The Remarkable Role of Triosephosphate Isomerase in Diabetes Pathophysiology
Abstract
1. Introduction
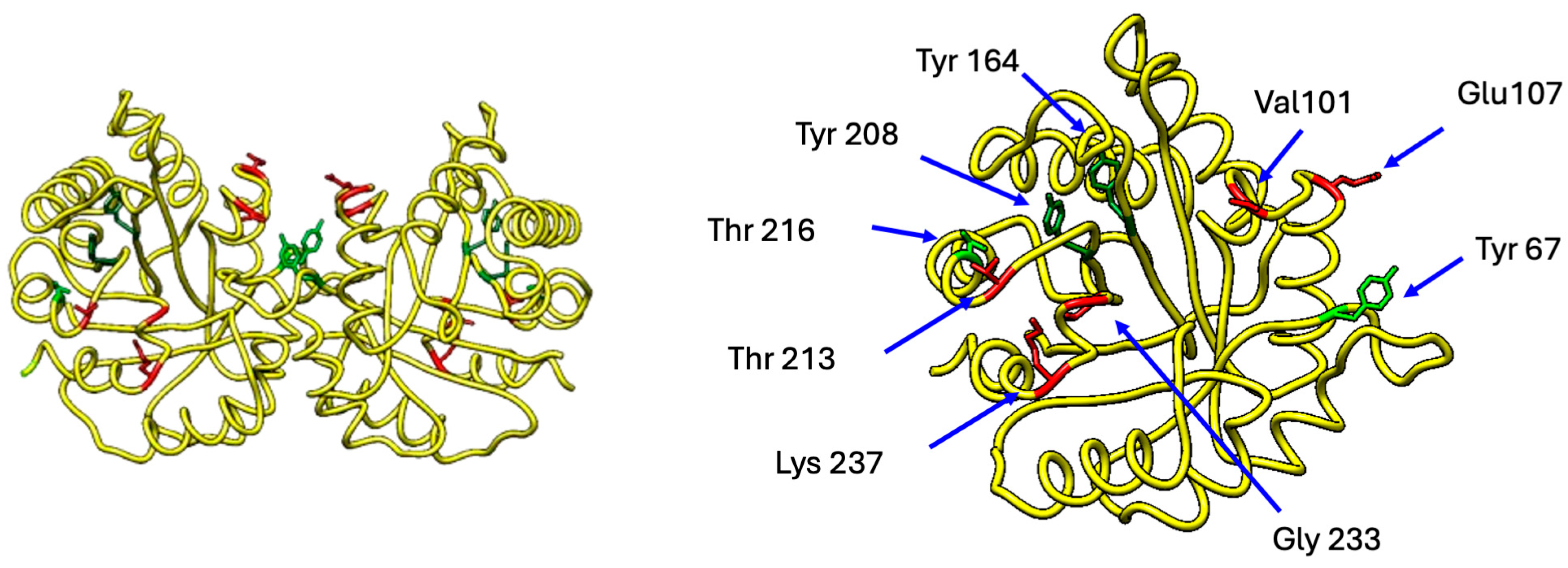
2. Triosephosphate Isomerase
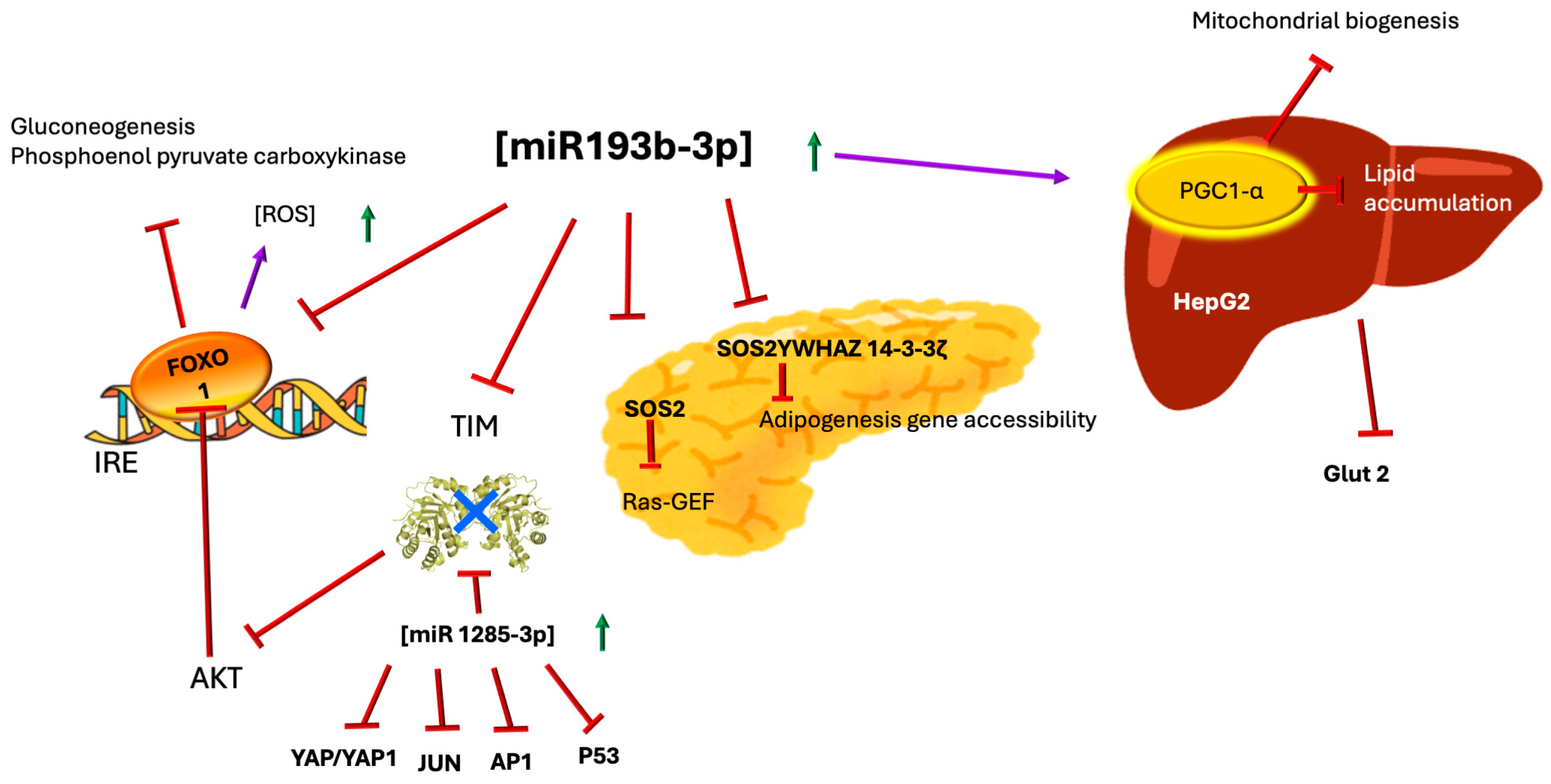
3. MicroRNAs—miR-193b-3p, miR-1285-3p, and TIM
4. Regulation of Insulin Secretion

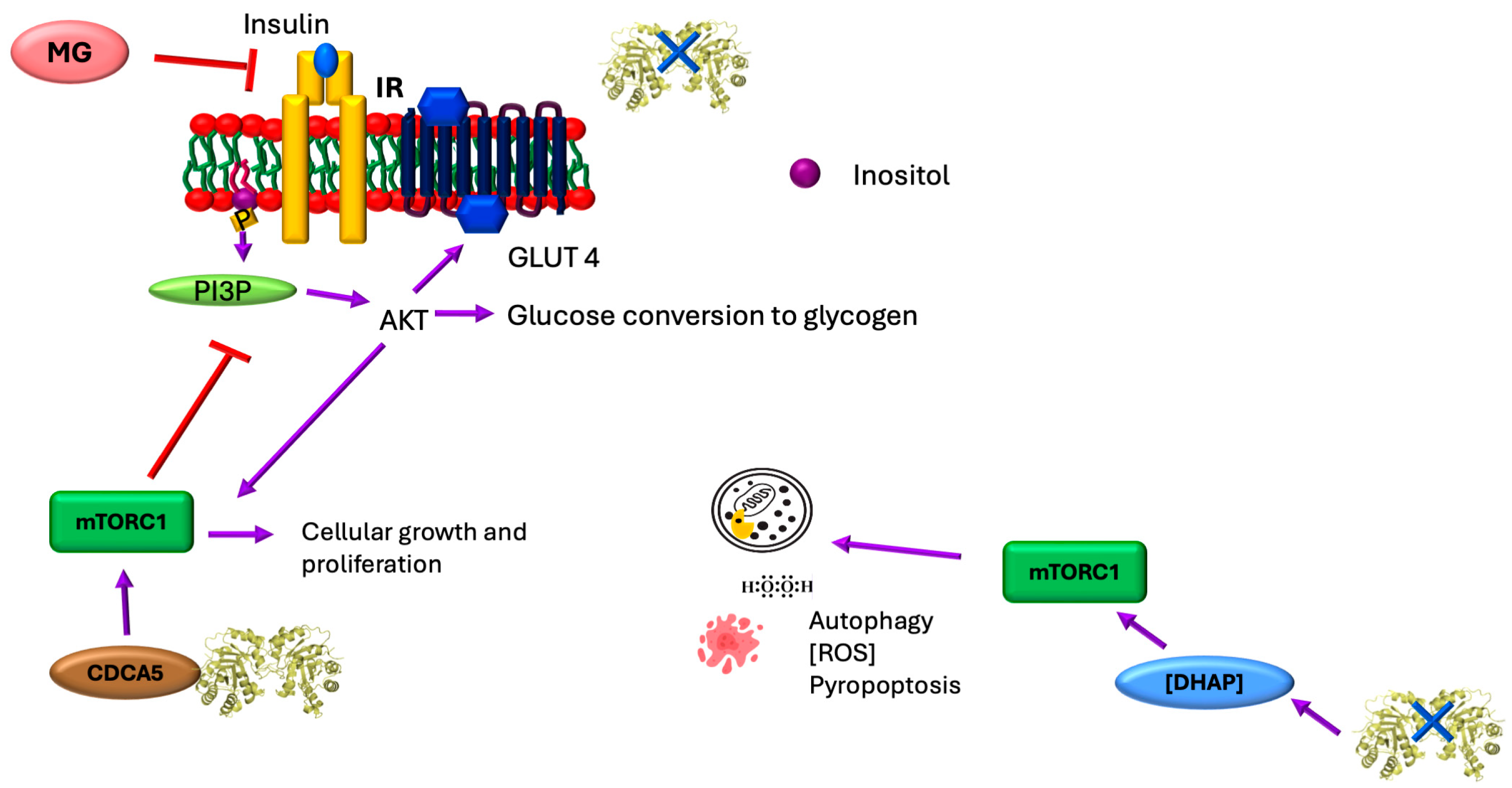
5. TIM, Sororin, and the mTORC1 Pathway
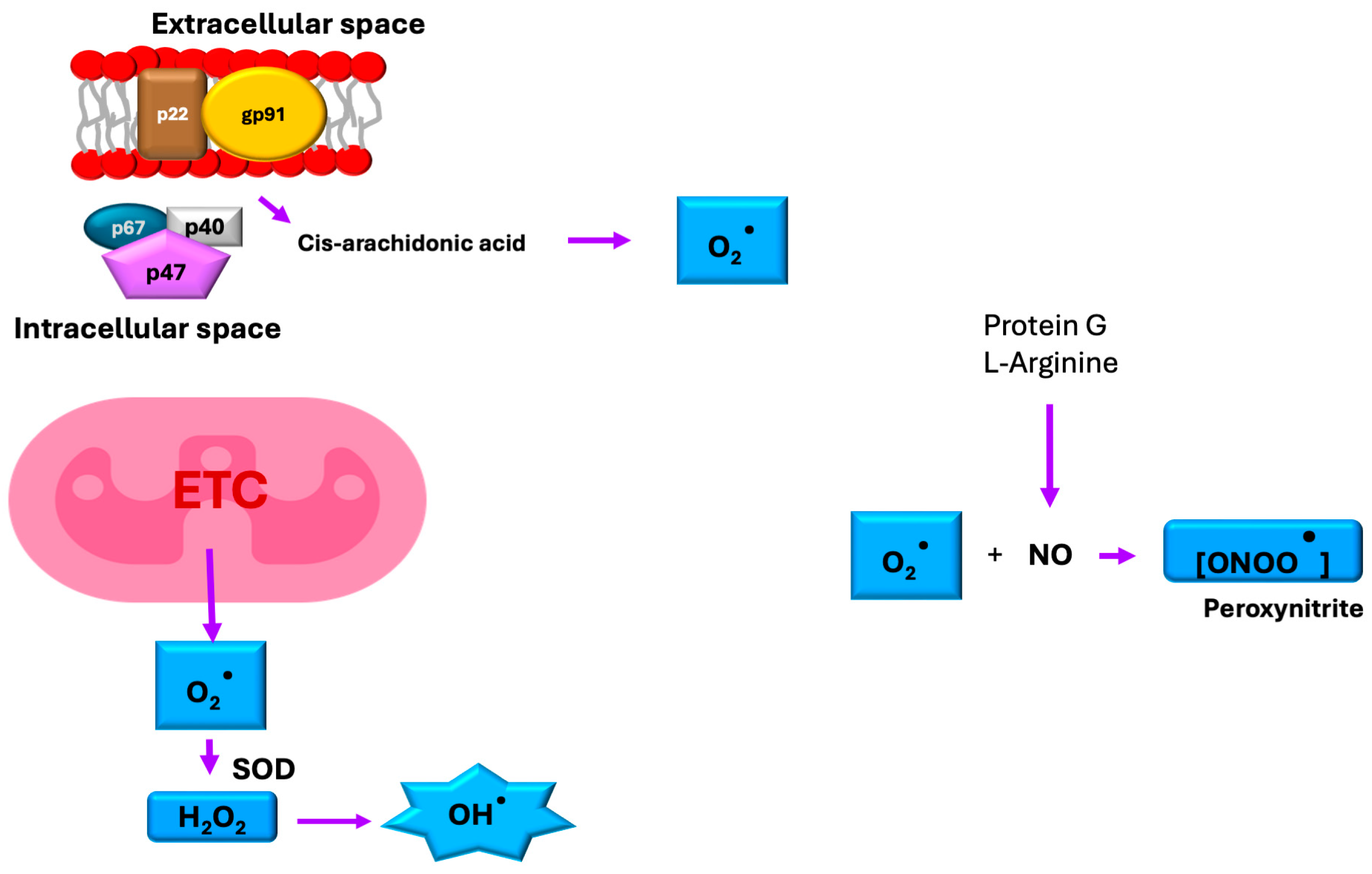
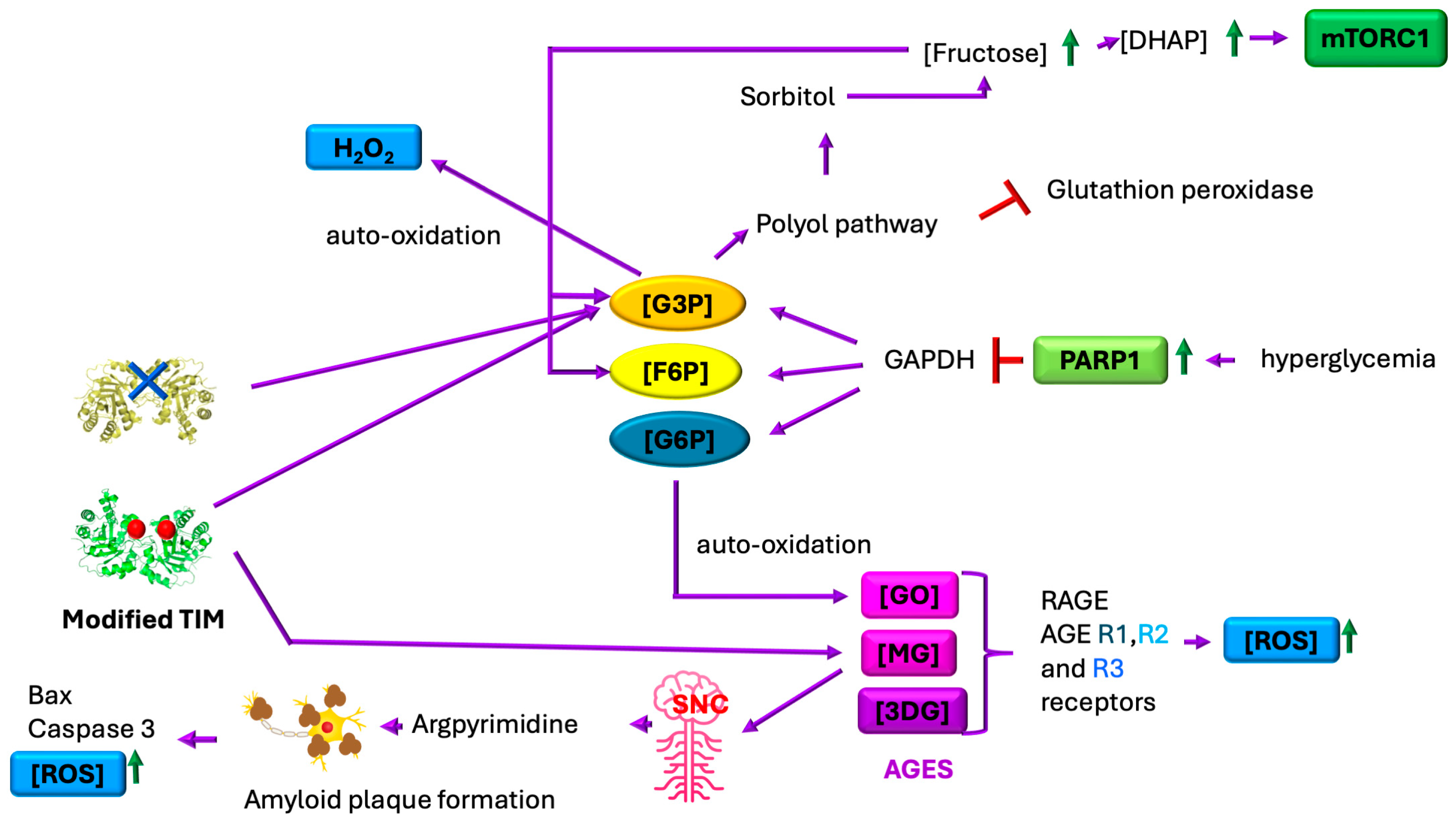
6. Oxidative Stress and TIM
7. TIM and the Fluidity of Cell Membranes
8. Discussion
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3DG | 3-deoxyglucosone; |
| ABC | ATP-binding cassette; |
| AGE | advanced glycation end product; |
| AKT | protein kinase B; |
| AP1 | activator protein 1; |
| CDCA5 | cell division cycle associated 5 or sororin; |
| c-Myc | MYC proto-oncogene; |
| DHAP | dihydroxyacetone phosphate; |
| F6P | fructose 6-phosphate; |
| FOXO1 | forkhead box protein O1; |
| G3P | glyceraldehyde 3-phosphate; |
| G6P | glucose 6-phosphate; |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase; |
| GATOR complex | regulator of the Rag GTPases; |
| GLUT1 | glucose transporter 1; |
| GLUT2 | glucose transporter 2; |
| GLUT4 | glucose transporter 4; |
