Bacteroides fragilis and Microbacterium as Microbial Signatures in Hashimoto’s Thyroiditis
Abstract
1. Introduction
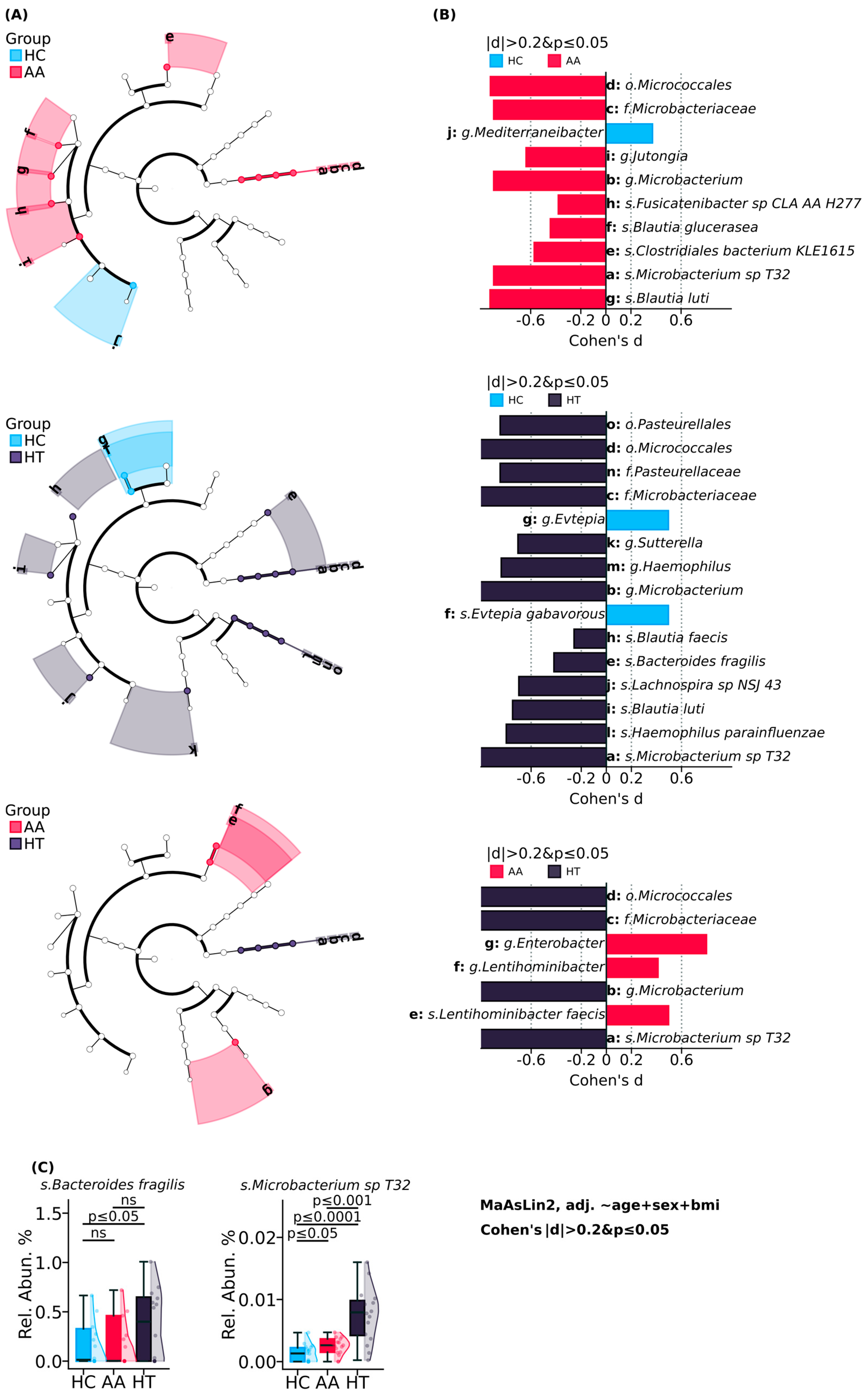
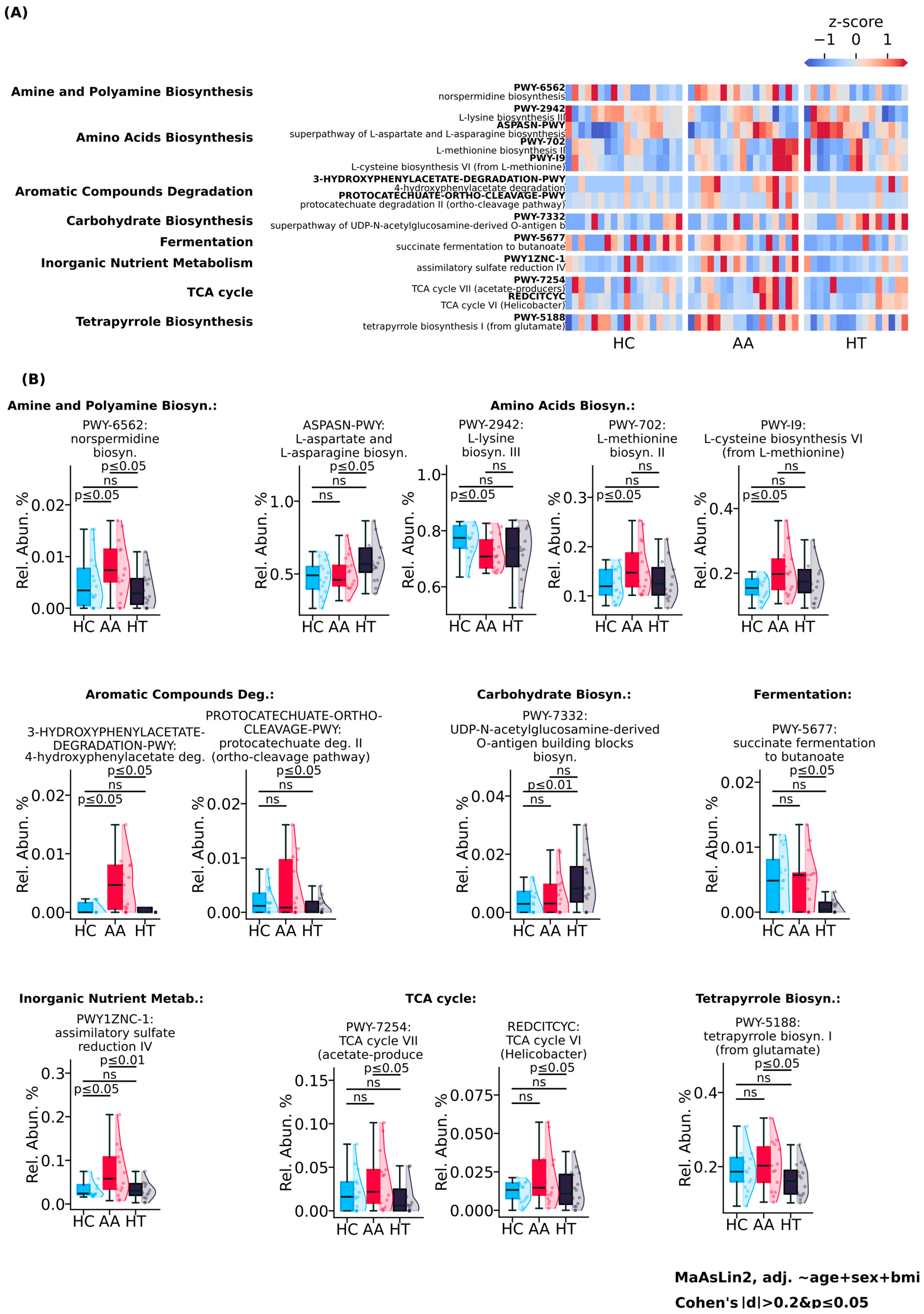
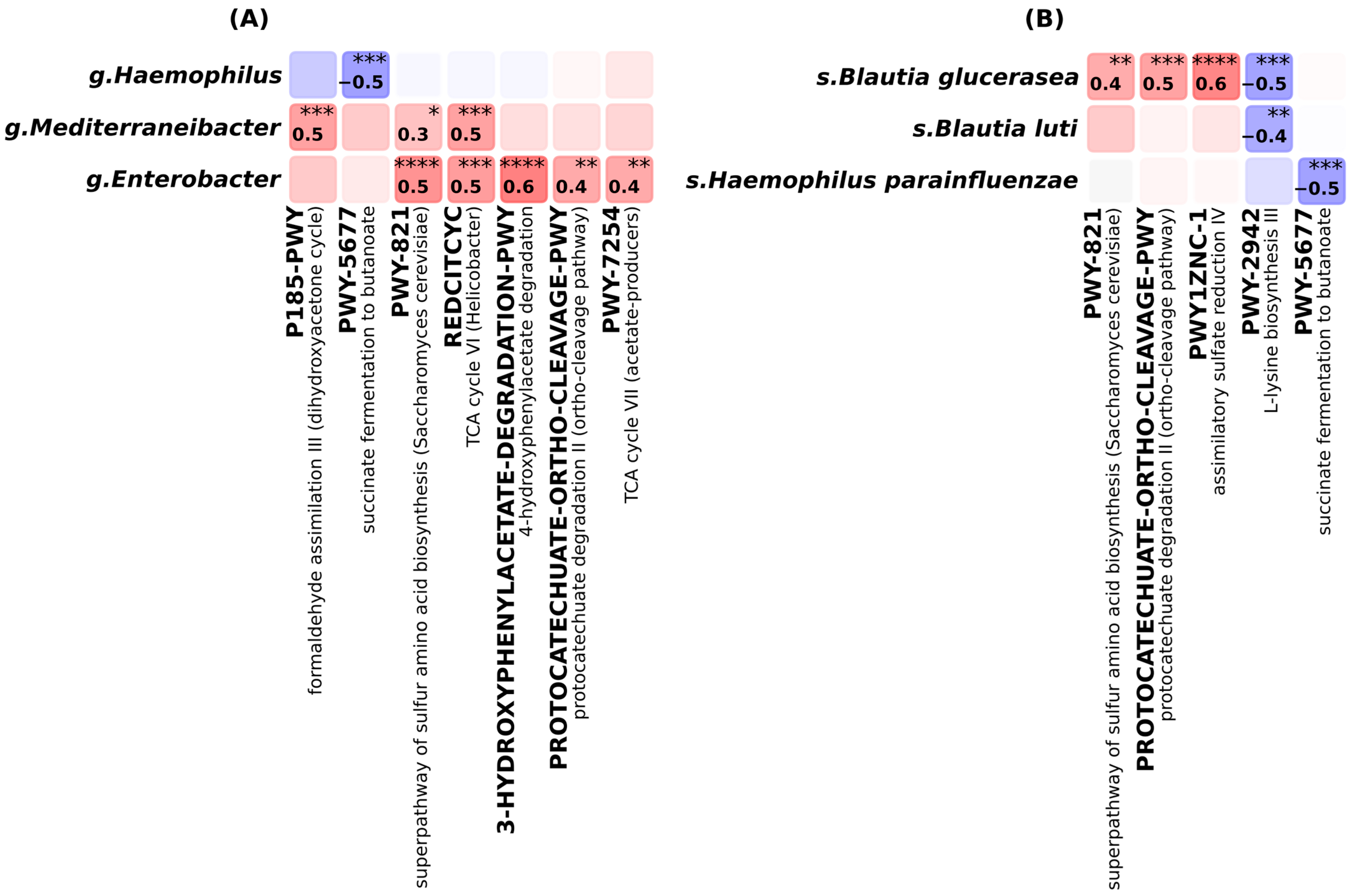
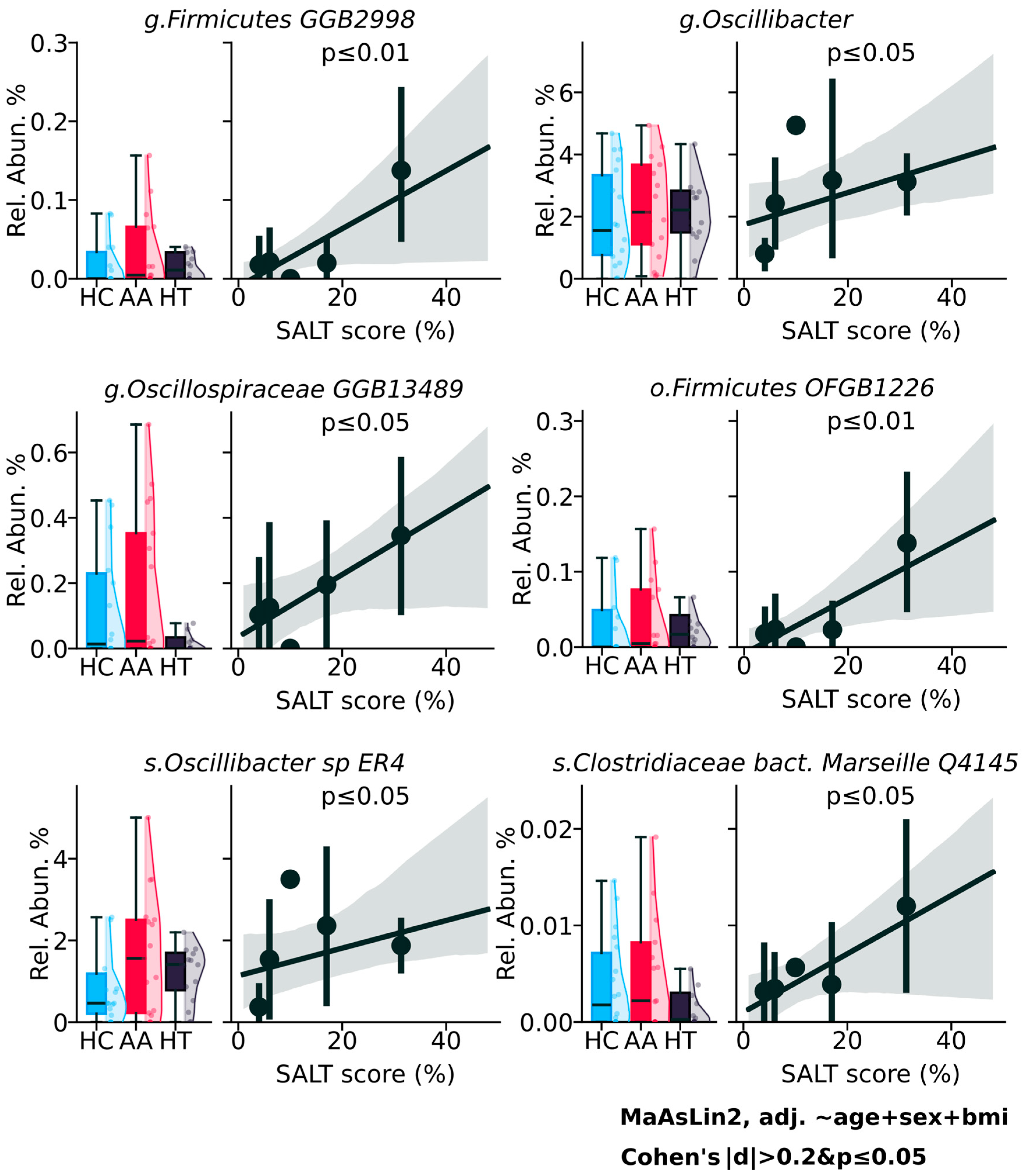
2. Results
3. Discussion
3.1. Known and Novel Gut Microbial Contributors to HT: Bacteroides fragilis and Microbacterium
3.2. Elevated Microbial Metabolic Activity and Functional Shifts in Alopecia Areata
3.3. Potential but Inconclusive Microbial Correlates of AA Severity
