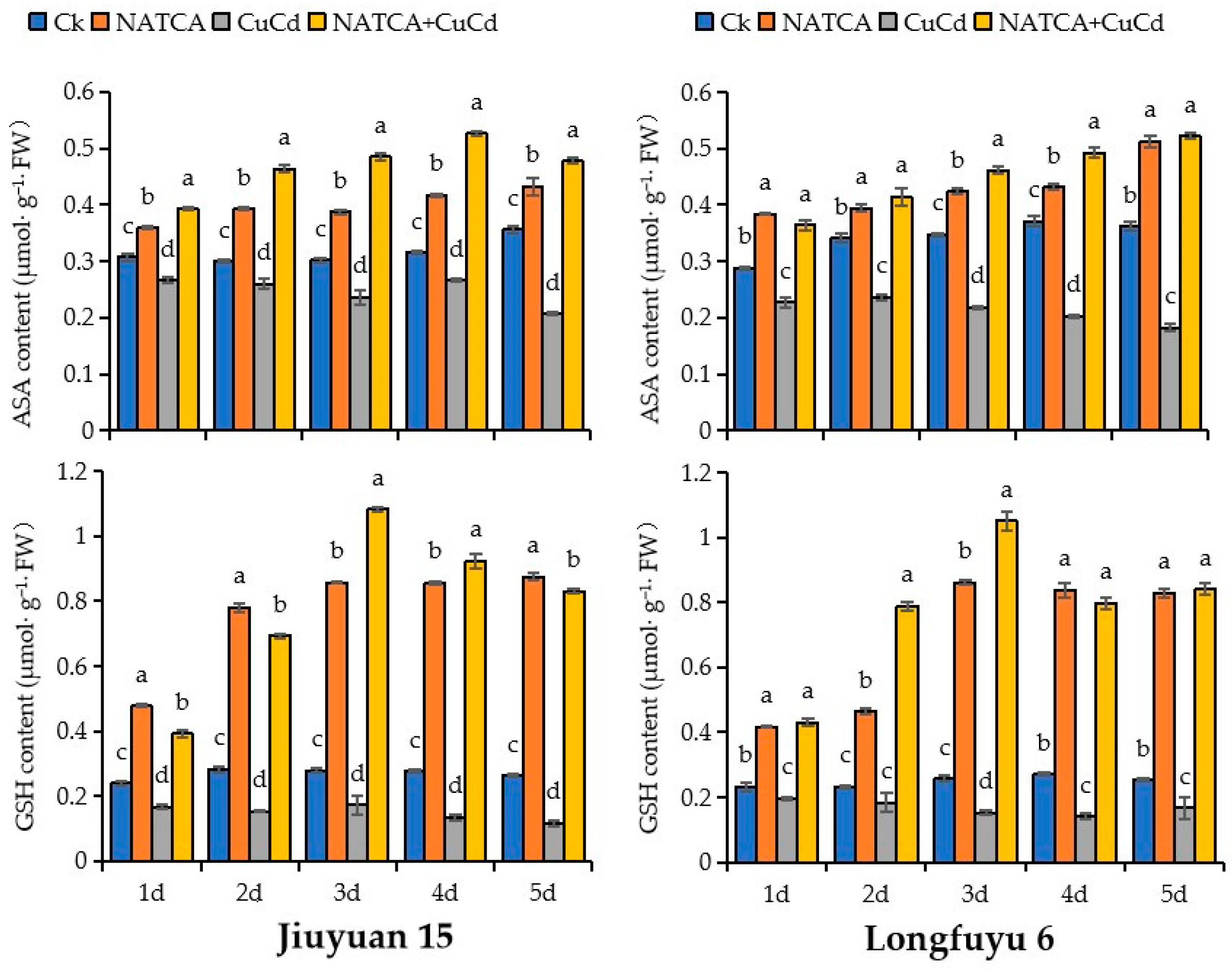
2.5. Content of AsA and GSH
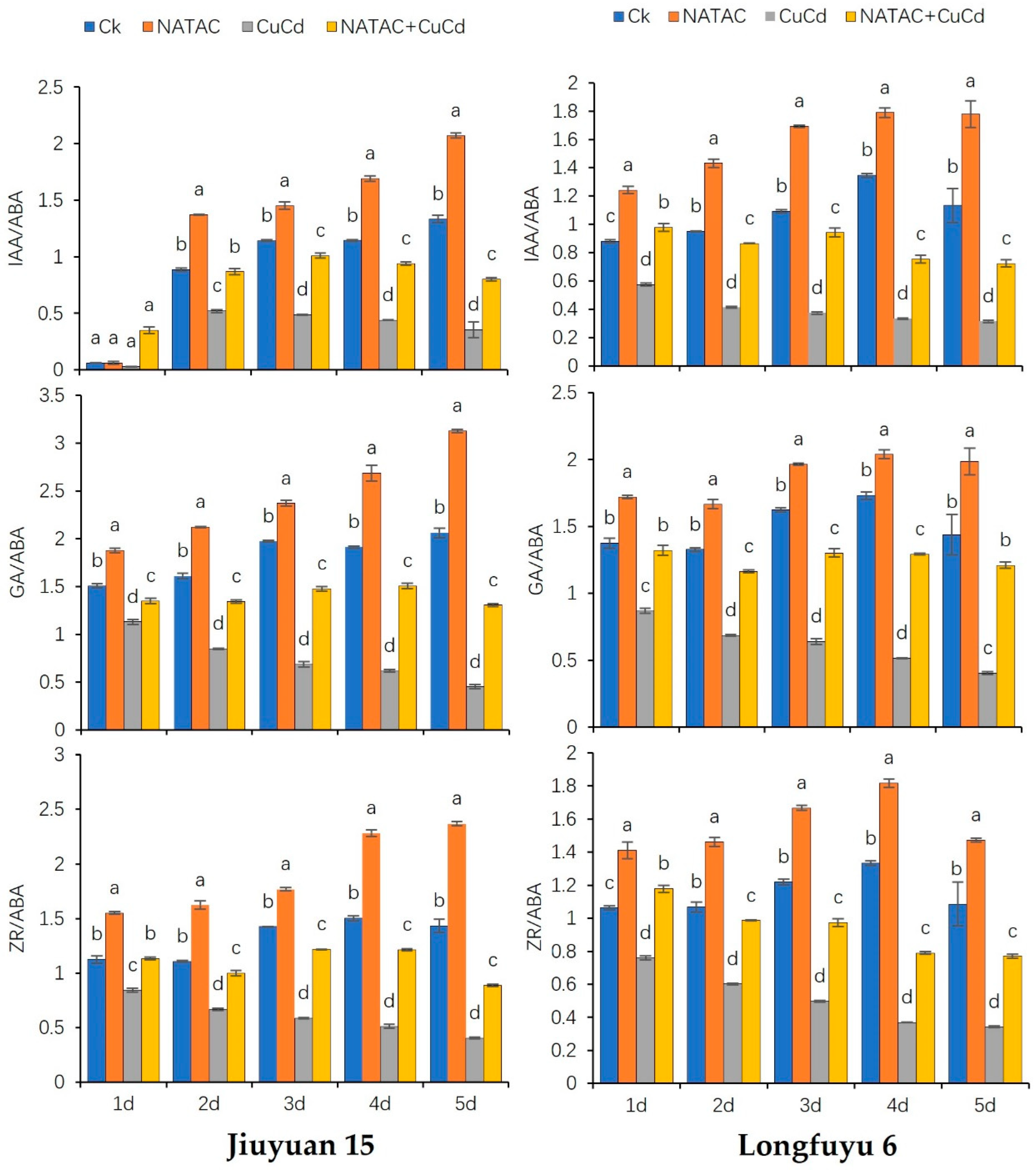
2.6. Contents of Endogenous Hormones
2.7. Balance of Endogenous Hormones
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Condition
- (1)
- Control group (CK), 1/2 Hoagland nutrient solution;
- (2)
- NATCA treatment (NATCA), add 20 mg·L−1 NATCA to 1/2 Hoagland nutrient solution;
- (3)
- (4)
- Copper and cadmium stress combined with NATCA treatment (NATCA + CuCd), 20 mg·L−1 NATCA, 80 mg·L−1 CdCl2, and 100 mg·L−1 CuSO4 were added to 1/2 Hoagland nutrient solution.
4.2. Determination of RuBPCase (Ribulose-1,5-Bisphosphate Carboxylase) and PEPCase (Phosphoenolpyruvate Carboxylase)
4.3. Determination of O2·− and H2O2
4.4. Determination of Electrolyte Leakage (EL)
4.5. Determination of Glutathione Reductase Activity (GR)
4.6. Determination of Ascorbate Peroxidase Activity (APX)
4.7. Determination of Dehydroascorbate Reductase Activity (DHAR)
4.8. Determination of Monodehydroascorbate Reductase Activity (MDHAR)
4.9. Determination of Ascorbate (AsA) Content
4.10. Determination of Glutathione (GSH) Content
4.11. Determination of Endogenous Hormones Content
4.12. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NATCA | folcisteine |
| Cu | copper |
| Cd | cadmium |
| ROS | reactive oxygen species |
| RUBPCase | ribulose-1,5-bisphosphate carboxylase |
| PEPCase | phosphoenolpyruvate carboxylase |
| AsA | ascorbate |
| GSH | glutathione |
| IAA | auxin |
| GA | gibberellin |
| ZR | zeatin nucleoside |
| ABA | abscisic acid. |
| EL | electrolyte leakage |
| GR | glutathione reductase activity |
| APX | ascorbate peroxidase activity |
| DHAR | dehydroascorbate reductase activity |
| MDHAR | monodehydroascorbate reductase activity |
| O2− | superoxide anion |
| H2O2 | hydrogen peroxide |
References
- Dai, W. Effects of Heavy Metals on Growth and Physiological Biochemical Characteristics of Maize. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2019. [Google Scholar]
- Li, Y. Differences of Cadmium Accumulation and Transport in Different Maize Varieties and Its Mechanism. Master’s Thesis, Shenyang Normal University, Shenyang, China, 2021. [Google Scholar]
- Wang, S.; Wu, W.; Liu, F.; Liao, R.; Hu, Y. Accumulation of Heavy Metals in Soil-Crop Systems: A Review for Wheat and Corn. Environ. Sci. Pollut. Res. 2017, 24, 15209–15225. [Google Scholar] [CrossRef]
- Wang, R.; Qian, C.; Wu, M.; Cheng, L. Study on heavy metals in soil mineralized by bacteria. J. Funct. Mater. 2007, 38, 1523–1526, 1530. [Google Scholar] [CrossRef]
- Gao, F.; Wang, X.; Han, J.; Liv, M.; Guo, X. Heavy metal pollution characteristics and its health risk assessment in a mollisol watershed of Northeast China: Taking Haigou watershed as study case. J. China Agric. Univ. 2020, 25, 73–83. [Google Scholar] [CrossRef]
- Zhang, W.; Long, J.; Zhang, X.; Shen, W.; Wei, Z. Pollution and Ecological Risk Evaluation of Heavy Metals in the Soil and Sediment around the HTM Tailings Pond, Northeastern China. Int. J. Environ. Res. Public Health 2020, 17, 7072. [Google Scholar] [CrossRef]
- Armienta, M.A.; Beltrán, M.; Martínez, S.; Labastida, I. Heavy Metal Assimilation in Maize (Zea mays L.) Plants Growing near Mine Tailings. Environ. Geochem. Health 2020, 42, 2361–2375. [Google Scholar] [CrossRef]
- Wei, X.; Zhou, Y.; Jiang, Y.; Tsang, D.C.W.; Zhang, C.; Liu, J.; Zhou, Y.; Yin, M.; Wang, J.; Shen, N.; et al. Health Risks of Metal(Loid)s in Maize (Zea mays L.) in an Artisanal Zinc Smelting Zone and Source Fingerprinting by Lead Isotope. Sci. Total Environ. 2020, 742, 140321. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.A.; Naveed, M.; Ahmad, Z.; Gao, B.; Mustafa, A.; Núñez-Delgado, A. Combined Application of Biochar and Sulfur Regulated Growth, Physiological, Antioxidant Responses and Cr Removal Capacity of Maize (Zea mays L.) in Tannery Polluted Soils. J. Environ. Manag. 2020, 259, 110051. [Google Scholar] [CrossRef]
- Dou, X.; Li, B.; Cui, J.; Li, G.; Wang, Y. Assessment of heavy metal pollution and risk of farmland soil and agricultural products around a smelter in Liaoning. J. Agro-Environ. Sci. 2020, 39, 2249–2258. [Google Scholar] [CrossRef]
