VBIT-4 Rescues Mitochondrial Dysfunction and Reduces Skeletal Muscle Degeneration in a Severe Model of Duchenne Muscular Dystrophy
Abstract
1. Introduction
2. Results
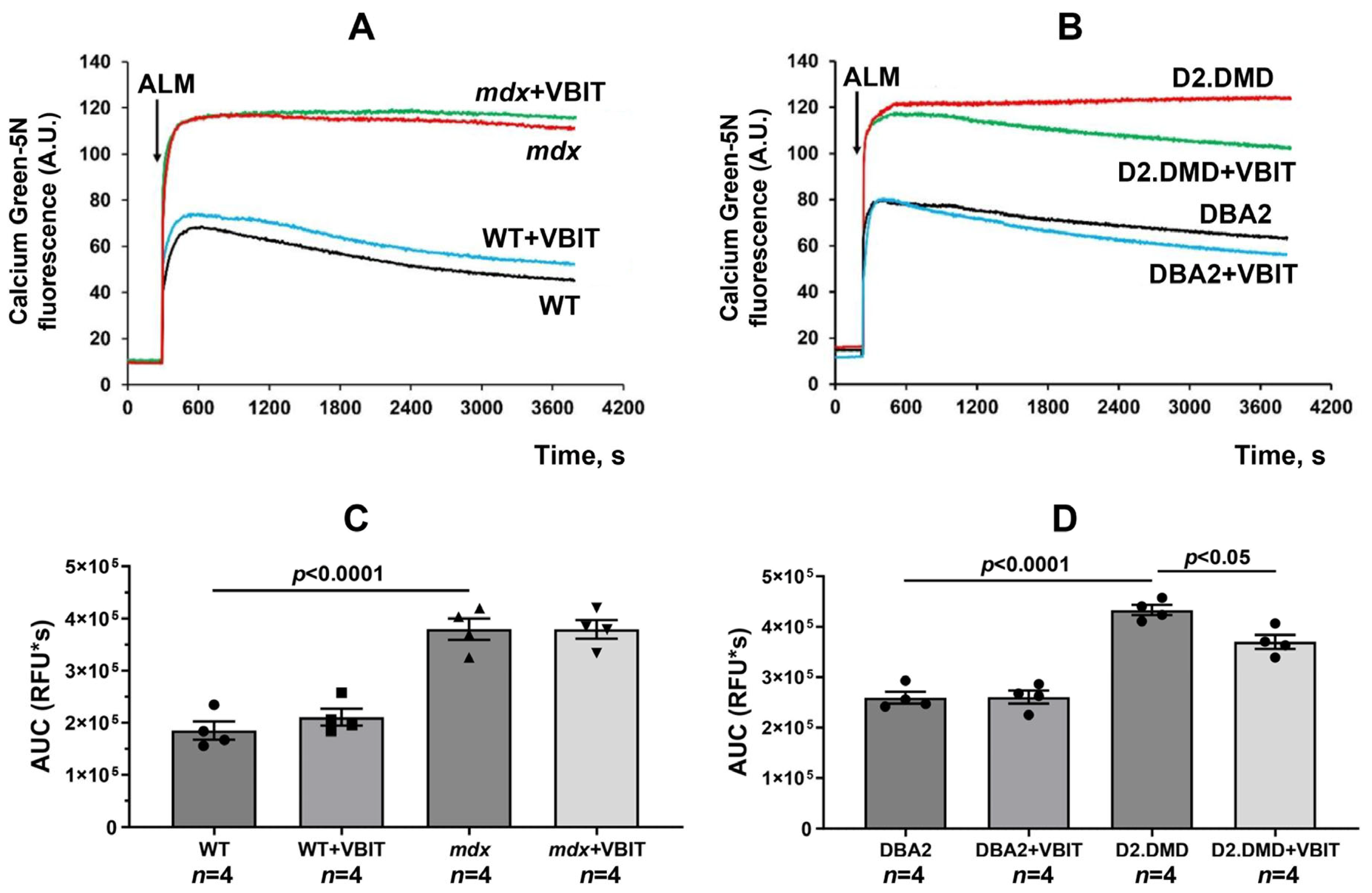
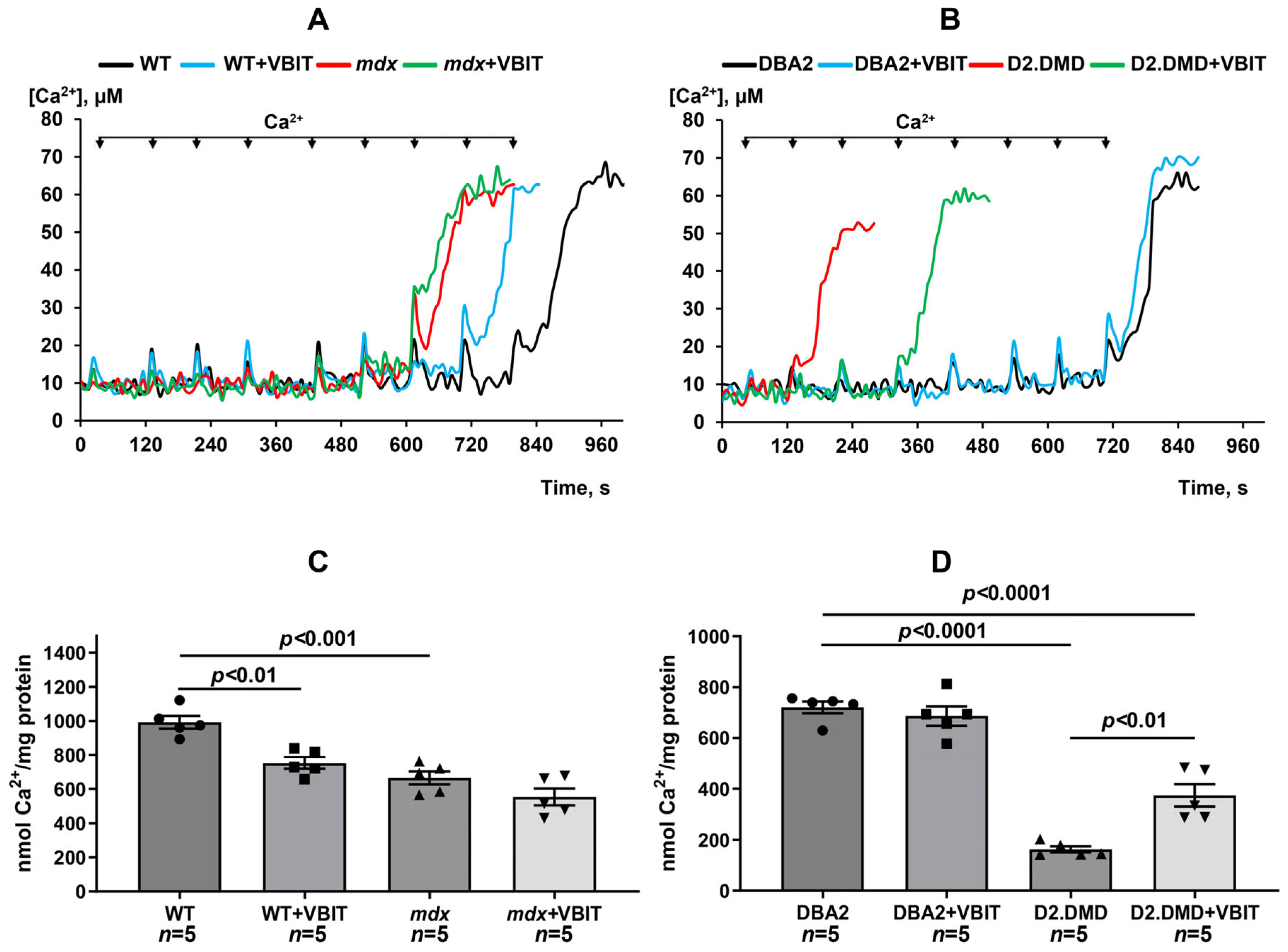
2.1. VBIT-4 Reduces Calcium Overload and Enhances Resistance to MPT Pore Induction in the Quadriceps Mitochondria of D2.DMDel8-34 Mice but Not in Mdx Mice
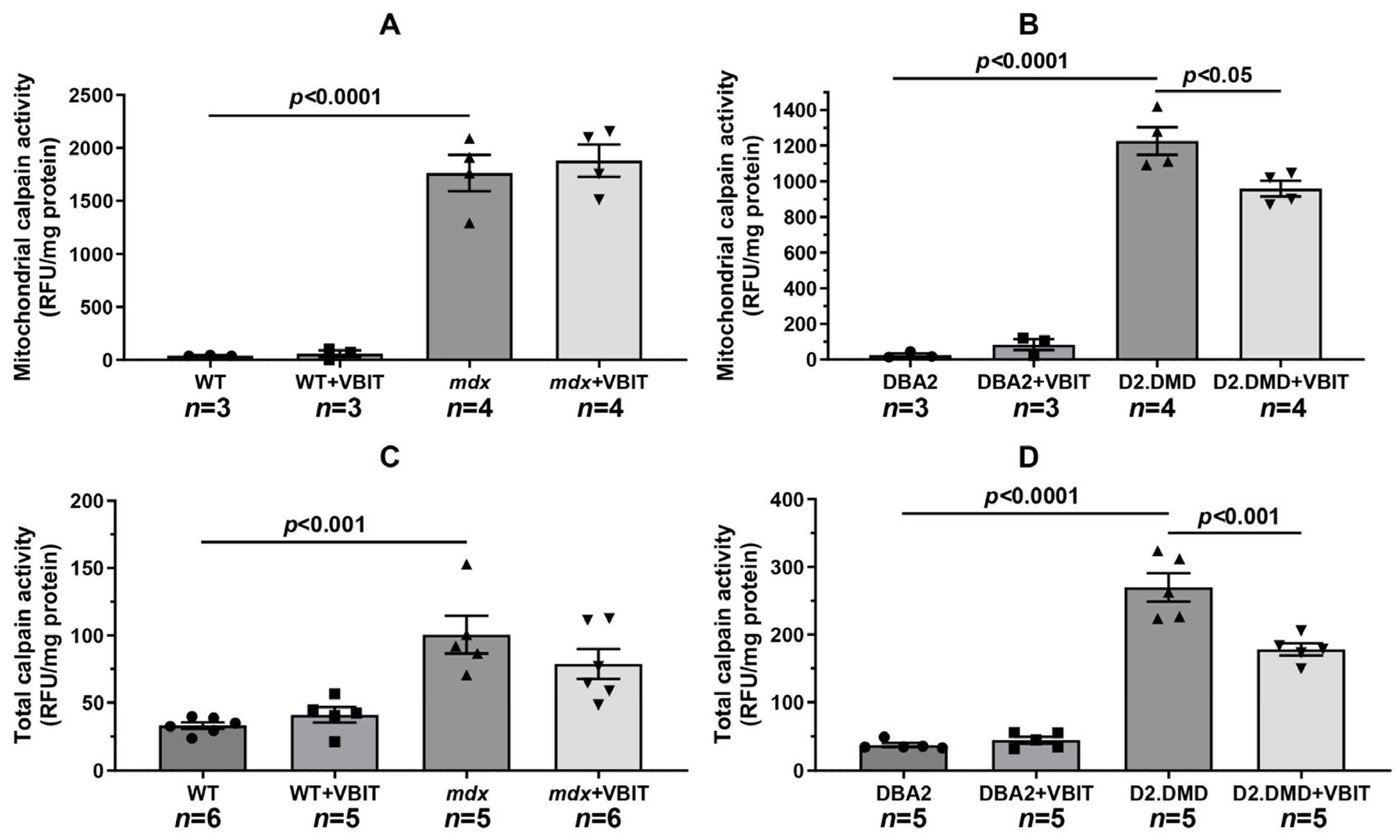
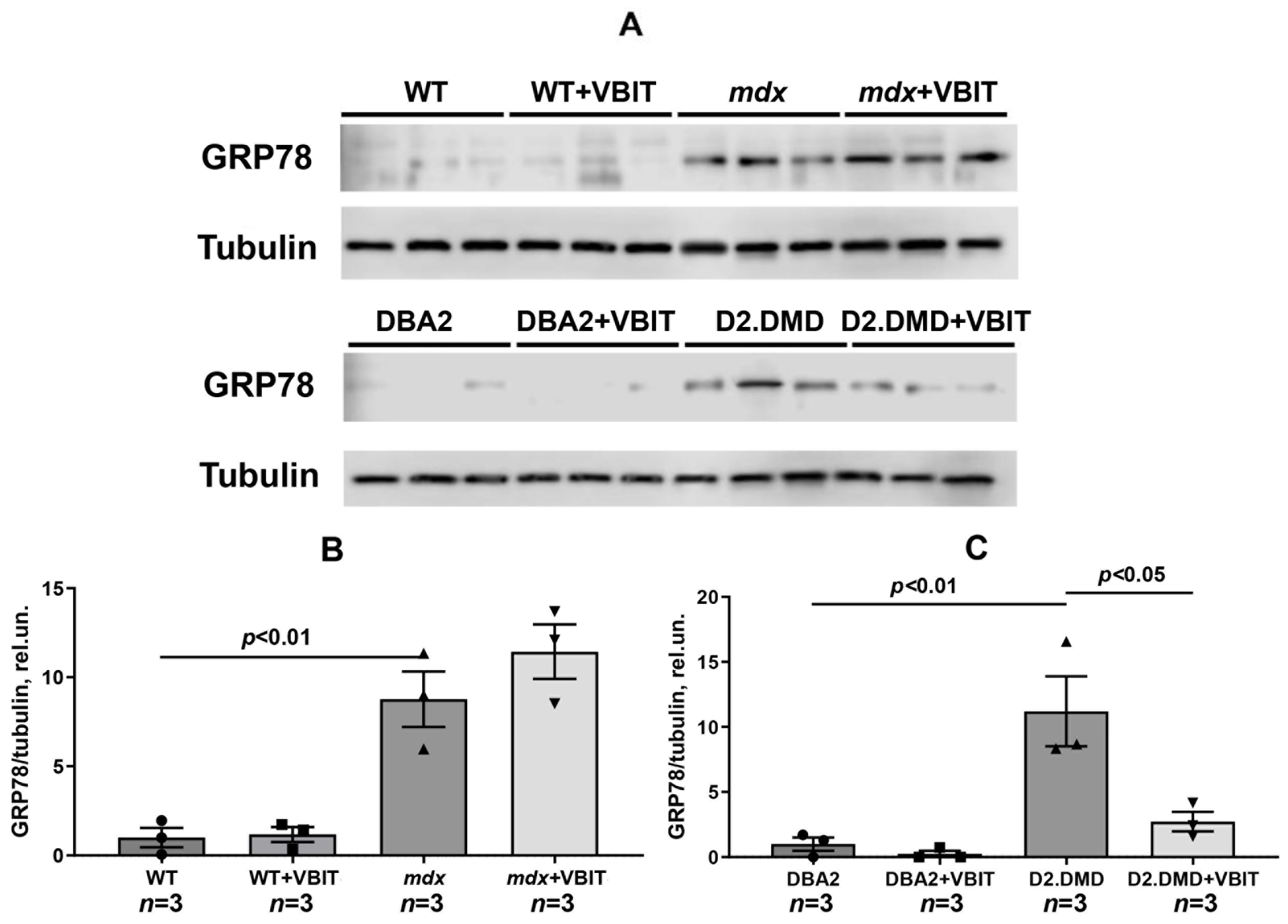
2.2. VBIT-4 Reduces the Activity of Both the Mitochondrial and Tissue Fractions of Calpains, as Well as SR Stress Levels in the Quadriceps Muscles of D2.DMDel8-34 Mice but Not in Mdx Mice
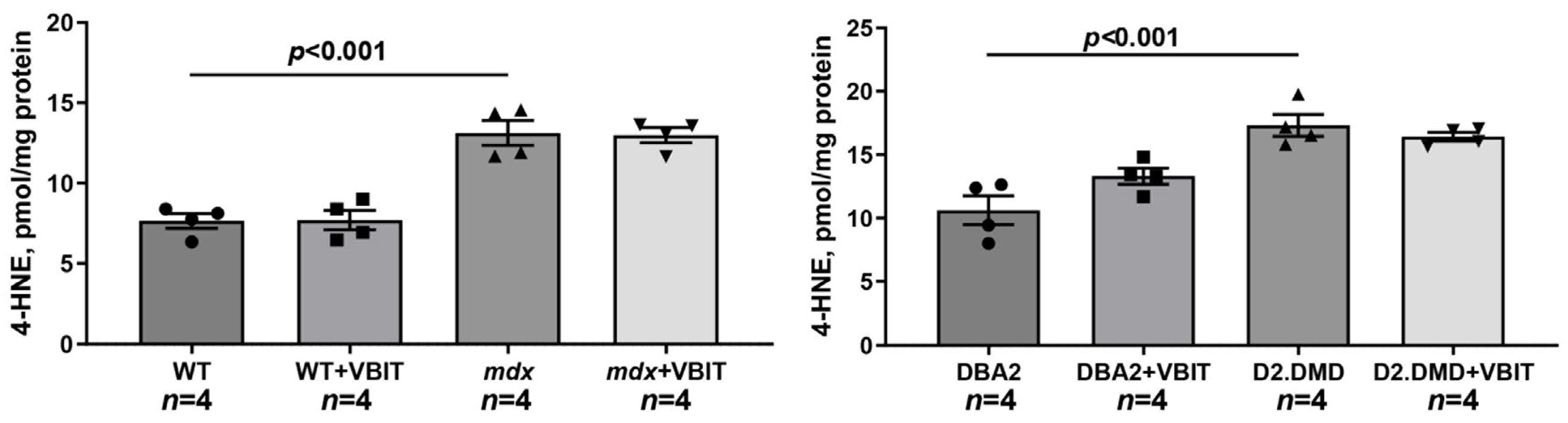
2.3. VBIT-4 Treatment Failed to Rescue Either the Deficient Oxidative Phosphorylation Capacity in the Quadriceps Mitochondria or the Elevated Oxidative Stress Observed in the Dystrophin-Deficient Muscle Tissue
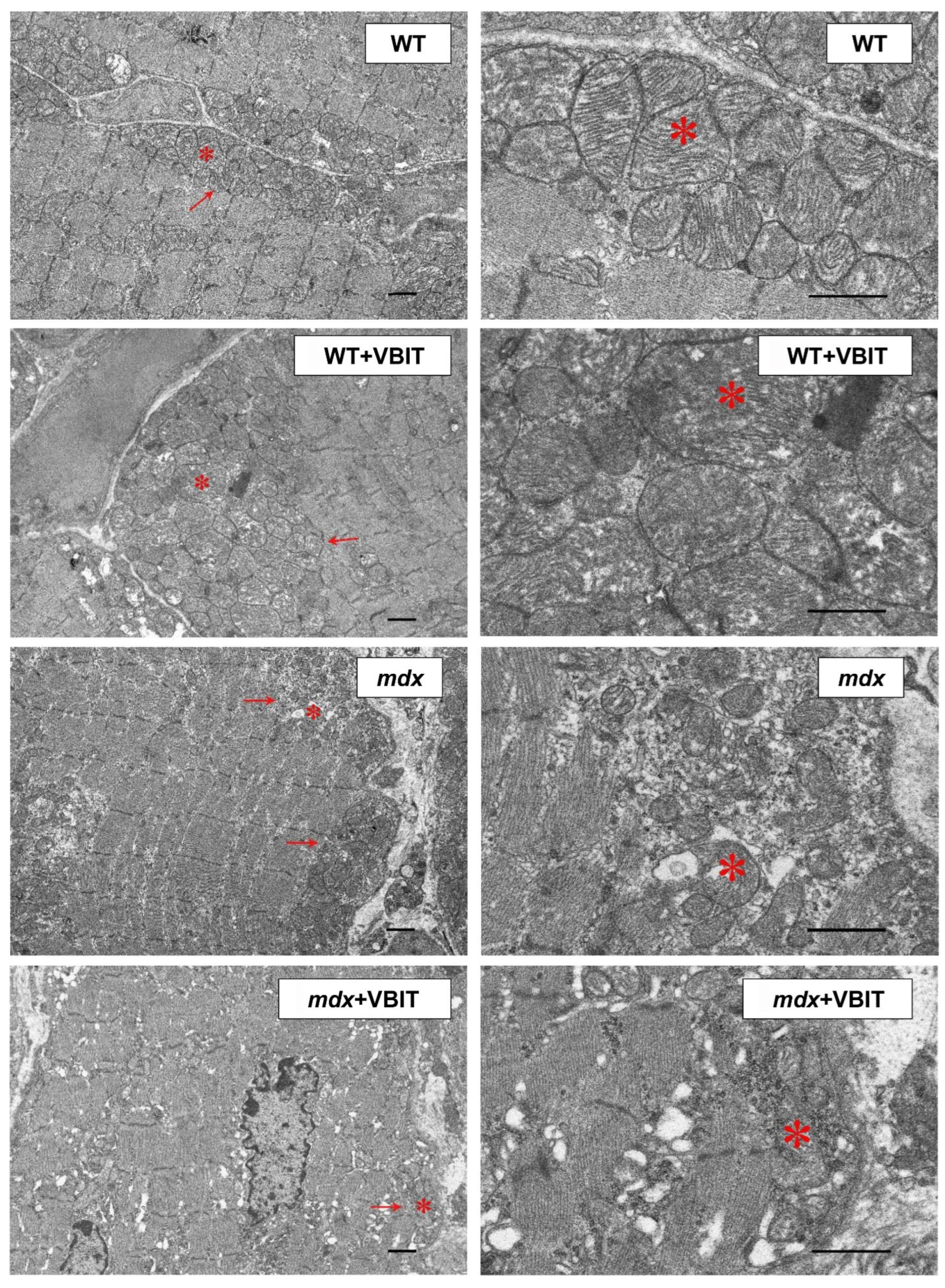
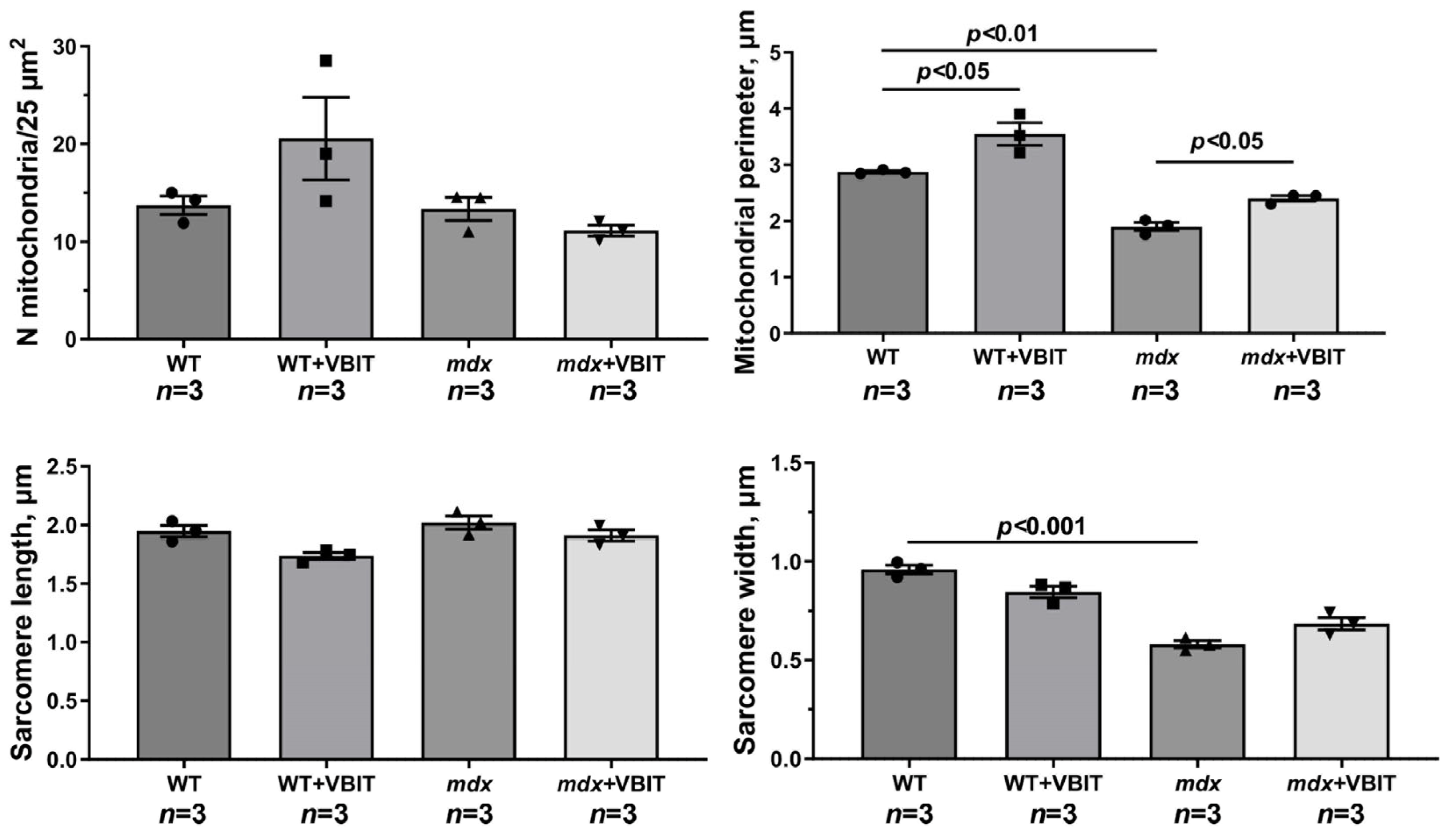
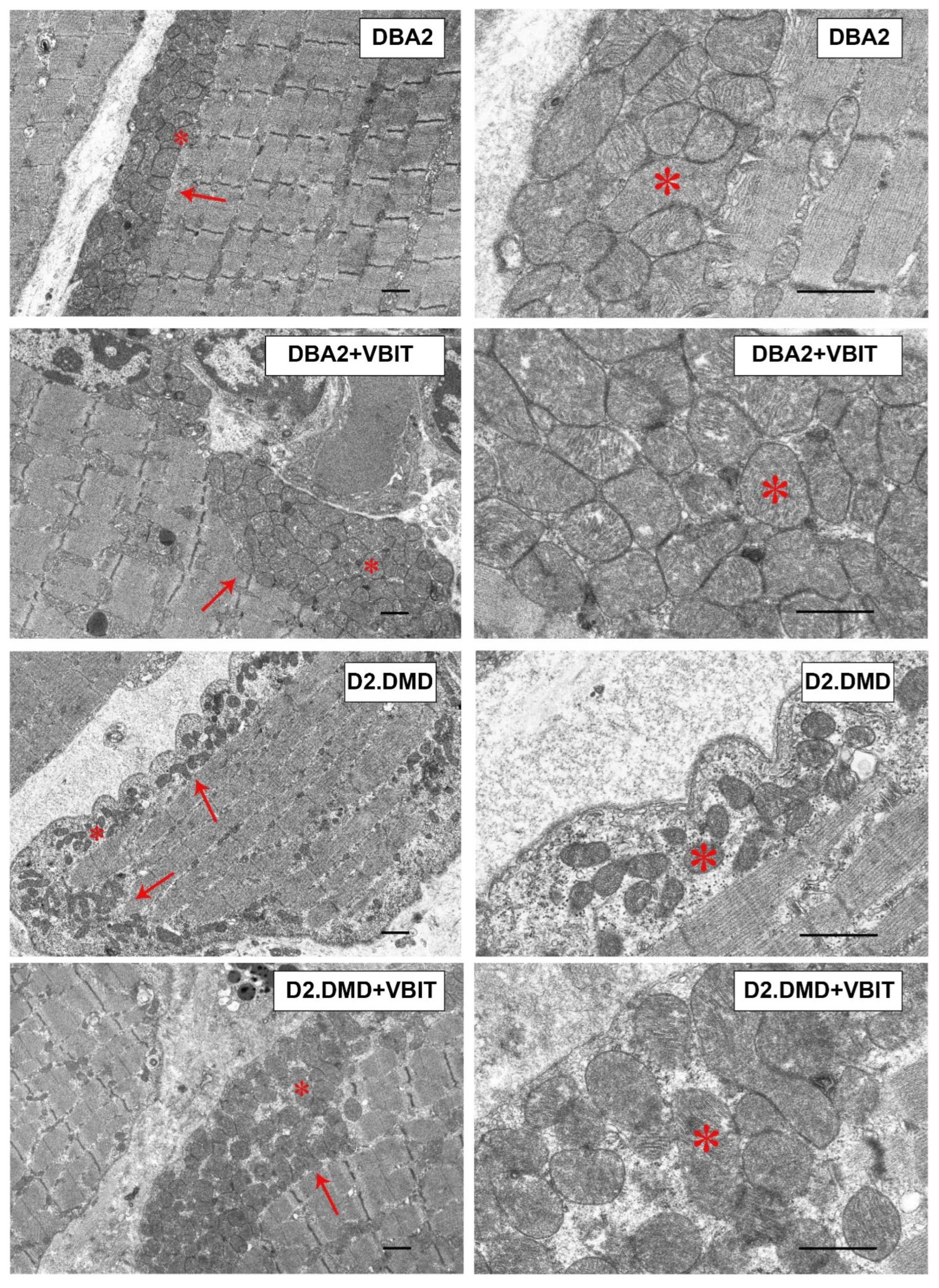
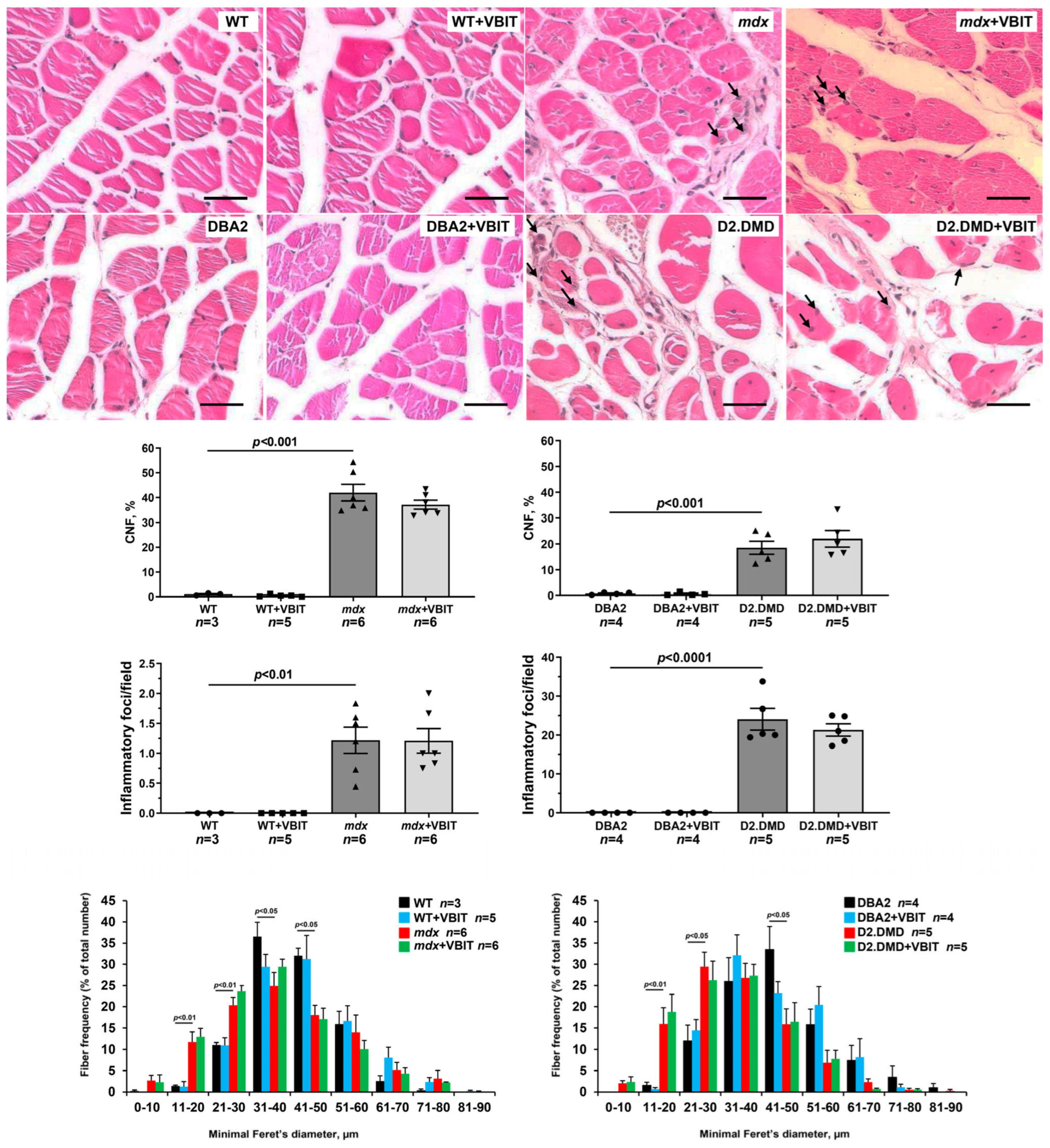
2.4. VBIT-4 Improves the Mitochondrial Ultrastructure in D2.DMDel8-34 Mouse Quadriceps, with No Effect in Mdx Mice, but Impairs the Ultrastructure in Healthy Controls
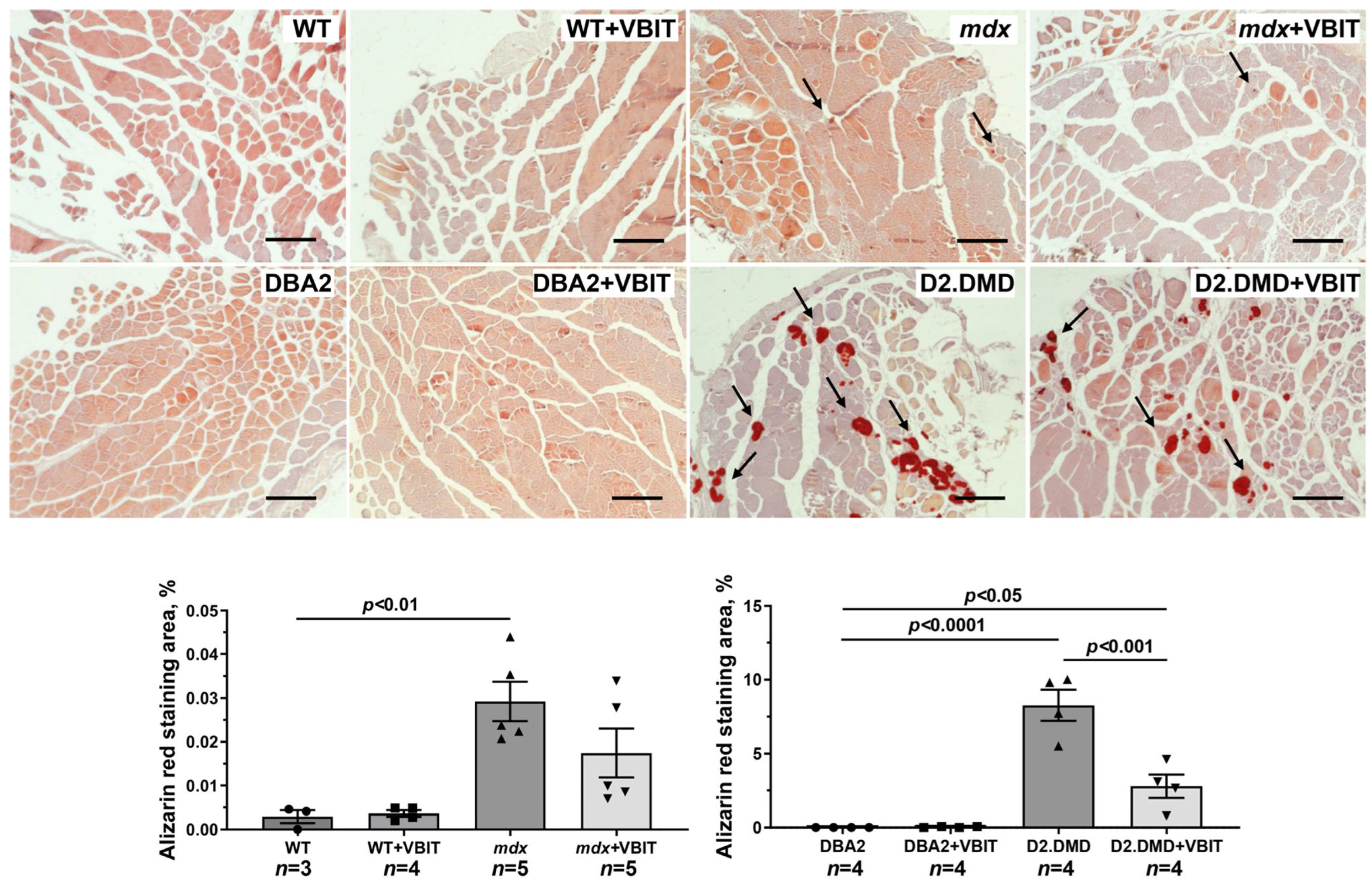
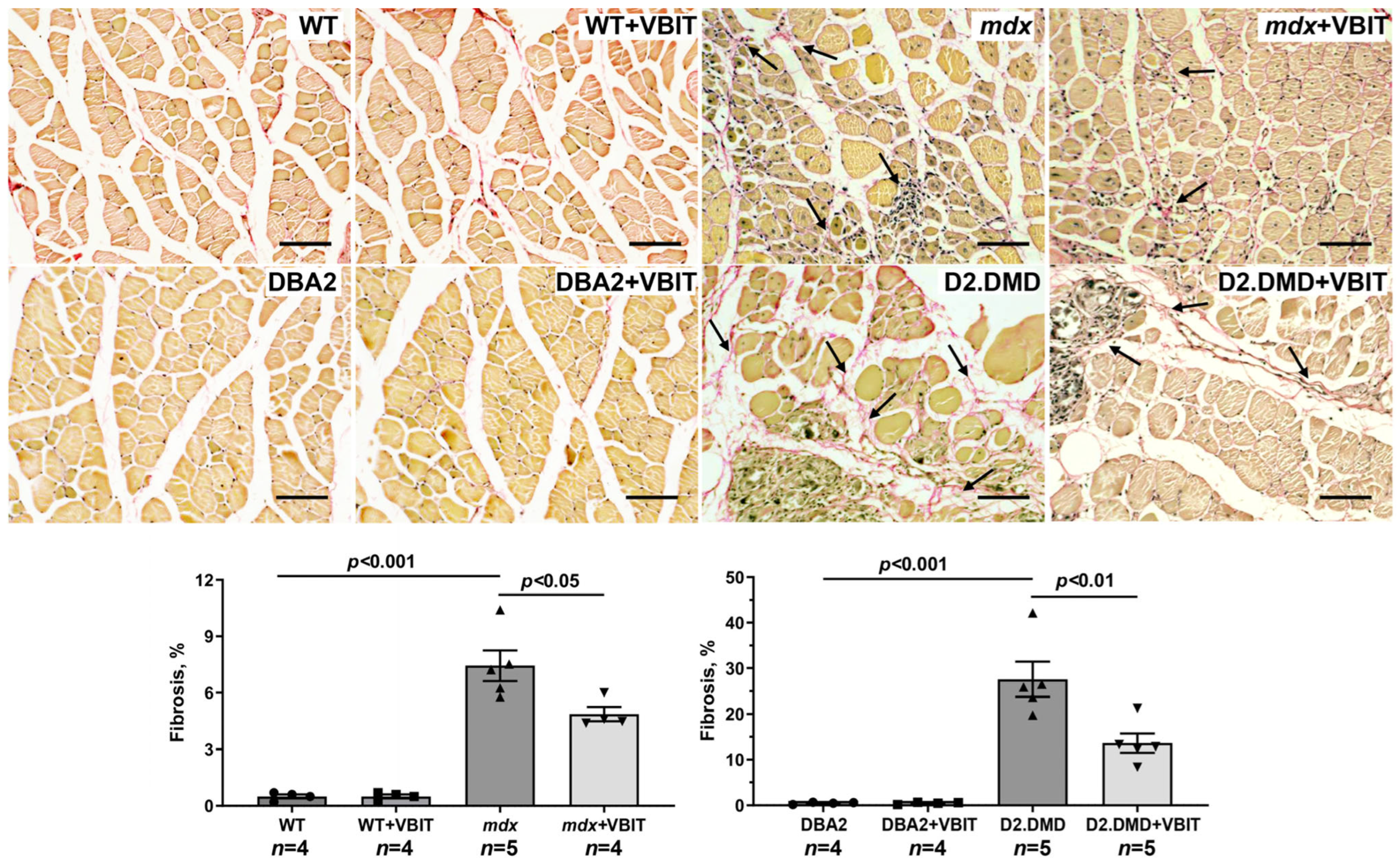
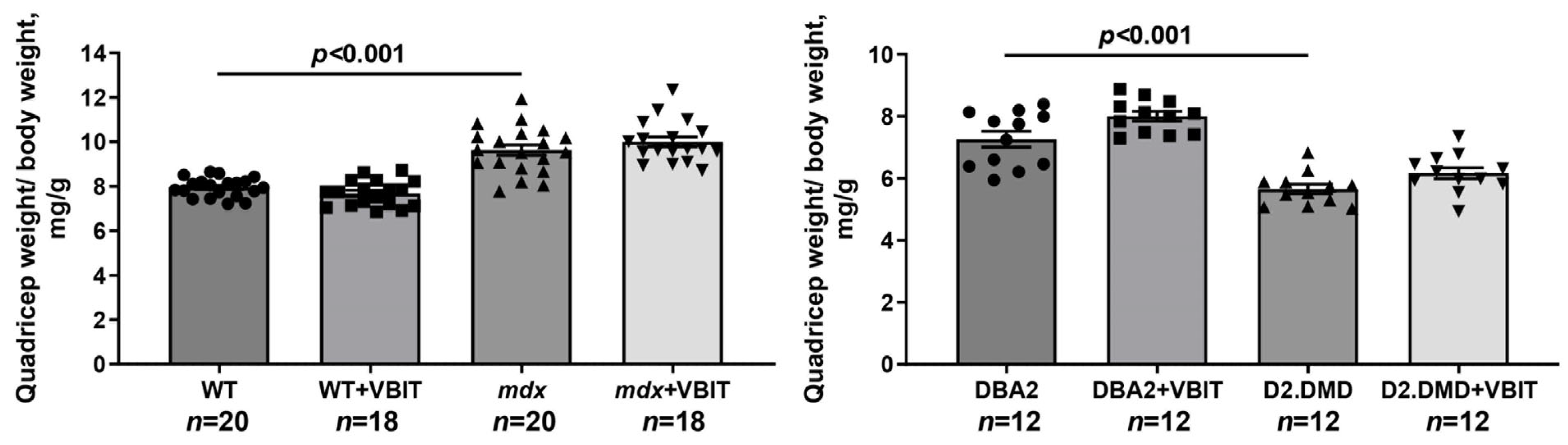
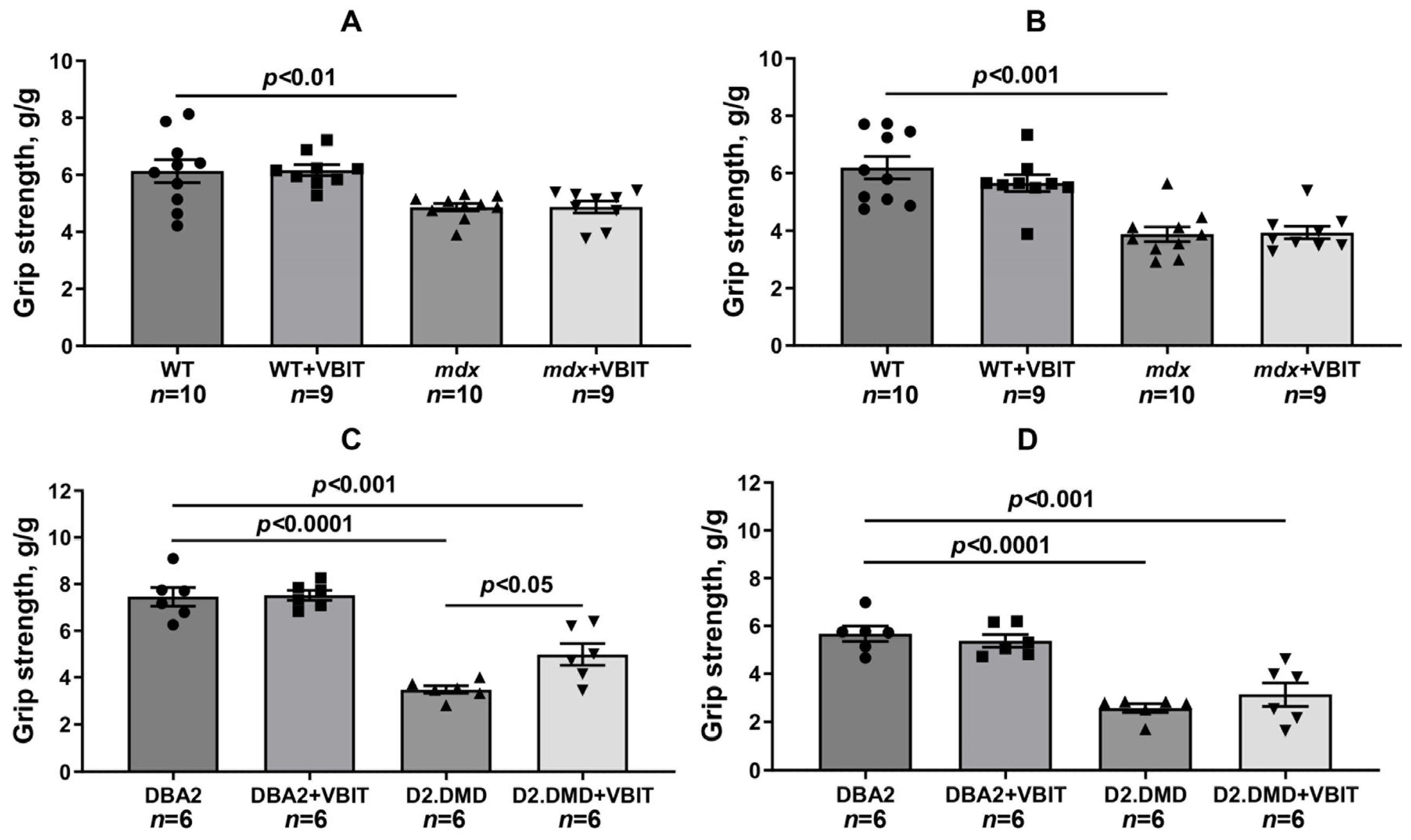
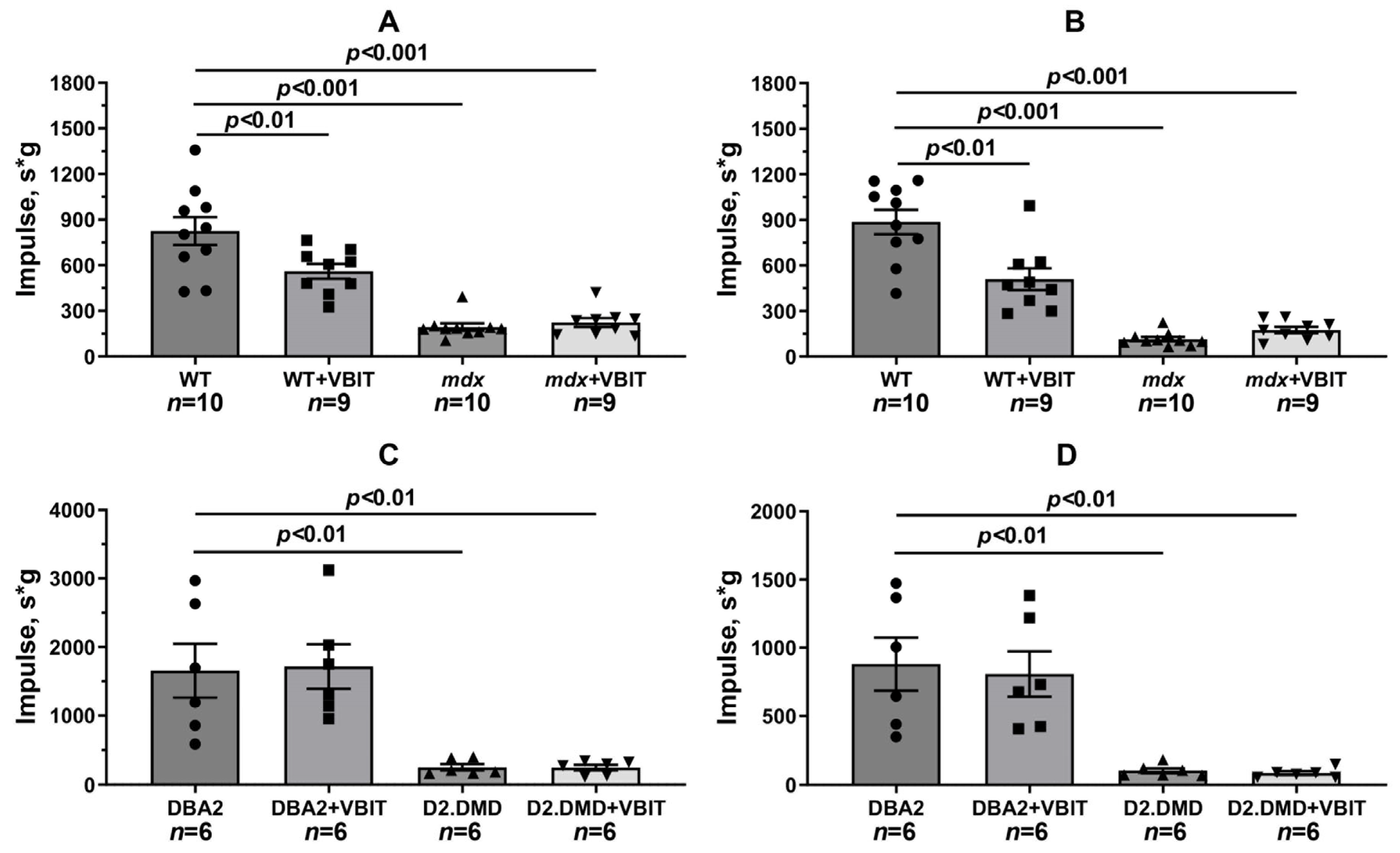
2.5. VBIT-4 Reduces Skeletal Muscle Calcification and Fibrosis in D2.DMD Mice and Exerts a Limited Beneficial Effect on Muscle Strength in These Animals
3. Discussion
4. Materials and Methods
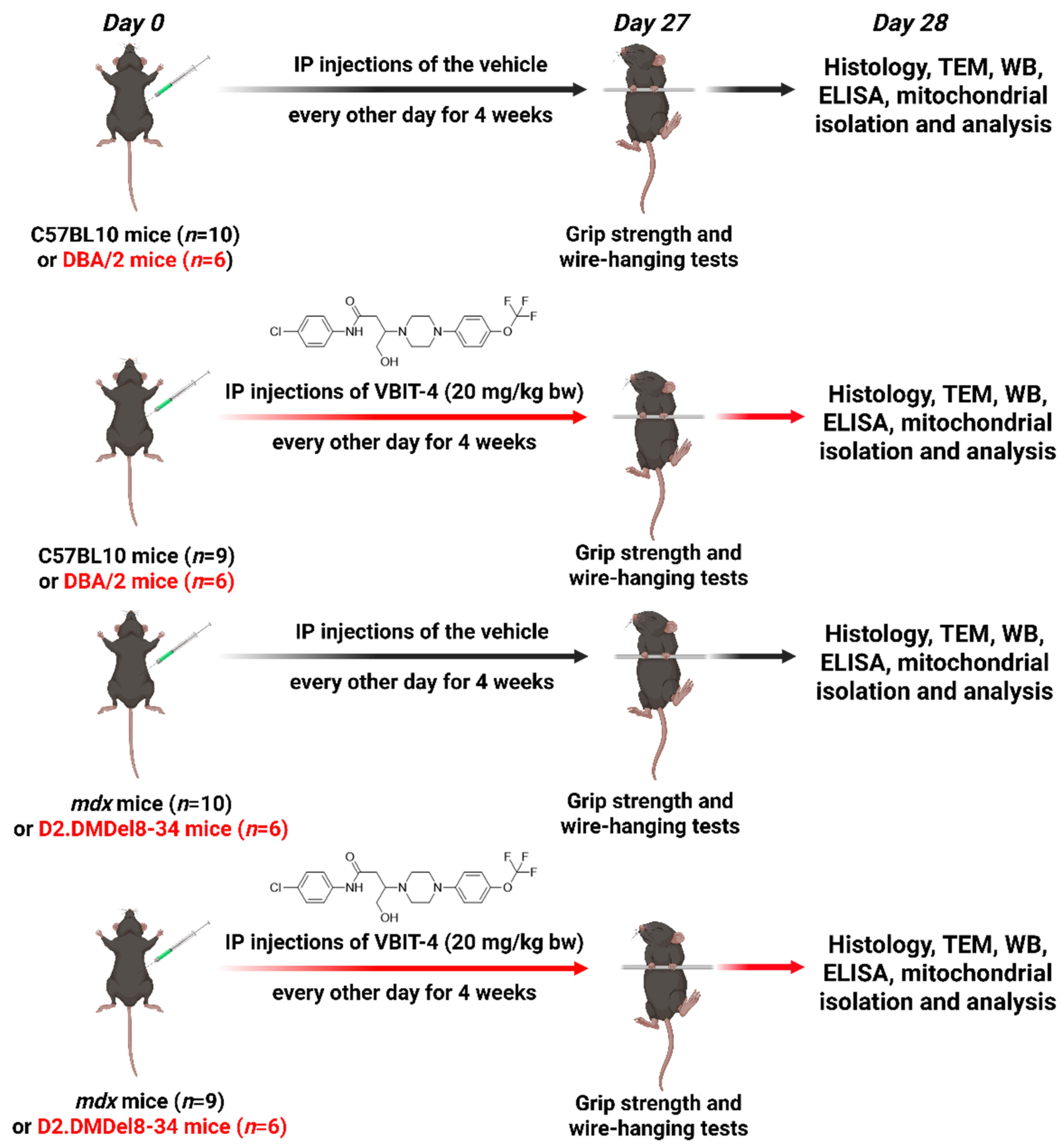
4.1. Animals
4.2. Grip Strength and Wire-Hanging Tests
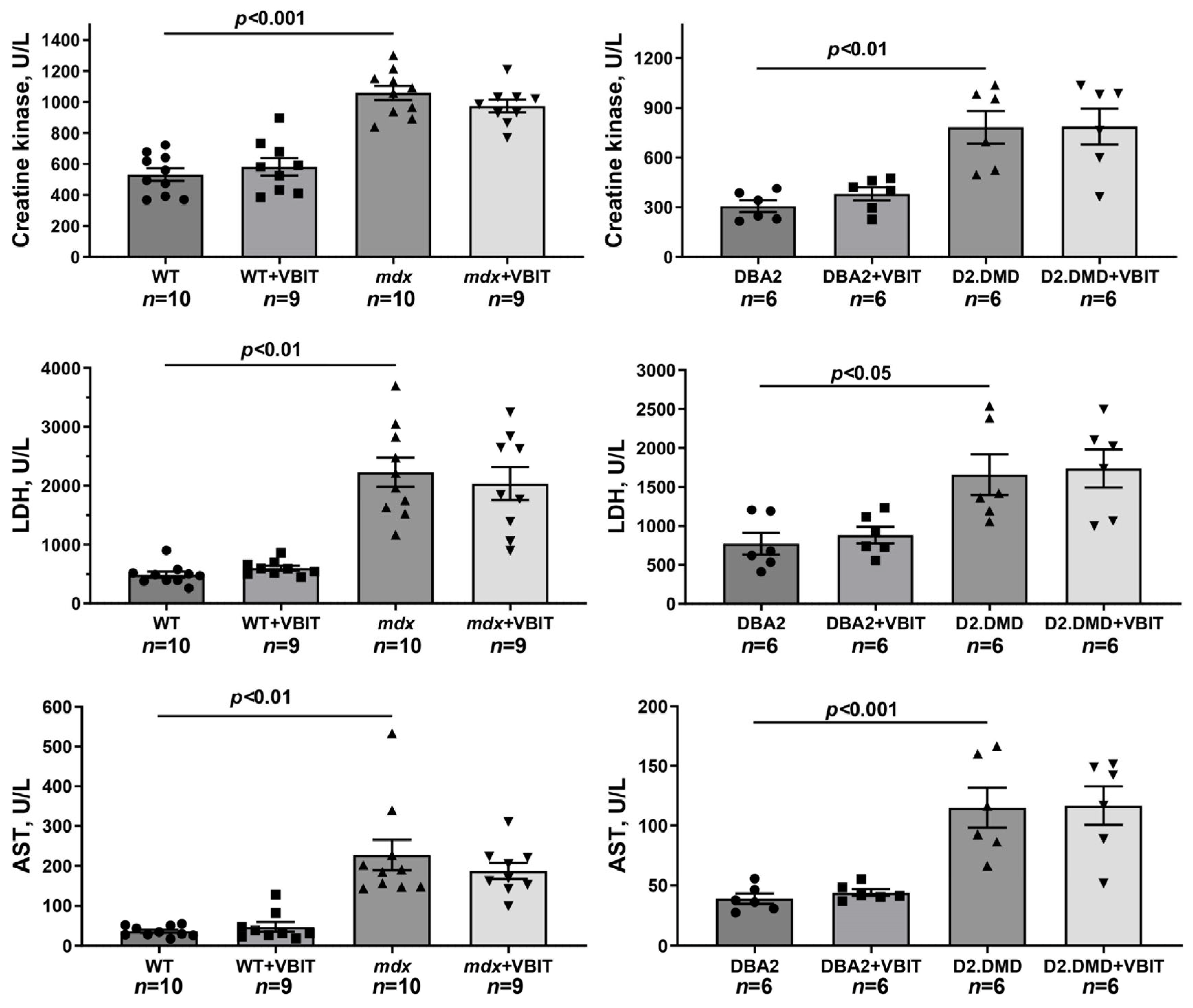
4.3. Creatine Kinase, LDH, and AST Assays
4.4. Transmission Electron Microscopy
4.5. Histological Analysis
4.6. Electrophoresis and Western Blotting
4.7. Measurement of 4-HNE in the Quadriceps Tissue
4.8. Isolation of Skeletal Muscle Mitochondria and Their Functional Analysis
4.9. Measurement of Calpain Activity in the Tissue and Mitochondria
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DMD | Duchenne muscular dystrophy |
| VDAC | Voltage-dependent anion channel |
| MPT | Mitochondrial permeability transition |
| SR | Sarcoplasmic reticulum |
| CRC | Calcium retention capacity |
| 4-HNE | 4-hydroxy-trans-2-nonenal |
| GRP78 | Glucose-regulated protein 78 |