| GO | glyoxal; |
| Hep G2 | human liver cancer cell line; |
| HIF1α | hypoxia inducible factor 1 alpha; |
| HIPPO | Salvador-Warts-Hippo (SWH) pathway; |
| IRS | insulin receptor substrate; |
| K ATP | ATP-sensitive potassium channels; |
| MG | methylglyoxal; |
| miRNA | microRNA; |
| mTOR | mammalian target of rapamycin; |
| mTORC1 | mammalian target of rapamycin complex 1; |
| mTORC2 | mammalian target of rapamycin complex 2; |
| NLRP3 | nucleotide-binding domain leucine-rich repeat family pyrin domain containing 3; |
| NOX | NADPH oxidase; |
| PARP1 | poly(ADP-ribose) polymerase 1; |
| PI3K | phosphoinositide 3-kinase; |
| PK | pyruvate kinase; |
| PPARGC1A | peroxisome proliferator receptor gamma coactivator 1 alpha; |
| RAGE | receptor for advanced glycation end products; |
| RCS | reactive dicarbonyl molecules; |
| RNS | reactive nitrogen species; |
| ROS | reactive oxygen species; |
| SOD | superoxide dismutase; |
| SOS2 | Son of Sevenless 2; |
| SUR1 | sulfonylurea receptor 1; |
| SUR2 | sulfonylurea receptor 2; |
| TIM | triosephosphate isomerase; |
| UTR | untranslated region; |
| YAP/YAP1 | yes associated protein 1; |
| YWHAZ/14-3-3ζ | gene coding for tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta/14-3-3 protein zeta. |
