Targeting the Unfolded Protein Response with Natural Products: Therapeutic Potential in ER Stress-Related Diseases
Abstract
1. Introduction
1.1. The Onset of Endoplasmic Reticulum (ER) Stress
1.2. Rationale for Investigating Natural Products as Therapeutic Modulators
2. The Endoplasmic Reticulum and the Unfolded Protein Response (UPR)
2.1. ER Homeostasis and Stress Inducers
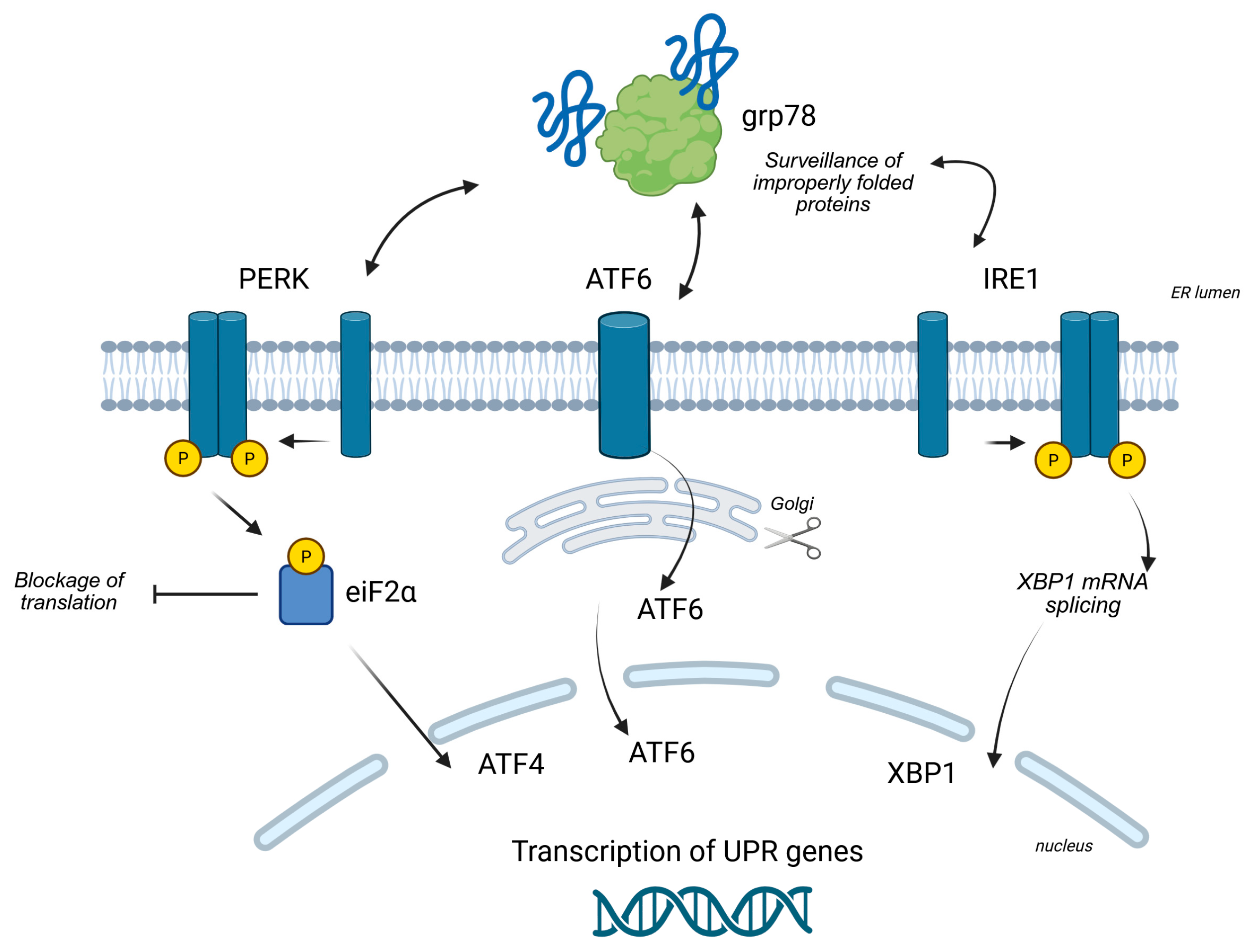
2.2. Key UPR Signaling Pathways
2.2.1. The PERK Pathway
2.2.2. The IRE1α Pathway
2.2.3. The ATF6 Pathway
2.3. The Dual Nature of the UPR
3. ER Stress in Disease Pathogenesis
3.1. Cancer
3.2. Neurodegenerative Diseases
3.3. Metabolic Disorders
3.4. Inflammatory and Autoimmune Diseases
3.5. Cardiovascular Diseases
3.6. Respiratory Diseases
3.7. Viral Infections
| Disease | Role of Endoplasmic Reticulum Stress |
|---|---|
| Cancer | Cancer cells hijack the UPR to promote survival, apoptosis resistance, and chemoresistance. The PERK-eIF2α-ATF4 and IRE1α pathways are often activated to adapt to the hostile tumor environment [27]. |
| Neurodegenerative Diseases | Chronic accumulation of misfolded proteins (e.g., Aβ, α-synuclein, mutant Huntingtin protein) overloads the ER, triggering stress. The activation of the pro-apoptotic CHOP arm contributes to progressive neuronal cell death [30]. |
| Metabolic Disorders (Type 2 Diabetes, Obesity) | In pancreatic β-cells, ER stress leads to dysfunction and apoptosis. In the liver and adipose tissue, it induces inflammation and insulin resistance by activating kinases like JNK [33]. |
| Inflammatory and Autoimmune Diseases | ER stress activates the NF-κB transcription factor, leading to the production of pro-inflammatory cytokines. UPR dysregulation in immune cells can contribute to the production of autoantibodies [37]. |
| Cardiovascular Diseases | Promotes atherosclerotic plaque formation and contributes to cardiomyocyte dysfunction and apoptosis during ischemia/reperfusion. It can also impair calcium handling and cardiac function [41]. |
| Respiratory Diseases | Cigarette smoke-induced ER stress activates the PERK-CHOP pathway [45]. |
| Viral Infections | Viruses exploit the ER for their protein synthesis, inducing ER stress. They manipulate UPR signaling pathways to enhance their replication and evade the host’s immune response [47]. |
4. Natural Products Modulating ER Stress
4.1. Mechanisms of Action on ER Stress
4.2. Key Examples of Natural Products and Their Effects
4.2.1. Flavonoids and Polyphenols
4.2.2. Alkaloids and Saponins
4.2.3. Terpenoids and Other Compounds
| Natural Compound | Primary Mechanisms of Action | |
|---|---|---|
| Curcumin |  | Inhibits the pro-survival PERK-eIF2α-ATF4 pathway, reduces oxidative stress and inflammation by blocking NF-κB [52]. |
| Resveratrol |  | Activates the SIRT1 and AMPK pathways to improve protein folding and energy homeostasis, acts as an antioxidant [58]. |
| EGCG |  | Acts as a chemical chaperone, inhibits the PERK-eIF2α pathway and promotes IRE1α/XBP1s, has strong antioxidant and anti-inflammatory properties [63]. |
| Quercetin | 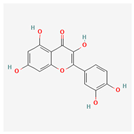 | Potent antioxidant, suppresses the pro-apoptotic CHOP pathway, and modulates inflammatory signaling by inhibiting NF-κB [66]. |
| Oleuropein |  | Powerful antioxidant that reduces ER stress and protects cells from free radicals [68]. |