- Duan, L. The Remediation Research of Cu and Cd Contaminated in Wastewater Irrigation Farmland. Master’s Thesis, Beijing Jiaotong University, Beijing, China, 2012. [Google Scholar]
- Liu, P.; Zhang, Y.; Feng, N.; Zhu, M.; Tian, J. Potentially Toxic Element (PTE) Levels in Maize, Soil, and Irrigation Water and Health Risks through Maize Consumption in Northern Ningxia, China. BMC Public Health 2020, 20, 1729. [Google Scholar] [CrossRef]
- Hua, X.; Cheng, B.; Zhao, R.; Huo, X.; Guo, X. Effect of Steel Slag Application on Maize Production in Farmland. J. Shanxi Agric. Sci. 2015, 43, 43–46. [Google Scholar] [CrossRef]
- Carbonell, G.; Imperial, R.M.D.; Torrijos, M.; Delgado, M.; Rodriguez, J.A. Effects of Municipal Solid Waste Compost and Mineral Fertilizer Amendments on Soil Properties and Heavy Metals Distribution in Maize Plants (Zea mays L.). Chemosphere 2011, 85, 1614–1623. [Google Scholar] [CrossRef]
- Huang, S.; Song, Q.; Li, Q.; Zhang, H.; Luo, X.; Zheng, Z. Damage of Heavy Metals to Vallisneria natans (V. natans) and Characterization of Microbial Community in Biofilm. Aquat. Toxicol. 2020, 225, 105515. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, Y.; Zheng, Y.; Li, J. Effects of Cadmium and Lead Stress on Growth and Photosynthetic Physiology of Populus yunnanensis Seedlings. J. Ecol. Rural Environ. 2021, 37, 1331–1340. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, H. The Effect of Copper Stress on Pigment Content, Photosynthetic Characteristics and Osmotic Regulation System of Luffa cylindrica Seedlings. Tianjin Agric. Sci. 2021, 27, 9–12. [Google Scholar] [CrossRef]
- Hu, M. Effect of FA on the Amelioration of Tomato Under the Stress of Copper, Cadmium. Master’s Thesis, Shandong Agricultural University, Taian, China, 2018. [Google Scholar]
- Han, T.-W.; Tseng, C.-C.; Cai, M.; Chen, K.; Cheng, S.-Y.; Wang, J. Effects of Cadmium on Bioaccumulation, Bioabsorption, and Photosynthesis in Sarcodia suiae. Int. J. Environ. Res. Public Health 2020, 17, 1294. [Google Scholar] [CrossRef]
- Gao, M.; Yang, Y.; Song, Z. Effects of Graphene Oxide on Cadmium Uptake and Photosynthesis Performance in Wheat Seedlings. Ecotoxicol. Environ. Saf. 2019, 173, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, A.; Sell, C. Enhanced Stress-Induced Senescence-Response May Increase Species Lifespan. Aging 2021, 13, 15694–15696. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Dai, J.; Shen, X.; Wang, L.; Cui, Q.; Zhu, Y. The effect of water stress on the photosynthetic performance and yield of maize. Acta Agron. Sin. 1995, 21, 356–363. [Google Scholar]
- Song, M. Study on the photosynthetic characteristics and changes in starch synthesis related enzyme activities of regenerated maize hairy root plants. J. Tonghua Norm. Univ. 2021, 42, 86–89. [Google Scholar] [CrossRef]
- Taylor, T.C.; Andersson, I. The Structure of the Complex between Rubisco and Its Natural Substrate Ribulose 1,5-Bisphosphate. J. Mol. Biol. 1997, 265, 432–444. [Google Scholar] [CrossRef]
- Portis, A.R. Regulation of Ribulose 1,5-Bisphosphate Carboxylase/Oxygenase Activity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 415–437. [Google Scholar] [CrossRef]
- Hussain, I.; Iqbal, M.; Qurat-Ul-Ain, S.; Rasheed, R.; Mahmood, S.; Wahid, A. Cadmium Dose and Exposure-Time Dependent Alterations in Growth and Physiology of Maize (Zea mays). Int. J. Agric. Biol. 2012, 14, 959–964. [Google Scholar] [CrossRef]
- Horváth, G.; Droppa, M.; Oravecz, Á.; Raskin, V.I.; Marder, J.B. Formation of the Photosynthetic Apparatus during Greening of Cadmium-Poisoned Barley Leaves. Planta 1996, 199, 238–243. [Google Scholar] [CrossRef]
- Khairy, A.I.H.; Oh, M.J.; Lee, S.M.; Kim, D.S.; Roh, K.S. Nitric Oxide Overcomes Cd and Cu Toxicity in in Vitro-Grown Tobacco Plants through Increasing Contents and Activities of Rubisco and Rubisco Activase. Biochim. Open 2016, 2, 41–51. [Google Scholar] [CrossRef]
- Peco, J.D.; Campos, J.A.; Romero-Puertas, M.C.; Olmedilla, A.; Higueras, P.; Sandalio, L.M. Characterization of Mechanisms Involved in Tolerance and Accumulation of Cd in Biscutella auriculata L. Ecotoxicol. Environ. Saf. 2020, 201, 110784. [Google Scholar] [CrossRef]
- Hu, Z. The Responses of Corn Seedlings to Copper Stress as well as Comparison with Responses to Cadmium Stress. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2008. [Google Scholar]
