Joint Tissues: Convergence and Divergence of the Pathogenetic Mechanisms of Rheumatoid Arthritis and Osteoarthritis
Abstract
1. Introduction
2. Comparison of RA and OA Genetic Backgrounds
3. Signaling Pathways in RA and OA Pathogenesis
4. Individual and Environmental RA and OA Triggers
| Adipokine | Immune Functions | Cartilage | Estrogens/ Testosterone | Female/ Male | Age | Lean/Obese | RA | OA |
|---|---|---|---|---|---|---|---|---|
| Adiponectin | Multidirectional [176] | Promoted aggrecan degradation [177] | ⇑/⇓ [178,179,180] | f > m [178,179,180,181] | ⇑ 66–80 years vs. 51–65 years = 36–50 years [182] | ⇓/⇑ [183] ⇑/⇓ [184,185] | ⇑ CRP and ESR [186,187,188] | ⇑ cartilage damage [177,189] |
| Omentin | Multidirectional (⇑ IL-4, ⇑ IL-1β) [190] | Blocks cartilage degradation, bone erosion, chondrocyte senescence via suppressing the proinflammatory cytokines [191,192] | ⇑/? [193] | f < m [194] | ⇑ [195] | ⇑/⇓ [190,196,197] | ⇓ MMP-3 levels, RA activity CDAI, ESR [198] | ⇓ in synovia [199] ⇓ OA progression [191] |
| Apelin | Anti-inflammatory [200,201] | In total catabolic: stimulated chondrocyte proliferation, yet increased expression of MMP and IL-1beta and decreased collagen II level [202] | ⇑/⇓ apelin and APJ expression are up-regulated by estrogen [203] Inverse association with testosterone levels [204] | No sexual dimorphism [205] | ⇓ apelin and its receptor (APJ) expression [206,207] | ⇑/⇓ No difference [183] ⇓/⇑ [208,209] | ⇓ [210] Promotes neoangio-genesis [211] | ⇑ progression via stimulation of neoangio-genesis [212] |
| Vaspin | Anti-inflammatory [213] | Promoted differentiation and chondrocyte survival, and ECM formation [214] | No association [215] | f > m [216,217] | ⇑ [182] | ⇓/⇑ [217] | ⇓ eRA activity (DAS28), ESR, CRP levels [218] | ⇓ in serum ⇑ in synovia [219] |
| Adipsin | Proinflammatory [190] | Promoted cartilage volume loss [220] | ⇑/? expression of adipsin gene [221] | f > m [222] | ⇑ [223,224] | ⇓/⇑ [223] | ⇑ clinical activity in early RA [225] | ⇑ +OA progression [220] |
| Leptin | Proinflammatory [190] | Promoted chondrocyte apoptosis [226], degradation ECM [227], cartilage volume loss [228] | ⇑/⇓ [193,229,230] | f > m [180,231,232,233] | ⇑ In male ? In female leptin resistance due to reduced expression of leptin receptor [234] | ⇓/⇑ [185,190,235] | ⇑ [186,187] Direct link with CRP levels [188] | ⇑ [193] Prediction of early-onset post-traumatic OA [236] |
| Resistin | Proinflammatory [190] | Promoted proteoglycan loss due to inhibition of proteoglycan synthesis in chondrocytes [237,238] | ⇑/⇓ [215,239] | f < m [240] f = m [241] | Associated with combination of age-related comorbidities but not with age itself [242,243] | ⇓/⇑ [190,244] | ⇑ [186] Direct link with CRP levels [188] | ⇑ [193,245] Prediction of early-onset post-traumatic OA [236] |
| Visfatin | Proinflammatory [190] | Promoted collagen II and aggrecan degradation [246] | ⇑/⇑ [247,248] | f = m [249,250] | ⇑ In female [250] | ⇓/⇑ [190,251] | ⇑ Direct link with DAS28 and CRP [187,252] | ⇑ [193] Direct link joint damage [246] Prediction of early-onset post-traumatic OA [236] |
| Chemerin | Proinflammatory [190] | ECM degradation due to stimulation of pro-catabolic cytokine and metalloproteinase production [253] | ⇑/? [193,254] | f > m [254] f < m [255] f = m [256] | ⇑ [257] | ⇓/⇑ [190,235,258] | ⇑ Direct link with DAS28, ESR, CRP [259] | ⇑ [193,245] Prediction of early-onset post-traumatic OA [236] |
| RA | OA | |
|---|---|---|
| Adiponectin | Polymorphisms rs266729, rs2241766, rs2082940, and rs1063539 in the adiponectin gene—no association with RA. Adiponectin gene rs1063539 locus was possibly associated with anti-CCP in RA female patients [260]. No significant genetic correlation between adiponectin levels and RA [261]. | The ADIPOQ gene rs1501299 (+276G/T) polymorphism was not associated with KOA severity or vulnerability [262]. Polymorphisms +45T/G and +276G/T of the ADIPOQ gene might not be responsible for OA susceptibility among Thais [263]. The SNP rs182052 in the ADIPOQ gene may potentially modify individual susceptibility to knee OA in the Chinese population [264]. Associations may exist between ADIPOQ rs2241766 and knee OA in Asians’ DOI [265]. The ADIPOQ gene rs1501299 polymorphism intensifies the risk of knee OA in this Chinese Han population [266]. |
| Omentin | Revealed the association between omentin rs2274907 and RA susceptibility [267]. | The Val109Asp polymorphism of the omentin-1 gene may not be the primary pathogenic factor of KOA in Chinese individuals. The Val/Val genotype can be regarded as a potential biomarker for the risk of KOA progression [268]. ITLN1 (intelectin-1, also known as omentin) polymorphism rs2274908 was related to KOA risk in the Han population [269]. |
| Leptin | Leptin gene (rs10244329, rs2071045, and rs2167270) polymorphisms are not associated with RA genetic susceptibility and its clinical features in the Chinese population [270]. | In normal weight and overweight Han Chinese individuals, LEP polymorphisms (three SNPs of leptin—rs11761556, rs12706832, rs2071045) were associated with knee OA [271]. |
| Resistin | There were no significant differences for the distribution of allele and genotype frequencies of three resistin SNPs (rs1862513, rs3745368, and rs3745367) between RA patients and normal controls (all p > 0.05). The genotype effects of dominant and recessive models were also analyzed, and no significant association was detected (all p > 0.05). Haplotype analysis suggested that the frequency of haplotype GAA was notably lower in RA patients in comparison with normal controls. Thus, resistin gene polymorphisms might affect the genetic predisposition of RA in the Chinese population [272]. C allele of the resistin SNP rs7408174 as well as those with the AG allele or who had at least one A allele of the SNP rs3219175 are at greater risk of developing RA disease compared with wild-type carriers [273]. | Weak associations between resistin genes and hand OA in Finnish women, and that the associations are modified by BMI [274]. Resistin −420/+299 alleles haplotype analysis demonstrated that mutant alleles were more prevalent in knee OA-affected individuals compared to healthy subjects (p < 0.05) in Pakistani population [275]. SNP rs3745368 from resistin was identified as being related to an increased risk of HOA [276]. |
| Visfatin | X | SNP rs4730153 was significantly associated with decreased risk of OA in an additive genetic model (p < 0.05), while rs16872158 showed an increased risk of developing OA (p < 0.05) in the Chinese population [277]. Limited data revealed that associations may exist between visfatin rs4730153 and knee OA in Asians, and between visfatin rs16872158 and knee OA in Asians [265]. |
| Chemerin | Chemerin rs17173608 polymorphism were associated with increased susceptibility to RA [267]. | X |
| Apelin | No association between apelin rs2235306 and RA [267]. | X |
5. Comparison of Pathogenesis of ACCP-Positive and ACCP-Negative RA and OA Through the Prism of Joint Tissue Processes
5.1. ACCP-Positive RA: Initial Processes
5.2. ACCP-Negative RA: Initial Processes
5.3. OA: Initial Processes
6. ACCP-Positive and ACCP-Negative RA in Full Swing
7. OA in Full Swing
8. The Efficiency of Conventional Synthetic and Biologic DMARDs in RA and OA
| OA Phenotype | Efficacy |
|---|---|
| Methotrexate | |
| symptomatic, radiographic (Kellgren–Lawrence grades 3 to 4), painful inadequate response to current medication Knee OA, n = 207, 6 months, up to 25 mg [433] | ⇓ pain, MS, ⇑ function |
| Knee OA, n = 160, 6 months, up to 25 mg radiograph (X-ray) tibiofemoral OA within the last 2 years, Kellgren–Lawrence grades 3 to 4 [11] | ⇓ pain |
| Knee OA, n = 58, 4 months, 7.5 mg DS OA 2 years, Kellgren–Lawrence 2–3, synovitis [434] | no effect on pain, no difference in paracetamol consumption |
| Knee OA with insufficient pain relief from, or inability to tolerate, traditional analgesics including NSAIDs and opioids with synovitis, average duration 4 year, K/L score 1–4 (n = 20 II score 67%, n = 3 III score—10%), n = 24 weeks, up to 20 mg Erosions, 51.6; osteophytes, 68.9% [432] | 13/30 (43%) achieved ≥30% reduction in pain VAS, 7 (23%) achieved ≥50% reduction, and 4 (13%) had worsened. All had synovitis (effusion or synovial hypertrophy 52 mm) at baseline and 25/30 demonstrated both pathologies. US at the final study visit (including three participants who withdrew after 12 weeks) demonstrated synovitis in 22 people. There was a median (IQR) reduction in total synovial thickness of 1.3 mm (0.7 to 3.8) (n = 26) and a median (IQR) reduction in total effusion measurement of 0.6 mm (1.3 to 3.6) (n = 26) (p > 0.05). Baseline synovitis or effusion (whether total values summated across the three knee compartments or maximum individual compartment scores) were not substantively correlated with baseline pain or change in 48-h pain VAS at 24 weeks (r < 0.2). Changes in synovitis and effusion at 24 weeks were similarly not substantively correlated with changes in pain. |
| Moderate to severe knee OA, Kellgren–Lawrence score of III to IV, n = 100, 6 months, 7.5 mg up to 15 mg [435] | reduced pain severity and improved functional status and quality of life |
| Clinical and radiographic knee OA, n = 155, 50% K-L grade 3–4, 12 months, 10 mg up to 25 mg [436] | ⇓ pain (contradictory results) and stiffness, ⇑ function No change in synovial volume (MRI) |
| Knee OA with pain resistant to paracetamol, Kellgren–Lawrence II–III, n = 58, experience 2 years, 4 months, 7.5 mg [434] | no amelioration of symptoms functional status, tendency to reduce consumption of analgesics |
| Knee OA with effusion-synovitis, n = 215, Kellgren–Lawrence score II-22 (21%), III-39 (37%), IV-44 (42%), 52 weeks, up to 15 mg [437] | VAS pain and effusion-synovitis and maximal area, cartilage defects—no difference with placebo |
| Knee and hand OA, n = 465, 6 months, metanalysis [438] | reduced knee and hand stiffness at the end of follow-up knee and hand stiffness at 6 months of follow-up |
| Erosive hand OA, n = 64, 6 and 12 months, 10 mg [10] Verbruggen–Veys anatomical score [439] and Ghent University Score System (GUSS) scores [440] | Comparable effect of MTX and placebo on pain, functional disability, joint damage progression vs. placebo Joints with space loss appeared to be eroding less in the MTX group compared to the placebo group. Only serum IL-6 level and presence of synovitis at inclusion (but not pain, sex, age adipokines) were associated with a higher risk of erosive evolution in the non-erosive joints using the GUSS score at 12 months in the entire population. |
| Hand OA, n = 202, Kellgren and Lawrence grade ≥ 2 with synovitis, experience 6 years, 6 months, 20 mg [441] | moderate effect on reducing pain, but not function |
| Hand OA refractory to usual treatments [10] | |
| Erosive hand OA, 2 months, 10 mg [11] | decreased pain and morning stiffness, but not functional indices, number of tender and swollen joints |
| Biologics | |
| Tocilizumab anti-IL6 receptor | |
| Symptomatic hand OA with synovitis, Kellgren–Lawrence grade ≥ 2, experience 9 years, n = 104 [9] | no more effective than placebo for pain relief, number of painful and swollen joints, duration of morning stiffness, patients’ and physicians’ global assessment and function scores |
| Hip, knee, and hand OA (late in the most analyzed studies) Meta-analysis [13] | Anti-TNFa, n = 427, experience 6–14 (Anakinra, Adalimumab, Etanercept, Infliximab) no effect on pain and function |
| Anti-IL-1, n = 404, experience (when specified) 5–11 years (AMG108, Canakinumab, ABT981, Lutikizumab) no effect on pain and function | |
| Anti-NGF (nerve growth factor), n = 1749, experience 3–7 (Tanezumab, Fulranumab, Fasinumab, AMG403) ⇓ pain; ⇑ function | |
| Anti-IL-1 | |
| Knee OA, n = 1240, Kellgren–Lawrence grades II–III (50/50%, when specified) meta-analysis [442] | superior to placebo in terms of pain relief and functional improvement (ABT981, AMG 108, Orthokine, ABT-981, Anakinra, Canakinumab, Diacerein) |
| Diacerein Knee OA n = 1277 meta-analysis [443] | pain and function—short-term residual effectiveness |
| Diacerein Knee OA n = 1732 meta-analysis [444] | ⇓ pain |
| Diacerein Knee OA n = 1533 [445] | ⇑ function |
| Diacerein knee and/or hip meta-analysis [446] | ⇓ pain, ⇑ function, ⇑ escape medication use |
| TNF inhibitors | |
| Adalimumab | |
| Hand OA, n = 276, erosive inflammatory phenotype Meta-analysis (Etanercept, Adalimumab), n = 276 [447] | no effect on pain at 4–6 weeks and 24–26 weeks and on grip strength at 12 months reducing progression of structural outcomes (X-ray, ultrasonography, or MRI) in hand OA with of inflammation but not in those without inflammation at 12 months |
| hand OA refractory to analgesics, n = 85, 13 years [448] Kellgren–Lawrence grade and Verbruggen–Veys anatomical scores—progression was not analyzed in dynamic | no difference to placebo for pain decrease in the number of swollen joints adalimumab group |
| erosive hand OA with synovitis, n = 43, MRI-detected synovitis [449] | No effect pain, function, and stiffness subscales from baseline to 4, 8 and 12 weeks, no effect on MRI-detected synovitis and bone marrow lesions pain and inflammation are not responsive to TNF α inhibition |
| erosive hand OA (on radiology), n = 60, experience > 6 years [450] Verbruggen–Veys anatomical scores Exploration of potential risk factors for more erosive disease—disease duration, palpable effusion at baseline | Effect on progression of joint damage in joints with soft tissue swelling compared to placebo. Risk factors for progression were then identified and the presence of palpable soft tissue swelling at baseline was recognized as the strongest predictor for erosive progression. In this subpopulation at risk, statistically significant less erosive evolution on the radiological image (3.7%) was seen in the adalimumab treated group compared to the placebo group |
| Etanercept | |
| symptomatic erosive inflammatory hand osteoarthritis, n = 90, experience 8 years [451] Verbruggen–Veys score and MRI | did not relieve pain effectively after 24 weeks in erosive osteoarthritis. Small subgroup analyses showed a signal for effects on subchondral bone in actively inflamed joints, but future studies to confirm this are warranted less MRI bone marrow lesions in more pronounced inflammatory joint group |
| erosive (≥ 1 IPJ with radiographic pre(erosive) anatomical phase (“J”/“E”) according to Verbruggen–Veys system) inflammatory (≥1 IPJ with soft swelling/erythema and with positive power Doppler at US) symptomatic (VAS pain > 30/100 on NSAID use, flare after NSAID washout) OA were included [452] quantitative Ghent University Scoring System | No effect-VAS pain, hand function (FIHOA), quality of life (SF-36), no. of tender joints and grip strength, radiographic progression after 4, 8, 12, 24, 36 weeks, and 1 year Symptomatic and inflammatory patients completing the study ETN was superior over placebo both on pain and structural damage assessed by GUSS; ETN was especially effective in joints with signs of inflammation |
9. Synthesis of the Key Findings on RA and OA Convergence and Divergence of the Pathogenetic Mechanisms
- Shared mutations of 29 genes, encoding molecules involved in immunoinflammatory processes and ECM production.
- Unidirectional association of non-genetic factors with OA and ACCP-negative RA; signaling pathway overactivation with the same consequences for RA and OA.
- Serum ACCPs were rarely detected in OA (ACCP-negative RA exists as well!).
- For a clearer understanding, studies of OA variants with potentially different mechanisms are needed. Erosive hand OA is especially interesting.
- Innate and adaptive immune responses (although less aggressive than in RA) are involved in OA development.
- Identical to those in RA, lymphoid nodular aggregates (but not GCs) were revealed in 30% of OA synovial samples. On the other hand, GCs were not revealed in all RA synovial ‘pathotypes’, but only in lymphoid ones, while myeloid and especially pauci-immune and fibroid pathotypes look quite acceptable for OA.