| Baicalein | 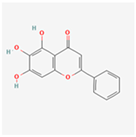 | Alleviates ER stress and modulates UPR based on context (pro-survival vs. pro-apoptotic) [73]. |
| Berberine |  | Activates the AMPK pathway, which reduces metabolic ER stress and improves glucose and lipid metabolism [74,76]. |
| Ginsenosides | 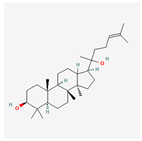 | Protects cells from ER stress-induced apoptosis by inhibiting the PERK-eIF2α-CHOP pathway [81]. |
| Piperine |  | Exerts anti-inflammatory and antioxidant effects, modulates ER stress pathways, and inhibits NF-κB [80]. |
| Astragaloside IV | 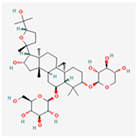 | Inhibits pro-apoptotic pathways, enhances pro-survival GRP78/BiP expression [94]. |
| Honokiol |  | Inhibits the IRE1α branch of the UPR, suppressing pro-survival signals in cancer cells [87]. |
| Ursolic Acid |  | Alleviates ER stress by down-regulating pro-apoptotic UPR proteins and promoting the expression of protective genes [89]. |
| Camphene |  | Induces ER stress, modulates UPR [92]. |
| Sulforaphane |  | Activates the Nrf2 pathway, a master regulator of antioxidant and detoxifying genes, thereby indirectly alleviating ER stress [72]. |
5. Challenges and Future Directions
5.1. Challenges in Research and Development
5.2. Future Perspectives
| Aspect | Challenges | Future Solutions |
|---|---|---|
| Natural Compounds | Low bioavailability, variability, complex mechanisms of action. | Semi-synthetic derivatives, advanced delivery systems (nanotechnology). |
| Research | Poor reproducibility, difficulty isolating specific mechanisms. | High-throughput screening, SAR (structure–activity relationship) studies. |
| Therapeutic Development | Lack of robust clinical trials and clear regulatory pathways. | Combination therapies, rigorous and standardized clinical validation. |
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hetz, C.; Papa, F.R. The Unfolded Protein Response and Cell Fate Control. Mol. Cell 2018, 69, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Martinon, F.; Rodriguez, D.; Glimcher, L.H. The unfolded protein response: Integrating stress signals through the stress sensor IRE1α. Physiol. Rev. 2011, 91, 1219–1243. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ackerman, S.L. Endoplasmic reticulum stress in health and disease. Curr. Opin. Cell Biol. 2006, 18, 444–452. [Google Scholar] [CrossRef]
- Carreras-Sureda, A.; Pihán, P.; Hetz, C. Calcium signaling at the endoplasmic reticulum: Fine-tuning stress responses. Cell Calcium 2018, 70, 24–31. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef]
- Makio, T.; Chen, J.; Simmen, T. ER stress as a sentinel mechanism for ER Ca. Cell Calcium 2024, 124, 102961. [Google Scholar] [CrossRef]
- Pontisso, I.; Ornelas-Guevara, R.; Combettes, L.; Dupont, G. A journey in UPR modelling. Biol. Cell 2023, 115, 2200111. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Lindholm, D.; Ren, J.; Pratico, D. ER stress and UPR in Alzheimer’s disease: Mechanisms, pathogenesis, treatments. Cell Death Dis. 2022, 13, 706. [Google Scholar] [CrossRef] [PubMed]