| RCR | Respiratory control ratio |
| DNP | 2,4-dinitrophenol |
| CNF | Centrally nucleated fibers |
| AST | Aspartate aminotransferase |
| LDH | Lactate dehydrogenase |
| UPR | Unfolded protein response |
| ROS | Reactive oxygen species |
References
- Duan, D.; Goemans, N.; Takeda, S.; Mercuri, E.; Aartsma-Rus, A. Duchenne muscular dystrophy. Nat. Rev. Dis. Primers 2021, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Dubinin, M.V.; Belosludtsev, K.N. Ion Channels of the Sarcolemma and Intracellular Organelles in Duchenne Muscular Dystrophy: A Role in the Dysregulation of Ion Homeostasis and a Possible Target for Therapy. Int. J. Mol. Sci. 2023, 24, 2229. [Google Scholar] [CrossRef] [PubMed]
- Altamirano, F.; Valladares, D.; Henríquez-Olguín, C.; Casas, M.; López, J.R.; Allen, P.D.; Jaimovich, E. Nifedipine treatment reduces resting calcium concentration, oxidative and apoptotic gene expression, and improves muscle function in dystrophic mdx mice. PLoS ONE 2013, 8, e81222. [Google Scholar] [CrossRef]
- Edwards, J.N.; Friedrich, O.; Cully, T.R.; von Wegner, F.; Murphy, R.M.; Launikonis, B.S. Upregulation of store-operated Ca2+ entry in dystrophic mdx mouse muscle. Am. J. Physiol. Cell Physiol. 2010, 299, C42–C50. [Google Scholar] [CrossRef]
- Cartes-Saavedra, B.; Ghosh, A.; Hajnóczky, G. The roles of mitochondria in global and local intracellular calcium signalling. Nat. Rev. Mol. Cell Biol. 2025, 26, 456–475. [Google Scholar] [CrossRef]
- Jackson, M.J.; Jones, D.A.; Edwards, R.H. Measurements of calcium and other elements in muscle biopsy samples from patients with Duchenne muscular dystrophy. Clin. Chim. Acta 1985, 147, 215–221. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Johnson, P.L.; Thakar, J.H. Reversal of impaired oxidative phosphorylation and calcium overloading in the in vitro cardiac mitochondria of CHF-146 dystrophic hamsters with hereditary muscular dystrophy. J. Neurol. Sci. 1993, 120, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.K.; Johnson, P.L.; Thakar, J.H. Reversal of impaired oxidative phosphorylation and calcium overloading in the skeletal muscle mitochondria of CHF-146 dystrophic hamsters. Mol. Chem. Neuropathol. 1998, 34, 53–77. [Google Scholar] [CrossRef]
- Nylen, E.G.; Wrogemann, K. Mitochondrial calcium content and oxidative phosphorylation in heart and skeletal muscle of dystrophic mice. Exp. Neurol. 1983, 80, 69–80. [Google Scholar] [CrossRef]
