A Comprehensive Review of the Triangular Relationship Among Diet, Gut Microbiota, and Aging
Abstract
1. Introduction
2. Methodology
3. Gut Microbiome—Overview
3.1. Complexity and Composition of Gut Microbiota
3.2. Dominant Bacterial Groups
3.3. Early-Life Microbiota Development
3.4. Functional Roles of Microbiota in Adults
3.5. Dysbiosis and Its Link to Diseases
3.6. Changes in Microbiota Composition
4. Diet as a Modulator of the Gut Microbiota
5. Impact of Dietary Patterns on Gut Microbiota
5.1. Mediterranean Diet
5.2. Western Diet
5.3. Plant-Based Diets (Vegetarian/Vegan)
6. Role of Specific Nutrients in Microbiota–Aging Interactions
6.1. Carbohydrates and Fiber
6.2. Fats
6.3. Proteins
6.4. Micronutrients and Bioactive Compounds (Vitamins, Polyphenols, Minerals)
6.4.1. Vitamins
6.4.2. Minerals
6.4.3. Polyphenols
6.5. Probiotics and Prebiotics
6.5.1. Probiotics
6.5.2. Prebiotics
7. Gut Microbiota and Aging: Mechanistic Insights
7.1. Mechanisms of Gut Microbiota–Aging Interaction
7.2. Gut Barrier Function and Permeability
7.3. Production of Metabolites and Their Effects on Aging
8. Diet and Aging
8.1. Diet and Biology of Aging
8.2. Diet and Physical/Cognitive Function of Aging
Effect of DASH and MIND Diet
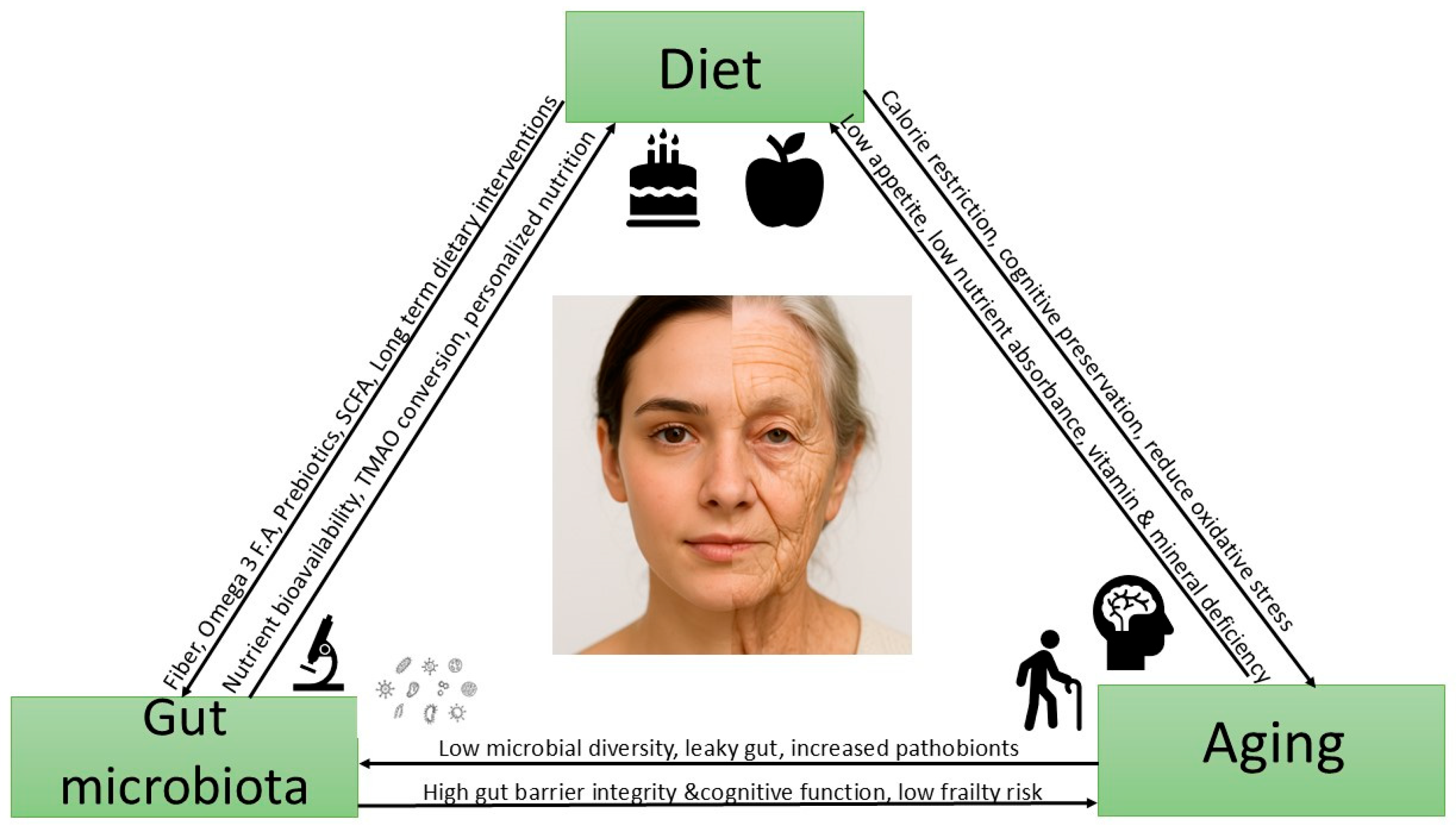
9. The Interconnected Triangle: Diet, Gut Microbiota, and Aging
9.1. Interconnection of Gut Microbiota and Aging
9.2. Interconnection of Diet and Aging
10. Research Gaps and Future Research Directions
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SCFA | Short-Chain Fatty Acid |
| MD | Mediterranean Diet |
| PUFA | Polyunsaturated Fatty Acid |
| MUFA | Monounsaturated Fatty Acid |
| TMA | Trimethylamine |
| TMAO | Trimethylamine-N-oxide |
| LPS | Lipopolysaccharide |
| MMKD | Modified Mediterranean Ketogenic Diet |
| BMI | Body Mass Index |
| DNA | Deoxyribonucleic Acid |
| ROS | Reactive Oxygen Species |
| NO | Nitric Oxide |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| CALERIE | Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy |
| DASH | Dietary Approaches to Stop Hypertension |
| MIND | Mediterranean-DASH Intervention for Neurodegenerative Delay |
| ITF | Inulin-Type Fructan |
| GOS | Galactooligosaccharide |
| AP | Aloe Polysaccharide |
| FXR | Farnesoid X Receptor |
| TGR5 | Takeda G Protein-Coupled Receptor 5 |
| IgA | Immunoglobulin A |
| TJP2 | Tight Junction Protein 2 |
| HDAC | Histone Deacetylase |
| BDNF | Brain-Derived Neurotrophic Factor |
| AMPK | AMP-Activated Protein Kinase |
| mTOR | Mechanistic Target of Rapamycin |
| MACs | Microbiota-Accessible Carbohydrates |
| UNESCO | United Nations Educational, Scientific, and Cultural Organization |
References
- O’Toole, P.W.; Claesson, M.J. Gut Microbiota: Changes throughout the Lifespan from Infancy to Elderly. Int. Dairy J. 2010, 20, 281–291. [Google Scholar] [CrossRef]
- Donati Zeppa, S.; Agostini, D.; Ferrini, F.; Gervasi, M.; Barbieri, E.; Bartolacci, A.; Piccoli, G.; Saltarelli, R.; Sestili, P.; Stocchi, V. Interventions on Gut Microbiota for Healthy Aging. Cells 2023, 12, 34. [Google Scholar] [CrossRef]
- Jeste, D.V.; Nguyen, T.T. The Gut Microbiome, Aging, and Longevity: A Systematic Review. Nutrients 2020, 12, 3759. [Google Scholar] [CrossRef] [PubMed]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet versus Mediterranean Diet at the Gut Microbiota-Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Khan, M.R. An Insight into Gut Microbiota and Its Functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, J.; Real, J.M.F.; Guarner, F.; Gueimonde, M.; Rodríguez, J.M.; Saenz de Pipaon, M.; Sanz, Y. Gut Microbes and Health. Gastroenterol. Y Hepatol. (Engl. Ed.) 2021, 44, 519–535. [Google Scholar] [CrossRef]
- Kelso, A.L. The Effects of Aging on Gut Microbiome Composition and Association with Age-Related Disease States: A Literature Review. Undergrad. Res. Nat. Clin. Sci. Technol. J. 2024, 8, 1–10. [Google Scholar] [CrossRef]
- Andoh, A. Physiological Role of Gut Microbiota for Maintaining Human Health. Digestion 2016, 93, 176–181. [Google Scholar] [CrossRef]
- Pushpanathan, P.; Mathew, G.; Selvarajan, S.; Seshadri, K.; Srikanth, P. Gut Microbiota and Its Mysteries. Indian J. Med. Microbiol. 2019, 37, 268–277. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Butel, M.J.; Waligora-Dupriet, A.J.; Wydau-Dematteis, S. The Developing Gut Microbiota and Its Consequences for Health. J. Dev. Orig. Health Dis. 2018, 9, 590–597. [Google Scholar] [CrossRef]
- Randeni, N.; Bordiga, M.; Xu, B. A Comprehensive Review of the Triangular Relationship among Diet–Gut Microbiota–Inflammation. Int. J. Mol. Sci. 2024, 25, 9366. [Google Scholar] [CrossRef] [PubMed]
- Bibbò, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The Role of Diet on Gut Microbiota Composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar] [PubMed]
- Jardon, K.M.; Canfora, E.E.; Goossens, G.H.; Blaak, E.E. Dietary Macronutrients and the Gut Microbiome: A Precision Nutrition Approach to Improve Cardiometabolic Health. Gut 2022, 71, 1214–1226. [Google Scholar] [CrossRef] [PubMed]
- Bolte, L.A.; Vich Vila, A.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.E.; Fu, J.; et al. Long-Term Dietary Patterns Are Associated with pro-Inflammatory and Anti-Inflammatory Features of the Gut Microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Roy, C.I.L. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef]
- Del Chierico, F.; Vernocchi, P.; Dallapiccola, B.; Putignani, L. Mediterranean Diet and Health: Food Effects on Gut Microbiota and Disease Control. Int. J. Mol. Sci. 2014, 15, 11678–11699. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The Role of the Gut Microbiota in Nutrition and Health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef]
- Flint, H.J. The Impact of Nutrition on the Human Microbiome. Nutr. Rev. 2012, 70, S10–S13. [Google Scholar] [CrossRef]
- Toward, R.E.; Walton, G.E.; Gibson, G.R. Immunosenescence and the Gut Microbiota: The Role of Probiotics and Prebiotics. Nutr. Aging 2012, 1, 167–180. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-Level Adherence to a Mediterranean Diet Beneficially Impacts the Gut Microbiota and Associated Metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Mitsou, E.K.; Kakali, A.; Antonopoulou, S.; Mountzouris, K.C.; Yannakoulia, M.; Panagiotakos, D.B.; Kyriacou, A. Adherence to the Mediterranean Diet Is Associated with the Gut Microbiota Pattern and Gastrointestinal Characteristics in an Adult Population. Br. J. Nutr. 2017, 117, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2021, 13, 7. [Google Scholar] [CrossRef]
- Suriano, F.; Vieira-Silva, S.; Falony, G.; de Wouters d’Oplinter, A.; Paone, P.; Delzenne, N.M.; Everard, A.; Raes, J.; Van Hul, M.; Cani, P.D. Fat and Not Sugar as the Determining Factor for Gut Microbiota Changes, Obesity, and Related Metabolic Disorders in Mice. Am. J. Physiol. Endocrinol. Metab. 2023, 324, E85–E96. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef] [PubMed]
