Orange Peel-Mediated Green Synthesis of ZnO and CuO Nanoparticles: Evaluation for Antimicrobial Activity and Biocompatibility in Tissue Engineering
Abstract
1. Introduction
2. Results and Discussion
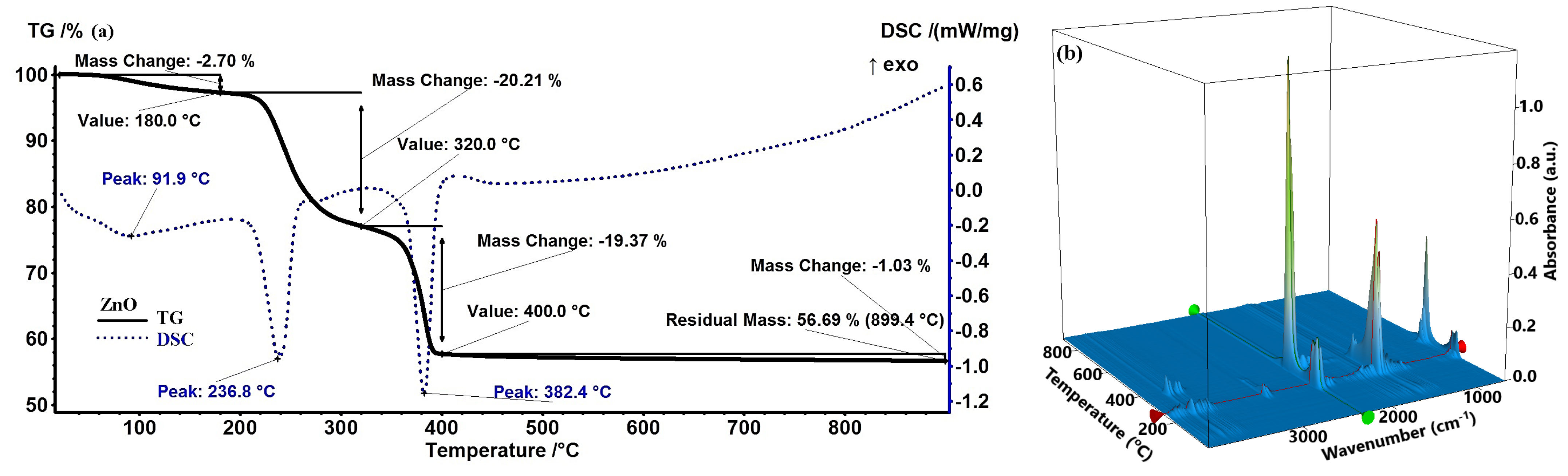
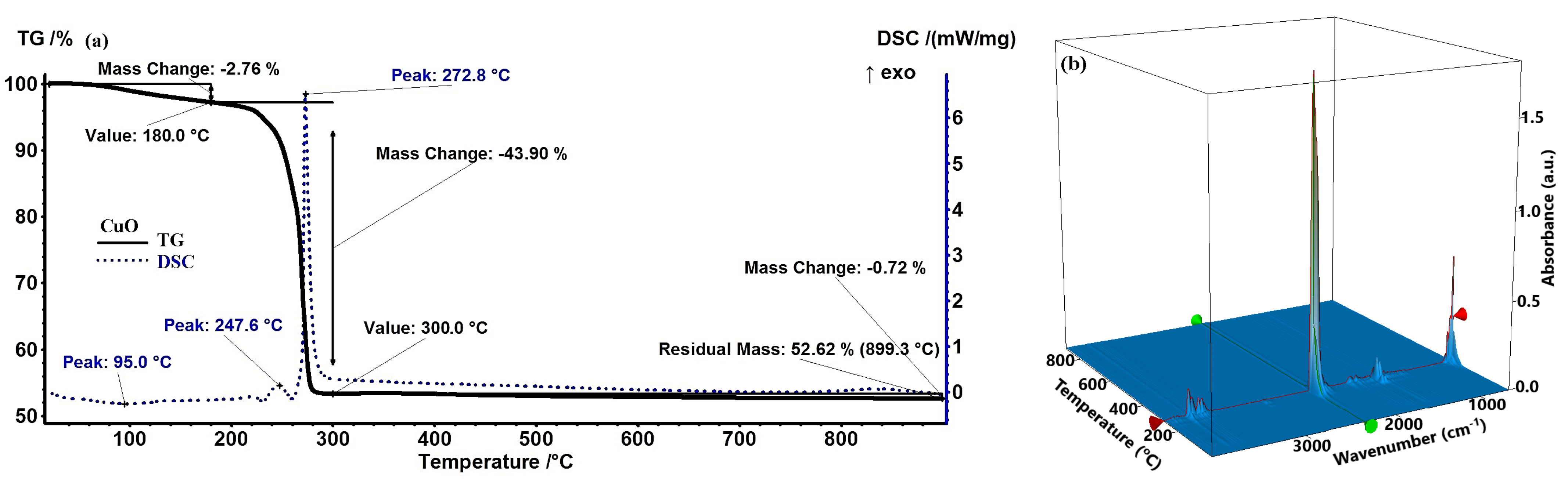
2.1. Thermogravimetric Analysis Results
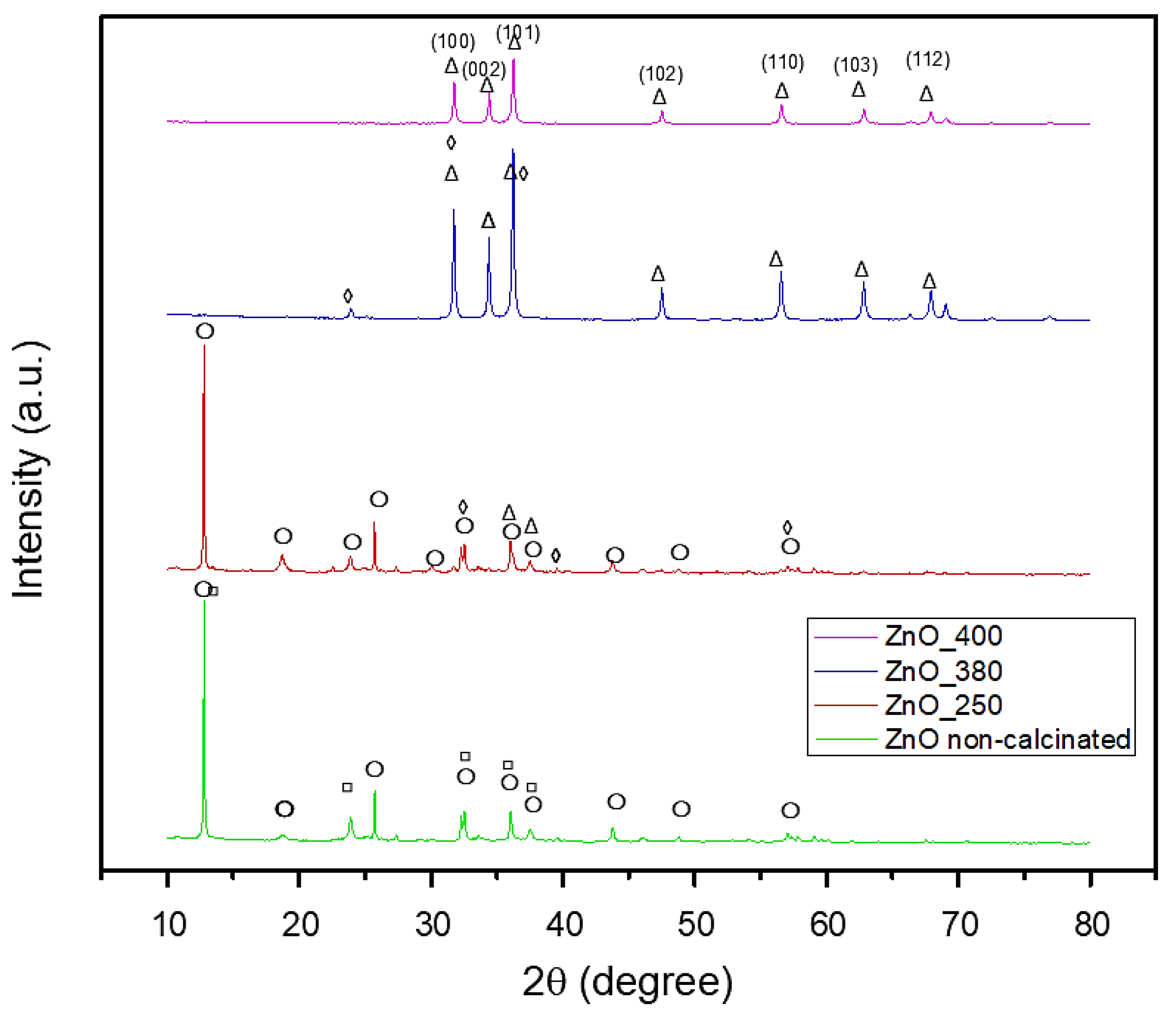
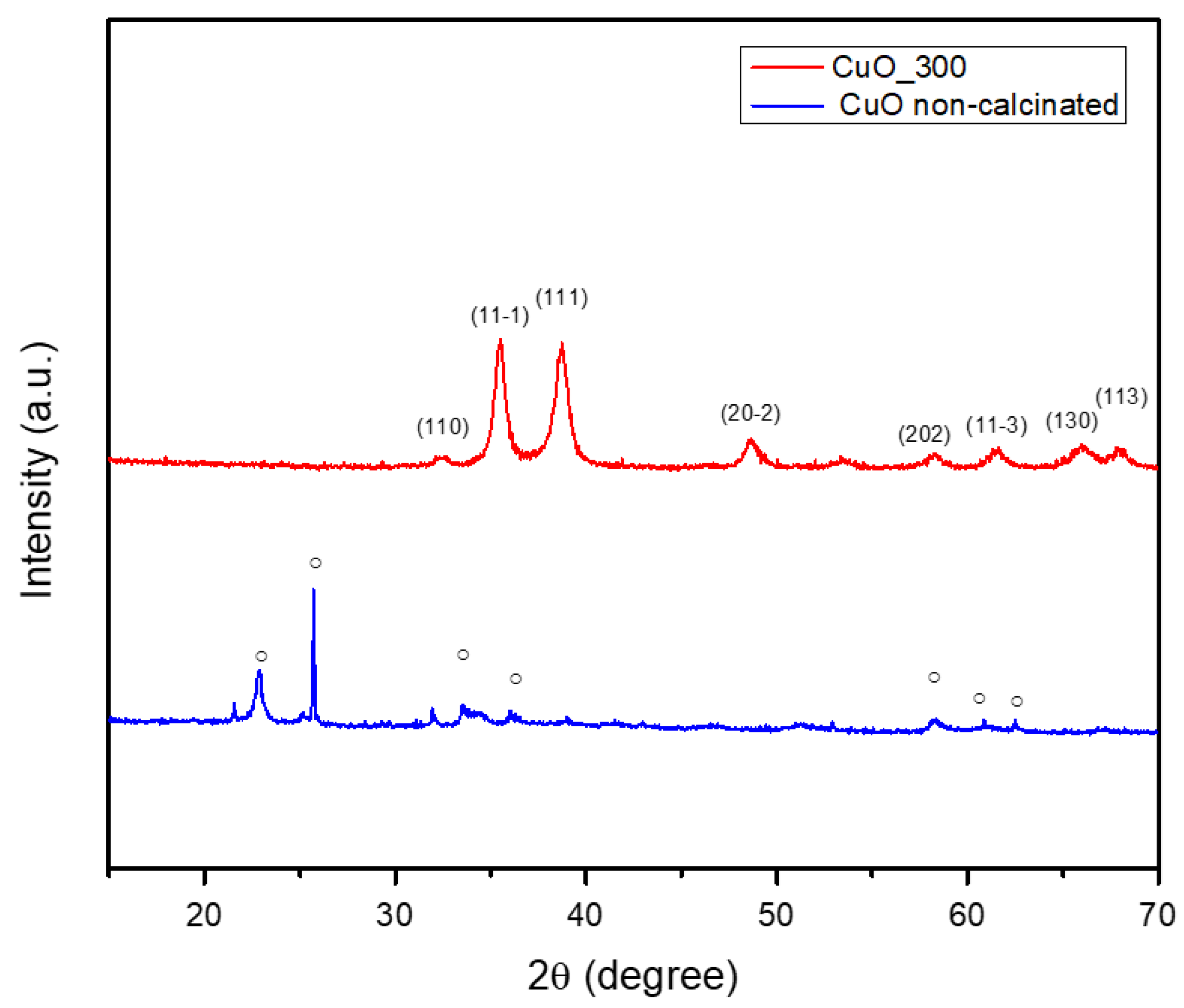
2.2. X-Ray Diffraction (XRD) Analysis Results
2.3. Scanning Electron Microscopy (SEM) Analysis Results
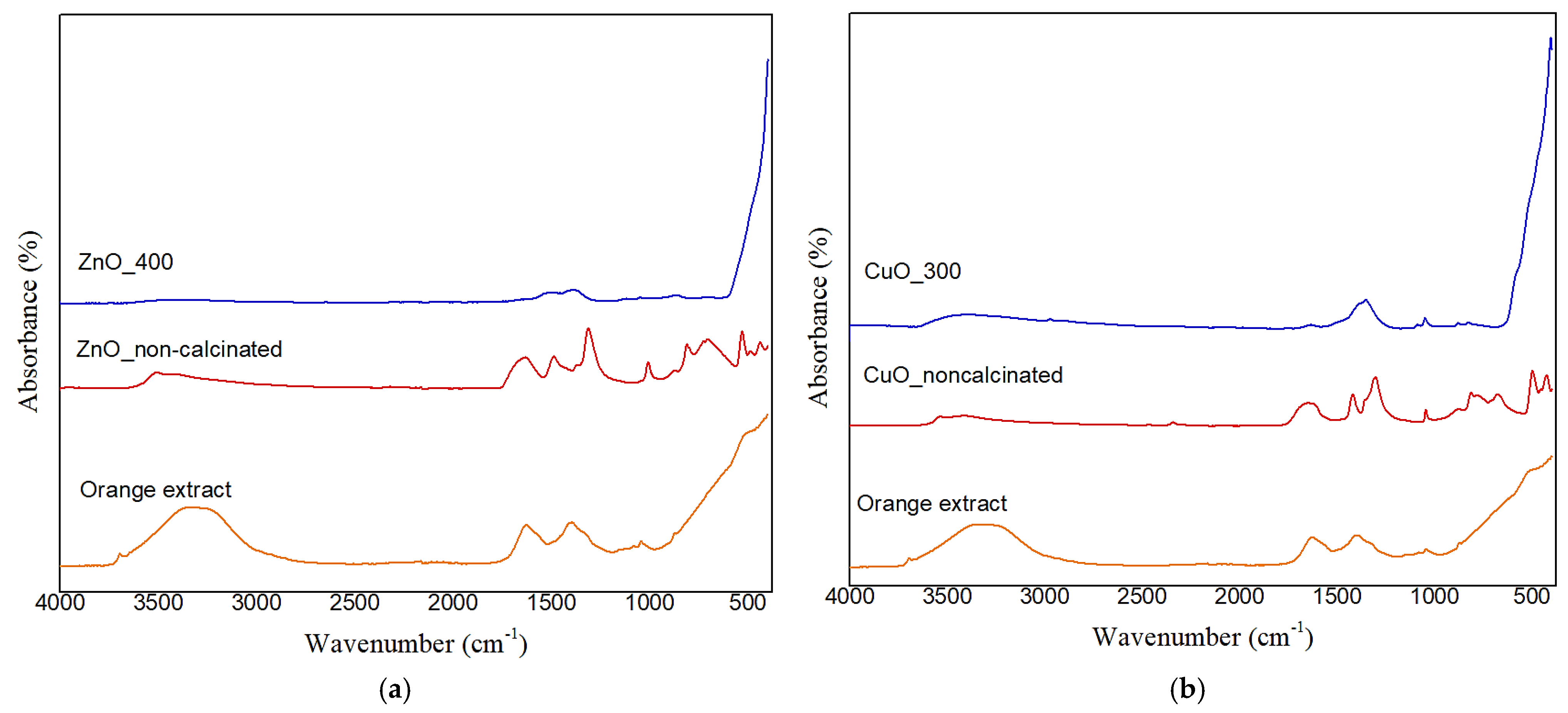
2.4. Fourier-Transform Infrared (FTIR) Spectroscopy Analysis Results
2.5. GC-MS Analysis Results
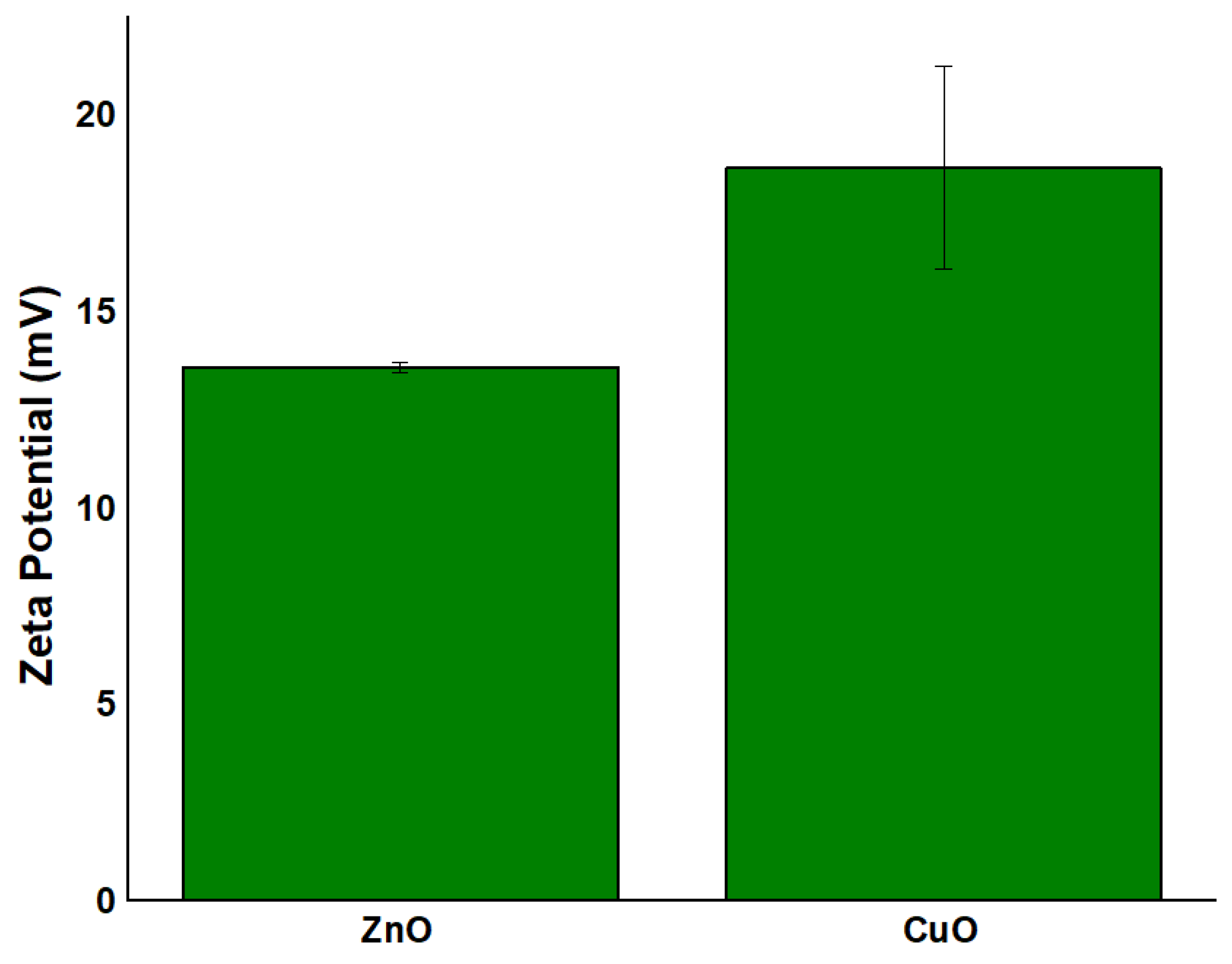
2.6. Zeta Potential Analysis Results
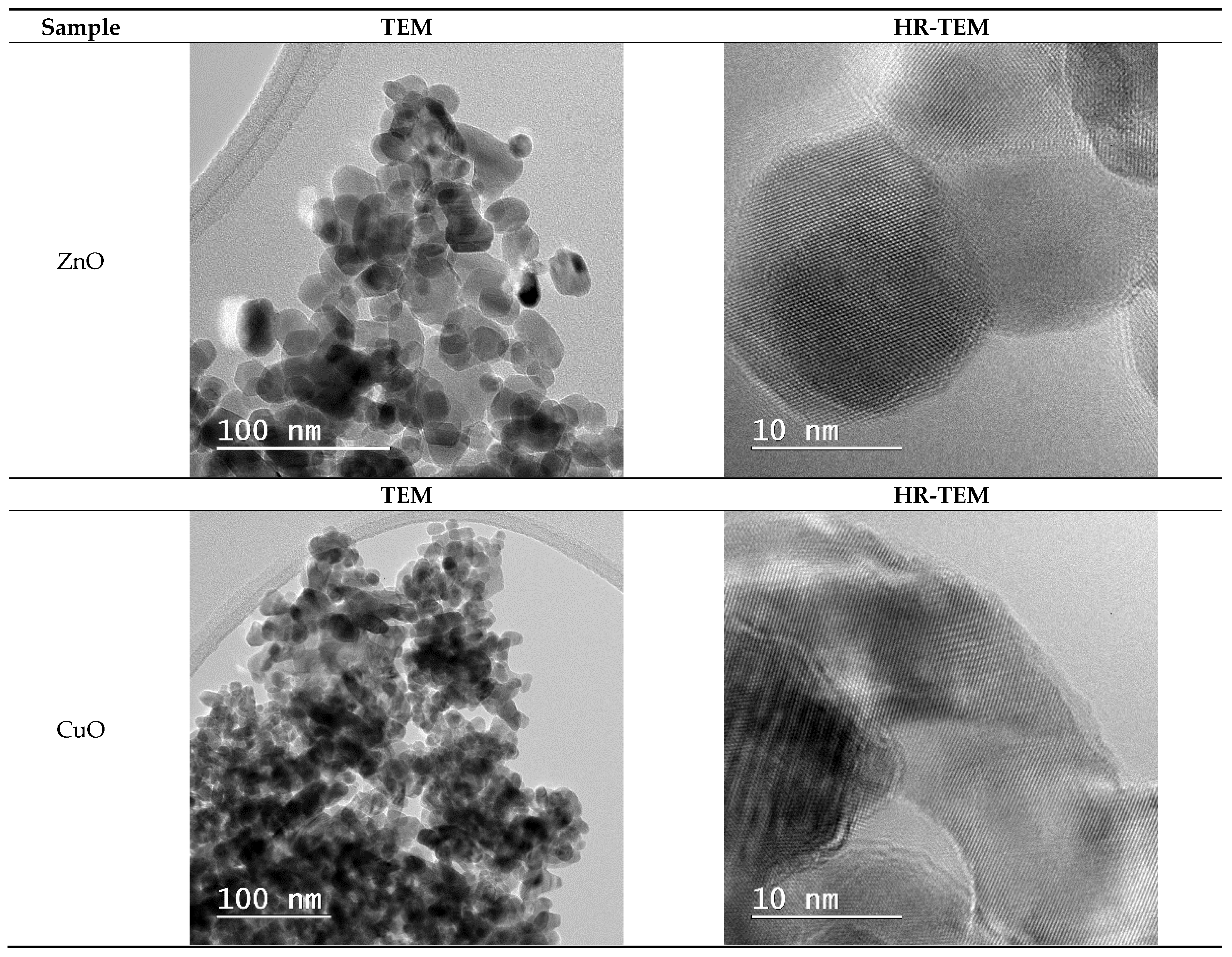
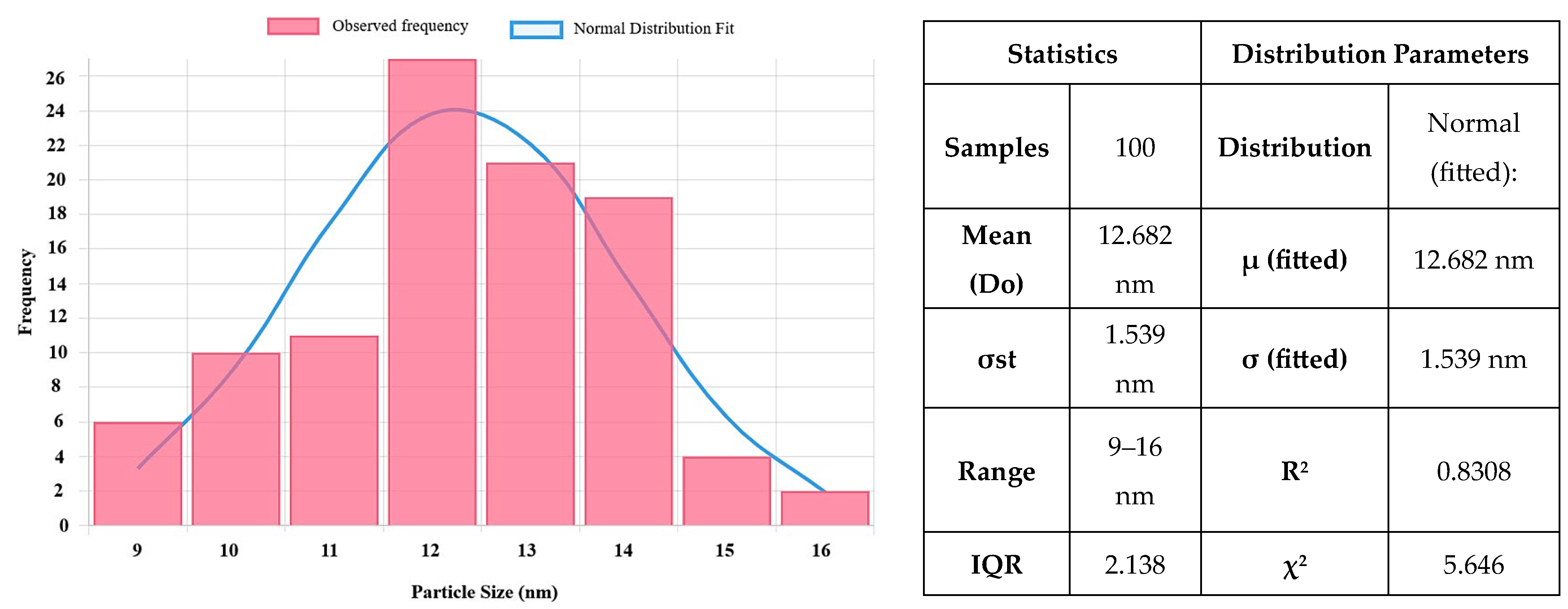
2.7. Transmission Electron Microscopy (TEM) Analysis Results
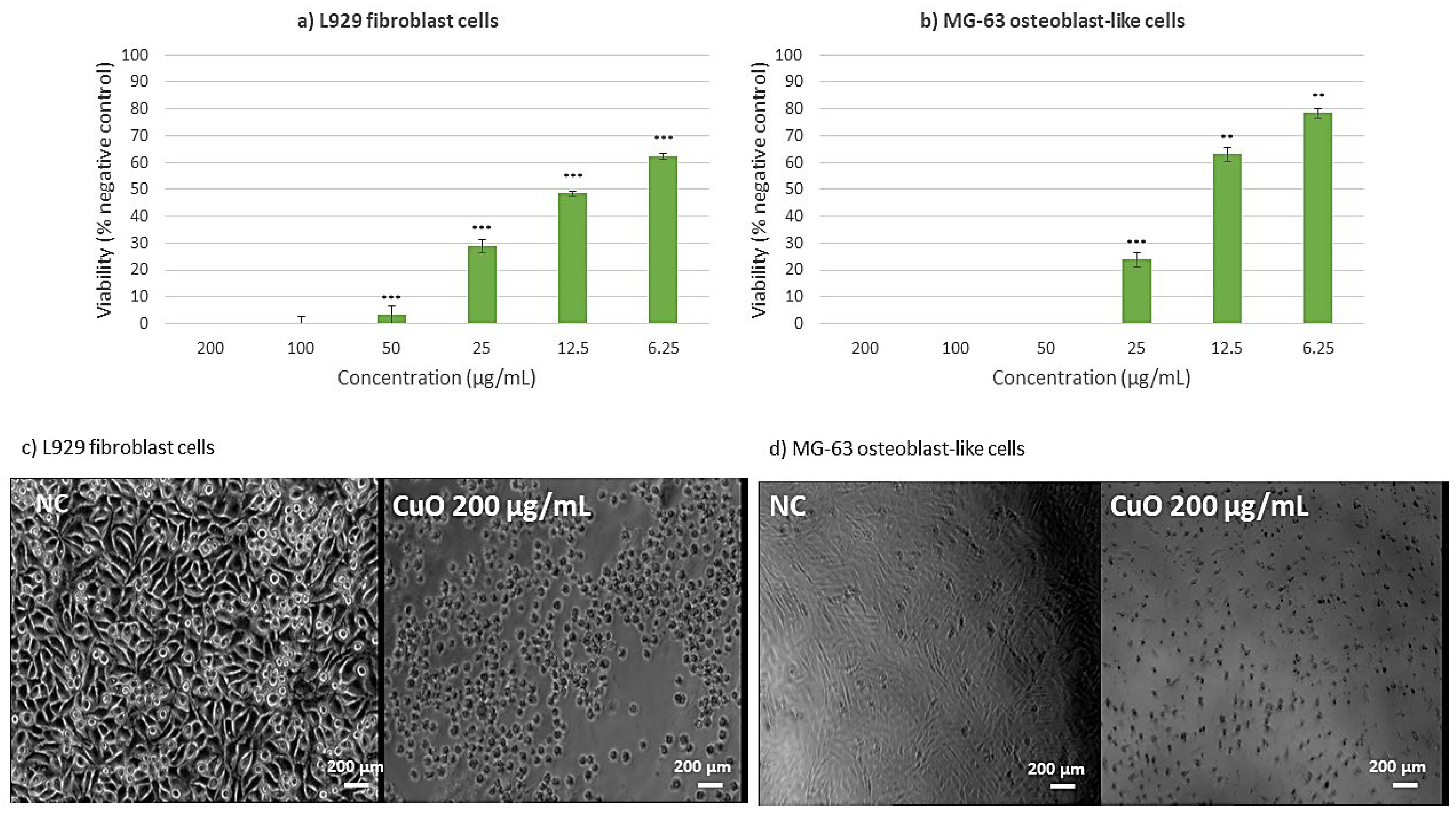
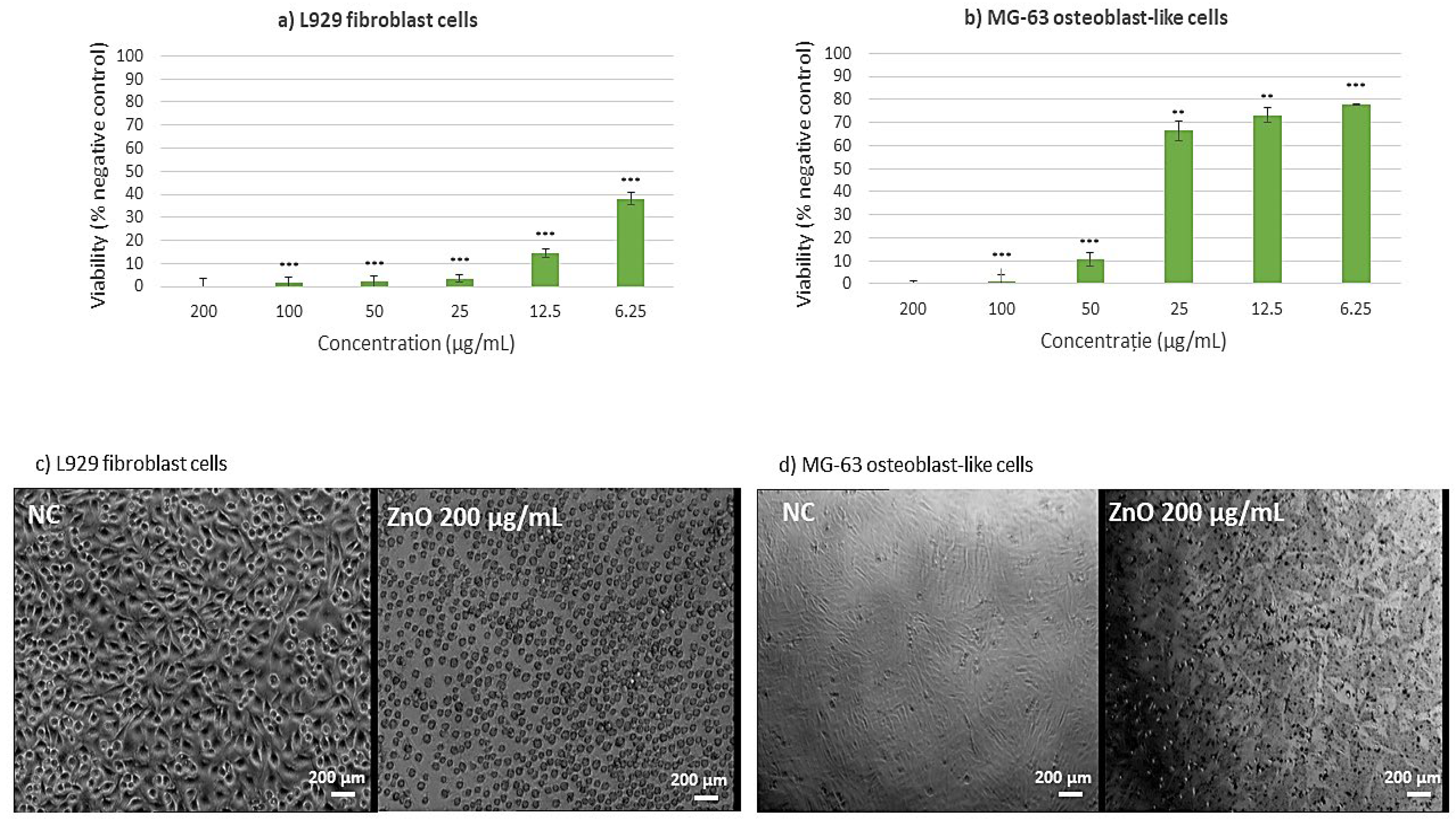
2.8. Cell Viability Assessment Results
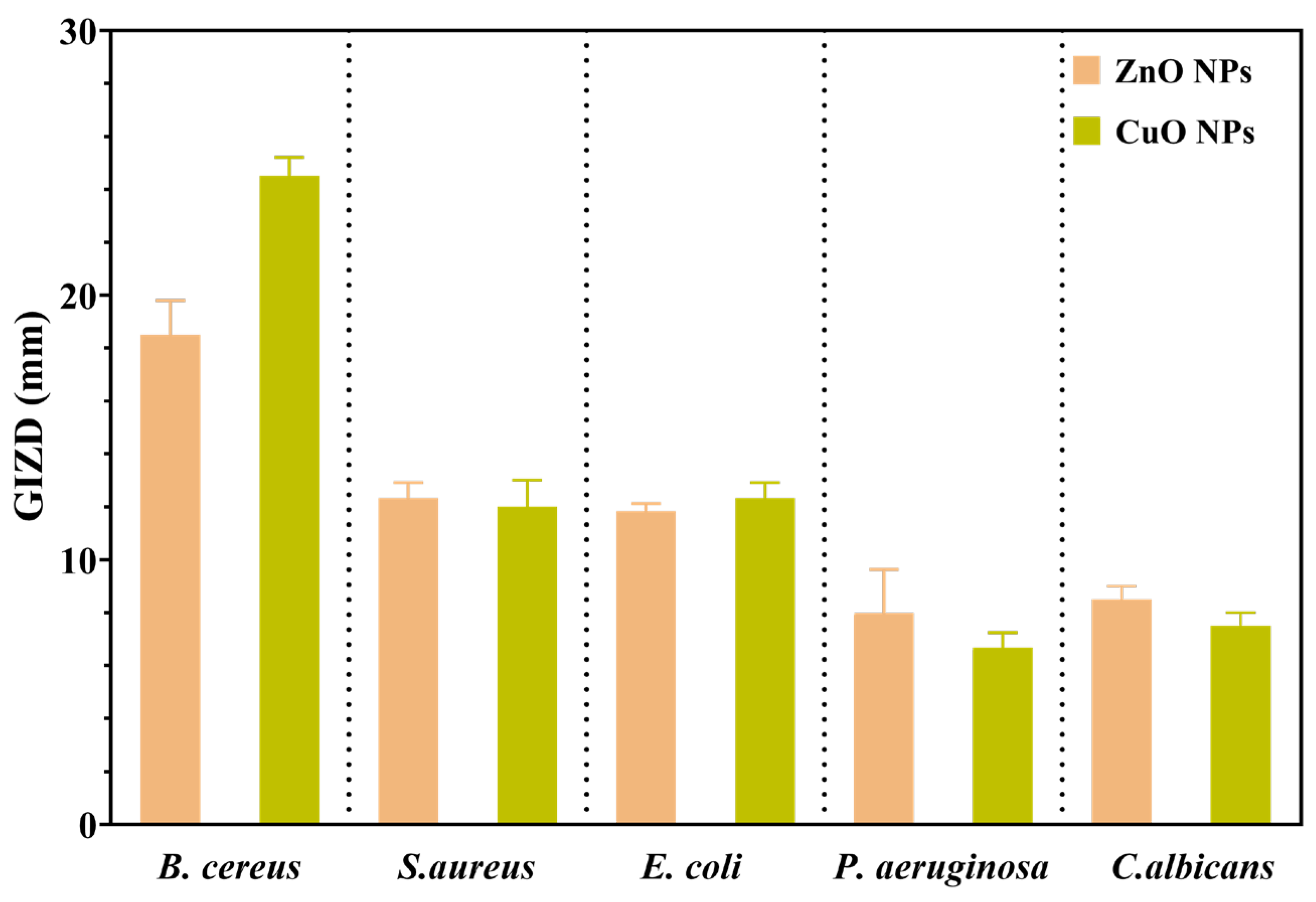
2.9. Qualitative Evaluation of Antimicrobial Activity Assessment
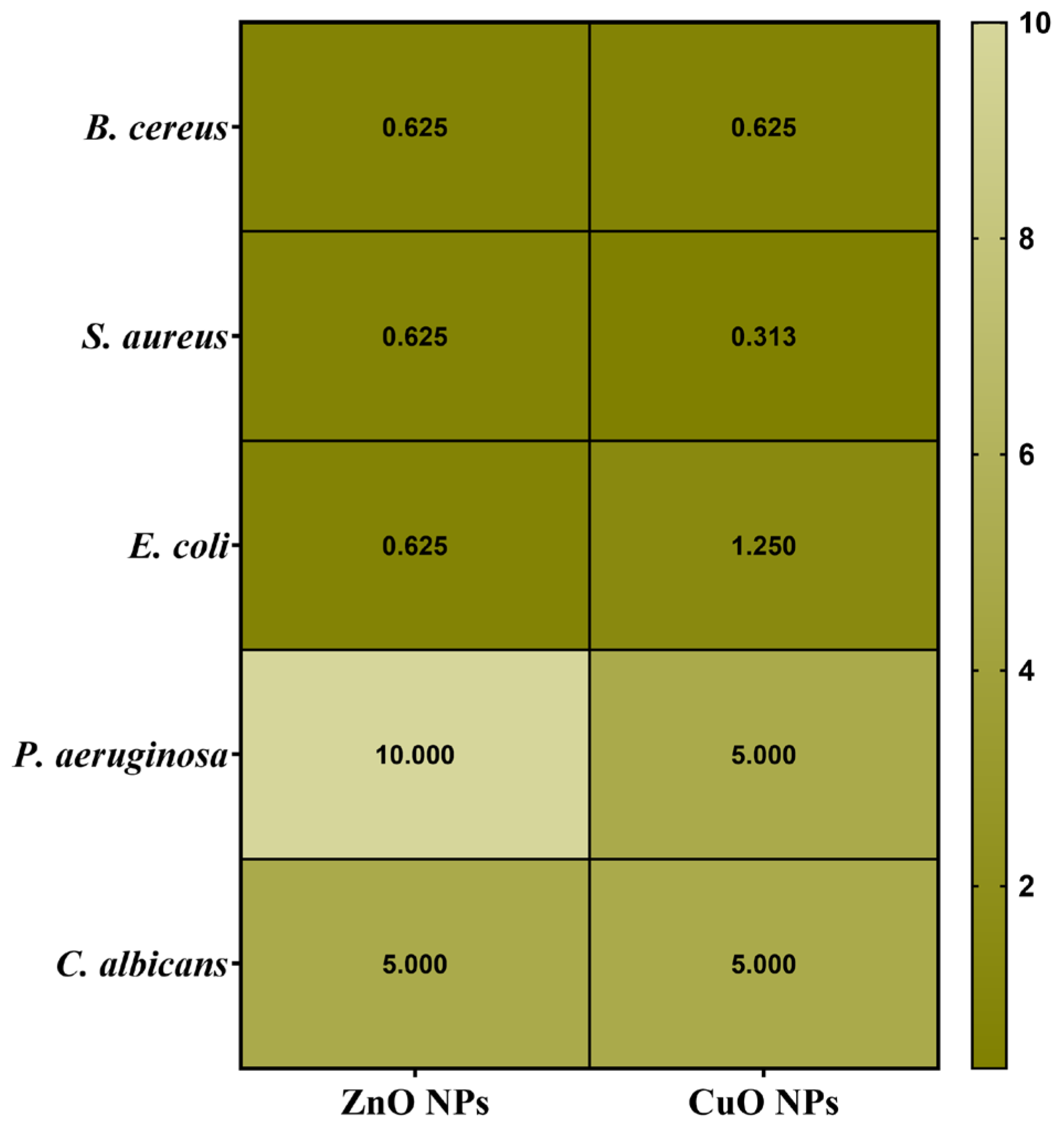
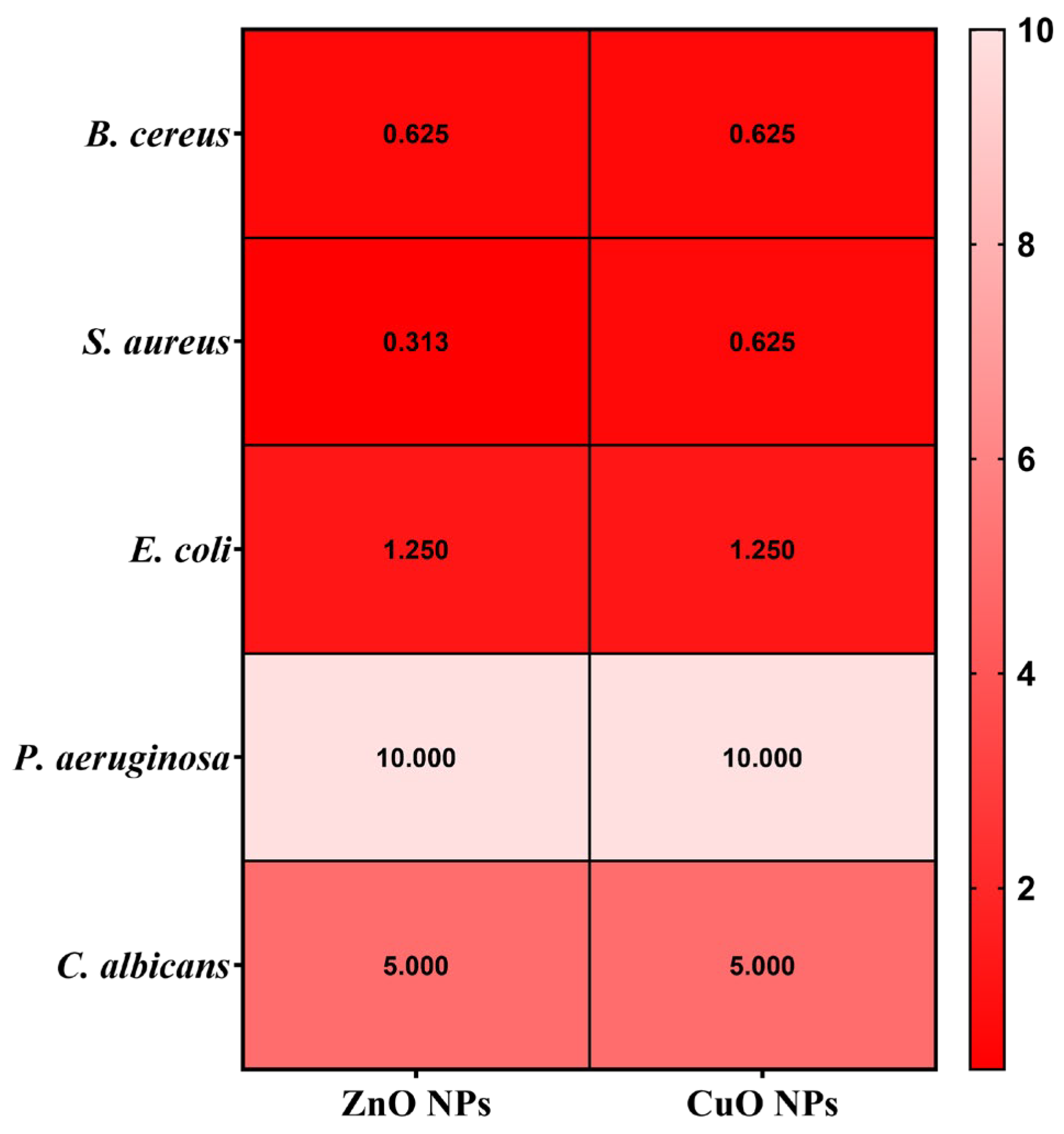
2.10. Quantitative Evaluation of Antimicrobial Activity Assessment
2.11. Semiquantitative Assessment of Microbial Adherence to the Inert Substratum Results
3. Discussion
Limitations and Future Perspectives
4. Materials and Methods
4.1. Materials
4.2. Preparation of Orange Peel Extract
4.3. Green Synthesis of Nanoparticles
4.4. Thermogravimetric Analysis
4.5. X-Ray Diffraction (XRD) Analysis
4.6. Scanning Electron Microscopy (SEM) Analysis
4.7. Fourier-Transform Infrared (FTIR) Spectroscopy Analysis
4.8. GC-MS Analysis
4.9. Zeta Potential Analysis
4.10. Transmission Electron Microscopy (TEM) Analysis
4.11. Biological Analysis
4.11.1. Cell Culture Preparation
4.11.2. Cell Viability Assessment
4.11.3. Qualitative Evaluation of Antimicrobial Activity
4.11.4. Quantitative Evaluation of Antimicrobial Activity
4.11.5. Semiquantitative Assessment of Microbial Adherence to the Inert Substratum
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, G.; Zhen, C.; Yang, J.; Wang, J.; Wang, S.; Fang, Y.; Shang, P. Recent advances of nanoparticles on bone tissue engineering and bone cells. Nanoscale Adv. 2024, 6, 1957–1973. [Google Scholar] [CrossRef]
- Karbowniczek, J.E.; Berniak, K.; Knapczyk-Korczak, J.; Williams, G.; Bryant, J.A.; Nikoi, N.D.; Banzhaf, M.; de Cogan, F.; Stachewicz, U. Strategies of nanoparticles integration in polymer fibers to achieve antibacterial effect and enhance cell proliferation with collagen production in tissue engineering scaffolds. J. Colloid Interface Sci. 2023, 650, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Tejashwini, D.M.; Harini, H.V.; Nagaswarupa, H.P.; Naik, R.; Deshmukh, V.V.; Basavaraju, N. An in-depth exploration of eco-friendly synthesis methods for metal oxide nanoparticles and their role in photocatalysis for industrial dye degradation. Chem. Phys. Impact 2023, 7, 100355. [Google Scholar] [CrossRef]
- Nadaf, S.J.; Jadhav, N.R.; Naikwadi, H.S.; Savekar, P.L.; Sapkal, I.D.; Kambli, M.M.; Desai, I.A. Green synthesis of gold and silver nanoparticles: Updates on research, patents, and future prospects. OpenNano 2022, 8, 100076. [Google Scholar] [CrossRef]
- Álvarez-Chimal, R.; Arenas-Alatorre, J.Á.; Álvarez-Pérez, M.A. Nanoparticle-polymer composite scaffolds for bone tissue engineering. A review. Eur. Polym. J. 2024, 213, 113093. [Google Scholar] [CrossRef]