- Mercado, G.; Castillo, V.; Soto, P.; Sidhu, A. ER stress and Parkinson’s disease: Pathological inputs that converge into the secretory pathway. Brain Res. 2016, 1648, 626–632. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J. Endoplasmic Reticulum (ER) Stress and Its Role in Pancreatic β-Cell Dysfunction and Senescence in Type 2 Diabetes. Int. J. Mol. Sci. 2022, 23, 4843. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, M.; Chen, W.; Zhao, T.; Wei, Y. Cancer and ER stress: Mutual crosstalk between autophagy, oxidative stress and inflammatory response. Biomed. Pharmacother. 2019, 118, 109249. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, G.; Martinotti, S.; Ranzato, E. Endoplasmic Reticulum Stress and Cancer: Could Unfolded Protein Response Be a Druggable Target for Cancer Therapy? Int. J. Mol. Sci. 2023, 24, 1566. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; He, M.; Xiong, S.; Xia, X. Endoplasmic reticulum stress: Molecular mechanism and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 352. [Google Scholar] [CrossRef]
- Kim, C.; Kim, B. Anti-Cancer Natural Products and Their Bioactive Compounds Inducing ER Stress-Mediated Apoptosis: A Review. Nutrients 2018, 10, 1021. [Google Scholar] [CrossRef]
- Almanza, A.; Carlesso, A.; Chintha, C.; Creedican, S.; Doultsinos, D.; Leuzzi, B.; Luís, A.; McCarthy, N.; Montibeller, L.; More, S.; et al. Endoplasmic reticulum stress signalling—From basic mechanisms to clinical applications. FEBS J. 2019, 286, 241–278. [Google Scholar] [CrossRef]
- Mei, Y.; Thompson, M.D.; Cohen, R.A.; Tong, X. Endoplasmic Reticulum Stress and Related Pathological Processes. J. Pharmacol. Biomed. Anal. 2013, 1, 1000107. [Google Scholar]
- Groenendyk, J.; Agellon, L.B.; Michalak, M. Calcium signaling and endoplasmic reticulum stress. Int. Rev. Cell Mol. Biol. 2021, 363, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Umashankar, B.; Eliasson, L.; Ooi, C.Y.; Kim, K.W.; Shaw, J.A.M.; Waters, S.A. Beyond insulin: Unraveling the complex interplay of ER stress, oxidative damage, and CFTR modulation in CFRD. J. Cyst. Fibros. 2024, 23, 842–852. [Google Scholar] [CrossRef]
- Li, S.; Kong, L.; Yu, X. The expanding roles of endoplasmic reticulum stress in virus replication and pathogenesis. Crit. Rev. Microbiol. 2015, 41, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Read, A.; Schröder, M. The Unfolded Protein Response: An Overview. Biology 2021, 10, 384. [Google Scholar] [CrossRef]
- Hollien, J.; Lin, J.H.; Li, H.; Stevens, N.; Walter, P.; Weissman, J.S. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J. Cell Biol. 2009, 186, 323–331. [Google Scholar] [CrossRef]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- Ong, G.; Ragetli, R.; Mnich, K.; Doble, B.W.; Kammouni, W.; Logue, S.E. IRE1 signaling increases PERK expression during chronic ER stress. Cell Death Dis. 2024, 15, 276. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.; Luzuriaga, J.; Maxwell, E.L.; West, P.K.; Bensellam, M.; Laybutt, D.R. The balance between adaptive and apoptotic unfolded protein responses regulates β-cell death under ER stress conditions through XBP1, CHOP and JNK. Mol. Cell Endocrinol. 2015, 413, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, K.R.; Riaz, T.A.; Kim, H.R.; Chae, H.J. The aftermath of the interplay between the endoplasmic reticulum stress response and redox signaling. Exp. Mol. Med. 2021, 53, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Bhattacharya, A.; Qi, L. Endoplasmic reticulum quality control in cancer: Friend or foe. Semin. Cancer Biol. 2015, 33, 25–33. [Google Scholar] [CrossRef]