- González Olmo, B.M.; Butler, M.J.; Barrientos, R.M. Evolution of the Human Diet and Its Impact on Gut Microbiota, Immune Responses, and Brain Health. Nutrients 2021, 13, 196. [Google Scholar] [CrossRef]
- Horne, R.G.; Yu, Y.; Zhang, R.; Abdalqadir, N.; Rossi, L.; Surette, M.; Sherman, P.M.; Adeli, K. High Fat-High Fructose Diet-Induced Changes in the Gut Microbiota Associated with Dyslipidemia in Syrian Hamsters. Nutrients 2020, 12, 3557. [Google Scholar] [CrossRef]
- Djekic, D.; Shi, L.; Brolin, H.; Carlsson, F.; Särnqvist, C.; Savolainen, O.; Cao, Y.; Bäckhed, F.; Tremaroli, V.; Landberg, R.; et al. Effects of a Vegetarian Diet on Cardiometabolic Risk Factors, Gut Microbiota, and Plasma Metabolome in Subjects with Ischemic Heart Disease: A Randomized, Crossover Study. J. Am. Heart Assoc. 2020, 9, e016518. [Google Scholar] [CrossRef]
- do Rosario, V.A.; Fernandes, R.; de Trindade, E.B.S.M. Vegetarian Diets and Gut Microbiota: Important Shifts in Markers of Metabolism and Cardiovascular Disease. Nutr. Rev. 2016, 74, 444–454. [Google Scholar] [CrossRef]
- Wong, M.W.; Yi, C.H.; Liu, T.T.; Lei, W.Y.; Hung, J.S.; Lin, C.L.; Lin, S.Z.; Chen, C.L. Impact of Vegan Diets on Gut Microbiota: An Update on the Clinical Implications. Tzu Chi Med. J. 2018, 30, 200–203. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean Diet Intervention Alters the Gut Microbiome in Older People Reducing Frailty and Improving Health Status: The NU-AGE 1-Year Dietary Intervention across Five European Countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Effects of the Mediterranean Diet on Health and Gut Microbiota. Nutrients 2023, 15, 2150. [Google Scholar] [CrossRef]
- Las Heras, V.; Melgar, S.; MacSharry, J.; Gahan, C.G.M. The Influence of the Western Diet on Microbiota and Gastrointestinal Immunity. Annu. Rev. Food Sci. Technol. 2022, 13, 489–512. [Google Scholar] [CrossRef] [PubMed]
- Martinez, K.B.; Leone, V.; Chang, E.B. Western Diets, Gut Dysbiosis, and Metabolic Diseases: Are They Linked? Gut Microbes 2017, 8, 130–142. [Google Scholar] [CrossRef] [PubMed]
- De Siena, M.; Raoul, P.; Costantini, L.; Scarpellini, E.; Cintoni, M.; Gasbarrini, A.; Rinninella, E.; Mele, M.C. Food Emulsifiers and Metabolic Syndrome: The Role of the Gut Microbiota. Foods 2022, 11, 2205. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z. Gut Microbiota: An Important Link between Western. Nutrients 2019, 11, 10–12. [Google Scholar] [CrossRef]
- Rodriguez-Castaño, G.P.; Caro-Quintero, A.; Reyes, A.; Lizcano, F. Advances in Gut Microbiome Research, Opening New Strategies to Cope with a Western Lifestyle. Front. Genet. 2017, 7, 224. [Google Scholar] [CrossRef]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef]
- Shen, X.; Tilves, C.; Kim, H.; Tanaka, T.; Spira, A.P.; Chia, C.W.; Talegawkar, S.A.; Ferrucci, L.; Mueller, N.T. Plant-Based Diets and the Gut Microbiome: Findings from the Baltimore Longitudinal Study of Aging. Am. J. Clin. Nutr. 2024, 119, 628–638. [Google Scholar] [CrossRef]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef]
- Xiao, W.; Zhang, Q.; Yu, L.; Tian, F.; Chen, W.; Zhai, Q. Effects of Vegetarian Diet-Associated Nutrients on Gut Microbiota and Intestinal Physiology. Food Sci. Hum. Wellness 2022, 11, 208–217. [Google Scholar] [CrossRef]
- Trefflich, I.; Jabakhanji, A.; Menzel, J.; Blaut, M.; Michalsen, A.; Lampen, A.; Abraham, K.; Weikert, C. Is a Vegan or a Vegetarian Diet Associated with the Microbiota Composition in the Gut? Results of a New Cross-Sectional Study and Systematic Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2990–3004. [Google Scholar] [CrossRef] [PubMed]
- Losasso, C.; Eckert, E.M.; Mastrorilli, E.; Villiger, J.; Mancin, M.; Patuzzi, I.; Di Cesare, A.; Cibin, V.; Barrucci, F.; Pernthaler, J.; et al. Assessing the Influence of Vegan, Vegetarian and Omnivore Oriented Westernized Dietary Styles on Human Gut Microbiota: A Cross Sectional Study. Front. Microbiol. 2018, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Losno, E.A.; Sieferle, K.; Perez-Cueto, F.J.A.; Ritz, C. Vegan Diet and the Gut Microbiota Composition in Healthy Adults. Nutrients 2021, 13, 2402. [Google Scholar] [CrossRef]
- Sakkas, H.; Bozidis, P.; Touzios, C.; Kolios, D.; Athanasiou, G.; Athanasopoulou, E.; Gerou, I.; Gartzonika, C. Nutritional Status and the Influence of the Vegan Diet on the Gut Microbiota and Human Health. Medicina 2020, 56, 88. [Google Scholar] [CrossRef]
- Glick-Bauer, M.; Yeh, M.C. The Health Advantage of a Vegan Diet: Exploring the Gut Microbiota Connection. Nutrients 2014, 6, 4822–4838. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Neyrinck, A.M.; Delzenne, N.M. Changes in Gut Microbiota Control Metabolic Diet–Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Mujico, J.R.; Baccan, G.C.; Gheorghe, A.; Díaz, L.E.; Marcos, A. Changes in Gut Microbiota Due to Supplemented Fatty Acids in Diet-Induced Obese Mice. Br. J. Nutr. 2013, 110, 711–720. [Google Scholar] [CrossRef]
- De La Serre, C.B.; Ellis, C.L.; Lee, J.; Hartman, A.L.; Rutledge, J.C.; Raybould, H.E. Propensity to High-Fat Diet-Induced Obesity in Rats Is Associated with Changes in the Gut Microbiota and Gut Inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, 440–448. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, X.; Luo, T.; Wang, D.; Sun, Y.; Dai, J. Effects of Short-Term Dietary Fiber Intervention on Gut Microbiota in Young Healthy People. Diabetes Metab. Syndr. Obes. 2021, 14, 3507–3516. [Google Scholar] [CrossRef] [PubMed]
- Tap, J.; Furet, J.P.; Bensaada, M.; Philippe, C.; Roth, H.; Rabot, S.; Lakhdari, O.; Lombard, V.; Henrissat, B.; Corthier, G.; et al. Gut Microbiota Richness Promotes Its Stability upon Increased Dietary Fibre Intake in Healthy Adults. Environ. Microbiol. 2015, 17, 4954–4964. [Google Scholar] [CrossRef] [PubMed]
- Drabińska, N.; Jarocka-Cyrta, E.; Markiewicz, L.H.; Krupa-Kozak, U. The Effect of Oligofructose-Enriched Inulin on Faecal Bacterial Counts and Microbiota-Associated Characteristics in Celiac Disease Children Following a Gluten-Free Diet: Results of a Randomized, Placebo-Controlled Trial. Nutrients 2018, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Lei, O.K.; Nie, J.; Shi, Q.; Xu, Y.; Kong, Z. Effects of Low-Carbohydrate Diet and Exercise Training on Gut Microbiota. Front. Nutr. 2022, 9, 884550. [Google Scholar] [CrossRef]
- Kostovcikova, K.; Coufal, S.; Galanova, N.; Fajstova, A.; Hudcovic, T.; Kostovcik, M.; Prochazkova, P.; Zakostelska, Z.J.; Cermakova, M.; Sediva, B.; et al. Diet Rich in Animal Protein Promotes Pro-Inflammatory Macrophage Response and Exacerbates Colitis in Mice. Front. Immunol. 2019, 10, 919. [Google Scholar] [CrossRef]
- Vidal-Lletjós, S.; Andriamihaja, M.; Blais, A.; Grauso, M.; Lepage, P.; Davila, A.M.; Viel, R.; Gaudichon, C.; Leclerc, M.; Blachier, F.; et al. Dietary Protein Intake Level Modulates Mucosal Healing and Mucosa-Adherent Microbiota in Mouse Model of Colitis. Nutrients 2019, 11, 514. [Google Scholar] [CrossRef]
- Kiilerich, P.; Myrmel, L.S.; Fjære, E.; Hao, Q.; Hugenholtz, F.; Sonne, S.B.; Derrien, M.; Pedersen, L.M.; Petersen, R.K.; Mortensen, A.; et al. Effect of a Long-Term High-Protein Diet on Survival, Obesity Development, and Gut Microbiota in Mice. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E886–E899. [Google Scholar] [CrossRef]