- Indistinguishable from that in RA, pannuses were found in OA articular tissues.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The Pathogenesis of Rheumatoid Arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Roškar, S.; Hafner-Bratkovič, I. The Role of Inflammasomes in Osteoarthritis and Secondary Joint Degeneration Diseases. Life 2022, 12, 731. [Google Scholar] [CrossRef] [PubMed]
- Starodubtseva, I.A. Prevalence of secondary osteoarthritis in patients with rheumatoid arthritis and risk factors for its progression. Adv. Gerontol. 2014, 27, 693–698. [Google Scholar] [PubMed]
- Fujikawa, K.; Kawakami, A.; Tamai, M.; Uetani, M.; Takao, S.; Arima, K.; Iwamoto, N.; Aramaki, T.; Kawashiri, S.; Ichinose, K.; et al. High Serum Cartilage Oligomeric Matrix Protein Determines the Subset of Patients with Early-Stage Rheumatoid Arthritis with High Serum C-Reactive Protein, Matrix Metalloproteinase-3, and MRI-Proven Bone Erosion. J. Rheumatol. 2009, 36, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Niki, Y.; Takeuchi, T.; Nakayama, M.; Nagasawa, H.; Kurasawa, T.; Yamada, H.; Toyama, Y.; Miyamoto, T. Clinical Significance of Cartilage Biomarkers for Monitoring Structural Joint Damage in Rheumatoid Arthritis Patients Treated with Anti-TNF Therapy. PLoS ONE 2012, 7, e37447. [Google Scholar] [CrossRef] [PubMed]
- Serdyuk, I.L.; Valeeva, A.R.; Korovina, M.O.; Renaudineau, Y.; Arleevskaya, M.I. Triggering role of environmental and individual factors in rheumatoid arthritis and preclinical joint symptoms. Kazan Med. J. 2024, 106, 51–61. [Google Scholar] [CrossRef]
- Wenham, C.Y.J.; Hensor, E.M.A.; Grainger, A.J.; Hodgson, R.; Balamoody, S.; Dore, C.J.; Emery, P.; Conaghan, P.G. A randomized, double-blind, placebo-controlled trial of low-dose oral prednisolone for treating painful hand osteoarthritis. Rheumatology 2012, 51, 2286–2294. [Google Scholar] [CrossRef]
- Richette, P.; Latourte, A.; Sellam, J.; Wendling, D.; Piperno, M.; Goupille, P.; Pers, Y.-M.; Eymard, F.; Ottaviani, S.; Ornetti, P.; et al. Efficacy of tocilizumab in patients with hand osteoarthritis: Double blind, randomised, placebo-controlled, multicentre trial. Ann. Rheum. Dis. 2021, 80, 349–355. [Google Scholar] [CrossRef]
- Ferrero, S.; Wittoek, R.; Allado, E.; Cruzel, C.; Fontas, E.; Breuil, V.; Ziegler, L.; Kremer, J.; Loeuille, D.; Roux, C.H. Methotrexate treatment in hand osteoarthritis refractory to usual treatments: A randomised, double-blind, placebo-controlled trial. Semin. Arthritis Rheum. 2021, 51, 831–838. [Google Scholar] [CrossRef]
- Kingsbury, S.R.; Tharmanathan, P.; Arden, N.K.; Batley, M.; Birrell, F.; Cocks, K.; Doherty, M.; Edwards, C.J.; Garrood, T.; Grainger, A.J.; et al. Pain reduction with oral methotrexate in knee osteoarthritis, a pragmatic phase iii trial of treatment effectiveness (PROMOTE): Study protocol for a randomized controlled trial. Trials 2015, 16, 77. [Google Scholar] [CrossRef]
- Schieker, M.; Conaghan, P.G.; Mindeholm, L.; Praestgaard, J.; Solomon, D.H.; Scotti, C.; Gram, H.; Thuren, T.; Roubenoff, R.; Ridker, P.M. Effects of Interleukin-1β Inhibition on Incident Hip and Knee Replacement: Exploratory Analyses from a Randomized, Double-Blind, Placebo-Controlled Trial. Ann. Intern. Med. 2020, 173, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Li, H.; Feng, H.; Long, H.; Yang, Z.; Li, J.; Wang, Y.; Xie, D. Efficacy and safety of biologic agents for the treatment of osteoarthritis: A meta-analysis of randomized placebo-controlled trials. Ther. Adv. Musculoskelet. 2022, 14, 1759720X221080377. [Google Scholar] [CrossRef]
- Nwankwo, E.C.; Labaran, L.A.; Athas, V.; Olson, S.; Adams, S.B. Pathogenesis of Posttraumatic Osteoarthritis of the Ankle. Orthop. Clin. N. Am. 2019, 50, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, G.; Cobo-Molinos, J.; Antich, C.; López-Ruiz, E. Osteoarthritis: Trauma vs Disease. In Osteochondral Tissue Engineering; Oliveira, J.M., Pina, S., Reis, R.L., San Roman, J., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2018; Volume 1059, pp. 63–83. ISBN 978-3-319-76734-5. [Google Scholar]
- Revell, P.A.; Mayston, V.; Lalor, P.; Mapp, P. The synovial membrane in osteoarthritis: A histological study including the characterisation of the cellular infiltrate present in inflammatory osteoarthritis using monoclonal antibodies. Ann. Rheum. Dis. 1988, 47, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Sinkeviciute, D.; He, Y.; Karsdal, M.; Henrotin, Y.; Mobasheri, A.; Önnerfjord, P.; Bay-Jensen, A. The minor collagens in articular cartilage. Protein Cell 2017, 8, 560–572. [Google Scholar] [CrossRef]
- Czarny-Ratajczak, M.; Lohiniva, J.; Rogala, P.; Kozlowski, K.; Perälä, M.; Carter, L.; Spector, T.D.; Kolodziej, L.; Seppänen, U.; Glazar, R.; et al. A Mutation in COL9A1 Causes Multiple Epiphyseal Dysplasia: Further Evidence for Locus Heterogeneity. Am. J. Hum. Genet. 2001, 69, 969–980. [Google Scholar] [CrossRef]
- Escudero-Esparza, A.; Kalchishkova, N.; Kurbasic, E.; Jiang, W.G.; Blom, A.M. The novel complement inhibitor human CUB and Sushi multiple domains 1 (CSMD1) protein promotes factor I-mediated degradation of C4b and C3b and inhibits the membrane attack complex assembly. FASEB J. 2013, 27, 5083–5093. [Google Scholar] [CrossRef]
- Huang, T.; Liang, Y.; Zhang, H.; Chen, X.; Wei, H.; Sun, W.; Wang, Y. CSMD1 Mutations Are Associated with Increased Mutational Burden, Favorable Prognosis, and Anti-Tumor Immunity in Gastric Cancer. Genes 2021, 12, 1715. [Google Scholar] [CrossRef]
- Lin, S.; Li, M.; Zhou, Y.; Chen, L.; Wang, Y.; Zhuang, Z.; Zhao, H.; Yang, R. Annexin A3 accelerates osteoclast differentiation by promoting the level of RANK and TRAF6. Bone 2023, 172, 116758. [Google Scholar] [CrossRef]
- Chen, Y.; Di, M.; Tang, Y.; Zhao, J.; Wang, Q.; Guo, Z.; Li, Y.; Ouyang, D.; Yang, J.; Chen, H.; et al. Epstein-Barr virus causes vascular abnormalities in epithelial malignancies through upregulating ANXA3-HIF-1α-VEGF pathway. Oncogene 2024, 43, 2143–2159. [Google Scholar] [CrossRef]
- Loo, W.J.; Turchin, I.; Prajapati, V.H.; Gooderham, M.J.; Grewal, P.; Hong, C.; Sauder, M.; Vender, R.B.; Maari, C.; Papp, K.A. Clinical Implications of Targeting the JAK-STAT Pathway in Psoriatic Disease: Emphasis on the TYK2 Pathway. J. Cutan. Med. Surg. 2023, 27, 3S–24S. [Google Scholar] [CrossRef]
- Li, W.; Cao, T.; Luo, C.; Cai, J.; Zhou, X.; Xiao, X.; Liu, S. Crosstalk between ER stress, NLRP3 inflammasome, and inflammation. Appl. Microbiol. Biotechnol. 2020, 104, 6129–6140. [Google Scholar] [CrossRef]
- Marcinkowski, M.; Pilžys, T.; Garbicz, D.; Steciuk, J.; Zugaj, D.; Mielecki, D.; Sarnowski, T.J.; Grzesiuk, E. Human and Arabidopsis alpha-ketoglutarate-dependent dioxygenase homolog proteins—New players in important regulatory processes. IUBMB Life 2020, 72, 1126–1144. [Google Scholar] [CrossRef] [PubMed]
- Azzam, S.K.; Alsafar, H.; Sajini, A.A. FTO m6A Demethylase in Obesity and Cancer: Implications and Underlying Molecular Mechanisms. Int. J. Mol. Sci. 2022, 23, 3800. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; She, X.; Gu, C.; Hu, Y.; Ma, M.; Qiu, Q.; Sun, T.; Xu, X.; Chen, H.; Zheng, Z. FTO fuels diabetes-induced vascular endothelial dysfunction associated with inflammation by erasing m6A methylation of TNIP1. J. Clin. Investig. 2023, 133, e160517. [Google Scholar] [CrossRef]
- Bjune, J.-I.; Lawrence-Archer, L.; Røsland, G.V.; Tronstad, K.J.; Njølstad, P.R.; Sagen, J.V.; Dankel, S.N.; Mellgren, G. The homeobox factor Irx3 maintains adipogenic identity. Metabolism 2020, 103, 154014. [Google Scholar] [CrossRef]
- Sanghera, D.K.; Ortega, L.; Han, S.; Singh, J.; Ralhan, S.K.; Wander, G.S.; Mehra, N.K.; Mulvihill, J.J.; Ferrell, R.E.; Nath, S.K.; et al. Impact of nine common type 2 diabetes risk polymorphisms in Asian Indian Sikhs: PPARG2 (Pro12Ala), IGF2BP2, TCF7L2 and FTOvariants confer a significant risk. BMC Med. Genet. 2008, 9, 59. [Google Scholar] [CrossRef]
- Hunt, L.E.; Noyvert, B.; Bhaw-Rosun, L.; Sesay, A.K.; Paternoster, L.; Nohr, E.A.; Davey Smith, G.; Tommerup, N.; Sørensen, T.I.A.; Elgar, G. Complete re-sequencing of a 2Mb topological domain encompassing the FTO/IRXB genes identifies a novel obesity-associated region upstream of IRX5. Genome Med. 2015, 7, 126. [Google Scholar] [CrossRef]
- Tachmazidou, I.; Hatzikotoulas, K.; Southam, L.; Esparza-Gordillo, J.; Haberland, V.; Zheng, J.; Johnson, T.; Koprulu, M.; Zengini, E.; Steinberg, J.; et al. Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK Biobank data. Nat. Genet. 2019, 51, 230–236. [Google Scholar] [CrossRef]
- Boer, C.G.; Hatzikotoulas, K.; Southam, L.; Stefánsdóttir, L.; Zhang, Y.; Coutinho De Almeida, R.; Wu, T.T.; Zheng, J.; Hartley, A.; Teder-Laving, M.; et al. Deciphering osteoarthritis genetics across 826,690 individuals from 9 populations. Cell 2021, 184, 4784–4818.e17. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Zhang, J.; Yang, J.; Lv, Q.; Zhong, C. Overexpression of FTO alleviates osteoarthritis by regulating the processing of miR-515-5p and the TLR4/MyD88/NF-κB axis. Int. Immunopharmacol. 2023, 114, 109524. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Jiang, T.; Zheng, W.; Zhang, J.; Li, A.; Lu, C.; Lin, Z. FTO-mediated m6A demethylation of pri-miR-3591 alleviates osteoarthritis progression. Arthritis Res. Ther. 2023, 25, 53. [Google Scholar] [CrossRef]
- Massey, J.; Plant, D.; Hyrich, K.; Morgan, A.W.; Wilson, A.G.; Spiliopoulou, A.; Colombo, M.; McKeigue, P.; Isaacs, J.; Cordell, H.; et al. Genome-wide association study of response to tumour necrosis factor inhibitor therapy in rheumatoid arthritis. Pharmacogenom. J. 2018, 18, 657–664. [Google Scholar] [CrossRef]
- Jin, L.; Chen, Q.; Hu, K.; Fan, D.; Zhang, H.; Deng, J.; Qi, W.; Yu, Q. The FTO-CMPK2 Pathway in Fibroblast-like Synoviocytes Modulates Rheumatoid Arthritis Synovial Inflammation and Cartilage Homeostasis via mtDNA Regulation. Int. J. Biol. Sci. 2024, 20, 1617–1633. [Google Scholar] [CrossRef]
- Henkel, C.; Styrkársdóttir, U.; Thorleifsson, G.; Stefánsdóttir, L.; Björnsdóttir, G.; Banasik, K.; Brunak, S.; Erikstrup, C.; Dinh, K.M.; Hansen, T.F.; et al. Genome-wide association meta-analysis of knee and hip osteoarthritis uncovers genetic differences between patients treated with joint replacement and patients without joint replacement. Ann. Rheum. Dis. 2023, 82, 384–392. [Google Scholar] [CrossRef]
- McDonald, M.-L.N.; Lakshman Kumar, P.; Srinivasasainagendra, V.; Nair, A.; Rocco, A.P.; Wilson, A.C.; Chiles, J.W.; Richman, J.S.; Pinson, S.A.; Dennis, R.A.; et al. Novel genetic loci associated with osteoarthritis in multi-ancestry analyses in the Million Veteran Program and UK Biobank. Nat. Genet. 2022, 54, 1816–1826. [Google Scholar] [CrossRef]
- Nakajima, M.; Takahashi, A.; Kou, I.; Rodriguez-Fontenla, C.; Gomez-Reino, J.J.; Furuichi, T.; Dai, J.; Sudo, A.; Uchida, A.; Fukui, N.; et al. New Sequence Variants in HLA Class II/III Region Associated with Susceptibility to Knee Osteoarthritis Identified by Genome-Wide Association Study. PLoS ONE 2010, 5, e9723. [Google Scholar] [CrossRef]
- Saevarsdottir, S.; Stefansdottir, L.; Sulem, P.; Thorleifsson, G.; Ferkingstad, E.; Rutsdottir, G.; Glintborg, B.; Westerlind, H.; Grondal, G.; Loft, I.C.; et al. Multiomics analysis of rheumatoid arthritis yields sequence variants that have large effects on risk of the seropositive subset. Ann. Rheum. Dis. 2022, 81, 1085–1095. [Google Scholar] [CrossRef]
- Ishigaki, K.; Sakaue, S.; Terao, C.; Luo, Y.; Sonehara, K.; Yamaguchi, K.; Amariuta, T.; Too, C.L.; Laufer, V.A.; Scott, I.C.; et al. Multi-ancestry genome-wide association analyses identify novel genetic mechanisms in rheumatoid arthritis. Nat. Genet. 2022, 54, 1640–1651. [Google Scholar] [CrossRef]
- Liu, R.; Shang, X.; Fu, Y.; Wang, Y.; Wang, P.; Yan, S. Shared genetic architecture between hypothyroidism and rheumatoid arthritis: A large-scale cross-trait analysis. Mol. Immunol. 2024, 168, 17–24. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Ma, R.; Guo, X.; Zhang, G.; Liu, S.; Zhu, W.; Liu, H.; Gao, P. ETS-1-activated LINC01016 over-expression promotes tumor progression via suppression of RFFL-mediated DHX9 ubiquitination degradation in breast cancers. Cell Death Dis. 2023, 14, 507. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Li, W.; Wu, X.; Qian, Z.; Ying, J.; Gao, S.; He, J. Integrated analysis of single-cell and bulk RNA-sequencing identifies a signature based on B cell marker genes to predict prognosis and immunotherapy response in lung adenocarcinoma. Cancer Immunol. Immunother. 2022, 71, 2341–2354. [Google Scholar] [CrossRef] [PubMed]
- Alfaisal University; Dvornyk, V. Integrated in-depth bioinformatic analysis suggests RELCH/KIAA1468, LINC02341, and AKAP11 as candidate genes for ages at menarche and menopause. RRB 2021, 7, 220–231. [Google Scholar] [CrossRef]
- Franceschini, A.; Szklarczyk, D.; Frankild, S.; Kuhn, M.; Simonovic, M.; Roth, A.; Lin, J.; Minguez, P.; Bork, P.; Von Mering, C.; et al. STRING v9.1: Protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2012, 41, D808–D815. [Google Scholar] [CrossRef]
- Vitales-Noyola, M.; Hernández-Castro, B.; Alvarado-Hernández, D.; Baranda, L.; Bernal-Silva, S.; Abud-Mendoza, C.; Niño-Moreno, P.; González-Amaro, R. Levels of Pathogenic Th17 and Th22 Cells in Patients with Rheumatoid Arthritis. J. Immunol. Res. 2022, 2022, 5398743. [Google Scholar] [CrossRef]
- Yamada, H.; Nakashima, Y.; Okazaki, K.; Mawatari, T.; Fukushi, J.-I.; Oyamada, A.; Fujimura, K.; Iwamoto, Y.; Yoshikai, Y. Preferential Accumulation of Activated Th1 Cells Not Only in Rheumatoid Arthritis But Also in Osteoarthritis Joints. J. Rheumatol. 2011, 38, 1569–1575. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Sig. Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef]
- Liu, S.; Ma, H.; Zhang, H.; Deng, C.; Xin, P. Recent advances on signaling pathways and their inhibitors in rheumatoid arthritis. Clin. Immunol. 2021, 230, 108793. [Google Scholar] [CrossRef]
- Kovács, B.; Vajda, E.; Nagy, E.E. Regulatory Effects and Interactions of the Wnt and OPG-RANKL-RANK Signaling at the Bone-Cartilage Interface in Osteoarthritis. Int. J. Mol. Sci. 2019, 20, 4653. [Google Scholar] [CrossRef]
- Ostojic, M.; Zevrnja, A.; Vukojevic, K.; Soljic, V. Immunofluorescence Analysis of NF-κB and iNOS Expression in Different Cell Populations during Early and Advanced Knee Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 6461. [Google Scholar] [CrossRef]
- Sohn, D.H.; Sokolove, J.; Sharpe, O.; Erhart, J.C.; Chandra, P.E.; Lahey, L.J.; Lindstrom, T.M.; Hwang, I.; Boyer, K.A.; Andriacchi, T.P.; et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res. Ther. 2012, 14, R7. [Google Scholar] [CrossRef]
- Horváth, E.; Sólyom, Á.; Székely, J.; Nagy, E.E.; Popoviciu, H. Inflammatory and Metabolic Signaling Interfaces of the Hypertrophic and Senescent Chondrocyte Phenotypes Associated with Osteoarthritis. Int. J. Mol. Sci. 2023, 24, 16468. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, D.; Bai, X. Macrophages regulate the progression of osteoarthritis. Osteoarthr. Cartil. 2020, 28, 555–561. [Google Scholar] [CrossRef]
- Arra, M.; Swarnkar, G.; Ke, K.; Otero, J.E.; Ying, J.; Duan, X.; Maruyama, T.; Rai, M.F.; O’Keefe, R.J.; Mbalaviele, G.; et al. LDHA-mediated ROS generation in chondrocytes is a potential therapeutic target for osteoarthritis. Nat. Commun. 2020, 11, 3427. [Google Scholar] [CrossRef]
- Liu, B.; Xian, Y.; Chen, X.; Shi, Y.; Dong, J.; Yang, L.; An, X.; Shen, T.; Wu, W.; Ma, Y.; et al. Inflammatory Fibroblast-Like Synoviocyte-Derived Exosomes Aggravate Osteoarthritis via Enhancing Macrophage Glycolysis. Adv. Sci. 2024, 11, 2307338. [Google Scholar] [CrossRef]
- Van Den Bosch, M.H.J. Inflammation in osteoarthritis: Is it time to dampen the alarm(in) in this debilitating disease? Clin. Exp. Immunol. 2019, 195, 153–166. [Google Scholar] [CrossRef]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. 2013, 5, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lee, H.; Lee, C.; Fang, W.; Chen, H.; Salter, D.M.; Su, S. Association of a functional polymorphism in the promoter region of TLR-3 with osteoarthritis: A two-stage case–control study. J. Orthop. Res. 2013, 31, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Xu, E.; Xiao, Y.; Cai, X. Evaluation of the Relationship Between Common Variants in the TLR-9 Gene and Hip Osteoarthritis Susceptibility. Genet. Test. Mol. Biomark. 2019, 23, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.-T.; Lv, Z.-T.; Sheng, W.-B. The association between rs12901499 polymorphism in SMAD3 gene and risk of osteoarthritis: A meta-analysis. Ther. Clin. Risk Manag. 2018, 14, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Faber, B.G.; Frysz, M.; Boer, C.G.; Evans, D.S.; Ebsim, R.; Flynn, K.A.; Lundberg, M.; Southam, L.; Hartley, A.; Saunders, F.R.; et al. The identification of distinct protective and susceptibility mechanisms for hip osteoarthritis: Findings from a genome-wide association study meta-analysis of minimum joint space width and Mendelian randomisation cluster analyses. eBioMedicine 2023, 95, 104759. [Google Scholar] [CrossRef] [PubMed]
