A Pre-Clinical Study on the Use of the Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitor PEP 2-8 to Mitigate Ischemic Injury in a Rat Marginal Donor Model
Abstract
1. Introduction
2. Results
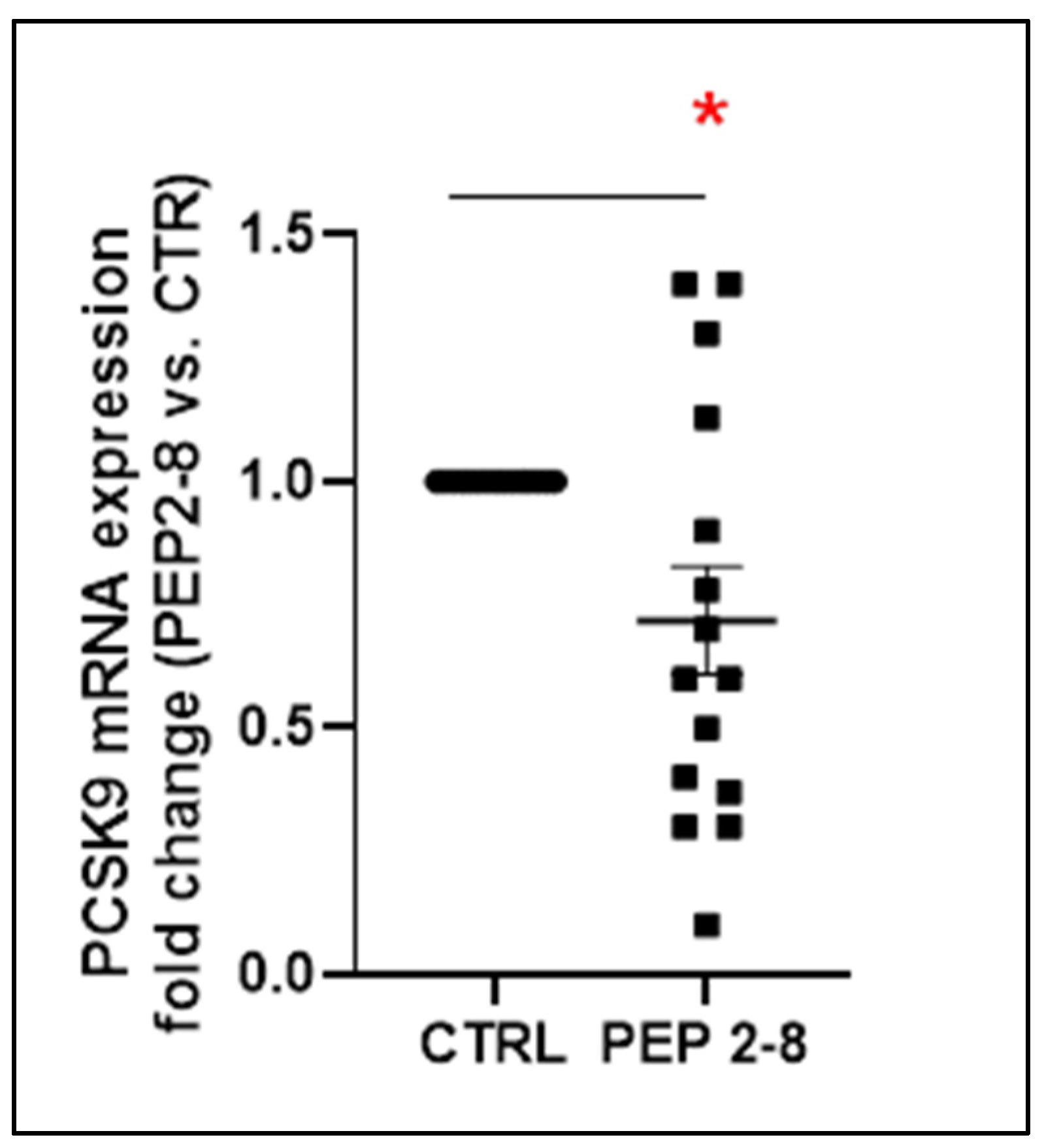
2.1. Reduced PCSK9 Gene Expression After Renal Conditioning with PEP 2-8
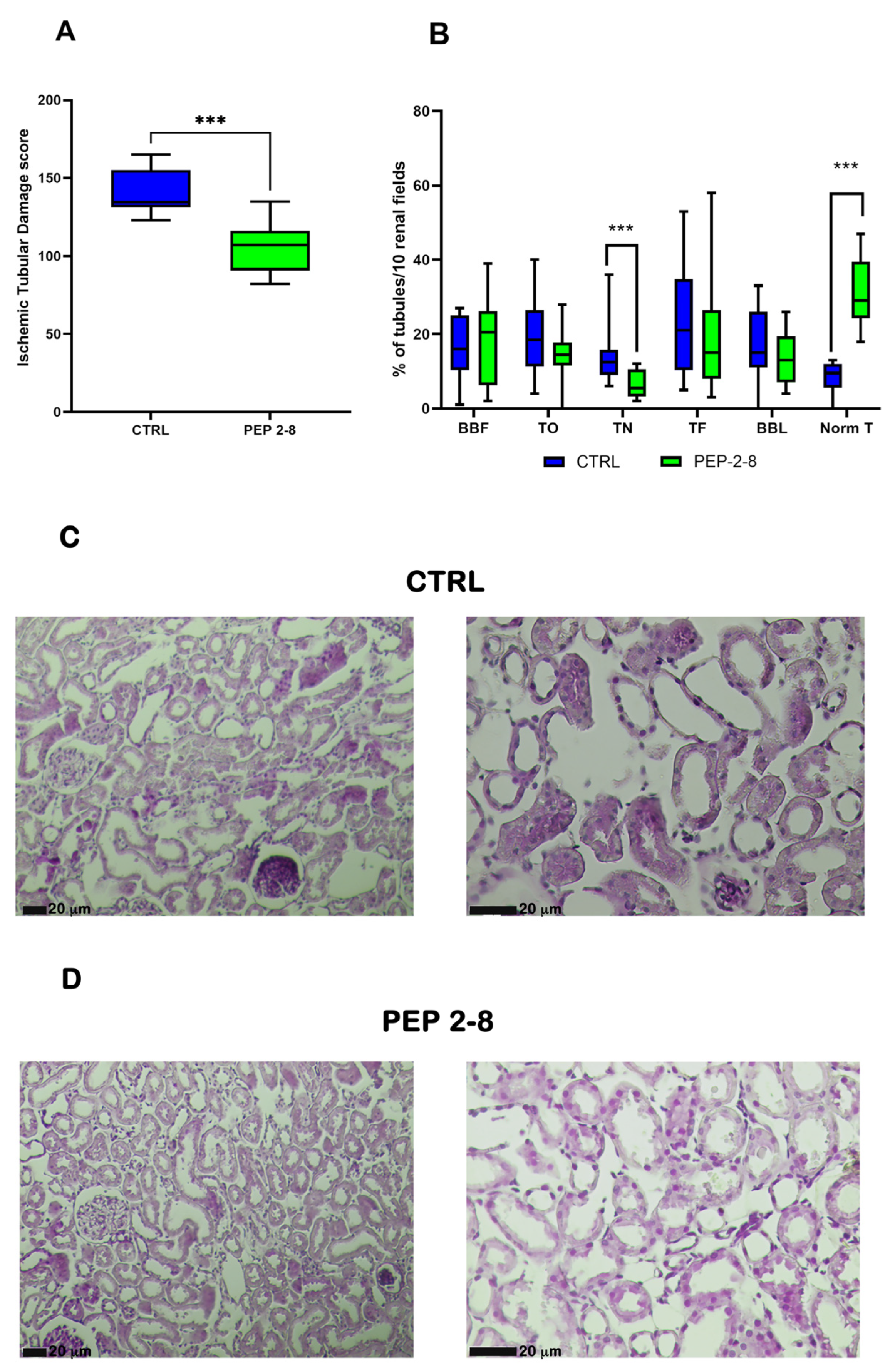
2.2. Reduction in Ischemic Tubular Damage in Kidneys Treated with PCSK9 Inhibitors
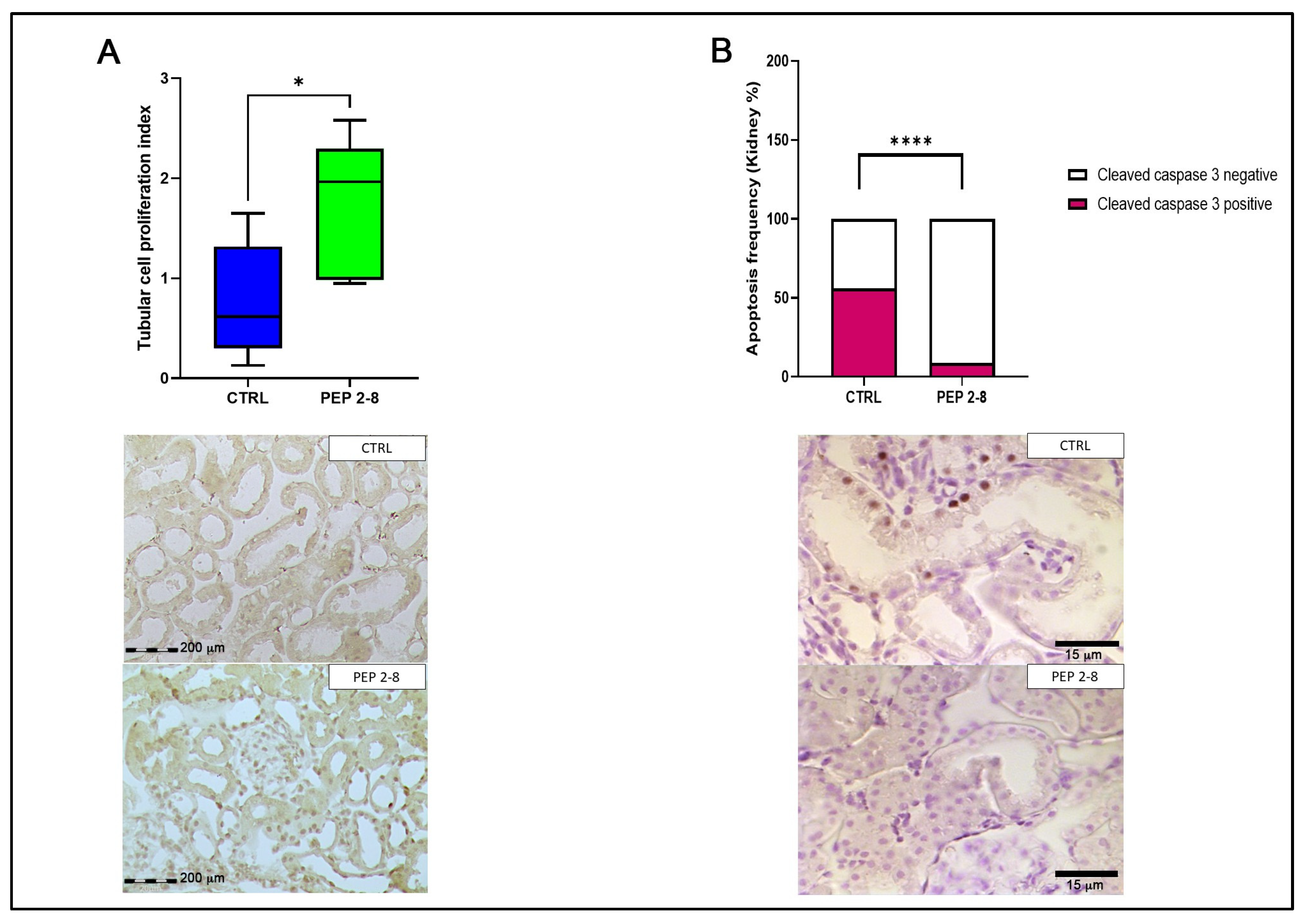
2.3. Preserved Tubular Regeneration and Reduced Apoptosis in Kidneys Treated with PEP 2-8
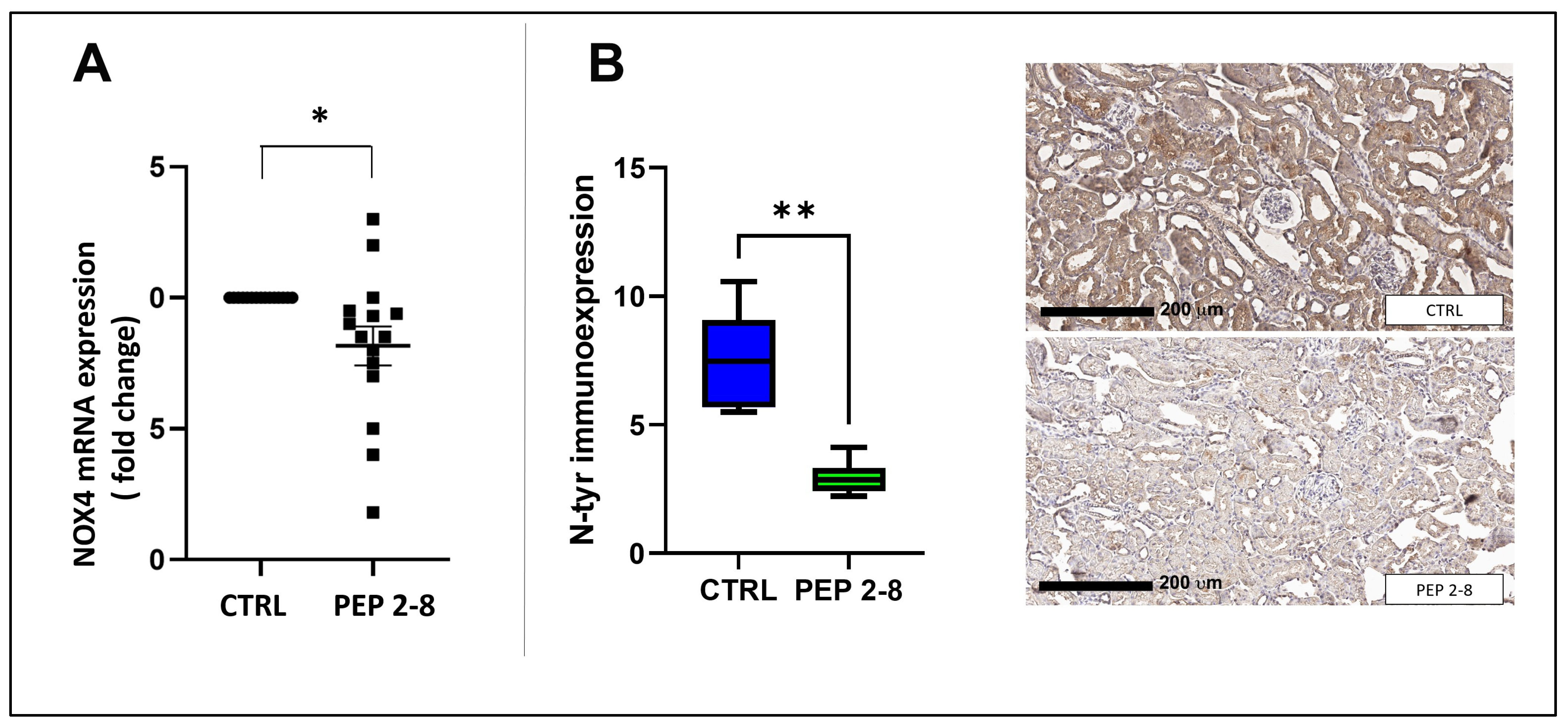
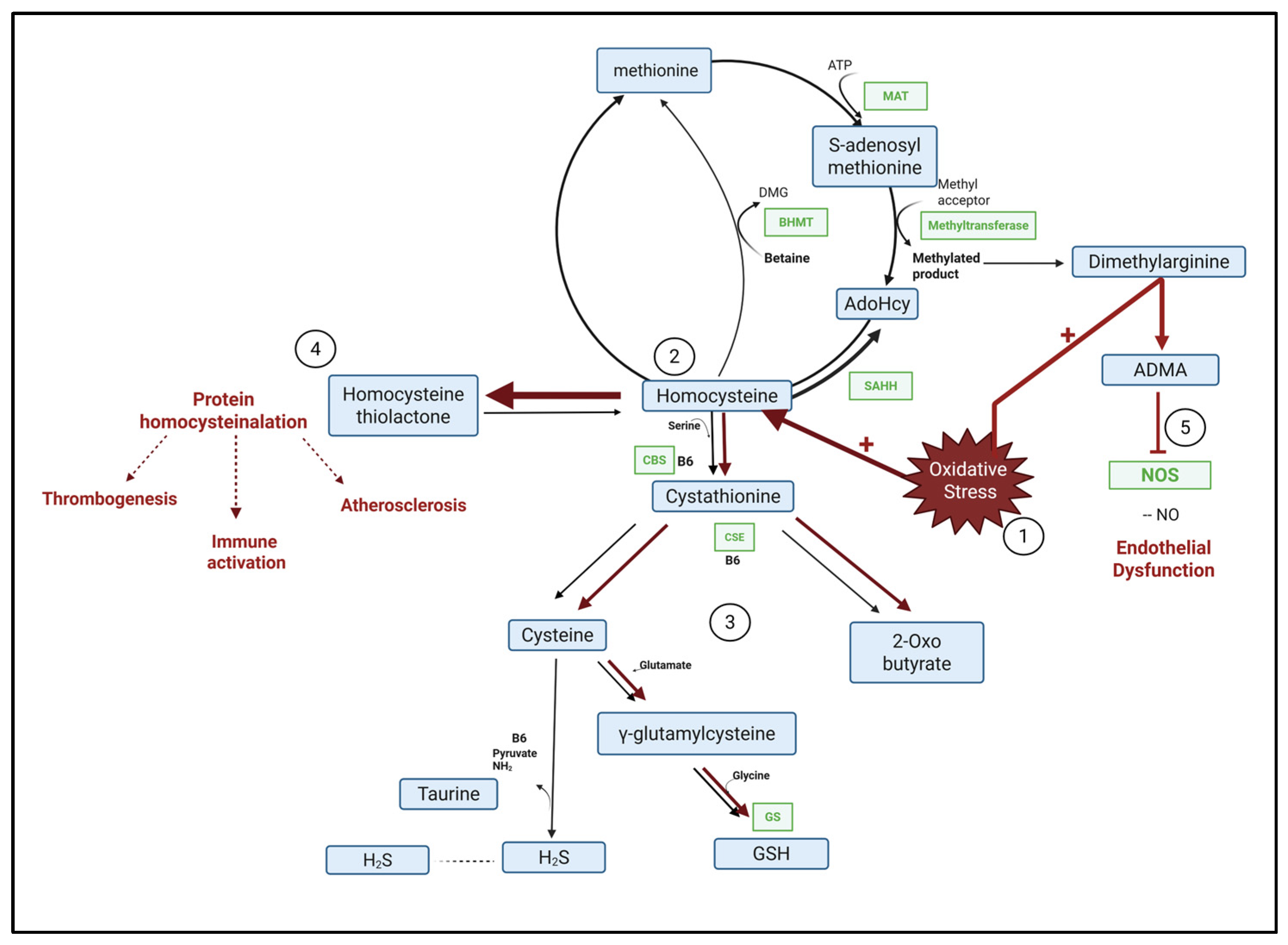
2.4. Reduced Oxidative Stress in Kidneys Treated with PEP 2-8
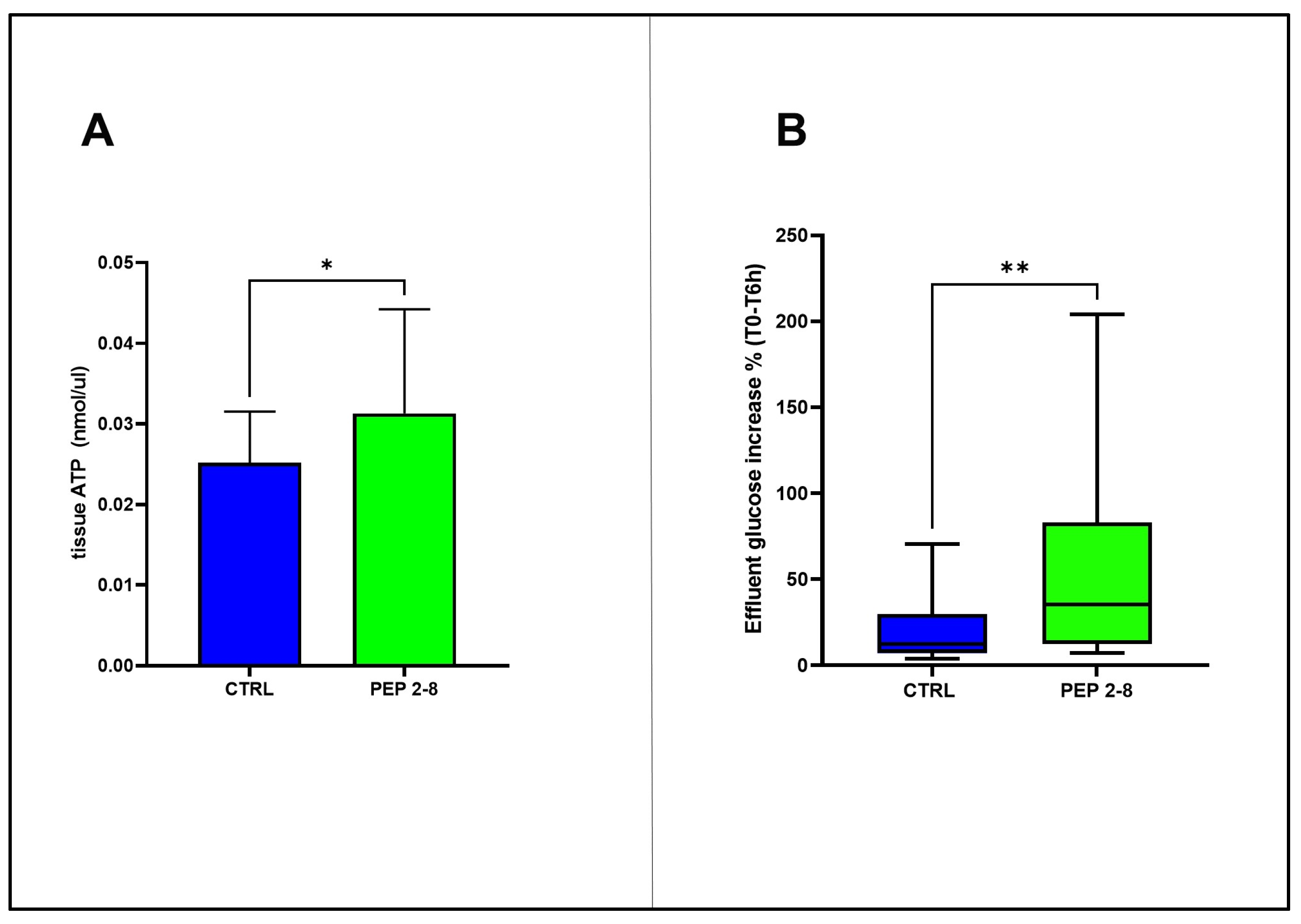
2.5. Sustained Metabolic Activity in Kidneys Treated with PEP 2-8
2.6. Metabolomics Profiles of Treated and Control Kidneys
3. Discussion
Limitations and Strengths of the Study
4. Materials and Methods
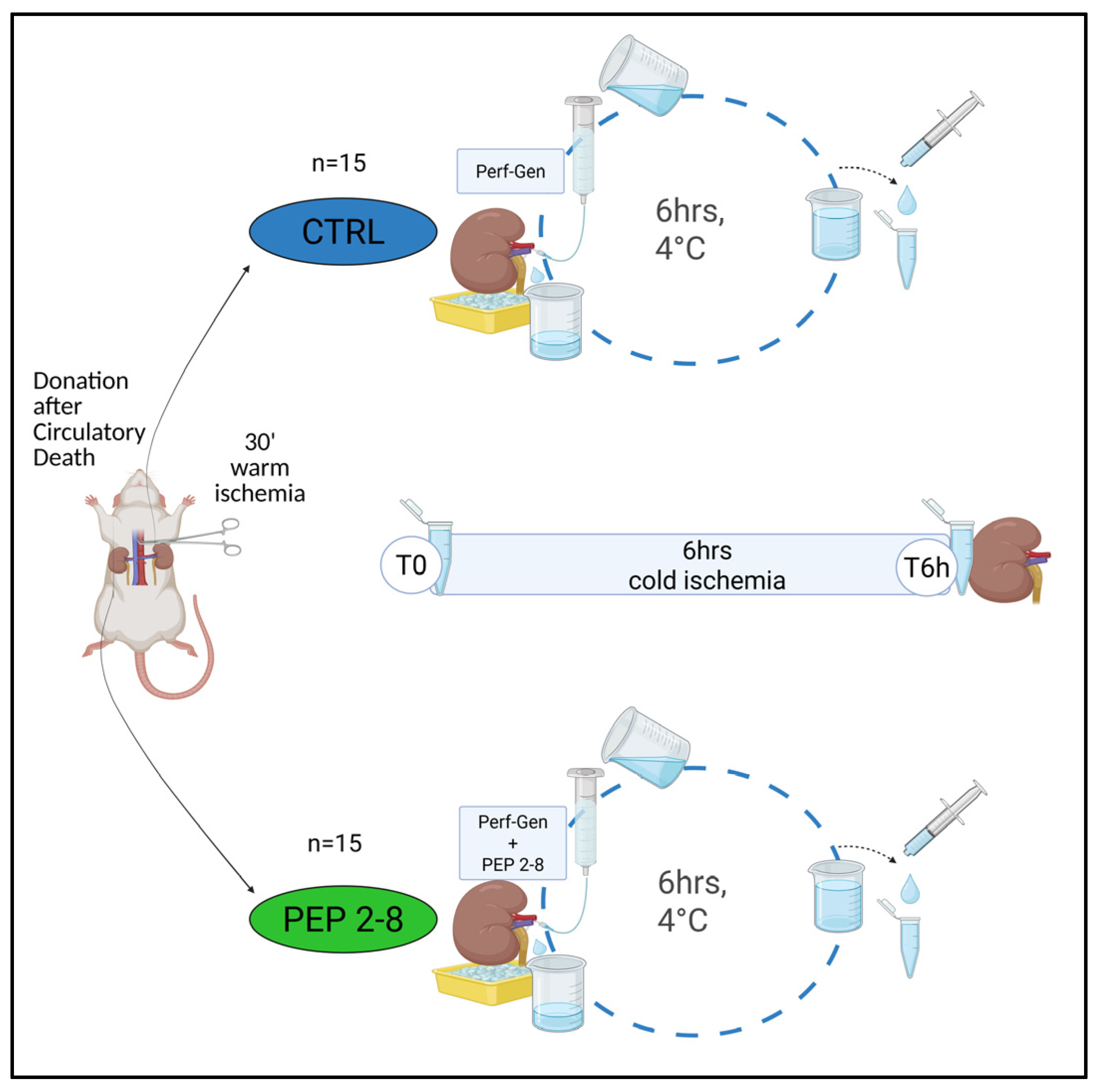
4.1. Animal DCD Model
- (A)
- CTRL (control group, n = 15): Left kidneys perfused with Perf-Gen solution (IGL Group, Lissieu, France).
- (B)
- PEP 2-8 (treated group, n = 15): Right kidneys perfused with Perf-Gen solution supplemented with PCSK9 inhibitor.