- Bround, M.J.; Havens, J.R.; York, A.J.; Sargent, M.A.; Karch, J.; Molkentin, J.D. ANT-dependent MPTP underlies necrotic myofiber death in muscular dystrophy. Sci. Adv. 2023, 9, eadi2767. [Google Scholar] [CrossRef]
- Dubinin, M.V.; Mikheeva, I.B.; Stepanova, A.E.; Igoshkina, A.D.; Cherepanova, A.A.; Semenova, A.A.; Sharapov, V.A.; Kireev, I.I.; Belosludtsev, K.N. Mitochondrial Transplantation Therapy Ameliorates Muscular Dystrophy in mdx Mouse Model. Biomolecules 2024, 14, 316. [Google Scholar] [CrossRef]
- Dubinin, M.V.; Talanov, E.Y.; Tenkov, K.S.; Starinets, V.S.; Mikheeva, I.B.; Sharapov, M.G.; Belosludtsev, K.N. Duchenne muscular dystrophy is associated with the inhibition of calcium uniport in mitochondria and an increased sensitivity of the organelles to the calcium-induced permeability transition. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165674. [Google Scholar] [CrossRef]
- Valladares, D.; Utreras-Mendoza, Y.; Campos, C.; Morales, C.; Diaz-Vegas, A.; Contreras-Ferrat, A.; Westermeier, F.; Jaimovich, E.; Marchi, S.; Pinton, P.; et al. IP3 receptor blockade restores autophagy and mitochondrial function in skeletal muscle fibers of dystrophic mice. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3685–3695. [Google Scholar] [CrossRef]
- Dubinin, M.V.; Stepanova, A.E.; Igoshkina, A.D.; Mikheeva, I.B.; Talanov, E.Y.; Cherepanova, A.A.; Belosludtsev, K.N. Effect of 2-Aminoethoxydiphenyl Borate on the State of Skeletal Muscles in Dystrophin-Deficient mdx Mice. Front. Biosci. (Landmark Ed.) 2024, 29, 428. [Google Scholar] [CrossRef] [PubMed]
- Dubinin, M.V.; Stepanova, A.E.; Mikheeva, I.B.; Igoshkina, A.D.; Cherepanova, A.A.; Talanov, E.Y.; Khoroshavina, E.I.; Belosludtsev, K.N. Reduction of Mitochondrial Calcium Overload via MKT077-Induced Inhibition of Glucose-Regulated Protein 75 Alleviates Skeletal Muscle Pathology in Dystrophin-Deficient mdx Mice. Int. J. Mol. Sci. 2024, 25, 9892. [Google Scholar] [CrossRef]
- Millay, D.P.; Sargent, M.A.; Osinska, H.; Baines, C.P.; Barton, E.R.; Vuagniaux, G.; Sweeney, H.L.; Robbins, J.; Molkentin, J.D. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat. Med. 2008, 14, 442–447. [Google Scholar] [CrossRef]
- Dubinin, M.V.; Starinets, V.S.; Talanov, E.Y.; Mikheeva, I.B.; Belosludtseva, N.V.; Belosludtsev, K.N. Alisporivir Improves Mitochondrial Function in Skeletal Muscle of mdx Mice but Suppresses Mitochondrial Dynamics and Biogenesis. Int. J. Mol. Sci. 2021, 22, 9780. [Google Scholar] [CrossRef]