- Fels, D.R.; Koumenis, C. The PERK/eIF2alpha/ATF4 module of the UPR in hypoxia resistance and tumor growth. Cancer Biol. Ther. 2006, 5, 723–728. [Google Scholar] [CrossRef]
- Fusakio, M.E.; Willy, J.A.; Wang, Y.; Mirek, E.T.; Al Baghdadi, R.J.; Adams, C.M.; Anthony, T.G.; Wek, R.C. Transcription factor ATF4 directs basal and stress-induced gene expression in the unfolded protein response and cholesterol metabolism in the liver. Mol. Biol. Cell 2016, 27, 1536–1551. [Google Scholar] [CrossRef]
- Tam, A.B.; Mercado, E.L.; Hoffmann, A.; Niwa, M. ER stress activates NF-κB by integrating functions of basal IKK activity, IRE1 and PERK. PLoS ONE 2012, 7, e45078. [Google Scholar] [CrossRef]
- Sweeney, P.; Park, H.; Baumann, M.; Dunlop, J.; Frydman, J.; Kopito, R.; McCampbell, A.; Leblanc, G.; Venkateswaran, A.; Nurmi, A.; et al. Protein misfolding in neurodegenerative diseases: Implications and strategies. Transl. Neurodegener. 2017, 6, 6. [Google Scholar] [CrossRef]
- Costa, C.A.D.; Manaa, W.E.; Duplan, E.; Checler, F. The Endoplasmic Reticulum Stress/Unfolded Protein Response and Their Contributions to Parkinson’s Disease Physiopathology. Cells 2020, 9, 2495. [Google Scholar] [CrossRef]
- Maity, S.; Komal, P.; Kumar, V.; Saxena, A.; Tungekar, A.; Chandrasekar, V. Impact of ER Stress and ER-Mitochondrial Crosstalk in Huntington’s Disease. Int. J. Mol. Sci. 2022, 23, 780. [Google Scholar] [CrossRef]
- Ghemrawi, R.; Battaglia-Hsu, S.F.; Arnold, C. Endoplasmic Reticulum Stress in Metabolic Disorders. Cells 2018, 7, 63. [Google Scholar] [CrossRef]
- Alotaibi, G.; Alkhammash, A. Pharmacological landscape of endoplasmic reticulum stress: Uncovering therapeutic avenues for metabolic diseases. Eur. J. Pharmacol. 2025, 998, 177509. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Z.; Ren, F.; Deng, H.; Wen, J.; Xiang, Q.; Zhou, Z.; Yang, X.; Rao, C. Endoplasmic reticulum stress and unfolded protein response in renal lipid metabolism. Exp. Cell Res. 2025, 446, 114463. [Google Scholar] [CrossRef] [PubMed]
- Ajoolabady, A.; Liu, S.; Klionsky, D.J.; Lip, G.Y.H.; Tuomilehto, J.; Kavalakatt, S.; Pereira, D.M.; Samali, A.; Ren, J. ER stress in obesity pathogenesis and management. Trends Pharmacol. Sci. 2022, 43, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, S.Z.; Lourie, R.; Das, I.; Chen, A.C.; McGuckin, M.A. The interplay between endoplasmic reticulum stress and inflammation. Immunol. Cell Biol. 2012, 90, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Eugene, S.P.; Reddy, V.S.; Trinath, J. Endoplasmic Reticulum Stress and Intestinal Inflammation: A Perilous Union. Front. Immunol. 2020, 11, 543022. [Google Scholar] [CrossRef]
- Aragon, I.V.; Barrington, R.A.; Jackowski, S.; Mori, K.; Brewer, J.W. The specialized unfolded protein response of B lymphocytes: ATF6α-independent development of antibody-secreting B cells. Mol. Immunol. 2012, 51, 347–355. [Google Scholar] [CrossRef]
- Zhu, H.; Jiang, C.; Kaufman, R.J.; Li, H.; Singh, N. In Vitro Stimulation of IRE1α/XBP1-Deficient B Cells with LPS. In Methods in Molecular Biology; Humana: New York, NY, USA, 2022; Volume 2378, pp. 221–231. [Google Scholar] [CrossRef]
- Hong, J.; Kim, K.; Kim, J.H.; Park, Y. The Role of Endoplasmic Reticulum Stress in Cardiovascular Disease and Exercise. Int. J. Vasc. Med. 2017, 2017, 2049217. [Google Scholar] [CrossRef]
