Keratoconjunctivitis Sicca in Sjögren Disease: Diagnostic Challenges and Therapeutic Advances
Abstract
1. Introduction
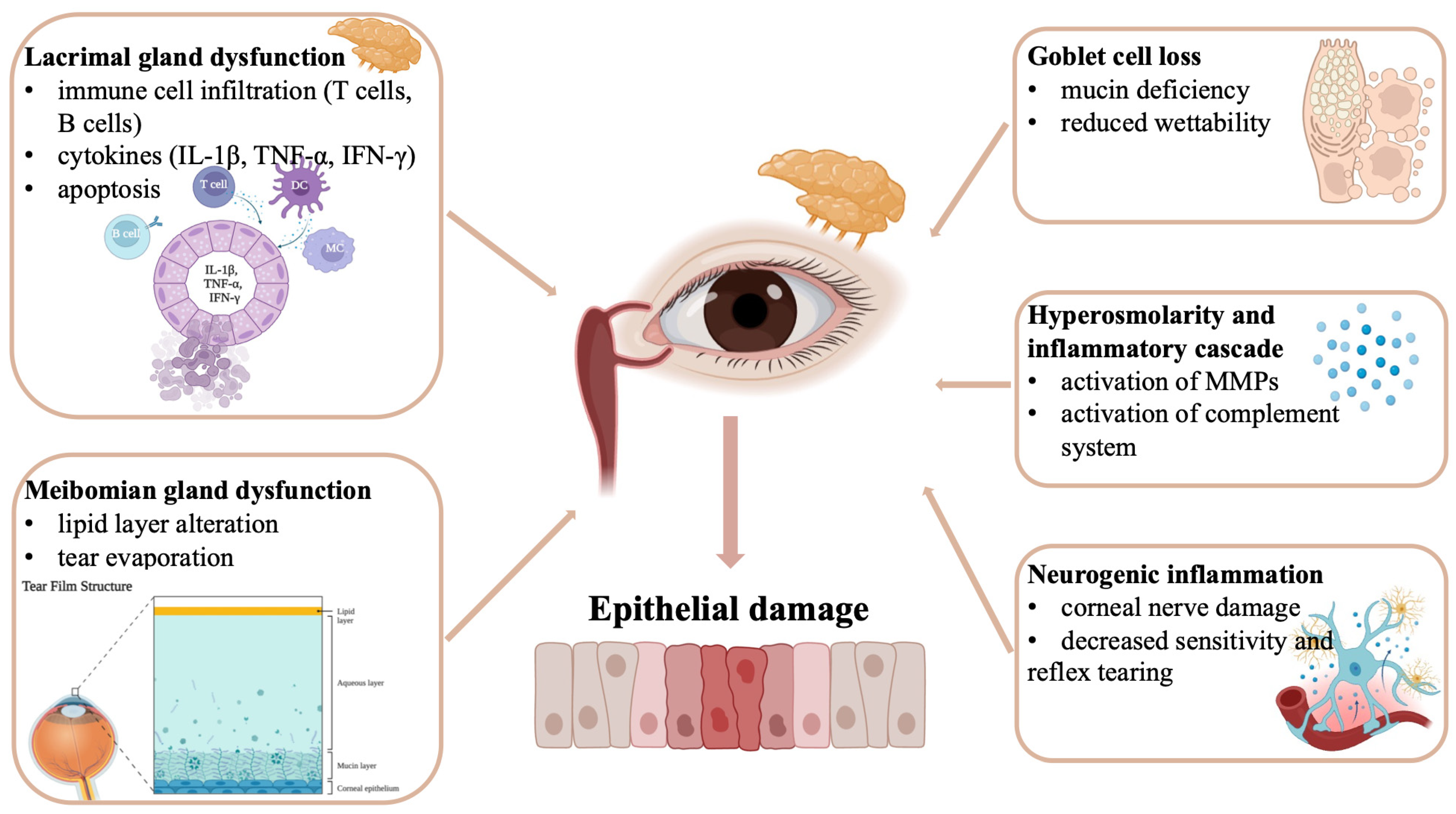
2. Pathophysiology of Keratoconjunctivitis Sicca in Sjögren Disease
2.1. Autoimmune Mechanisms and Glandular Dysfunction
2.2. Tear Film Abnormalities and Ocular Surface Changes
2.3. Inflammatory Cascade and Ocular Surface Damage
3. Diagnostic Challenges in Keratoconjunctivitis Sicca
3.1. Clinical Presentation and Symptom Variability
3.2. Diagnostic Testing Limitations and Interpretation
3.3. Differential Diagnosis Considerations
4. Advanced Diagnostic Approaches
4.1. Imaging Technologies and Biomarkers
4.2. Functional Assessment Techniques
5. Current Therapeutic Approaches
5.1. Artificial Tears and Lubricants
5.2. Anti-Inflammatory Therapies
5.3. Secretagogues and Tear Stimulants
5.4. Procedural Interventions
6. Emerging Therapeutic Strategies
6.1. Biologic Therapies and Targeted Interventions
6.2. Regenerative Medicine Approaches
6.3. Gene Therapy and Molecular Interventions
7. Future Directions and Research Priorities
7.1. Personalized Medicine Approaches
7.2. Combination Therapy Strategies
8. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AS-OCT | Anterior Segment Optical Coherence Tomography |
| BAFF | B-cell Activating Factor |
| CD | Cluster of Differentiation |
| DED | Dry Eye Disease |
| DEQ | Dry Eye Questionnaire |
| HLA | Human Leukocyte Antigen |
| IFN-γ | Interferon gamma |
| IFN-I | Type I Interferon |
| IL-1β | Interleukin-1 beta |
| IVCM | In Vivo Confocal Microscopy |
| KCS | Keratoconjunctivitis Sicca |
| MGD | Meibomian Gland Dysfunction |
| MMP-9 | Matrix Metalloproteinase-9 |
| MSC | Mesenchymal Stem Cell |
| NSAID | Nonsteroidal Anti-Inflammatory Drug |
| OSDI | Ocular Surface Disease Index |
| PRP | Platelet-Rich Plasma |
| SD | Sjögren’s Disease |
| sIgA | Secretory Immunoglobulin A |
| SSA/Ro | Sjögren Syndrome Antigen A (Ro) |
| SSB/La | Sjögren Syndrome Antigen B (La) |
| SS | Sjögren’s Syndrome |
| TBUT | Tear Break-Up Time |
| TGF-β | Transforming Growth Factor-beta |
| TFOS DEWS II | Tear Film and Ocular Surface Society Dry Eye Workshop II |
| TNF-α | Tumor Necrosis Factor-alpha |
References
- Qin, B.; Wang, J.; Yang, Z.; Yang, M.; Ma, N.; Huang, F.; Zhong, R. Epidemiology of primary Sjogren’s syndrome: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zeron, P.; Siso-Almirall, A.; Bosch, X. Primary Sjogren syndrome. BMJ 2012, 344, e3821. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; de Paiva, C.S. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology 2017, 124, S4–S13. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510, Erratum in Ocul. Surf. 2019, 17, 842. [Google Scholar] [CrossRef]
- Mavragani, C.P.; Moutsopoulos, H.M. Sjogren’s syndrome. Annu. Rev. Pathol. 2014, 9, 273–285. [Google Scholar] [CrossRef]
- Patel, R.; Shahane, A. The epidemiology of Sjogren’s syndrome. Clin. Epidemiol. 2014, 6, 247–255. [Google Scholar]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Galor, A.; Feuer, W.; Lee, D.J.; Florez, H.; Carter, D.; Pouyeh, B.; Prunty, W.J.; Perez, V.L. Prevalence and risk factors of dry eye syndrome in a United States veterans affairs population. Am. J. Ophthalmol. 2011, 152, 377–384.e2. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Solans, R.; Rosas, J.; Camps, M.T.; Gil, A.; Del Pino-Montes, J.; Calvo-Alen, J.; Jiménez-Alonso, J.; Micó, M.-L.; Beltrán, J.; et al. Primary Sjogren syndrome in Spain: Clinical and immunologic expression in 1010 patients. Medicine 2008, 87, 210–219. [Google Scholar] [CrossRef]
- Akpek, E.K.; Klimava, A.; Thorne, J.E.; Martin, D.; Lekhanont, K.; Ostrovsky, A. Evaluation of patients with dry eye for presence of underlying Sjogren syndrome. Cornea 2009, 28, 493–497. [Google Scholar] [CrossRef]
- Caffery, B.E.; Richter, D.; Simpson, T.; Fonn, D.; Doughty, M.; Gordon, K. CANDEES. The Canadian Dry Eye Epidemiology Study. Adv. Exp. Med. Biol. 1998, 438, 805–806. [Google Scholar]
- Miljanovic, B.; Dana, R.; Sullivan, D.A.; Schaumberg, D.A. Impact of dry eye syndrome on vision-related quality of life. Am. J. Ophthalmol. 2007, 143, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Lessard, C.J.; Li, H.; Adrianto, I.; Ice, J.A.; Rasmussen, A.; Grundahl, K.M.; Kelly, J.A.; Dozmorov, M.G.; Miceli-Richard, C.; Bowman, S.; et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjogren’s syndrome. Nat. Genet. 2013, 45, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Igoe, A.; Scofield, R.H. Autoimmunity and infection in Sjogren’s syndrome. Curr. Opin. Rheumatol. 2013, 25, 480–487. [Google Scholar] [CrossRef]
- Baudouin, C.; Messmer, E.M.; Aragona, P.; Geerling, G.; Akova, Y.A.; Benítez-Del-Castillo, J.; Boboridis, K.G.; Merayo-Lloves, J.; Rolando, M.; Labetoulle, M. Revisiting the vicious circle of dry eye disease: A focus on the pathophysiology of meibomian gland dysfunction. Br. J. Ophthalmol. 2016, 100, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef]
- Manoussakis, M.N.; Boiu, S.; Korkolopoulou, P.; Kapsogeorgou, E.K.; Kavantzas, N.; Ziakas, P.; Patsouris, E.; Moutsopoulos, H.M. Rates of infiltration by macrophages and dendritic cells and expression of interleukin-18 and interleukin-12 in the chronic inflammatory lesions of Sjogren’s syndrome: Correlation with certain features of immune hyperactivity and factors associated with high risk of lymphoma development. Arthritis Rheum. 2007, 56, 3977–3988. [Google Scholar]
- Christodoulou, M.I.; Kapsogeorgou, E.K.; Moutsopoulos, H.M. Characteristics of the minor salivary gland infiltrates in Sjogren’s syndrome. J. Autoimmun. 2010, 34, 400–407. [Google Scholar] [CrossRef]
- Scofield, R.H.; Farris, A.D.; Horsfall, A.C.; Harley, J.B. Fine specificity of the autoimmune response to the Ro/SSA and La/SSB ribonucleoproteins. Arthritis Rheum. 1999, 42, 199–209. [Google Scholar] [CrossRef]
- Bacman, S.; Berra, A.; Sterin-Borda, L.; Borda, E. Muscarinic acetylcholine receptor antibodies as a new marker of dry eye Sjogren syndrome. Investig. Opthalmol. Vis. Sci. 2001, 42, 321–327. [Google Scholar]
- Peri, Y.; Agmon-Levin, N.; Theodor, E.; Shoenfeld, Y. Sjogren’s syndrome, the old and the new. Best Pract. Res. Clin. Rheumatol. 2012, 26, 105–117. [Google Scholar] [CrossRef]
- Wahren-Herlenius, M.; Dorner, T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet 2013, 382, 819–831. [Google Scholar] [CrossRef]