- Huda, M.N.; Ahmad, S.M.; Kalanetra, K.M.; Taft, D.H.; Alam, M.J.; Khanam, A.; Raqib, R.; Underwood, M.A.; Mills, D.A.; Stephensen, C.B. Neonatal Vitamin A Supplementation and Vitamin A Status Are Associated with Gut Microbiome Composition in Bangladeshi Infants in Early Infancy and at 2 Years of Age. J. Nutr. 2019, 149, 1075–1088. [Google Scholar] [CrossRef]
- Garg, M.; Hendy, P.; Ding, J.N.; Shaw, S.; Hold, G.; Hart, A. The Effect of Vitamin D on Intestinal Inflammation and Faecal Microbiota in Patients with Ulcerative Colitis. J. Crohn’s Colitis 2018, 12, 963–972. [Google Scholar] [CrossRef]
- Trautvetter, U.; Camarinha-Silva, A.; Jahreis, G.; Lorkowski, S.; Glei, M. High Phosphorus Intake and Gut-Related Parameters-Results of a Randomized Placebo-Controlled Human Intervention Study. Nutr. J. 2018, 17, 1–11. [Google Scholar] [CrossRef]
- Shen, H.; Han, J.; Li, Y.; Lu, C.; Zhou, J.; Li, Y.; Su, X. Different Host-Specific Responses in Thyroid Function and Gut Microbiota Modulation between Diet-Induced Obese and Normal Mice given the Same Dose of Iodine. Appl. Microbiol. Biotechnol. 2019, 103, 3537–3547. [Google Scholar] [CrossRef]
- Marques, C.; Fernandes, I.; Meireles, M.; Faria, A.; Spencer, J.P.E.; Mateus, N.; Calhau, C. Gut Microbiota Modulation Accounts for the Neuroprotective Properties of Anthocyanins. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Q.; Zheng, M.; Hao, S.; Lum, J.S.; Chen, X.; Huang, X.F.; Yu, Y.; Zheng, K. Supplement of Microbiota-Accessible Carbohydrates Prevents Neuroinflammation and Cognitive Decline by Improving the Gut Microbiota-Brain Axis in Diet-Induced Obese Mice. J. Neuroinflam. 2020, 17, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Bautista, S.; Omaña-Covarrubias, A.; Nez-Castro, A.T.; López-Pontigo, L.; Pimentel-Pérez, M.; Chávez-Mejía, A. Impact of Gut Microbiota on Aging and Frailty: A Narrative Review of the Literature. Geriatrics 2024, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.I.; Isobe, K.; Martiny, J.B.H. Short-Term Dietary Fiber Interventions Produce Consistent Gut Microbiome Responses across Studies. mSystems 2024, 9, e0013324. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef]
- Jia, X.; Chen, Q.; Wu, H.; Liu, H.; Jing, C.; Gong, A.; Zhang, Y. Exploring a Novel Therapeutic Strategy: The Interplay between Gut Microbiota and High-Fat Diet in the Pathogenesis of Metabolic Disorders. Front. Nutr. 2023, 10, 1291853. [Google Scholar] [CrossRef]
- Hryckowian, A.J.; Van Treuren, W.; Smits, S.A.; Davis, N.M.; Gardner, J.O.; Bouley, D.M.; Sonnenburg, J.L. Microbiota-Accessible Carbohydrates Suppress Clostridium Difficile Infection in a Murine Model. Nat. Microbiol. 2018, 3, 662–669. [Google Scholar] [CrossRef]
- Moskalev, A. Nutritional Regulation of Aging and Longevity; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; ISBN 9783030830175. [Google Scholar]
- Rinninella, E.; Mele, M.C.; Merendino, N.; Cintoni, M.; Anselmi, G.; Caporossi, A.; Gasbarrini, A.; Minnella, A.M. The Role of Diet, Micronutrients and the Gut Microbiota in Age-Related Macular Degeneration: New Perspectives from the Gut–Retina Axis. Nutrients 2018, 10, 1677. [Google Scholar] [CrossRef]
- Takesh, S.; Parvani, M.; Banitalebi, E. The Impact of Gut Microbiome Changes on Health and Disease in Older Adults. Life Sci. Stud. J. 2025, 3, 13–32. [Google Scholar] [CrossRef]
- Perler, B.K.; Friedman, E.S.; Wu, G.D. The Role of the Gut Microbiota in the Relationship Between Diet and Human Health. Annu. Rev. Physiol. 2023, 85, 449–468. [Google Scholar] [CrossRef]
- Aziz, T.; Hussain, N.; Hameed, Z.; Lin, L. Elucidating the Role of Diet in Maintaining Gut Health to Reduce the Risk of Obesity, Cardiovascular and Other Age-Related Inflammatory Diseases: Recent Challenges and Future Recommendations. Gut Microbes 2024, 16, 2297864. [Google Scholar] [CrossRef] [PubMed]
- Newman, T.M.; Clear, K.Y.J.; Wilson, A.S.; Soto-Pantoja, D.R.; Ochs-Balcom, H.M.; Cook, K.L. Early-Life Dietary Exposures Mediate Persistent Shifts in the Gut Microbiome and Visceral Fat Metabolism. Am. J. Physiol. Cell Physiol. 2023, 324, C644–C657. [Google Scholar] [CrossRef] [PubMed]
- Yadav, G.V. Dietary Habits and Gut Microbiome: An Influence on Gut Brain Axis. J. Infect. Dis. Microbiol. 2023, 1, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-Ketogenic Diet Modulates Gut Microbiome and Short-Chain Fatty Acids in Association with Alzheimer’s Disease Markers in Subjects with Mild Cognitive Impairment. eBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Di Somma, C.; Maisto, M.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine N-Oxide, Mediterranean Diet, and Nutrition in Healthy, Normal-Weight Adults: Also a Matter of Sex? Nutrition 2019, 62, 7–17. [Google Scholar] [CrossRef]
- Zhang, X.; Gérard, P. Diet-Gut Microbiota Interactions on Cardiovascular Disease. Comput. Struct. Biotechnol. J. 2022, 20, 1528–1540. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z.; Yu, H.; Lu, Y.; Yu, W.; Miao, M.; Shi, H. Anti-Aging Effects of a Functional Food via the Action of Gut Microbiota. Aging 2021, 13, 17880–17900. [Google Scholar] [CrossRef]
- Kadyan, S.; Sharma, A.; Arjmandi, B.H.; Singh, P.; Nagpal, R. Prebiotic Potential of Dietary Beans and Pulses and Their Resistant Starch for Aging-Associated Gut and Metabolic Health. Nutrients 2022, 14, 1726. [Google Scholar] [CrossRef]
- Graf, D.; Monk, J.M.; Lepp, D.; Wu, W.; McGillis, L.; Roberton, K.; Brummer, Y.; Tosh, S.M.; Power, K.A. Cooked Red Lentils Dose-Dependently Modulate the Colonic Microenvironment in Healthy C57Bl,/6 Male Mice. Nutrients 2019, 11, 1853. [Google Scholar] [CrossRef]
- Boachie, R.T.; Capuano, E.; Oliviero, T.; Udenigwe, C.C.; Fogliano, V. Undigested Glycated Lentil Proteins Modulate the Gut Microbiota Profile but Not the Metabolites in Vitro. J. Funct. Foods 2023, 107, 105667. [Google Scholar] [CrossRef]
- Dostal Webster, A.; Staley, C.; Hamilton, M.J.; Huang, M.; Fryxell, K.; Erickson, R.; Kabage, A.J.; Sadowsky, M.J.; Khoruts, A. Influence of Short-Term Changes in Dietary Sulfur on the Relative Abundances of Intestinal Sulfate-Reducing Bacteria. Gut Microbes 2019, 10, 447–457. [Google Scholar] [CrossRef]
- Uebanso, T.; Shimohata, T.; Mawatari, K.; Takahashi, A. Functional Roles of B-Vitamins in the Gut and Gut Microbiome. Mol. Nutr. Food Res. 2020, 64, 2000426. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Mafra, D.; Shiels, P.G.; Hackeng, T.M.; Stenvinkel, P.; Schurgers, L.J. Vitamin K and Hallmarks of Ageing: Focus on Diet and Gut Microbiome. Nutrients 2023, 15, 2727. [Google Scholar] [CrossRef] [PubMed]