- Yang, S.; Wu, M.; Li, X.; Zhao, R.; Zhao, Y.; Liu, L.; Wang, S. Role of Endoplasmic Reticulum Stress in Atherosclerosis and Its Potential as a Therapeutic Target. Oxid. Med. Cell Longev. 2020, 2020, 9270107. [Google Scholar] [CrossRef] [PubMed]
- Mainali, N.; Li, X.; Wang, X.; Balasubramaniam, M.; Ganne, A.; Kore, R.; Shmookler Reis, R.J.; Mehta, J.L.; Ayyadevara, S. Myocardial infarction elevates endoplasmic reticulum stress and protein aggregation in heart as well as brain. Mol. Cell Biochem. 2024, 479, 2741–2753. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.; Terentyev, D. ER stress and calcium-dependent arrhythmias. Front. Physiol. 2022, 13, 1041940. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Liu, K.; Li, X.; Zeng, X.L.; Liu, X.J. Melatonin ameliorates PM2.5-induced airway inflammation and apoptosis by PERK/eIF2α/ATF4/CHOP in chronic obstructive pulmonary disease mice. Toxicol. Appl. Pharmacol. 2025, 499, 117314. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, J.; Andreu-Martínez, R.; Pérez-Sánchez, L.; Hernández-García, A.; Muñoz-Calleja, C.; Cogolludo, Á.; Calzada, M.J. CSE-Induced ER-Mitochondria Crosstalk Promotes Oxidative Stress and Impairs Bronchial Contractile Response. Antioxidants 2025, 14, 703. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, A. Virus-induced ER stress and the unfolded protein response. Front. Plant Sci. 2012, 3, 293. [Google Scholar] [CrossRef]
- Asha, K.; Sharma-Walia, N. Virus and tumor microenvironment induced ER stress and unfolded protein response: From complexity to therapeutics. Oncotarget 2018, 9, 31920–31936. [Google Scholar] [CrossRef]
- Ranzato, E.; Bonsignore, G.; Martinotti, S. ER Stress Response and Induction of Apoptosis in Malignant Pleural Mesothelioma: The Achilles Heel Targeted by the Anticancer Ruthenium Drug BOLD-100. Cancers 2022, 14, 4126. [Google Scholar] [CrossRef]
- Calfon, M.; Zeng, H.; Urano, F.; Till, J.H.; Hubbard, S.R.; Harding, H.P.; Clark, S.G.; Ron, D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 2002, 415, 92–96. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Feng, K.; Ge, Y.; Chen, Z.; Li, X.; Liu, Z.; Li, H.; Tang, T.; Yang, F.; Wang, X. Curcumin Inhibits the PERK-eIF2. Oxid. Med. Cell Longev. 2019, 2019, 8574386. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Neuroprotective Effects of Curcumin in Neurodegenerative Diseases. Foods 2024, 13, 1774. [Google Scholar] [CrossRef]
- Buhrmann, C.; Mobasheri, A.; Busch, F.; Aldinger, C.; Stahlmann, R.; Montaseri, A.; Shakibaei, M. Curcumin modulates nuclear factor kappaB (NF-kappaB)-mediated inflammation in human tenocytes in vitro: Role of the phosphatidylinositol 3-kinase/Akt pathway. J. Biol. Chem. 2011, 286, 28556–28566. [Google Scholar] [CrossRef]
- Panknin, T.M.; Howe, C.L.; Hauer, M.; Bucchireddigari, B.; Rossi, A.M.; Funk, J.L. Curcumin Supplementation and Human Disease: A Scoping Review of Clinical Trials. Int. J. Mol. Sci. 2023, 24, 4476. [Google Scholar] [CrossRef]
- Martinotti, S.; Bonsignore, G.; Patrone, M.; Ranzato, E. Mediterranean Diet Polyphenols: Anthocyanins and their Implications for Health. Mini Rev. Med. Chem. 2020, 21, 1692–1700. [Google Scholar] [CrossRef]