- Yiannaki, E.E.; Tzioufas, A.G.; Bachmann, M.; Hantoumi, J.; Tsikaris, V.; Sakarellos-Daitsiotis, M.; Sakarellos, C.; Moutsopoulos, H.M. The value of synthetic linear epitope analogues of La/SSB for the detection of autoantibodies to La/SSB; specificity, sensitivity and comparison of methods. Clin. Exp. Immunol. 1998, 112, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Galletti, J.G.; de Paiva, C.S. The ocular surface immune system through the eyes of aging. Ocul. Surf. 2021, 20, 139–162. [Google Scholar] [CrossRef]
- Kroneld, U.; Jonsson, R.; Carlsten, H.; Bremell, T.; Johannessen, A.C.; Tarkowski, A. Expression of the mucosal lymphocyte integrin alphaEbeta7 and its ligand E-cadherin in salivary glands of patients with Sjogren’s syndrome. Scand. J. Rheumatol. 1998, 27, 215–218. [Google Scholar] [PubMed]
- Song, Y.; Li, J.; Wu, Y. Evolving understanding of autoimmune mechanisms and new therapeutic strategies of autoimmune disorders. Signal Transduct. Target. Ther. 2024, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Cusick, M.F.; Libbey, J.E.; Fujinami, R.S. Molecular mimicry as a mechanism of autoimmune disease. Clin. Rev. Allergy Immunol. 2012, 42, 102–111. [Google Scholar] [CrossRef]
- Baer, A.N.; Maynard, J.W.; Shaikh, F.; Magder, L.S.; Petri, M. Secondary Sjogren’s syndrome in systemic lupus erythematosus defines a distinct disease subset. J. Rheumatol. 2010, 37, 1143–1149. [Google Scholar] [CrossRef]
- Tsunawaki, S.; Sporn, M.; Ding, A.; Nathan, C. Deactivation of macrophages by transforming growth factor-beta. Nature 1988, 334, 260–262. [Google Scholar] [CrossRef]
- Kapsogeorgou, E.K.; Christodoulou, M.I.; Panagiotakos, D.B.; Paikos, S.; Tassidou, A.; Tzioufas, A.G.; Moutsopoulos, H.M. Minor salivary gland inflammatory lesions in Sjogren syndrome: Do they evolve? J. Rheumatol. 2013, 40, 1566–1571. [Google Scholar] [CrossRef]
- Båve, U.; Nordmark, G.; Lövgren, T.; Rönnelid, J.; Cajander, S.; Eloranta, M.; Alm, G.V.; Rönnblom, L. Activation of the type I interferon system in primary Sjogren’s syndrome: A possible etiopathogenic mechanism. Arthritis Rheum. 2005, 52, 1185–1195. [Google Scholar] [CrossRef]
- Gottenberg, J.-E.; Cagnard, N.; Lucchesi, C.; Letourneur, F.; Mistou, S.; Lazure, T.; Jacques, S.; Ba, N.; Ittah, M.; Lepajolec, C.; et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjogren’s syndrome. Proc. Natl. Acad. Sci. USA 2006, 103, 2770–2775, Erratum in Proc. Natl. Acad. Sci. USA 2006, 103, 5242. [Google Scholar] [CrossRef] [PubMed]
- Imgenberg-Kreuz, J.; Sandling, J.K.; Almlöf, J.C.; Nordlund, J.; Signér, L.; Norheim, K.B.; Omdal, R.; Rönnblom, L.; Eloranta, M.-L.; Syvänen, A.-C.; et al. Genome-wide DNA methylation analysis in multiple tissues in primary Sjogren’s syndrome reveals regulatory effects at interferon-induced genes. Ann. Rheum. Dis. 2016, 75, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Delcroix, V.; Mauduit, O.; Lee, H.S.; Ivanova, A.; Umazume, T.; Knox, S.M.; de Paiva, C.S.; Dartt, D.A.; Makarenkova, H.P. The First Transcriptomic Atlas of the Adult Lacrimal Gland Reveals Epithelial Complexity and Identifies Novel Progenitor Cells in Mice. Cells 2023, 12, 1435. [Google Scholar] [CrossRef] [PubMed]
- Bjordal, O.; Norheim, K.B.; Rodahl, E.; Jonsson, R.; Omdal, R. Primary Sjogren’s syndrome and the eye. Surv. Ophthalmol. 2020, 65, 119–132. [Google Scholar] [CrossRef]
- Hayashi, T. Dysfunction of lacrimal and salivary glands in Sjogren’s syndrome: Nonimmunologic injury in preinflammatory phase and mouse model. J. Biomed. Biotechnol. 2011, 2011, 407031. [Google Scholar] [CrossRef]
- Nguyen, C.Q.; Cha, S.R.; Peck, A.B. Sjogren’s syndrome (SjS)-like disease of mice: The importance of B lymphocytes and autoantibodies. Front. Biosci. 2007, 12, 1767–1789. [Google Scholar] [CrossRef][Green Version]
- Tzioufas, A.G.; Kapsogeorgou, E.K.; Moutsopoulos, H.M. Pathogenesis of Sjogren’s syndrome: What we know and what we should learn. J. Autoimmun. 2012, 39, 4–8. [Google Scholar] [CrossRef]
- Hernandez-Molina, G.; Leal-Alegre, G.; Michel-Peregrina, M. The meaning of anti-Ro and anti-La antibodies in primary Sjogren’s syndrome. Autoimmun. Rev. 2011, 10, 123–125. [Google Scholar] [CrossRef]
- Baimpa, E.; Dahabreh, I.J.; Voulgarelis, M.; Moutsopoulos, H.M. Hematologic manifestations and predictors of lymphoma development in primary Sjogren syndrome: Clinical and pathophysiologic aspects. Medicine 2009, 88, 284–293. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brito-Zerón, P.; Kostov, B.; Sisó-Almirall, A.; Bosch, X.; Buss, D.; Trilla, A.; Stone, J.H.; Khamashta, M.A.; Shoenfeld, Y. Google-driven search for big data in autoimmune geoepidemiology: Analysis of 394,827 patients with systemic autoimmune diseases. Autoimmun. Rev. 2015, 14, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Chivasso, C.; D’Agostino, C.; Parisis, D.; Soyfoo, M.S.; Delporte, C. Involvement of aquaporin 5 in Sjogren’s syndrome. Autoimmun. Rev. 2023, 22, 103268. [Google Scholar] [CrossRef] [PubMed]
- Hermida-Prieto, M.; Garcia-Castro, J.; Marinas-Pardo, L. Systemic Treatment of Immune-Mediated Keratoconjunctivitis Sicca with Allogeneic Stem Cells Improves the Schirmer Tear Test Score in a Canine Spontaneous Model of Disease. J. Clin. Med. 2021, 10, 5981. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Feulner, L.; Djonov, V.; Pavlovic, D.; Volarevic, V. The Molecular Mechanisms Responsible for Tear Hyperosmolarity-Induced Pathological Changes in the Eyes of Dry Eye Disease Patients. Cells 2023, 12, 2755. [Google Scholar] [CrossRef]
- De Paiva, C.S.; Pflugfelder, S.C. Rationale for anti-inflammatory therapy in dry eye syndrome. Arq. Bras. Oftalmol. 2008, 71 (Suppl. 6), 89–95. [Google Scholar] [CrossRef]
- Stern, M.E.; Schaumburg, C.S.; Pflugfelder, S.C. Dry eye as a mucosal autoimmune disease. Int. Rev. Immunol. 2013, 32, 19–41. [Google Scholar] [CrossRef]
- Yoon, S.P.; Yu, Z.; Pflugfelder, S.C.; de Paiva, C.S. Differentially Expressed Tear Proteins in Sjogren’s Syndrome Keratoconjunctivitis Sicca. Transl. Vis. Sci. Technol. 2023, 12, 8. [Google Scholar] [CrossRef]
- Carnahan, M.C.; Goldstein, D.A. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr. Opin. Ophthalmol. 2000, 11, 478–483. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Tseng, S.C.; Sanabria, O.; Kell, H.; Garcia, C.G.; Felix, C.; Feuer, W.; Reis, B.L. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea 1998, 17, 38–56. [Google Scholar] [CrossRef]
- Lemp, M.A.; Crews, L.A.; Bron, A.J.; Foulks, G.N.; Sullivan, B.D. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: A retrospective study. Cornea 2012, 31, 472–478. [Google Scholar] [CrossRef]
- Caffery, B.; Joyce, E.; Boone, A.; Slomovic, A.; Simpson, T.; Jones, L.; Senchyna, M. Tear lipocalin and lysozyme in Sjogren and non-Sjogren dry eye. Optom. Vis. Sci. 2008, 85, 661–667. [Google Scholar] [CrossRef]