References
- Cloete, L. Diabetes mellitus: An overview of the types, symptoms, complications and management. Nurs. Stand. 2022, 37, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Defeudis, G.; Mazzilli, R.; Tenuta, M.; Rossini, G.; Zamponi, V.; Olana, S.; Faggiano, A.; Pozzilli, P.; Isidori, A.M.; Gianfrilli, D. Erectile dysfunction and diabetes: A melting pot of circumstances and treatments. Diabetes Metab. Res. 2022, 38, e3494. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhao, M.; Li, Z.; Nie, H.; He, J.; Chen, Z.; Yuan, J.; Guo, H.; Zhang, X.; Yang, H.; et al. Plasma miR-193b-3p is elevated in type 2 diabetes and could impair glucose metabolism. Front. Endocrinol. 2022, 13, 814347. [Google Scholar] [CrossRef]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef]
- Luo, A.; Xie, Z.; Wang, Y.; Wang, X.; Li, S.; Yan, J.; Zhan, G.; Zhou, Z.; Li, S. Type 2 diabetes mellitus-associated cognitive dysfunction: Advances in potential mechanisms and therapies. Neurosci. Biobehav. Rev. 2022, 137, 104642. [Google Scholar] [CrossRef]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative stress: Pathogenetic role in diabetes mellitus and its complications and therapeutic approaches to correction. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef]