4.2. Samples Collection
4.3. Tubular Ischemic Damage Score
4.4. Tubular Proliferation Index and N-Tyrosine Staining
4.5. Apoptosis
4.6. RNA Extraction, Reverse Transcriptase, and Polymerase Chain Reaction
| Gene | Accession Number | Primer Forward 5′→3′ | Primer Reverse 5′→3′ |
| Pcsk9 | NM_199253 | CAT GGA ACC TGG AGC GGA TT | ACC TGG CTA CTT CCG TCA GG |
| NOX4 | NM_053524.1 | TTT CTC AGG TGT GCA TGT AGC | GCG TAG GTA GAA GCT GTA ACC A |
| B2M | NM_004048.4 | GGG ACT AAA CCT CCA GCC AC | CTA CAG CAC ACG CAG TCT GA |
4.7. Biochemical Assays
4.8. Metabolomics Studies
4.8.1. Extraction of Polar Metabolites
4.8.2. NMR Spectra Acquisition and Processing
4.8.3. Metabolite Identification and Quantification Were Performed Using ASICS
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADMA | Asymmetric dimethylarginine |
| Adohcy | S-adenosyl homocysteine |
| ATP | Adenosine Triphosphate |
| B6 | Vitamin B6 |
| BBF | Bleb formation |
| BBL | Brush border loss |
| BHMT | Betaine homocysteine methyltransferase |
| CBS | Cystathionine βsynthase |
| cDNA | Complementary deoxyribonucleic acid |
| CSE | Cystathionine γ-lyase, |