- Boukes, R.J.; Boonstra, A.; Breebaart, A.C.; Reits, D.; Glasius, E.; Luyendyk, L.; Kijlstra, A. Analysis of human tear protein profiles using high performance liquid chromatography (HPLC). Doc. Ophthalmol. 1987, 67, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Villani, E.; Beretta, S.; De Capitani, M.; Galimberti, D.; Viola, F.; Ratiglia, R. In vivo confocal microscopy of meibomian glands in Sjogren’s syndrome. Investig. Ophthalmol. Vis. Sci. 2011, 52, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Goto, E.; Matsumoto, Y.; Kamoi, M.; Endo, K.; Ishida, R.; Dogru, M.; Kaido, M.; Kojima, T.; Tsubota, K. Tear evaporation rates in Sjogren syndrome and non-Sjogren dry eye patients. Am. J. Ophthalmol. 2007, 144, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; Tiffany, J.M.; Gouveia, S.M.; Yokoi, N.; Voon, L.W. Functional aspects of the tear film lipid layer. Exp. Eye Res. 2004, 78, 347–360. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Huang, A.J.; Feuer, W.; Chuchovski, P.T.; Pereira, I.C.; Tseng, S.C. Conjunctival cytologic features of primary Sjogren’s syndrome. Ophthalmology 1990, 97, 985–991. [Google Scholar] [CrossRef]
- Mathers, W.D.; Stovall, D.; Lane, J.A.; Zimmerman, M.B.; Johnson, S. Menopause and tear function: The influence of prolactin and sex hormones on human tear production. Cornea 1998, 17, 353–358. [Google Scholar] [CrossRef]
- Lam, H.; Bleiden, L.; de Paiva, C.S.; Farley, W.; Stern, M.E.; Pflugfelder, S.C. Tear cytokine profiles in dysfunctional tear syndrome. Am. J. Ophthalmol. 2009, 147, 198–205.e1. [Google Scholar] [CrossRef]
- Stern, M.E.; Gao, J.; Siemasko, K.F.; Beuerman, R.W.; Pflugfelder, S.C. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp. Eye Res. 2004, 78, 409–416. [Google Scholar] [CrossRef]
- De Paiva, C.S.; Villarreal, A.L.; Corrales, R.M.; Rahman, H.T.; Chang, V.Y.; Farley, W.J.; Stern, M.E.; Niederkorn, J.Y.; Li, D.-Q.; Pflugfelder, S.C. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2553–2560. [Google Scholar] [CrossRef]
- Huang, D.; Li, Z. Multidimensional immunotherapy for dry eye disease: Current status and future directions. Front. Ophthalmol. 2024, 4, 1449283. [Google Scholar] [CrossRef]
- Baudouin, C.; Aragona, P.; Messmer, E.M.; Tomlinson, A.; Calonge, M.; Boboridis, K.G.; Akova, Y.A.; Geerling, G.; Labetoulle, M.; Rolando, M. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: Proceedings of the OCEAN group meeting. Ocul. Surf. 2013, 11, 246–258. [Google Scholar] [CrossRef]
- Versura, P.; Profazio, V.; Campos, E.C. Performance of tear osmolarity compared to previous diagnostic tests for dry eye diseases. Curr. Eye Res. 2010, 35, 553–564. [Google Scholar] [CrossRef]
- Lemp, M.A.; Bron, A.J.; Baudouin, C.; del Castillo, J.M.B.; Geffen, D.; Tauber, J.; Foulks, G.N.; Pepose, J.S.; Sullivan, B.D. Tear osmolarity in the diagnosis and management of dry eye disease. Am. J. Ophthalmol. 2011, 151, 792–798.e1. [Google Scholar] [CrossRef]
- Zheng, Q.; Ren, Y.; Reinach, P.S.; Xiao, B.; Lu, H.; Zhu, Y.; Qu, J.; Chen, W. Reactive oxygen species activated NLRP3 inflammasomes initiate inflammation in hyperosmolarity stressed human corneal epithelial cells and environment-induced dry eye patients. Exp. Eye Res. 2015, 134, 133–140. [Google Scholar] [CrossRef]
- Chi, W.; Li, F.; Chen, H.; Wang, Y.; Zhu, Y.; Yang, X.; Zhu, J.; Wu, F.; Ouyang, H.; Ge, J.; et al. Caspase-8 promotes NLRP1/NLRP3 inflammasome activation and IL-1beta production in acute glaucoma. Proc. Natl. Acad. Sci. USA 2014, 111, 11181–11186. [Google Scholar] [CrossRef] [PubMed]
- Bian, F.; Qi, H.; Ma, P.; Zhang, L.; Yoon, K.-C.; Pflugfelder, S.C.; Li, D.-Q. An immunoprotective privilege of corneal epithelial stem cells against Th17 inflammatory stress by producing glial cell-derived neurotrophic factor. Stem Cells 2010, 28, 2172–2181. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Hattori, T.; Park, E.Y.; Stevenson, W.; Chauhan, S.K.; Dana, R. Expression of toll-like receptor 4 contributes to corneal inflammation in experimental dry eye disease. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5632–5640. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, D.Q.; Doshi, A.; Farley, W.; Corrales, R.M.; Pflugfelder, S.C. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4293–4301. [Google Scholar] [CrossRef]
- Li, D.Q.; Chen, Z.; Song, X.J.; Luo, L.; Pflugfelder, S.C. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4302–4311. [Google Scholar] [CrossRef]
- Yagci, A.; Gurdal, C. The role and treatment of inflammation in dry eye disease. Int. Ophthalmol. 2014, 34, 1291–1301. [Google Scholar] [CrossRef]
- De Paiva, C.S.; Corrales, R.M.; Villarreal, A.L.; Farley, W.J.; Li, D.-Q.; Stern, M.E.; Pflugfelder, S.C. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp. Eye Res. 2006, 83, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Kunert, K.S.; Tisdale, A.S.; Stern, M.E.; Smith, J.A.; Gipson, I.K. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: Effect on conjunctival lymphocytes. Arch. Ophthalmol. 2000, 118, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Schwalb, T.A.; Addeo, J.V.; Ghosn, C.R.; Stern, M.E. The role of apoptosis in the pathogenesis of canine keratoconjunctivitis sicca: The effect of topical Cyclosporin A therapy. Cornea 1998, 17, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ogawa, Y.; Dogru, M.; Kawai, M.; Tatematsu, Y.; Uchino, M.; Okada, N.; Igarashi, A.; Kujira, A.; Fujishima, H.; et al. Ocular surface and tear functions after topical cyclosporine treatment in dry eye patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2008, 41, 293–302. [Google Scholar] [CrossRef]
- Chauhan, S.K.; El Annan, J.; Ecoiffier, T.; Goyal, S.; Zhang, Q.; Saban, D.R.; Dana, R. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J. Immunol. 2009, 182, 1247–1252. [Google Scholar] [CrossRef]
- Pelegrino, F.S.; Volpe, E.A.; Gandhi, N.B.; Li, D.Q.; Pflugfelder, S.C.; de Paiva, C.S. Deletion of interferon-gamma delays onset and severity of dacryoadenitis in CD25KO mice. Arthritis Res. Ther. 2012, 14, R234. [Google Scholar] [CrossRef]
- Zoukhri, D.; Fix, A.; Alroy, J.; Kublin, C.L. Mechanisms of murine lacrimal gland repair after experimentally induced inflammation. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4399–4406. [Google Scholar] [CrossRef]