- Agrawal, R.; Reno, C.M.; Sharma, S.; Christensen, C.; Huang, Y.; Fisher, S.J. Insulin action in the brain regulates both central and peripheral functions. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E156–E163. [Google Scholar] [CrossRef]
- Formiga, F.; Pérez-Maraver, M. Diabetes mellitus tipo 3. ¿El renacer de la insulina inhalada? Endocrinol. Nutr. 2014, 61, 173–175. [Google Scholar] [CrossRef]
- Janoutová, J.; Machaczka, O.; Zatloukalová, A.; Janout, V. Is Alzheimer’s disease a type 3 diabetes? A review. Cent. Eur. J. Public Health 2022, 30, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. 2024. Available online: https://www.cdc.gov/diabetes/prevention-type-2/index.html (accessed on 1 July 2025).
- Medina-Pérez, E.A.; Sánchez-Reyes, A.; Hernández-Peredo, A.R.; Martínez-López, M.A.; Jiménez-Flores, C.N.; Serrano-Ortiz, I.; Maqueda-Pineda, A.V.; Islas-Cruz, D.N.; Cruz-González, M. Diabetes gestacional. Diagnóstico y tratamiento en el primer nivel de atención. Med. Interna México 2017, 33, 91–98. Available online: https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0186-48662017000100091 (accessed on 1 July 2025).