| CTRL | Control group. Kidneys perfused with Perf-Gen solution |
| DCD | Donation after circulatory death |
| DDAH | Dimethylarginine dimethylaminohydrolase |
| DMSO | Dimethyl sulfoxide |
| DNA | Deoxyribonucleic acid |
| ECD | Expanded criteria donors |
| ELISA | Enzyme-linked immunosorbent assay |
| FC | Fold change |
| FFPE | Formalin-fixed paraffin-embedded |
| GS | Glutathione synthase |
| GSH | Glutathione |
| H2S | Hydrogen sulfide |
| HP | Hypothermic perfusion |
| HRP | Horseradish peroxidase |
| HUVECs | Human umbilical vein endothelial cells. |
| IQR | interquartile range |
| IRI | Ischemia–reperfusion injury |
| LDH | Lactate dehydrogenase |
| LDL | Low-density lipoprotein |
| MAT | Methionine adenosyltransferase |
| MP | Machine perfusion |
| mRNA | Messenger ribonucleic acid |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NMR | Nuclear magnetic resonance |
| NO | Nitric oxide |
| Norm T | Normal tubules |
| NOS | Nitric oxide synthase |
| NOX4 | NADPH oxidase 4 |
| N-Tyr | Nitrotyrosine |
| OCT | Optimal cutting temperature compound |
| PBS | Phosphate-buffered saline |
| PCA | Principal component analysis |
| PCNA | Proliferation cell nuclear antigen |
