Molecular Signatures Related to Inflammation and Angiogenesis in Patients with Lower Extremity Artery Disease, Abdominal Aortic Aneurysm, and Varicose Veins: Shared and Distinct Pathways
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Study Groups
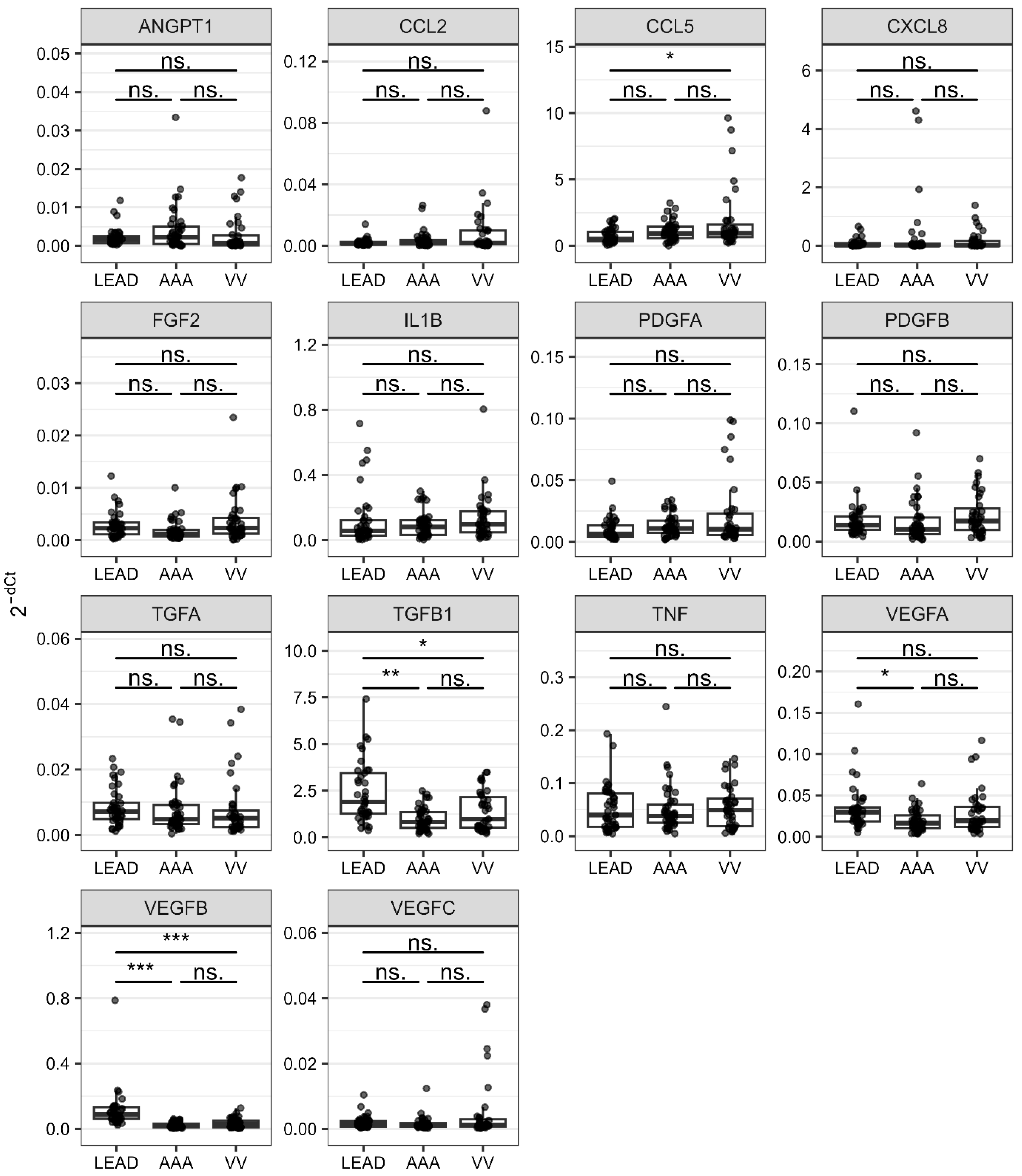
2.2. Dysregulatios of Genes Related to Angiogenesis and Inflammation Between LEAD, AAA, and VV
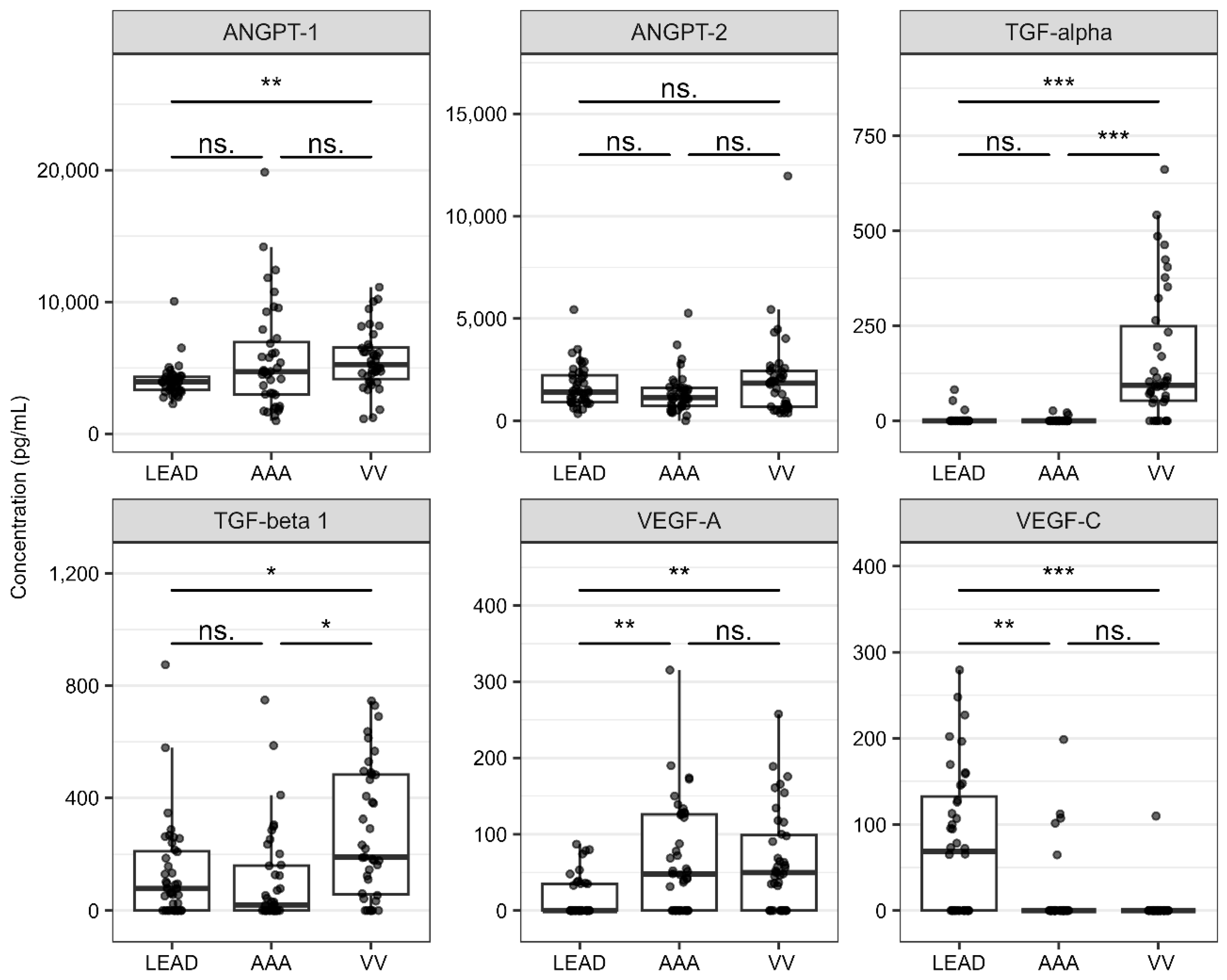
2.3. Differences in Plasma Concentrations of Angiogenesis-Related Proteins in LEAD, AAA, and VV
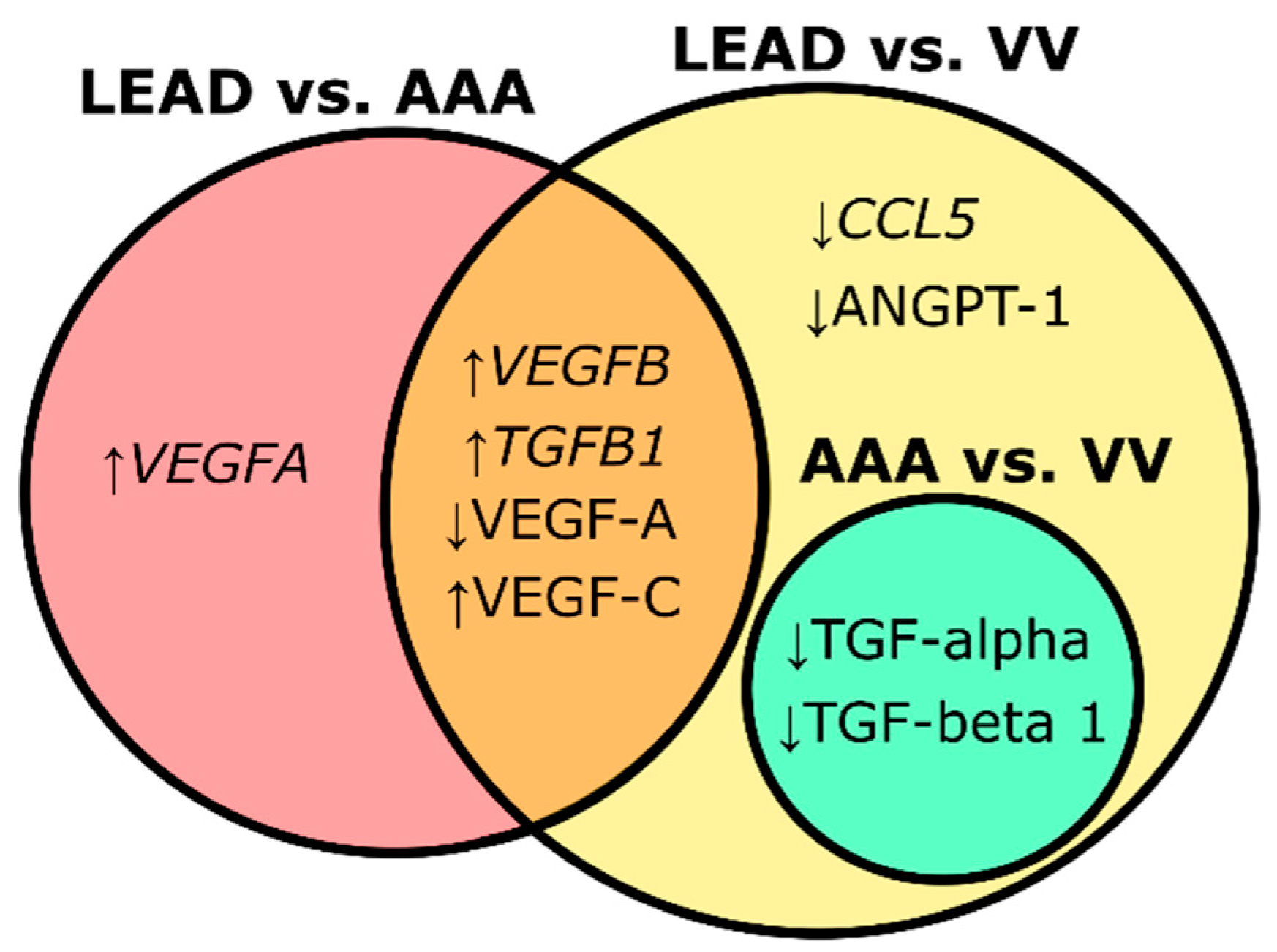
2.4. Relationships Between Identified Molecular Signatures and Characteristics of the Study Groups
2.5. Coexpression of Selected Genes and Proteins
2.6. Identification of Transcription Factors (TFs) Potentially Involved in the Observed Changes in Gene Expression
2.7. Identification of miRNA Potentially Involved in the Observed Changes in Expression of Selected Genes and TFs
2.8. Identification of Biological Processes Related to Identified Gene and Protein Signatures
3. Discussion
4. Materials and Methods
4.1. Outline of the Study Design
4.2. Gene Expression Dataset Analysis
4.3. Plasma Protein Levels Data Analysis
4.4. RNA-Seq Dataset Analysis
4.5. miRNA-Seq Dataset Analysis
4.6. Statistical Analysis and Modeling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAA | Abdominal aortic aneurysm |
| FDR | False discovery rate |
| LEAD | Lower extremity artery disease |
| miRNAs | microRNAs |
| OR | Odds ratio |
| PBMC | peripheral blood mononuclear cells |
| PCA | Principal component analysis |
| ROC | Receiver operating characteristic |
| ROC-AUC | Area under receiver operating characteristic curve |
| TFs | Transcription factors |
| VV | Varicose veins |
References
- Criqui, M.H.; Matsushita, K.; Aboyans, V.; Hess, C.N.; Hicks, C.W.; Kwan, T.W.; McDermott, M.M.; Misra, S.; Ujueta, F. Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e171–e191, Correction in Circulation 2021, 144, e193. [Google Scholar] [CrossRef]
- Townsend, N.; Kazakiewicz, D.; Lucy Wright, F.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of Cardiovascular Disease in Europe. Nat. Rev. Cardiol. 2022, 19, 133–143. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3032, Correction in J. Am. Coll. Cardiol. 2021, 77, 1958–1959. [Google Scholar] [CrossRef]
- Song, P.; Fang, Z.; Wang, H.; Cai, Y.; Rahimi, K.; Zhu, Y.; Fowkes, F.G.R.; Fowkes, F.J.I.; Rudan, I. Global and Regional Prevalence, Burden, and Risk Factors for Carotid Atherosclerosis: A Systematic Review, Meta-Analysis, and Modelling Study. Lancet Glob. Health 2020, 8, e721–e729. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.-B.; Bartelink, M.-L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in Collaboration with the European Society for Vascular Surgery (ESVS): Document Covering Atherosclerotic Disease of Extracranial Carotid and Vertebral, Mesenteric, Renal, Upper and Lower Extremity arteries Endorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Golledge, J. Update on the Pathophysiology and Medical Treatment of Peripheral Artery Disease. Nat. Rev. Cardiol. 2022, 19, 456–474. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; de Borst, G.J.; Hinchliffe, R.; Teraa, M. Peripheral Artery Disease: Underappreciated Impact and Residual Cardiovascular Risk Despite Revascularization. Clin. Ther. 2023, 45, 1019–1022. [Google Scholar] [CrossRef]