- Baldi, S.; Pagliai, G.; Di Gloria, L.; Pallecchi, M.; Barca, F.; Pieri, B.; Bartolucci, G.; Ramazzotti, M.; Amedei, A.; Palendri, G.; et al. Beneficial Effects of Micronutrient Supplementation in Restoring the Altered Microbiota and Gut-Retina Axis in Patients with Neovascular Age-Related Macular Degeneration-A Randomized Clinical Trial. Nutrients 2024, 16, 3971. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, B.S.; Marimuthu, M.M.C.; Sundaram, V.A.; Saravanan, B.; Chandrababu, P.; Chopra, H.; Malik, T. Micro Nutrients as Immunomodulators in the Ageing Population: A Focus on Inflammation and Autoimmunity. Immun. Ageing 2024, 21, 88. [Google Scholar] [CrossRef]
- Reyes-gavil, C.G.D.L.; Gonzalez, S.; Gueimonde, M. Age-Associated Changes in Gut Microbiota and Dietary Components Related with the Immune System. Nutrients 2019, 11, 1765. [Google Scholar] [CrossRef]
- Davis II, E.; Wong, C.; Bouranis, J.; Sharpton, T.; Ho, E. Zinc Status Elicits Age-Dependent Effects in the Gut Microbiome. Curr. Dev. Nutr. 2020, 4, nzaa062_009. [Google Scholar] [CrossRef]
- Zhang, F.; Ye, J.; Zhu, X.; Wang, L.; Gao, P.; Shu, G.; Jiang, Q.; Wang, S. Anti-Obesity Effects of Dietary Calcium: The Evidence and Possible Mechanisms. Int. J. Mol. Sci. 2019, 20, 3072. [Google Scholar] [CrossRef]
- Rahbar Saadat, Y.; Niknafs, B.; Hosseiniyan Khatibi, S.M.; Ardalan, M.; Majdi, H.; Bahmanpoor, Z.; Abediazar, S.; Zununi Vahed, S. Gut Microbiota; an Overlooked Effect of Phosphate Binders. Eur. J. Pharmacol. 2020, 868, 172892. [Google Scholar] [CrossRef]
- Fang, S.; Zhuo, Z.; Yu, X.; Wang, H.; Feng, J. Oral Administration of Liquid Iron Preparation Containing Excess Iron Induces Intestine and Liver Injury, Impairs Intestinal Barrier Function and Alters the Gut Microbiota in Rats. J. Trace Elem. Med. Biol. 2018, 47, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, F.; Moranta, D.; Tejada, S.; Jiménez, M.; Esteban, S. Impact of Gut Microbiota in Brain Ageing: Polyphenols as Beneficial Modulators. Antioxidants 2023, 12, 812. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Stasiak, M.; Oniszczuk, A. Beneficial Effects of Phenolic Compounds on Gut Microbiota and Metabolic Syndrome. Int. J. Mol. Sci. 2021, 22, 3715. [Google Scholar] [CrossRef] [PubMed]
- Pereira, Q.C.; Fortunato, I.M.; Oliveira, F.d.S.; Alvarez, M.C.; dos Santos, T.W.; Ribeiro, M.L. Polyphenolic Compounds: Orchestrating Intestinal Microbiota Harmony during Aging. Nutrients 2024, 16, 1066. [Google Scholar] [CrossRef]
- Pradhan, S.; Blanton, C.; Ochoa-Reparaz, J.; Bhattarai, N.; Sharma, K. Herbs and Spices: Modulation of Gut Microbiota for Healthy Aging. Gastroenterol. Insights 2024, 15, 447–458. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, M.; Tu, X.; Mo, X.; Zhang, L.; Yang, B.; Wang, F.; Kim, Y.-B.; Huang, C.; Chen, L.; et al. Dietary Polyphenols as Anti-Aging Agents: Targeting the Hallmarks of Aging. Nutrients 2024, 16, 3305. [Google Scholar] [CrossRef]
- Ross, F.C.; Mayer, D.E.; Horn, J.; Cryan, J.F.; Del Rio, D.; Randolph, E.; Gill, C.I.R.; Gupta, A.; Ross, R.P.; Stanton, C.; et al. Potential of Dietary Polyphenols for Protection from Age-Related Decline and Neurodegeneration: A Role for Gut Microbiota? Nutr. Neurosci. 2024, 27, 1058–1076. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Parise, A.; Meschi, T. Accounting Gut Microbiota as the Mediator of Beneficial Effects of Dietary (Poly)Phenols on Skeletal Muscle in Aging. Nutrients 2023, 15, 2367. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of Probiotics on Gut Microbiota: An Overview. Int. J. Mol. Sci. 2024, 25, 6022. [Google Scholar] [CrossRef]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, Gut Microbiota, and Their Influence on Host Health and Disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Butel, M.J. Probiotics, Gut Microbiota and Health. Med. Mal. Infect. 2014, 44, 1–8. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. Biomed Res. Int. 2018, 2018, 9478630. [Google Scholar] [CrossRef]
- Hemarajata, P.; Versalovic, J. Effects of Probiotics on Gut Microbiota: Mechanisms of Intestinal Immunomodulation and Neuromodulation. Ther. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Lin, C.S.; Chang, C.J.; Lu, C.C.; Martel, J.; Ojcius, D.M.; Ko, Y.F.; Young, J.D.; Lai, H.C. Impact of the Gut Microbiota, Prebiotics, and Probiotics on Human Health and Disease. Biomed. J. 2014, 37, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Du, P.; Guo, Y.; Xie, Y.; Yu, H.; Yao, W.; Cheng, Y.; Qian, H. Extraction, Characterization of Aloe Polysaccharides and the in-Depth Analysis of Its Prebiotic Effects on Mice Gut Microbiota. Carbohydr. Polym. 2021, 261, 117874. [Google Scholar] [CrossRef] [PubMed]
- Toward, R.E.; Montandon, S.L.; Walton, G.E.; Gibson, G.R. Effect of Prebiotics on the Human Gut Microbiota of Elderly Persons. Gut Microbes 2012, 3, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Paone, P.; Suriano, F.; Jian, C.; Korpela, K.; Delzenne, N.M.; Van Hul, M.; Salonen, A.; Cani, P.D. Prebiotic Oligofructose Protects against High-Fat Diet-Induced Obesity by Changing the Gut Microbiota, Intestinal Mucus Production, Glycosylation and Secretion. Gut Microbes 2022, 14, 2152307. [Google Scholar] [CrossRef]
- Leyrolle, Q.; Cserjesi, R.; Mulders, M.D.; Zamariola, G.; Hiel, S.; Gianfrancesco, M.A.; Portheault, D.; Amadieu, C.; Bindels, L.B.; Leclercq, S.; et al. Prebiotic Effect on Mood in Obese Patients Is Determined by the Initial Gut Microbiota Composition: A Randomized, Controlled Trial. Brain Behav. Immun. 2021, 94, 289–298. [Google Scholar] [CrossRef]
- Mello, A.M.; Paroni, G.; Daragjati, J.; Pilotto, A. Gastrointestinal Microbiota and Their Contribution to Healthy Aging. Dig. Dis. 2016, 34, 194–201. [Google Scholar] [CrossRef]
- Rasouli-Saravani, A.; Jahankhani, K.; Moradi, S.; Gorgani, M.; Shafaghat, Z.; Mirsanei, Z.; Mehmandar, A.; Mirzaei, R. Role of Microbiota Short-Chain Fatty Acid Chains in the Pathogenesis of Autoimmune Diseases. Biomed. Pharmacother. 2023, 162, 114620. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef]
- Zhao, M.; Chu, J.; Feng, S.; Guo, C.; Xue, B.; He, K.; Li, L. Immunological Mechanisms of Inflammatory Diseases Caused by Gut Microbiota Dysbiosis: A Review. Biomed. Pharmacother. 2023, 164, 114985. [Google Scholar] [CrossRef] [PubMed]
- Caldarelli, M.; Rio, P.; Marrone, A.; Giambra, V.; Gasbarrini, A.; Gambassi, G.; Cianci, R. Inflammaging: The Next Challenge—Exploring the Role of Gut Microbiota, Environmental Factors, and Sex Differences. Biomedicines 2024, 12, 1716. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, D.; Ostrov, B.E. The Impact of the Microbiome on Immunosenescence. Immunol. Investig. 2018, 47, 801–811. [Google Scholar] [CrossRef] [PubMed]
- De Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut Microbiome and Health: Mechanistic Insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Paray, B.A.; Albeshr, M.F.; Jan, A.T.; Rather, I.A. Leaky Gut and Autoimmunity: An Intricate Balance in Individuals Health and the Diseased State. Int. J. Mol. Sci. 2020, 21, 9770. [Google Scholar] [CrossRef]