- Perfus-Barbeoch, L.; Leonhardt, N.; Vavasseur, A.; Forestier, C. Heavy Metal Toxicity: Cadmium Permeates through Calcium Channels and Disturbs the Plant Water Status. Plant J. 2002, 32, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, W.; Lian, J.; Shen, M.; Huo, X. Effects of Electric Fields on Cd Accumulation and Photosynthesis in Zea mays Seedlings. J. Environ. Manag. 2020, 276, 111328. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Zia Ur Rehman, M.; Adrees, M.; Arshad, M.; Qayyum, M.F.; Ali, L.; Hussain, A.; Chatha, S.A.S.; Imran, M. Alleviation of Cadmium Accumulation in Maize (Zea mays L.) by Foliar Spray of Zinc Oxide Nanoparticles and Biochar to Contaminated Soil. Environ. Pollut. 2019, 248, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-C.; Zhang, S.-L.; Wu, K.-J.; Li, R.; He, X.-R.; He, D.-N.; Huang, C.; Wei, H. The Effects of Exogenous Organic Acids on the Growth, Photosynthesis and Cellular Ultrastructure of Salix variegata Franch. Under Cd Stress. Ecotoxicol. Environ. Saf. 2020, 187, 109790. [Google Scholar] [CrossRef]
- Dorta, D.J.; Leite, S.; DeMarco, K.C.; Prado, I.M.R.; Rodrigues, T.; Mingatto, F.E.; Uyemura, S.A.; Santos, A.C.; Curti, C. A Proposed Sequence of Events for Cadmium-Induced Mitochondrial Impairment. J. Inorg. Biochem. 2003, 97, 251–257. [Google Scholar] [CrossRef]
- Tanwir, K.; Javed, M.T.; Abbas, S.; Shahid, M.; Akram, M.S.; Chaudhary, H.J.; Iqbal, M. Serratia Sp. CP-13 Alleviates Cd Toxicity by Morpho-Physio-Biochemical Improvements, Antioxidative Potential and Diminished Cd Uptake in Zea mays L. Cultivars Differing in Cd Tolerance. Ecotoxicol. Environ. Saf. 2021, 208, 111584. [Google Scholar] [CrossRef] [PubMed]
- Casalino, E.; Sblano, C.; Landriscina, C. Enzyme Activity Alteration by Cadmium Administration to Rats: The Possibility of Iron Involvement in Lipid Peroxidation. Arch. Biochem. Biophys. 1997, 346, 171–179. [Google Scholar] [CrossRef]
- Rizvi, A.; Khan, M.S. Heavy Metal Induced Oxidative Damage and Root Morphology Alterations of Maize (Zea mays L.) Plants and Stress Mitigation by Metal Tolerant Nitrogen Fixing Azotobacter Chroococcum. Ecotoxicol. Environ. Saf. 2018, 157, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y. Effects Of Cadmium On Physiological and Metabolic Characteristics of Fuyou 9 and Shenyu 33 Maize During Germination. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2020. [Google Scholar]
- Zhang, F.; Hao, S.; Chen, K. Effect of Copper Stress on Seedling Biomass, Photosynthetic Pigment and Oxygen Metabolism in Petunia hybrida Vilm. J. West China For. Sci. 2017, 46, 97–102. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Zhu, R. Effects of Exogenous Brassinolide on Root Development of Kentucky Bluegrass Under Cadmium Stres. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2021. [Google Scholar]
- Luo, J. The Study of Endogenous Hormone on the Leaf of Different Rice Materials Under Cadmium Stress. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2013. [Google Scholar]
- Guo, Y. The Effects of Low Temperature Stress on Corn Growth. Guangdong Seric. 2021, 55, 15–16. [Google Scholar] [CrossRef]
- Han, X. Alleviating Effect of Spraying Salicylic Acid and 6-Benzylamino Adenine on High Temperature Stress of Summer Maize. Master’s Thesis, Shandong Agricultural University, Taian, China, 2022. [Google Scholar]
- Leng, X. Study on the Molecular Mechanism of Grape in Response to Copper Stress. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2017. [Google Scholar]
- Wang, Q. Effects of Exogenous Nitric Oxide and Salhicvhic Acid on Physiological Characteristics of Ryegrass Under Copper, Lead and Cadmium Stress. Master’s Thesis, Shandong Agricultural University, Taian, China, 2013. [Google Scholar]
- López-Ruiz, B.A.; Zluhan-Martínez, E.; Sánchez, M.D.L.P.; Álvarez-Buylla, E.R.; Garay-Arroyo, A. Interplay between Hormones and Several Abiotic Stress Conditions on Arabidopsis Thaliana Primary Root Development. Cells 2020, 9, 2576. [Google Scholar] [CrossRef]
- Shu, K.; Liu, X.; Xie, Q.; He, Z. Two Faces of One Seed: Hormonal Regulation of Dormancy and Germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef]