- Kessler, V.; Klopf, J.; Eilenberg, W.; Neumayer, C.; Brostjan, C. AAA Revisited: A Comprehensive Review of Risk Factors, Management, and Hallmarks of Pathogenesis. Biomedicines 2022, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Sakalihasan, N.; Michel, J.-B.; Katsargyris, A.; Kuivaniemi, H.; Defraigne, J.-O.; Nchimi, A.; Powell, J.T.; Yoshimura, K.; Hultgren, R. Abdominal Aortic Aneurysms. Nat. Rev. Dis. Primers 2018, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Murad, M.H.; Nguyen, L.L.; et al. The Society for Vascular Surgery Practice Guidelines on the Care of Patients with an Abdominal Aortic Aneurysm. J. Vasc. Surg. 2018, 67, 2–77.e2. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Álvarez-Mon, M.A.; Chaowen, C.; Ruiz-Grande, F.; Pekarek, L.; Monserrat, J.; Asúnsolo, A.; García-Honduvilla, N.; et al. Understanding Chronic Venous Disease: A Critical Overview of Its Pathophysiology and Medical Management. J. Clin. Med. 2021, 10, 3239. [Google Scholar] [CrossRef]
- Nicolaides, A.; Kakkos, S.; Baekgaard, N.; Comerota, A.; de Maeseneer, M.; Eklof, B.; Giannoukas, A.D.; Lugli, M.; Maleti, O.; Myers, K.; et al. Management of Chronic Venous Disorders of the Lower Limbs. Guidelines According to Scientific Evidence. Part I. Int. Angiol. 2018, 37, 181–254. [Google Scholar] [CrossRef]
- Raffetto, J.D. Pathophysiology of Chronic Venous Disease and Venous Ulcers. Surg. Clin. North. Am. 2018, 98, 337–347. [Google Scholar] [CrossRef]
- Poredos, P.; Kozak, M.; Antignani, P.L.; Jezovnik, M.K. From Varicose Veins to Venous Thromboembolic Events. Int. Angiol. 2023, 42, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, Z.; Gui, L.; Wu, Z.; Miao, Y.; Gao, Q.; Diao, Y.; Li, Y. Varicose Veins and Risk of Venous Thromboembolic Diseases: A Two-Sample-Based Mendelian Randomization Study. Front. Cardiovasc. Med. 2022, 9, 849027. [Google Scholar] [CrossRef]
- Wołkowski, K.; Urbanek, T. A Review of the Risk of Deep Vein Thrombosis in Surgical and Minimally Invasive Treatment of Varicose Veins. Phlebol. Rev. 2018, 26, 44–53. [Google Scholar] [CrossRef]
- Stanek, A.; Mosti, G.; Nematillaevich, T.S.; Valesky, E.M.; Planinšek Ručigaj, T.; Boucelma, M.; Marakomichelakis, G.; Liew, A.; Fazeli, B.; Catalano, M.; et al. No More Venous Ulcers-What More Can We Do? J. Clin. Med. 2023, 12, 6153. [Google Scholar] [CrossRef]
- Davies, A.H. The Seriousness of Chronic Venous Disease: A Review of Real-World Evidence. Adv. Ther. 2019, 36, 5–12. [Google Scholar] [CrossRef]
- Golledge, J. Abdominal Aortic Aneurysm: Update on Pathogenesis and Medical Treatments. Nat. Rev. Cardiol. 2019, 16, 225–242. [Google Scholar] [CrossRef]
- Ganta, V.C.; Choi, M.; Farber, C.R.; Annex, B.H. Antiangiogenic VEGF165b Regulates Macrophage Polarization via S100A8/S100A9 in Peripheral Artery Disease. Circulation 2019, 139, 226–242. [Google Scholar] [CrossRef]
- Ganta, V.C.; Choi, M.; Kutateladze, A.; Annex, B.H. VEGF165b Modulates Endothelial VEGFR1-STAT3 Signaling Pathway and Angiogenesis in Human and Experimental Peripheral Arterial Disease. Circ. Res. 2017, 120, 282–295. [Google Scholar] [CrossRef]
- Kikuchi, R.; Nakamura, K.; MacLauchlan, S.; Ngo, D.T.-M.; Shimizu, I.; Fuster, J.J.; Katanasaka, Y.; Yoshida, S.; Qiu, Y.; Yamaguchi, T.P.; et al. An Antiangiogenic Isoform of VEGF-A Contributes to Impaired Vascularization in Peripheral Artery Disease. Nat. Med. 2014, 20, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Kuppuswamy, S.; Annex, B.H.; Ganta, V.C. Targeting Anti-Angiogenic VEGF165b-VEGFR1 Signaling Promotes Nitric Oxide Independent Therapeutic Angiogenesis in Preclinical Peripheral Artery Disease Models. Cells 2022, 11, 2676. [Google Scholar] [CrossRef] [PubMed]
- Annex, B.H.; Cooke, J.P. New Directions in Therapeutic Angiogenesis and Arteriogenesis in Peripheral Arterial Disease. Circ. Res. 2021, 128, 1944–1957. [Google Scholar] [CrossRef] [PubMed]
- Djahanpour, N.; Ahsan, N.; Li, B.; Khan, H.; Connelly, K.; Leong-Poi, H.; Qadura, M. A Systematic Review of Interleukins as Diagnostic and Prognostic Biomarkers for Peripheral Artery Disease. Biomolecules 2023, 13, 1640. [Google Scholar] [CrossRef]