- Styrkarsdottir, U.; Stefansdottir, L.; Thorleifsson, G.; Stefansson, O.A.; Saevarsdottir, S.; Lund, S.H.; Rafnar, T.; Hoshijima, K.; Novak, K.; Oreiro, N.; et al. Meta-analysis of erosive hand osteoarthritis identifies four common variants that associate with relatively large effect. Ann. Rheum. Dis. 2023, 82, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, S.; Cai, H.; Lin, Y.; Zheng, X.; Zhang, B.; Xia, C. Overexpression of microRNA-634 suppresses survival and matrix synthesis of human osteoarthritis chondrocytes by targeting PIK3R1. Sci. Rep. 2016, 6, 23117. [Google Scholar] [CrossRef]
- Nakajima, M.; Shi, D.; Dai, J.; Tsezou, A.; Zheng, M.; Norman, P.E.; Chou, C.; Lee, M.T.M.; Hwang, J.; Kim, D.; et al. A large-scale replication study for the association of rs17039192 in HIF-2α with knee osteoarthritis. J. Orthop. Res. 2012, 30, 1244–1248. [Google Scholar] [CrossRef]
- Fernández-Torres, J.; Hernández-Díaz, C.; Espinosa-Morales, R.; Camacho-Galindo, J.; Galindo-Sevilla, N.D.C.; López-Macay, Á.; Zamudio-Cuevas, Y.; Martínez-Flores, K.; Santamaría-Olmedo, M.G.; Pineda, C.; et al. Polymorphic variation of hypoxia inducible factor-1 A (HIF1A) gene might contribute to the development of knee osteoarthritis: A pilot study. BMC Musculoskelet. Disord. 2015, 16, 218. [Google Scholar] [CrossRef]
- Philip, A. TGF-b signaling in cartilage homeostasis and osteoarthritis. Front. Biosci. 2012, S4, 251–268. [Google Scholar] [CrossRef]
- Thielen, N.; Van Der Kraan, P.; Van Caam, A. TGFβ/BMP Signaling Pathway in Cartilage Homeostasis. Cells 2019, 8, 969. [Google Scholar] [CrossRef]
- Zhai, G.; Doré, J.; Rahman, P. TGF-β signal transduction pathways and osteoarthritis. Rheumatol. Int. 2015, 35, 1283–1292. [Google Scholar] [CrossRef]
- Chan, B.Y.; Fuller, E.S.; Russell, A.K.; Smith, S.M.; Smith, M.M.; Jackson, M.T.; Cake, M.A.; Read, R.A.; Bateman, J.F.; Sambrook, P.N.; et al. Increased chondrocyte sclerostin may protect against cartilage degradation in osteoarthritis. Osteoarthr. Cartil. 2011, 19, 874–885. [Google Scholar] [CrossRef]
- Guo, H.; Huang, J.; Liang, Y.; Wang, D.; Zhang, H. Focusing on the hypoxia-inducible factor pathway: Role, regulation, and therapy for osteoarthritis. Eur. J. Med. Res. 2022, 27, 288. [Google Scholar] [CrossRef] [PubMed]
- Blaney Davidson, E.N.; Vitters, E.L.; Van Der Kraan, P.M.; Van Den Berg, W.B. Expression of transforming growth factor-β (TGFβ) and the TGFβ signalling molecule SMAD-2P in spontaneous and instability-induced osteoarthritis: Role in cartilage degradation, chondrogenesis and osteophyte formation. Ann. Rheum. Dis. 2006, 65, 1414–1421. [Google Scholar] [CrossRef]
- Van Der Kraan, P.M.; Van Den Berg, W.B. Osteophytes: Relevance and biology. Osteoarthr. Cartil. 2007, 15, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Liu, G.; Liu, X.; Zhou, Y.; Sun, Q.; Zhen, G.; Wang, X.; Hu, Y.; Gao, P.; Demehri, S.; et al. Angiogenesis stimulated by elevated PDGF-BB in subchondral bone contributes to osteoarthritis development. JCI Insight 2020, 5, e135446. [Google Scholar] [CrossRef]
- Ashraf, S.; Walsh, D.A. Angiogenesis in osteoarthritis. Curr. Opin. Rheumatol. 2008, 20, 573–580. [Google Scholar] [CrossRef]
- Roman-Blas, J.A.; Jimenez, S.A. Targeting NF-κB: A Promising Molecular Therapy in Inflammatory Arthritis. Int. Rev. Immunol. 2008, 27, 351–374. [Google Scholar] [CrossRef]
- De Rooy, D.P.C.; Yeremenko, N.G.; Wilson, A.G.; Knevel, R.; Lindqvist, E.; Saxne, T.; Krabben, A.; Leijsma, M.K.; Daha, N.A.; Tsonaka, S.; et al. Genetic studies on components of the Wnt signalling pathway and the severity of joint destruction in rheumatoid arthritis. Ann. Rheum. Dis. 2013, 72, 769–775, Correction in Ann. Rheum. Dis. 2014, 74, 319. https://doi.org/10.1136/annrheumdis-2012-202184corr1. [Google Scholar] [CrossRef]
- Ruyssen-Witrand, A.; Degboé, Y.; Cantagrel, A.; Nigon, D.; Lukas, C.; Scaramuzzino, S.; Allanore, Y.; Vittecoq, O.; Schaeverbeke, T.; Morel, J.; et al. Association between RANK, RANKL and OPG polymorphisms with ACPA and erosions in rheumatoid arthritis: Results from a meta-analysis involving three French cohorts. RMD Open 2016, 2, e000226. [Google Scholar] [CrossRef]
- Wielińska, J.; Kolossa, K.; Świerkot, J.; Dratwa, M.; Iwaszko, M.; Bugaj, B.; Wysoczańska, B.; Chaszczewska-Markowska, M.; Jeka, S.; Bogunia-Kubik, K. Polymorphisms within the RANK and RANKL Encoding Genes in Patients with Rheumatoid Arthritis: Association with Disease Progression and Effectiveness of the Biological Treatment. Arch. Immunol. Ther. Exp. 2020, 68, 24. [Google Scholar] [CrossRef]
- Furuya, T.; Hakoda, M.; Ichikawa, N.; Higami, K.; Nanke, Y.; Yago, T.; Kamatani, N.; Kotake, S. Associations between HLA-DRB1, RANK, RANKL, OPG, and IL-17 genotypes and disease severity phenotypes in Japanese patients with early rheumatoid arthritis. Clin. Rheumatol. 2007, 26, 2137–2141. [Google Scholar] [CrossRef]
- Iriyoda, T.M.V.; Flauzino, T.; Costa, N.T.; Lozovoy, M.A.B.; Reiche, E.M.V.; Simão, A.N.C. TGFB1 (rs1800470 and rs1800469) variants are independently associated with disease activity and autoantibodies in rheumatoid arthritis patients. Clin. Exp. Med. 2022, 22, 37–45. [Google Scholar] [CrossRef]
- Hussein, Y.M.; Mohamed, R.H.; El-Shahawy, E.E.; Alzahrani, S.S. Interaction between TGF-β1 (869C/T) polymorphism and biochemical risk factor for prediction of disease progression in rheumatoid arthritis. Gene 2014, 536, 393–397. [Google Scholar] [CrossRef]
- Paradowska-Gorycka, A.; Romanowska-Próchnicka, K.; Haladyj, E.; Manczak, M.; Maslinski, S.; Olesinska, M. Association of the Smad3 and NFATc2 gene polymorphisms and their serum levels with susceptibility to rheumatoid arthritis in Polish cohorts. Clin. Exp. Immunol. 2015, 179, 444–453. [Google Scholar] [CrossRef][Green Version]
- Elshazli, R.; Settin, A. Association of PTPN22 rs2476601 and STAT4 rs7574865 polymorphisms with rheumatoid arthritis: A meta-analysis update. Immunobiology 2015, 220, 1012–1024. [Google Scholar] [CrossRef]
- Bravo-Villagra, K.M.; Muñoz-Valle, J.F.; Baños-Hernández, C.J.; Cerpa-Cruz, S.; Navarro-Zarza, J.E.; Parra-Rojas, I.; Aguilar-Velázquez, J.A.; García-Arellano, S.; López-Quintero, A. STAT4 Gene Variant rs7574865 Is Associated with Rheumatoid Arthritis Activity and Anti-CCP Levels in the Western but Not in the Southern Population of Mexico. Genes 2024, 15, 241. [Google Scholar] [CrossRef] [PubMed]
- Arleevskaya, M.I.; Larionova, R.V.; Brooks, W.H.; Bettacchioli, E.; Renaudineau, Y. Toll-Like Receptors, Infections, and Rheumatoid Arthritis. Clin. Rev. Allergy Immunol. 2020, 58, 172–181. [Google Scholar] [CrossRef]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL–RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef]
- Schett, G.; Zwerina, J.; David, J.-P. The role of Wnt proteins in arthritis. Nat. Rev. Rheumatol. 2008, 4, 473–480. [Google Scholar] [CrossRef]
- Sen, M.; Reifert, J.; Lauterbach, K.; Wolf, V.; Rubin, J.S.; Corr, M.; Carson, D.A. Regulation of fibronectin and metalloproteinase expression by Wnt signaling in rheumatoid arthritis synoviocytes. Arthritis Rheum. 2002, 46, 2867–2877. [Google Scholar] [CrossRef]
- Sen, M.; Lauterbach, K.; El-Gabalawy, H.; Firestein, G.S.; Corr, M.; Carson, D.A. Expression and function of wingless and frizzled homologs in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2000, 97, 2791–2796. [Google Scholar] [CrossRef]
- Strunk, J. A new approach to studying angiogenesis in rheumatoid arthritis by means of power Doppler ultrasonography and measurement of serum vascular endothelial growth factor. Rheumatology 2004, 43, 1480–1483. [Google Scholar] [CrossRef][Green Version]
- Wang, S.-Y.; Liu, Y.-Y.; Ye, H.; Guo, J.-P.; Li, R.; Liu, X.; Li, Z.-G. Circulating Dickkopf-1 Is Correlated with Bone Erosion and Inflammation in Rheumatoid Arthritis. J. Rheumatol. 2011, 38, 821–827. [Google Scholar] [CrossRef]
- Kong, Y.-Y.; Feige, U.; Sarosi, I.; Bolon, B.; Tafuri, A.; Morony, S.; Capparelli, C.; Li, J.; Elliott, R.; McCabe, S.; et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 1999, 402, 304–309. [Google Scholar] [CrossRef]
- Lubberts, E.; Van Den Bersselaar, L.; Oppers-Walgreen, B.; Schwarzenberger, P.; Coenen-de Roo, C.J.J.; Kolls, J.K.; Joosten, L.A.B.; Van Den Berg, W.B. IL-17 Promotes Bone Erosion in Murine Collagen-Induced Arthritis Through Loss of the Receptor Activator of NF-κB Ligand/Osteoprotegerin Balance. J. Immunol. 2003, 170, 2655–2662. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Wu, W.; Nan, Y.; Sun, W.; Wang, Y. SMAD2 inhibits pyroptosis of fibroblast-like synoviocytes and secretion of inflammatory factors via the TGF-β pathway in rheumatoid arthritis. Arthritis Res. Ther. 2023, 25, 144. [Google Scholar] [CrossRef] [PubMed]
- Ba, X.; Huang, Y.; Shen, P.; Huang, Y.; Wang, H.; Han, L.; Lin, W.J.; Yan, H.J.; Xu, L.J.; Qin, K.; et al. WTD Attenuating Rheumatoid Arthritis via Suppressing Angiogenesis and Modulating the PI3K/AKT/mTOR/HIF-1α Pathway. Front. Pharmacol. 2021, 12, 696802. [Google Scholar] [CrossRef] [PubMed]
- Hayer, S.; Pundt, N.; Peters, M.A.; Wunrau, C.; Kühnel, I.; Neugebauer, K.; Strietholt, S.; Zwerina, J.; Korb, A.; Penninger, J.; et al. PI3Kγ regulates cartilage damage in chronic inflammatory arthritis. FASEB J. 2009, 23, 4288–4298. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, L.; Bian, Q.; Xie, L.; Liu, W.; Zhen, G.; Crane, J.L.; Zhou, X.; Cao, X. Aberrant Activation of TGF-β in Subchondral Bone at the Onset of Rheumatoid Arthritis Joint Destruction. J. Bone Miner. Res. 2015, 30, 2033–2043. [Google Scholar] [CrossRef]
- Guo, X.; Chen, G. Hypoxia-Inducible Factor Is Critical for Pathogenesis and Regulation of Immune Cell Functions in Rheumatoid Arthritis. Front. Immunol. 2020, 11, 1668. [Google Scholar] [CrossRef]
- Guan, S.-Y.; Leng, R.-X.; Tao, J.-H.; Li, X.-P.; Ye, D.-Q.; Olsen, N.; Zheng, S.G.; Pan, H.-F. Hypoxia-inducible factor-1α: A promising therapeutic target for autoimmune diseases. Expert Opin. Ther. Targets 2017, 21, 715–723. [Google Scholar] [CrossRef]
- Ibrahim, S.S.A.; Huttunen, K.M. Orchestrated modulation of rheumatoid arthritis via crosstalking intracellular signaling pathways. Inflammopharmacology 2021, 29, 965–974. [Google Scholar] [CrossRef]
- Traylor, M.; Knevel, R.; Cui, J.; Taylor, J.; Harm-Jan, W.; Conaghan, P.G.; Cope, A.P.; Curtis, C.; Emery, P.; Newhouse, S.; et al. Genetic associations with radiological damage in rheumatoid arthritis: Meta-analysis of seven genome-wide association studies of 2,775 cases. PLoS ONE 2019, 14, e0223246. [Google Scholar] [CrossRef]
- Hu, L.; Liu, R.; Zhang, L. Advance in bone destruction participated by JAK/STAT in rheumatoid arthritis and therapeutic effect of JAK/STAT inhibitors. Int. Immunopharmacol. 2022, 111, 109095. [Google Scholar] [CrossRef] [PubMed]
- Goldblatt, F.; O’Neill, S.G. Clinical aspects of autoimmune rheumatic diseases. Lancet 2013, 382, 797–808. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.; Gershwin, M.E. Human autoimmune diseases: A comprehensive update. J. Intern. Med. 2015, 278, 369–395. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.; Watt, F.E.; Vincent, T.L.; Dziedzic, K. Hand osteoarthritis: Clinical phenotypes, molecular mechanisms and disease management. Nat. Rev. Rheumatol. 2018, 14, 641–656. [Google Scholar] [CrossRef]
- Dório, M.; Deveza, L.A. Phenotypes in Osteoarthritis. Clin. Geriatr. Med. 2022, 38, 273–286. [Google Scholar] [CrossRef]
- Ye, J.; Xie, D.; Li, X.; Lu, N.; Zeng, C.; Lei, G.; Wei, J.; Li, J. Phenotypes of osteoarthritis-related knee pain and their transition over time: Data from the osteoarthritis initiative. BMC Musculoskelet. Disord. 2024, 25, 173. [Google Scholar] [CrossRef]
- Frisell, T.; Holmqvist, M.; Källberg, H.; Klareskog, L.; Alfredsson, L.; Askling, J. Familial Risks and Heritability of Rheumatoid Arthritis: Role of Rheumatoid Factor/Anti–Citrullinated Protein Antibody Status, Number and Type of Affected Relatives, Sex, and Age. Arthritis Rheum. 2013, 65, 2773–2782. [Google Scholar] [CrossRef]
- Riyazi, N.; Meulenbelt, I.; Kroon, H.M.; Ronday, K.H.; Hellio Le Graverand, M.-P.; Rosendaal, F.R.; Breedveld, F.C.; Slagboom, P.E.; Kloppenburg, M. Evidence for familial aggregation of hand, hip, and spine but not knee osteoarthritis in siblings with multiple joint involvement: The GARP study. Ann. Rheum. Dis. 2005, 64, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.; Khan, O.Y.; Zehra, S.; Azhar, A.; Fatima, S. Association between obesity and risk of knee osteoarthritis. Pak. J. Pharm. Sci. 2020, 33, 295–298. [Google Scholar] [PubMed]
- Bengtsson, C.; Malspeis, S.; Orellana, C.; Sparks, J.A.; Costenbader, K.H.; Karlson, E.W. Association Between Menopausal Factors and the Risk of Seronegative and Seropositive Rheumatoid Arthritis: Results from the Nurses’ Health Studies. Arthritis Care Res. 2017, 69, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Bikbov, M.M.; Kazakbaeva, G.M.; Gilmanshin, T.R.; Zainullin, R.M.; Rakhimova, E.M.; Fakhretdinova, A.A.; Tuliakova, A.M.; Rusakova, I.A.; Panda-Jonas, S.; Nuriev, I.F.; et al. Prevalence and associated factors of osteoarthritis in the Ural Eye and Medical Study and the Ural Very Old Study. Sci. Rep. 2022, 12, 12607. [Google Scholar] [CrossRef]
- Scublinsky, D.; Venarotti, H.; Citera, G.; Messina, O.D.; Scheines, E.; Rillo, O.; Arturi, A.; Hofman, J.; Somma, L.F.; Casado, G.; et al. The Prevalence of Rheumatoid Arthritis in Argentina: A Capture-Recapture Study in a City of Buenos Aires Province. JCR J. Clin. Rheumatol. 2010, 16, 317–321. [Google Scholar] [CrossRef]
- Ro, J.; Kim, S.H.; Kim, H.-R.; Lee, S.-H.; Min, H.K. Impact of lifestyle and comorbidities on seropositive rheumatoid arthritis risk from Korean health insurance data. Sci. Rep. 2022, 12, 2201. [Google Scholar] [CrossRef]
- Srikanth, V.K.; Fryer, J.L.; Zhai, G.; Winzenberg, T.M.; Hosmer, D.; Jones, G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr. Cartil. 2005, 13, 769–781. [Google Scholar] [CrossRef]
- Haugen, I.K.; Englund, M.; Aliabadi, P.; Niu, J.; Clancy, M.; Kvien, T.K.; Felson, D.T. Prevalence, incidence and progression of hand osteoarthritis in the general population: The Framingham Osteoarthritis Study. Ann. Rheum. Dis. 2011, 70, 1581–1586, Correction in Ann. Rheum. Dis. 2018, 77, 1546. https://doi.org/10.1136/ard.2011.150078corr1. [Google Scholar] [CrossRef]
- Parazzini, F. Menopausal status, hormone replacement therapy use and risk of self-reported physician-diagnosed osteoarthritis in women attending menopause clinics in Italy. Maturitas 2003, 46, 207–212. [Google Scholar] [CrossRef]
- Tan, X.; Mei, Y.; Zhou, Y.; Liao, Z.; Zhang, P.; Liu, Y.; Han, Y.; Wang, D. Causal association of menstrual reproductive factors on the risk of osteoarthritis: A univariate and multivariate Mendelian randomization study. PLoS ONE 2024, 19, e0307958. [Google Scholar] [CrossRef]
- Orellana, C.; Saevarsdottir, S.; Klareskog, L.; Karlson, E.W.; Alfredsson, L.; Bengtsson, C. Postmenopausal hormone therapy and the risk of rheumatoid arthritis: Results from the Swedish EIRA population-based case-control study. Eur. J. Epidemiol. 2015, 30, 449–457. [Google Scholar] [CrossRef]
- Jung, J.H.; Bang, C.H.; Song, G.G.; Kim, C.; Kim, J.-H.; Choi, S.J. Knee osteoarthritis and menopausal hormone therapy in postmenopausal women: A nationwide cross-sectional study. Menopause 2019, 26, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Sandmark, H.; Hogstedt, C.; Lewold, S.; Vingård, E. Osteoarthrosis of the knee in men and women in association with overweight, smoking, and hormone therapy. Ann. Rheum. Dis. 1999, 58, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.; Seo, J.; Shin, Y.; Han, G.H.; Yoon, S.-H.; Noh, J.H.; Kim, M.H.; Yuk, J.-S. Menopausal Hormone Therapy and Osteoarthritis Risk: Retrospective Population-Based Study in South Korea. J. Menopausal. Med. 2024, 30, 78. [Google Scholar] [CrossRef] [PubMed]
- Von Mühlen, D.; Morton, D.; Von Mühlen, C.A.; Barrett-Connor, E. Postmenopausal Estrogen and Increased Risk of Clinical Osteoarthritis at the Hip, Hand, and Knee in Older Women. J. Women’s Health Gend.-Based Med. 2002, 11, 511–518. [Google Scholar] [CrossRef]
- Jørgensen, K.T.; Pedersen, B.V.; Jacobsen, S.; Biggar, R.J.; Frisch, M. National cohort study of reproductive risk factors for rheumatoid arthritis in Denmark: A role for hyperemesis, gestational hypertension and pre-eclampsia? Ann. Rheum. Dis. 2010, 69, 358–363. [Google Scholar] [CrossRef]
- Orellana, C.; Wedrén, S.; Källberg, H.; Holmqvist, M.; Karlson, E.W.; Alfredsson, L.; Bengtsson, C. Parity and the risk of developing rheumatoid arthritis: Results from the Swedish Epidemiological Investigation of Rheumatoid Arthritis study. Ann. Rheum. Dis. 2014, 73, 752–755. [Google Scholar] [CrossRef]
- Pikwer, M.; Orellana, C.; Källberg, H.; Pikwer, A.; Turesson, C.; Klareskog, L.; Alfredsson, L.; Saevarsdottir, S.; Bengtsson, C. Parity influences the severity of ACPA-negative early rheumatoid arthritis: A cohort study based on the Swedish EIRA material. Arthritis Res. Ther. 2015, 17, 358. [Google Scholar] [CrossRef]
- Ham, D.; Bae, S. Associations of breastfeeding duration and the total number of children breastfed with self-reported osteoarthritis in Korea women 50 years and older: A cross-sectional study. Epidemiol. Health 2023, 45, e2023044. [Google Scholar] [CrossRef]