- Tajik, N.; Frech, M.; Schulz, O.; Schälter, F.; Lucas, S.; Azizov, V.; Dürholz, K.; Steffen, F.; Omata, Y.; Rings, A.; et al. Targeting Zonulin and Intestinal Epithelial Barrier Function to Prevent Onset of Arthritis. Nat. Commun. 2020, 11, 1995. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’sullivan, O.; et al. Gut Microbiota Composition Correlates with Diet and Health in the Elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in Elderly People. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Gieryńska, M.; Szulc-Dąbrowska, L.; Struzik, J.; Mielcarska, M.B.; Gregorczyk-Zboroch, K.P. Integrity of the Intestinal Barrier: The Involvement of Epithelial Cells and Microbiota—A Mutual Relationship. Animals 2022, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.-X.; Ma, C.; Irge, D.D.; Li, S.-M.; Chen, S.-M.; Zhou, S.-X.; Zhao, X.-X.; Li, H.-Y.; Li, J.-Y.; Yang, Y.-M.; et al. Gastrodia Elata and Parishin Ameliorate Aging Induced ‘Leaky Gut’ in Mice: Correlation with Gut Microbiota. Biomed. J. 2023, 46, 100547. [Google Scholar] [CrossRef] [PubMed]
- Schoultz, I.; Keita, Å.V. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Gao, Y.; Zeng, B.; Fan, X.; Yang, D.; Yang, M. Effects of Anti-Aging Interventions on Intestinal Microbiota. Gut Microbes 2021, 13, 1994835. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, K.; Wei, J.; Ding, Y.; Wang, X.; Hou, H.; Wu, J.; Liu, T.; Wang, B.; Cao, H. Gut Microbiota-Derived Short-Chain Fatty Acids Regulate Gastrointestinal Tumor Immunity: A Novel Therapeutic Strategy? Front. Immunol. 2023, 14, 1158200. [Google Scholar] [CrossRef]
- Capurso, A.; Crepaldi, G.; Capurso, C. The Mediterranean Diet: A Pathway to Successful Aging. Aging Clin. Exp. Res. 2020, 32, 1187–1188. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Orfanos, P.; Norat, T.; Bueno-de-Mesquita, B.; Ocké, M.C.; Peeters, P.H.M.; van der Schouw, Y.T.; Boeing, H.; Hoffmann, K.; Boffetta, P.; et al. Modified Mediterranean Diet and Survival: EPIC-Elderly Prospective Cohort Study. Br. Med. J. 2005, 330, 991–995. [Google Scholar] [CrossRef]
- Edirisinghe, I.; Burton-Freeman, B.M. Age Associated Endothelial Dysfunction: Role of Oxidative Stress, Inflammation and Western Diet. Nutr. Aging 2014, 2, 197–211. [Google Scholar] [CrossRef]
- Andreo-López, M.C.; Contreras-Bolívar, V.; Muñoz-Torres, M.; García-Fontana, B.; García-Fontana, C. Influence of the Mediterranean Diet on Healthy Aging. Int. J. Mol. Sci. 2023, 24, 4491. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Raubenheimer, D.; Solon-Biet, S.; de Cabo, R.; Simpson, S.J. Does Diet Influence Aging? Evidence from Animal Studies. J. Intern. Med. 2024, 295, 400–415. [Google Scholar] [CrossRef]
- Yeung, S.S.Y.; Kwan, M.; Woo, J. Healthy Diet for Healthy Aging. Nutrients 2021, 13, 4310. [Google Scholar] [CrossRef]
- Racine, E.; Troyer, J.L.; Warren-Findlow, J.; McAuley, W.J. The Effect of Medical Nutrition Therapy on Changes in Dietary Knowledge and DASH Diet Adherence in Older Adults with Cardiovascular Disease. J. Nutr. Health Aging 2011, 15, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Key, M.N.; Szabo-Reed, A.N. Impact of Diet and Exercise Interventions on Cognition and Brain Health in Older Adults: A Narrative Review. Nutrients 2023, 15, 2495. [Google Scholar] [CrossRef] [PubMed]
- Daniel, G.D.; Chen, H.; Bertoni, A.G.; Rapp, S.R.; Fitzpatrick, A.L.; Luchsinger, J.A.; Wood, A.C.; Hughes, T.M.; Burke, G.L.; Hayden, K.M. DASH Diet Adherence and Cognitive Function: Multi-Ethnic Study of Atherosclerosis. Clin. Nutr. ESPEN 2021, 46, 223–231. [Google Scholar] [CrossRef] [PubMed]
- McGrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and Inflammation in Cognitive Ageing and Alzheimer’s Disease [Dieta e Inflamación En El Envejecimiento Cognitivo y La Enfermedad de Alzheimer]. Curr. Nutr. Rep. 2019, 8, 53–65. [Google Scholar] [CrossRef]
- Huang, L.; Tao, Y.; Chen, H.; Chen, X.; Shen, J.; Zhao, C.; Xu, X.; He, M.; Zhu, D.; Zhang, R.; et al. Mediterranean-Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay (MIND) Diet and Cognitive Function and Its Decline: A Prospective Study and Meta-Analysis of Cohort Studies. Am. J. Clin. Nutr. 2023, 118, 174–182. [Google Scholar] [CrossRef]
- Holthaus, T.A.; Kashi, M.; Cannavale, C.N.; Edwards, C.G.; Aguiñaga, S.; Walk, A.D.; Burd, N.A.; Holscher, H.D.; Khan, N.A. MIND Dietary Pattern Adherence Is Selectively Associated with Cognitive Processing Speed in Middle-Aged Adults. J. Nutr. 2022, 152, 2941–2949. [Google Scholar] [CrossRef]
- Barnes, L.L.; Dhana, K.; Liu, X.; Carey, V.J.; Ventrelle, J.; Johnson, K.; Hollings, C.S.; Bishop, L.; Laranjo, N.; Stubbs, B.J.; et al. Trial of the MIND Diet for Prevention of Cognitive Decline in Older Persons. N. Engl. J. Med. 2023, 389, 602–611. [Google Scholar] [CrossRef]
- Ward, N.A.; Reid-Mccann, R.; Brennan, L.; Cardwell, C.R.; De Groot, C.; Maggi, S.; McCaffrey, N.; McGuinness, B.; McKinley, M.C.; Noale, M.; et al. Effects of PROtein Enriched MEDiterranean Diet and EXercise on Nutritional Status and Cognition in Adults at Risk of Undernutrition and Cognitive Decline: The PROMED-EX Randomised Controlled Trial. BMJ Open 2023, 13, e070689. [Google Scholar] [CrossRef]
- Hu, F.B. Diet Strategies for Promoting Healthy Aging and Longevity: An Epidemiological Perspective. J. Intern. Med. 2024, 295, 508–531. [Google Scholar] [CrossRef]
- Devranis, P.; Vassilopoulou, Ε.; Tsironis, V.; Sotiriadis, P.M.; Chourdakis, M.; Aivaliotis, M.; Tsolaki, M. Mediterranean Diet, Ketogenic Diet or MIND Diet for Aging Populations with Cognitive Decline: A Systematic Review. Life 2023, 13, 173. [Google Scholar] [CrossRef]
- Liu, X.; Morris, M.C.; Dhana, K.; Ventrelle, J.; Johnson, K.; Bishop, L.; Hollings, C.S.; Boulin, A.; Laranjo, N.; Stubbs, B.J.; et al. Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Study: Rationale, Design and Baseline Characteristics of a Randomized Control Trial of the MIND Diet on Cognitive Decline. Contemp. Clin. Trials 2021, 102, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Hur, T.-Y.; Hong, Y. Influence of Altered Gut Microbiota Composition on Aging and Aging-Related Diseases. J. Lifestyle Med. 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mancabelli, L.; Milani, C.; De Biase, R.; Bocchio, F.; Fontana, F.; Lugli, G.A.; Alessandri, G.; Tarracchini, C.; Viappiani, A.; De Conto, F.; et al. Taxonomic and Metabolic Development of the Human Gut Microbiome across Life Stages: A Worldwide Metagenomic Investigation. mSystems 2024, 9, e0129423. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Bastos, P.; Fontes-Villalba, M.; O’Keefe, J.; Lindeberg, S.; Cordain, L. The Western Diet and Lifestyle and Diseases of Civilization. Res. Reports Clin. Cardiol. 2011, 2, 2–15. [Google Scholar] [CrossRef]
- Wachtel-Galor, S.; Siu, P.M.; Benzie, I.F.F. Antioxidants, Vegetarian Diets and Aging. Aging Oxidative Stress Diet. Antioxidants 2014, 81–91. [Google Scholar] [CrossRef]
- Moschen, A.R.; Wieser, V.; Tilg, H. Dietary Factors: Major Regulators of the Gut’s Microbiota. Gut Liver 2012, 6, 411–416. [Google Scholar] [CrossRef]

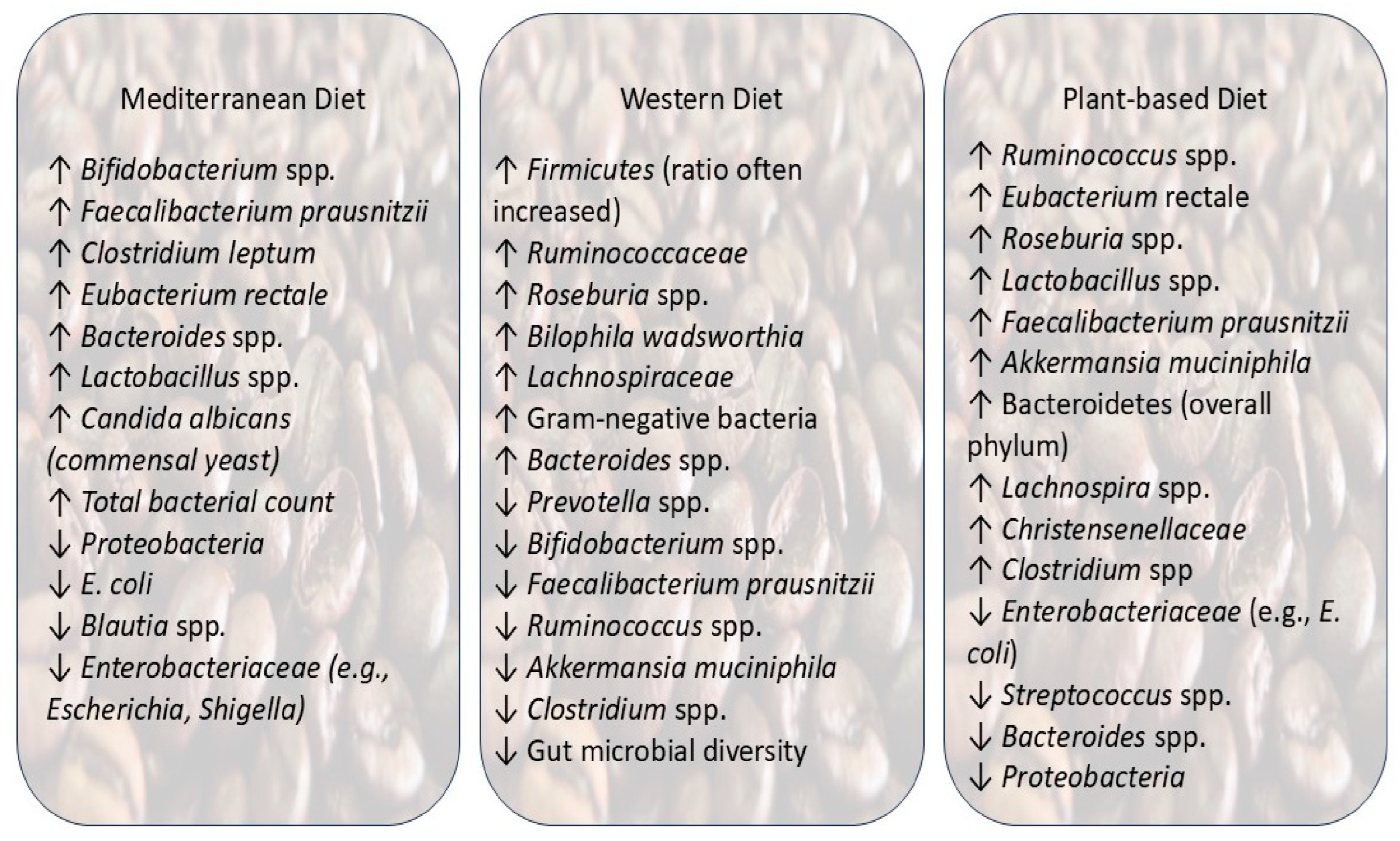


| Diet Pattern | Impact on Gut Microbiota | Production of Metabolites | Impact on Health | References |
|---|---|---|---|---|
| Mediterranean diet | Increased
|
|
| [22] |
|
|
| [23] | |
|
|
| [24] | |
|
|
| [18] | |
| Western diet | Increased
|
|