- Upadhyay, K.; Tamrakar, R.K.; Thomas, S.; Kumar, M. Surface functionalized nanoparticles: A boon to biomedical science. Chem.-Biol. Interact. 2023, 380, 110537. [Google Scholar] [CrossRef]
- Nour-Djihane Mazouzi, K.B.; Haddad, A. Effect of adding magnesium oxide nanoparticles on hydroxyl methyl cellulose based films: Physico-chemical, mechanical and rheological properties. Rom. J. Mater. 2015, 54, 22–28. [Google Scholar]
- Tyagi, P.K.; Quispe, C.; Herrera-Bravo, J.; Tyagi, S.; Barbhai Mrunal, D.; Kumar, M.; Dablool, A.S.; Alghamdi, S.; Batiha, G.E.-S.; Sharifi-Rad, J.; et al. Synthesis of Silver and Gold Nanoparticles: Chemical and Green Synthesis Method and Its Toxicity Evaluation against Pathogenic Bacteria Using the ToxTrak Test. J. Nanomater. 2021, 2021, 3773943. [Google Scholar] [CrossRef]
- Eddy, D.R.; Rahmawati, D.; Permana, M.D.; Takei, T.; Solihudin; Suryana; Noviyanti, A.R.; Rahayu, I. A review of recent developments in green synthesis of TiO2 nanoparticles using plant extract: Synthesis, characterization and photocatalytic activity. Inorg. Chem. Commun. 2024, 165, 112531. [Google Scholar] [CrossRef]
- Meher, A.; Tandi, A.; Moharana, S.; Chakroborty, S.; Mohapatra, S.S.; Mondal, A.; Dey, S.; Chandra, P. Silver nanoparticle for biomedical applications: A review. Hybrid Adv. 2024, 6, 100184. [Google Scholar] [CrossRef]
- Saeed, Z.; Pervaiz, M.; Ejaz, A.; Hussain, S.; Shaheen, S.; Shehzad, B.; Younas, U. Garlic and ginger extracts mediated green synthesis of silver and gold nanoparticles: A review on recent advancements and prospective applications. Biocatal. Agric. Biotechnol. 2023, 53, 102868. [Google Scholar] [CrossRef]
- Canales, D.A.; Piñones, N.; Saavedra, M.; Loyo, C.; Palza, H.; Peponi, L.; Leonés, A.; Baier, R.V.; Boccaccini, A.R.; Grünelwald, A.; et al. Fabrication and assessment of bifunctional electrospun poly(l-lactic acid) scaffolds with bioglass and zinc oxide nanoparticles for bone tissue engineering. Int. J. Biol. Macromol. 2023, 228, 78–88. [Google Scholar] [CrossRef]
- Murugan, B.; Rahman, M.Z.; Fatimah, I.; Anita Lett, J.; Annaraj, J.; Kaus, N.H.M.; Al-Anber, M.A.; Sagadevan, S. Green synthesis of CuO nanoparticles for biological applications. Inorg. Chem. Commun. 2023, 155, 111088. [Google Scholar] [CrossRef]
- Irina Elena Doicin, A.-D.G.; Neacsu, I.A.; Ene Alexandra Cătălina Bîrcă, V.L.; Holban, A.M.; Andronescu, E. Spin-coating deposition of antimicrobial zno nanoparticles on cotton fabrics for medical applications. Rom. J. Mater. 2024, 54, 189. [Google Scholar]
- Nandhini, S.N.; Sisubalan, N.; Vijayan, A.; Karthikeyan, C.; Gnanaraj, M.; Gideon, D.A.M.; Jebastin, T.; Varaprasad, K.; Sadiku, R. Recent advances in green synthesized nanoparticles for bactericidal and wound healing applications. Heliyon 2023, 9, e13128. [Google Scholar] [CrossRef]
- Ghazal, H.; Waqar, A.; Yaseen, F.; Shahid, M.; Sultana, M.; Tariq, M.; Bashir, M.K.; Tahseen, H.; Raza, T.; Ahmad, F. Role of nanoparticles in enhancing chemotherapy efficacy for cancer treatment. Next Mater. 2024, 2, 100128. [Google Scholar] [CrossRef]