- Wang, A.; Zawadzki, N.; Hedlin, H.; LeBlanc, E.; Budrys, N.; Van Horn, L.; Gass, M.; Westphal, L.; Stefanick, M. Reproductive history and osteoarthritis in the Women’s Health Initiative. Scand. J. Rheumatol. 2021, 50, 58–67. [Google Scholar] [CrossRef]
- Pedersen, M.; Jacobsen, S.; Klarlund, M.; Pedersen, B.V.; Wiik, A.; Wohlfahrt, J.; Frisch, M. Environmental risk factors differ between rheumatoid arthritis with and without auto-antibodies against cyclic citrullinated peptides. Arthritis Res. Ther. 2006, 8, R133. [Google Scholar] [CrossRef] [PubMed]
- Orellana, C.; Saevarsdottir, S.; Klareskog, L.; Karlson, E.W.; Alfredsson, L.; Bengtsson, C. Oral contraceptives, breastfeeding and the risk of developing rheumatoid arthritis: Results from the Swedish EIRA study. Ann. Rheum. Dis. 2017, 76, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- Karlson, E.W.; Mandl, L.A.; Hankinson, S.E.; Grodstein, F. Do breast-feeding and other reproductive factors influence future risk of rheumatoid arthritis?: Results from the Nurses’ Health Study. Arthritis Rheum. 2004, 50, 3458–3467. [Google Scholar] [CrossRef] [PubMed]
- Jacobsson, L.T.H. Perinatal characteristics and risk of rheumatoid arthritis. BMJ 2003, 326, 1068–1069. [Google Scholar] [CrossRef][Green Version]
- Kim, M.-Y.; Kim, H.-J.; Noh, J.-H.; Kim, S.-A.; Hwang, D.-S.; Lee, C.-H.; Ha, I.-H. Relationship of breastfeeding duration with joint pain and knee osteoarthritis in middle-aged Korean women: A cross-sectional study using the Korea National Health and Nutrition Examination Survey. BMC Women’s Health 2020, 20, 213. [Google Scholar] [CrossRef]
- Yoshida, K.; Wang, J.; Malspeis, S.; Marchand, N.; Lu, B.; Prisco, L.C.; Martin, L.W.; Ford, J.A.; Costenbader, K.H.; Karlson, E.W.; et al. Passive Smoking Throughout the Life Course and the Risk of Incident Rheumatoid Arthritis in Adulthood Among Women. Arthritis Rheumatol. 2021, 73, 2219–2228. [Google Scholar] [CrossRef]
- Carlens, C.; Jacobsson, L.; Brandt, L.; Cnattingius, S.; Stephansson, O.; Askling, J. Perinatal characteristics, early life infections and later risk of rheumatoid arthritis and juvenile idiopathic arthritis. Ann. Rheum. Dis. 2009, 68, 1159–1164. [Google Scholar] [CrossRef]
- Mandl, L.A.; Costenbader, K.H.; Simard, J.F.; Karlson, E.W. Is birthweight associated with risk of rheumatoid arthritis? Data from a large cohort study. Ann. Rheum. Dis. 2009, 68, 514–518. [Google Scholar] [CrossRef]
- Jordan, K.M.; Syddall, H.; Dennison, E.M.; Cooper, C.; Arden, N.K. Birthweight, vitamin D receptor gene polymorphism, and risk of lumbar spine osteoarthritis. J. Rheumatol. 2005, 32, 678–683. [Google Scholar]
- Hussain, S.M.; Wang, Y.; Wluka, A.E.; Shaw, J.E.; Magliano, D.J.; Graves, S.; Cicuttini, F.M. Association of Low Birth Weight and Preterm Birth with the Incidence of Knee and Hip Arthroplasty for Osteoarthritis. Arthritis Care Res. 2015, 67, 502–508. [Google Scholar] [CrossRef]
- Clynes, M.A.; Parsons, C.; Edwards, M.H.; Jameson, K.A.; Harvey, N.C.; Aihie Sayer, A.; Cooper, C.; Dennison, E.M. Further evidence of the developmental origins of osteoarthritis: Results from the Hertfordshire Cohort Study. J. Dev. Orig. Health Dis. 2014, 5, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Wesley, A.; Bengtsson, C.; Elkan, A.; Klareskog, L.; Alfredsson, L.; Wedrén, S.; for the Epidemiological Investigation of Rheumatoid Arthritis Study Group. Association between body mass index and anti–citrullinated protein antibody–positive and anti–citrullinated protein antibody–negative rheumatoid arthritis: Results from a population-based case–control study. Arthritis Care Res. 2013, 65, 107–112. [Google Scholar] [CrossRef]
- Lahiri, M.; Luben, R.N.; Morgan, C.; Bunn, D.K.; Marshall, T.; Lunt, M.; Verstappen, S.M.M.; Symmons, D.P.M.; Khaw, K.-T.; Wareham, N.; et al. Using lifestyle factors to identify individuals at higher risk of inflammatory polyarthritis (results from the European Prospective Investigation of Cancer-Norfolk and the Norfolk Arthritis Register—The EPIC-2-NOAR Study). Ann. Rheum. Dis. 2014, 73, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Rong, J.; Wang, Y.; Hu, F.; Bao, C.; Li, X.; Zhao, Y. The relationship between body mass index and hip osteoarthritis: A systematic review and meta-analysis. Jt. Bone Spine 2011, 78, 150–155. [Google Scholar] [CrossRef]
- Jiang, L.; Tian, W.; Wang, Y.; Rong, J.; Bao, C.; Liu, Y.; Zhao, Y.; Wang, C. Body mass index and susceptibility to knee osteoarthritis: A systematic review and meta-analysis. Jt. Bone Spine 2012, 79, 291–297. [Google Scholar] [CrossRef]
- Holliday, K.L.; McWilliams, D.F.; Maciewicz, R.A.; Muir, K.R.; Zhang, W.; Doherty, M. Lifetime body mass index, other anthropometric measures of obesity and risk of knee or hip osteoarthritis in the GOAL case-control study. Osteoarthr. Cartil. 2011, 19, 37–43. [Google Scholar] [CrossRef]
- Grotle, M.; Hagen, K.B.; Natvig, B.; Dahl, F.A.; Kvien, T.K. Obesity and osteoarthritis in knee, hip and/or hand: An epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet. Disord. 2008, 9, 132. [Google Scholar] [CrossRef]
- Jiang, L.; Xie, X.; Wang, Y.; Wang, Y.; Lu, Y.; Tian, T.; Chu, M.; Shen, Y. Body mass index and hand osteoarthritis susceptibility: An updated meta-analysis. Int. J. Rheum. Dis. 2016, 19, 1244–1254. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, J.; Chen, L.; Xiong, Y.; Wu, T.; Shen, S.; Wang, X.; Meng, X.; Lu, Y.; Lei, X. Causal Association of Coffee Consumption and Total, Knee, Hip and Self-Reported Osteoarthritis: A Mendelian Randomization Study. Front. Endocrinol. 2021, 12, 768529. [Google Scholar] [CrossRef]
- Bang, C.H.; Kim, C.; Kim, J.-H.; Choi, S.J.; Song, G.G.; Jung, J.H. Is knee osteoarthritis related to coffee drinking? A nationwide cross-sectional observational study. Clin. Rheumatol. 2019, 38, 817–825. [Google Scholar] [CrossRef]
- Sundström, B.; Johansson, I.; Rantapää-Dahlqvist, S. Diet and alcohol as risk factors for rheumatoid arthritis: A nested case–control study. Rheumatol. Int. 2015, 35, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, K.; Mathiessen, A.; Hammer, H.; Kvien, T.; Slatkowsky-Christensen, B.; Natvig, B.; Hagen, K.; Østerås, N.; Haugen, I. Smoking and alcohol use are associated with structural and inflammatory hand osteoarthritis features. Scand. J. Rheumatol. 2017, 46, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Haugen, I.K.; Magnusson, K.; Turkiewicz, A.; Englund, M. The Prevalence, Incidence, and Progression of Hand Osteoarthritis in Relation to Body Mass Index, Smoking, and Alcohol Consumption. J. Rheumatol. 2017, 44, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Klareskog, L.; Stolt, P.; Lundberg, K.; Källberg, H.; Bengtsson, C.; Grunewald, J.; Rönnelid, J.; Erlandsson Harris, H.; Ulfgren, A.; Rantapää-Dahlqvist, S.; et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA–DR (shared epitope)–restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006, 54, 38–46. [Google Scholar] [CrossRef]
- Yahya, A.; Bengtsson, C.; Lai, T.C.; Larsson, P.T.; Mustafa, A.N.; Abdullah, N.A.; Muhamad, N.; Hussein, H.; Klareskog, L.; Alfredsson, L.; et al. Smoking is associated with an increased risk of developing ACPA-positive but not ACPA-negative rheumatoid arthritis in Asian populations: Evidence from the Malaysian MyEIRA case–control study. Mod. Rheumatol. 2012, 22, 524–531. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, C.; Li, H.; Yang, T.; Deng, Z.; Yang, Y.; Ding, X.; Xie, D.; Wang, Y.; Lei, G. Relationship between cigarette smoking and radiographic knee osteoarthritis in Chinese population: A cross-sectional study. Rheumatol. Int. 2015, 35, 1211–1217. [Google Scholar] [CrossRef]
- Kong, L.; Wang, L.; Meng, F.; Cao, J.; Shen, Y. Association between smoking and risk of knee osteoarthritis: A systematic review and meta-analysis. Osteoarthr. Cartil. 2017, 25, 809–816. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, S.Y. Correlation between radiographic knee osteoarthritis and lifetime cigarette smoking amount in a Korean population: A cross-sectional study. Medicine 2020, 99, e20839. [Google Scholar] [CrossRef]
- Olsson, Å.R.; Skogh, T.; Wingren, G. Comorbidity and lifestyle, reproductive factors, and environmental exposures associated with rheumatoid arthritis. Ann. Rheum. Dis. 2001, 60, 934–939, Correction in Ann. Rheum. Dis. 2001, 60, 1161. https://doi.org/10.1136/ard.60.12.1161. [Google Scholar] [CrossRef]
- Vallerand, I.A.; Lewinson, R.T.; Frolkis, A.D.; Lowerison, M.W.; Kaplan, G.G.; Swain, M.G.; Bulloch, A.G.M.; Patten, S.B.; Barnabe, C. Depression as a risk factor for the development of rheumatoid arthritis: A population-based cohort study. RMD Open 2018, 4, e000670. [Google Scholar] [CrossRef]
- Sparks, J.A.; Malspeis, S.; Hahn, J.; Wang, J.; Roberts, A.L.; Kubzansky, L.D.; Costenbader, K.H. Depression and Subsequent Risk for Incident Rheumatoid Arthritis Among Women. Arthritis Care Res. 2021, 73, 78–89. [Google Scholar] [CrossRef]
- Jung, J.H.; Seok, H.; Kim, J.; Song, G.G.; Choi, S.J. Association between osteoarthritis and mental health in a Korean population: A nationwide study. Int. J. Rheum. Dis. 2018, 21, 611–619. [Google Scholar] [CrossRef]
- Park, H.; Kim, H.; Lee, Y. Knee osteoarthritis and its association with mental health and health-related quality of life: A nationwide cross-sectional study. Geriatr. Gerontol. Int. 2020, 20, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, N.; Sasaki, S.; Iwasaki, K.; Danjoh, S.; Kinoshita, H.; Yasuda, T.; Tamaki, T.; Hashimoto, T.; Kellingray, S.; Croft, P.; et al. Occupational lifting is associated with hip osteoarthritis: A Japanese case-control study. J. Rheumatol. 2000, 27, 434–440. [Google Scholar] [PubMed]
- Coggon, D.; Croft, P.; Kellingray, S.; Barrett, D.; McLaren, M.; Cooper, C. Occupational physical activities and osteoarthritis of the knee. Arthritis Rheum. 2000, 43, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Arleevskaya, M.; Takha, E.; Petrov, S.; Kazarian, G.; Renaudineau, Y.; Brooks, W.; Larionova, R.; Korovina, M.; Valeeva, A.; Shuralev, E.; et al. Interplay of Environmental, Individual and Genetic Factors in Rheumatoid Arthritis Provocation. Int. J. Mol. Sci. 2022, 23, 8140. [Google Scholar] [CrossRef]
- Bolduc, J.A.; Collins, J.A.; Loeser, R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef]
- Loeser, R.F. Aging and osteoarthritis: The role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthr. Cartil. 2009, 17, 971–979. [Google Scholar] [CrossRef]
- Zamudio-Cuevas, Y.; Martínez-Flores, K.; Martínez-Nava, G.A.; Clavijo-Cornejo, D.; Fernández-Torres, J.; Sánchez-Sánchez, R. Rheumatoid arthritis and oxidative stress, a review of a decade. Cell. Mol. Biol. 2022, 68, 174–184. [Google Scholar] [CrossRef]
- Darrah, E.; Andrade, F. Editorial: Citrullination, and Carbamylation, and Malondialdehyde-Acetaldehyde! Oh My! Entering the Forest of Autoantigen Modifications in Rheumatoid Arthritis. Arthritis Rheumatol. 2015, 67, 604–608. [Google Scholar] [CrossRef]
- Verheul, M.K.; Böhringer, S.; Van Delft, M.A.M.; Jones, J.D.; Rigby, W.F.C.; Gan, R.W.; Holers, V.M.; Edison, J.D.; Deane, K.D.; Janssen, K.M.J.; et al. Triple Positivity for Anti–Citrullinated Protein Autoantibodies, Rheumatoid Factor, and Anti–Carbamylated Protein Antibodies Conferring High Specificity for Rheumatoid Arthritis: Implications for Very Early Identification of At-Risk Individuals. Arthritis Rheumatol. 2018, 70, 1721–1731. [Google Scholar] [CrossRef]
- Takha, E.A.; Larionova, R.V.; Petrov, S.V.; Kazarian, G.G.; Valeeva, A.R.; Korovina, M.O.; Shamaev, N.D.; Pipchenko, A.P.; Renaudineau, Y.; Kravtsova, O.A.; et al. Possible mechanism of the implementing the trigger role of air pollution in rheumatoid arthritis (preliminary data). Hyg. Sanit. 2022, 101, 139–145. [Google Scholar] [CrossRef]
- Weyand, C.M.; Goronzy, J.J. Aging of the Immune System. Mechanisms and Therapeutic Targets. Ann. Am. Thorac. Soc. 2016, 13, S422–S428. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.H.; Lenz, K.L.; Pollitt, E.N.; Ferguson, D.; Hutson, I.; Springer, L.E.; Oestreich, A.K.; Tang, R.; Choi, Y.-R.; Meyer, G.A.; et al. Adipose tissue is a critical regulator of osteoarthritis. Proc. Natl. Acad. Sci. USA 2021, 118, e2021096118. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef]
- Hao, D.; Li, M.; Wu, Z.; Duan, Y.; Li, D.; Qiu, G. Synovial fluid level of adiponectin correlated with levels of aggrecan degradation markers in osteoarthritis. Rheumatol. Int. 2011, 31, 1433–1437. [Google Scholar] [CrossRef]
- Xu, A.; Chan, K.W.; Hoo, R.L.C.; Wang, Y.; Tan, K.C.B.; Zhang, J.; Chen, B.; Lam, M.C.; Tse, C.; Cooper, G.J.S.; et al. Testosterone Selectively Reduces the High Molecular Weight Form of Adiponectin by Inhibiting Its Secretion from Adipocytes. J. Biol. Chem. 2005, 280, 18073–18080. [Google Scholar] [CrossRef]
- Nishizawa, H.; Shimomura, I.; Kishida, K.; Maeda, N.; Kuriyama, H.; Nagaretani, H.; Matsuda, M.; Kondo, H.; Furuyama, N.; Kihara, S.; et al. Androgens Decrease Plasma Adiponectin, an Insulin-Sensitizing Adipocyte-Derived Protein. Diabetes 2002, 51, 2734–2741. [Google Scholar] [CrossRef]
- Laughlin, G.A.; Barrett-Connor, E.; May, S. Sex–specific association of the androgen to oestrogen ratio with adipocytokine levels in older adults: The Rancho Bernardo Study. Clin. Endocrinol. 2006, 65, 506–513. [Google Scholar] [CrossRef]
- Boyne, M.S.; Bennett, N.R.; Cooper, R.S.; Royal-Thomas, T.Y.; Bennett, F.I.; Luke, A.; Wilks, R.J.; Forrester, T.E. Sex-differences in adiponectin levels and body fat distribution: Longitudinal observations in Afro-Jamaicans. Diabetes Res. Clin. Pract. 2010, 90, e33–e36. [Google Scholar] [CrossRef]
- Xu, X.; Wen, J.; Lu, Y.; Ji, H.; Zhuang, J.; Su, Y.; Liu, B.; Li, H.; Xu, Y. Impact of age on plasma vaspin concentration in a group of normal Chinese people. J. Endocrinol. Investig. 2017, 40, 143–151. [Google Scholar] [CrossRef]
- Bellissimo, M.P.; Hsu, E.; Hao, L.; Easley, K.; Martin, G.S.; Ziegler, T.R.; Alvarez, J.A. Relationships between plasma apelin and adiponectin with normal weight obesity, body composition, and cardiorespiratory fitness in working adults. J. Clin. Transl. Endocrinol. 2021, 24, 100257. [Google Scholar] [CrossRef]
- Cruz-Mejía, S.; Durán López, H.H.; Navarro Meza, M.; Xochihua Rosas, I.; De La Peña, S.; Arroyo Helguera, O.E. Body mass index is associated with interleukin-1, adiponectin, oxidative stress and ioduria levels in healthy adults. Nutr. Hosp. 2018, 35, 841. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Feng, D.; Qu, X.; Fu, J.; Wang, Y.; Li, L.; Li, L.; Han, L.; Esangbedo, I.C.; Li, M.; et al. Role of adipokines FGF21, leptin and adiponectin in self-concept of youths with obesity. Eur. Neuropsychopharmacol. 2018, 28, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Vasileiadis, G.K.; Lundell, A.-C.; Zhang, Y.; Andersson, K.; Gjertsson, I.; Rudin, A.; Maglio, C. Adipocytokines in Untreated Newly Diagnosed Rheumatoid Arthritis: Association with Circulating Chemokines and Markers of Inflammation. Biomolecules 2021, 11, 325. [Google Scholar] [CrossRef]
- Otero, M.; Lago, R.; Gomez, R.; Lago, F.; Dieguez, C.; Gómez-Reino, J.J.; Gualillo, O. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2006, 65, 1198–1201. [Google Scholar] [CrossRef]
- Yoshino, T.; Kusunoki, N.; Tanaka, N.; Kaneko, K.; Kusunoki, Y.; Endo, H.; Hasunuma, T.; Kawai, S. Elevated Serum Levels of Resistin, Leptin, and Adiponectin are Associated with C-reactive Protein and also Other Clinical Conditions in Rheumatoid Arthritis. Intern. Med. 2011, 50, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Nugzar, O.; Zandman-Goddard, G.; Oz, H.; Lakstein, D.; Feldbrin, Z.; Shargorodsky, M. The role of ferritin and adiponectin as predictors of cartilage damage assessed by arthroscopy in patients with symptomatic knee osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2018, 32, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.B. The complex role of adipokines in obesity, inflammation, and autoimmunity. Clin. Sci. 2021, 135, 731–752. [Google Scholar] [CrossRef]
- Ko, C.-Y.; Lin, Y.-Y.; Achudhan, D.; Chang, J.-W.; Liu, S.-C.; Lai, C.-Y.; Huang, Y.-L.; Tsai, C.-H.; Fong, Y.-C.; Chen, H.-T.; et al. Omentin-1 ameliorates the progress of osteoarthritis by promoting IL-4-dependent anti-inflammatory responses and M2 macrophage polarization. Int. J. Biol. Sci. 2023, 19, 5275–5289. [Google Scholar] [CrossRef]
- Chai, B.; Zheng, Z.-H.; Liao, X.; Li, K.-Y.; Liang, J.-S.; Huang, Y.-X.; Tong, C.-J.; Ou, D.-J.; Lu, J. The protective role of omentin-1 in IL-1β-induced chondrocyte senescence. Artif. Cells Nanomed. Biotechnol. 2020, 48, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Economou, A.; Mallia, I.; Fioravanti, A.; Gentileschi, S.; Nacci, F.; Bellando Randone, S.; Lepri, G.; Guiducci, S. The Role of Adipokines between Genders in the Pathogenesis of Osteoarthritis. Int. J. Mol. Sci. 2024, 25, 10865. [Google Scholar] [CrossRef] [PubMed]
- Valencak, T.G.; Osterrieder, A.; Schulz, T.J. Sex matters: The effects of biological sex on adipose tissue biology and energy metabolism. Redox Biol. 2017, 12, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Alissa, E.M.; Al-Salmi, M.M.; Alama, N.A.; Ferns, G.A. Role of omentin-1 and C-reactive protein in obese subjects with subclinical inflammation. J. Clin. Transl. Endocrinol. 2016, 3, 7–11. [Google Scholar] [CrossRef]