- Schiavone, M.; Zulian, A.; Menazza, S.; Petronilli, V.; Argenton, F.; Merlini, L.; Sabatelli, P.; Bernardi, P. Alisporivir rescues defective mitochondrial respiration in Duchenne muscular dystrophy. Pharmacol. Res. 2017, 125, 122–131. [Google Scholar] [CrossRef]
- Reutenauer, J.; Dorchies, O.M.; Patthey-Vuadens, O.; Vuagniaux, G.; Ruegg, U.T. Investigation of Debio 025, a cyclophilin inhibitor, in the dystrophic mdx mouse, a model for Duchenne muscular dystrophy. Br. J. Pharmacol. 2008, 155, 574–584. [Google Scholar] [CrossRef]
- Wissing, E.R.; Millay, D.P.; Vuagniaux, G.; Molkentin, J.D. Debio-025 is more effective than prednisone in reducing muscular pathology in mdx mice. Neuromuscul. Disord. 2010, 20, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Stocco, A.; Smolina, N.; Sabatelli, P.; Šileikytė, J.; Artusi, E.; Mouly, V.; Cohen, M.; Forte, M.; Schiavone, M.; Bernardi, P. Treatment with a triazole inhibitor of the mitochondrial permeability transition pore fully corrects the pathology of sapje zebrafish lacking dystrophin. Pharmacol. Res. 2021, 165, 105421. [Google Scholar] [CrossRef]
- Szabadkai, G.; Bianchi, K.; Várnai, P.; De Stefani, D.; Wieckowski, M.R.; Cavagna, D.; Nagy, A.I.; Balla, T.; Rizzuto, R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 2006, 175, 901–911. [Google Scholar] [CrossRef]
- Colombini, M.; Blachly-Dyson, E.; Forte, M. VDAC, a channel in the outer mitochondrial membrane. In Ion Channels; Narahashi, T., Ed.; Springer: Boston, MA, USA, 1996; Volume 4, pp. 1–20. [Google Scholar]
- Wu, W.; Song, X.; Li, B. Identification of VDAC1 as a mitochondria-related target of Duchenne muscular dystrophy based on bioinformatics analysis and in vitro experiments. Int. Immunopharmacol. 2025, 158, 114836. [Google Scholar] [CrossRef] [PubMed]
- Bellissimo, C.A.; Delfinis, L.J.; Hughes, M.C.; Turnbull, P.C.; Gandhi, S.; DiBenedetto, S.N.; Rahman, F.A.; Tadi, P.; Amaral, C.A.; Dehghani, A.; et al. Mitochondrial creatine sensitivity is lost in the D2.mdx model of Duchenne muscular dystrophy and rescued by the mitochondrial-enhancing compound Olesoxime. Am. J. Physiol. Cell Physiol. 2023, 324, C1141–C1157. [Google Scholar] [CrossRef] [PubMed]
- Ben-Hail, D.; Begas-Shvartz, R.; Shalev, M.; Shteinfer-Kuzmine, A.; Gruzman, A.; Reina, S.; De Pinto, V.; Shoshan-Barmatz, V. Novel Compounds Targeting the Mitochondrial Protein VDAC1 Inhibit Apoptosis and Protect against Mitochondrial Dysfunction. J. Biol. Chem. 2016, 291, 24986–25003. [Google Scholar] [CrossRef]