- Grizzanti, J.; Moritz, W.R.; Pait, M.C.; Stanley, M.; Kaye, S.D.; Carroll, C.M.; Constantino, N.J.; Deitelzweig, L.J.; Snipes, J.A.; Kellar, D.; et al. KATP channels are necessary for glucose-dependent increases in amyloid-β and Alzheimer’s disease–related pathology. JCI Insight 2023, 8, e162454. [Google Scholar] [CrossRef]
- Ohtsuki, S. Insulin receptor at the blood–brain barrier: Transport and signaling. Vitam. Horm. 2024, 126, 113–124. [Google Scholar] [CrossRef]
- Mesarosova, L.; Scheper, M.; Iyer, A.; Anink, J.J.; Mills, J.D.; Aronica, E. miR-193b-3p/PGC-1α pathway regulates an insulin dependent anti-inflammatory response in Parkinson’s disease. Neurobiol. Dis. 2024, 199, 106587. [Google Scholar] [CrossRef]
- Hernández-Alcántara, G.; Garza-Ramos, G.; Hernández, G.M.; Gómez-Puyou, A.; Perez-Montfort, R. Catalysis and stability of triosephosphate isomerase from Trypanosoma brucei with different residues at position 14 of the dimer interface. Characterization of a catalytically competent monomeric enzyme. Biochemistry 2002, 41, 4230–4238. [Google Scholar] [CrossRef] [PubMed]
- Katebi, A.R.; Jernigan, R.L. The critical role of the loops of triosephosphate isomerase for its oligomerization, dynamics, and functionality. Prot. Sci. 2014, 23, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.R. Enzyme catalysis: Not different, just better. Nature 1991, 350, 121–124. [Google Scholar] [CrossRef]
- Wierenga, R.K.; Kapetaniou, E.G.; Venkatesan, R. Triosephosphate isomerase: A highly evolved biocatalyst. Cell Mol. Life Sci. 2010, 67, 3961–3982. [Google Scholar] [CrossRef] [PubMed]
- Bar-Even, A.; Flamholz, A.; Noor, E.; Milo, R. Rethinking glycolysis: On the biochemical logic of metabolic pathways. Nat. Chem. Biol. 2012, 8, 509–517. [Google Scholar] [CrossRef]
- Helliwell, J.R. Triosephosphate isomerase: The perfect enzyme, but how does it work? IUCrJ 2021, 8 Pt 4, 480–481. [Google Scholar] [CrossRef]
- Karg, E.; Németh, I.; Horányi, M.; Pintér, S.; Vécsei, L.; Hollán, S. Diminished blood levels of reduced glutathione and α-tocopherol in two triosephosphate isomerase-deficient brothers. Blood Cells Mol. Dis. 2000, 26, 91–100. [Google Scholar] [CrossRef]
- Sun, T.; Tan, L.; Liu, M.; Zeng, L.; Zhao, K.; Cai, Z.; Sun, S.; Li, Z.; Liu, R. Tilianin improves cognition in a vascular dementia rodent model by targeting miR-193b-3p/CaM- and miR-152-3p/CaMKIIα-mediated inflammatory and apoptotic pathways. Front. Immunol. 2023, 14, 1118808. [Google Scholar] [CrossRef]
- Orosz, F.; Oláh, J.; Ovádi, J. Triosephosphate isomerase deficiency: New insights into an enigmatic disease. Biochim. Biophys. Acta Mol. Basis Dis. 2009, 1792, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, J.; Mohrenweiser, H.W. Characterization of two new electrophoretic variants of human triosephosphate isomerase: Stability, kinetic, and immunological properties. Biochem. Genet. 1982, 20, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Perry, B.A.; Mohrenweiser, H.W. Human triosephosphate isomerase: Substitution of Arg for Gly at position 122 in a thermolabile electromorph variant, TPI-Manchester. Hum. Genet. 1992, 88, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Zingg, B.C.; Mohrenweiser, H.W. Molecular analysis of a series of alleles in humans with reduced activity at the triosephosphate isomerase locus. Am. J. Hum. Genet. 1996, 58, 308–316. [Google Scholar] [PubMed] [PubMed Central]
- Hollán, S.; Magócsi, M.; Fodor, E.; Horányi, M.; Harsányi, V.; Farkas, T. Search for the pathogenesis of the differing phenotype in two compound heterozygote Hungarian brothers with the same genotypic triosephosphate isomerase deficiency. Proc. Natl. Acad. Sci. USA 1997, 94, 10362–10366. [Google Scholar] [CrossRef]
- Arya, R.; Lalloz, M.R.A.; Bellingham, A.J.; Layton, D.M. Evidence for founder effect of the glu104asp substitution and identification of new mutations in triosephosphate isomerase deficiency. Hum. Mutat. 1997, 10, 290–294. [Google Scholar] [CrossRef]
- Ationu, A.; Humphries, A.; Lalloz, M.R.A.; Arya, R.; Wild, B.; Warrilow, J.; Morgan, J.; Bellingham, A.J.; Layton, D.M. Reversal of metabolic block in glycolysis by enzyme replacement in triosephosphate isomerase–deficient cells. Blood 1999, 94, 3193–3198. [Google Scholar] [CrossRef]
- Ahmed, N.; Battah, S.; Karachalias, N.; Babaei-Jadidi, R.; Horányi, M.; Baróti, K.; Hollán, S.; Thornalley, P.J. Increased formation of methylglyoxal and protein glycation, oxidation and nitrosation in triosephosphate isomerase deficiency. Biochim. Biophys. Acta Mol. Basis Dis. 2003, 1639, 121–132. [Google Scholar] [CrossRef]
- Oláh, J.; Orosz, F.; Puskás, L.G.; Hackler, L.; Horányi, M.; Polgár, L.; Hollán, S.; Ovadi, J. Triosephosphate isomerase deficiency: Consequences of an inherited mutation at mRNA, protein and metabolic levels. Biochem. J. 2005, 392, 675–683. [Google Scholar] [CrossRef]
- Ralser, M.; Heeren, G.; Breitenbach, M.; Lehrach, H.; Krobitsch, S. Triose phosphate isomerase deficiency is caused by altered dimerization–not catalytic inactivity–of the mutant enzymes. PLoS ONE 2006, 1, e30. [Google Scholar] [CrossRef]