- Çimen, A. Serum Omentin-1 Levels and Endothelial Dysfunction in Obesity. Acta Endo. 2017, 13, 138–143. [Google Scholar] [CrossRef]
- Oświęcimska, J.; Suwała, A.; Świętochowska, E.; Ostrowska, Z.; Gorczyca, P.; Ziora-Jakutowicz, K.; Machura, E.; Szczepańska, M.; Kukla, M.; Stojewska, M.; et al. Serum Omentin Levels in Adolescent Girls with Anorexia Nervosa and Obesity. Physiol. Res. 2015, 64, 701–709. [Google Scholar] [CrossRef]
- Robinson, C.; Tsang, L.; Solomon, A.; Woodiwiss, A.J.; Gunter, S.; Millen, A.M.E.; Norton, G.R.; Fernandez-Lopez, M.J.; Hollan, I.; Dessein, P.H. Omentin concentrations are independently associated with those of matrix metalloproteinase-3 in patients with mild but not severe rheumatoid arthritis. Rheumatol. Int. 2017, 37, 3–11. [Google Scholar] [CrossRef]
- Li, Z.-G.; Zhao, D.-W.; Xia, C.-J.; Wang, T.-N.; Liu, Y.-P.; Zhang, Y.; Wang, B.-J. Decreased synovial fluid omentin-1 concentrations reflect symptomatic severity in patients with knee osteoarthritis. Scand. J. Clin. Lab. Investig. 2012, 72, 623–628. [Google Scholar] [CrossRef]
- Chi, Y.; Chai, J.; Xu, C.; Luo, H.; Zhang, Q. Apelin inhibits the activation of the nucleotide-binding domain and the leucine-rich, repeat-containing family, pyrin-containing 3 (NLRP3) inflammasome and ameliorates insulin resistance in severely burned rats. Surgery 2015, 157, 1142–1152. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Li, P.; Zheng, Y.; Yang, Y.; Ji, S. Apelin/APJ system in inflammation. Int. Immunopharmacol. 2022, 109, 108822. [Google Scholar] [CrossRef]
- Hu, P.-F.; Chen, W.-P.; Tang, J.-L.; Bao, J.-P.; Wu, L.-D. Apelin plays a catabolic role on articular cartilage: In vivo and in vitro studies. Int. J. Mol. Med. 2010, 26, 357–363. [Google Scholar] [CrossRef]
- Anima, B.; Gurusubramanian, G.; Roy, V.K. Hormonal dependent expression of apelin and apelin receptor in the ovary and uterus of mice. Reprod. Biol. 2024, 24, 100918. [Google Scholar] [CrossRef] [PubMed]
- Tekin, S.; Erden, Y.; Sandal, S.; Etem Onalan, E.; Ozyalin, F.; Ozen, H.; Yilmaz, B. Effects of apelin on reproductive functions: Relationship with feeding behavior and energy metabolism. Arch. Physiol. Biochem. 2017, 123, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Butruille, L.; Drougard, A.; Knauf, C.; Moitrot, E.; Valet, P.; Storme, L.; Deruelle, P.; Lesage, J. The apelinergic system: Sexual dimorphism and tissue-specific modulations by obesity and insulin resistance in female mice. Peptides 2013, 46, 94–101. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, L.; Tang, M.; Guo, Y.; Li, L. Apelin/APJ system: A novel promising target for anti-aging intervention. Clin. Chim. Acta 2018, 487, 233–240. [Google Scholar] [CrossRef]
- Rai, R.; Ghosh, A.K.; Eren, M.; Mackie, A.R.; Levine, D.C.; Kim, S.-Y.; Cedernaes, J.; Ramirez, V.; Procissi, D.; Smith, L.H.; et al. Downregulation of the Apelinergic Axis Accelerates Aging, whereas Its Systemic Restoration Improves the Mammalian Healthspan. Cell Rep. 2017, 21, 1471–1480. [Google Scholar] [CrossRef]
- Soriguer, F.; Garrido-Sanchez, L.; Garcia-Serrano, S.; Garcia-Almeida, J.M.; Garcia-Arnes, J.; Tinahones, F.J.; Garcia-Fuentes, E. Apelin Levels Are Increased in Morbidly Obese Subjects with Type 2 Diabetes Mellitus. Obes. Surg. 2009, 19, 1574–1580. [Google Scholar] [CrossRef]
- Castan-Laurell, I.; Vítkova, M.; Daviaud, D.; Dray, C.; Kováčiková, M.; Kovacova, Z.; Hejnova, J.; Stich, V.; Valet, P. Effect of hypocaloric diet-induced weight loss in obese women on plasma apelin and adipose tissue expression of apelin and APJ. Eur. J. Endocrinol. 2008, 158, 905–910. [Google Scholar] [CrossRef]
- Di Franco, M.; Spinelli, F.R.; Metere, A.; Gerardi, M.C.; Conti, V.; Boccalini, F.; Iannuccelli, C.; Ciciarello, F.; Agati, L.; Valesini, G. Serum Levels of Asymmetric Dimethylarginine and Apelin as Potential Markers of Vascular Endothelial Dysfunction in Early Rheumatoid Arthritis. Mediat. Inflamm. 2012, 2012, 347268. [Google Scholar] [CrossRef]
- Chang, T.-K.; Zhong, Y.-H.; Liu, S.-C.; Huang, C.-C.; Tsai, C.-H.; Lee, H.-P.; Wang, S.-W.; Hsu, C.-J.; Tang, C.-H. Apelin Promotes Endothelial Progenitor Cell Angiogenesis in Rheumatoid Arthritis Disease via the miR-525-5p/Angiopoietin-1 Pathway. Front. Immunol. 2021, 12, 737990. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Kuo, S.-J.; Liu, S.-C.; Wang, S.-W.; Tsai, C.-H.; Fong, Y.-C.; Tang, C.-H. Apelin Affects the Progression of Osteoarthritis by Regulating VEGF-Dependent Angiogenesis and miR-150-5p Expression in Human Synovial Fibroblasts. Cells 2020, 9, 594. [Google Scholar] [CrossRef] [PubMed]
- Heiker, J.T. Vaspin (serpinA12) in obesity, insulin resistance, and inflammation: Molecular mechanisms of vaspin function. J. Pept. Sci. 2014, 20, 299–306. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, K.; Zhang, S.; Guan, Z. Vaspin promotes chondrogenic differentiation of BMSCs via Akt activation in osteoarthritis. BMC Musculoskelet. Disord. 2022, 23, 344. [Google Scholar] [CrossRef] [PubMed]
- Wyskida, K.; Franik, G.; Wikarek, T.; Owczarek, A.; Delroba, A.; Chudek, J.; Sikora, J.; Olszanecka-Glinianowicz, M. The levels of adipokines in relation to hormonal changes during the menstrual cycle in young, normal-weight women. Endocr. Connect. 2017, 6, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Esteghamati, A.; Mousavizadeh, M.; Noshad, S.; Zandieh, A.; Zarei, H.; Nakhjavani, M. Gender-dependent Effects of Metformin on Vaspin and Adiponectin in Type 2 Diabetes Patients: A Randomized Clinical Trial. Horm. Metab. Res. 2012, 45, 319–325. [Google Scholar] [CrossRef]
- Youn, B.-S.; Klöting, N.; Kratzsch, J.; Lee, N.; Park, J.W.; Song, E.-S.; Ruschke, K.; Oberbach, A.; Fasshauer, M.; Stumvoll, M.; et al. Serum Vaspin Concentrations in Human Obesity and Type 2 Diabetes. Diabetes 2008, 57, 372–377. [Google Scholar] [CrossRef]
- Chamorro-Melo, Y.; Calixto, O.-J.; Bello-Gualtero, J.; Bautista-Molano, W.; Beltran-Ostos, A.; Romero-Sánchez, C. Evaluation of the adipokine profile (adiponectin, resistin, adipsin, vaspin, and leptin) in patients with early rheumatoid arthritis and its correlation with disease activity. Reumatologia 2022, 60, 192–199. [Google Scholar] [CrossRef]
- Bao, J.-P.; Jiang, L.-F.; Chen, W.-P.; Hu, P.-F.; Wu, L.-D. Expression of vaspin in the joint and the levels in the serum and synovial fluid of patients with osteoarthritis. Int. J. Clin. Exp. Med. 2014, 7, 3447–3453. [Google Scholar]
- Martel-Pelletier, J.; Raynauld, J.-P.; Dorais, M.; Abram, F.; Pelletier, J.-P. The levels of the adipokines adipsin and leptin are associated with knee osteoarthritis progression as assessed by MRI and incidence of total knee replacement in symptomatic osteoarthritis patients: Apost hocanalysis. Rheumatology 2016, 55, 680–688. [Google Scholar] [CrossRef]
- Bitirim, C.V.; Ozer, Z.B.; Akcali, K.C. Estrogen receptor alpha regulates the expression of adipogenic genes genetically and epigenetically in rat bone marrow-derived mesenchymal stem cells. PeerJ 2021, 9, e12071. [Google Scholar] [CrossRef]
- Ramirez, M.F.; Pan, A.S.; Parekh, J.K.; Owunna, N.; Courchesne, P.; Larson, M.G.; Levy, D.; Murabito, J.M.; Ho, J.E.; Lau, E.S. Sex Differences in Protein Biomarkers and Measures of Fat Distribution. J. Am. Heart Assoc. 2024, 13, e000223. [Google Scholar] [CrossRef]
- Milek, M.; Moulla, Y.; Kern, M.; Stroh, C.; Dietrich, A.; Schön, M.R.; Gärtner, D.; Lohmann, T.; Dressler, M.; Kovacs, P.; et al. Adipsin Serum Concentrations and Adipose Tissue Expression in People with Obesity and Type 2 Diabetes. Int. J. Mol. Sci. 2022, 23, 2222. [Google Scholar] [CrossRef]
- Maity, S.K.; Das Sharma, A.; Sarkar, J.; Chaudhuri, T.; Tantia, O.; Chakrabarti, P. Adipose tissue–derived adipsin marks human aging in non-type 2 diabetes population. BMJ Open Diab. Res. Care 2024, 12, e004179. [Google Scholar] [CrossRef] [PubMed]
- Romero-Sánchez, C.; De Avila, J.; Ramos-Casallas, A.; Chila-Moreno, L.; Delgadillo, N.A.; Chalem-Choueka, P.; Pacheco-Tena, C.; Bello-Gualtero, J.M.; Bautista-Molano, W. High Levels of Leptin and Adipsin Are Associated with Clinical Activity in Early Rheumatoid Arthritis Patients with Overweight and Periodontal Infection. Diagnostics 2023, 13, 1126. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Barreal, A.; González-Rodríguez, M.; Ruiz-Fernández, C.; Eldjoudi, D.A.; AbdElHafez, Y.R.F.; Lago, F.; Conde, J.; Gómez, R.; González-Gay, M.A.; Mobasheri, A.; et al. An Update on the Role of Leptin in the Immuno-Metabolism of Cartilage. Int. J. Mol. Sci. 2021, 22, 2411. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, A.; Vuolteenaho, K.; Nieminen, R.; Moilanen, T.; Moilanen, E. Leptin enhances MMP-1, MMP-3 and MMP-13 production in human osteoarthritic cartilage and correlates with MMP-1 and MMP-3 in synovial fluid from OA patients. Clin. Exp. Rheumatol. 2011, 29, 57–64. [Google Scholar]
- Berry, P.A.; Jones, S.W.; Cicuttini, F.M.; Wluka, A.E.; Maciewicz, R.A. Temporal relationship between serum adipokines, biomarkers of bone and cartilage turnover, and cartilage volume loss in a population with clinical knee osteoarthritis. Arthritis Rheum. 2011, 63, 700–707. [Google Scholar] [CrossRef]
- Luukkaa, V.; Pesonen, U.; Huhtaniemi, I.; Lehtonen, A.; Tilvis, R.; Tuomilehto, J.; Koulu, M.; Huupponen, R. Inverse Correlation between Serum Testosterone and Leptin in Men1. J. Clin. Endocrinol. Metab. 1998, 83, 3243–3246. [Google Scholar] [CrossRef][Green Version]
- Elbers, J.M.H.; Asscheman, H.; Seidell, J.C.; Frölich, M.; Meinders, A.E.; Gooren, L.J.G. Reversal of the Sex Difference in Serum Leptin Levels upon Cross-Sex Hormone Administration in Transsexuals. J. Clin. Endocrinol. Metab. 1997, 82, 3267–3270. [Google Scholar] [CrossRef]
- Guerra, B.; Fuentes, T.; Delgado-Guerra, S.; Guadalupe-Grau, A.; Olmedillas, H.; Santana, A.; Ponce-Gonzalez, J.G.; Dorado, C.; Calbet, J.A.L. Gender Dimorphism in Skeletal Muscle Leptin Receptors, Serum Leptin and Insulin Sensitivity. PLoS ONE 2008, 3, e3466. [Google Scholar] [CrossRef]
- Seyfart, T.; Friedrich, N.; Kische, H.; Bülow, R.; Wallaschofski, H.; Völzke, H.; Nauck, M.; Keevil, B.G.; Haring, R. Association of sex hormones with physical, laboratory, and imaging markers of anthropometry in men and women from the general population. PLoS ONE 2018, 13, e0189042. [Google Scholar] [CrossRef]
- Hellström, L.; Wahrenberg, H.; Hruska, K.; Reynisdottir, S.; Arner, P. Mechanisms behind gender differences in circulating leptin levels. J. Intern. Med. 2000, 247, 457–462. [Google Scholar] [CrossRef]
- Roszkowska-Gancarz, M.; Jonas, M.; Owczarz, M.; Kurylowicz, A.; Polosak, J.; Franek, E.; Slusarczyk, P.; Mossakowska, M.; Puzianowska-Kuznicka, M. Age-related changes of leptin and leptin receptor variants in healthy elderly and long-lived adults. Geriatr. Gerontol. Int. 2015, 15, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Würfel, M.; Breitfeld, J.; Gebhard, C.; Scholz, M.; Baber, R.; Riedel-Heller, S.G.; Blüher, M.; Stumvoll, M.; Kovacs, P.; Tönjes, A. Interplay between adipose tissue secreted proteins, eating behavior and obesity. Eur. J. Nutr. 2022, 61, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Kluzek, S.; Arden, N.K.; Newton, J. Adipokines as potential prognostic biomarkers in patients with acute knee injury. Biomarkers 2015, 20, 519–525. [Google Scholar] [CrossRef]
- Lee, J.H.; Ort, T.; Ma, K.; Picha, K.; Carton, J.; Marsters, P.A.; Lohmander, L.S.; Baribaud, F.; Song, X.-Y.R.; Blake, S. Resistin is elevated following traumatic joint injury and causes matrix degradation and release of inflammatory cytokines from articular cartilage in vitro. Osteoarthr. Cartil. 2009, 17, 613–620. [Google Scholar] [CrossRef]
- Zhao, C.-W.; Song, W.-X.; Liu, B.; Gao, Y.-H.; Ding, L.; Huang, Y.-F.; Qi, X. Resistin induces chemokine and matrix metalloproteinase production via CAP1 receptor and activation of p38-MAPK and NF-κB signalling pathways in human chondrocytes. Pathog. Rheum. Arthritis One Year Rev. 2022, 40, 501–513. [Google Scholar] [CrossRef]
- Siemińska, L.; Cichoń-Lenart, A.; Kajdaniuk, D.; Kos-Kudła, B.; Marek, B.; Lenart, J.; Nowak, M. Sex hormones and adipocytokines in postmenopausal women. Pol. Merkur. Lek. 2006, 20, 727–730. [Google Scholar]
- Massengale, M.; Lu, B.; Pan, J.J.; Katz, J.N.; Solomon, D.H. Adipokine Hormones and Hand Osteoarthritis: Radiographic Severity and Pain. PLoS ONE 2012, 7, e47860. [Google Scholar] [CrossRef]
- Presle, N.; Pottie, P.; Dumond, H.; Guillaume, C.; Lapicque, F.; Pallu, S.; Mainard, D.; Netter, P.; Terlain, B. Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis. Contribution of joint tissues to their articular production. Osteoarthr. Cartil. 2006, 14, 690–695. [Google Scholar] [CrossRef]
- Ohmori, R.; Momiyama, Y.; Kato, R.; Taniguchi, H.; Ogura, M.; Ayaori, M.; Nakamura, H.; Ohsuzu, F. Associations Between Serum Resistin Levels and Insulin Resistance, Inflammation, and Coronary Artery Disease. J. Am. Coll. Cardiol. 2005, 46, 379–380. [Google Scholar] [CrossRef] [PubMed]
- Vozarova De Courten, B.; Degawa-Yamauchi, M.; Considine, R.V.; Tataranni, P.A. High Serum Resistin Is Associated with an Increase in Adiposity But Not a Worsening of Insulin Resistance in Pima Indians. Diabetes 2004, 53, 1279–1284, Erratum in Diabetes 2004, 53, 2518. https://doi.org/10.2337/diabetes.53.9.2518. [Google Scholar] [CrossRef] [PubMed]
- Onyemelukwe, O.U.; Ogoina, D.; Onyemelukwe, G.C. Effect of Obesity on Resistin Concentrations in Normal, Pre-Obese and Obese Apparently Healthy Nigerian-Africans. West Afr. J. Med. 2022, 39, 702–791. [Google Scholar]
- Vasileva, E.; Stankova, T.; Batalov, K.; Staynova, R.; Nonchev, B.; Bivolarska, A.; Karalilova, R. Association of serum and synovial adipokines (chemerin and resistin) with inflammatory markers and ultrasonographic evaluation scores in patients with knee joint osteoarthritis—A pilot study. Rheumatol. Int. 2024, 44, 1997–2005. [Google Scholar] [CrossRef]
- Duan, Y.; Hao, D.; Li, M.; Wu, Z.; Li, D.; Yang, X.; Qiu, G. Increased synovial fluid visfatin is positively linked to cartilage degradation biomarkers in osteoarthritis. Rheumatol. Int. 2012, 32, 985–990. [Google Scholar] [CrossRef]
- Annie, L.; Gurusubramanian, G.; Roy, V.K. Estrogen and progesterone dependent expression of visfatin/NAMPT regulates proliferation and apoptosis in mice uterus during estrous cycle. J. Steroid Biochem. Mol. Biol. 2019, 185, 225–236. [Google Scholar] [CrossRef]
- Rempuia, V.; Gurusubramanian, G.; Roy, V.K. Intra-testicular visfatin inhibition disrupts androgen and estrogen signalling in the mouse testis. Reprod. Biol. 2024, 24, 100956. [Google Scholar] [CrossRef]
- Chen, W.; Bao, J.; Feng, J.; Hu, P.; Shi, Z.; Wu, L. Increased serum concentrations of visfatin and its production by different joint tissues in patients with osteoarthritis. Clin. Chem. Lab. Med. 2010, 48, 1141–1145. [Google Scholar] [CrossRef]
- Jurdana, M.; Petelin, A.; Černelič Bizjak, M.; Bizjak, M.; Biolo, G.; Jenko-Pražnikar, Z. Increased serum visfatin levels in obesity and its association with anthropometric/biochemical parameters, physical inactivity and nutrition. e-SPEN J. 2013, 8, e59–e67. [Google Scholar] [CrossRef]
- Ugur, K.; Erman, F.; Turkoglu, S.; Aydin, Y.; Aksoy, A.; Lale, A.; Karagöz, Z.K.; Ugur, İ.; Akkoc, R.F.; Yalniz, M. Asprosin, visfatin and subfatin as new biomarkers of obesity and metabolic syndrome. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2124–2133. [Google Scholar] [CrossRef]
- Lee, Y.H.; Bae, S. Circulating adiponectin and visfatin levels in rheumatoid arthritis and their correlation with disease activity: A meta-analysis. Int. J. Rheum. Dis. 2018, 21, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Iannone, F.; Lapadula, G. Chemerin/ChemR23 pathway: A system beyond chemokines. Arthritis Res. Ther. 2011, 13, 104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, L.; Ma, P.; Huang, C.; Liu, Y.; Zhang, Y.; Gao, C.; Xiao, T.; Ren, P.-G.; Zabel, B.A.; Zhang, J.V. Expression of chemerin and its receptors in rat testes and its action on testosterone secretion. J. Endocrinol. 2014, 220, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, Y.; Wan, S.; Wei, Q.; Shang, W.; Huang, G.; Fang, P.; Min, W. Loss of chemerin triggers bone remodeling in vivo and in vitro. Mol. Metab. 2021, 53, 101322. [Google Scholar] [CrossRef]
- Stejskal, D.; Karpisek, M.; Hanulova, Z.; Svestak, M. Chemerin is an independent marker of the metabolic syndrome in a caucasian population—A pilot study. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2008, 152, 217–221. [Google Scholar] [CrossRef]
- Aronis, K.N.; Sahin-Efe, A.; Chamberland, J.P.; Spiro, A.; Vokonas, P.; Mantzoros, C.S. Chemerin levels as predictor of acute coronary events: A case–control study nested within the veterans affairs normative aging study. Metabolism 2014, 63, 760–766. [Google Scholar] [CrossRef]
- Chang, S.; Eisenberg, D.; Zhao, L.; Adams, C.; Leib, R.; Morser, J.; Leung, L. Chemerin activation in human obesity. Obesity 2016, 24, 1522–1529. [Google Scholar] [CrossRef]
- Dessein, P.H.; Tsang, L.; Woodiwiss, A.J.; Norton, G.R.; Solomon, A. Circulating Concentrations of the Novel Adipokine Chemerin Are Associated with Cardiovascular Disease Risk in Rheumatoid Arthritis. J. Rheumatol. 2014, 41, 1746–1754. [Google Scholar] [CrossRef]