- Zhang, Q.; Gong, M.; Liu, K.; Chen, Y.; Yuan, J.; Chang, Q. Rhizoglomus intraradices Improves Plant Growth, Root Morphology and Phytohormone Balance of Robinia pseudoacacia in Arsenic-Contaminated Soils. Front. Microbiol. 2020, 11, 1428. [Google Scholar] [CrossRef]
- Peleg, Z.; Blumwald, E. Hormone Balance and Abiotic Stress Tolerance in Crop Plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.P.; Zhu, J.; Wang, P.; Zeng, J.; Tan, R.; Yang, Y.Z.; Liu, Z.M. Effect of Cd on Growth, Physiological Response, Cd Subcellular Distribution and Chemical Forms of Koelreuteria paniculata. Ecotoxicol. Environ. Saf. 2018, 160, 10–18. [Google Scholar] [CrossRef]
- Yan, H.; Filardo, F.; Hu, X.; Zhao, X.; Fu, D. Cadmium Stress Alters the Redox Reaction and Hormone Balance in Oilseed Rape (Brassica napus L.) Leaves. Environ. Sci. Pollut. Res. 2016, 23, 3758–3769. [Google Scholar] [CrossRef]
- Yan, H.; Yang, X.; Lai, F. The effect of copper stress on endogenous growth factors in rapeseed leaves. Hubei Agric. Sci 2018, 57, 23–26. [Google Scholar] [CrossRef]
- Tan, M.; Tang, J.; He, Z.; Ju, L. Effects of grafting on growth and endogenous hormones of melon (Cucumis melo L.) seedlings under copper stress. J. Northwest Sci-Tech Univ. Agric. For. (Nat. Sci. Ed.) 2016, 44, 113–120. [Google Scholar] [CrossRef]
- Wang, M.; Lin, W.; Ma, B.; Li, M.; Tian, P. Effect of endophyte infection on growth and endogenous hormones of Festuca sinensis under Zn and Cd treatments. Pratac. Sci. 2019, 36, 2250–2258. [Google Scholar] [CrossRef]
- Ni, S.; Wang, W.; Chen, H. A Plant Growth Regulator Composition, Its Preparation Method, and Application 2020. Patent, China Application Number CN 202010693241.4, 17 July 2020. [Google Scholar]
- Zheng, X.; Wan, Q.; Yao, F.; Liu, J. A Crop Growth Regulator Composition and Its Application. Patent, China Application Number CN 201911273679.0, 12 December 2019. [Google Scholar]
- Liu, R.; Wang, C.; Han, A.; Li, D.; Xu, L.; Zhou, F.; Zhang, P.; Li, J.; Zhang, B. A Growth Regulator Composition Containing Sodium Salicylate and Hemiphyllin 2021. Patent, China Application Number CN 202110711097.7, 25 June 2021. [Google Scholar]
- Yao, F.; Wan, C.; Liu, J.; Xu, W.; Zheng, X. Research on Biological Activity of Acetyl-Thiazolidine-4-Carboxylic Acid in Seedling Stage of Several Crops. Mod. Agrochem. 2021, 20, 62–64. [Google Scholar] [CrossRef]
- Hota, D.; Bhoyar, M.G.; Sharma, D. Analysis of Vegetative Growth by Spraying of Forchlorfenuron and N-Acetyl Thiazolidine 4-Carboxylic Acid on of Apricot (Prunus armeniaca L.) Cv. New Castle. Int. J. Chem. Stud. 2017, 5, 2182–2185. [Google Scholar]
- Hota, D.; Sharma, D.P.; Sharma, N. Effect of Forchlorfenuron and N-Acetyl Thiazolidine 4-Carboxylic Acid on Vegetative Growth and Fruit Set of Apricot (Prunus armeniaca L.) Cv. New Castle. J. Pharmacogn. Phytochem. 2017, 6, 279–282. [Google Scholar]
- Hota, D.; Sharma, D.P.; Sharma, N.; Mishra, G.; Singh Solanki, S.P.; Priyadarshi, V. Effect of Forchlorfenuron and N-Acetyl Thiazolidine 4-Carboxylic Acid on Size and Yield of Apricot (Prunus armeniaca L.) Cv. New Castle. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1852–1860. [Google Scholar] [CrossRef]
- Hota, D.; Sharma, D.P.; Singh, N. Effect of Forchlorfenuron and N-Acetyl Thiazolidine 4-Carboxylic Acid on Fruit Drop of Apricot (Prunus armeniaca L.) Cv. New Castle. Int. J. Pure Appl. BioSci. 2017, 5, 1123–1127. [Google Scholar] [CrossRef]
- Hota, D.; Sharma, D.P.; Sahoo, T. Effect of Forchlorfenuron and N-Acetyl Thiazolidine 4-Carboxylic Acid on Chemical Parameter of Apricot (Prunus armeniaca L.) Cv. New Castle. Curr. J. Appl. Sci. Technol. 2018, 31, 1–6. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy Metal Hyperaccumulating Plants: How and Why Do They Do It? And What Makes Them so Interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef]
- Jhanji, S.; Setia, R.C.; Kaur, N.; Kaur, P.; Setia, N. Role of Nitric Oxide in Cadmium-Induced Stress on Growth, Photosynthetic Components and Yield of Brassica napus L. J. Environ. Biol. 2012, 33, 1027–1032. [Google Scholar] [CrossRef]
- Sage, R.F.; Sage, T.L.; Kocacinar, F. Photorespiration and the Evolution of C4 Photosynthesis. Annu. Rev. Plant Biol. 2012, 63, 19–47. [Google Scholar] [CrossRef]