- Lee, J.; Hong, S.W.; Kwon, H.; Park, S.E.; Rhee, E.J.; Park, C.Y.; Oh, K.W.; Park, S.W.; Lee, W.Y. Resveratrol, an activator of SIRT1, improves ER stress by increasing clusterin expression in HepG2 cells. Cell Stress. Chaperones 2019, 24, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, Y.; Shu, L.; Song, G.; Ma, H. Resveratrol reduces liver endoplasmic reticulum stress and improves insulin sensitivity in vivo and in vitro. Drug Des. Devel Ther. 2019, 13, 1473–1485. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Ramírez, N.; Alquisiras-Burgos, I.; Ortiz-Plata, A.; Ruiz-Tachiquín, M.E.; Espinoza-Rojo, M.; Aguilera, P. Resveratrol Activates Neuronal Autophagy Through AMPK in the Ischemic Brain. Mol. Neurobiol. 2020, 57, 1055–1069. [Google Scholar] [CrossRef]
- Brown, K.; Theofanous, D.; Britton, R.G.; Aburido, G.; Pepper, C.; Sri Undru, S.; Howells, L. Resveratrol for the Management of Human Health: How Far Have We Come? A Systematic Review of Resveratrol Clinical Trials to Highlight Gaps and Opportunities. Int. J. Mol. Sci. 2024, 25, 747. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, B.I.; Ruiz-Ramos, M.; Pedraza-Chaverri, J.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Effect of Resveratrol on Markers of Oxidative Stress and Sirtuin 1 in Elderly Adults with Type 2 Diabetes. Int. J. Mol. Sci. 2023, 24, 7422. [Google Scholar] [CrossRef] [PubMed]
- Chakrawarti, L.; Agrawal, R.; Dang, S.; Gupta, S.; Gabrani, R. Therapeutic effects of EGCG: A patent review. Expert. Opin. Ther. Pat. 2016, 26, 907–916. [Google Scholar] [CrossRef]
- Md Nesran, Z.N.; Shafie, N.H.; Ishak, A.H.; Mohd Esa, N.; Ismail, A.; Md Tohid, S.F. Induction of Endoplasmic Reticulum Stress Pathway by Green Tea Epigallocatechin-3-Gallate (EGCG) in Colorectal Cancer Cells: Activation of PERK/p-eIF2. Biomed. Res. Int. 2019, 2019, 3480569. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Ranzato, E.; Burlando, B. (-)- Epigallocatechin-3-gallate induces GRP78 accumulation in the ER and shifts mesothelioma constitutive UPR into proapoptotic ER stress. J. Cell Physiol. 2018, 233, 7082–7090. [Google Scholar] [CrossRef]
- Mereles, D.; Hunstein, W. Epigallocatechin-3-gallate (EGCG) for clinical trials: More pitfalls than promises? Int. J. Mol. Sci. 2011, 12, 5592–5603. [Google Scholar] [CrossRef]
- Topçu-Tarladaçalışır, Y.; Sapmaz-Metin, M.; Mercan, Z.; Erçetin, D. Quercetin Attenuates Endoplasmic Reticulum Stress and Apoptosis in TNBS-Induced Colitis by Inhibiting the Glucose Regulatory Protein 78 Activation. Balkan Med. J. 2024, 41, 30–37. [Google Scholar] [CrossRef]
- Mirza, M.A.; Mahmood, S.; Hilles, A.R.; Ali, A.; Khan, M.Z.; Zaidi, S.A.A.; Iqbal, Z.; Ge, Y. Quercetin as a Therapeutic Product: Evaluation of Its Pharmacological Action and Clinical Applications-A Review. Pharmaceuticals 2023, 16, 1631. [Google Scholar] [CrossRef]
- Elmazoglu, Z.; Ergin, V.; Sahin, E.; Kayhan, H.; Karasu, C. Oleuropein and rutin protect against 6-OHDA-induced neurotoxicity in PC12 cells through modulation of mitochondrial function and unfolded protein response. Interdiscip. Toxicol. 2017, 10, 129–141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Butt, M.S.; Tariq, U.; Iahtisham-Ul-Haq; Naz, A.; Rizwan, M. Neuroprotective effects of oleuropein: Recent developments and contemporary research. J. Food Biochem. 2021, 45, e13967. [Google Scholar] [CrossRef]