- Zhao, Y.-L.; Zhang, T.-P.; Wu, J.; Li, B.-Z.; Li, X.-M.; Pan, H.-F.; Ye, D.-Q. Association of adiponectin and adiponectin receptor gene polymorphisms with rheumatoid arthritis in a Chinese population. Postgrad. Med. J. 2020, 96, 149–155. [Google Scholar] [CrossRef]
- Chen, H.; Mi, S.; Zhu, J.; Jin, W.; Li, Y.; Wang, T.; Li, Y.; Fan, C. No Causal Association Between Adiponectin and the Risk of Rheumatoid Arthritis: A Mendelian Randomization Study. Front. Genet. 2021, 12, 670282. [Google Scholar] [CrossRef]
- Elnemr, R.; El Hamid, M.M.A.; Taleb, R.S.Z.; Khalil, N.F.W.; El-Sherif, S.M. Study of adiponectin gene (rs1501299) polymorphism and serum adiponectin level in patients with primary knee osteoarthritis. Hum. Genom. 2024, 18, 105. [Google Scholar] [CrossRef]
- Zhan, D.; Thumtecho, S.; Tanavalee, A.; Yuktanandana, P.; Anomasiri, W.; Honsawek, S. Association of adiponectin gene polymorphisms with knee osteoarthritis. World J. Orthop. 2017, 8, 719. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhu, X.; Rong, J.; Xing, B.; Wang, S.; Liu, A.; Chu, M.; Huang, G. Obesity, osteoarthritis and genetic risk: The rs182052 polymorphism in the ADIPOQ gene is potentially associated with risk of knee osteoarthritis. Bone Jt. Res. 2018, 7, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Meng, F.; Wu, J.; Long, H.; Li, J.; Wu, Z.; He, H.; Wang, H.; Wang, N.; Xie, D. Associations between adipokines gene polymorphisms and knee osteoarthritis: A meta-analysis. BMC Musculoskelet. Disord. 2022, 23, 166. [Google Scholar] [CrossRef]
- Shang, H.; Hao, Y.; Hu, W.; Hu, X.; Jin, Q. Association between ADIPOQ gene variants and knee osteoarthritis in a Chinese population. Biosci. Rep. 2019, 39, BSR20182104. [Google Scholar] [CrossRef]
- Wahba, A.S.; Ibrahim, M.E.; Abo-elmatty, D.M.; Mehanna, E.T. Association of the Adipokines Chemerin, Apelin, Vaspin and Omentin and Their Functional Genetic Variants with Rheumatoid Arthritis. J. Pers. Med. 2021, 11, 976. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, Y.; Xu, H.; Hu, H.; Chen, M.; Shuai, Z. Val109Asp Polymorphism of the Omentin-1 Gene and Incidence of Knee Osteoarthritis in a Chinese Han Population: A Correlation Analysis. Drug Des. Dev. Ther. 2021, 15, 5075–5086. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, R. The effect of ITLN1, XCL2 and DOT1L variants on knee osteoarthritis risk in the Han population. Arch. Orthop. Trauma. Surg. 2023, 143, 4821–4831. [Google Scholar] [CrossRef]
- Tao, S.-S.; Dan, Y.-L.; Wu, G.-C.; Zhang, Q.; Zhang, T.-P.; Fan, Y.-G.; Pan, H.-F. Association of Leptin Gene Polymorphisms with Rheumatoid Arthritis in a Chinese Population. BioMed Res. Int. 2020, 2020, 3789319. [Google Scholar] [CrossRef]
- Qin, J.; Shi, D.; Dai, J.; Zhu, L.; Tsezou, A.; Jiang, Q. Association of the leptin gene with knee osteoarthritis susceptibility in a Han Chinese population: A case–control study. J. Hum. Genet. 2010, 55, 704–706. [Google Scholar] [CrossRef]
- Li, H.; Zhang, T.; Li, X.; Pan, H.; Ma, D. Association of single nucleotide polymorphisms in resistin gene with rheumatoid arthritis in a Chinese population. Clin. Lab. Anal. 2018, 32, e22595. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, C.-H.; Lu, T.; Sun, Y.; Xu, G.; Huang, C.-C.; Yang, S.-F.; Su, C.-M. Resistin polymorphisms are associated with rheumatoid arthritis susceptibility in Chinese Han subjects. Medicine 2018, 97, e0177. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, S.; Solovieva, S.; Vehmas, T.; Hirvonen, A.; Leino-Arjas, P. Adipokine genes and radiographic hand osteoarthritis in Finnish women: A cross-sectional study. Scand. J. Rheumatol. 2018, 47, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.K.B.; Murtaza, I.; Javed, Q. Role of resistin genetic variations in knee osteoarthritis pathogenesis, a cross sectional study. Mol. Biol. Rep. 2019, 46, 2657–2663. [Google Scholar] [CrossRef]
- Yan, S.; Liu, H.; Nie, H.; Bu, G.; Yuan, W.; Wang, S. Common variants of RARRES2 and RETN contribute to susceptibility to hand osteoarthritis and related pain. Biomark. Med. 2022, 16, 731–738. [Google Scholar] [CrossRef]
- Chu, M.; Rong, J.; Wang, Y.; Zhu, L.; Xing, B.; Tao, Y.; Zhuang, X.; Zhao, Y.; Jiang, L. Strong association of the polymorphisms in PBEF1 and knee OA risk: A two-stage population-based study in China. Sci. Rep. 2016, 6, 19094. [Google Scholar] [CrossRef]
- Pratt, A.G.; Isaacs, J.D. Seronegative rheumatoid arthritis: Pathogenetic and therapeutic aspects. Best Pract. Res. Clin. Rheumatol. 2014, 28, 651–659. [Google Scholar] [CrossRef]
- Malmström, V.; Catrina, A.I.; Klareskog, L. The immunopathogenesis of seropositive rheumatoid arthritis: From triggering to targeting. Nat. Rev. Immunol. 2017, 17, 60–75, Correction in Nat. Rev. Immunol. 2022, 22, 459. https://doi.org/10.1038/s41577-022-00741-0. [Google Scholar] [CrossRef]
- Arleevskaya, M.; Takha, E.; Petrov, S.; Kazarian, G.; Novikov, A.; Larionova, R.; Valeeva, A.; Shuralev, E.; Mukminov, M.; Bost, C.; et al. Causal risk and protective factors in rheumatoid arthritis: A genetic update. J. Transl. Autoimmun. 2021, 4, 100119. [Google Scholar] [CrossRef]
- Arleevskaya, M.I.; Kravtsova, O.A.; Lemerle, J.; Renaudineau, Y.; Tsibulkin, A.P. How Rheumatoid Arthritis Can Result from Provocation of the Immune System by Microorganisms and Viruses. Front. Microbiol. 2016, 7, 1296. [Google Scholar] [CrossRef]
- De Hair, M.J.H.; Van De Sande, M.G.H.; Ramwadhdoebe, T.H.; Hansson, M.; Landewé, R.; Van Der Leij, C.; Maas, M.; Serre, G.; Van Schaardenburg, D.; Klareskog, L.; et al. Features of the Synovium of Individuals at Risk of Developing Rheumatoid Arthritis: Implications for Understanding Preclinical Rheumatoid Arthritis. Arthritis Rheumatol. 2014, 66, 513–522. [Google Scholar] [CrossRef]
- Magalhães, R.; Stiehl, P.; Morawietz, L.; Berek, C.; Krenn, V. Morphological and molecular pathology of the B cell response in synovitis of rheumatoid arthritis. Virchows Arch. 2002, 441, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Musters, A.; Balzaretti, G.; Van Schaik, B.D.C.; Jongejan, A.; Van Der Weele, L.; Tas, S.W.; Van Kampen, A.H.C.; De Vries, N. In rheumatoid arthritis inflamed joints share dominant patient-specific B-cell clones. Front. Immunol. 2022, 13, 915687. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, A.; Ytterberg, A.J.; Sun, M.; Sakuraba, K.; Steen, J.; Joshua, V.; Tarasova, N.K.; Malmström, V.; Wähämaa, H.; Réthi, B.; et al. Citrullination Controls Dendritic Cell Transdifferentiation into Osteoclasts. J. Immunol. 2019, 202, 3143–3150. [Google Scholar] [CrossRef] [PubMed]
- Wigerblad, G.; Bas, D.B.; Fernades-Cerqueira, C.; Krishnamurthy, A.; Nandakumar, K.S.; Rogoz, K.; Kato, J.; Sandor, K.; Su, J.; Jimenez–Andrade, J.M.; et al. Autoantibodies to citrullinated proteins induce joint pain independent of inflammation via a chemokine-dependent mechanism. Ann. Rheum. Dis. 2016, 75, 730–738, Erratum in Ann. Rheum. Dis. 2019, 78, 865. https://doi.org/10.1136/annrheumdis-2015-208094corr1. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Joshua, V.; Haj Hensvold, A.; Jin, T.; Sun, M.; Vivar, N.; Ytterberg, A.J.; Engström, M.; Fernandes-Cerqueira, C.; Amara, K.; et al. Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss. Ann. Rheum. Dis. 2016, 75, 721–729, Correction in Ann. Rheum. Dis. 2019, 78, 866. [Google Scholar] [CrossRef]
- Harre, U.; Georgess, D.; Bang, H.; Bozec, A.; Axmann, R.; Ossipova, E.; Jakobsson, P.-J.; Baum, W.; Nimmerjahn, F.; Szarka, E.; et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Investig. 2012, 122, 1791–1802. [Google Scholar] [CrossRef]
- Romão, V.C.; Fonseca, J.E. Disease mechanisms in preclinical rheumatoid arthritis: A narrative review. Front. Med. 2022, 9, 689711. [Google Scholar] [CrossRef]
- Cantaert, T.; Brouard, S.; Thurlings, R.M.; Pallier, A.; Salinas, G.F.; Braud, C.; Klarenbeek, P.L.; De Vries, N.; Zhang, Y.; Soulillou, J.; et al. Alterations of the synovial T cell repertoire in anti–citrullinated protein antibody–positive rheumatoid arthritis. Arthritis Rheum. 2009, 60, 1944–1956. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Jin, S.; Wang, M.; Jiao, Y.; Yang, B.; Lu, X.; Ji, X.; Fei, Y.; Yang, H.; et al. Single-cell sequencing of immune cells from anticitrullinated peptide antibody positive and negative rheumatoid arthritis. Nat. Commun. 2021, 12, 4977. [Google Scholar] [CrossRef]
- Floudas, A.; Canavan, M.; McGarry, T.; Mullan, R.; Nagpal, S.; Veale, D.J.; Fearon, U. ACPA Status Correlates with Differential Immune Profile in Patients with Rheumatoid Arthritis. Cells 2021, 10, 647. [Google Scholar] [CrossRef]
- Alivernini, S.; Bruno, D.; Tolusso, B.; Bui, L.; Petricca, L.; Gigante, M.R.; Birra, D.; Fedele, A.L.; Peluso, G.; Federico, F.; et al. Differential synovial tissue biomarkers among psoriatic arthritis and rheumatoid factor/anti-citrulline antibody-negative rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 116. [Google Scholar] [CrossRef] [PubMed]
- Dennis, G.; Holweg, C.T.; Kummerfeld, S.K.; Choy, D.F.; Setiadi, A.F.; Hackney, J.A.; Haverty, P.M.; Gilbert, H.; Lin, W.Y.; Diehl, L.; et al. Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis Res. Ther. 2014, 16, R90. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, L.; D’Onofrio, B.; Manzo, A.; Montecucco, C.; Bugatti, S. The Genetic, Environmental, and Immunopathological Complexity of Autoantibody-Negative Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 12386. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.; Watad, A.; Savic, S. Mechanistic immunological based classification of rheumatoid arthritis. Autoimmun. Rev. 2018, 17, 1115–1123. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, Z. Comparative study on the level of B lymphocyte stimulator (B ly S) and frequency of lymphocytes between sero-negative and sero-positive rheumatoid arthritis patients. Int. J. Rheum. Dis. 2012, 15, 478–485. [Google Scholar] [CrossRef]
- Grunz, J.-P.; Gietzen, C.H.; Christopoulos, G.; Van Schoonhoven, J.; Goehtz, F.; Schmitt, R.; Hesse, N. Osteoarthritis of the Wrist: Pathology, Radiology, and Treatment. Semin. Musculoskelet. Radiol. 2021, 25, 294–303. [Google Scholar] [CrossRef]
- Gleason, B.; Chisari, E.; Parvizi, J. Osteoarthritis Can Also Start in the Gut: The Gut–Joint Axis. Indian J. Orthop. 2022, 56, 1150–1155. [Google Scholar] [CrossRef]
- Andersson, J.K.; Hagert, E.; Brittberg, M. Cartilage Injuries and Posttraumatic Osteoarthritis in the Wrist: A Review. Cartilage 2021, 13, 156S–168S. [Google Scholar] [CrossRef]
- Watt, F.E. Hand osteoarthritis, menopause and menopausal hormone therapy. Maturitas 2016, 83, 13–18. [Google Scholar] [CrossRef]
- Egloff, C.; Hügle, T.; Valderrabano, V. Biomechanics and pathomechanisms of osteoarthritis. Swiss Med. Wkly. 2012, 142, w13583. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef]
- Ene, R.; Sinescu, R.D.; Ene, P.; Cîrstoiu, M.M.; Cîrstoiu, F.C. Synovial inflammation in patients with different stages of knee osteoarthritis. Rom. J. Morphol. Embryol. 2015, 56, 169–173. [Google Scholar]
- Sellam, J.; Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef]
- Ponchel, F.; Burska, A.N.; Hensor, E.M.A.; Raja, R.; Campbell, M.; Emery, P.; Conaghan, P.G. Changes in peripheral blood immune cell composition in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1870–1878. [Google Scholar] [CrossRef]
- Hu, W.; Chen, Y.; Dou, C.; Dong, S. Microenvironment in subchondral bone: Predominant regulator for the treatment of osteoarthritis. Ann. Rheum. Dis. 2021, 80, 413–422. [Google Scholar] [CrossRef]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Y.; Jin, K.; Wen, Z.; Cao, W.; Wu, B.; Wen, R.; Tian, L.; Berry, G.J.; Goronzy, J.J.; et al. The DNA Repair Nuclease MRE11A Functions as a Mitochondrial Protector and Prevents T Cell Pyroptosis and Tissue Inflammation. Cell Metab. 2019, 30, 477–492.e6. [Google Scholar] [CrossRef] [PubMed]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Sci. Transl. Med. 2013, 5, 178ra40. [Google Scholar] [CrossRef] [PubMed]
- Zec, K.; Schonfeldova, B.; Ai, Z.; Van Grinsven, E.; Pirgova, G.; Eames, H.L.; Berthold, D.L.; Attar, M.; Compeer, E.B.; Arnon, T.I.; et al. Macrophages in the synovial lining niche initiate neutrophil recruitment and articular inflammation. J. Exp. Med. 2023, 220, e20220595. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, L.J.; Oliveira, C.B.; Sandoval-Heglund, D.; Barrera-Vargas, A.; Merayo-Chalico, J.; Aguirre-Aguilar, E.; Kaplan, M.J.; Carmona-Rivera, C. Anti-Carbamylated LL37 Antibodies Promote Pathogenic Bone Resorption in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 715997. [Google Scholar] [CrossRef]
- Wright, H.L.; Moots, R.J.; Edwards, S.W. The multifactorial role of neutrophils in rheumatoid arthritis. Nat. Rev. Rheumatol. 2014, 10, 593–601. [Google Scholar] [CrossRef]
- Feldman, M.; Ginsburg, I. A Novel Hypothetical Approach to Explain the Mechanisms of Pathogenicity of Rheumatic Arthritis. Mediterr. J. Rheumatol. 2021, 32, 112. [Google Scholar] [CrossRef]
- Bromley, M.; Woolley, D.E. Histopathology of the rheumatoid lesion. Arthritis Rheum. 1984, 27, 857–863. [Google Scholar] [CrossRef]
- Kraan, M.C.; Haringman, J.J.; Post, W.J.; Versendaal, J.; Breedveld, F.C.; Tak, P.P. Immunohistological analysis of synovial tissue for differential diagnosis in early arthritis. Rheumatology 1999, 38, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Bery, A.I.; Shepherd, H.M.; Li, W.; Krupnick, A.S.; Gelman, A.E.; Kreisel, D. Role of tertiary lymphoid organs in the regulation of immune responses in the periphery. Cell. Mol. Life Sci. 2022, 79, 359. [Google Scholar] [CrossRef] [PubMed]
- Thurlings, R.M.; Wijbrandts, C.A.; Mebius, R.E.; Cantaert, T.; Dinant, H.J.; Van Der Pouw-Kraan, T.C.T.M.; Verweij, C.L.; Baeten, D.; Tak, P.P. Synovial lymphoid neogenesis does not define a specific clinical rheumatoid arthritis phenotype. Arthritis Rheum. 2008, 58, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Klimiuk, P.A.; Sierakowski, S.; Latosiewicz, R.; Cylwik, J.P.; Cylwik, B.; Skowronski, J.; Chwiecko, J. Soluble adhesion molecules (ICAM-1, VCAM-1, and E-selectin) and vascular endothelial growth factor (VEGF) in patients with distinct variants of rheumatoid synovitis. Ann. Rheum. Dis. 2002, 61, 804–809. [Google Scholar] [CrossRef]
- Klimiuk, P.A.; Goronzy, J.J.; Björnsson, J.; Beckenbaugh, R.D.; Weyand, C.M. Tissue cytokine patterns distinguish variants of rheumatoid synovitis. Am. J. Pathol. 1997, 151, 1311–1319. [Google Scholar]
- Humby, F.; Bombardieri, M.; Manzo, A.; Kelly, S.; Blades, M.C.; Kirkham, B.; Spencer, J.; Pitzalis, C. Ectopic Lymphoid Structures Support Ongoing Production of Class-Switched Autoantibodies in Rheumatoid Synovium. PLoS Med. 2009, 6, e1. [Google Scholar] [CrossRef]
- Rosengren, S.; Wei, N.; Kalunian, K.C.; Zvaifler, N.J.; Kavanaugh, A.; Boyle, D.L. Elevated autoantibody content in rheumatoid arthritis synovia with lymphoid aggregates and the effect of rituximab. Arthritis Res. Ther. 2008, 10, R105. [Google Scholar] [CrossRef] [PubMed]
- Corsiero, E.; Bombardieri, M.; Carlotti, E.; Pratesi, F.; Robinson, W.; Migliorini, P.; Pitzalis, C. Single cell cloning and recombinant monoclonal antibodies generation from RA synovial B cells reveal frequent targeting of citrullinated histones of NETs. Ann. Rheum. Dis. 2016, 75, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Corsiero, E.; Jagemann, L.; Perretti, M.; Pitzalis, C.; Bombardieri, M. Characterization of a Synovial B Cell–Derived Recombinant Monoclonal Antibody Targeting Stromal Calreticulin in the Rheumatoid Joints. J. Immunol. 2018, 201, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Randen, I.; Mellbye, O.J.; Førre, Ø.; Natvig, J.B. The Identification of Germinal Centres and Follicular Dendritic Cell Networks in Rheumatoid Synovial Tissue. Scand. J. Immunol. 1995, 41, 481–486. [Google Scholar] [CrossRef]
- Krenn, V.; Morawietz, L.; Häupl, T.; Neidel, J.; Petersen, I.; König, A. Grading of Chronic Synovitis—A Histopathological Grading System for Molecular and Diagnostic Pathology. Pathol.-Res. Pract. 2002, 198, 317–325. [Google Scholar] [CrossRef]
- Klaasen, R.; Thurlings, R.M.; Wijbrandts, C.A.; Van Kuijk, A.W.; Baeten, D.; Gerlag, D.M.; Tak, P.P. The relationship between synovial lymphocyte aggregates and the clinical response to infliximab in rheumatoid arthritis: A prospective study. Arthritis Rheum. 2009, 60, 3217–3224. [Google Scholar] [CrossRef]
- Tak, P.P.; Smeets, T.J.M.; Daha, M.R.; Kluin, P.M.; Meijers, K.A.E.; Brand, R.; Meinders, A.E.; Breedveld, F.C. Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum. 1997, 40, 217–225. [Google Scholar] [CrossRef]
- Cañete, J.D.; Celis, R.; Moll, C.; Izquierdo, E.; Marsal, S.; Sanmartí, R.; Palacín, A.; Lora, D.; De La Cruz, J.; Pablos, J.L. Clinical significance of synovial lymphoid neogenesis and its reversal after anti-tumour necrosis factor α therapy in rheumatoid arthritis. Ann. Rheum. Dis. 2009, 68, 751–756. [Google Scholar] [CrossRef]
- Sen, M.; Chamorro, M.; Reifert, J.; Corr, M.; Carson, D.A. Blockade of Wnt-5A/Frizzled 5 signaling inhibits rheumatoid synoviocyte activation. Arthritis Rheum. 2001, 44, 772–781. [Google Scholar] [CrossRef]
- Korb-Pap, A.; Bertrand, J.; Sherwood, J.; Pap, T. Stable activation of fibroblasts in rheumatic arthritis—Causes and consequences. Rheumatology 2016, 55, ii64–ii67. [Google Scholar] [CrossRef]