- Mantelli, F.; Massaro-Giordano, M.; Macchi, I.; Lambiase, A.; Bonini, S. The cellular mechanisms of dry eye: From pathogenesis to treatment. J. Cell. Physiol. 2013, 228, 2253–2256. [Google Scholar] [CrossRef]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Benitez-Del-Castillo, J.M.; Acosta, M.C.; Wassfi, M.A.; Diaz-Valle, D.; Gegundez, J.A.; Fernandez, C.; Garcia-SanChez, J. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Investig. Ophthalmol. Vis. Sci. 2007, 48, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Tuisku, I.S.; Konttinen, Y.T.; Konttinen, L.M.; Tervo, T.M. Alterations in corneal sensitivity and nerve morphology in patients with primary Sjogren’s syndrome. Exp. Eye Res. 2008, 86, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.D.; Lee, S.J.; Kim, J.H.; Lee, S.M. The Role of Neuropeptides in Pathogenesis of Dry Dye. J. Clin. Med. 2021, 10, 4248. [Google Scholar] [CrossRef] [PubMed]
- Bourcier, T.; Acosta, M.C.; Borderie, V.; Borra’s, F.; Gallar, J.; Bury, T.; Laroche, L.; Belmonte, C. Decreased corneal sensitivity in patients with dry eye. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2341–2345. [Google Scholar] [CrossRef]
- McDonald, M.; Patel, D.A.; Keith, M.S.; Snedecor, S.J. Economic and Humanistic Burden of Dry Eye Disease in Europe, North America, and Asia: A Systematic Literature Review. Ocul. Surf. 2016, 14, 144–167. [Google Scholar] [CrossRef]
- Uchino, M.; Schaumberg, D.A. Dry Eye Disease: Impact on Quality of Life and Vision. Curr. Ophthalmol. Rep. 2013, 1, 51–57. [Google Scholar] [CrossRef]
- Bartlett, J.D.; Keith, M.S.; Sudharshan, L.; Snedecor, S.J. Associations between signs and symptoms of dry eye disease: A systematic review. Clin. Ophthalmol. 2015, 9, 1719–1730. [Google Scholar] [CrossRef]
- McMonnies, C.W. The clinical and experimental significance of blinking behavior. J. Optom. 2020, 13, 74–80. [Google Scholar] [CrossRef]
- Korb, D.R.; Greiner, J.V.; Glonek, T.; Esbah, R.; Finnemore, V.M.; Whalen, A.C. Effect of periocular humidity on the tear film lipid layer. Cornea 1996, 15, 129–134. [Google Scholar] [CrossRef]
- Knop, E.; Knop, N.; Millar, T.; Obata, H.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1938–1978. [Google Scholar] [CrossRef]
- Sullivan, B.D.; Whitmer, D.; Nichols, K.K.; Tomlinson, A.; Foulks, G.N.; Geerling, G.; Pepose, J.S.; Kosheleff, V.; Porreco, A.; Lemp, M.A. An objective approach to dry eye disease severity. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6125–6130. [Google Scholar] [CrossRef]
- Belmonte, C.; Nichols, J.J.; Cox, S.M.; Brock, J.A.; Begley, C.G.; Bereiter, D.A.; Dartt, D.A.; Galor, A.; Hamrah, P.; Ivanusic, J.J.; et al. TFOS DEWS II pain and sensation report. Ocul. Surf. 2017, 15, 404–437. [Google Scholar] [CrossRef]
- Galor, A.; Levitt, R.C.; Felix, E.R.; Martin, E.R.; Sarantopoulos, C.D. Neuropathic ocular pain: An important yet underevaluated feature of dry eye. Eye 2015, 29, 301–312. [Google Scholar] [CrossRef]
- Nettune, G.R.; Pflugfelder, S.C. Post-LASIK tear dysfunction and dysesthesia. Ocul. Surf. 2010, 8, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Nichols, K.K.; Mitchell, G.L.; Zadnik, K. The repeatability of clinical measurements of dry eye. Cornea 2004, 23, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Willcox, M.D.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef] [PubMed]
- Wolkoff, P.; Nojgaard, J.K.; Troiano, P.; Piccoli, B. Eye complaints in the office environment: Precorneal tear film integrity influenced by eye blinking efficiency. Occup. Environ. Med. 2005, 62, 4–12. [Google Scholar] [CrossRef]
- Portello, J.K.; Rosenfield, M.; Bababekova, Y.; Estrada, J.M.; Leon, A. Computer-related visual symptoms in office workers. Ophthalmic Physiol. Opt. 2012, 32, 375–382. [Google Scholar] [CrossRef]
- Blehm, C.; Vishnu, S.; Khattak, A.; Mitra, S.; Yee, R.W. Computer vision syndrome: A review. Surv. Ophthalmol. 2005, 50, 253–262. [Google Scholar] [CrossRef]
- Rosenfield, M. Computer vision syndrome: A review of ocular causes and potential treatments. Ophthalmic Physiol. Opt. 2011, 31, 502–515. [Google Scholar] [CrossRef]
- Galor, A.; Feuer, W.; Lee, D.J.; Florez, H.; Faler, A.L.; Zann, K.L.; Perez, V.L. Depression, post-traumatic stress disorder, and dry eye syndrome: A study utilizing the national United States Veterans Affairs administrative database. Am. J. Ophthalmol. 2012, 154, 340–346.e2. [Google Scholar] [CrossRef]
- Labbe, A.; Wang, Y.X.; Jie, Y.; Baudouin, C.; Jonas, J.B.; Xu, L. Dry eye disease, dry eye symptoms and depression: The Beijing Eye Study. Br. J. Ophthalmol. 2013, 97, 1399–1403. [Google Scholar] [CrossRef]
- Fernandez, C.A.; Galor, A.; Arheart, K.L.; Musselman, D.L.; Venincasa, V.D.; Florez, H.J.; Lee, D.J. Dry eye syndrome, posttraumatic stress disorder, and depression in an older male veteran population. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3666–3672. [Google Scholar] [CrossRef] [PubMed]
- Vehof, J.; Kozareva, D.; Hysi, P.G.; Hammond, C.J. Prevalence and risk factors of dry eye disease in a British female cohort. Br. J. Ophthalmol. 2014, 98, 1712–1717. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gong, L.; Sun, X.; Chapin, W.J. Anxiety and depression in patients with dry eye syndrome. Curr. Eye Res. 2011, 36, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ayaki, M.; Kawashima, M.; Negishi, K.; Tsubota, K. High prevalence of sleep and mood disorders in dry eye patients: Survey of 1000 eye clinic visitors. Neuropsychiatr. Dis. Treat. 2015, 11, 889–894. [Google Scholar] [CrossRef]
- Ayaki, M.; Tsubota, K.; Kawashima, M.; Kishimoto, T.; Mimura, M.; Negishi, K. Sleep Disorders are a Prevalent and Serious Comorbidity in Dry Eye. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES143–DES150. [Google Scholar] [CrossRef]
- Srinivasan, S.; Menzies, K.; Sorbara, L.; Jones, L. Infrared imaging of meibomian gland structure using a novel keratograph. Optom. Vis. Sci. 2012, 89, 788–794. [Google Scholar] [CrossRef]
- Tavakoli, M.; Hossain, P.; Malik, R.A. Clinical applications of corneal confocal microscopy. Clin. Ophthalmol. 2008, 2, 435–445. [Google Scholar] [CrossRef]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef]
- Begley, C.G.; Chalmers, R.L.; Abetz, L.; Venkataraman, K.; Mertzanis, P.; Caffery, B.A.; Snyder, C.; Edrington, T.; Nelson, D.; Simpson, T. The relationship between habitual patient-reported symptoms and clinical signs among patients with dry eye of varying severity. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4753–4761. [Google Scholar] [CrossRef] [PubMed]