- Saenz-Pipaon, G.; Martinez-Aguilar, E.; Orbe, J.; González Miqueo, A.; Fernandez-Alonso, L.; Paramo, J.A.; Roncal, C. The Role of Circulating Biomarkers in Peripheral Arterial Disease. Int. J. Mol. Sci. 2021, 22, 3601. [Google Scholar] [CrossRef]
- Kokje, V.B.C.; Gäbel, G.; Dalman, R.L.; Koole, D.; Northoff, B.H.; Holdt, L.M.; Hamming, J.F.; Lindeman, J.H.N. CXCL8 Hyper-Signaling in the Aortic Abdominal Aneurysm. Cytokine 2018, 108, 96–104. [Google Scholar] [CrossRef]
- Middleton, R.K.; Bown, M.J.; Lloyd, G.M.; Jones, J.L.; London, N.J.; Sayers, R.D. Characterisation of Interleukin-8 and Monocyte Chemoattractant Protein-1 Expression within the Abdominal Aortic Aneurysm and Their Association with Mural Inflammation. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 46–55. [Google Scholar] [CrossRef]
- Zalewski, D.; Chmiel, P.; Kołodziej, P.; Borowski, G.; Feldo, M.; Kocki, J.; Bogucka-Kocka, A. Dysregulations of Key Regulators of Angiogenesis and Inflammation in Abdominal Aortic Aneurysm. Int. J. Mol. Sci. 2023, 24, 12087. [Google Scholar] [CrossRef]
- Kunecki, M.; Danilewicz, M.; Nawrocka-Kunecka, A. Usefulness of Serum VEGF Concentration Measurement to Estimate Aortic Aneurysm Risk of Rupture. Acta Angiol. 2006, 12, 7–15. [Google Scholar]
- Iwańczyk, S.; Lehmann, T.; Cieślewicz, A.; Radziemski, A.; Malesza, K.; Wrotyński, M.; Jagodziński, P.P.; Grygier, M.; Lesiak, M.; Araszkiewicz, A. Involvement of Angiogenesis in the Pathogenesis of Coronary Aneurysms. Biomedicines 2021, 9, 1269. [Google Scholar] [CrossRef] [PubMed]
- Poredos, P.; Spirkoska, A.; Rucigaj, T.; Fareed, J.; Jezovnik, M.K. Do Blood Constituents in Varicose Veins Differ from the Systemic Blood Constituents? Eur. J. Vasc. Endovasc. Surg. 2015, 50, 250–256. [Google Scholar] [CrossRef]
- Jacob, M.-P.; Cazaubon, M.; Scemama, A.; Prié, D.; Blanchet, F.; Guillin, M.-C.; Michel, J.-B. Plasma Matrix Metalloproteinase-9 as a Marker of Blood Stasis in Varicose Veins. Circulation 2002, 106, 535–538. [Google Scholar] [CrossRef]
- Kowalewski, R.; Małkowski, A.; Sobolewski, K.; Gacko, M. Vascular Endothelial Growth Factor and Its Receptors in the Varicose Vein Wall. Acta Angiol. 2011, 17, 141–149. [Google Scholar]
- Lee, J.-D.; Lai, C.-H.; Yang, W.-K.; Lee, T.-H. Increased Expression of Hypoxia-Inducible Factor-1α and Metallothionein in Varicocele and Varicose Veins. Phlebology 2012, 27, 409–415. [Google Scholar] [CrossRef]
- Zalewski, D.; Chmiel, P.; Kołodziej, P.; Kocki, M.; Feldo, M.; Kocki, J.; Bogucka-Kocka, A. Key Regulators of Angiogenesis and Inflammation Are Dysregulated in Patients with Varicose Veins. Int. J. Mol. Sci. 2024, 25, 6785. [Google Scholar] [CrossRef]
- Liska, O.; Bohár, B.; Hidas, A.; Korcsmáros, T.; Papp, B.; Fazekas, D.; Ari, E. TFLink: An Integrated Gateway to Access Transcription Factor–Target Gene Interactions for Multiple Species. Database 2022, 2022, baac083. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Zhou, G.; Soufan, O.; Xia, J. miRNet 2.0: Network-Based Visual Analytics for miRNA Functional Analysis and Systems Biology. Nucleic Acids Res. 2020, 48, W244–W251. [Google Scholar] [CrossRef] [PubMed]
- Bogucka-Kocka, A.; Zalewski, D.P.; Ruszel, K.P.; Stępniewski, A.; Gałkowski, D.; Bogucki, J.; Komsta, Ł.; Kołodziej, P.; Zubilewicz, T.; Feldo, M.; et al. Dysregulation of MicroRNA Regulatory Network in Lower Extremities Arterial Disease. Front. Genet. 2019, 10, 1200. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, D.P.; Ruszel, K.P.; Stępniewski, A.; Gałkowski, D.; Bogucki, J.; Komsta, Ł.; Kołodziej, P.; Chmiel, P.; Zubilewicz, T.; Feldo, M.; et al. Dysregulation of microRNA Modulatory Network in Abdominal Aortic Aneurysm. J. Clin. Med. 2020, 9, 1974. [Google Scholar] [CrossRef]