- Bustamante, M.F.; Garcia-Carbonell, R.; Whisenant, K.D.; Guma, M. Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res. Ther. 2017, 19, 110. [Google Scholar] [CrossRef]
- Jamora, C.; Fuchs, E. Intercellular adhesion, signalling and the cytoskeleton. Nat. Cell. Biol. 2002, 4, E101–E108. [Google Scholar] [CrossRef]
- Sutton, S.; Clutterbuck, A.; Harris, P.; Gent, T.; Freeman, S.; Foster, N.; Barrett-Jolley, R.; Mobasheri, A. The contribution of the synovium, synovial derived inflammatory cytokines and neuropeptides to the pathogenesis of osteoarthritis. Vet. J. 2009, 179, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mobasheri, A.; Mozafari, M. Inflammatory mediators in osteoarthritis: A critical review of the state-of-the-art, current prospects, and future challenges. Bone 2016, 85, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Benito, M.J.; Veale, D.J.; FitzGerald, O.; Van Den Berg, W.B.; Bresnihan, B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 2005, 64, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Galushko, E.A.; Bolshakova, T.Y.; Vinogradova, I.B.; Ivanova, O.N.; Lesnyak, O.M.; Menshikova, L.V.; Petrachkova, T.N.; Erdes, S.F. Structure of rheumatic diseases among adult population of Russia according to data of an epidemiological study (preliminary results). Rheumatol. Sci. Pract. 2009, 47, 11–17. [Google Scholar] [CrossRef][Green Version]
- Van Oosterhout, M.; Bajema, I.; Levarht, E.W.N.; Toes, R.E.M.; Huizinga, T.W.J.; Van Laar, J.M. Differences in synovial tissue infiltrates between anti–cyclic citrullinated peptide–positive rheumatoid arthritis and anti–cyclic citrullinated peptide–negative rheumatoid arthritis. Arthritis Rheum. 2008, 58, 53–60. [Google Scholar] [CrossRef]
- Christensen, A.F.; Lindegaard, H.; Hørslev-Petersen, K.; Hetland, M.L.; Ejbjerg, B.; Stengaard-Pedersen, K.; Jacobsen, S.; Lottenburger, T.; Ellingsen, T.; Andersen, L.S.; et al. Cartilage Oligomeric Matrix Protein Associates Differentially with Erosions and Synovitis and Has a Different Temporal Course in Cyclic Citrullinated Peptide Antibody (Anti-CCP)-positive versus Anti-CCP-negative Early Rheumatoid Arthritis. J. Rheumatol. 2011, 38, 1563–1568. [Google Scholar] [CrossRef][Green Version]
- Pitzalis, C.; Kelly, S.; Humby, F. New learnings on the pathophysiology of RA from synovial biopsies. Curr. Opin. Rheumatol. 2013, 25, 334–344. [Google Scholar] [CrossRef]
- Kasperkovitz, P.V.; Timmer, T.C.G.; Smeets, T.J.; Verbeet, N.L.; Tak, P.P.; Van Baarsen, L.G.M.; Baltus, B.; Huizinga, T.W.J.; Pieterman, E.; Fero, M.; et al. Fibroblast-like synoviocytes derived from patients with rheumatoid arthritis show the imprint of synovial tissue heterogeneity: Evidence of a link between an increased myofibroblast-like phenotype and high-inflammation synovitis. Arthritis Rheum. 2005, 52, 430–441. [Google Scholar] [CrossRef]
- Van Der Pouw Kraan, T.C.T.M.; Van Gaalen, F.A.; Huizinga, T.W.J.; Pieterman, E.; Breedveld, F.C.; Verweij, C.L. Discovery of distinctive gene expression profiles in rheumatoid synovium using cDNA microarray technology: Evidence for the existence of multiple pathways of tissue destruction and repair. Genes Immun. 2003, 4, 187–196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Delft, M.A.M.; Van Beest, S.; Kloppenburg, M.; Trouw, L.A.; Ioan-Facsinay, A. Presence of Autoantibodies in Erosive Hand Osteoarthritis and Association with Clinical Presentation. J. Rheumatol. 2019, 46, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Dolzani, P.; Assirelli, E.; Pulsatelli, L.; Addimanda, O.; Mancarella, L.; Peri, G.; Mantovani, A.; Facchini, A.; Meliconi, R. Systemic inflammation and antibodies to citrullinated peptides in hand osteoarthritis. Clin. Exp. Rheumatol. 2011, 29, 1006–1009. [Google Scholar] [PubMed]
- Glant, T.T.; Ocsko, T.; Markovics, A.; Szekanecz, Z.; Katz, R.S.; Rauch, T.A.; Mikecz, K. Characterization and Localization of Citrullinated Proteoglycan Aggrecan in Human Articular Cartilage. PLoS ONE 2016, 11, e0150784. [Google Scholar] [CrossRef]
- Ahmed, U.; Anwar, A.; Savage, R.S.; Costa, M.L.; Mackay, N.; Filer, A.; Raza, K.; Watts, R.A.; Winyard, P.G.; Tarr, J.; et al. Biomarkers of early stage osteoarthritis, rheumatoid arthritis and musculoskeletal health. Sci. Rep. 2015, 5, 9259. [Google Scholar] [CrossRef]
- Xie, X.; Van Delft, M.A.M.; Shuweihdi, F.; Kingsbury, S.R.; Trouw, L.A.; Doody, G.M.; Conaghan, P.G.; Ponchel, F. Auto-antibodies to post-translationally modified proteins in osteoarthritis. Osteoarthr. Cartil. 2021, 29, 924–933. [Google Scholar] [CrossRef]
- Lopes, E.B.P.; Filiberti, A.; Husain, S.A.; Humphrey, M.B. Immune Contributions to Osteoarthritis. Curr. Osteoporos. Rep. 2017, 15, 593–600. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef]
- Smith, M.D.; Triantafillou, S.; Parker, A.; Youssef, P.P.; Coleman, M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J. Rheumatol. 1997, 24, 365–371. [Google Scholar]
- Orita, S.; Koshi, T.; Mitsuka, T.; Miyagi, M.; Inoue, G.; Arai, G.; Ishikawa, T.; Hanaoka, E.; Yamashita, K.; Yamashita, M.; et al. Associations between proinflammatory cytokines in the synovial fluid and radiographic grading and pain-related scores in 47 consecutive patients with osteoarthritis of the knee. BMC Musculoskelet. Disord. 2011, 12, 144. [Google Scholar] [CrossRef]
- Livshits, G.; Zhai, G.; Hart, D.J.; Kato, B.S.; Wang, H.; Williams, F.M.K.; Spector, T.D. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: The Chingford study. Arthritis Rheum. 2009, 60, 2037–2045. [Google Scholar] [CrossRef]
- Stannus, O.; Jones, G.; Cicuttini, F.; Parameswaran, V.; Quinn, S.; Burgess, J.; Ding, C. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr. Cartil. 2010, 18, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.; Rogers, V.E.; Henriksen, V.T.; Aguirre, D.; Trawick, R.H.; Rasmussen, G.L.; Momberger, N.G. Serum cytokines are increased and circulating micronutrients are not altered in subjects with early compared to advanced knee osteoarthritis. Cytokine 2014, 68, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Barreto, G.; Sandelin, J.; Salem, A.; Nordström, D.C.; Waris, E. Toll-like receptors and their soluble forms differ in the knee and thumb basal osteoarthritic joints. Acta Orthop. 2017, 88, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Takakubo, Y.; Hirayama, T.; Konttinen, Y.T.; Goodman, S.B.; Yamakawa, M.; Takagi, M. Expression of Toll-like Receptors and Their Signaling Pathways in Rheumatoid Synovitis. J. Rheumatol. 2011, 38, 810–820. [Google Scholar] [CrossRef]
- Takakubo, Y.; Barreto, G.; Konttinen, Y.T.; Oki, H.; Takagi, M. Role of Innate Immune Sensors, TLRs, and NALP3 in Rheumatoid Arthritis and Osteoarthritis. J. Long Term Eff. Med. Implant. 2014, 24, 243–251. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Martel-Pelletier, J.; Pelletier, J.-P. The role of cytokines in osteoarthritis pathophysiology. Biorheology 2002, 39, 237–246. [Google Scholar] [CrossRef]
- Rosenberg, J.H.; Rai, V.; Dilisio, M.F.; Agrawal, D.K. Damage-associated molecular patterns in the pathogenesis of osteoarthritis: Potentially novel therapeutic targets. Mol. Cell. Biochem. 2017, 434, 171–179. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, H.; Wei, J.; Lin, S.; Zong, Z.; Gong, F.; Huang, X.; Sun, J.; Li, P.; Lin, H.; et al. Inhibition of Nrf2/HO-1 signaling leads to increased activation of the NLRP3 inflammasome in osteoarthritis. Arthritis Res. Ther. 2019, 21, 300. [Google Scholar] [CrossRef]
- Yan, Z.; Qi, W.; Zhan, J.; Lin, Z.; Lin, J.; Xue, X.; Pan, X.; Zhou, Y. Activating Nrf2 signalling alleviates osteoarthritis development by inhibiting inflammasome activation. J. Cell. Mol. Med. 2020, 24, 13046–13057. [Google Scholar] [CrossRef]
- Abdel Galil, S.M.; Ezzeldin, N.; Fawzy, F.; El-Boshy, M. The single-nucleotide polymorphism (SNP) of tumor necrosis factor α −308G/A gene is associated with early-onset primary knee osteoarthritis in an Egyptian female population. Clin. Rheumatol. 2017, 36, 2525–2530. [Google Scholar] [CrossRef]
- Fernandes, M.T.P.; Fernandes, K.B.P.; Anibal, F.F.; Shimoya-Bittencourt, W.; Santos, V.M.; De Oliveira Perrucini, P.D.; Poli-Frederico, R.C. Functional status and severity of osteoarthritis in elderly is associated to the polymorphism of TNFA gene. Adv. Rheumatol. 2019, 59, 25. [Google Scholar] [CrossRef]
- Stern, A.G.; De Carvalho, M.R.C.; Buck, G.A.; Adler, R.A.; Rao, T.P.S.; Disler, D.; Moxley, G. Association of erosive hand osteoarthritis with a single nucleotide polymorphism on the gene encoding interleukin-1 beta. Osteoarthr. Cartil. 2003, 11, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Solovieva, S.; Kämäräinen, O.-P.; Hirvonen, A.; Hämäläinen, S.; Laitala, M.; Vehmas, T.; Luoma, K.; Näkki, A.; Riihimäki, H.; Ala-Kokko, L.; et al. Association Between Interleukin 1 Gene Cluster Polymorphisms and Bilateral Distal Interphalangeal Osteoarthritis. J. Rheumatol. 2009, 36, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Moxley, G.; Meulenbelt, I.; Chapman, K.; Van Diujn, C.M.; Eline Slagboom, P.; Neale, M.C.; Smith, A.J.P.; Carr, A.J.; Loughlin, J. Interleukin-1 region meta-analysis with osteoarthritis phenotypes. Osteoarthr. Cartil. 2010, 18, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, O.; Williams, F.M.K.; Valdes, A.; Hart, D.J.; Malkin, I.; Spector, T.D.; Livshits, G. Association of interleukin-6 gene polymorphisms with hand osteoarthritis and hand osteoporosis. Cytokine 2014, 69, 94–101. [Google Scholar] [CrossRef]
- Milaras, C.; Lepetsos, P.; Dafou, D.; Potoupnis, M.; Tsiridis, E. Association of Matrix Metalloproteinase (MMP) Gene Polymorphisms with Knee Osteoarthritis: A Review of the Literature. Cureus 2021, 13, e18607. [Google Scholar] [CrossRef]
- Vrgoc, G.; Vrbanec, J.; Eftedal, R.K.; Dembic, P.L.; Balen, S.; Dembic, Z.; Jotanovic, Z. Interleukin-17 and Toll-like Receptor 10 genetic polymorphisms and susceptibility to large joint osteoarthritis. J. Orthop. Res. 2018, 36, 1684–1693. [Google Scholar] [CrossRef]
- Zheng, M.; Shi, S.; Zheng, Q.; Wang, Y.; Ying, X.; Jin, Y. Association between TLR-9 gene rs187084 polymorphism and knee osteoarthritis in a Chinese population. Biosci. Rep. 2017, 37, BSR20170844. [Google Scholar] [CrossRef]
- Xi, X.; Mehmood, A.; Niu, P.; Yang, J.; Wang, Y.; Zhou, H.; Han, X.; Ma, L.; Jin, S.; Wu, Y. Association of X-linked TLR-7 gene polymorphism with the risk of knee osteoarthritis: A case–control study. Sci. Rep. 2022, 12, 7243. [Google Scholar] [CrossRef]
- Stefik, D.; Vranic, V.; Ivkovic, N.; Velikic, G.; Maric, D.M.; Abazovic, D.; Vojvodic, D.; Maric, D.L.; Supic, G. Potential Impact of Polymorphisms in Toll-like Receptors 2, 3, 4, 7, 9, miR-146a, miR-155, and miR-196a Genes on Osteoarthritis Susceptibility. Biology 2023, 12, 458. [Google Scholar] [CrossRef] [PubMed]
- Huss, R.S.; Huddleston, J.I.; Goodman, S.B.; Butcher, E.C.; Zabel, B.A. Synovial tissue–infiltrating natural killer cells in osteoarthritis and periprosthetic inflammation. Arthritis Rheum. 2010, 62, 3799–3805. [Google Scholar] [CrossRef] [PubMed]
- Platzer, H.; Trauth, R.; Nees, T.A.; Tripel, E.; Gantz, S.; Schiltenwolf, M.; Moradi, B.; Rosshirt, N. CD8+ T Cells in OA Knee Joints Are Differentiated into Subsets Depending on OA Stage and Compartment. J. Clin. Med. 2022, 11, 2814. [Google Scholar] [CrossRef] [PubMed]
- Jaime, P.; García-Guerrero, N.; Estella, R.; Pardo, J.; García-Álvarez, F.; Martinez-Lostao, L. CD56+/CD16− Natural Killer cells expressing the inflammatory protease granzyme A are enriched in synovial fluid from patients with osteoarthritis. Osteoarthr. Cartil. 2017, 25, 1708–1718. [Google Scholar] [CrossRef]
- Pawłowska, J.; Mikosik, A.; Soroczynska-Cybula, M.; Jóźwik, A.; Łuczkiewicz, P.; Mazurkiewicz, S.; Lorczyński, A.; Witkowski, J.M.; Bryl, E. Different distribution of CD4 and CD8 T cells in synovial membrane and peripheral blood of rheumatoid arthritis and osteoarthritis patients. Folia Histochem. Cytobiol. 2010, 47, 627–632. [Google Scholar] [CrossRef]
- Yamin, R.; Berhani, O.; Peleg, H.; Aamar, S.; Stein, N.; Gamliel, M.; Hindi, I.; Scheiman-Elazary, A.; Gur, C. High percentages and activity of synovial fluid NK cells present in patients with advanced stage active Rheumatoid Arthritis. Sci. Rep. 2019, 9, 1351. [Google Scholar] [CrossRef]
- Haywood, L.; McWilliams, D.F.; Pearson, C.I.; Gill, S.E.; Ganesan, A.; Wilson, D.; Walsh, D.A. Inflammation and angiogenesis in osteoarthritis. Arthritis Rheum. 2003, 48, 2173–2177. [Google Scholar] [CrossRef]
- Pessler, F.; Chen, L.X.; Dai, L.; Gomez-Vaquero, C.; Diaz-Torne, C.; Paessler, M.E.; Scanzello, C.; Çakir, N.; Einhorn, E.; Schumacher, H.R. A histomorphometric analysis of synovial biopsies from individuals with Gulf War Veterans’ Illness and joint pain compared to normal and osteoarthritis synovium. Clin. Rheumatol. 2008, 27, 1127–1134. [Google Scholar] [CrossRef]
- Sakkas, L.I.; Scanzello, C.; Johanson, N.; Burkholder, J.; Mitra, A.; Salgame, P.; Katsetos, C.D.; Platsoucas, C.D. T Cells and T-Cell Cytokine Transcripts in the Synovial Membrane in Patients with Osteoarthritis. Clin. Diagn. Lab. Immunol. 1998, 5, 430–437. [Google Scholar] [CrossRef]
- Krenn, V.; Hensel, F.; Kim, H.J.; Souto Carneiro, M.M.; Starostik, P.; Ristow, G.; König, A.; Vollmers, H.P.; Müller-Hermelink, H.K. Molecular IgV(H) analysis demonstrates highly somatic mutated B cells in synovialitis of osteoarthritis: A degenerative disease is associated with a specific, not locally generated immune response. Lab. Investig. 1999, 79, 1377–1384. [Google Scholar]
- Kouskoff, V.; Lacaud, G.; Nemazee, D. T Cell-Independent Rescue of B Lymphocytes from Peripheral Immune Tolerance. Science 2000, 287, 2501–2503. [Google Scholar] [CrossRef] [PubMed]
- Takemura, S.; Braun, A.; Crowson, C.; Kurtin, P.J.; Cofield, R.H.; O’Fallon, W.M.; Goronzy, J.J.; Weyand, C.M. Lymphoid Neogenesis in Rheumatoid Synovitis. J. Immunol. 2001, 167, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Rosshirt, N.; Trauth, R.; Platzer, H.; Tripel, E.; Nees, T.A.; Lorenz, H.-M.; Tretter, T.; Moradi, B. Proinflammatory T cell polarization is already present in patients with early knee osteoarthritis. Arthritis Res. Ther. 2021, 23, 37. [Google Scholar] [CrossRef] [PubMed]
- Nees, T.A.; Rosshirt, N.; Zhang, J.A.; Platzer, H.; Sorbi, R.; Tripel, E.; Reiner, T.; Walker, T.; Schiltenwolf, M.; Lorenz, H.-M.; et al. T Helper Cell Infiltration in Osteoarthritis-Related Knee Pain and Disability. J. Clin. Med. 2020, 9, 2423. [Google Scholar] [CrossRef]
- Deleuran, B.W.; Chu, C.Q.; Field, M.; Brennan, F.M.; Katsikis, P.; Feldmann, M.; Maini, R.N. Localization of interleukin-1α, type 1 interleukin-1 receptor and interleukin-1 receptor antagonist in the synovial membrane and cartilage/pannus junction in rheumatoid arthritis. Rheumatology 1992, 31, 801–809. [Google Scholar] [CrossRef]
- Johnell, O.; Hulth, A.; Henricson, A. T-Lymphocyte Subsets and HLA-DR-Expressing Cells in the Osteoarthritic Synovialis. Scand. J. Rheumatol. 1985, 14, 259–264. [Google Scholar] [CrossRef]
- De Jong, H.; Berlo, S.E.; Hombrink, P.; Otten, H.G.; Van Eden, W.; Lafeber, F.P.; Heurkens, A.H.M.; Bijlsma, J.W.J.; Glant, T.T.; Prakken, B.J. Cartilage proteoglycan aggrecan epitopes induce proinflammatory autoreactive T-cell responses in rheumatoid arthritis and osteoarthritis. Ann. Rheum. Dis. 2010, 69, 255–262. [Google Scholar] [CrossRef]
- Sakata, M.; Masuko-Hongo, K.; Nakamura, H.; Onuma, H.; Tsuruha, J.I.; Aoki, H.; Nishioka, K.; Kato, T. Osteoarthritic articular chondrocytes stimulate autologous T cell responses in vitro. Clin. Exp. Rheumatol. 2003, 21, 704–710. [Google Scholar]
- Nees, T.A.; Zhang, J.A.; Platzer, H.; Walker, T.; Reiner, T.; Tripel, E.; Moradi, B.; Rosshirt, N. Infiltration Profile of Regulatory T Cells in Osteoarthritis-Related Pain and Disability. Biomedicines 2022, 10, 2111. [Google Scholar] [CrossRef]
- Moradi, B.; Schnatzer, P.; Hagmann, S.; Rosshirt, N.; Gotterbarm, T.; Kretzer, J.P.; Thomsen, M.; Lorenz, H.-M.; Zeifang, F.; Tretter, T. CD4+CD25+/highCD127low/- regulatory T cells are enriched in rheumatoid arthritis and osteoarthritis joints—Analysis of frequency and phenotype in synovial membrane, synovial fluid and peripheral blood. Arthritis Res. Ther. 2014, 16, R97. [Google Scholar] [CrossRef]
- Ueno, H.; Banchereau, J.; Vinuesa, C.G. Pathophysiology of T follicular helper cells in humans and mice. Nat. Immunol. 2015, 16, 142–152. [Google Scholar] [CrossRef]
- Chu, Y.; Wang, F.; Zhou, M.; Chen, L.; Lu, Y. A preliminary study on the characterization of follicular helper T (Tfh) cells in rheumatoid arthritis synovium. Acta Histochem. 2014, 116, 539–543. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, P.; Zhang, Q.; Che, N.; Xu, L.; Qian, J.; Tan, W.; Zhang, M. Adiponectin promotes fibroblast-like synoviocytes producing IL-6 to enhance T follicular helper cells response in rheumatoid arthritis. Clin. Exp. Rheumatol. 2020, 38, 11–18. [Google Scholar] [PubMed]