| PCSK9 | Proprotein convertase subtilisin/Kexin type 9 |
| PEP 2-8 | Treated group. Kidneys perfused with Perf-Gen solution supplemented with a PCSK9 inhibitor. |
| PIPOX | Pipecolate oxidase |
| qPCR | Quantitative polymerase chain reaction |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| RT-PCR | Real time polymerase chain reaction |
| SAHH | S-adenosyl homocysteine hydrolase |
| SD | Standard deviation |
| SEM | Standard error of the mean |
| TF | Tubular epithelial cell flattening |
| TID | Tubular ischemic damage |
| TN | Tubular necrosis |
| TO | Tubular lumen obstruction |
| TPI | Tubular cell proliferation index |
| TSP | 3-trimethylsilyl)propionic-2,2,3,3-d4 acid sodium salt |
| β2-m | β2-microglobulin |
References
- Bon, D.; Chatauret, N.; Giraud, S.; Thuillier, R.; Favreau, F.; Hauet, T. New Strategies to Optimize Kidney Recovery and Preservation in Transplantation. Nat. Rev. Nephrol. 2012, 8, 339–347. [Google Scholar] [CrossRef]
- Padilla, S. 2025 International Registry in Organ Donation and Transplantation. 2025. Available online: https://dtifoundation.com/wp-content/uploads/2025/08/2025-International-Registry-in-Organ-Donation-and-Transplantation-1.pdf (accessed on 31 July 2025).