| [22] |
|
|
| [25] | |
|
|
| [26] | |
|
|
| [27] | |
|
|
| [28] | |
| Plant-based diet |
|
|
| [29] |
|
|
| [30] | |
|
|
| [31] |
| Basic Nutrient | Intervention Description | Test Model | Treatment Conditions | Outcome | Reference |
|---|---|---|---|---|---|
| Dietary fat | A high-fat diet comprising 72% fat (from corn oil and lard), 28% protein, 1% carbohydrate | Male C57bl6/J mice and ob/ob mice (C57bl6 background) | High-fat diet (72% fat) and/or antibiotic treatment (1 g/L ampicillin + 0.5 g/L neomycin in drinking water), n = 13–17 depending on group. Duration: 4 weeks. Location: Université Catholique de Louvain, Belgium, and Rangueil Institute of Molecular Medicine, Toulouse, France | High-fat diet altered gut microbiota, increased intestinal permeability, and elevated plasma lipopolysaccharides (metabolic endotoxemia), leading to inflammation, oxidative stress, adipocyte hypertrophy, insulin resistance, and glucose intolerance | [48] |
| Total groups: control diet (CD), high-fat diet (HFD), HFD + oleic acid-derived compound (HFD-S1), HFD + omega-3 fatty acids (HFD-S2) | Female ICR (CD-1) outbred mice, 8 weeks old at study | HFD-S1: 1500 mg/kg/day oleic acid-derived compound HFD-S2: 3000 mg/kg/day EPA + DHA (omega-3 fatty acids) n = 6–8 mice per cage, 3 cages per group Duration: 8-week HFD induction followed by 7 weeks of supplementation Location: Institute of Food Science, Technology, and Nutrition (ICTAN-CSIC), Madrid, Spain | HFD alone increased gut dysbiosis (increased Firmicutes, Enterobacteriales; reduced Bacteroidetes, Bifidobacterium spp.) HFD-S1 (oleic acid compound) restored Bacteroidetes and Bifidobacterium levels and decreased clostridial cluster XIVa HFD-S2 (omega-3) increased Lactobacillus but had no effect on weight or other microbiota alterations | [49] | |
| Grouped as LF, low-fat diet; DIO-R, diet-induced obesity resistant; DIO-P, diet-induced obesity-prone diets | Male Sprague Dawley rats (initial weight ~262 g) | LF diet: 70% carbohydrate, 20% protein, 10% fat (SAT 25.1%, MUFA 34.7%, PUFA 40.2%), 3.85 kcal/g HF diet: 35% carbohydrate, 20% protein, 45% fat (SAT 36.3%, MUFA 45.3%, PUFA 18.5%), 4.73 kcal/g Duration: 8 or 12 weeks Location: University of California, Davis, CA, USA | Elevated gut inflammation markers in DIO-P Increased intestinal permeability and plasma lipopolysaccharides in DIO-P HF diet altered gut microbiota in all groups (increased Bacteroidales and Clostridiales; reduced total bacterial load), but only DIO-P showed increased Enterobacteriales | [50] | |
| Dietary fiber | Randomized, crossover clinical trial Mixed fiber supplement from Revilife (Nantong Richen Bioengineering Co., Ltd., Nantong City, China) | 12 healthy young adults (6 males, 6 females) aged 22–32 years | 20 g/day mixed dietary fiber (polyglucan, inulin, resistant malt dextrin) for 4 days 4-day washout Total study duration: 16 days (including washout and baseline) Location: Xinjiang Medical University, Urumqi, China | Fiber intervention increased the abundance of Alloprevotella, Parabacteroides, and Parasutterella Decreased the abundance of Adlercreutzia, Anaerovorax, Enterococcus, Intestinibacter, and Ruminococcus2 | [51] |
| Meals are lyophilized and pre-packaged to control fiber intake; fiber source unspecified | Human participants: 19 healthy young adults (9 males, 10 females) aged 19–25 years | 10 g/day or 40 g/day dietary fiber for 5 days, separated by 2-week washout periods Crossed over after the first phase (10 participants: 10 g → 40 g; 9 participants: 40 g → 10 g) All meals prepared and standardized (same ingredients, same soluble/insoluble fiber ratio) Total duration: 6 weeks Location: Grenoble University Hospital and INRA, Jouy-en-Josas, France | Increased Prevotella, Coprococcus, Dorea species and elevated short-chain fatty acids, like caproate and valerate Low richness participants showed more variable microbiota shifts with fiber intervention | [52] | |
| Carbohydrates | Carbohydrate (in the form of oligofructose-enriched inulin). All were on a gluten-free diet (GFD) for at least 6 months prior to the trial | Human participants: 34 pediatric celiac disease patients (62% female), mean age 10 years | Oligofructose-enriched inulin (Synergy 1) (10 g/day), n = 18, and placebo (maltodextrin; 7 g/day), n = 16, as reference/clinical trials (34 pediatric celiac disease patients, 62% females, on a gluten-free diet) Synergy 1 (Orafti®) and placebo supplements were administered orally once daily Duration: 3 months. Location: University of Warmia and Mazury, Olsztyn, Poland | Significant increase in Bifidobacterium count in Synergy 1 group; stable Clostridium leptum count vs. decline in placebo; reduced Lactobacillus in both groups Metabolites: Synergy 1 group showed increased fecal acetate and butyrate, with total SCFAs rising by 31% from baseline | [53] |
| LC (low-carbohydrate) group (n = 11) LC + HIIT: high-intensity interval training (LC-HIIT, n = 13): 10 sprints of 6 s with 9 s rests LC + MICT: moderate-intensity continuous training (LC-MICT, n = 12): 30 min cycling at 50–60% VO2 peak | 50 overweight/obese young Chinese females, age 22.2 ± 3.3 years, BMI 25.1 ± 3.1 kg/m2 | Low-carbohydrate diet (9% carbs, 23% protein, 68% fat of daily energy) maintained for 4 weeks, total daily intake ~1900 kcal LC-HIIT: 20 sessions over 4 weeks (2.5 min/session, sprint cycling) LC-MICT: 30 min continuous cycling at increasing intensity over 4 weeks. Location: University of Macau, China (Participants kept stable daily energy intake; food diaries monitored) | LC increase Phascolarctobacterium LC-HIIT decreased Bifidobacterium Both LC-HIIT and LC-MICT increased Blautia and reduced Alistipes (linked to type 2 diabetes)Changes in genera, like Sutterella and Enterobacter, correlated with body composition metrics Blood pressure changes associated positively with Ruminococcus, Eubacterium, Roseburia and negatively with Faecalibacterium, Bacteroides, and Parabacteroides | [54] | |
| Protein | Mice were weaned directly onto the synthetic diets; a subset received the diet in the parental generation to assess long-term effects | Male BALB/c and RAG2 knockout mice and germ-free mice | aCD (animal protein control): 176 g/kg casein aHPD (animal high protein): 514 g/kg casein pCD (plant protein control): 173 g/kg wheat gluten pHPD (plant high protein): 500 g/kg wheat gluten DSS-induced colitis: 3% dextran sulfate sodium Chronic colitis: 3 cycles of DSS (5 days DSS + 9 days water each) Duration: 3 weeks of diet pre-treatment + acute/chronic colitis phases Location: Institute of Microbiology of the CAS, Prague, Czech Republic | aHPD-fed mice showed increased severity of both acute and chronic DSS colitis, altered microbiota: increased Escherichia, Enterococcus, Streptococcus, reduced Lactobacillus, Bifidobacterium, and increased abundance of Candida tropicalis aHPD mice had reduced bacterial alpha diversity and shifted functional microbial genes (reduced barrier function pathways, increased motility/secretion) | [55] |