- Habibzadeh, F.; Sadraei, S.M.; Mansoori, R.; Singh Chauhan, N.P.; Sargazi, G. Nanomaterials supported by polymers for tissue engineering applications: A review. Heliyon 2022, 8, e12193. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Namakka, M.; Rahman, M.R.; Said, K.A.M.B.; Abdul Mannan, M.; Patwary, A.M. A review of nanoparticle synthesis methods, classifications, applications, and characterization. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100900. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Osibote, O.A. Green synthesis of metal oxide nanoparticles, and their various applications. J. Hazard. Mater. Adv. 2024, 13, 100401. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Oladzadabbasabadi, N.; Aziz, A.A.; Khaniabadi, P.M.; Al-ouqaili, M.T.S.; Jameel, M.S.; Braim, F.S.; Mehrdel, B.; Ghasemlou, M. Recent advances of plant-mediated metal nanoparticles: Synthesis, properties, and emerging applications for wastewater treatment. J. Environ. Chem. Eng. 2024, 12, 112345. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Herdade, A.M.; Guerra, C.; Peixoto, D.; Pereira-Silva, M.; Zeinali, M.; Mascarenhas-Melo, F.; Paranhos, A.; Veiga, F. Plant-mediated green synthesis of metal-based nanoparticles for dermopharmaceutical and cosmetic applications. Int. J. Pharm. 2021, 597, 120311. [Google Scholar] [CrossRef] [PubMed]
- Tijani, N.A.; Hokello, J.; Awojobi, K.O.; Marnadu, R.; Shkir, M.; Ahmad, Z.; Afolabi, A.O.; Adewinbi, S.A.; Adebayo, I.A. Recent advances in Mushroom-mediated nanoparticles: A critical review of mushroom biology, nanoparticles synthesis, types, characteristics and applications. J. Drug Deliv. Sci. Technol. 2024, 96, 105695. [Google Scholar] [CrossRef]
- Wen, J.; Gao, F.; Liu, H.; Wang, J.; Xiong, T.; Yi, H.; Zhou, Y.; Yu, Q.; Zhao, S.; Tang, X. Metallic nanoparticles synthesized by algae: Synthetic route, action mechanism, and the environmental catalytic applications. J. Environ. Chem. Eng. 2024, 12, 111742. [Google Scholar] [CrossRef]
- Motelica, L.; Vasile, B.-S.; Ficai, A.; Surdu, A.-V.; Ficai, D.; Oprea, O.-C.; Andronescu, E.; Mustățea, G.; Ungureanu, E.L.; Dobre, A.A. Antibacterial Activity of Zinc Oxide Nanoparticles Loaded with Essential Oils. Pharmaceutics 2023, 15, 2470. [Google Scholar] [CrossRef]
- Maneva, M.; Petrov, N. On the thermal decomposition of Zn(NO3)2·6H2O and its deuterated analogue. J. Therm. Anal. 1989, 35, 2297–2303. [Google Scholar] [CrossRef]
- Ghose, J.; Kanungo, A. Studies on the thermal decomposition of Cu(NO3)2·3 H2O. J. Therm. Anal. 1981, 20, 459–462. [Google Scholar] [CrossRef]
- Harish, V.; Ansari, M.M.; Tewari, D.; Gaur, M.; Yadav, A.B.; García-Betancourt, M.-L.; Abdel-Haleem, F.M.; Bechelany, M.; Barhoum, A. Nanoparticle and Nanostructure Synthesis and Controlled Growth Methods. Nanomaterials 2022, 12, 3226. [Google Scholar] [CrossRef]
- Altammar, K.A. A review on nanoparticles: Characteristics, synthesis, applications, and challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef]
- Motelica, L.; Vasile, B.-S.; Ficai, A.; Surdu, A.-V.; Ficai, D.; Oprea, O.-C.; Andronescu, E.; Jinga, D.C.; Holban, A.M. Influence of the Alcohols on the ZnO Synthesis and Its Properties: The Photocatalytic and Antimicrobial Activities. Pharmaceutics 2022, 14, 2842. [Google Scholar] [CrossRef]
- Doan Thi, T.U.; Nguyen, T.T.; Thi, Y.D.; Ta Thi, K.H.; Phan, B.T.; Pham, K.N. Green synthesis of ZnO nanoparticles using orange fruit peel extract for antibacterial activities. RSC Adv. 2020, 10, 23899–23907. [Google Scholar] [CrossRef]
- Bratovcic, A.; Dautovic, A. Green Synthesis of Silver Nanoparticles Using Aqueous Orange and Lemon Peel Extract and Evaluation of Their Antimicrobial Properties. Adv. Nanopart. 2024, 13, 11–28. [Google Scholar] [CrossRef]