- Noble, J.; Jouve, T.; Malvezzi, P.; Süsal, C.; Rostaing, L. Transplantation of Marginal Organs: Immunological Aspects and Therapeutic Perspectives in Kidney Transplantation. Front. Immunol. 2019, 10, 3142. [Google Scholar] [CrossRef] [PubMed]
- Ariyamuthu, V.K.; Qannus, A.A.; Tanriover, B. How Do We Increase Deceased Donor Kidney Utilization and Reduce Discard? Curr. Opin. Organ Transplant. 2025, 30, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Eckle, T. Ischemia and Reperfusion—From Mechanism to Translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Farías, J.G.; Herrera, E.A.; Carrasco-Pozo, C.; Sotomayor-Zárate, R.; Cruz, G.; Morales, P.; Castillo, R.L. Pharmacological Models and Approaches for Pathophysiological Conditions Associated with Hypoxia and Oxidative Stress. Pharmacol. Ther. 2016, 158, 1–23. [Google Scholar] [CrossRef]
- Kataria, A.; Magoon, S.; Makkar, B.; Gundroo, A. Machine Perfusion in Kidney Transplantation. Curr. Opin. Organ Transplant. 2019, 24, 378–384. [Google Scholar] [CrossRef]
- Esposito, P.; Grosjean, F.; Rampino, T.; Libetta, C.; Gregorini, M.; Fasoli, G.; Marchi, G.; Sileno, G.; Montagna, F.; Dal Canton, A. Costimulatory Pathways in Kidney Transplantation: Pathogenetic Role, Clinical Significance and New Therapeutic Opportunities. Int. Rev. Immunol. 2014, 33, 212–233. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Q.; Meng, H.; Duan, H.; Liu, X.; Wu, J.; Gao, F.; Wang, S.; Tan, R.; Yuan, J. Ischemia-Reperfusion Injury: Molecular Mechanisms and Therapeutic Targets. Signal Transduct. Target. Ther. 2024, 9, 12. [Google Scholar] [CrossRef]
- Barisione, C.; Verzola, D.; Garibaldi, S.; Ferrari, P.F.; Garibotto, G.; Ameri, P.; Pane, B.; Spinella, G.; Pratesi, G.; Palombo, D. Renal Ischemia/Reperfusion Early Induces Myostatin and PCSK9 Expression in Rat Kidneys and HK-2 Cells. Int. J. Mol. Sci. 2021, 22, 9884. [Google Scholar] [CrossRef]
- Ortona, S.; Barisione, C.; Ferrari, P.F.; Palombo, D.; Pratesi, G. PCSK9 and Other Metabolic Targets to Counteract Ischemia/Reperfusion Injury in Acute Myocardial Infarction and Visceral Vascular Surgery. J. Clin. Med. 2022, 11, 3638. [Google Scholar] [CrossRef]
- Huang, G.; Lu, X.; Duan, Z.; Zhang, K.; Xu, L.; Bao, H.; Xiong, X.; Lin, M.; Li, C.; Li, Y.; et al. PCSK9 Knockdown Can Improve Myocardial Ischemia/Reperfusion Injury by Inhibiting Autophagy. Cardiovasc. Toxicol. 2022, 22, 951–961. [Google Scholar] [CrossRef]
- Huang, G.; Lu, X.; Zhou, H.; Li, R.; Huang, Q.; Xiong, X.; Luo, Z.; Li, W. PCSK9 Inhibition Protects against Myocardial Ischemia-Reperfusion Injury via Suppressing Autophagy. Microvasc. Res. 2022, 142, 104371. [Google Scholar] [CrossRef]
- Andreadou, I.; Tsoumani, M.; Vilahur, G.; Ikonomidis, I.; Badimon, L.; Varga, Z.V.; Ferdinandy, P.; Schulz, R. PCSK9 in Myocardial Infarction and Cardioprotection: Importance of Lipid Metabolism and Inflammation. Front. Physiol. 2020, 11, 602497. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Jia, C.; Yu, W.; Li, X.; Xia, N.; Nie, H.; Wikana, L.P.; Chen, M.; Ni, Y.; et al. Blockade of Hepatocyte PCSK9 Ameliorates Hepatic Ischemia-Reperfusion Injury by Promoting Pink1-Parkin-Mediated Mitophagy. Cell. Mol. Gastroenterol. Hepatol. 2024, 17, 149–169. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, W.; Yang, Z.; Xing, X. E3 Ubiquitin Ligase MARCH1 Reduces Inflammation and Pyroptosis in Cerebral Ischemia-Reperfusion Injury via PCSK9 Downregulation. Mamm. Genome 2024, 35, 346–361. [Google Scholar] [CrossRef]
- Apaijai, N.; Moisescu, D.M.; Palee, S.; McSweeney, C.M.; Saiyasit, N.; Maneechote, C.; Boonnag, C.; Chattipakorn, N.; Chattipakorn, S.C. Pretreatment With PCSK9 Inhibitor Protects the Brain Against Cardiac Ischemia/Reperfusion Injury Through a Reduction of Neuronal Inflammation and Amyloid Beta Aggregation. J. Am. Heart Assoc. 2019, 8, e010838. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Jia, C.; Li, Z.; Zang, Y. Protective Mechanism of Proprotein Convertase Subtilisin-Like Kexin Type 9 Inhibitor on Rats with Middle Cerebral Artery Occlusion-Induced Cerebral Ischemic Infarction. Comput. Intell. Neurosci. 2022, 2022, 4964262. [Google Scholar] [CrossRef]
- Seidah, N.G.; Prat, A. The Multifaceted Biology of PCSK9. Endocr. Rev. 2022, 43, 558–582. [Google Scholar] [CrossRef]
- Shapiro, M.D.; Fazio, S. Dyslipidaemia: The PCSK9 Adventure—Humanizing Extreme LDL Lowering. Nat. Rev. Cardiol. 2017, 14, 319–320. [Google Scholar] [CrossRef]
- Melendez, Q.M.; Krishnaji, S.T.; Wooten, C.J.; Lopez, D. Hypercholesterolemia: The Role of PCSK9. Arch. Biochem. Biophys. 2017, 625–626, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Zaid, A.; Roubtsova, A.; Essalmani, R.; Marcinkiewicz, J.; Chamberland, A.; Hamelin, J.; Tremblay, M.; Jacques, H.; Jin, W.; Davignon, J.; et al. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9): Hepatocyte-Specific Low-Density Lipoprotein Receptor Degradation and Critical Role in Mouse Liver Regeneration. Hepatology 2008, 48, 646–654. [Google Scholar] [CrossRef]
- Lambert, G.; Sjouke, B.; Choque, B.; Kastelein, J.J.P.; Hovingh, G.K. The PCSK9 Decade. J. Lipid Res. 2012, 53, 2515–2524. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Liang, Y.; Chang, H.; Cai, T.; Feng, B.; Gordon, K.; Zhu, Y.; Shi, H.; He, Y.; Xie, L. Targeting Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9): From Bench to Bedside. Signal Transduct. Target. Ther. 2024, 9, 13. [Google Scholar] [CrossRef]
- Dutka, M.; Zimmer, K.; Ćwiertnia, M.; Ilczak, T.; Bobiński, R. The Role of PCSK9 in Heart Failure and Other Cardiovascular Diseases-Mechanisms of Action beyond Its Effect on LDL Cholesterol. Heart Fail. Rev. 2024, 29, 917–937. [Google Scholar] [CrossRef]