- Markoulli, M.; Kolanu, S. Contact lens wear and dry eyes: Challenges and solutions. Clin. Optom. 2017, 9, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Itoh, K.; Inoue, K.; Amano, S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology 2008, 115, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.T.M.; Power, B.; Xue, A.L.; Kim, J.S.; Craig, J.P. Diagnostic performance of qualitative and quantitative methods of meibomian gland dropout evaluation in dry eye disease: An investigator-masked, randomised crossover study. Cont Lens Anterior Eye 2025, 48, 102324. [Google Scholar] [CrossRef]
- Daniel, E.; Maguire, M.G.; Pistilli, M.; Bunya, V.Y.; Massaro-Giordano, G.M.; Smith, E.; Kadakia, P.A.; Asbell, P.A. Grading and baseline characteristics of meibomian glands in meibography images and their clinical associations in the Dry Eye Assessment and Management (DREAM) study. Ocul. Surf. 2019, 17, 491–501. [Google Scholar] [CrossRef]
- Pult, H.; Riede-Pult, B.H. Non-contact meibography: Keep it simple but effective. Cont Lens Anterior Eye 2012, 35, 77–80. [Google Scholar] [CrossRef]
- Gupta, P.K.; Karpecki, P. Comprehensive Assessment of the Meibomian Glands by Meibography: Why the Upper Eyelids Matter. Cornea 2025, 44, 128–135. [Google Scholar] [CrossRef]
- Ban, Y.; Shimazaki-Den, S.; Tsubota, K.; Shimazaki, J. Morphological evaluation of meibomian glands using noncontact infrared meibography. Ocul. Surf. 2013, 11, 47–53. [Google Scholar] [CrossRef]
- Lin, X.; Fu, Y.; Li, L.; Chen, C.; Chen, X.; Mao, Y.; Lian, H.; Yang, W.; Dai, Q. A Novel Quantitative Index of Meibomian Gland Dysfunction, the Meibomian Gland Tortuosity. Transl. Vis. Sci. Technol. 2020, 9, 34. [Google Scholar] [CrossRef]
- Arita, R.; Itoh, K.; Maeda, S.; Maeda, K.; Furuta, A.; Fukuoka, S.; Tomidokoro, A.; Amano, S. Proposed diagnostic criteria for obstructive meibomian gland dysfunction. Ophthalmology 2009, 116, 2058–2063.e1. [Google Scholar] [CrossRef]
- Llorens-Quintana, C.; Rico-Del-Viejo, L.; Syga, P.; Madrid-Costa, D.; Iskander, D.R. A Novel Automated Approach for Infrared-Based Assessment of Meibomian Gland Morphology. Transl. Vis. Sci. Technol. 2019, 8, 17. [Google Scholar] [CrossRef]
- Nichols, J.J.; Berntsen, D.A.; Mitchell, G.L.; Nichols, K.K. An assessment of grading scales for meibography images. Cornea 2005, 24, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, A.; Bron, A.J.; Korb, D.R.; Amano, S.; Paugh, J.R.; Pearce, E.I.; Yee, R.; Yokoi, N.; Arita, R.; Dogru, M. The international workshop on meibomian gland dysfunction: Report of the diagnosis subcommittee. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2006–2049. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, J.; Sakata, M.; Tsubota, K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch. Ophthalmol. 1995, 113, 1266–1270. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Mossa, F.; Tiffany, J.M.; Bron, A.J. Assessment of meibomian gland function in dry eye using meibometry. Arch. Ophthalmol. 1999, 117, 723–729. [Google Scholar] [CrossRef]
- Liu, S.; Richards, S.M.; Lo, K.; Hatton, M.; Fay, A.; Sullivan, D.A. Changes in gene expression in human meibomian gland dysfunction. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2727–2740. [Google Scholar] [CrossRef]
- Nichols, K.K.; Foulks, G.N.; Bron, A.J.; Glasgow, B.J.; Dogru, M.; Tsubota, K.; Lemp, M.A.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Executive summary. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1922–1929. [Google Scholar] [CrossRef]
- Koh, Y.W.; Celik, T.; Lee, H.K.; Petznick, A.; Tong, L. Detection of meibomian glands and classification of meibography images. J. Biomed. Opt. 2012, 17, 086008. [Google Scholar] [CrossRef]
- Xiao, J.; Adil, M.Y.; Chen, X.; Utheim, Ø.A.; Ræder, S.; Tønseth, K.A.; Lagali, N.S.; Dartt, D.A.; Utheim, T.P. Functional and Morphological Evaluation of Meibomian Glands in the Assessment of Meibomian Gland Dysfunction Subtype and Severity. Am. J. Ophthalmol. 2020, 209, 160–167. [Google Scholar] [CrossRef]
- Özer, H.; Yılmaz, S.; Bozkurt, B.; Tezcan, D.; Yazol, M.; Hakbilen, S.; Topaloğlu, Ö.F.; Durmaz, M.S. Assessment of lacrimal gland involvement in primary Sjogren’s syndrome using gray-scale ultrasonography and shear wave elastography. Eur. Radiol. 2023, 33, 9368–9377. [Google Scholar] [CrossRef]
- Zhong, W.; Zhang, H.; Ran, H. Advances in imaging of the lacrimal gland in Sjogren’s syndrome: A narrative review. J. Clin. Ultrasound. 2024, 52, 68–77. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, J.; Sheng, J. Systematic review and meta-analysis of the diagnostic performance of lacrimal gland ultrasound elastography in primary Sjogren’s syndrome. Int. Ophthalmol. 2024, 44, 271. [Google Scholar] [CrossRef]
- Cornec, D.; Jousse-Joulin, S.; Pers, J.; Marhadour, T.; Cochener, B.; Boisramé-Gastrin, S.; Nowak, E.; Youinou, P.; Saraux, A.; Devauchelle-Pensec, V. Contribution of salivary gland ultrasonography to the diagnosis of Sjogren’s syndrome: Toward new diagnostic criteria? Arthritis Rheum. 2013, 65, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Villani, E.; Magnani, F.; Viola, F.; Santaniello, A.; Scorza, R.; Nucci, P.; Ratiglia, R. In vivo confocal evaluation of the ocular surface morpho-functional unit in dry eye. Optom. Vis. Sci. 2013, 90, 576–586. [Google Scholar] [CrossRef]
- Aragona, P.; Aguennouz, M.; Rania, L.; Postorino, E.; Sommario, M.S.; Roszkowska, A.M.; De Pasquale, M.G.; Pisani, A.; Puzzolo, D. Matrix metalloproteinase 9 and transglutaminase 2 expression at the ocular surface in patients with different forms of dry eye disease. Ophthalmology 2015, 122, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Ban, Y.; Ogawa, Y.; Ibrahim, O.M.; Tatematsu, Y.; Kamoi, M.; Uchino, M.; Yaguchi, S.; Dogru, M.; Tsubota, K. Morphologic evaluation of meibomian glands in chronic graft-versus-host disease using in vivo laser confocal microscopy. Mol. Vis. 2011, 17, 2533–2543. [Google Scholar] [PubMed]
- Ibrahim, O.M.; Matsumoto, Y.; Dogru, M.; Adan, E.S.; Wakamatsu, T.H.; Goto, T.; Negishi, K.; Tsubota, K. The efficacy, sensitivity, and specificity of in vivo laser confocal microscopy in the diagnosis of meibomian gland dysfunction. Ophthalmology 2010, 117, 665–672. [Google Scholar] [CrossRef]
- Sambursky, R.; Davitt, W.F., 3rd; Latkany, R.; Tauber, S.; Starr, C.; Friedberg, M.; Dirks, M.S.; McDonald, M. Sensitivity and specificity of a point-of-care matrix metalloproteinase 9 immunoassay for diagnosing inflammation related to dry eye. JAMA Ophthalmol. 2013, 131, 24–28. [Google Scholar] [CrossRef]
- Sall, K.; Stevenson, O.D.; Mundorf, T.K.; Reis, B.L. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology 2000, 107, 631–639. [Google Scholar] [CrossRef]