- Skiba, M.I.; Vorobyova, V.I. Synthesis of Silver Nanoparticles Using Orange Peel Extract Prepared by Plasmochemical Extraction Method and Degradation of Methylene Blue under Solar Irradiation. Adv. Mater. Sci. Eng. 2019, 2019, 8306015. [Google Scholar] [CrossRef]
- Maqbool, Z.; Khalid, W.; Atiq, H.T.; Koraqi, H.; Javaid, Z.; Alhag, S.K.; Al-Shuraym, L.A.; Bader, D.M.D.; Almarzuq, M.; Afifi, M.; et al. Citrus Waste as Source of Bioactive Compounds: Extraction and Utilization in Health and Food Industry. Molecules 2023, 28, 1636. [Google Scholar] [CrossRef]
- Shehata, M.G.; Awad, T.S.; Asker, D.; El Sohaimy, S.A.; Abd El-Aziz, N.M.; Youssef, M.M. Antioxidant and antimicrobial activities and UPLC-ESI-MS/MS polyphenolic profile of sweet orange peel extracts. Curr. Res. Food Sci. 2021, 4, 326–335. [Google Scholar] [CrossRef]
- Matei, A.; Stoian, M.; Crăciun, G.; Țucureanu, V. Chemical Synthesis and Characterization of Fatty Acid-Capped ZnO Nanoparticles. J. Compos. Sci. 2024, 8, 429. [Google Scholar] [CrossRef]
- Bouttier-Figueroa, D.C.; Cortez-Valadez, J.M.; Flores-Acosta, M.; Robles-Zepeda, R.E., Dr. Synthesis of Metallic Nanoparticles Using Plant’s Natural Extracts: Synthesis Mechanisms and Applications: Synthesis of Metallic Nanoparticles Using Plant’s Natural Extracts. Biotecnia 2023, 25, 125–139. [Google Scholar] [CrossRef]
- Bhattacharjee, R.; Negi, A.; Bhattacharya, B.; Dey, T.; Mitra, P.; Preetam, S.; Kumar, L.; Kar, S.; Das, S.S.; Iqbal, D.; et al. Nanotheranostics to target antibiotic-resistant bacteria: Strategies and applications. OpenNano 2023, 11, 100138. [Google Scholar] [CrossRef]
- Dananjaya, S.H.S.; Kumar, R.S.; Yang, M.; Nikapitiya, C.; Lee, J.; De Zoysa, M. Synthesis, characterization of ZnO-chitosan nanocomposites and evaluation of its antifungal activity against pathogenic Candida albicans. Int. J. Biol. Macromol. 2018, 108, 1281–1288. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Vasile, O.R.; Serdaru, I.; Andronescu, E.; Truşcă, R.; Surdu, V.A.; Oprea, O.; Ilie, A.; Vasile, B.Ş. Influence of the size and the morphology of ZnO nanoparticles on cell viability. Comptes Rendus Chim. 2015, 18, 1335–1343. [Google Scholar] [CrossRef]
- Dwivedi, S.; Wahab, R.; Khan, F.; Mishra, Y.K.; Musarrat, J.; Al-Khedhairy, A.A. Reactive Oxygen Species Mediated Bacterial Biofilm Inhibition via Zinc Oxide Nanoparticles and Their Statistical Determination. PLoS ONE 2014, 9, e111289. [Google Scholar] [CrossRef]
- Motelica, L.; Oprea, O.-C.; Vasile, B.-S.; Ficai, A.; Ficai, D.; Andronescu, E.; Holban, A.M. Antibacterial Activity of Solvothermal Obtained ZnO Nanoparticles with Different Morphology and Photocatalytic Activity against a Dye Mixture: Methylene Blue, Rhodamine B and Methyl Orange. Int. J. Mol. Sci. 2023, 24, 5677. [Google Scholar] [CrossRef] [PubMed]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Ali, D.; Muneer, I.; Pirzada, M.; Ejaz, T.; Yasmeen, F.; Bashir, F.; Hanif, M.; Aziz, M.H.; Wahab, R. Green-mediated sol-gel fabrication of CuO/ZnO nanoparticles from orange peel extract for environmental remediation: Insights from x-ray diffraction analysis using various models. Phys. Scr. 2025, 100, 015980. [Google Scholar] [CrossRef]
- Ananda Murthy, H.C.; Zeleke, T.D.; Tan, K.B.; Ghotekar, S.; Alam, M.W.; Balachandran, R.; Chan, K.-Y.; Sanaulla, P.F.; Anil Kumar, M.R.; Ravikumar, C.R. Enhanced multifunctionality of CuO nanoparticles synthesized using aqueous leaf extract of Vernonia amygdalina plant. Results Chem. 2021, 3, 100141. [Google Scholar] [CrossRef]