- Zalewski, D.P.; Ruszel, K.P.; Stępniewski, A.; Gałkowski, D.; Bogucki, J.; Komsta, Ł.; Kołodziej, P.; Chmiel, P.; Zubilewicz, T.; Feldo, M.; et al. Dysregulations of MicroRNA and Gene Expression in Chronic Venous Disease. J. Clin. Med. 2020, 9, 1251. [Google Scholar] [CrossRef] [PubMed]
- Camaré, C.; Pucelle, M.; Nègre-Salvayre, A.; Salvayre, R. Angiogenesis in the Atherosclerotic Plaque. Redox Biol. 2017, 12, 18–34. [Google Scholar] [CrossRef]
- Azimi-Nezhad, M.; Stathopoulou, M.G.; Bonnefond, A.; Rancier, M.; Saleh, A.; Lamont, J.; Fitzgerald, P.; Ndiaye, N.C.; Visvikis-Siest, S. Associations of Vascular Endothelial Growth Factor (VEGF) with Adhesion and Inflammation Molecules in a Healthy Population. Cytokine 2013, 61, 602–607. [Google Scholar] [CrossRef]
- Kaneko, H.; Anzai, T.; Takahashi, T.; Kohno, T.; Shimoda, M.; Sasaki, A.; Shimizu, H.; Nagai, T.; Maekawa, Y.; Yoshimura, K.; et al. Role of Vascular Endothelial Growth Factor-A in Development of Abdominal Aortic Aneurysm. Cardiovasc. Res. 2011, 91, 358–367. [Google Scholar] [CrossRef]
- Xu, J. Progress on Angiogenic and Antiangiogenic Agents in the Tumor Microenvironment. Front. Oncol. 2024, 14, 1491099. [Google Scholar] [CrossRef]
- Mamer, S.B.; Wittenkeller, A.; Imoukhuede, P.I. VEGF-A Splice Variants Bind VEGFRs with Differential Affinities. Sci. Rep. 2020, 10, 14413. [Google Scholar] [CrossRef]
- Peach, C.J.; Mignone, V.W.; Arruda, M.A.; Alcobia, D.C.; Hill, S.J.; Kilpatrick, L.E.; Woolard, J. Molecular Pharmacology of VEGF-A Isoforms: Binding and Signalling at VEGFR2. Int. J. Mol. Sci. 2018, 19, 1264. [Google Scholar] [CrossRef]
- White, A.L.; Bix, G.J. VEGFA Isoforms as Pro-Angiogenic Therapeutics for Cerebrovascular Diseases. Biomolecules 2023, 13, 702. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, P.A.; McCarty, J.H.; Guerrero, P.A.; McCarty, J.H. TGF-β Activation and Signaling in Angiogenesis. In Physiologic and Pathologic Angiogenesis—Signaling Mechanisms and Targeted Therapy; IntechOpen: London, UK, 2017; ISBN 978-953-51-3024-6. [Google Scholar]
- Ismaeel, A.; Miserlis, D.; Papoutsi, E.; Haynatzki, G.; Bohannon, W.T.; Smith, R.S.; Eidson, J.L.; Casale, G.P.; Pipinos, I.I.; Koutakis, P. Endothelial Cell-Derived pro-Fibrotic Factors Increase TGF-Β1 Expression by Smooth Muscle Cells in Response to Cycles of Hypoxia-Hyperoxia. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166278. [Google Scholar] [CrossRef]
- Yin, L.; Wang, L.; Shi, Z.; Ji, X.; Liu, L. The Role of Peroxisome Proliferator-Activated Receptor Gamma and Atherosclerosis: Post-Translational Modification and Selective Modulators. Front. Physiol. 2022, 13, 826811. [Google Scholar] [CrossRef]
- Zheng, Y.; Shao, M.; Zheng, Y.; Sun, W.; Qin, S.; Sun, Z.; Zhu, L.; Guan, Y.; Wang, Q.; Wang, Y.; et al. PPARs in Atherosclerosis: The Spatial and Temporal Features from Mechanism to Druggable Targets. J. Adv. Res. 2025, 69, 225–244. [Google Scholar] [CrossRef]
- Song, Y.; Li, S.; He, C. PPARγ Gene Polymorphisms, Metabolic Disorders, and Coronary Artery Disease. Front. Cardiovasc. Med. 2022, 9, 808929. [Google Scholar] [CrossRef]
- Luan, J.; Ji, X.; Liu, L. PPARγ in Atherosclerotic Endothelial Dysfunction: Regulatory Compounds and PTMs. Int. J. Mol. Sci. 2023, 24, 14494. [Google Scholar] [CrossRef]
- Ramirez, A.; Ballard, E.N.; Roman, J. TGFβ1 Controls PPARγ Expression, Transcriptional Potential, and Activity, in Part, through Smad3 Signaling in Murine Lung Fibroblasts. PPAR Res. 2012, 2012, 375876. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Y.; Du, Y.; Li, Y. MiRNA-130a Promotes Inflammation to Accelerate Atherosclerosis via the Regulation of Proliferator-Activated Receptor γ (PPARγ) Expression. Anatol. J. Cardiol. 2021, 25, 630–637. [Google Scholar] [CrossRef]