- Verma, A.; Shteinfer-Kuzmine, A.; Kamenetsky, N.; Pittala, S.; Paul, A.; Nahon Crystal, E.; Ouro, A.; Chalifa-Caspi, V.; Pandey, S.K.; Monsonego, A.; et al. Targeting the overexpressed mitochondrial protein VDAC1 in a mouse model of Alzheimer’s disease protects against mitochondrial dysfunction and mitigates brain pathology. Transl. Neurodegener. 2022, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Gupta, R.; Blanco, L.P.; Yang, S.; Shteinfer-Kuzmine, A.; Wang, K.; Zhu, J.; Yoon, H.E.; Wang, X.; Kerkhofs, M.; et al. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science 2019, 366, 1531–1536. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Serov, D.A.; Ilzorkina, A.I.; Starinets, V.S.; Dubinin, M.V.; Talanov, E.Y.; Karagyaur, M.N.; Primak, A.L.; Belosludtseva, N.V. Pharmacological and Genetic Suppression of VDAC1 Alleviates the Development of Mitochondrial Dysfunction in Endothelial and Fibroblast Cell Cultures upon Hyperglycemic Conditions. Antioxidants 2023, 12, 1459. [Google Scholar] [CrossRef]
- Klapper-Goldstein, H.; Verma, A.; Elyagon, S.; Gillis, R.; Murninkas, M.; Pittala, S.; Paul, A.; Shoshan-Barmatz, V.; Etzion, Y. VDAC1 in the diseased myocardium and the effect of VDAC1-interacting compound on atrial fibrosis induced by hyperaldosteronism. Sci. Rep. 2020, 10, 22101. [Google Scholar] [CrossRef]
- Coley, W.D.; Bogdanik, L.; Vila, M.C.; Yu, Q.; Van Der Meulen, J.H.; Rayavarapu, S.; Novak, J.S.; Nearing, M.; Quinn, J.L.; Saunders, A.; et al. Effect of genetic background on the dystrophic phenotype in mdx mice. Hum. Mol. Genet. 2016, 25, 130–145. [Google Scholar] [CrossRef]
- Hammers, D.W.; Hart, C.C.; Matheny, M.K.; Wright, L.A.; Armellini, M.; Barton, E.R.; Sweeney, H.L. The D2.mdx mouse as a preclinical model of the skeletal muscle pathology associated with Duchenne muscular dystrophy. Sci. Rep. 2020, 10, 14070. [Google Scholar] [CrossRef]
- Egorova, T.V.; Zotova, E.D.; Reshetov, D.A.; Polikarpova, A.V.; Vassilieva, S.G.; Vlodavets, D.V.; Gavrilov, A.A.; Ulianov, S.V.; Buchman, V.L.; Deykin, A.V. CRISPR/Cas9-generated mouse model of Duchenne muscular dystrophy recapitulating a newly identified large 430 kb deletion in the human DMD gene. Dis. Model. Mech. 2019, 12, dmm037655. [Google Scholar] [CrossRef]