- Tauber, J.; Karpecki, P.; Latkany, R.; Luchs, J.; Martel, J.; Sall, K.; Raychaudhuri, A.; Smith, V.; Semba, C.P.; OPUS-2 Investigators. Lifitegrast Ophthalmic Solution 5.0% versus Placebo for Treatment of Dry Eye Disease: Results of the Randomized Phase III OPUS-2 Study. Ophthalmology 2015, 122, 2423–2431. [Google Scholar] [CrossRef]
- Sheppard, J.D.; Torkildsen, G.L.; Lonsdale, J.D.; D’Ambrosio, F.A., Jr.; McLaurin, E.B.; Eiferman, R.A.; Kennedy, K.S.; Semba, C.P.; OPUS-1 Study Group. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: Results of the OPUS-1 phase 3 study. Ophthalmology 2014, 121, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Vivino, F.B.; Al-Hashimi, I.; Khan, Z.; LeVeque, F.G.; Salisbury, P.L., 3rd; Tran-Johnson, T.K.; Muscoplat, C.C.; Trivedi, M.; Goldlust, B.; Gallagher, S.C. Pilocarpine tablets for the treatment of dry mouth and dry eye symptoms in patients with Sjogren syndrome: A randomized, placebo-controlled, fixed-dose, multicenter trial. P92-01 Study Group. Arch. Intern. Med. 1999, 159, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Petrone, D.; Condemi, J.J.; Fife, R.; Gluck, O.; Cohen, S.; Dalgin, P. A double-blind, randomized, placebo-controlled study of cevimeline in Sjogren’s syndrome patients with xerostomia and keratoconjunctivitis sicca. Arthritis Rheum. 2002, 46, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Ervin, A.M.; Law, A.; Pucker, A.D. Punctal occlusion for dry eye syndrome. Cochrane Database Syst. Rev. 2017, 6, CD006775. [Google Scholar] [CrossRef]
- Ervin, A.M.; Wojciechowski, R.; Schein, O. Punctal occlusion for dry eye syndrome. Cochrane Database Syst. Rev. 2010, CD006775. [Google Scholar] [CrossRef]
- Rosenthal, P.; Cotter, J.M.; Baum, J. Treatment of persistent corneal epithelial defect with extended wear of a fluid-ventilated gas-permeable scleral contact lens. Am. J. Ophthalmol. 2000, 130, 33–41. [Google Scholar] [CrossRef]
- Meijer, J.M.; Meiners, P.M.; Vissink, A.; Spijkervet, F.K.L.; Abdulahad, W.; Kamminga, N.; Brouwer, E.; Kallenberg, C.G.M.; Bootsma, H. Effectiveness of rituximab treatment in primary Sjogren’s syndrome: A randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010, 62, 960–968. [Google Scholar] [CrossRef]
- Dass, S.; Bowman, S.J.; Vital, E.M.; Ikeda, K.; Pease, C.T.; Hamburger, J.; Richards, A.; Rauz, S.; Emery, P. Reduction of fatigue in Sjogren syndrome with rituximab: Results of a randomised, double-blind, placebo-controlled pilot study. Ann. Rheum. Dis. 2008, 67, 1541–1544. [Google Scholar] [CrossRef]
- Zandbelt, M.M.; de Wilde, P.; van Damme, P.; Hoyng, C.B.; van de Putte, L.; van den Hoogen, F. Etanercept in the treatment of patients with primary Sjogren’s syndrome: A pilot study. J. Rheumatol. 2004, 31, 96–101. [Google Scholar]
- Shiozawa, S.; Tanaka, Y.; Shiozawa, K. Single-blinded controlled trial of low-dose oral IFN-alpha for the treatment of xerostomia in patients with Sjogren’s syndrome. J. Interferon Cytokine Res. 1998, 18, 255–262. [Google Scholar] [CrossRef]
- Adler, S.; Korner, M.; Forger, F.; Huscher, D.; Caversaccio, M.D.; Villiger, P.M. Evaluation of histologic, serologic, and clinical changes in response to abatacept treatment of primary Sjogren’s syndrome: A pilot study. Arthritis Care Res. 2013, 65, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Geerling, G.; Maclennan, S.; Hartwig, D. Autologous serum eye drops for ocular surface disorders. Br. J. Ophthalmol. 2004, 88, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Harthan, J.S.; Shorter, E. Therapeutic uses of scleral contact lenses for ocular surface disease: Patient selection and special considerations. Clin. Optom. 2018, 10, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, J.; Sensebe, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016, 25, 829–848. [Google Scholar] [CrossRef]
- Garg, A.; Zhang, X. Lacrimal gland development: From signaling interactions to regenerative medicine. Dev. Dyn. 2017, 246, 970–980. [Google Scholar] [CrossRef]
- Miletich, I. Molecular regulation of ocular gland development. Semin. Cell Dev. Biol. 2019, 91, 66–74. [Google Scholar] [CrossRef]
- Khan, S.; Fitch, S.; Knox, S.; Arora, R. Exocrine gland structure-function relationships. Development 2022, 149, dev197657. [Google Scholar] [CrossRef]
- Clayton, J.A. Dry Eye. N. Engl. J. Med. 2018, 378, 2212–2223. [Google Scholar] [CrossRef]
- Alio, J.L.; Rodriguez, A.E.; Martinez, L.M.; Rio, A.L. Autologous fibrin membrane combined with solid platelet-rich plasma in the management of perforated corneal ulcers: A pilot study. JAMA Ophthalmol. 2013, 131, 745–751. [Google Scholar] [CrossRef]
- Freire, V.; Andollo, N.; Etxebarria, J.; Hernaez-Moya, R.; Duran, J.A.; Morales, M.C. Corneal wound healing promoted by 3 blood derivatives: An in vitro and in vivo comparative study. Cornea 2014, 33, 614–620. [Google Scholar] [CrossRef]
- Anitua, E.; Muruzabal, F.; Tayebba, A.; Riestra, A.; Perez, V.L.; Merayo-Lloves, J.; Orive, G. Autologous serum and plasma rich in growth factors in ophthalmology: Preclinical and clinical studies. Acta Ophthalmol. 2015, 93, e605-14. [Google Scholar] [CrossRef]
- Lopez-Plandolit, S.; Morales, M.C.; Freire, V.; Etxebarria, J.; Duran, J.A. Plasma rich in growth factors as a therapeutic agent for persistent corneal epithelial defects. Cornea 2010, 29, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Luo, Z.; Yao, X.; Lu, D.; Hong, T.; Zhu, X.; Chen, M.; Wang, X. Identification of Epigenetic Alteration of the IFI44L Gene in B Cells of Sjogren’s Syndrome as a Clinical Biomarker and Molecular Significance. J. Inflamm. Res. 2025, 18, 2499–2512. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.; Wang, W.; Liu, Q.; Ke, N.; Li, H.; Sun, F.; Zhang, J.; Zhu, Z. Autoimmune disease: A view of epigenetics and therapeutic targeting. Front. Immunol. 2024, 15, 1482728. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Chen, D.; Li, Q.; Sun, X.; Song, Y.; Wang, C. Curcumin inhibits inflammatory response and bone loss during experimental periodontitis in rats. Acta Odontol. Scand. 2013, 71, 349–356. [Google Scholar] [CrossRef]
- Ice, J.A.; Li, H.; Adrianto, I.; Lin, P.C.; Kelly, J.A.; Montgomery, C.G.; Lessard, C.J.; Moser, K.L. Genetics of Sjogren’s syndrome in the genome-wide association era. J. Autoimmun. 2012, 39, 57–63. [Google Scholar] [CrossRef]
- Konsta, O.D.; Thabet, Y.; Le Dantec, C.; Brooks, W.H.; Tzioufas, A.G.; Pers, J.-O.; Renaudineau, Y. The contribution of epigenetics in Sjogren’s Syndrome. Front. Genet. 2014, 5, 71. [Google Scholar] [CrossRef]