- Wang, J.; Xu, X.; Li, P.; Zhang, B.; Zhang, J. HDAC3 Protects against Atherosclerosis through Inhibition of Inflammation via the microRNA-19b/PPARγ/NF-κB Axis. Atherosclerosis 2021, 323, 1–12. [Google Scholar] [CrossRef]
- Meryet-Figuiere, M.; Vernon, M.; Andrianteranagna, M.; Lambert, B.; Brochen, C.; Issartel, J.-P.; Guttin, A.; Gauduchon, P.; Brotin, E.; Dingli, F.; et al. Network-Based Integration of Multi-Omics Data Identifies the Determinants of miR-491-5p Effects. Cancers 2021, 13, 3970. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.-C.; Tsai, P.-J.; Chen, J.-Y.; Lai, C.-H.; Wang, K.-C.; Teng, S.-H.; Lin, S.-C.; Chang, A.Y.W.; Jiang, M.-J.; Li, Y.-H.; et al. PPARγ Level Contributes to Structural Integrity and Component Production of Elastic Fibers in the Aorta. Hypertension 2016, 67, 1298–1308. [Google Scholar] [CrossRef]
- Tang, W.; Guo, Z.-D.; Chai, W.-N.; Du, D.-L.; Yang, X.-M.; Cao, L.; Chen, H.; Zhou, C.; Cheng, C.-J.; Sun, X.-C.; et al. Downregulation of miR-491-5p Promotes Neovascularization after Traumatic Brain Injury. Neural Regen. Res. 2021, 17, 577–586. [Google Scholar] [CrossRef]
- Zeng, G.-G.; Zhou, J.; Jiang, W.-L.; Yu, J.; Nie, G.-Y.; Li, J.; Zhang, S.-Q.; Tang, C.-K. A Potential Role of NFIL3 in Atherosclerosis. Curr. Probl. Cardiol. 2024, 49, 102096. [Google Scholar] [CrossRef]
- Liu, F.; Huang, Y.; Liu, F.; Wang, H. Identification of Immune-Related Genes in Diagnosing Atherosclerosis with Rheumatoid Arthritis through Bioinformatics Analysis and Machine Learning. Front. Immunol. 2023, 14, 1126647. [Google Scholar] [CrossRef]
- Chen, L.; Shangguan, Z.; Dong, Z.; Deng, Q.; Ding, Y.; Yang, S. NFIL3 Aggravates Human Coronary Artery Endothelial Cell Injury by Promoting ITGAM Transcription in Kawasaki Disease. Hematology 2023, 28, 2277502. [Google Scholar] [CrossRef]
- Zhou, K.; Luo, W.; Gui, D.-D.; Ren, Z.; Wei, D.-H.; Liu, L.-S.; Li, G.-H.; Tang, Z.-H.; Xiong, W.-H.; Hu, H.-J.; et al. Hydrogen Sulfide Attenuates Atherosclerosis Induced by Low Shear Stress by Sulfhydrylating Endothelium NFIL3 to Restrain MEST Mediated Endothelial Mesenchymal Transformation. Nitric Oxide 2024, 142, 47–57. [Google Scholar] [CrossRef]
- Marques, R.E.; Guabiraba, R.; Russo, R.C.; Teixeira, M.M. Targeting CCL5 in Inflammation. Expert Opin. Ther. Targets 2013, 17, 1439–1460. [Google Scholar] [CrossRef]
- del Rio Solá, L.; Aceves, M.; Dueñas, A.I.; González-Fajardo, J.A.; Vaquero, C.; Sanchez Crespo, M.; García-Rodríguez, C. Varicose Veins Show Enhanced Chemokine Expression. Eur. J. Vasc. Endovasc. Surg. 2009, 38, 635–641. [Google Scholar] [CrossRef]
- Tisato, V.; Zauli, G.; Gianesini, S.; Menegatti, E.; Brunelli, L.; Manfredini, R.; Zamboni, P.; Secchiero, P. Modulation of Circulating Cytokine-Chemokine Profile in Patients Affected by Chronic Venous Insufficiency Undergoing Surgical Hemodynamic Correction. J. Immunol. Res. 2014, 2014, 473765. [Google Scholar] [CrossRef]
- Nasri Nasrabadi, P.; Martin, D.; Gharib, E.; Robichaud, G.A. The Pleiotropy of PAX5 Gene Products and Function. Int. J. Mol. Sci. 2022, 23, 10095. [Google Scholar] [CrossRef]
- Shadrina, A.S.; Sharapov, S.Z.; Shashkova, T.I.; Tsepilov, Y.A. Varicose Veins of Lower Extremities: Insights from the First Large-Scale Genetic Study. PLoS Genet. 2019, 15, e1008110. [Google Scholar] [CrossRef]
- Gnanamony, M.; Demirkhanyan, L.; Ge, L.; Sojitra, P.; Bapana, S.; Norton, J.A.; Gondi, C.S. Circular Dumbbell miR-34a-3p and −5p Suppresses Pancreatic Tumor Cell-Induced Angiogenesis and Activates Macrophages. Oncol. Lett. 2021, 21, 75. [Google Scholar] [CrossRef]