- Spencer, M.J.; Croall, D.E.; Tidball, J.G. Calpains are activated in necrotic fibers from mdx dystrophic mice. J. Biol. Chem. 1995, 270, 10909–10914. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, G.; Peng, T. Calpain-Mediated Mitochondrial Damage: An Emerging Mechanism Contributing to Cardiac Disease. Cells 2021, 10, 2024. [Google Scholar] [CrossRef]
- Pauly, M.; Angebault-Prouteau, C.; Dridi, H.; Notarnicola, C.; Scheuermann, V.; Lacampagne, A.; Matecki, S.; Fauconnier, J. ER stress disturbs SR/ER-mitochondria Ca2+ transfer: Implications in Duchenne muscular dystrophy. Biochim. Biophys. Acta. Mol. Basis Dis. 2017, 1863, 2229–2239. [Google Scholar] [CrossRef]
- Percival, J.M.; Siegel, M.P.; Knowels, G.; Marcinek, D.J. Defects in mitochondrial localization and ATP synthesis in the mdx mouse model of Duchenne muscular dystrophy are not alleviated by PDE5 inhibition. Hum. Mol. Genet. 2013, 22, 153–167. [Google Scholar] [CrossRef]
- Rybalka, E.; Timpani, C.A.; Cooke, M.B.; Williams, A.D.; Hayes, A. Defects in mitochondrial ATP synthesis in dystrophin-deficient mdx skeletal muscles may be caused by complex I insufficiency. PLoS ONE 2014, 9, e115763. [Google Scholar] [CrossRef]
- Tidball, J.G.; Wehling-Henricks, M. The role of free radicals in the pathophysiology of muscular dystrophy. J. Appl. Physiol. 2007, 102, 1677–1686. [Google Scholar] [CrossRef]
- Young, C.N.J.; Gosselin, M.R.F.; Rumney, R.; Oksiejuk, A.; Chira, N.; Bozycki, L.; Matryba, P.; Łukasiewicz, K.; Kao, A.P.; Dunlop, J.; et al. Total Absence of Dystrophin Expression Exacerbates Ectopic Myofiber Calcification and Fibrosis and Alters Macrophage Infiltration Patterns. Am. J. Pathol. 2020, 190, 190–205. [Google Scholar] [CrossRef]
- Romero, N.B.; Bitoun, M. Centronuclear myopathies. Semin. Pediatr. Neurol. 2011, 18, 250–256. [Google Scholar] [CrossRef]
- Bulfield, G.; Siller, W.G.; Wight, P.A.; Moore, K.J. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc. Natl. Acad. Sci. USA 1984, 81, 1189–1192. [Google Scholar] [CrossRef]
- Shintani-Ishida, K.; Yoshida, K. Mitochondrial m-calpain opens the mitochondrial permeability transition pore in ischemia-reperfusion. Int. J. Cardiol. 2015, 197, 26–32. [Google Scholar] [CrossRef]
- Coluccino, G.; Negro, A.; Filippi, A.; Bean, C.; Muraca, V.P.; Gissi, C.; Canetti, D.; Mimmi, M.C.; Zamprogno, E.; Ciscato, F.; et al. N-terminal cleavage of cyclophilin D boosts its ability to bind F-ATP synthase. Commun. Biol. 2024, 7, 1486. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Ilzorkina, A.I.; Matveeva, L.A.; Chulkov, A.V.; Semenova, A.A.; Dubinin, M.V.; Belosludtseva, N.V. Effect of VBIT-4 on the functional activity of isolated mitochondria and cell viability. Biochim. Biophys. Acta Biomembr. 2024, 1866, 184329. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, N.; Wu, B.; Wu, S.; Zhang, Y.; Sun, Y. The Role of Mitochondria in Vascular Calcification. J. Transl. Int. Med. 2020, 8, 80–90. [Google Scholar] [CrossRef]