| P14: 14% protein diet P30: 30% protein diet P53: 53% protein diet | Male C57BL/6 mice (n = 132 DSS-treated, plus 12 healthy controls) | Isocaloric diets differing only in protein content (milk protein: casein + whey) Treatment started on day 7 (post-inflammation peak) and continued for 3, 6, or 21 days Food and water provided ad libitum Outcomes assessed on days 10, 13, and 28 Location: AgroParisTech and INRA labs, Palaiseau, France | P30 diet improved mucosal healing, reduced intestinal permeability, increased epithelial proliferation P30-fed mice showed increased colonization by butyrate-producing bacteria during resolution phase | [56] | |
| Female C57BL/6J mice | REF: low-fat control diet HFS: high-fat diet (25%) with high sucrose (43%) HFP: high-fat diet (25%) with high protein (43%) Ad libitum feeding from 3 weeks of age until study endpoints (up to ~95 weeks) REF (9% fat, 33% sucrose, 33% protein); HFS (25% fat, 43% sucrose); HFP (25% fat, 43% protein) | Gut microbiota changed significantly with age, especially at 16 months, and aging is linked to decreased firmicutes to Bacteroidetes ratio (in REF and HFP groups) Phylotypes driving age-related shifts included Akkermansia muciniphila, Sphingomonas, Desulfovibrio, and Olsenella Gut microbial changes with age were more prominent than changes due to the protein/sucrose ratio. Survival was lower in HFS mice; HFP mice maintained better longevity, metabolic profile, and microbial diversity—bacterial diversity declined with age across all diet groups | [57] | ||
| Vitamin | Vitamin A | 306 human infants | 50,000 IU vitamin A or placebo, orally within 48 h of birth—no additional interventions during early infancy; follow-up through 15 weeks and again at 2 years—location: Dhaka, Bangladesh | At 2 years, plasma retinol was positively associated with Actinobacteria (especially Bifidobacterium) and Akkermansia in girls Vitamin A supplementation increased Bifidobacterium abundance in boys during early infancy but not in girls Gut microbiota diversity changed with age, and supplementation had sex-specific effects persisting into toddlerhood | [58] |
| Vitamin D | 25 human participants with low vitamin D levels (25(OH)D < 50 nmol/L): 8 with active ulcerative colitis (UC) 9 with inactive UC 8 non-IBD controls | 40,000 IU vitamin D3 (cholecalciferol) per week for 8 weeks Total dose: 320,000 IU over study period Participants assessed pre- and post-intervention for inflammatory markers and gut microbiota composition Location: Harrow, UK | Increase in Enterobacteriaceae abundance after supplementation. No significant shifts in Ruminococcus gnavus, Akkermansia, Bifidobacteria, or SCFA-producing Clostridia. Trends suggested reduced R. gnavus after treatment, but not statistically significant | [59] | |
| Minerals | Phosphorus | 62 healthy adult participants (30 men, 32 women), mean age 29 ± 7 years, mean BMI 24 ± 3 kg/m2 | 2-week placebo run-in for all groups 3 intervention arms for 8 weeks: P1000/Ca0: 1000 mg phosphorus/day P1000/Ca500: 1000 mg phosphorus + 500 mg calcium/day P1000/Ca1000: 1000 mg phosphorus + 1000 mg calcium/day Phosphorus as monosodium phosphate; calcium as calcium carbonate Intake via sherbet powder diluted in water, twice daily | P1000/Ca1000 group (men only) showed a significantly altered gut microbial community compared to Ca0 and Ca500 Clostridium XVIII was more abundant in men in the Ca1000 group No significant changes in microbial diversity in women | [60] |
| Iodine | Female ICR mice (3 weeks old), n = 6 per group Groups: control (standard diet) control + iodine (KIO3) high-fat diet (HFD) HFD + iodine (KIO3) | 18 μg/kg/day potassium iodate (KIO3) via daily oral gavage HFD: 34.9% fat, 5.21 kcal/g; control diet: 4.62% fat, 3.45 kcal/g. Duration: 8 weeks. Iodine supplementation following HFD induction | Iodine increased pathogenic bacteria in obese mice (e.g., Clostridium, Enterococcus, Fusobacterium nucleatum) and reduced probiotics (Lactobacillus, Bifidobacterium, F. prausnitzii) Iodine had opposite effects in normal mice, increasing beneficial microbes and lowering inflammatory bacteria | [61] | |
| Polyphenols | Anthocyanins (blackberry anthocyanin-rich extract/BE) | Male Wistar rats, n = 24 total Groups: control diet (C) control + BE (C + BE) high-fat diet (HF) high-fat diet + BE (HF + BE) | BE: 25 mg/kg/day, delivered in food pellets Duration: 17 weeks total Diets: standard or high-fat diet (60% calories from fat) | BE altered gut microbiota and attenuated neuroinflammation, a hallmark of cognitive aging BE restored microbial diversity disrupted by HF diet, increased Pseudoflavonifractor and Oscillobacter, and reduced pro-inflammatory bacteria (e.g., Ruminococcus). BE also reduced fecal LPS (lipopolysaccharide), hinting at better gut barrier function | [62] |
| Feature | Mediterranean Diet (MD) | DASH Diet | MIND Diet |
|---|---|---|---|
| Overall Aim | Promote longevity and reduce chronic disease risk through balanced, traditional dietary patterns | Prevent and control hypertension while supporting cardiovascular health | Protect brain health and reduce the risk of dementia/Alzheimer’s disease |
| Core Principles | Plant-forward diet, daily olive oil, moderate fish/poultry, limited red meat, moderate wine | High intake of fruits, vegetables, whole grains, low-fat dairy, lean protein, reduced sodium | Hybrid of DASH and MD with targeted emphasis on brain-protective foods |
| Primary Food Sources | Vegetables, fruits, legumes, nuts, whole grains, olive oil, fish, moderate dairy, and wine | Fruits, vegetables, whole grains, low-fat dairy, nuts, seeds, poultry, fish | Leafy greens, berries, nuts, whole grains, olive oil, beans, fish, poultry |
| Restricted Foods | Red/processed meat, refined grains, added sugars, butter, cream | High-sodium foods, red/processed meat, sweets, sugar-sweetened drinks | Red meat, butter/margarine, cheese, pastries, fried/fast food |
| Key Nutrients | Monounsaturated fats, fiber, antioxidants, polyphenols, omega-3s | Potassium, calcium, magnesium, fiber, lean proteins, low sodium | Vitamin E, folate, omega-3s, antioxidants (especially from berries and greens) |
| Lifestyle Factors | Encourages communal eating, seasonal/local foods, physical activity | Portion control, sodium restriction, balanced nutrient intake | Focus on consistent intake of neuroprotective foods rather than calorie restriction |
| Evidence-Based Health Outcomes | Lower risk of cardiovascular disease, diabetes, cancer; improved longevity | Clinically proven to lower blood pressure and cardiovascular risk | Slower cognitive decline, reduced risk of Alzheimer’s and neurodegeneration |
| Diet type | Study Details | Effect on Aging | References |
|---|---|---|---|