- Abomuti, M.A.; Danish, E.Y.; Firoz, A.; Hasan, N.; Malik, M.A. Green Synthesis of Zinc Oxide Nanoparticles Using Salvia officinalis Leaf Extract and Their Photocatalytic and Antifungal Activities. Biology 2021, 10, 1075. [Google Scholar] [CrossRef]
- Denisa-Maria Radulescu, I.A.N.; Vasile, B.S.; Vasile-Adrian Surdu, E.A. Green Synthesis of Copper, Zinc, and Magnesium Oxide Nanoparticles Using Orange Peel Extract. U.P.B. Sci. Bull. 2025, 87, 1–12. [Google Scholar]
- Agarwal, H.; Venkat Kumar, S.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles—An eco-friendly approach. Resour. Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Said, Z.; Pandey, A.K.; Tiwari, A.K.; Kalidasan, B.; Jamil, F.; Thakur, A.K.; Tyagi, V.V.; Sarı, A.; Ali, H.M. Nano-enhanced phase change materials: Fundamentals and applications. Prog. Energy Combust. Sci. 2024, 104, 101162. [Google Scholar] [CrossRef]
- Keabadile, O.P.; Aremu, A.O.; Elugoke, S.E.; Fayemi, O.E. Green and Traditional Synthesis of Copper Oxide Nanoparticles-Comparative Study. Nanomaterials 2020, 10, 2502. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Sanaeimehr, Z.; Javadi, I.; Namvar, F. Antiangiogenic and antiapoptotic effects of green-synthesized zinc oxide nanoparticles using Sargassum muticum algae extraction. Cancer Nanotechnol. 2018, 9, 3. [Google Scholar] [CrossRef]
- Saranya, S.; Vijayaranai, K.; Pavithra, S.; Raihana, N.; Kumanan, K. In vitro cytotoxicity of zinc oxide, iron oxide and copper nanopowders prepared by green synthesis. Toxicol. Rep. 2017, 4, 427–430. [Google Scholar] [CrossRef]
- Ahamed, M.; Siddiqui, M.A.; Akhtar, M.J.; Ahmad, I.; Pant, A.B.; Alhadlaq, H.A. Genotoxic potential of copper oxide nanoparticles in human lung epithelial cells. Biochem. Biophys. Res. Commun. 2010, 396, 578–583. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Möller, L. Copper Oxide Nanoparticles Are Highly Toxic: A Comparison between Metal Oxide Nanoparticles and Carbon Nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef]
- Vranic, S.; Boggetto, N.; Contremoulins, V.; Mornet, S.; Reinhardt, N.; Marano, F.; Baeza-Squiban, A.; Boland, S. Deciphering the mechanisms of cellular uptake of engineered nanoparticles by accurate evaluation of internalization using imaging flow cytometry. Part. Fibre Toxicol. 2013, 10, 2. [Google Scholar] [CrossRef]
- Jo, M.-R.; Chung, H.-E.; Kim, H.-J.; Bae, S.-H.; Go, M.-R.; Yu, J.; Choi, S.-J. Effects of zinc oxide nanoparticle dispersants on cytotoxicity and cellular uptake. Mol. Cell. Toxicol. 2016, 12, 281–288. [Google Scholar] [CrossRef]
- Mittag, A.; Hoera, C.; Kämpfe, A.; Westermann, M.; Kuckelkorn, J.; Schneider, T.; Glei, M. Cellular Uptake and Toxicological Effects of Differently Sized Zinc Oxide Nanoparticles in Intestinal Cells. Toxics 2021, 9, 96. [Google Scholar] [CrossRef]
- Velumani, P.; Palani, N.; Antalin Casmie, A.; Senthilvel, R.; Parthasarthy, V. Cellular and chromosomal interaction of bio-synthesized copper oxide nanoparticles—Induced nano-cytotoxicity and genotoxicity. Toxicol. Vitr. 2025, 104, 106000. [Google Scholar] [CrossRef]
- Wang, Z.; Li, N.; Zhao, J.; White, J.C.; Qu, P.; Xing, B. CuO Nanoparticle Interaction with Human Epithelial Cells: Cellular Uptake, Location, Export, and Genotoxicity. Chem. Res. Toxicol. 2012, 25, 1512–1521. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Zerboni, A.; Bengalli, R.; Baeri, G.; Fiandra, L.; Catelani, T.; Mantecca, P. Mixture Effects of Diesel Exhaust and Metal Oxide Nanoparticles in Human Lung A549 Cells. Nanomaterials 2019, 9, 1302. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Lakshmi Prasanna, V.; Vijayaraghavan, R. Insight into the Mechanism of Antibacterial Activity of ZnO: Surface Defects Mediated Reactive Oxygen Species Even in the Dark. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef]