- Jobe, T.O.; Zenzen, I.; Rahimzadeh Karvansara, P.; Kopriva, S. Integration of Sulfate Assimilation with Carbon and Nitrogen Metabolism in Transition from C3 to C4 Photosynthesis. J. Environ. Biol. 2019, 70, 4211–4221. [Google Scholar] [CrossRef]
- Meng, Y. Mitigation Effect and Physiological and Ecological Regulation Mechanism of Exogenous Hemin on Maize Seedling under Cadmium Stress. Ph.D. Thesis, Northeast Agricultural University, Harbin, China, 2021. [Google Scholar]
- Li, G.; Zhang, S.; Liu, P.; Gao, H.; Wang, J.; Liu, C.; Dong, S.; Zhang, J. Effect of Cadmium on Photosystem Activities of Maize (Zea mays L.) Leaves. Sci. Agric. Sin. 2011, 44, 3118–3126. [Google Scholar] [CrossRef]
- Gao, F.; Yang, R.; Tian, K.; Zhong, Y. Effects of Copper in Pig Feces on Photosynthetic Characteristics of Maize. J. Agro-Environ. Sci. 2008, 27, 1033–1037. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, H.; Li, C.; Liang, Y.; Lu, X. Effects of exogenous Ca2+ on growth, photosynthetic characteristics and photosystem II function of maize seedlings under cadmium stress. Pratac. Sci. 2016, 25, 40–48. [Google Scholar] [CrossRef]
- McCord, J.M. The Evolution of Free Radicals and Oxidative Stress. Am. J. Med. 2000, 108, 652–659. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, Oxidative Stress and the Biology of Ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Exogenous Silicon Attenuates Cadmium-Induced Oxidative Stress in Brassica Napus L. by Modulating AsA-GSH Pathway and Glyoxalase System. Front. Plant Sci. 2017, 8, 1061. [Google Scholar] [CrossRef]
- Lin, R.; Du, W.; Wang, X.; Guo, H. Free Radical Metabolism and Response of Antioxidant Enzymes in Wheat Seedlings (Triticum aestivum L.) Exposed to Soil Cadmium. J. Agro-Environ. Sci. 2008, 27, 23–29. [Google Scholar] [CrossRef]
- Abreu, I.A.; Saraiva, L.M.; Soares, C.M.; Teixeira, M.; Cabelli, D.E. The Mechanism of Superoxide Scavenging by Archaeoglobus fulgidus Neelaredoxin. J. Biol. Chem. 2001, 276, 38995–39001. [Google Scholar] [CrossRef]
- Jonak, C. Complexity, Cross Talk and Integration of Plant MAP Kinase Signalling. Curr. Opin. Plant Biol. 2002, 5, 415–424. [Google Scholar] [CrossRef]
- Nzengue, Y.; Steiman, R.; Rachidi, W.; Favier, A.; Guiraud, P. Oxidative Stress Induced by Cadmium in the C6 Cell Line: Role of Copper and Zinc. Biol. Trace Elem. Res. 2012, 146, 410–419. [Google Scholar] [CrossRef]
- Krężel, A.; Maret, W. Different Redox States of Metallothionein/Thionein in Biological Tissue. Biochem. J. 2007, 402, 551–558. [Google Scholar] [CrossRef]
- Bulgakova, N.M.; Bulgakov, A.V. Pulsed Laser Ablation of Solids: Transition from Normal Vaporization to Phase Explosion. Appl. Phys. A 2001, 73, 199–208. [Google Scholar] [CrossRef]
- Zhao, H. The Effect of Exogenous Nitric Oxide on Typha angustifolia Seedling Under Copper. Cadmium and Combined Stress. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2017. [Google Scholar]
- Tousi, S.; Zoufan, P.; Ghahfarrokhie, A.R. Alleviation of Cadmium-Induced Phytotoxicity and Growth Improvement by Exogenous Melatonin Pretreatment in Mallow (Malva parviflora) Plants. Ecotoxicol. Environ. Saf. 2020, 206, 111403. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Rizwan, M.; Farid, M.; Shakoor, M.B.; Gill, R.A.; Najeeb, U.; Iqbal, N.; Ahmad, R. Citric Acid Assisted Phytoremediation of Copper by Brassica napus L. Ecotoxicol. Environ. Saf. 2015, 120, 310–317. [Google Scholar] [CrossRef]
- Zouari, M.; Ben Ahmed, C.; Elloumi, N.; Bellassoued, K.; Delmail, D.; Labrousse, P.; Ben Abdallah, F.; Ben Rouina, B. Impact of Proline Application on Cadmium Accumulation, Mineral Nutrition and Enzymatic Antioxidant Defense System of Olea europaea L. cv Chemlali Exposed to Cadmium Stress. Ecotoxicol. Environ. Saf. 2016, 128, 195–205. [Google Scholar] [CrossRef]
- Xu, X.; Liu, C.; Zhao, X.; Li, R.; Deng, W. Involvement of an Antioxidant Defense System in the Adaptive Response to Cadmium in Maize Seedlings (Zea mays L.). Bull. Environ. Contam. Toxicol. 2014, 93, 618–624. [Google Scholar] [CrossRef]
- Hayat, K.; Menhas, S.; Bundschuh, J.; Zhou, P.; Niazi, N.K.; Amna; Hussain, A.; Hayat, S.; Ali, H.; Wang, J.; et al. Plant Growth Promotion and Enhanced Uptake of Cd by Combinatorial Application of Bacillus pumilus and EDTA on Zea mays L. Int. J. Phytorem. 2020, 22, 1372–1384. [Google Scholar] [CrossRef]