- Ou, X.; Gao, J.-H.; He, L.-H.; Yu, X.-H.; Wang, G.; Zou, J.; Zhao, Z.-W.; Zhang, D.-W.; Zhou, Z.; Tang, C.-K. Angiopoietin-1 Aggravates Atherosclerosis by Inhibiting Cholesterol Efflux and Promoting Inflammatory Response. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158535. [Google Scholar] [CrossRef]
- Ahmad, S.; Cudmore, M.J.; Wang, K.; Hewett, P.; Potluri, R.; Fujisawa, T.; Ahmed, A. Angiopoietin-1 Induces Migration of Monocytes in a Tie-2 and Integrin-Independent Manner. Hypertension 2010, 56, 477–483. [Google Scholar] [CrossRef]
- Kimura, M.; Moteki, H.; Ogihara, M. Role of Hepatocyte Growth Regulators in Liver Regeneration. Cells 2023, 12, 208. [Google Scholar] [CrossRef]
- Sasaki, Y.; Kawamoto, A.; Iwano, M.; Kurioka, H.; Takase, E.; Kawata, H.; Tsujimura, S.; Fukuhara, S.; Akai, Y.; Hashimoto, T.; et al. Vascular Endothelial Growth Factor mRNA Synthesis by Peripheral Blood Mononuclear Cells in Patients with Acute Myocardial Infarction. Int. J. Cardiol. 2001, 81, 51–60. [Google Scholar] [CrossRef]
- Hojo, Y.; Ikeda, U.; Zhu, Y.; Okada, M.; Ueno, S.; Arakawa, H.; Fujikawa, H.; Katsuki, T.; Shimada, K. Expression of Vascular Endothelial Growth Factor in Patients with Acute Myocardial Infarction. JACC J. 2000, 35, 968–973. [Google Scholar] [CrossRef]
- Dańczak-Pazdrowska, A.; Kowalczyk, M.J.; Szramka-Pawlak, B.; Gornowicz-Porowska, J.; Szewczyk, A.; Silny, W.; Molińska-Glura, M.; Olewicz-Gawlik, A.; Żaba, R.; Pazdrowski, J.; et al. Transforming Growth Factor-Β1 in Plaque Morphea. Postep. Dermatol. Alergol. 2013, 30, 337–342. [Google Scholar] [CrossRef]
- Meyer, A.; Wang, W.; Qu, J.; Croft, L.; Degen, J.L.; Coller, B.S.; Ahamed, J. Platelet TGF-Β1 Contributions to Plasma TGF-Β1, Cardiac Fibrosis, and Systolic Dysfunction in a Mouse Model of Pressure Overload. Blood 2012, 119, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Debette, S.; Visvikis-Siest, S.; Chen, M.-H.; Ndiaye, N.-C.; Song, C.; Destefano, A.; Safa, R.; Nezhad, M.A.; Sawyer, D.; Marteau, J.-B.; et al. Identification of Cis and Trans Acting Genetic Variants Explaining up to Half the Variation in Circulating VEGF Levels. Circ. Res. 2011, 109, 554–563. [Google Scholar] [CrossRef]
- Juarez, I.; Gutierrez, A.; Vaquero-Yuste, C.; Molanes-López, E.M.; López, A.; Lasa, I.; Gómez, R.; Martin-Villa, J.M. TGFB1 Polymorphisms and TGF-Β1 Plasma Levels Identify Gastric Adenocarcinoma Patients with Lower Survival Rate and Disseminated Disease. J. Cell Mol. Med. 2021, 25, 774–783. [Google Scholar] [CrossRef]
- Zalewski, D.P.; Ruszel, K.P.; Stępniewski, A.; Gałkowski, D.; Bogucki, J.; Kołodziej, P.; Szymańska, J.; Płachno, B.J.; Zubilewicz, T.; Feldo, M.; et al. Identification of Transcriptomic Differences between Lower Extremities Arterial Disease, Abdominal Aortic Aneurysm and Chronic Venous Disease in Peripheral Blood Mononuclear Cells Specimens. Int. J. Mol. Sci. 2021, 22, 3200. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, D.P.; Ruszel, K.P.; Stępniewski, A.; Gałkowski, D.; Feldo, M.; Kocki, J.; Bogucka-Kocka, A. miRNA Regulatory Networks Associated with Peripheral Vascular Diseases. J. Clin. Med. 2022, 11, 3470. [Google Scholar] [CrossRef]
- Zalewski, D.; Bogucka-Kocka, A. RQdeltaCT: An Open-Source R Package for Relative Quantification of Gene Expression Using Delta Ct Methods. Sci. Rep. 2025, 15, 29762. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Hothorn, T.; Hornik, K.; van de Wiel, M.A.; Zeileis, A. Implementing a Class of Permutation Tests: The Coin Package. J. Stat. Softw. 2008, 28, 1–23. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An Open-Source Package for R and S+ to Analyze and Compare ROC Curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]