- Hwang, C.; Choi, M.-H.; Kim, H.-E.; Jeong, S.-H.; Park, J.-U. Reactive oxygen species-generating hydrogel platform for enhanced antibacterial therapy. NPG Asia Mater. 2022, 14, 72, Correction in NPG Asia Mater. 2022, 14, 77. [Google Scholar] [CrossRef]
- Applerot, G.; Lellouche, J.; Lipovsky, A.; Nitzan, Y.; Lubart, R.; Gedanken, A.; Banin, E. Understanding the antibacterial mechanism of CuO nanoparticles: Revealing the route of induced oxidative stress. Small 2012, 8, 3326–3337. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Fomina, P.A.; Validov, S.Z.; Kozlov, V.A. Antibacterial Properties of Copper Oxide Nanoparticles (Review). Int. J. Mol. Sci. 2024, 25, 11563. [Google Scholar] [CrossRef]
- Jiang, W.; Mashayekhi, H.; Xing, B. Bacterial toxicity comparison between nano- and micro-scaled oxide particles. Environ. Pollut. 2009, 157, 1619–1625. [Google Scholar] [CrossRef]
- Hetta, H.F.; Ramadan, Y.N.; Al-Harbi, A.I.; Ahmed, E.A.; Battah, B.; Abd Ellah, N.H.; Zanetti, S.; Donadu, M.G. Nanotechnology as a Promising Approach to Combat Multidrug Resistant Bacteria: A Comprehensive Review and Future Perspectives. Biomedicines 2023, 11, 413. [Google Scholar] [CrossRef]
- Lima, S.L.; Colombo, A.L.; de Almeida Junior, J.N. Fungal Cell Wall: Emerging Antifungals and Drug Resistance. Front. Microbiol. 2019, 10, 2573. [Google Scholar] [CrossRef]
- Ahmed, B.; Ameen, F.; Rizvi, A.; Ali, K.; Sonbol, H.; Zaidi, A.; Khan, M.S.; Musarrat, J. Destruction of Cell Topography, Morphology, Membrane, Inhibition of Respiration, Biofilm Formation, and Bioactive Molecule Production by Nanoparticles of Ag, ZnO, CuO, TiO2, and Al2O3 toward Beneficial Soil Bacteria. ACS Omega 2020, 5, 7861–7876. [Google Scholar] [CrossRef]
- Mountcastle, S.E.; Vyas, N.; Villapun, V.M.; Cox, S.C.; Jabbari, S.; Sammons, R.L.; Shelton, R.M.; Walmsley, A.D.; Kuehne, S.A. Biofilm viability checker: An open-source tool for automated biofilm viability analysis from confocal microscopy images. npj Biofilm. Microbiomes 2021, 7, 44. [Google Scholar] [CrossRef]
- Mhade, S.; Kaushik, K.S. Tools of the Trade: Image Analysis Programs for Confocal Laser-Scanning Microscopy Studies of Biofilms and Considerations for Their Use by Experimental Researchers. ACS Omega 2023, 8, 20163–20177. [Google Scholar] [CrossRef]
- El-Sawaf, A.K.; El-Moslamy, S.H.; Kamoun, E.A.; Hossain, K. Green synthesis of trimetallic CuO/Ag/ZnO nanocomposite using Ziziphus spina-christi plant extract: Characterization, statistically experimental designs, and antimicrobial assessment. Sci. Rep. 2024, 14, 19718. [Google Scholar] [CrossRef]
- Lahiri, D.; Ray, R.R.; Sarkar, T.; Upadhye, V.J.; Ghosh, S.; Pandit, S.; Pati, S.; Edinur, H.A.; Abdul Kari, Z.; Nag, M.; et al. Anti-biofilm efficacy of green-synthesized ZnO nanoparticles on oral biofilm: In vitro and in silico study. Front. Microbiol. 2022, 13, 939390. [Google Scholar] [CrossRef]
- Ghobadian, M.; Nabiuni, M.; Parivar, K.; Fathi, M.; Pazooki, J. Toxic effects of magnesium oxide nanoparticles on early developmental and larval stages of zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2015, 122, 260–267. [Google Scholar] [CrossRef]