- Wu, C.; Lin, D.; Ji, J.; Jiang, Y.; Jiang, F.; Wang, Y. PCSK9 Inhibition Regulates Infarction-Induced Cardiac Myofibroblast Transdifferentiation via Notch1 Signaling. Cell Biochem. Biophys. 2023, 81, 359–369. [Google Scholar] [CrossRef]
- Ma, M.; Hou, C.; Liu, J. Effect of PCSK9 on Atherosclerotic Cardiovascular Diseases and Its Mechanisms: Focus on Immune Regulation. Front. Cardiovasc. Med. 2023, 10, 1148486. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, B.; Tai, S.; Zhou, S.; Zheng, X.-L. PCSK9: Associated with Cardiac Diseases and Their Risk Factors? Arch. Biochem. Biophys. 2021, 704, 108717. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, Y.-M.; He, N.-Q.; Gao, R.; Xu, W.-X.; Zhuo, X.-J.; Ren, Z.; Wu, C.-Y.; Liu, L.-S. PCSK9: A Emerging Participant in Heart Failure. Biomed. Pharmacother. 2023, 158, 114106. [Google Scholar] [CrossRef] [PubMed]
- Momtazi-Borojeni, A.A.; Banach, M.; Ruscica, M.; Sahebkar, A. The Role of PCSK9 in NAFLD/NASH and Therapeutic Implications of PCSK9 Inhibition. Expert. Rev. Clin. Pharmacol. 2022, 15, 1199–1208. [Google Scholar] [CrossRef]
- Han, L.; Wu, L.; Yin, Q.; Li, L.; Zheng, X.; Du, S.; Huang, X.; Bai, L.; Wang, Y.; Bian, Y. A Promising Therapy for Fatty Liver Disease: PCSK9 Inhibitors. Phytomedicine 2024, 128, 155505. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Żak, J.; Malinowski, B.; Popek, G.; Grześk, G. PCSK9 Signaling Pathways and Their Potential Importance in Clinical Practice. EPMA J. 2017, 8, 391–402. [Google Scholar] [CrossRef]
- Ding, Z.; Pothineni, N.V.K.; Goel, A.; Lüscher, T.F.; Mehta, J.L. PCSK9 and Inflammation: Role of Shear Stress, pro-Inflammatory Cytokines, and LOX-1. Cardiovasc. Res. 2020, 116, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Walley, K.R. Role of Lipoproteins and Proprotein Convertase Subtilisin/Kexin Type 9 in Endotoxin Clearance in Sepsis. Curr. Opin. Crit. Care 2016, 22, 464–469. [Google Scholar] [CrossRef]
- Amput, P.; Palee, S.; Arunsak, B.; Pratchayasakul, W.; Thonusin, C.; Kerdphoo, S.; Jaiwongkam, T.; Chattipakorn, S.C.; Chattipakorn, N. PCSK9 Inhibitor and Atorvastatin Reduce Cardiac Impairment in Ovariectomized Prediabetic Rats via Improved Mitochondrial Function and Ca2+ Regulation. J. Cell. Mol. Med. 2020, 24, 9189–9203. [Google Scholar] [CrossRef]
- Li, X.; Dai, F.; Wang, H.; Wei, G.; Jiang, Q.; Yin, P.; Wang, S.; Ge, J.; Yang, C.; Wu, J.; et al. PCSK9 Participates in Oxidized-Low Density Lipoprotein-Induced Myocardial Injury through Mitochondrial Oxidative Stress and Drp1-Mediated Mitochondrial Fission. Clin. Transl. Med. 2022, 12, e729. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, S.; Wang, X.; Mathur, P.; Dai, Y.; Theus, S.; Deng, X.; Fan, Y.; Mehta, J.L. Cross-Talk Between PCSK9 and Damaged mtDNA in Vascular Smooth Muscle Cells: Role in Apoptosis. Antioxid. Redox Signal. 2016, 25, 997–1008. [Google Scholar] [CrossRef]
- Xu, X.; Cui, Y.; Cao, L.; Zhang, Y.; Yin, Y.; Hu, X. PCSK9 Regulates Apoptosis in Human Lung Adenocarcinoma A549 Cells via Endoplasmic Reticulum Stress and Mitochondrial Signaling Pathways. Exp. Ther. Med. 2017, 13, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, S.; Fang, Y.; Zou, Y.; Song, D.; Zhang, S.; Cai, Y. Proprotein Convertase Subtilisin/Kexin Type 9 Promotes Gastric Cancer Metastasis and Suppresses Apoptosis by Facilitating MAPK Signaling Pathway Through HSP70 Up-Regulation. Front. Oncol. 2020, 10, 609663. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Li, T.; Luo, J.; Zhang, X.; Wang, T.; Wang, Y.; Ma, Y.; Yang, B.; Jia, J.; Dmytriw, A.A.; et al. PCSK9 Increases Vulnerability of Carotid Plaque by Promoting Mitochondrial Dysfunction and Apoptosis of Vascular Smooth Muscle Cells. CNS Neurosci. Ther. 2024, 30, e14640. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Liu, S.; Brickell, A.N.; Zhang, J.; Wu, Z.; Zhou, S.; Ding, Z. PCSK9 Regulates Pyroptosis via mtDNA Damage in Chronic Myocardial Ischemia. Basic. Res. Cardiol. 2020, 115, 66. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Y.; Qiu, Y.; Sun, J.; He, J.; Liu, Z.; Shi, J.; Wei, W.; Wu, G.; Liang, J. PCSK9 Promotes Hypoxia-Induced EC Pyroptosis by Regulating Smac Mitochondrion-Cytoplasm Translocation in Critical Limb Ischemia. JACC Basic. Transl. Sci. 2023, 8, 1060–1077. [Google Scholar] [CrossRef]
- Ivan, L.; Uyy, E.; Suica, V.I.; Boteanu, R.M.; Cerveanu-Hogas, A.; Hansen, R.; Antohe, F. Hepatic Alarmins and Mitochondrial Dysfunction under Residual Hyperlipidemic Stress Lead to Irreversible NAFLD. J. Clin. Transl. Hepatol. 2023, 11, 284–294. [Google Scholar] [CrossRef]
- Wu, D.; Zhou, Y.; Pan, Y.; Li, C.; Wang, Y.; Chen, F.; Chen, X.; Yang, S.; Zhou, Z.; Liao, Y.; et al. Vaccine Against PCSK9 Improved Renal Fibrosis by Regulating Fatty Acid β-Oxidation. J. Am. Heart Assoc. 2020, 9, e014358. [Google Scholar] [CrossRef]
- Palee, S.; McSweeney, C.M.; Maneechote, C.; Moisescu, D.M.; Jaiwongkam, T.; Kerdphoo, S.; Chattipakorn, S.C.; Chattipakorn, N. PCSK9 Inhibitor Improves Cardiac Function and Reduces Infarct Size in Rats with Ischaemia/Reperfusion Injury: Benefits beyond Lipid-Lowering Effects. J. Cell. Mol. Med. 2019, 23, 7310–7319. [Google Scholar] [CrossRef]
- Zhang, Y.; Eigenbrot, C.; Zhou, L.; Shia, S.; Li, W.; Quan, C.; Tom, J.; Moran, P.; Di Lello, P.; Skelton, N.J.; et al. Identification of a Small Peptide That Inhibits PCSK9 Protein Binding to the Low Density Lipoprotein Receptor. J. Biol. Chem. 2014, 289, 942–955. [Google Scholar] [CrossRef]