- Omar, S.H. Oleuropein in olive and its pharmacological effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Kostov, R.V.; Kensler, T.W. KEAP1 and Done? Targeting the NRF2 Pathway with Sulforaphane. Trends Food Sci. Technol. 2017, 69, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Kang, N.I.; Lee, H.K.; Jang, K.Y.; Park, J.W.; Park, B.H. Sulforaphane protects kidneys against ischemia-reperfusion injury through induction of the Nrf2-dependent phase 2 enzyme. Biochem. Pharmacol. 2008, 75, 2214–2223. [Google Scholar] [CrossRef] [PubMed]
- Üstüner, M.C.; Tanrikut, C.; Üstüner, D.; Kolaç, U.K.; Köroğlu, Z.; Burukoğlu, D.; Entok, E. The effect of baicalein on endoplasmic reticulum stress and autophagy on liver damage. Hum. Exp. Toxicol. 2021, 40, 1624–1633. [Google Scholar] [CrossRef]
- Wang, Z.S.; Lu, F.E.; Xu, L.J.; Dong, H. Berberine reduces endoplasmic reticulum stress and improves insulin signal transduction in Hep G2 cells. Acta Pharmacol. Sin. 2010, 31, 578–584. [Google Scholar] [CrossRef]
- Khater, S.I.; Almanaa, T.N.; Fattah, D.M.A.; Khamis, T.; Seif, M.M.; Dahran, N.; Alqahtani, L.S.; Metwally, M.M.M.; Mostafa, M.; Albedair, R.A.; et al. Liposome-Encapsulated Berberine Alleviates Liver Injury in Type 2 Diabetes via Promoting AMPK/mTOR-Mediated Autophagy and Reducing ER Stress: Morphometric and Immunohistochemical Scoring. Antioxidants 2023, 12, 1220. [Google Scholar] [CrossRef]
- Guo, H.H.; Shen, H.R.; Wang, L.L.; Luo, Z.G.; Zhang, J.L.; Zhang, H.J.; Gao, T.L.; Han, Y.X.; Jiang, J.D. Berberine is a potential alternative for metformin with good regulatory effect on lipids in treating metabolic diseases. Biomed. Pharmacother. 2023, 163, 114754. [Google Scholar] [CrossRef] [PubMed]
- Hammad, A.S.; Ravindran, S.; Khalil, A.; Munusamy, S. Structure-activity relationship of piperine and its synthetic amide analogs for therapeutic potential to prevent experimentally induced ER stress in vitro. Cell Stress. Chaperones 2017, 22, 417–428. [Google Scholar] [CrossRef]
- Duan, Z.; Xie, H.; Yu, S.; Wang, S.; Yang, H. Piperine Derived from. Foods 2022, 11, 2990. [Google Scholar] [CrossRef] [PubMed]
- Pratti, V.L.; Thomas, M.; Bhoite, R.; Satyavrat, V. Investigating Bioavailability of Curcumin and Piperine Combination in Comparison to Turmeric Rhizomes: An in vitro Study. J. Exp. Pharmacol. 2024, 16, 37–47. [Google Scholar] [CrossRef]
- Baito, Q.N.; Jaafar, H.M.; Mohammad, T.A.M. Piperine suppresses inflammatory fibroblast-like synoviocytes derived from rheumatoid arthritis patients Via NF-κB inhibition. Cell Immunol. 2023, 391–392, 104752. [Google Scholar] [CrossRef]
- He, B.; Chen, D.; Zhang, X.; Yang, R.; Yang, Y.; Chen, P.; Shen, Z. Oxidative Stress and Ginsenosides: An Update on the Molecular Mechanisms. Oxid. Med. Cell Longev. 2022, 2022, 9299574. [Google Scholar] [CrossRef]
- Chunarkar-Patil, P.; Kaleem, M.; Mishra, R.; Ray, S.; Ahmad, A.; Verma, D.; Bhayye, S.; Dubey, R.; Singh, H.N.; Kumar, S. Anticancer Drug Discovery Based on Natural Products: From Computational Approaches to Clinical Studies. Biomedicines 2024, 12, 201. [Google Scholar] [CrossRef]
- Ancajas, C.M.F.; Oyedele, A.S.; Butt, C.M.; Walker, A.S. Advances, opportunities, and challenges in methods for interrogating the structure activity relationships of natural products. Nat. Prod. Rep. 2024, 41, 1543–1578. [Google Scholar] [CrossRef]
- Lv, Y.; Li, W.; Liao, W.; Jiang, H.; Liu, Y.; Cao, J.; Lu, W.; Feng, Y. Nano-Drug Delivery Systems Based on Natural Products. Int. J. Nanomedicine 2024, 19, 541–569. [Google Scholar] [CrossRef]