- Duvvuri, B.; Pachman, L.M.; Hermanson, P.; Wang, T.; Moore, R.; Ding-Hwa Wang, D.; Long, A.; Morgan, G.A.; Doty, S.; Tian, R.; et al. Role of mitochondria in the myopathy of juvenile dermatomyositis and implications for skeletal muscle calcinosis. J. Autoimmun. 2023, 138, 103061. [Google Scholar] [CrossRef]
- Duddy, W.; Duguez, S.; Johnston, H.; Cohen, T.V.; Phadke, A.; Gordish-Dressman, H.; Nagaraju, K.; Gnocchi, V.; Low, S.; Partridge, T. Muscular dystrophy in the mdx mouse is a severe myopathy compounded by hypotrophy, hypertrophy and hyperplasia. Skelet. Muscle 2015, 5, 16. [Google Scholar] [CrossRef]
- Heydemann, A. Skeletal Muscle Metabolism in Duchenne and Becker Muscular Dystrophy-Implications for Therapies. Nutrients 2018, 10, 796. [Google Scholar] [CrossRef]
- Rostovtseva, T.K.; Bezrukov, S.M.; Hoogerheide, D.P. Regulation of Mitochondrial Respiration by VDAC Is Enhanced by Membrane-Bound Inhibitors with Disordered Polyanionic C-Terminal Domains. Int. J. Mol. Sci. 2021, 22, 7358. [Google Scholar] [CrossRef]
- Van Putten, M.; Aartsma-Rus, A.; Louvain-la-Neuve, L. The Use of Hanging Wire Tests to Monitor Muscle Strength and Condition over Time; TREAT-NMD Neuromscular Network/Wellstone Muscular Dystrophy Center: Washington, DC, USA, 2011; Volume 2, pp. 1–11. [Google Scholar]
- Aartsma-Rus, A.; van Putten, M. Assessing functional performance in the mdx mouse model. J. Vis. Exp. 2014, 85, 51303. [Google Scholar] [CrossRef]
- Kumar, A.; Accorsi, A.; Rhee, Y.; Girgenrath, M. Do’s and don’ts in the preparation of muscle cryosections for histological analysis. J. Vis. Exp. 2015, 99, e52793. [Google Scholar] [CrossRef]
- Frezza, C.; Cipolat, S.; Scorrano, L. Organelle isolation: Functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat. Protoc. 2007, 2, 287–295. [Google Scholar] [CrossRef]
- Chance, B.; Williams, G.R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J. Biol. Chem. 1955, 217, 383–393. [Google Scholar] [CrossRef]


| Group | Respiratory Rate, nmol O2/min per 1 mg of Protein | RCR (Relative Units) | |||
|---|---|---|---|---|---|
| State 2 | State 3 | State 4 | State 3UDNP | ||