- Huang, L.; Li, Y.; Cheng, Z.; Lv, Z.; Luo, S.; Xia, Y. PCSK9 Promotes Endothelial Dysfunction During Sepsis Via the TLR4/MyD88/NF-κB and NLRP3 Pathways. Inflammation 2023, 46, 115–128. [Google Scholar] [CrossRef]
- Debik, J.; Sangermani, M.; Wang, F.; Madssen, T.S.; Giskeødegård, G.F. Multivariate Analysis of NMR-Based Metabolomic Data. NMR Biomed. 2022, 35, e4638. [Google Scholar] [CrossRef] [PubMed]
- Schreckenberg, R.; Wolf, A.; Szabados, T.; Gömöri, K.; Szabó, I.A.; Ágoston, G.; Brenner, G.; Bencsik, P.; Ferdinandy, P.; Schulz, R.; et al. Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) Deletion but Not Inhibition of Extracellular PCSK9 Reduces Infarct Sizes Ex Vivo but Not In Vivo. Int. J. Mol. Sci. 2022, 23, 6512. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Tang, Z.-H.; Jiang, L.; Li, X.-F.; Jiang, Z.-S.; Liu, L.-S. PCSK9 siRNA Inhibits HUVEC Apoptosis Induced by Ox-LDL via Bcl/Bax-Caspase9-Caspase3 Pathway. Mol. Cell. Biochem. 2012, 359, 347–358. [Google Scholar] [CrossRef]
- Jaén, R.I.; Povo-Retana, A.; Rosales-Mendoza, C.; Capillas-Herrero, P.; Sánchez-García, S.; Martín-Sanz, P.; Mojena, M.; Prieto, P.; Boscá, L. Functional Crosstalk between PCSK9 Internalization and Pro-Inflammatory Activation in Human Macrophages: Role of Reactive Oxygen Species Release. Int. J. Mol. Sci. 2022, 23, 9114. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Liu, S.; Wang, X.; Theus, S.; Deng, X.; Fan, Y.; Zhou, S.; Mehta, J.L. PCSK9 Regulates Expression of Scavenger Receptors and Ox-LDL Uptake in Macrophages. Cardiovasc. Res. 2018, 114, 1145–1153. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, S.; Wang, X.; Deng, X.; Fan, Y.; Sun, C.; Wang, Y.; Mehta, J.L. Hemodynamic Shear Stress via ROS Modulates PCSK9 Expression in Human Vascular Endothelial and Smooth Muscle Cells and along the Mouse Aorta. Antioxid. Redox Signal. 2015, 22, 760–771. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Holterman, C.E.; Read, N.C.; Kennedy, C.R.J. Nox and Renal Disease. Clin. Sci. 2015, 128, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Thamilselvan, V.; Menon, M.; Thamilselvan, S. Oxalate-Induced Activation of PKC-Alpha and -Delta Regulates NADPH Oxidase-Mediated Oxidative Injury in Renal Tubular Epithelial Cells. Am. J. Physiol.-Ren. Physiol. 2009, 297, F1399–F1410. [Google Scholar] [CrossRef]
- Liu, S.; Wu, J.; Stolarz, A.; Zhang, H.; Boerma, M.; Byrum, S.D.; Rusch, N.J.; Ding, Z. PCSK9 Attenuates Efferocytosis in Endothelial Cells and Promotes Vascular Aging. Theranostics 2023, 13, 2914–2929. [Google Scholar] [CrossRef]
- Safaeian, L.; Mirian, M.; Bahrizadeh, S. Evolocumab, a PCSK9 Inhibitor, Protects Human Endothelial Cells against H2O2-Induced Oxidative Stress. Arch. Physiol. Biochem. 2022, 128, 1681–1686. [Google Scholar] [CrossRef]
- Lankin, V.Z.; Tikhaze, A.K.; Viigimaa, M.; Chazova, I.E. PCSK9 Inhibitor Causes a Decrease in the Level of Oxidatively Modified Low-Density Lipoproteins in Patients with Coronary Artery Diseases. Ter. Arkh. 2018, 90, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Cammisotto, V.; Baratta, F.; Castellani, V.; Bartimoccia, S.; Nocella, C.; D’Erasmo, L.; Cocomello, N.; Barale, C.; Scicali, R.; Di Pino, A.; et al. Proprotein Convertase Subtilisin Kexin Type 9 Inhibitors Reduce Platelet Activation Modulating Ox-LDL Pathways. Int. J. Mol. Sci. 2021, 22, 7193. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Mukhopadhyay, P.; Matyas, C.; Trojnar, E.; Paloczi, J.; Yang, Y.R.; Blank, B.A.; Savage, C.; Sorokin, A.V.; Mehta, N.N.; et al. PCSK9 Inhibition as a Novel Therapeutic Target for Alcoholic Liver Disease. Sci. Rep. 2019, 9, 17167. [Google Scholar] [CrossRef]
- Bajic, Z.; Sobot, T.; Skrbic, R.; Stojiljkovic, M.P.; Ponorac, N.; Matavulj, A.; Djuric, D.M. Homocysteine, Vitamins B6 and Folic Acid in Experimental Models of Myocardial Infarction and Heart Failure—How Strong Is That Link? Biomolecules 2022, 12, 536. [Google Scholar] [CrossRef]
- Oliva-Damaso, E.; Oliva-Damaso, N.; Rodriguez-Esparragon, F.; Payan, J.; Baamonde-Laborda, E.; Gonzalez-Cabrera, F.; Santana-Estupiñan, R.; Rodriguez-Perez, J.C. Asymmetric (ADMA) and Symmetric (SDMA) Dimethylarginines in Chronic Kidney Disease: A Clinical Approach. Int. J. Mol. Sci. 2019, 20, 3668. [Google Scholar] [CrossRef] [PubMed]
- Kurata, H.; Fujii, T.; Tsutsui, H.; Katayama, T.; Ohkita, M.; Takaoka, M.; Tsuruoka, N.; Kiso, Y.; Ohno, Y.; Fujisawa, Y.; et al. Renoprotective Effects of l-Carnosine on Ischemia/Reperfusion-Induced Renal Injury in Rats. J. Pharmacol. Exp. Ther. 2006, 319, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Westhof, T.; Vaziri, N.D.; Ingrosso, D.; Perna, A.F. Gases as Uremic Toxins: Is There Something in the Air? Semin. Nephrol. 2014, 34, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, S. The Biochemistry, Measurement and Current Clinical Significance of Asymmetric Dimethylarginine. Ann. Clin. Biochem. 2010, 47, 17–28. [Google Scholar] [CrossRef]
- Ruberti, O.M.; Sousa, A.S.; Viana, L.R.; Pereira Gomes, M.F.; Medeiros, A.; Gomes Marcondes, M.C.C.; Borges, L.d.F.; Crestani, C.C.; Mostarda, C.; Moraes, T.F.d.C.; et al. Aerobic Training Prevents Cardiometabolic Changes Triggered by Myocardial Infarction in Ovariectomized Rats. J. Cell. Physiol. 2021, 236, 1105–1115. [Google Scholar] [CrossRef]
- Joncquel, M.; Labasque, J.; Demaret, J.; Bout, M.-A.; Hamroun, A.; Hennart, B.; Tronchon, M.; Defevre, M.; Kim, I.; Kerckhove, A.; et al. Targeted Metabolomics Analysis Suggests That Tacrolimus Alters Protection against Oxidative Stress. Antioxidants 2023, 12, 1412. [Google Scholar] [CrossRef]