- Sauter, E.R. Cancer prevention and treatment using combination therapy with natural compounds. Expert. Rev. Clin. Pharmacol. 2020, 13, 265–285. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.P.; Lee, W.L.; Tang, Y.Q.; Yap, W.H. Honokiol: A Review of Its Anticancer Potential and Mechanisms. Cancers 2019, 12, 48. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wu, C.L.; Liu, J.F.; Fong, Y.C.; Hsu, S.F.; Li, T.M.; Su, Y.C.; Liu, S.H.; Tang, C.H. Honokiol induces cell apoptosis in human chondrosarcoma cells through mitochondrial dysfunction and endoplasmic reticulum stress. Cancer Lett. 2010, 291, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: An Update. Int. J. Mol. Sci. 2020, 21, 5920. [Google Scholar] [CrossRef]
- Zheng, Q.Y.; Li, P.P.; Jin, F.S.; Yao, C.; Zhang, G.H.; Zang, T.; Ai, X. Ursolic acid induces ER stress response to activate ASK1-JNK signaling and induce apoptosis in human bladder cancer T24 cells. Cell Signal 2013, 25, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X.; Guo, J.; Zhang, Y.; Fan, J.; Chen, J.; Jiang, J.; Yu, B.; Zhang, K.; Zhou, B. Ursolic acid ameliorates cerebral ischemia-reperfusion injury by inhibiting NF-κB/NLRP3-mediated microglia pyroptosis and neuroinflammation. Front. Pharmacol. 2025, 16, 1622131. [Google Scholar] [CrossRef]
- Stamatiou, R.; Anagnostopoulou, M.; Ioannidou-Kabouri, K.; Rapti, C.; Lazou, A. Camphene as a Protective Agent in Myocardial Ischemia/Reperfusion Injury. Antioxidants 2024, 13, 405. [Google Scholar] [CrossRef]
- Martucciello, S.; Masullo, M.; Cerulli, A.; Piacente, S. Natural Products Targeting ER Stress, and the Functional Link to Mitochondria. Int. J. Mol. Sci. 2020, 21, 1905. [Google Scholar] [CrossRef] [PubMed]
- Dana, A.H.; Alejandro, S.P. Role of sulforaphane in endoplasmic reticulum homeostasis through regulation of the antioxidant response. Life Sci. 2022, 299, 120554. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.T.; Wang, Y.; Peng, F. Astragaloside IV sensitizes non-small cell lung cancer cells to cisplatin by suppressing endoplasmic reticulum stress and autophagy. J. Thorac. Dis. 2020, 12, 3715–3724. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Song, L.; Hua, S. Perspectives and controversies regarding the use of natural products for the treatment of lung cancer. Cancer Med. 2021, 10, 2396–2422. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinotti, S.; Ranzato, E. Targeting the Unfolded Protein Response with Natural Products: Therapeutic Potential in ER Stress-Related Diseases. Int. J. Mol. Sci. 2025, 26, 8814. https://doi.org/10.3390/ijms26188814
Martinotti S, Ranzato E. Targeting the Unfolded Protein Response with Natural Products: Therapeutic Potential in ER Stress-Related Diseases. International Journal of Molecular Sciences. 2025; 26(18):8814. https://doi.org/10.3390/ijms26188814
Chicago/Turabian StyleMartinotti, Simona, and Elia Ranzato. 2025. "Targeting the Unfolded Protein Response with Natural Products: Therapeutic Potential in ER Stress-Related Diseases" International Journal of Molecular Sciences 26, no. 18: 8814. https://doi.org/10.3390/ijms26188814
APA StyleMartinotti, S., & Ranzato, E. (2025). Targeting the Unfolded Protein Response with Natural Products: Therapeutic Potential in ER Stress-Related Diseases. International Journal of Molecular Sciences, 26(18), 8814. https://doi.org/10.3390/ijms26188814