- Ramezani Farani, M.; Farsadrooh, M.; Zare, I.; Gholami, A.; Akhavan, O. Green Synthesis of Magnesium Oxide Nanoparticles and Nanocomposites for Photocatalytic Antimicrobial, Antibiofilm and Antifungal Applications. Catalysts 2023, 13, 642. [Google Scholar] [CrossRef]
- Liang, Y.-P.; Chan, Y.-B.; Aminuzzaman, M.; Shahinuzzaman, M.; Djearamane, S.; Thiagarajah, K.; Leong, S.-Y.; Wong, L.-S.; Tey, L.-H. Green Synthesis and Characterization of Copper Oxide Nanoparticles from Durian (Durio zibethinus) Husk for Environmental Applications. Catalysts 2025, 15, 275. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanopart. Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Memic, A. Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and -negative bacterial strains. Int. J. Nanomed. 2012, 7, 3527–3535. [Google Scholar] [CrossRef]
- Dörner, L.; Cancellieri, C.; Rheingans, B.; Walter, M.; Kägi, R.; Schmutz, P.; Kovalenko, M.V.; Jeurgens, L.P.H. Cost-effective sol-gel synthesis of porous CuO nanoparticle aggregates with tunable specific surface area. Sci. Rep. 2019, 9, 11758. [Google Scholar] [CrossRef]
- Bekele, B.; Degefa, A.; Tesgera, F.; Jule, L.T.; Shanmugam, R.; Priyanka Dwarampudi, L.; Nagaprasad, N.; Ramasamy, K. Green versus Chemical Precipitation Methods of Preparing Zinc Oxide Nanoparticles and Investigation of Antimicrobial Properties. J. Nanomater. 2021, 2021, 9210817. [Google Scholar] [CrossRef]
- Subhadip Jana, P.B.; Banerjee, S. Comparative Evaluation of Synthesized Zinc Oxide Nanoparticles by Chemical Precipitation and Green Chemistry in Removal of Methylene Blue. Lett. Appl. NanoBioScience 2024, 13, 67. [Google Scholar] [CrossRef]
- Sabeena, G.; Rajaduraipandian, S.; Pushpalakshmi, E.; Alhadlaq, H.A.; Mohand, R.; Annadurai, G.; Ahamed, M. Green and chemical synthesis of CuO nanoparticles: A comparative study for several in vitro bioactivities and in vivo toxicity in zebrafish embryos. J. King Saud Univ.-Sci. 2022, 34, 102092. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Aramesh, N.; Hasnain, M.S.; Nayak, A.K.; Varma, R.S. Greener fabrication of metal nanoparticles using plant materials: A review. Chem. Phys. Impact 2023, 7, 100255. [Google Scholar] [CrossRef]
- Radulescu, D.-M.; Andronescu, E.; Vasile, O.R.; Ficai, A.; Vasile, B.S. Silk fibroin-based scaffolds for wound healing applications with metal oxide nanoparticles. J. Drug Deliv. Sci. Technol. 2024, 96, 105689. [Google Scholar] [CrossRef]
- El-Khawaga, A.M.; Elsayed, M.A.; Gobara, M.; Suliman, A.A.; Hashem, A.H.; Zaher, A.A.; Mohsen, M.; Salem, S.S. Green synthesized ZnO nanoparticles by Saccharomyces cerevisiae and their antibacterial activity and photocatalytic degradation. Biomass Convers. Biorefin. 2023, 15, 2673–2684, Correction in Biomass Convers. Biorefin. 2023, 15, 2673–2684. [Google Scholar] [CrossRef]
- SelvaKumar, M.; Jeyamurugan, R.; Jose, P.A.; Ponvel, K.M.; Sundarajan, M. Green Synthesis, Characterization, and Biological Evaluation of CuO–ZnO Nanoparticles Using Cyphostemma Setosum Leaf Extract. ChemistrySelect 2025, 10, e06062. [Google Scholar] [CrossRef]
- Vindhya, P.S.; Kavitha, V.T. Comparative study of antibacterial activity of zinc oxide and copper oxide nanoparticles synthesized by green method. AIP Conf. Proc. 2021, 2369, 020195. [Google Scholar] [CrossRef]
- Uthra, C.; Nagaraj, K.; Wadaan, M.A.; Karuppiah, C.; Maity, P.; Baabbad, A.; Kaliyaperumal, R.; Venkatachalapathy, R.; Shah, F.; Kumar, P. Zinc and Copper Oxide Nanoparticles: Pioneering Antibacterial and Antibiofilm Strategies for Environmental Restoration against Antibiotic-Resistant Bacteria. Materials 2024, 17, 3444. [Google Scholar] [CrossRef]