- de Andrade, R.B.; Gemelli, T.; Rojas, D.B.; Kim, T.D.H.; Zanatta, Â.; Schmitz, F.; Rodrigues, A.F.; Wyse, A.T.S.; Wajner, M.; Dutra-Filho, C.S.; et al. Evaluation of Oxidative Stress Parameters and Energy Metabolism in Cerebral Cortex of Rats Subjected to Sarcosine Administration. Mol. Neurobiol. 2017, 54, 4496–4506. [Google Scholar] [CrossRef]
- Hipkiss, A.R. Energy Metabolism, Proteotoxic Stress and Age-Related Dysfunction—Protection by Carnosine. Mol. Asp. Med. 2011, 32, 267–278. [Google Scholar] [CrossRef]
- Sajid, M.I.; Nunez, F.J.; Amirrad, F.; Roosan, M.R.; Vojtko, T.; McCulloch, S.; Alachkar, A.; Nauli, S.M. Untargeted Metabolomics Analysis on Kidney Tissues from Mice Reveals Potential Hypoxia Biomarkers. Sci. Rep. 2023, 13, 17516. [Google Scholar] [CrossRef]
- Neubauer, J.A.; Sunderram, J. Heme Oxygenase-1 and Chronic Hypoxia. Respir. Physiol. Neurobiol. 2012, 184, 178–185. [Google Scholar] [CrossRef]
- Maines, M.D.; Gibbs, P.E.M. 30 Some Years of Heme Oxygenase: From a “Molecular Wrecking Ball” to a “Mesmerizing” Trigger of Cellular Events. Biochem. Biophys. Res. Commun. 2005, 338, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Grignano, M.A.; Bruno, S.; Viglio, S.; Avanzini, M.A.; Tapparo, M.; Ramus, M.; Croce, S.; Valsecchi, C.; Pattonieri, E.F.; Ceccarelli, G.; et al. CD73-Adenosinergic Axis Mediates the Protective Effect of Extracellular Vesicles Derived from Mesenchymal Stromal Cells on Ischemic Renal Damage in a Rat Model of Donation after Circulatory Death. Int. J. Mol. Sci. 2022, 23, 10681. [Google Scholar] [CrossRef]
- Crowe, A.R.; Yue, W. Semi-Quantitative Determination of Protein Expression UsingImmunohistochemistry Staining and Analysis: An Integrated Protocol. Bio-Protocol 2019, 9, e3465. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Bustin, S.A. Improving the Quality of Quantitative Polymerase Chain Reaction Experiments: 15 Years of MIQE. Mol. Asp. Med. 2024, 96, 101249. [Google Scholar] [CrossRef]
- Lefort, G.; Liaubet, L.; Marty-Gasset, N.; Canlet, C.; Vialaneix, N.; Servien, R. Joint Automatic Metabolite Identification and Quantification of a Set of 1H NMR Spectra. Anal. Chem. 2021, 93, 2861–2870. [Google Scholar] [CrossRef]
- Lefort, G.; Liaubet, L.; Canlet, C.; Tardivel, P.; Père, M.-C.; Quesnel, H.; Paris, A.; Iannuccelli, N.; Vialaneix, N.; Servien, R. ASICS: An R Package for a Whole Analysis Workflow of 1D 1H NMR Spectra. Bioinformatics 2019, 35, 4356–4363. [Google Scholar] [CrossRef]
- Mckay, R.T. How the 1D-NOESY Suppresses Solvent Signal in Metabonomics NMR Spectroscopy: An Examination of the Pulse Sequence Components and Evolution. Concepts Magn. Reson. Part A Bridg. Educ. Res. 2011, 38A, 197–220. [Google Scholar] [CrossRef]
- Silva, J.G.; Tavares, L.; Belew, G.D.; Rodrigues, J.A.; Araújo, R.; Gil, A.M.; Jones, J.G. Impact of High-Fat Diet-Induced Metabolic Dysfunction-Associated Steatotic Liver Disease on Heart, Kidney, and Skeletal Muscle Metabolomes in Wild-Type Mice. J. Proteome Res. 2025, 24, 2491–2504. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic Quotient Normalization as Robust Method to Account for Dilution of Complex Biological Mixtures. Application in 1H NMR Metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Low, B.; Wang, Y.; Zhao, T.; Yu, H.; Huan, T. Closing the Knowledge Gap of Post-Acquisition Sample Normalization in Untargeted Metabolomics. ACS Meas. Sci. Au 2024, 4, 702–711. [Google Scholar] [CrossRef] [PubMed]
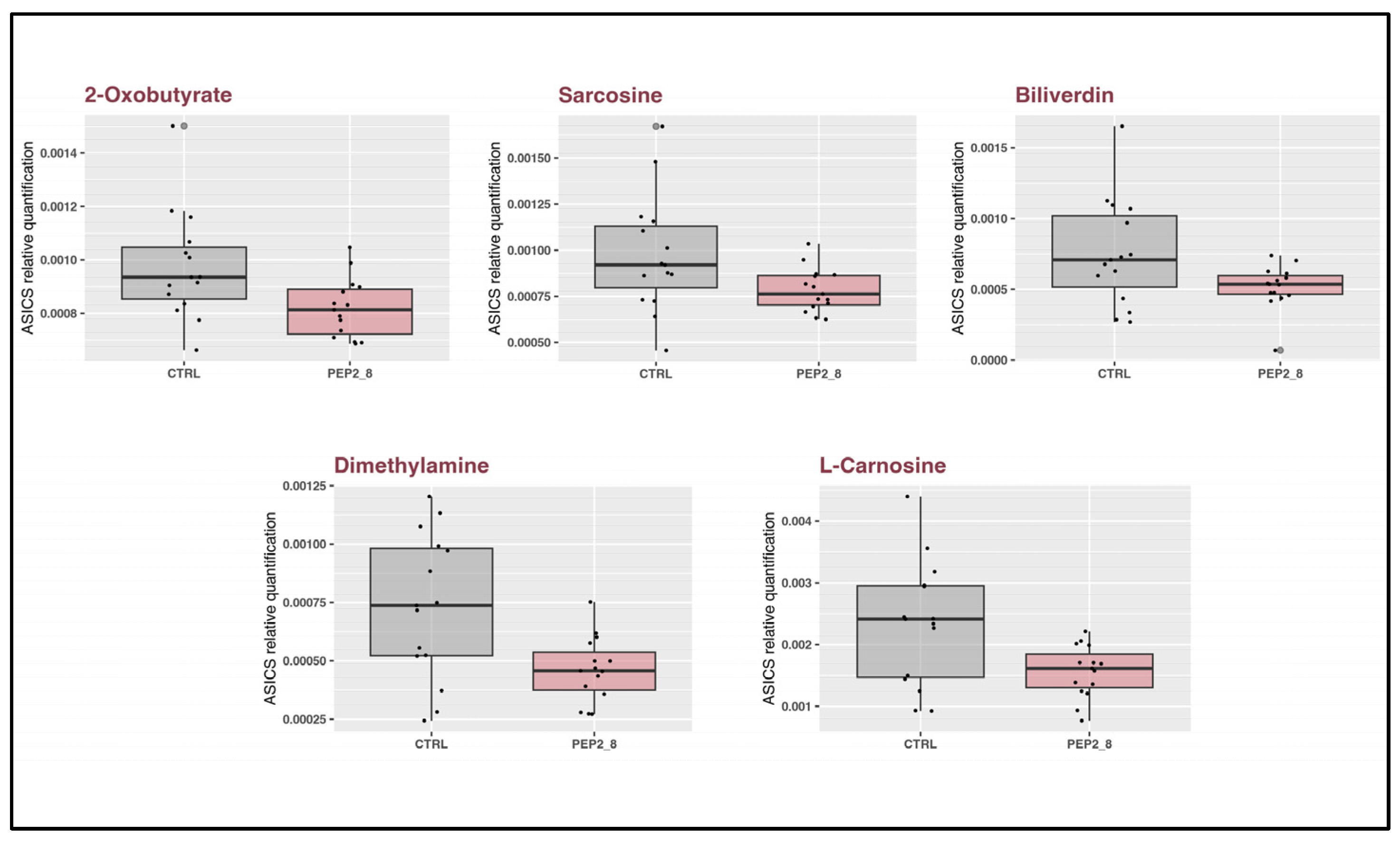
| Metabolite | Kruskall Wallis p Value | T Test p Value |
|---|---|---|
| Dimethylamine | 0.0136 | 0.0047 |
| 2-Oxobutyrate | 0.0152 | 0.0150 |
| L-Carnosine | 0.0213 | 0.0104 |
| Sarcosine | 0.0362 | 0.0358 |
| Biliverdin | 0.0488 | 0.0322 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grignano, M.A.; Gregorini, M.; Barisione, C.; Ivaldo, C.; Verzola, D.; Rumeo, N.; Malabarba, S.; Mimmi, M.C.; Montatixe Fonseca, E.C.; Viglio, S.; et al. A Pre-Clinical Study on the Use of the Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitor PEP 2-8 to Mitigate Ischemic Injury in a Rat Marginal Donor Model. Int. J. Mol. Sci. 2025, 26, 8937. https://doi.org/10.3390/ijms26188937
Grignano MA, Gregorini M, Barisione C, Ivaldo C, Verzola D, Rumeo N, Malabarba S, Mimmi MC, Montatixe Fonseca EC, Viglio S, et al. A Pre-Clinical Study on the Use of the Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitor PEP 2-8 to Mitigate Ischemic Injury in a Rat Marginal Donor Model. International Journal of Molecular Sciences. 2025; 26(18):8937. https://doi.org/10.3390/ijms26188937
Chicago/Turabian StyleGrignano, Maria Antonietta, Marilena Gregorini, Chiara Barisione, Caterina Ivaldo, Daniela Verzola, Noemi Rumeo, Stefano Malabarba, Maria Chiara Mimmi, Elizabeth Carolina Montatixe Fonseca, Simona Viglio, and et al. 2025. "A Pre-Clinical Study on the Use of the Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitor PEP 2-8 to Mitigate Ischemic Injury in a Rat Marginal Donor Model" International Journal of Molecular Sciences 26, no. 18: 8937. https://doi.org/10.3390/ijms26188937
APA StyleGrignano, M. A., Gregorini, M., Barisione, C., Ivaldo, C., Verzola, D., Rumeo, N., Malabarba, S., Mimmi, M. C., Montatixe Fonseca, E. C., Viglio, S., Iadarola, P., Islami, T., Pattonieri, E. F., Ceccarelli, G., Picciotto, D., Pratesi, G., Viazzi, F., Stea, E. D., Arbustini, E., ... Rampino, T. (2025). A Pre-Clinical Study on the Use of the Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitor PEP 2-8 to Mitigate Ischemic Injury in a Rat Marginal Donor Model. International Journal of Molecular Sciences, 26(18), 8937. https://doi.org/10.3390/ijms26188937