| Characteristic | LEAD Group (n = 40) | AAA Group (n = 40) | VV Group (n = 40) | p 1 |
|---|---|---|---|---|
| Age | 60.3 ± 7.56 (45–76) | 59.2 ± 9.47 (45–80) | 53.7 ± 8.00 (39–72) | 2.058 × 10−3 |
| Sex male/female | 25 (62.5%)/15 (37.5%) | 34 (85%)/6 (15%) | 27 (67.5%)/13 (32.5%) | >0.05 |
| Body mass index (BMI) | 26.2 ± 3.06 (20.8–32.9) | 26.8 ± 4.27 (19.5–35.1) | 25.8 ± 3.40 (17.6–32.5) | >0.05 |
| Smoking | 35 (87.5%) | 15 (37.5%) | 0 (0%) | 2.367 × 10−17 |
| Hypertension | 38 (95%) | 7 (17.5%) | 1 (2.5%) | 1.151 × 10−21 |
| LDL (mg/dL) | 109.9 ± 21.04 (73–167) | 108.9 ± 14.16 (79–151) | 96.6 ± 14.23 (71–121) | 1.513 × 10−3 |
| HDL (mg/dL) | 41.93 ± 2.90 (35–47) | 40.70 ± 3.70 (31–46) | 41.70 ± 3.52 (33–48) | >0.05 |
| Cholesterol (mg/dL) | 201.1 ± 11.2 (176–231) | 206.1 ± 22.3 (143–302) | 203.2 ± 18.2 (167–242) | >0.05 |
| Creatinine (mg/dL) | 0.87 ± 0.12 (0.67–1.09) | 0.80 ± 0.16 (0.38–1.08) | 0.63 ± 0.13 (0.34–0.89) | 2.366 × 10−10 |
| Urea (mg/dL) | 33.0 ± 6.21 (21–56) | 35.5 ± 5.71 (25–44) | 31.2 ± 6.51 (21–45) | 6.432 × 10−3 |
| C-reactive protein (mg/L) | 4.68 ± 1.40 (2.0–9.1) | 4.34 ± 1.31 (1.1–6.8) | 2.54 ± 1.06 (0.8–5.2) | 1.779 × 10−10 |
| Fibrinogen (mg/dL) | 265.1 ± 60.3 (189–467) | 177.5 ± 45.4 (121–316) | 162.7 ± 33.5 (109–261) | 8.834 × 10−14 |
| Homocysteine (µmol/L) | 6.92 ± 1.58 (4.99–12.7) | 8.00 ± 2.04 (3.56–13.8) | 6.44 ± 1.35 (3.89–8.9) | 2.772 × 10−4 |
| Comparison | Gene Symbol | Gene Name | Differential Expression | ROC | Univariate Logistic Regression | ||
|---|---|---|---|---|---|---|---|
| Fold Change | FDR | ROC-AUC | OR | FDR | |||
| LEAD vs. AAA | TGFB1 | Transforming growth factor beta 1 | 2.494 | 2.704 × 10−5 | 0.806 | 9.997 | 1.297 × 10−3 |
| VEGFA | Vascular endothelial growth factor A | 1.858 | 1.490 × 10−3 | 0.739 | 4.432 | 1.995 × 10−2 | |
| VEGFB | Vascular endothelial growth factor B | 5.057 | 1.099 × 10−10 | 0.953 | 526.126 | 4.312 × 10−4 | |
| LEAD vs. VV | CCL5 | C-C motif chemokine ligand 5 | 0.405 | 3.143 × 10−2 | 0.686 | 0.351 | 0.127 |
| TGFB1 | Transforming growth factor beta 1 | 1.716 | 3.143 × 10−2 | 0.695 | 3.089 | 4.201 × 10−2 | |
| VEGFB | Vascular endothelial growth factor B | 3.248 | 5.914 × 10−7 | 0.876 | 26.394 | 5.374 × 10−4 | |
| Comparison | Protein Symbol | Protein Name | Mean Concentration (pg/mL) ± SD | FDR | ROC-AUC | Univariate Logistic Regression | ||
|---|---|---|---|---|---|---|---|---|
| LEAD | AAA | OR | FDR | |||||
| LEAD vs. AAA | VEGF-A | Vascular endothelial growth factor A | 15.92 ± 27.01 | 66.40 ± 71.97 | 4.969 × 10−4 | 0.726 | 0.978 | 2.452 × 10−4 |
| VEGF-C | Vascular endothelial growth factor C | 76.28 ± 83.13 | 14.60 ± 42.20 | 3.637 × 10−4 | 0.719 | 1.016 | 2.452 × 10−4 | |
| LEAD vs. VV | ANGPT-1 | Angiopoietin-1 | 4021.43 ± 1250.10 | 5592.54 ± 2309.60 | 1.069 × 10−4 | 0.764 | 0.999 | 3.085 × 10−3 |
| TGF-alpha | Protransforming growth factor alpha | 4.10 ± 15.79 | 165.05 ± 175.22 | 5.000 × 10−10 | 0.883 | 0.953 | 2.950 × 10−4 | |
| TGF-beta 1 | Transforming growth factor beta-1 proprotein | 132.38 ± 174.67 | 277.53 ± 236.20 | 6.833 × 10−3 | 0.679 | 0.996 | 6.571 × 10−3 | |
| VEGF-A | Vascular endothelial growth factor A | 15.92 ± 27.01 | 64.36 ± 65.08 | 1.111 × 10−4 | 0.742 | 0.975 | 1.871 × 10−3 | |
| VEGF-C | Vascular endothelial growth factor C | 76.28 ± 83.13 | 2.81 ± 17.56 | 1.529 × 10−6 | 0.763 | 1.034 | 3.085 × 10−3 | |
| AAA vs. VV | TGF-alpha | Protransforming growth factor alpha | 1.60 ± 5.83 | 165.05 ± 175.22 | 2.417 × 10−10 | 0.890 | 0.929 | 4.604 × 10−3 |
| TGF-beta 1 | Transforming growth factor beta-1 proprotein | 106.29 ± 171.70 | 277.53 ± 236.20 | 9.934 × 10−4 | 0.730 | 0.996 | 4.604 × 10−3 | |
| Comparison | TF Symbol | TF Name | Differential Expression | ROC | Selected Associated Genes | |