- Imgenberg-Kreuz, J.; Almlöf, J.C.; Leonard, D.; Alexsson, A.; Nordmark, G.; Eloranta, M.-L.; Rantapää-Dahlqvist, S.; Bengtsson, A.A.; Jönsen, A.; Padyukov, L.; et al. DNA methylation mapping identifies gene regulatory effects in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2018, 77, 736–743. [Google Scholar] [CrossRef]
- Charras, A.; Arvaniti, P.; Le Dantec, C.; Dalekos, G.N.; Zachou, K.; Bordron, A.; Renaudineau, Y. JAK Inhibitors and Oxidative Stress Control. Front. Immunol. 2019, 10, 2814. [Google Scholar] [CrossRef]
- Tsokos, G.C.; Lo, M.S.; Costa Reis, P.; Sullivan, K.E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2016, 12, 716–730. [Google Scholar] [CrossRef] [PubMed]
- Alunno, A.; Bistoni, O.; Bartoloni, E.; Caterbi, S.; Bigerna, B.; Tabarrini, A.; Mannucci, R.; Falini, B.; Gerli, R. IL-17-producing CD4-CD8- T cells are expanded in the peripheral blood, infiltrate salivary glands and are resistant to corticosteroids in patients with primary Sjogren’s syndrome. Ann. Rheum. Dis. 2013, 72, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Selvam, S.; Thomas, P.B.; Hamm-Alvarez, S.F.; Schechter, J.E.; Stevenson, D.; Mircheff, A.K.; Trousdale, M.D. Current status of gene delivery and gene therapy in lacrimal gland using viral vectors. Adv. Drug Deliv. Rev. 2006, 58, 1243–1257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cholkar, K.; Patel, S.P.; Vadlapudi, A.D.; Mitra, A.K. Novel strategies for anterior segment ocular drug delivery. J. Ocul. Pharmacol. Ther. 2013, 29, 106–123. [Google Scholar] [CrossRef]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef]
- Brito-Zeron, P.; Baldini, C.; Bootsma, H.; Bowman, S.J.; Jonsson, R.; Mariette, X.; Sivils, K.; Theander, E.; Tzioufas, A.; Ramos-Casals, M. Sjogren syndrome. Nat. Rev. Dis. Primers 2016, 2, 16047. [Google Scholar] [CrossRef]
- Imgenberg-Kreuz, J.; Sandling, J.K.; Nordmark, G. Epigenetic alterations in primary Sjogren’s syndrome—An overview. Clin. Immunol. 2018, 196, 12–20. [Google Scholar] [CrossRef]
- Li, P.; Han, M.; Zhao, X.; Ren, G.; Mei, S.; Zhong, C. Abnormal Epigenetic Regulations in the Immunocytes of Sjogren’s Syndrome Patients and Therapeutic Potentials. Cells 2022, 11, 1767. [Google Scholar] [CrossRef]
- Moreno-Montanes, J.; Bleau, A.M.; Jimenez, A.I. Tivanisiran, a novel siRNA for the treatment of dry eye disease. Expert Opin. Investig. Drugs 2018, 27, 421–426. [Google Scholar] [CrossRef]
- Xue, K.; MacLaren, R.E. Antisense oligonucleotide therapeutics in clinical trials for the treatment of inherited retinal diseases. Expert Opin. Investig. Drugs 2020, 29, 1163–1170. [Google Scholar] [CrossRef]
- Gupta, A.; Kafetzis, K.N.; Tagalakis, A.D.; Yu-Wai-Man, C. RNA therapeutics in ophthalmology—Translation to clinical trials. Exp. Eye Res. 2021, 205, 108482. [Google Scholar] [CrossRef]
- Ci, Z.; Wang, H.; Luo, J.; Wei, C.; Chen, J.; Wang, D.; Zhou, Y. Application of Nanomaterials Targeting Immune Cells in the Treatment of Chronic Inflammation. Int. J. Nanomed. 2024, 19, 13925–13946. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Mu, J.; Tang, Y.; Qin, D.; Duan, J.; Wu, A. Next-generation nanomaterials: Advancing ocular anti-inflammatory drug therapy. J. Nanobiotechnology 2023, 21, 282. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Jia, H.; Mo, Z.; Zheng, K.; Chen, S.; Ding, Y.; Zhang, Y.; Wen, Y.; Xie, Q.; Qiu, J.; et al. Cross-linked thermosensitive nanohydrogels for ocular drug delivery with a prolonged residence time and enhanced bioavailability. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111445. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.C.; Zeng, W.; Wong, C.Y.; Mifsud, E.J.; Williamson, N.A.; Ang, C.-S.; Vingrys, A.J.; Downie, L.E. Tear Interferon-Gamma as a Biomarker for Evaporative Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4824–4830. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, K.; Chen, H.; Sun, F.; Xu, J.; Wu, Z.; Li, P.; Zhang, L.; Du, Y.; Luan, H.; et al. A genome-wide association study in Han Chinese identifies a susceptibility locus for primary Sjogren’s syndrome at 7q11.23. Nat. Genet. 2013, 45, 1361–1365. [Google Scholar] [CrossRef]
- Gandolfo, S.; Bombardieri, M.; Pers, J.O.; Mariette, X.; Ciccia, F. Precision medicine in Sjogren’s disease. Lancet Rheumatol. 2024, 6, e636–e647. [Google Scholar] [CrossRef]
- Soret, P.; Le Dantec, C.; Desvaux, E.; Foulquier, N.; Chassagnol, B.; Hubert, S.; Jamin, C.; Barturen, G.; Desachy, G.; Devauchelle-Pensec, V.; et al. A new molecular classification to drive precision treatment strategies in primary Sjogren’s syndrome. Nat. Commun. 2021, 12, 3523. [Google Scholar] [CrossRef]
- Verstappen, G.M.; van Nimwegen, J.F.; Vissink, A.; Kroese, F.G.M.; Bootsma, H. The value of rituximab treatment in primary Sjogren’s syndrome. Clin. Immunol. 2017, 182, 62–71. [Google Scholar] [CrossRef]
- Kuo, M.-T.; Fang, P.-C.; Chao, T.-L.; Chen, A.; Lai, Y.-H.; Huang, Y.-T.; Tseng, C.-Y. Tear Proteomics Approach to Monitoring Sjogren Syndrome or Dry Eye Disease. Int. J. Mol. Sci. 2019, 20, 1932. [Google Scholar] [CrossRef]
- Urbanski, G.; Assad, S.; Chabrun, F.; de la Barca, J.M.C.; Blanchet, O.; Simard, G.; Lenaers, G.; Prunier-Mirebeau, D.; Gohier, P.; Lavigne, C.; et al. Tear metabolomics highlights new potential biomarkers for differentiating between Sjogren’s syndrome and other causes of dry eye. Ocul. Surf. 2021, 22, 110–116. [Google Scholar] [CrossRef]
- Retamozo, S.; Gheitasi, H.; Quartuccio, L.; Kostov, B.; Corazza, L.; Bové, A.; Sisó-Almirall, A.; Gandía, M.; Ramos-Casals, M.; De Vita, S.; et al. Cryoglobulinaemic vasculitis at diagnosis predicts mortality in primary Sjogren syndrome: Analysis of 515 patients. Rheumatology 2016, 55, 1443–1451. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Wang, X.; Jiang, X.; Jin, Y.; Han, Y.; Zhang, Z. Identification and verification of inflammatory biomarkers for primary Sjogren’s syndrome. Clin. Rheumatol. 2024, 43, 1335–1352. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Khamar, P.; Shirodkar, S.; Sethu, S.; Nair, A.P.; Ghosh, A. Patient stratification using point of care biomarkers in dry eye disease. Indian J. Ophthalmol. 2023, 71, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Hagan, S.; Martin, E.; Enriquez-de-Salamanca, A. Tear fluid biomarkers in ocular and systemic disease: Potential use for predictive, preventive and personalised medicine. EPMA J. 2016, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Mavragani, C.P.; Moutsopoulos, H.M. Sjogren’s syndrome: Old and new therapeutic targets. J. Autoimmun. 2020, 110, 102364. [Google Scholar] [CrossRef]