| WT (n = 5) | 33.2 ± 3.5 | 194.5 ± 5.4 | 30.3 ± 1.6 | 256.8 ± 11.8 | 6.5 ± 0.3 |
| WT + VBIT (n = 5) | 28.9 ± 2.4 | 146.0 ± 18.9 | 34.0 ± 2.3 | 188.3 ± 24 | 4.3 ± 0.4 ** |
| mdx (n = 5) | 31.2 ± 1.9 | 136.9 ± 10.8 * | 28.9 ± 1.9 | 173.4 ± 14.1 * | 4.7 ± 0.2 ** |
| mdx + VBIT (n = 5) | 31.6 ± 1.8 | 154.3 ± 11.8 | 35.5 ± 2.3 | 191.8 ± 19.5 | 4.4 ± 0.3 ** |
| DBA2 (n = 4) | 27.2 ± 0.8 | 145.5 ± 5.5 | 29.4 ± 1.8 | 176.3 ± 3.4 | 5.0 ± 0.2 |
| DBA2 + VBIT (n = 4) | 24.7 ± 1.9 | 136.4 ± 5.5 | 31.2 ± 3.3 | 161.4 ± 9.5 | 4.5 ± 0.4 |
| D2.DMD (n = 4) | 29.9 ± 1.4 | 61.0 ± 10.9 ** | 40.6 ± 3.4 | 83.0 ± 11.6 ** | 1.6 ± 0.4 ** |
| D2.DMD + VBIT (n = 4) | 27.4 ± 1.6 | 67.3 ± 9.8 ** | 31.6 ± 2.8 | 92.1 ± 11.8 ** | 2.1 ± 0.2 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubinin, M.V.; Stepanova, A.E.; Mikheeva, I.B.; Igoshkina, A.D.; Kraeva, E.N.; Cherepanova, A.A.; Talanov, E.Y.; Polikarpova, A.V.; Astashev, M.E.; Loginov, V.A.; et al. VBIT-4 Rescues Mitochondrial Dysfunction and Reduces Skeletal Muscle Degeneration in a Severe Model of Duchenne Muscular Dystrophy. Int. J. Mol. Sci. 2025, 26, 8845. https://doi.org/10.3390/ijms26188845
Dubinin MV, Stepanova AE, Mikheeva IB, Igoshkina AD, Kraeva EN, Cherepanova AA, Talanov EY, Polikarpova AV, Astashev ME, Loginov VA, et al. VBIT-4 Rescues Mitochondrial Dysfunction and Reduces Skeletal Muscle Degeneration in a Severe Model of Duchenne Muscular Dystrophy. International Journal of Molecular Sciences. 2025; 26(18):8845. https://doi.org/10.3390/ijms26188845
Chicago/Turabian StyleDubinin, Mikhail V., Anastasia E. Stepanova, Irina B. Mikheeva, Anastasia D. Igoshkina, Ekaterina N. Kraeva, Alena A. Cherepanova, Eugeny Yu. Talanov, Anna V. Polikarpova, Maxim E. Astashev, Vyacheslav A. Loginov, and et al. 2025. "VBIT-4 Rescues Mitochondrial Dysfunction and Reduces Skeletal Muscle Degeneration in a Severe Model of Duchenne Muscular Dystrophy" International Journal of Molecular Sciences 26, no. 18: 8845. https://doi.org/10.3390/ijms26188845
APA StyleDubinin, M. V., Stepanova, A. E., Mikheeva, I. B., Igoshkina, A. D., Kraeva, E. N., Cherepanova, A. A., Talanov, E. Y., Polikarpova, A. V., Astashev, M. E., Loginov, V. A., & Egorova, T. V. (2025). VBIT-4 Rescues Mitochondrial Dysfunction and Reduces Skeletal Muscle Degeneration in a Severe Model of Duchenne Muscular Dystrophy. International Journal of Molecular Sciences, 26(18), 8845. https://doi.org/10.3390/ijms26188845