- Tiryakioglu, M.; Eker, S.; Ozkutlu, F.; Husted, S.; Cakmak, I. Antioxidant Defense System and Cadmium Uptake in Barley Genotypes Differing in Cadmium Tolerance. J. Trace Elem. Med. Biol. 2006, 20, 181–189. [Google Scholar] [CrossRef]
- Jian, M. Enrichment and Proteomics of Cadmiumin Wheat Under Cadmium Stress. Master’s Thesis, Northwest A&F University, Xianyang, China, 2019. [Google Scholar]
- Szalai, G.; Tajti, J.; Hamow, K.Á.; Ildikó, D.; Khalil, R.; Vanková, R.; Dobrev, P.; Misheva, S.P.; Janda, T.; Pál, M. Molecular Background of Cadmium Tolerance in Rht Dwarf Wheat Mutant Is Related to a Metabolic Shift from Proline and Polyamine to Phytochelatin Synthesis. Environ. Sci. Pollut. Res. 2020, 27, 23664–23676. [Google Scholar] [CrossRef]
- Liu, J.; Wen, R.; Liu, J. Effects of Copper Stress on the Growth of Radix Isatis Seedlings. J. Shanxi Agric. Sci. 2017, 45, 1659–1661. [Google Scholar] [CrossRef]
- He, J. Studies on the Varieties of Corn and Sorghum with Cu, Cr Forms of Distribution Infuence and Response in Sewage Soil. Master’s Thesis, Shanxi Agricultural University, Taiyuan, China, 2015. [Google Scholar]
- Petrovic, D.; Krivokapic, S. The Effect of Cu, Zn, Cd, and Pb Accumulation on Biochemical Parameters (Proline, Chlorophyll) in the Water Caltrop (Trapa natans L.), Lake Skadar, Montenegro. Plants 2020, 9, 1287. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Hossain, M.S.; Mahmud, J.; Rahman, A.; Inafuku, M.; Oku, H.; Fujita, M. Coordinated Actions of Glyoxalase and Antioxidant Defense Systems in Conferring Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2017, 18, 200. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. The Role of Nitrate Reductase in Brassinosteroid-Induced Endogenous Nitric Oxide Generation to Improve Cadmium Stress Tolerance of Pepper Plants by Upregulating the Ascorbate-Glutathione Cycle. Ecotoxicol. Environ. Saf. 2020, 196, 110483. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Shahzad, B.; Ashraf, U.; Fahad, S.; Hassan, W.; Jan, S.; Khan, I.; Saleem, M.F.; et al. Osmoregulation and Antioxidant Production in Maize under Combined Cadmium and Arsenic Stress. Environ. Sci. Pollut. Res. 2016, 23, 11864–11875. [Google Scholar] [CrossRef]
- Yannarelli, G.G.; Fernández-Alvarez, A.J.; Santa-Cruz, D.M.; Tomaro, M.L. Glutathione Reductase Activity and Isoforms in Leaves and Roots of Wheat Plants Subjected to Cadmium Stress. Phytochemistry 2007, 68, 505–512. [Google Scholar] [CrossRef]
- Weng, N.; Zhou, D.; Wang, P.; Wang, D.; Chu, L. Influence of Sulfur on Subcellular Distribution, Uptake and Toxicity of Cu and Cd to Wheat Seadlings. Asian J. Ecotoxicol. 2011, 6, 87–93. [Google Scholar]
- Zhou, Y.; Huo, S.; Wang, L.; Meng, J.; Zhang, Z.; Xi, Z. Exogenous 24-Epibrassinolide Alleviates Oxidative Damage from Copper Stress in Grape (Vitis vinifera L.) Cuttings. Plant Physiol. Biochem. 2018, 130, 555–565. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, Z.; Jiao, J.; Ren, X.; Liu, Z.; Zuo, S.; Li, J. Effects of GA3 seed soaking on antioxidant enzymes and endogenous hormones of maize embryo under low temperature. Chin. J. Ecol. 2021, 40, 1340–1346. [Google Scholar] [CrossRef]
- Matayoshi, C.L.; Pena, L.B.; Arbona, V.; Gómez-Cadenas, A.; Gallego, S.M. Early Responses of Maize Seedlings to Cu Stress Include Sharp Decreases in Gibberellins and Jasmonates in the Root Apex. Protoplasma 2020, 257, 1243–1256. [Google Scholar] [CrossRef] [PubMed]
- Ben Massoud, M.; Sakouhi, L.; Karmous, I.; Zhu, Y.; El Ferjani, E.; Sheehan, D.; Chaoui, A. Protective Role of Exogenous Phytohormones on Redox Status in Pea Seedlings under Copper Stress. J. Plant Physiol. 2018, 221, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q. Physiological Response in Rice (Oryza sativa L.) Under Cadmium Stress Conditions. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2011. [Google Scholar]
- Yuan, Z.; Wu, Z. Effect of cadmium on antioxidative capability and phytohormone level in tobacco roots. Acta Ecol. Sin. 2010, 30, 4109–4118. [Google Scholar] [CrossRef]