- Spoială, A.; Ilie, C.-I.; Trușcă, R.-D.; Oprea, O.-C.; Surdu, V.-A.; Vasile, B.Ș.; Ficai, A.; Ficai, D.; Andronescu, E.; Dițu, L.-M. Zinc Oxide Nanoparticles for Water Purification. Materials 2021, 14, 4747. [Google Scholar] [CrossRef]
- Ilie, C.-I.; Spoiala, A.; Chircov, C.; Dolete, G.; Oprea, O.-C.; Vasile, B.-S.; Crainiceanu, S.A.; Nicoara, A.-I.; Marinas, I.C.; Stan, M.S.; et al. Antioxidant, Antitumoral, Antimicrobial, and Prebiotic Activity of Magnetite Nanoparticles Loaded with Bee Pollen/Bee Bread Extracts and 5-Fluorouracil. Antioxidants 2024, 13, 895. [Google Scholar] [CrossRef]
- CLSI. CLSI Supplement M100—Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2021; pp. 1–352. [Google Scholar]



| Class | Compounds | Functional Role | Effect on NP |
|---|---|---|---|
| Terpenoids | Linalool α-Terpineol Carvone Nootkatone | electron donation (reduction), chelation, antioxidant protection, and surface stabilization | facilitates metal ion reduction, controls nucleation, prevents oxidation, and minimizes agglomeration |
| Polymethoxyflavones | Tangeretin Nobiletin Heptamethoxyflavone | redox activity, chelation, and morphology optimization | initiates oxide formation, promotes size and shape uniformity |
| Fatty Acids | Palmitic Linoleic Acid Oleic Acid Stearic Acid | capping and micelle formation, provide colloidal stability, and adjust surface energy | enhances dispersion, controls particle growth and morphology |
| Amino Acids and Sugars | Derivatized forms | morphology optimization during the synthesis process | influences shape and size distribution |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radulescu, D.-M.; Neacsu, I.A.; Vasile, B.S.; Surdu, V.-A.; Oprea, O.-C.; Trusca, R.-D.; Chircov, C.; Popescu, R.C.; Ilie, C.-I.; Ditu, L.-M.; et al. Orange Peel-Mediated Green Synthesis of ZnO and CuO Nanoparticles: Evaluation for Antimicrobial Activity and Biocompatibility in Tissue Engineering. Int. J. Mol. Sci. 2025, 26, 8781. https://doi.org/10.3390/ijms26188781
Radulescu D-M, Neacsu IA, Vasile BS, Surdu V-A, Oprea O-C, Trusca R-D, Chircov C, Popescu RC, Ilie C-I, Ditu L-M, et al. Orange Peel-Mediated Green Synthesis of ZnO and CuO Nanoparticles: Evaluation for Antimicrobial Activity and Biocompatibility in Tissue Engineering. International Journal of Molecular Sciences. 2025; 26(18):8781. https://doi.org/10.3390/ijms26188781
Chicago/Turabian StyleRadulescu, Denisa-Maria, Ionela Andreea Neacsu, Bogdan Stefan Vasile, Vasile-Adrian Surdu, Ovidiu-Cristian Oprea, Roxana-Doina Trusca, Cristina Chircov, Roxana Cristina Popescu, Cornelia-Ioana Ilie, Lia-Mara Ditu, and et al. 2025. "Orange Peel-Mediated Green Synthesis of ZnO and CuO Nanoparticles: Evaluation for Antimicrobial Activity and Biocompatibility in Tissue Engineering" International Journal of Molecular Sciences 26, no. 18: 8781. https://doi.org/10.3390/ijms26188781
APA StyleRadulescu, D.-M., Neacsu, I. A., Vasile, B. S., Surdu, V.-A., Oprea, O.-C., Trusca, R.-D., Chircov, C., Popescu, R. C., Ilie, C.-I., Ditu, L.-M., Drumea, V., & Andronescu, E. (2025). Orange Peel-Mediated Green Synthesis of ZnO and CuO Nanoparticles: Evaluation for Antimicrobial Activity and Biocompatibility in Tissue Engineering. International Journal of Molecular Sciences, 26(18), 8781. https://doi.org/10.3390/ijms26188781