- Rodríguez-Almazán, C.; Arreola, R.; Rodríguez-Larrea, D.; Aguirre-López, B.; De Gómez-Puyou, M.T.; Perez-Montfort, R.; Costas, M.; Gómez-Puyou, A.; Torres-Larios, A. Structural basis of human triosephosphate isomerase deficiency. J. Biol. Chem. 2008, 283, 23254–23263. [Google Scholar] [CrossRef]
- Fermo, E.; Bianchi, P.; Vercellati, C.; Rees, D.C.; Marcello, A.P.; Barcellini, W.; Zanella, A. Triose phosphate isomerase deficiency associated with two novel mutations in TPI gene. Eur. J. Haematol. 2010, 85, 170–173. [Google Scholar] [CrossRef]
- Serdaroglu, G.; Aydinok, Y.; Yilmaz, S.; Manco, L.; Özer, E. Triosephosphate isomerase deficiency: A patient with Val231Met mutation. Pediatr. Neurol. 2011, 44, 139–142. [Google Scholar] [CrossRef]
- Roland, B.P.; Amrich, C.G.; Kammerer, C.J.; Stuchul, K.A.; Larsen, S.B.; Rode, S.; Aslam, A.A.; Heroux, A.; Wetzel, R.; VanDemark, A.P.; et al. Triosephosphate isomerase I170V alters catalytic site, enhances stability and induces pathology in a Drosophila model of TPI deficiency. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, N.; Torres-Larios, A.; García-Torres, I.; Enríquez-Flores, S.; Perez-Montfort, R. Differential effects on enzyme stability and kinetic parameters of mutants related to human triosephosphate isomerase deficiency. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, D.; Lei, M.; Guo, Y.; Cui, Y.; Chen, F.; Sun, W.; Chen, X. TPI1 activates the PI3K/AKT/mTOR signaling pathway to induce breast cancer progression by stabilizing CDCA5. J. Transl. Med. 2022, 20, 191. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, J.; Wang, F.; Wei, J.; Yang, Z.; Sun, M.; Liu, J.; Wen, M.; Huang, W.; Chen, Z.; et al. Protein modifications throughout the lung cancer proteome unravel the cancer-specific regulation of glycolysis. Cell Rep. 2021, 37, 110137. [Google Scholar] [CrossRef]
- De La Mora-de La Mora, I.; Torres-Larios, A.; Enríquez-Flores, S.; Méndez, S.T.; Castillo-Villanueva, A.; Gómez-Manzo, S.; López-Velázquez, G.; Marcial-Quino, J.; Torres-Arroyo, A.; García-Torres, I.; et al. Structural effects of protein aging: Terminal marking by deamidation in human triosephosphate isomerase. PLoS ONE 2015, 10, e0123379. [Google Scholar] [CrossRef]
- Hipkiss, A.R. Activity-induced deamidation of triose-phosphate isomerase may explain the deleterious effects of excessive glucose consumption. Int. J. Diabetes Clin. Res. 2016, 3, 066. [Google Scholar] [CrossRef]
- Mo, C.; Li, H.; Yan, M.; Xu, S.; Wu, J.; Li, J.; Yang, X.; Li, Y.; Yang, J.; Su, X.; et al. Dopaminylation of endothelial TPI1 suppresses ferroptotic angiocrine signals to promote lung regeneration over fibrosis. Cell Metab. 2024, 36, 1839–1857.e12. [Google Scholar] [CrossRef]
- Rodríguez-Bolaños, M.; Perez-Montfort, R. Medical and veterinary importance of the moonlighting functions of triosephosphate isomerase. Curr. Protein Pept. Sci. 2019, 20, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Myers, T.D.; Palladino, M.J. Newly discovered roles of triosephosphate isomerase including functions within the nucleus. Mol. Med. 2023, 29, 18. [Google Scholar] [CrossRef] [PubMed]
- Suksangrat, T.; Phannasil, P.; Jitrapakde, S. miRNA regulation of glucose and lipid metabolism in relation to diabetes and non-alcoholic fatty liver disease. In Reviews on Biomarker Studies of Metabolic and Metabolism-Related Disorders; Advances in Experimental Medicine and Biology; Guest, P., Ed.; Springer: Cham, Switzerland, 2019; Volume 1134, pp. 129–148. [Google Scholar] [CrossRef]
- Mollet, I.G.; Macedo, M.P. Pre-diabetes-linked miRNA miR-193b-3p targets PPARGC1A, disrupts metabolic gene expression profile and increases lipid accumulation in hepatocytes: Relevance for MAFLD. Int. J. Mol. Sci. 2023, 24, 3875. [Google Scholar] [CrossRef]
- Kolokotronis, T.; Majchrzak-Stiller, B.; Buchholz, M.; Mense, V.; Strotmann, J.; Peters, I.; Skrzypczyk, L.; Liffers, S.T.; Menkene, L.M.; Wagner, M.; et al. Differential miRNA and protein expression reveals miR-1285, its targets TGM2 and CDH-1, as well as CD166 and S100A13 as potential new biomarkers in patients with diabetes mellitus and pancreatic adenocarcinoma. Cancers 2024, 16, 2726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, J.; Xiong, D.; Mei, Y.; Zhu, Y.; Deng, P.; Duan, Y. Effect of miR-1285-3p as a diagnostic biomarker for chronic heart failure on vascular endothelial cells. J. Cardiothorac. Surg. 2025, 20, 53. [Google Scholar] [CrossRef]
- An, X.; Li, T.; Chen, N.; Wang, H.; Su, M.; Shi, H.; Duan, X.; Ma, Y. miR-1285-3p targets TPI1 to regulate the glycolysis metabolism signaling pathway of Tibetan sheep Sertoli cells. PLoS ONE 2022, 17, e0270364. [Google Scholar] [CrossRef]
- Olveira, F.P.; Martins, A.D.; Moreire, A.C.; Cheng, C.Y.; Alves, M.G. The Warburg effect revisited—Lesson from the Sertoli cell. Med. Res. Rev. 2014, 35, 126–151. [Google Scholar] [CrossRef]