- Murray-Brown, W.; Guo, Y.; Small, A.; Lowe, K.; Weedon, H.; Smith, M.D.; Lester, S.E.; Proudman, S.M.; Rao, N.L.; Hao, L.-Y.; et al. Differential expansion of T peripheral helper cells in early rheumatoid arthritis and osteoarthritis synovium. RMD Open 2022, 8, e002563. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Qi, C.; Liu, Y.; Gao, H.; Zhao, D.; Jiang, Y. Increased frequency of peripheral blood follicular helper T cells and elevated serum IL-21 levels in patients with knee osteoarthritis. Mol. Med. Rep. 2017, 15, 1095–1102. [Google Scholar] [CrossRef]
- Chichasova, N.V. Cartilage destruction in rheumatoid arthritis, its association with functional impairments. Mod. Rheumatol. J. 2014, 8, 60–71. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Qiu, F.; Zhu, X.; Mo, H.; Wu, Z.; Xiao, C. Pannus does not occur only in rheumatoid arthritis: A pathological observation of pannus of knee osteoarthritis. Nan Fang Yi Ke Da Xue Xue Bao 2019, 39, 747–750. [Google Scholar] [CrossRef]
- Shibakawa, A.; Aoki, H.; Masuko-Hongo, K.; Kato, T.; Tanaka, M.; Nishioka, K.; Nakamura, H. Presence of pannus-like tissue on osteoarthritic cartilage and its histological character. Osteoarthritis. Cartil. 2003, 11, 133–140. [Google Scholar] [CrossRef]
- Furuzawa-Carballeda, J.; Macip-Rodríguez, P.M.; Cabral, A.R. Osteoarthritis and rheumatoid arthritis pannus have similar qualitative metabolic characteristics and pro-inflammatory cytokine response. Clin. Exp. Rheumatol. 2008, 26, 554–560. [Google Scholar]
- Wernert, N.; Justen, H.-P.; Rothe, M.; Behrens, P.; Dreschers, S.; Neuhaus, T.; Florin, A.; Sachinidis, A.; Vetter, H.; Ko, Y. The Ets 1 transcription factor is upregulated during inflammatory angiogenesis in rheumatoid arthritis. J. Mol. Med. 2002, 80, 258–266. [Google Scholar] [CrossRef]
- Wang, C.; Chen, L.; Zhu, P.; Fan, C.; Wang, Y.; Jia, J. CD147 stimulates the angiogenesis in rheumatoid synovium via the activation of vascular endothelial growth factor. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2007, 23, 426–428. [Google Scholar]
- Kriegsmann, J.; Berndt, A.; Hansen, T.; Borsi, L.; Zardi, L.; Bräuer, R.; Petrow, P.K.; Otto, M.; Kirkpatrick, C.J.; Gay, S.; et al. Expression of fibronectin splice variants and oncofetal glycosylated fibronectin in the synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Rheumatol. Int. 2004, 24, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Pessler, F.; Ogdie, A.; Diaz-Torne, C.; Dai, L.; Yu, X.; Einhorn, E.; Gay, S.; Schumacher, H.R. Subintimal Ki-67 as a synovial tissue biomarker for inflammatory arthropathies. Ann. Rheum. Dis. 2008, 67, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Šenolt, L.; Grigorian, M.; Lukanidin, E.; Simmen, B.; Michel, B.A.; Pavelka, K.; Gay, R.E.; Gay, S.; Neidhart, M. S100A4 is expressed at site of invasion in rheumatoid arthritis synovium and modulates production of matrix metalloproteinases. Ann. Rheum. Dis. 2006, 65, 1645–1648. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, S.; Hedfors, E. Arthroscopic and immunohistologic characterization of knee joint synovitis in osteoarthritis. Arthritis Rheum. 1987, 30, 1081–1088. [Google Scholar] [CrossRef]
- Homandberg, G.A.; Hui, F. Association of Proteoglycan Degradation with Catabolic Cytokine and Stromelysin Release from Cartilage Cultured with Fibronectin Fragments. Arch. Biochem. Biophys. 1996, 334, 325–331. [Google Scholar] [CrossRef]
- Oehler, S.; Neureiter, D.; Meyer-Scholten, C.; Aigner, T. Subtyping of osteoarthritic synoviopathy. Clin. Exp. Rheumatol. 2002, 20, 633–640. [Google Scholar]
- Wood, M.J.; Leckenby, A.; Reynolds, G.; Spiering, R.; Pratt, A.G.; Rankin, K.S.; Isaacs, J.D.; Haniffa, M.A.; Milling, S.; Hilkens, C.M.U. Macrophage proliferation distinguishes 2 subgroups of knee osteoarthritis patients. JCI Insight 2019, 4, e125325. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 2017, 389, 2328–2337. [Google Scholar] [CrossRef]
- Favero, M.; Perino, G.; Valente, M.L.; Tiengo, C.; Ramonda, R. Radiological and histological analysis of two replaced interphalangeal joints with active subchondral bone resorption in erosive hand osteoarthritis: A novel mechanism? Skelet. Radiol. 2017, 46, 385–391. [Google Scholar] [CrossRef]
- Wittoek, R.; Cruyssen, B.V.; Verbruggen, G. Predictors of functional impairment and pain in erosive osteoarthritis of the interphalangeal joints: Comparison with controlled inflammatory arthritis. Arthritis Rheum. 2012, 64, 1430–1436. [Google Scholar] [CrossRef]
- Zhang, Y. Prevalence of Symptomatic Hand Osteoarthritis and Its Impact on Functional Status among the Elderly: The Framingham Study. Am. J. Epidemiol. 2002, 156, 1021–1027. [Google Scholar] [CrossRef]
- Kidd, K.L.; Peter, J.B. Erosive Osteoarthritis. Radiology 1966, 86, 640–647. [Google Scholar] [CrossRef]
- Favero, M.; Belluzzi, E.; Ortolan, A.; Lorenzin, M.; Oliviero, F.; Doria, A.; Scanzello, C.R.; Ramonda, R. Erosive hand osteoarthritis: Latest findings and outlook. Nat. Rev. Rheumatol. 2022, 18, 171–183. [Google Scholar] [CrossRef]
- Kloppenburg, M.; Kroon, F.P.; Blanco, F.J.; Doherty, M.; Dziedzic, K.S.; Greibrokk, E.; Haugen, I.K.; Herrero-Beaumont, G.; Jonsson, H.; Kjeken, I.; et al. 2018 update of the EULAR recommendations for the management of hand osteoarthritis. Ann. Rheum. Dis. 2019, 78, 16–24. [Google Scholar] [CrossRef]
- Dahaghin, S.; Bierma-zeinstra, S.M.A.; Hazes, J.M.W.; Koes, B.W. Clinical burden of radiographic hand osteoarthritis: A systematic appraisal. Arthritis Rheum. 2006, 55, 636–647. [Google Scholar] [CrossRef]
- Dell’Isola, A.; Allan, R.; Smith, S.L.; Marreiros, S.S.P.; Steultjens, M. Identification of clinical phenotypes in knee osteoarthritis: A systematic review of the literature. BMC Musculoskelet. Disord. 2016, 17, 425. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.C.; Carrino, J.A.; Schnitzer, T.J.; Guermazi, A.; Walsh, D.A.; White, A.; Nakajo, S.; Fountaine, R.J.; Hickman, A.; Pixton, G.; et al. Long-Term Safety and Efficacy of Subcutaneous Tanezumab Versus Nonsteroidal Antiinflammatory Drugs for Hip or Knee Osteoarthritis: A Randomized Trial. Arthritis Rheumatol. 2021, 73, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Sanga, P.; Katz, N.; Polverejan, E.; Wang, S.; Kelly, K.M.; Haeussler, J.; Thipphawong, J. Efficacy, safety, and tolerability of fulranumab, an anti-nerve growth factor antibody, in the treatment of patients with moderate to severe osteoarthritis pain. Pain 2013, 154, 1910–1919, Erratum in Pain 2014, 155, 204. https://doi.org/10.1016/j.pain.2013.10.020. [Google Scholar] [CrossRef]
- Buch, M.H. Defining refractory rheumatoid arthritis. Ann. Rheum. Dis. 2018, 77, 966–969. [Google Scholar] [CrossRef]
- Fonseca, J.E.; Canhão, H.; Tavares, N.J.; Cruz, M.; Branco, J.; Queiroz, M.V. Persistent low grade synovitis without erosive progression in magnetic resonance imaging of rheumatoid arthritis patients treated with infliximab over 1 year. Clin. Rheumatol. 2009, 28, 1213–1216. [Google Scholar] [CrossRef]
- Forslind, K.; Svensson, B. MRI evidence of persistent joint inflammation and progressive joint damage despite clinical remission during treatment of early rheumatoid arthritis. Scand. J. Rheumatol. 2016, 45, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Majorczyk, E.; Mazurek-Mochol, M.; Pawlik, A.; Kuśnierczyk, P. Clinical Factors and the Outcome of Treatment with Methotrexate in Rheumatoid Arthritis: Role of Rheumatoid Factor, Erosive Disease and High Level of Erythrocyte Sedimentation Rate. J. Clin. Med. 2022, 11, 6078. [Google Scholar] [CrossRef] [PubMed]
- Saevarsdottir, S.; Wallin, H.; Seddighzadeh, M.; Ernestam, S.; Geborek, P.; Petersson, I.F.; Bratt, J.; Van Vollenhoven, R.F. Predictors of response to methotrexate in early DMARD naïve rheumatoid arthritis: Results from the initial open-label phase of the SWEFOT trial. Ann. Rheum. Dis. 2011, 70, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Lukas, C.; Mary, J.; Debandt, M.; Daïen, C.; Morel, J.; Cantagrel, A.; Fautrel, B.; Combe, B. Predictors of good response to conventional synthetic DMARDs in early seronegative rheumatoid arthritis: Data from the ESPOIR cohort. Arthritis Res. Ther. 2019, 21, 243. [Google Scholar] [CrossRef]
- Watanabe, R.; Okano, T.; Gon, T.; Yoshida, N.; Fukumoto, K.; Yamada, S.; Hashimoto, M. Difficult-to-treat rheumatoid arthritis: Current concept and unsolved problems. Front. Med. 2022, 9, 1049875. [Google Scholar] [CrossRef]
- Novella-Navarro, M.; Plasencia, C.; Tornero, C.; Navarro-Compán, V.; Cabrera-Alarcón, J.L.; Peiteado-López, D.; Nuño, L.; Monjo-Henry, I.; Franco-Gómez, K.; Villalba, A.; et al. Clinical predictors of multiple failure to biological therapy in patients with rheumatoid arthritis. Arthritis Res. Ther. 2020, 22, 284. [Google Scholar] [CrossRef]
- Khader, Y.; Beran, A.; Ghazaleh, S.; Lee-Smith, W.; Altorok, N. Predictors of remission in rheumatoid arthritis patients treated with biologics: A systematic review and meta-analysis. Clin. Rheumatol. 2022, 41, 3615–3627. [Google Scholar] [CrossRef]
- Jung, J.-Y.; Lee, E.; Kim, J.-W.; Suh, C.-H.; Shin, K.; Kim, J.; Kim, H.-A. Unveiling difficult-to-treat rheumatoid arthritis: Long-term impact of biologic or targeted synthetic DMARDs from the KOBIO registry. Arthritis Res. Ther. 2023, 25, 174. [Google Scholar] [CrossRef]
- Hecquet, S.; Combier, A.; Steelandt, A.; Pons, M.; Wendling, D.; Molto, A.; Miceli-Richard, C.; Allanore, Y.; Avouac, J. Characteristics of patients with difficult-to-treat rheumatoid arthritis in a French single-centre hospital. Rheumatology 2023, 62, 3866–3874. [Google Scholar] [CrossRef]
- Wenham, C.Y.J.; Grainger, A.J.; Hensor, E.M.A.; Caperon, A.R.; Ash, Z.R.; Conaghan, P.G. Methotrexate for pain relief in knee osteoarthritis: An open-label study. Rheumatology 2013, 52, 888–892. [Google Scholar] [CrossRef]
- Kingsbury, S.R.; Tharmanathan, P.; Keding, A.; Watt, F.E.; Scott, D.L.; Roddy, E.; Birrell, F.; Arden, N.K.; Bowes, M.; Arundel, C.; et al. Pain Reduction with Oral Methotrexate in Knee Osteoarthritis: A Randomized, Placebo-Controlled Clinical Trial. Ann. Intern. Med. 2024, 177, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Holanda, H.T.D.; Pollak, D.F.; Pucinelli, M.L.C. Baixa dose de methotrexate comparado a placebo em osteoartrite de joelho. Rev. Bras. Reumatol. 2007, 47, 334–340. [Google Scholar] [CrossRef]
- Enteshari-Moghaddam, A.; Isazadehfar, K.; Habibzadeh, A.; Hemmati, M. Efficacy of Methotrexate on Pain Severity Reduction and Improvement of Quality of Life in Patients with Moderate to Severe Knee Osteoarthritis. Anesth. Pain Med. 2019, 9, e89990. [Google Scholar] [CrossRef] [PubMed]
- Kingsbury, S.R.; Tharmanathan, P.; Keding, A.; Corbacho, B.; Watt, F.E.; Scott, D.L.; Roddy, E.; Birrell, F.; Arden, N.K.; Bowes, M.A.; et al. Significant pain reduction with oral methotrexate in knee osteoarthritis; results from the promote randomised controlled phase iii trial of treatment effectiveness. Osteoarthr. Cartil. 2019, 27, S84–S85. [Google Scholar] [CrossRef]
- Zhu, Z.; Yu, Q.; Leng, X.; Xu, J.; Ren, L.; Wang, K.; Huang, C.; Pan, Y.; Zhao, Y.; Li, T.; et al. Low-Dose Methotrexate for the Treatment of Inflammatory Knee Osteoarthritis: A Randomized Clinical Trial. JAMA Intern. Med. 2025, 185, 808. [Google Scholar] [CrossRef]
- Queiroz, I.; Pimentel, T.; Gallo Ruelas, M.; Tavares, A.H.; Barbosa, L.M.; Defante, M.L.R.; Leandro, G.N.; Monteiro, A.R.; Pimentel, F.N. Methotrexate for osteoarthritis: A systematic review meta-analysis of randomized controlled trials. Inflammopharmacology 2025, 33, 135–144. [Google Scholar] [CrossRef]
- Verbruggen, G.; Veys, E.M. Numerical scoring systems for the anatomic evolution of osteoarthritis of the finger joints. Arthritis Rheum. 1996, 39, 308–320. [Google Scholar] [CrossRef]
- Verbruggen, G.; Wittoek, R.; Cruyssen, B.V.; Elewaut, D. Morbid anatomy of ‘erosive osteoarthritis’ of the interphalangeal finger joints: An optimised scoring system to monitor disease progression in affected joints. Ann. Rheum. Dis. 2010, 69, 862–867. [Google Scholar] [CrossRef]
- Wang, Y.; Jones, G.; Keen, H.I.; Hill, C.L.; Wluka, A.E.; Kasza, J.; Teichtahl, A.J.; Antony, B.; O’Sullivan, R.; Cicuttini, F.M. Methotrexate to treat hand osteoarthritis with synovitis (METHODS): An Australian, multisite, parallel-group, double-blind, randomised, placebo-controlled trial. Lancet 2023, 402, 1764–1772. [Google Scholar] [CrossRef]
- Yu, L.; Luo, R.; Qin, G.; Zhang, Q.; Liang, W. Efficacy and safety of anti-interleukin-1 therapeutics in the treatment of knee osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2023, 18, 100. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Z.; Ye, Y.; Li, H.; Luo, H.; Tang, K.; Lai, Y. Efficacy, residual effectiveness and safety of diacerein in the treatment of knee osteoarthritis: A meta-analysis of randomized placebo-controlled trials. Medicine 2022, 101, e31700. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Wang, K.; Duan, H.; Xu, X.; Kuang, G.; Lu, M. Diacerein versus non-steroidal anti-inflammatory drugs in the treatment of knee osteoarthritis: A meta-analysis. J. Orthop. Surg. Res. 2023, 18, 308. [Google Scholar] [CrossRef] [PubMed]
- Bartels, E.M.; Bliddal, H.; Schøndorff, P.K.; Altman, R.D.; Zhang, W.; Christensen, R. Symptomatic efficacy and safety of diacerein in the treatment of osteoarthritis: A meta-analysis of randomized placebo-controlled trials. Osteoarthr. Cartil. 2010, 18, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Rintelen, B.; Neumann, K.; Leeb, B.F. A Meta-analysis of Controlled Clinical Studies with Diacerein in the Treatment of Osteoarthritis. Arch. Intern. Med. 2006, 166, 1899. [Google Scholar] [CrossRef]
- Estee, M.M.; Cicuttini, F.M.; Page, M.J.; Wluka, A.E.; Wang, Y. Efficacy of tumor necrosis factor inhibitors in hand osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. Osteoarthr. Cartil. Open 2023, 5, 100404. [Google Scholar] [CrossRef]
- Chevalier, X.; Ravaud, P.; Maheu, E.; Baron, G.; Rialland, A.; Vergnaud, P.; Roux, C.; Maugars, Y.; Mulleman, D.; Lukas, C.; et al. Adalimumab in patients with hand osteoarthritis refractory to analgesics and NSAIDs: A randomised, multicentre, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 2015, 74, 1697–1705. [Google Scholar] [CrossRef]
- Aitken, D.; Laslett, L.L.; Pan, F.; Haugen, I.K.; Otahal, P.; Bellamy, N.; Bird, P.; Jones, G. A randomised double-blind placebo-controlled crossover trial of HUMira (adalimumab) for erosive hand OsteoaRthritis—The HUMOR trial. Osteoarthr. Cartil. 2018, 26, 880–887. [Google Scholar] [CrossRef]
- Verbruggen, G.; Wittoek, R.; Cruyssen, B.V.; Elewaut, D. Tumour necrosis factor blockade for the treatment of erosive osteoarthritis of the interphalangeal finger joints: A double blind, randomised trial on structure modification. Ann. Rheum. Dis. 2012, 71, 891–898. [Google Scholar] [CrossRef]
- Kloppenburg, M.; Ramonda, R.; Bobacz, K.; Kwok, W.-Y.; Elewaut, D.; Huizinga, T.W.J.; Kroon, F.P.B.; Punzi, L.; Smolen, J.S.; Vander Cruyssen, B.; et al. Etanercept in patients with inflammatory hand osteoarthritis (EHOA): A multicentre, randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 2018, 77, 1757–1764. [Google Scholar] [CrossRef]
- Kloppenburg, M.; Ramonda, R.; Kwok, W.-Y.; Bobacz, K.; Elewaut, D.; Frallonardo, P.; Huizinga, T.W.; Kroon, F.; Smolen, J.; Vander Cruyssen, B.; et al. OP0095 Randomized, Placebo-Controlled Trial to Evaluate Clinical Efficacy and Structure Modifying Properties of Subcutaneous Etanercept (ETN) in Patients with Erosive Inflammatory Hand Osteoarthritis (OA). Ann. Rheum. Dis. 2016, 75, 90–91. [Google Scholar] [CrossRef]
- Jonsson, H.; Helgason, J. Rheumatoid Arthritis in an Icelandic Textbook from 1782. Scand. J. Rheumatol. 1996, 25, 134–137. [Google Scholar] [CrossRef]
- Weyand, C.M.; Klimiuk, P.A.; Goronzy, J.J. Heterogeneity of rheumatoid arthritis: From phenotypes to genotypes. Springer Semin. Immunopathol. 1998, 20, 5–22. [Google Scholar] [CrossRef]
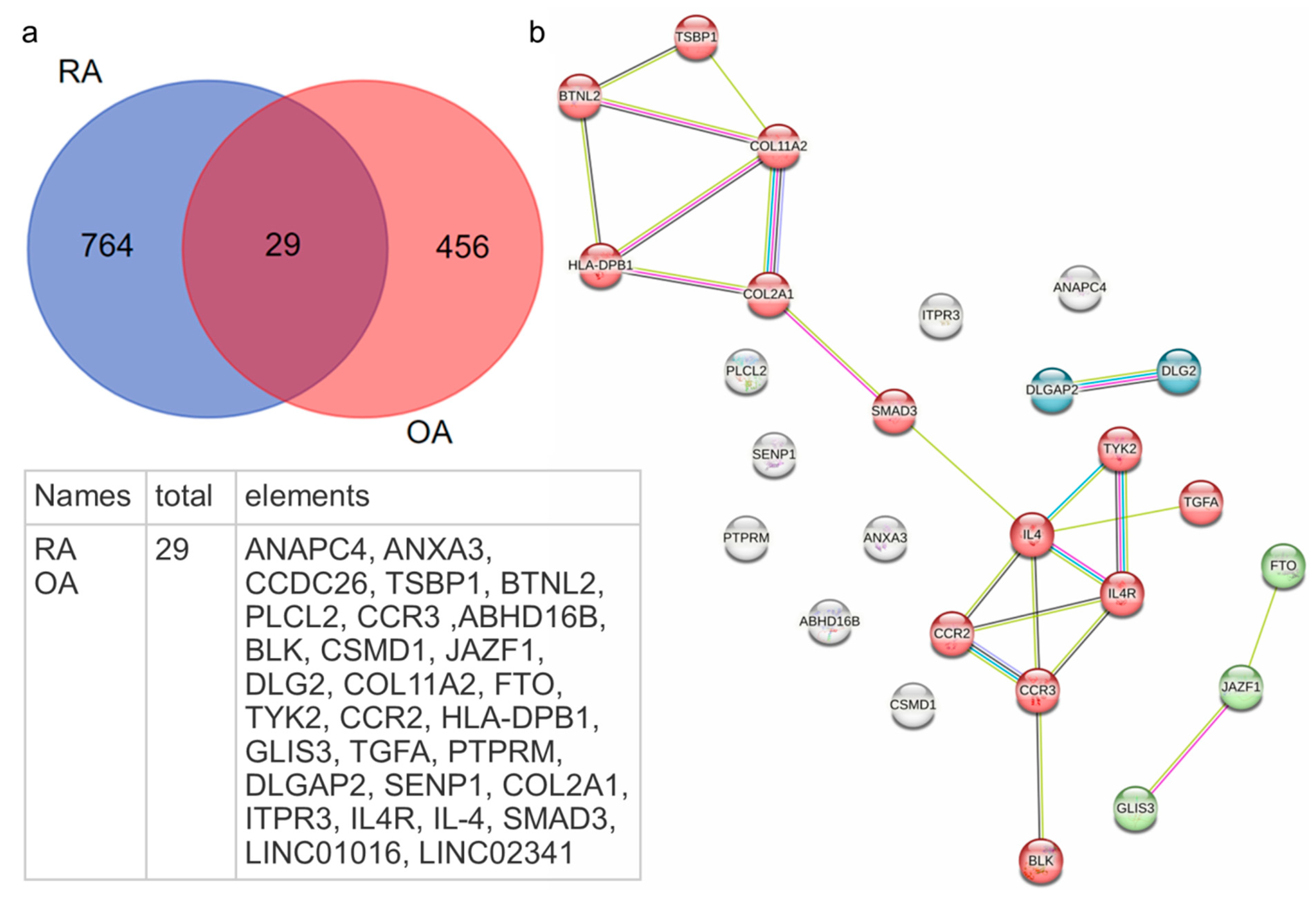
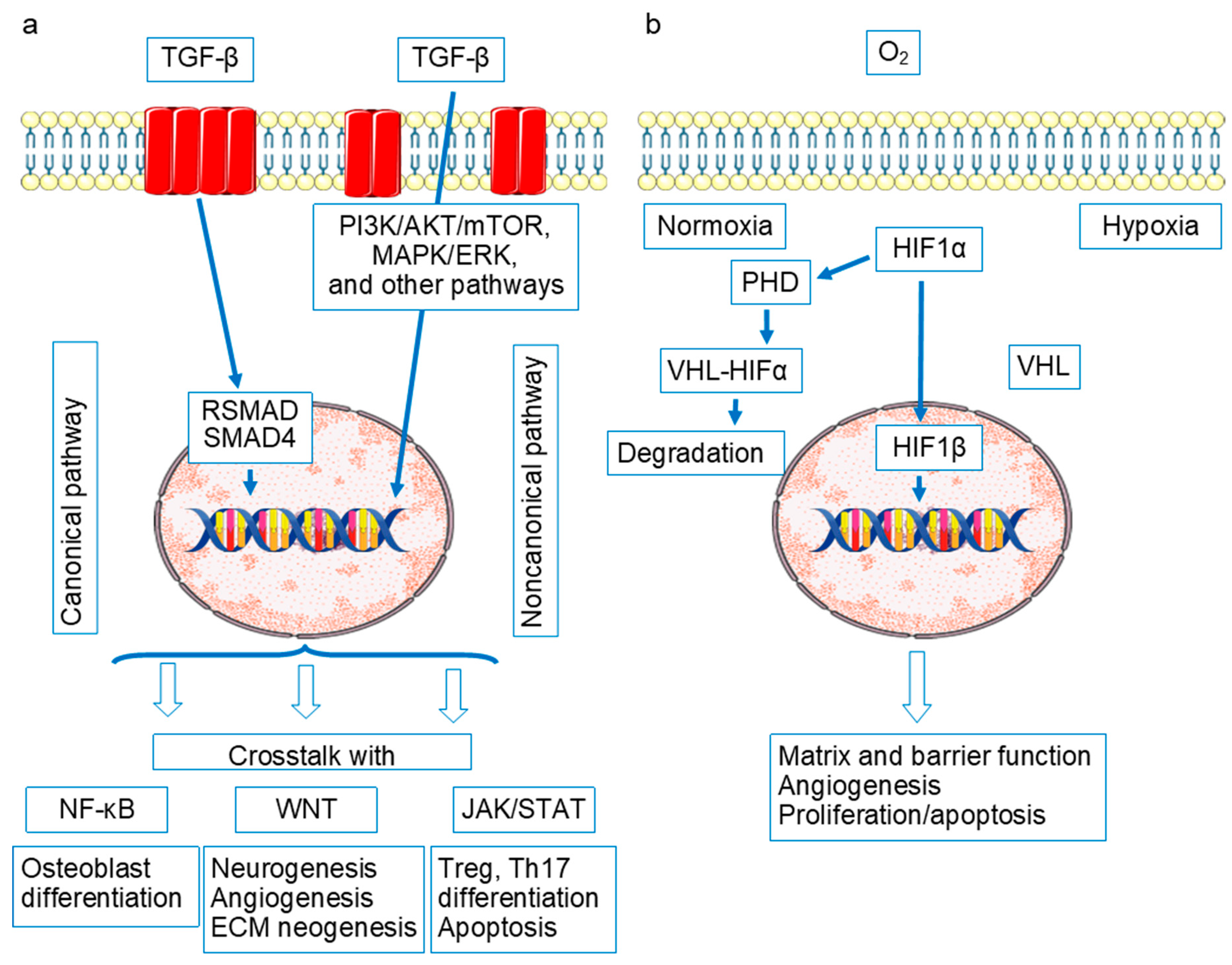

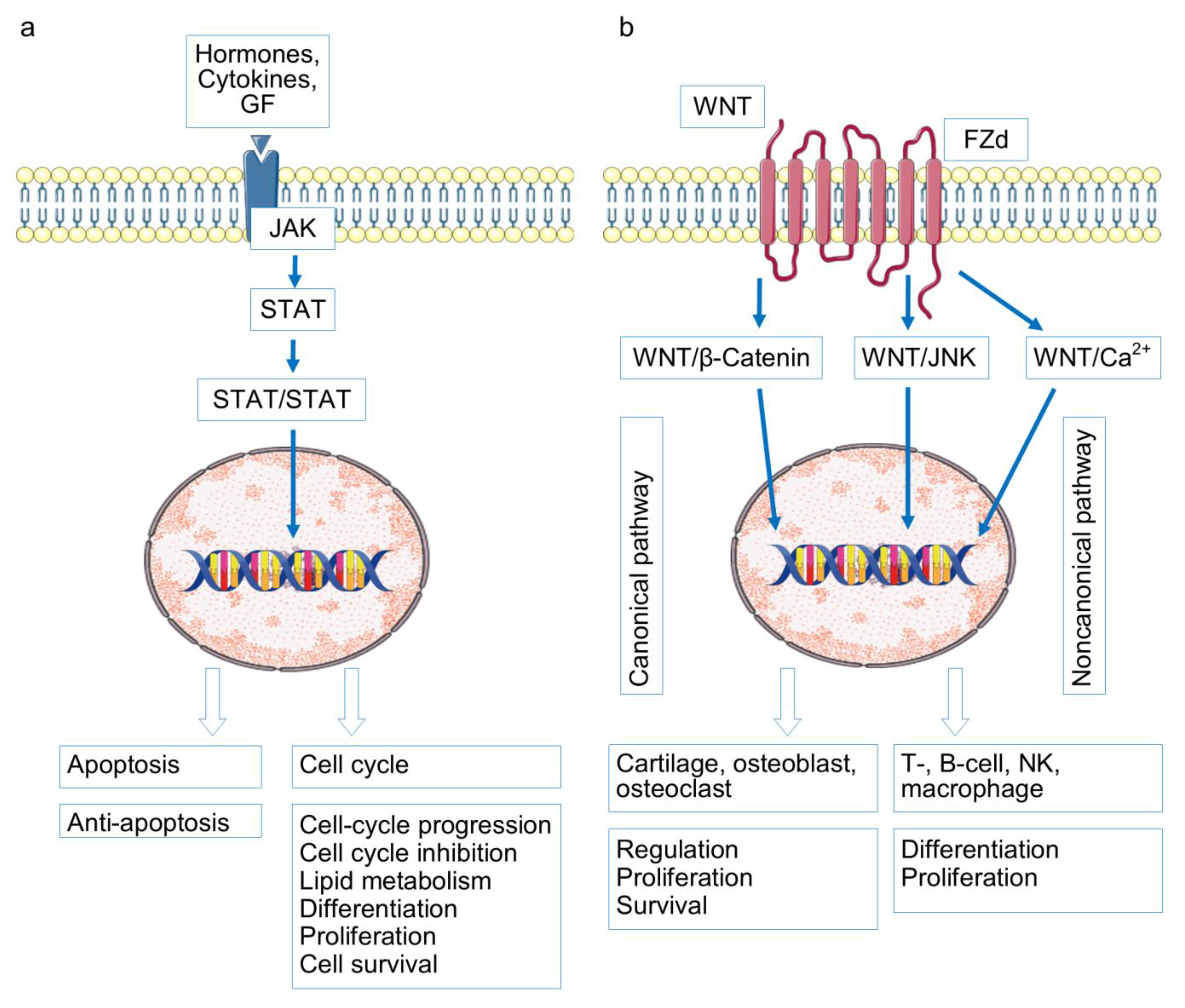
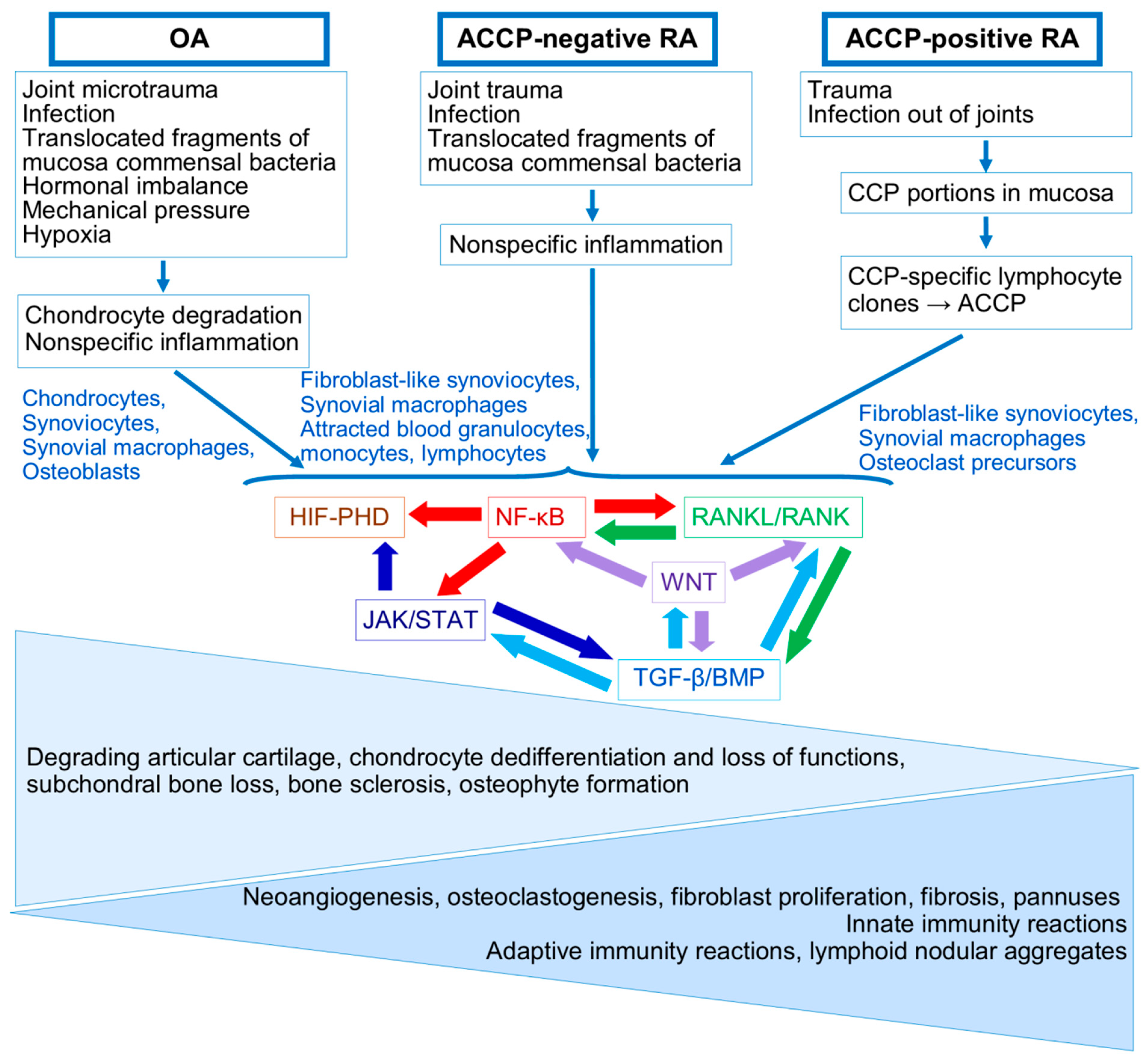
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korovina, M.O.; Valeeva, A.R.; Akhtyamov, I.F.; Brooks, W.; Renaudineau, Y.; Manukyan, G.; Arleevskaya, M.I. Joint Tissues: Convergence and Divergence of the Pathogenetic Mechanisms of Rheumatoid Arthritis and Osteoarthritis. Int. J. Mol. Sci. 2025, 26, 8742. https://doi.org/10.3390/ijms26178742
Korovina MO, Valeeva AR, Akhtyamov IF, Brooks W, Renaudineau Y, Manukyan G, Arleevskaya MI. Joint Tissues: Convergence and Divergence of the Pathogenetic Mechanisms of Rheumatoid Arthritis and Osteoarthritis. International Journal of Molecular Sciences. 2025; 26(17):8742. https://doi.org/10.3390/ijms26178742
Chicago/Turabian StyleKorovina, Marina O., Anna R. Valeeva, Ildar F. Akhtyamov, Wesley Brooks, Yves Renaudineau, Gayane Manukyan, and Marina I. Arleevskaya. 2025. "Joint Tissues: Convergence and Divergence of the Pathogenetic Mechanisms of Rheumatoid Arthritis and Osteoarthritis" International Journal of Molecular Sciences 26, no. 17: 8742. https://doi.org/10.3390/ijms26178742
APA StyleKorovina, M. O., Valeeva, A. R., Akhtyamov, I. F., Brooks, W., Renaudineau, Y., Manukyan, G., & Arleevskaya, M. I. (2025). Joint Tissues: Convergence and Divergence of the Pathogenetic Mechanisms of Rheumatoid Arthritis and Osteoarthritis. International Journal of Molecular Sciences, 26(17), 8742. https://doi.org/10.3390/ijms26178742