- Rabinovici, J.; Stewart, E.A. New Interventional Techniques for Adenomyosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 617–636. [Google Scholar] [CrossRef]
- Chaoui, A.; Jarrar, B.; El Ferjani, E. Effects of Cadmium and Copper on Peroxidase, NADH Oxidase and IAA Oxidase Activities in Cell Wall, Soluble and Microsomal Membrane Fractions of Pea Roots. J. Plant Physiol. 2004, 161, 1225–1234. [Google Scholar] [CrossRef]
- Lata, C.; Prasad, M. Role of DREBs in Regulation of Abiotic Stress Responses in Plants. J. Environ. Biol. 2011, 62, 4731–4748. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Effect of Abscisic Acid on Active Oxygen Species, Antioxidative Defence System and Oxidative Damage in Leaves of Maize Seedlings. Plant Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Scandalios, J.G. Two Structurally Similar Maize Cytosolic Superoxide Dismutase Genes, Sod4 and Sod4A, Respond Differentially to Abscisic Acid and High Osmoticum. Plant Physiol. 1998, 117, 217–224. [Google Scholar] [CrossRef]
- Hsu, Y.T.; Kao, C.H. Heat Shock-Mediated H2O2 Accumulation and Protection against Cd Toxicity in Rice Seedlings. Plant Soil 2007, 300, 137–147. [Google Scholar] [CrossRef]
- Deng, J. The Effect of Zinc or Cadmium Stress on Photosynthesis and Endogenous Hormone Levels of Sedum alfredii. Master’s Thesis, Guangxi University, Nanning, China, 2014. [Google Scholar]
- Wang, Z. Effects of Cadmium Pollution on Phytohormone Contents and Growth of Glycine max Plants. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2006. [Google Scholar]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Zambrzycka, E.; Godlewska-Żyłkiewicz, B. Phytohormones as Regulators of Heavy Metal Biosorption and Toxicity in Green Alga Chlorella vulgaris (Chlorophyceae). Plant Physiol. Biochem. 2012, 52, 52–65. [Google Scholar] [CrossRef]
- Zeng, H.; Yang, W.; Lu, C.; Lin, W.; Zou, M.; Zhang, H.; Wan, J.; Huang, X. Effect of CPPU on Carbohydrate and Endogenous Hormone Levels in Young Macadamia Fruit. PLoS ONE 2016, 11, e0158705. [Google Scholar] [CrossRef]
- Zhu, K. Preliminary Study on β -Aminobutyric Acid Induce Tobacco Against Copper, Cadmium Stress. Master’s Thesis, University of Science and Technology of China: Hefei, China, 2015. [Google Scholar]
- Zhang, C. The Physiological and Biochemical Response of Perennial Ryegrass to Cadmium Stress and the Regulation Effect of Exogenous CalciumTreatment. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2020. [Google Scholar]
- Qiu, D. The Physiological Mechanism of Chitosan Relieves Cadmium Toxicity in Maize Seedlings. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2019. [Google Scholar]
- Romero-Puertas, M.C.; Palma, J.M.; Gómez, M.; Del Río, L.A.; Sandalio, L.M. Cadmium Causes the Oxidative Modification of Proteins in Pea Plants. Plant Cell Environ. 2002, 25, 677–686. [Google Scholar] [CrossRef]
- Jambunathan, N. Determination and Detection of Reactive Oxygen Species (ROS), Lipid Peroxidation, and Electrolyte Leakage in Plants. In Plant Stress Tolerance; Sunkar, R., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 639, pp. 291–297. ISBN 978-1-60761-701-3. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, J.; Zhou, H.; Sun, Y.; Yin, Y.; Pei, D.; Ji, R.; Wu, J.; Wang, X. Elevated CO2 Levels Affects the Concentrations of Copper and Cadmium in Crops Grown in Soil Contaminated with Heavy Metals under Fully Open-Air Field Conditions. Environ. Sci. Technol. 2011, 45, 6997–7003. [Google Scholar] [CrossRef]
- Hao, Z.; Cang, J.; Xu, Z. Plant Physiology; Harbin Institute of Technology Press: Harbin, China, 2004; ISBN 978-7-5603-2086-1. [Google Scholar]
- Zhang, X.; Feng, B.; Wang, H.; Xu, X.; Shi, Y.; He, Y.; Chen, Z.; Sathe, A.P.; Shi, L.; Wu, J. A Substitution Mutation in OsPELOTA Confers Bacterial Blight Resistance by Activating the Salicylic Acid Pathway. J. Integr. Plant Biol. 2018, 60, 160–172. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Variety | Treatment | 1d | 2d | 3d | 4d |
|---|---|---|---|---|---|---|
| IAA (ng·g−1·FW) | Jiuyuan15 | Ck | 159.64 ± 0.20 c | 172.18 ± 4.11 c | 220.10 ± 2.36 c | 232.47 ± 1.54 b |
| NATCA | 230.22 ± 2.87 a | 234.30 ± 2.54 a | 251.78 ± 6.68 a | 258.56 ± 4.20 a | ||
| CuCd | 153.19 ± 1.58 d | 147.94 ± 2.11 d | 149.85 ± 1.35 d | 143.03 ± 1.57 d | ||