- Galardo, M.N.; Gorga, A.; Merlo, J.P.; Regueira, M.; Pellizzari, E.H.; Cigorraga, S.B.; Riera, M.F.; Meroni, S.B. Participation of HIFs in the regulation of Sertoli cell lactate production. Biochimie 2017, 132, 9–18. [Google Scholar] [CrossRef]
- Hermo, L.; Oliveira, R.L.; Smith, C.E.; Au, C.E.; Bergeron, J.J.M. Dark side of epididymis: Tails of sperm maturation. Andrology 2019, 7, 566–580. [Google Scholar] [CrossRef]
- Auer, J.; Camoin, L.; Courtot, A.; Hotellier, F.; De Almeida, M. Evidence that P36, a human sperm acrosomal antigen involved in the fertilization process is triosephosphate isomerase. Mol. Reprod. Dev. 2004, 68, 515–523. [Google Scholar] [CrossRef]
- Byrne, K.; Leahy, T.; McCulloch, R.; Colgrave, M.L.; Holland, M.K. Comprehensive mapping of the bull sperm surface proteome. Proteom. Syst. Biol. 2012, 12, 3559–3579. [Google Scholar] [CrossRef]
- Takei, G.L.; Miyashiro, D.; Mukai, C.; Okuno, M. Glycolisis plays an important role in energy transfer from the base to the distal end flagellum in mouse sperm. J. Exp. Biol. 2014, 217, 1876–1886. [Google Scholar] [CrossRef]
- Yang, L.; Shen, J.; Chen, J.; Li, W.; Xie, X. Reduced glycolysis contributed to inhibition of testis spermatogenesis in rats after chronic methamphetamine exposure. Med. Sci. Monit. 2019, 25, 5453–5464. [Google Scholar] [CrossRef] [PubMed]
- Auer, J.; Senechal, H.; Desvaux, F.X.; Albert, M.; De Almeida, M. Isolation and isolation of two sperm membrane proteins recognized by sperm-associated antibodies in infertile men. Mol. Reprod. Dev. 2000, 57, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Zangbar, M.S.; Keshtgar, S.; Zolghadri, J.; Gharesi-Fard, B. Antisperm protein targets in azoospermia men. J. Hum. Reprod. Sci. 2016, 9, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Daniel, B.; Livne, A.; Cohen, G.; Kahremany, S.; Sasson, S. Endothelial cell–derived triosephosphate isomerase attenuates insulin secretion from pancreatic beta cells of male rats. Endocrinology 2021, 162, bqaa234. [Google Scholar] [CrossRef]
- Martin, G.M.; Sung, M.W.; Yang, Z.; Innes, L.M.; Kandasamy, B.; David, L.L.; Yoshioka, C.; Shyng, S.L. Mechanism of pharmacochaperoning in a mammalian KATP channel revealed by cryo-EM. eLife 2019, 8, e46417. [Google Scholar] [CrossRef]
- Dhar-Chowdhury, P.; Harrell, M.D.; Han, S.Y.; Jankowska, D.; Parachuru, L.; Morrissey, A.; Srivastava, S.; Liu, W.; Malester, B.; Yoshida, H.; et al. The glycolytic enzymes, glyceraldehyde-3-phosphate dehydrogenase, triose-phosphate isomerase, and pyruvate kinase are components of the KATP channel macromolecular complex and regulate its junction. J. Biol. Chem. 2005, 280, 38464–38470. [Google Scholar] [CrossRef]
- Shi, Y.; Vaden, D.L.; Ju, S.; Ding, D.; Geiger, J.H.; Greenberg, M.L. Genetic perturbation of glycolysis results in inhibition of de novo inositol biosynthesis. J. Biol. Chem. 2005, 280, 41805–41810. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H. Myo-inositol for insulin resistance, metabolic syndrome, polycystic ovary syndrome and gestational diabetes. Open Heart 2022, 9, e001989. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling at a glance. J. Cell Sci. 2009, 122, 3589–3594. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Huang, Z.; Tian, Y.; Wang, H.; Ouyang, P.; Chen, H.; Wu, L.; Lin, B.; He, R. Role of triosephosphate isomerase and downstream functional genes on gastric cancer. Oncol. Rep. 2017, 38, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.; Liu, L.; Chen, H.; Qi, F.; Huang, Y.; Lin, J.; Sferra, T.J.; Sankararaman, S.; Wei, L.; Chu, J.; et al. Cell division cycle associated 5 promotes colorectal cancer progression by activating the ERK signaling pathway. Oncogenesis 2019, 8, 19. [Google Scholar] [CrossRef]
- Orozco, J.M.; Krawczyk, P.A.; Scaria, S.M.; Cangelosi, A.L.; Chan, S.H.; Kunchok, T.; Lewis, C.A.; Sabatini, D.M. Dihydroxyacetone phosphate signals glucose availability to mTORC1. Nat. Metab. 2020, 2, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Riboulet-Chavey, A.; Pierron, A.; Durand, I.; Murdaca, J.; Giudicelli, J.; Van Obberghen, E. Methylglyoxal impairs the insulin signaling pathways independently of the formation of intracellular reactive oxygen species. Diabetes 2006, 55, 1289–1299. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, H.; Luo, Q.; Yang, K.; Zou, Z.; Shi, M.; Liang, W. Dihydroxyacetone phosphate accumulation leads to podocyte pyroptosis in diabetic kidney disease. J. Cell. Mol. Med. 2023, 28, 18073. [Google Scholar] [CrossRef]
- Vergès, B. mTOR and cardiovascular diseases: Diabetes mellitus. Transplantation 2018, 102, S47–S49. [Google Scholar] [CrossRef]
- Tuo, Y.; Xiang, M. mTOR: A double-edged sword for diabetes. J. Leukoc. Biol. 2019, 106, 385–395. [Google Scholar] [CrossRef]
- Restaino, R.M.; Deo, S.H.; Parrish, A.R.; Fadel, P.J.; Padilla, J. Increased monocyte-derived reactive oxygen species in type 2 diabetes: Role of endoplasmic reticulum stress. Exp. Physiol. 2017, 102, 139–153. [Google Scholar] [CrossRef]