|---|---|---|---|---|---|---|
| Fold Change | FDR | ROC-AUC | ||||
| LEAD vs. AAA | PPARG | peroxisome proliferator activated receptor gamma | 4.034 | 3.247 × 10−2 | 0.857 | ↑ VEGFA, ↑ VEGFB, ↑ TGFB1 |
| LEAD vs. VV | EBF1 | EBF transcription factor 1 | 0.392 | 1.862 × 10−2 | 0.929 | ↑ VEGFB, ↑ TGFB1, ↓ CCL5 |
| NFIL3 | nuclear factor, interleukin 3 regulated | 2.119 | 1.306 × 10−2 | 0.929 | ↑ VEGFB | |
| PAX5 | paired box 5 | 0.346 | 8.957 × 10−3 | 0.875 | ↑ TGFB1, ↓ CCL5 | |
| SOX5 | SRY-box transcription factor 5 | 0.227 | 3.609 × 10−4 | 1.000 | ↑ TGFB1 | |
| Comparison | miRNA | Differential Expression | ROC | Associated Genes or TFs | |
|---|---|---|---|---|---|
| Fold Change | FDR | ROC-AUC | |||
| LEAD vs. AAA | hsa-miR-1301-3p | 0.741 | 1.398 × 10−4 | 0.815 | ↑ VEGFA |
| hsa-miR-326 | 0.767 | 1.278 × 10−2 | 0.739 | ↑ VEGFA | |
| hsa-miR-491-5p | 0.809 | 4.086 × 10−2 | 0.668 | ↑ PPARG | |
| LEAD vs. VV | hsa-miR-181b-5p | 1.441 | 8.738 × 10−4 | 0.752 | ↓ PAX5,↓ SOX5 |
| hsa-miR-3130-3p | 1.359 | 1.023 × 10−2 | 0.779 | ↓ PAX5 | |
| hsa-miR-193a-3p | 1.368 | 1.023 × 10−2 | 0.741 | ↓ SOX5 | |
| hsa-miR-1229-3p | 0.621 | 1.398 × 10−2 | 0.692 | ↑ NFIL3 | |
| hsa-miR-338-3p | 1.234 | 1.398 × 10−2 | 0.685 | ↓ EBF1 | |
| hsa-miR-7-5p | 1.255 | 1.945 × 10−2 | 0.688 | ↓ EBF1 | |
| hsa-miR-146a-5p | 0.811 | 2.289 × 10−2 | 0.735 | ↑ TGFB1 | |
| hsa-miR-3529-3p | 1.240 | 2.842 × 10−2 | 0.672 | ↓ SOX5 | |
| hsa-miR-491-5p | 0.795 | 2.915 × 10−2 | 0.657 | ↑ NFIL3 | |
| hsa-miR-625-3p | 0.727 | 3.691 × 10−2 | 0.672 | ↑ NFIL3 | |
| hsa-miR-335-5p | 0.761 | 3.691 × 10−2 | 0.691 | ↑ NFIL3 | |
| hsa-miR-374a-3p | 1.266 | 3.691 × 10−2 | 0.657 | ↓ SOX5 | |
| hsa-miR-582-5p | 1.318 | 4.509 × 10−2 | 0.667 | ↓ EBF1 | |
| hsa-miR-34a-5p | 1.279 | 4.890 × 10−2 | 0.726 | ↓ CCL5, ↓ EBF1 ↓ PAX5, ↓ SOX5 | |
| hsa-miR-21-3p | 1.286 | 4.960 × 10−2 | 0.682 | ↓ SOX5, ↓ CCL5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalewski, D.; Chmiel, P.; Kołodziej, P.; Feldo, M.; Stępniewski, A.; Ziaja-Sołtys, M.; Łuszczak, J.; Stanek, A.; Kocki, J.; Bogucka-Kocka, A. Molecular Signatures Related to Inflammation and Angiogenesis in Patients with Lower Extremity Artery Disease, Abdominal Aortic Aneurysm, and Varicose Veins: Shared and Distinct Pathways. Int. J. Mol. Sci. 2025, 26, 8786. https://doi.org/10.3390/ijms26188786
Zalewski D, Chmiel P, Kołodziej P, Feldo M, Stępniewski A, Ziaja-Sołtys M, Łuszczak J, Stanek A, Kocki J, Bogucka-Kocka A. Molecular Signatures Related to Inflammation and Angiogenesis in Patients with Lower Extremity Artery Disease, Abdominal Aortic Aneurysm, and Varicose Veins: Shared and Distinct Pathways. International Journal of Molecular Sciences. 2025; 26(18):8786. https://doi.org/10.3390/ijms26188786
Chicago/Turabian StyleZalewski, Daniel, Paulina Chmiel, Przemysław Kołodziej, Marcin Feldo, Andrzej Stępniewski, Marta Ziaja-Sołtys, Joanna Łuszczak, Agata Stanek, Janusz Kocki, and Anna Bogucka-Kocka. 2025. "Molecular Signatures Related to Inflammation and Angiogenesis in Patients with Lower Extremity Artery Disease, Abdominal Aortic Aneurysm, and Varicose Veins: Shared and Distinct Pathways" International Journal of Molecular Sciences 26, no. 18: 8786. https://doi.org/10.3390/ijms26188786
APA StyleZalewski, D., Chmiel, P., Kołodziej, P., Feldo, M., Stępniewski, A., Ziaja-Sołtys, M., Łuszczak, J., Stanek, A., Kocki, J., & Bogucka-Kocka, A. (2025). Molecular Signatures Related to Inflammation and Angiogenesis in Patients with Lower Extremity Artery Disease, Abdominal Aortic Aneurysm, and Varicose Veins: Shared and Distinct Pathways. International Journal of Molecular Sciences, 26(18), 8786. https://doi.org/10.3390/ijms26188786