| NATCA + CuCd | 210.38 ± 1.75 b | 199.33 ± 2.50 b | 236.07 ± 3.49 b | 208.81 ± 2.01 c | ||
| Longfuyu 6 | Ck | 208.45 ± 2.09 c | 242.94 ± 1.68 b | 252.68 ± 2.76 b | 294.48 ± 2.39 b | |
| NATCA | 274.22 ± 2.64 a | 314.91 ± 2.60 a | 337.72 ± 1.92 a | 348.69 ± 2.31 a | ||
| CuCd | 162.78 ± 0.86 d | 140.21 ± 2.91 d | 132.45 ± 1.28 d | 126.80 ± 1.85 d | ||
| NATCA + CuCd | 234.12 ± 3.41 b | 237.32 ± 2.46 c | 228.14 ± 4.39 c | 171.77 ± 5.73 c | ||
| GA (ng·g−1·FW) | Jiuyuan15 | Ck | 314.01 ± 9.62 b | 312.76 ± 5.19 b | 379.47 ± 2.09 b | 388.38 ± 2.68 b |
| NATCA | 354.85 ± 6.15 a | 361.99 ± 3.38 a | 410.63 ± 7.68 a | 409.78 ± 7.92 a | ||
| CuCd | 275.66 ± 2.17 c | 240.41 ± 1.59 c | 210.19 ± 7.29 d | 200.86 ± 4.24 d | ||
| NATCA + CuCd | 290.03 ± 4.29 c | 308.17 ± 3.31 b | 345.00 ± 3.63 c | 334.23 ± 5.39 c | ||
| Longfuyu 6 | Ck | 326.12 ± 6.27 b | 339.96 ± 1.25 b | 375.61 ± 0.95 b | 378.64 ± 4.13 b | |
| NATCA | 379.67 ± 1.89 a | 366.66 ± 4.46 a | 392.11 ± 2.48 a | 397.22 ± 3.45 a | ||
| CuCd | 246.64 ± 3.06 c | 233.02 ± 1.48 d | 227.02 ± 2.50 d | 196.18 ± 2.33 d | ||
| NATCA + CuCd | 316.14 ± 5.29 b | 319.43 ± 2.43 c | 315.37 ± 0.49 c | 295.26 ± 1.53 c | ||
| ZR (ng·g−1·FW) | Jiuyuan15 | Ck | 234.31 ± 2.57 c | 215.62 ± 1.39 c | 274.41 ± 1.43 c | 305.31 ± 4.45 b |
| NATCA | 293.33 ± 3.39 a | 277.17 ± 4.10 a | 306.52 ± 3.23 a | 348.35 ± 0.68 a | ||
| CuCd | 205.85 ± 1.96 d | 189.69 ± 1.68 d | 180.16 ± 1.24 d | 166.81 ± 5.30 d | ||
| NATCA + CuCd | 244.15 ± 1.98 b | 229.30 ± 1.71 b | 285.51 ± 2.74 b | 269.19 ± 1.19 c | ||
| Longfuyu 6 | Ck | 252.01 ± 1.64 c | 273.26 ± 5.39 b | 282.17 ± 1.34 b | 291.42 ± 2.88 b | |
| NATCA | 311.39 ± 7.83 a | 321.64 ± 3.43 a | 332.65 ± 3.46 a | 354.14 ± 1.98 a | ||
| CuCd | 215.59 ± 0.95 d | 204.52 ± 1.27 d | 176.93 ± 2.02 d | 140.68 ± 1.69 d | ||
| NATCA + CuCd | 281.88 ± 1.32 b | 270.46 ± 1.00 c | 235.82 ± 2.04 c | 180.14 ± 1.37 c | ||
| ABA (ng·g−1·FW) | Jiuyuan15 | Ck | 208.45 ± 5.03 b | 194.55 ± 2.10 c | 192.54 ± 0.82 c | 203.17 ± 0.37 c |
| NATCA | 189.07 ± 1.68 c | 170.85 ± 1.85 d | 173.35 ± 1.23 d | 152.68 ± 2.30 d | ||
| CuCd | 244.34 ± 5.01 a | 284.09 ± 4.35 a | 307.16 ± 1.53 a | 325.72 ± 2.00 a | ||
| NATCA + CuCd | 215.34 ± 1.58 b | 229.62 ± 4.48 b | 234.06 ± 2.03 b | 222.01 ± 1.35 b | ||
| Longfuyu 6 | Ck | 237.26 ± 2.69 b | 256.10 ± 2.24 c | 231.58 ± 2.17 b | 218.77 ± 0.97 c | |
| NATCA | 220.83 ± 2.54 c | 220.08 ± 3.80 d | 199.51 ± 0.41 c | 194.95 ± 2.41 d | ||
| CuCd | 283.62 ± 3.18 a | 339.86 ± 1.20 a | 355.76 ± 8.27 a | 380.63 ± 1.62 a | ||
| NATCA + CuCd | 239.50 ± 3.90 b | 274.36 ± 2.02 b | 242.58 ± 6.05 b | 228.28 ± 1.06 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.; Zhao, M.; Wei, J.; Fu, Y.; Xu, Z.; Xie, L.; Gu, W.; Zhou, Y. Folcisteine Safeguards Maize Against Copper–Cadmium Stress by Boosting the Activity of Photosynthesis-Related Enzymes and Antioxidant Defense Systems, Mediating Ascorbate–Glutathione Pathways and Hormonal Regulation. Int. J. Mol. Sci. 2025, 26, 8938. https://doi.org/10.3390/ijms26188938
Dong L, Zhao M, Wei J, Fu Y, Xu Z, Xie L, Gu W, Zhou Y. Folcisteine Safeguards Maize Against Copper–Cadmium Stress by Boosting the Activity of Photosynthesis-Related Enzymes and Antioxidant Defense Systems, Mediating Ascorbate–Glutathione Pathways and Hormonal Regulation. International Journal of Molecular Sciences. 2025; 26(18):8938. https://doi.org/10.3390/ijms26188938
Chicago/Turabian StyleDong, Ling, Meng Zhao, Jingwen Wei, Yiping Fu, Zihan Xu, Lihua Xie, Wanrong Gu, and Yu Zhou. 2025. "Folcisteine Safeguards Maize Against Copper–Cadmium Stress by Boosting the Activity of Photosynthesis-Related Enzymes and Antioxidant Defense Systems, Mediating Ascorbate–Glutathione Pathways and Hormonal Regulation" International Journal of Molecular Sciences 26, no. 18: 8938. https://doi.org/10.3390/ijms26188938
APA StyleDong, L., Zhao, M., Wei, J., Fu, Y., Xu, Z., Xie, L., Gu, W., & Zhou, Y. (2025). Folcisteine Safeguards Maize Against Copper–Cadmium Stress by Boosting the Activity of Photosynthesis-Related Enzymes and Antioxidant Defense Systems, Mediating Ascorbate–Glutathione Pathways and Hormonal Regulation. International Journal of Molecular Sciences, 26(18), 8938. https://doi.org/10.3390/ijms26188938