- Zochowska, D.; Wyzgal, J.; Paczek, L. Impact of CYP3A4*1B and CYP3A5*3 polymorphisms on the pharmacokinetics of cyclosporine and sirolimus in renal transplant recipients. Ann. Transplant. 2012, 17, 36–44. [Google Scholar]
- Li, R.-N.; Ou, T.-T.; Lin, C.-H.; Lin, Y.-Z.; Fang, T.-J.; Chen, Y.-J.; Tseng, C.-C.; Sung, W.-Y.; Wu, C.-C.; Yen, J.-H. NLRP3 Gene Polymorphisms in Rheumatoid Arthritis and Primary Sjogren’s Syndrome Patients. Diagnostics 2023, 13, 206. [Google Scholar] [CrossRef]
- Pecorelli, L.; Klein, K. Insights into Patient Heterogeneity in Sjogren’s Disease. Int. J. Mol. Sci. 2025, 26, 6367. [Google Scholar] [CrossRef]
- Nguyen, Y.; Nocturne, G.; Henry, J.; Ng, W.-F.; Belkhir, R.; Desmoulins, F.; Bergé, E.; Morel, J.; Perdriger, A.; Dernis, E.; et al. Identification of distinct subgroups of Sjogren’s disease by cluster analysis based on clinical and biological manifestations: Data from the cross-sectional Paris-Saclay and the prospective ASSESS cohorts. Lancet Rheumatol. 2024, 6, e216–e225. [Google Scholar] [CrossRef]
- Cummins, M.J.; Papas, A.; Kammer, G.M.; Fox, P.C. Treatment of primary Sjogren’s syndrome with low-dose human interferon alfa administered by the oromucosal route: Combined phase III results. Arthritis Rheum. 2003, 49, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Maitiyaer, M.; Tan, Q.; Huang, W.H.; Liu, Y.; Liu, Z.P.; Wen, Y.Q.; Zheng, Y.; Chen, X.; Chen, R.L.; et al. Emerging biologic frontiers for Sjogren’s syndrome: Unveiling novel approaches with emphasis on extra glandular pathology. Front. Pharmacol. 2024, 15, 1377055. [Google Scholar] [CrossRef] [PubMed]
- Anghel, S.A.; Dinu-Pirvu, C.-E.; Costache, M.-A.; Voiculescu, A.M.; Ghica, M.V.; Anuța, V.; Popa, L. Receptor Pharmacogenomics: Deciphering Genetic Influence on Drug Response. Int. J. Mol. Sci. 2024, 25, 9371. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Puche, A.B.; Salerno, L.C.; Versaci, F.; Romero, D.; Alio, J.L. Clinical evaluation of the repeatability of ocular aberrometry obtained with a new pyramid wavefront sensor. Eur. J. Ophthalmol. 2019, 29, 585–592. [Google Scholar] [CrossRef]
- Marvel, J.; Gargon, E.; Howse, C.; Chohan, A.; Mayhew, M.; Kenney, G.; Stone, L.; Fisher, B.A.; Steenackers, M.; Williamson, N.; et al. The Development and Content Validation of the Sjogren’s Related Quality of Life Instrument (SRQoL). Rheumatol. Ther. 2024, 11, 1591–1609. [Google Scholar] [CrossRef]
- Unger, J.; Mattsson, M.; Drăgoi, R.G.; Avram, C.; Boström, C.; Buttgereit, F.; Lackner, A.; Witte, T.; Raffeiner, B.; Peichl, P.; et al. The Experiences of Functioning and Health of Patients With Primary Sjogren’s Syndrome: A Multicenter Qualitative European Study. Front. Med. 2021, 8, 770422. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Ren, Y.; Xia, W.; Wu, J.; Yang, Z.; Jiang, Y.; Wen, Y.; Guo, Q.; Gu, J.; Yang, J.; Luo, J.; et al. Artificial intelligence-based prediction of organ involvement in Sjogren’s syndrome using labial gland biopsy whole-slide images. Clin. Rheumatol. 2025, 44, 2919–2927. [Google Scholar] [CrossRef]
| Test/Tool | Type | Target Parameter | Advantages | Limitations |
|---|---|---|---|---|
| Schirmer test | Functional | Tear volume | Simple, widely available | Poor specificity, variable reproducibility |
| TBUT | Functional | Tear film stability | Non-invasive, fast | Operator-dependent |
| Vital dye staining | Structural | Epithelial integrity | Detects surface damage | Variable correlation with symptoms |
| Tear osmolarity | Biomarker | Tear composition (osmolarity) | Quantifiable, point-of-care devices | Fluctuates; sensitive to environment |
| MMP-9 assay (InflammaDry) | Biomarker | Ocular surface inflammation | Fast, POC available | Binary output, not quantifiable |
| Meibography | Imaging | Meibomian gland morphology | Structural assessment | Limited availability |
| In vivo confocal microscopy | Imaging | Inflammatory cells, nerve density | High-resolution, detailed | Requires expertise, limited access |
| Anterior segment OCT | Imaging | Tear meniscus, conjunctiva | Objective, reproducible | Still not routine in many clinics |
| OSDI/DEQ | Symptom-based | Patient-reported symptom severity | Easy to administer | Subjective, no correlation with signs |
| Treatment Class | Examples | Mechanism of Action | Indication/Utility | Limitations/Notes |
|---|---|---|---|---|
| Artificial tears | Hyaluronic acid, CMC, lipid-based drops | Tear replacement, lubrication | First-line for all severities | Temporary relief, frequent use needed |
| Topical corticosteroids | Loteprednol, fluorometholone | Inhibit inflammation | Short-term flare control | Risk of IOP rise, cataract with prolonged use |
| Topical immunomodulators | Cyclosporine, lifitegrast | T-cell inhibition (calcineurin/LFA-1 pathways) | Chronic inflammation, maintenance therapy | Delayed onset; stinging on instillation |
| Oral secretagogues | Pilocarpine, cevimeline | Muscarinic receptor agonists | Residual gland function | Cholinergic side effects |
| Punctal occlusion | Silicone or collagen plugs | Reduces tear drainage | Moderate-to-severe aqueous deficiency | Epiphora, plug extrusion |
| Biologic therapies | Rituximab, abatacept, tocilizumab | Target B/T cells, cytokines (CD20, CD80/86, IL-6) | Refractory systemic and ocular disease | Off-label; systemic risks; cost |
| Regenerative therapy | Autologous serum, PRP, stem cells | Growth factors, epithelial healing | Severe epithelial damage, neurotrophic KCS | Access, standardization challenges |
| Procedural | IPL, scleral lenses | MGD treatment, tear reservoir creation | Severe or refractory cases | Requires expertise; cost |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soyfoo, M.; Motulsky, E.; Sarrand, J. Keratoconjunctivitis Sicca in Sjögren Disease: Diagnostic Challenges and Therapeutic Advances. Int. J. Mol. Sci. 2025, 26, 8824. https://doi.org/10.3390/ijms26188824
Soyfoo M, Motulsky E, Sarrand J. Keratoconjunctivitis Sicca in Sjögren Disease: Diagnostic Challenges and Therapeutic Advances. International Journal of Molecular Sciences. 2025; 26(18):8824. https://doi.org/10.3390/ijms26188824
Chicago/Turabian StyleSoyfoo, Muhammad, Elie Motulsky, and Julie Sarrand. 2025. "Keratoconjunctivitis Sicca in Sjögren Disease: Diagnostic Challenges and Therapeutic Advances" International Journal of Molecular Sciences 26, no. 18: 8824. https://doi.org/10.3390/ijms26188824
APA StyleSoyfoo, M., Motulsky, E., & Sarrand, J. (2025). Keratoconjunctivitis Sicca in Sjögren Disease: Diagnostic Challenges and Therapeutic Advances. International Journal of Molecular Sciences, 26(18), 8824. https://doi.org/10.3390/ijms26188824