| DASH diet | 147 participants, age ≥60, diagnosed with hypertension and/or hyperlipidemia, 12-month duration, randomized into MNT* vs. information-only group, 3 MNT sessions over one year, dietary recalls and knowledge assessments at baseline, 6 months, and 12 months, DASH adherence measured via nutrient scoring, study type: interventional, randomized controlled trial | ↓ blood pressure → ↓ stroke risk by 20–40%, ↓ LDL cholesterol by 1–20.9% → ↓ atherosclerosis progression, ↑ potassium intake → ↓ salt sensitivity, especially in older adults, ↓ saturated fat intake → ↓ cognitive decline risk by 15%, ↑ fiber intake → ↓ inflammation and improved vascular aging, ↓ sodium intake → ↓ arterial stiffness and improved brain perfusion, ↑ DASH adherence → ↑ cardiovascular resilience in aging | [133] |
| Older adults aged ≥60, studies span 6 months to 6 years, DASH diet assessed through food frequency questionnaires and nutrient scoring, cognitive outcomes measured using MMSE (mini-mental state examination), CASL (comprehensive assessment of spoken language), and memory tests | ↑ MMSE scores by 1.3–2.1 points in high adherence groups, ↓ risk of cognitive decline by 11–25% over 4–6 years, ↑ verbal memory performance by 15% with combined DASH and exercise, better vascular aging, ↓ risk of dementia by 20% in long-term adherence | [134] | |
| 4169 participants aged 45 to 84, multi-ethnic sample including White, African American, Hispanic, and Chinese American adults, free of cardiovascular disease at baseline, cognitive assessments conducted in 2011–2012 and 2016–2018, cognitive tests included digit symbol coding, cognitive abilities screening instrument, and digit span, study type was a prospective cohort | Improved processing speed, with digit symbol coding score rising by 1.3 points per 1 sd increase in DASH adherence, global cognition also improved, with cognitive abilities screening score increasing by 0.9 points per 1 sd, working memory showed no change, cognitive decline over five years was slower in participants with higher DASH adherence, aging effects were more pronounced in White and Chinese American groups | [135] | |
| MIND diet | 4066 participants, age ≥55, Chinese adults, median follow-up 3 years, cognitive tests conducted in 1997, 2000, 2004, and 2006, MIND diet score range 0–12, dietary intake assessed via 3-day 24 h recall and household weighing, cognitive function measured using telephone interview for cognitive status-modified, study type: observational, prospective cohort study, meta-analysis included 8 studies with 26,103 participants from China, the United States, and Spain | ↑ global cognitive z-score by 0.110 per 3-point increase in MIND score, ↑ verbal memory score by 0.102 per 3-point increase, ↑ cognitive function by 0.042 per sd increase in meta-analysis, ↓ annual cognitive decline by 0.010 units per sd increase, ↑ cognition equivalent to being 1 year younger per 3-point increase | [136] |
| 207 participants, age 34.1 ± 6.0 years, middle-aged adults, east-central Illinois, USA, cross-sectional study, data collected 2015–2020, MIND diet score range 3.0–12.5, average adherence ~49%, dietary intake assessed via dietary history questionnaire II (DHQII) | ↑ cognitive processing speed during incongruent trials, faster neural efficiency | [137] | |
| 604 participants, age ≥65, cognitively unimpaired, overweight (BMI ≥25), family history of dementia, suboptimal diet (MIND score ≤8), recruited from Chicago and Boston, randomized 1:1 to MIND diet vs. control diet (both with mild caloric restriction), intervention duration 3 years, dietary counseling provided to both groups, cognitive function assessed via 12-test battery (converted to z-scores), brain imaging (MRI) conducted in nonrandom subsample (n = 200), study type: two-site randomized controlled trial | ↑ global cognition score by 0.205 standardized units in MIND group vs. 0.170 in control group, mean difference = 0.035 (95% CI: −0.022 to 0.092, p = 0.23), no significant difference in cognitive domain scores, ↑ MIND diet score by 3.3 points in MIND group vs. 0.7 in control | [138] | |
| Mediterranean diet (MD) | 105 participants, age ≥60, at risk of undernutrition and cognitive decline, 6-month duration, 3 groups (diet + exercise, diet only, control), protein target 1.5 g/kg/day, energy 30 kcal/kg/day, exercise 2×/week 30–60 min, key foods delivered 3 months, personalized counseling, study type: interventional, randomized controlled trial | MNA score ↑ by 3.2 points, cognitive score ↑ by 11%, muscle mass ↑ by 8%, physical function ↑ by 9%, appetite score ↑ by 15%, inflammation markers ↓ by 12% | [139] |
| Large cohort studies (NHS, HPFS), follow-up duration >30 years, dietary patterns analyzed include Mediterranean, DASH, plant-based, Nordic, Okinawa, study type: observational, includes cohort and case–control studies | Lean body shape linked to 17% higher chance of healthy aging, replacing 5% saturated fat with polyunsaturated fat ↓ mortality risk by 15–27%, high nut intake ↓ death rate by 20%, high olive oil intake ↓ CVD risk by 16%, high anthocyanin intake ↓ cognitive decline odds by 24% | [140] | |
| 15 studies, age range mostly ≥60, sample sizes varied (30–1000+), duration ranged from 6 months to 4 years, diets assessed were Mediterranean, Ketogenic, MIND, cognitive decline or dementia as primary outcome, interventions included dietary counseling, food provision, or self-reported adherence, study type: observational and interventional, includes cohort studies, randomized controlled trials | Improved global cognition scores, ↓ Alzheimer’s disease risk by 53% with high adherence, ↑ memory retention and executive function, ↓ neuroinflammation markers, ↑ brain-derived neurotrophic factor (BDNF) levels, ↑ hippocampal volume in long-term adherence, ↓ cognitive decline rate over 4.5 years, ↑ antioxidant intake → better neuronal protection | [141] | |
| 604 participants, age 65–84, at risk for Alzheimer’s disease, 3-year duration, personalized dietary counseling, food provision included, cognitive assessments every 6 months, study type: interventional, randomized controlled trial | ↑ global cognition scores by 35% in high adherence group, ↓ Alzheimer’s disease risk by 53% with consistent adherence, ↑ memory and executive function by 20–25% over 3 years, ↓ oxidative stress markers by 30%, ↑ brain-derived neurotrophic factor (BDNF) levels by 25%, ↓ cognitive decline rate by 30% over 4.5 years, ↑ antioxidants | [142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramasinghe, C.; Bordiga, M.; Xu, B. A Comprehensive Review of the Triangular Relationship Among Diet, Gut Microbiota, and Aging. Int. J. Mol. Sci. 2025, 26, 8785. https://doi.org/10.3390/ijms26188785
Ramasinghe C, Bordiga M, Xu B. A Comprehensive Review of the Triangular Relationship Among Diet, Gut Microbiota, and Aging. International Journal of Molecular Sciences. 2025; 26(18):8785. https://doi.org/10.3390/ijms26188785
Chicago/Turabian StyleRamasinghe, Chapa, Matteo Bordiga, and Baojun Xu. 2025. "A Comprehensive Review of the Triangular Relationship Among Diet, Gut Microbiota, and Aging" International Journal of Molecular Sciences 26, no. 18: 8785. https://doi.org/10.3390/ijms26188785
APA StyleRamasinghe, C., Bordiga, M., & Xu, B. (2025). A Comprehensive Review of the Triangular Relationship Among Diet, Gut Microbiota, and Aging. International Journal of Molecular Sciences, 26(18), 8785. https://doi.org/10.3390/ijms26188785