- Bellier, J.; Nokin, M.J.; Lardé, E.; Karoyan, P.; Peulen, O.; Castronovo, V.; Bellahcène, A. Methylglyoxal, a potent inducer of AGEs, connects between diabetes and cancer. Diabetes Res. Clin. Pract. 2019, 148, 200–211. [Google Scholar] [CrossRef]
- Gao, W.; Zhao, J.; Li, H.; Gao, Z. Triosephosphate isomerase tyrosine nitration induced by heme–NaNO2–H2O2 or peroxynitrite: Effects of different natural phenolic compounds. J. Biochem. Mol. Toxicol. 2017, 31, e21893. [Google Scholar] [CrossRef] [PubMed]
- Tajes, M.; Eraso-Pichot, A.; Rubio-Moscardó, F.; Guivernau, B.; Bosch-Morató, M.; Valls-Comamala, V.; Muñoz, F.J. Methylglyoxal reduces mitochondrial potential and activates Bax and caspase-3 in neurons: Implications for Alzheimer’s disease. Neurosci. Lett. 2014, 580, 78–82. [Google Scholar] [CrossRef]
- Reyaz, A.; Alam, S.; Chandra, K.; Kohli, S.; Agarwal, S. Methylglyoxal and soluble RAGE in type 2 diabetes mellitus: Association with oxidative stress. J. Diabetes Metab. Disord. 2020, 19, 515–521. [Google Scholar] [CrossRef]
- Nigro, C.; Leone, A.; Raciti, G.; Longo, M.; Mirra, P.; Formisano, P.; Beguinot, F.; Miele, C. Methylglyoxal-glyoxalase 1 balance: The root of vascular damage. Int. J. Mol. Sci. 2017, 18, 188. [Google Scholar] [CrossRef] [PubMed]
- Margina, D.; Ilie, M.; Gradinaru, D.; Vladica, M.; Pencea, C.; Mitrea, N.; Katona, E. Redox status parameters and PBMC membrane fluidity in diabetes mellitus. Clin. Exp. Med. J. 2009, 3, 279–291. [Google Scholar] [CrossRef]
- Pilon, M. Revisiting the membrane-centric view of diabetes. Lipids Health Dis. 2016, 15, 167. [Google Scholar] [CrossRef]
- Bianchetti, G.; Cefalo, C.M.A.; Ferreri, C.; Sansone, A.; Vitale, M.; Serantoni, C.; Abeltino, A.; Mezza, T.; Ferraro, P.M.; De Spirito, M.; et al. Erythrocyte membrane fluidity: A novel biomarker of residual cardiovascular risk in type 2 diabetes. Eur. J. Clin. Investig. 2024, 54, e14121. [Google Scholar] [CrossRef]
- Merkle, S.; Pretsch, W. Characterization of triosephosphate isomerase mutants with reduced enzyme activity in Mus musculus. Genetics 1989, 123, 837–844. [Google Scholar] [CrossRef]
- Celotto, A.M.; Frank, A.C.; Seigle, J.L.; Palladino, M.J. Drosophila model of human inherited triosephosphate isomerase deficiency glycolytic enzymopathy. Genetics 2006, 174, 1237–1246. [Google Scholar] [CrossRef]
- Hrizo, S.L.; Eicher, S.L.; Myers, T.D.; McGrath, I.; Wodrich, A.P.K.; Venkastesh, H.; Manjooran, D.; Swoger, S.; Gagnon, K.; Bruskim, M.; et al. Identification of protein quality control regulators using a Drosophila model of TPI deficiency. Neurobiol. Dis. 2021, 152, 105299. [Google Scholar] [CrossRef]
- Stone, A.; Cujic, O.; Rowlett, A.; Aderhold, S.; Savage, E.; Graham, B.; Steinert, J.R. Triose-phosphate isomerase deficiency is associated with a dysregulation of synaptic vesicle recycling in Drosophila melanogaster. Front. Synaptic Neurosci. 2023, 15, 1124061. [Google Scholar] [CrossRef]
- Ginger, J.P.; Kreber, R.A.; Ganetzky, B. Wasted away, a Drosophila mutation in triosephosphate isomerase, causes paralysis, neurodegeneration, and early death. Proc. Natl. Acad. Sci. USA 2006, 103, 14987–14993. [Google Scholar] [CrossRef]
- Ralser, M.; Nebel, A.; Kleindorp, R.; Krobitsch, S.; Lehrach, H.; Schreiber, S.; Reinhardt, R.; Timmermann, B. Sequencing and genotypic analysis of the triosephosphate isomerase (TPI1) locus in a large sample of long-lived Germans. BMC Genet. 2008, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Li, Y.; Liu, F.; Wang, L. Generation and analysis of TPI deficiency zebrafish model. Yi Chuan 2024, 46, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Myers, T.; Ferguson, C.; Gliniak, E.; Homaniacs, E.G.; Palladino, M.J. Murine model of triosephosphate isomerase deficiency with anemia and severe neuromuscular dysfunction. Curr. Res. Neurobiol. 2022, 9, 100062. [Google Scholar] [CrossRef]
- Nieto-Martínez, R. Actividad física en la prevención y tratamiento de la diabetes. Rev. Venez. Endocrinol. Metab. 2010, 8, 40–45. Available online: https://ve.scielo.org/scielo.php?script=sci_arttext&pid=S1690-31102010000200003 (accessed on 1 July 2025).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Bolaños, M.; Perez-Montfort, R. The Remarkable Role of Triosephosphate Isomerase in Diabetes Pathophysiology. Int. J. Mol. Sci. 2025, 26, 8809. https://doi.org/10.3390/ijms26188809
Rodríguez-Bolaños M, Perez-Montfort R. The Remarkable Role of Triosephosphate Isomerase in Diabetes Pathophysiology. International Journal of Molecular Sciences. 2025; 26(18):8809. https://doi.org/10.3390/ijms26188809
Chicago/Turabian StyleRodríguez-Bolaños, Mónica, and Ruy Perez-Montfort. 2025. "The Remarkable Role of Triosephosphate Isomerase in Diabetes Pathophysiology" International Journal of Molecular Sciences 26, no. 18: 8809. https://doi.org/10.3390/ijms26188809
APA StyleRodríguez-Bolaños, M., & Perez-Montfort, R. (2025). The Remarkable Role of Triosephosphate Isomerase in Diabetes Pathophysiology. International Journal of Molecular Sciences, 26(18), 8809. https://doi.org/10.3390/ijms26188809