4. Materials and Methods
4.1. Study Subjects and Demographics
4.2. Sample Collection and Processing
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Alopecia areata |
| HT | Hashimoto’s thyroiditis |
| HC | Healthy control |
| IL-1β, IL-18, IL-10 | Interleukin 1 beta, 18, 10 |
| JAK-STAT | Janus kinase-signal transducer and activation of transcription |
| LPS | Lipopolysaccharide |
| PSA | Polysaccharide A |
| SALT | Severity of Alopecia areata |
| SCFA | Short-chain fatty acids |
| TCA | Tricarboxylic Acid |
| Th1, Th2, Th17, Treg | T helper cell types 1, 2, 17, Regulatory T cells |
References
- Caturegli, P.; De Remigis, A.; Rose, N.R. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef]
- Fröhlich, E.; Wahl, R. Thyroid Autoimmunity: Role of Anti-thyroid Antibodies in Thyroid and Extra-Thyroidal Diseases. Front. Immunol. 2017, 8, 521. [Google Scholar] [CrossRef]
- Gong, B.; Meng, F.; Wang, X.; Han, Y.; Yang, W.; Wang, C.; Shan, Z. Effects of iodine intake on gut microbiota and gut metabolites in Hashimoto thyroiditis-diseased humans and mice. Commun. Biol. 2024, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Wang, C.; Meng, F.; Wang, H.; Song, B.; Yang, Y.; Shan, Z. Association Between Gut Microbiota and Autoimmune Thyroid Disease: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 774362. [Google Scholar] [CrossRef] [PubMed]
- Pratt, C.H.; King, L.E., Jr.; Messenger, A.G.; Christiano, A.M.; Sundberg, J.P. Alopecia areata. Nat. Rev. Dis. Primers 2017, 3, 17011. [Google Scholar] [CrossRef]
- Shi, Q.; Duvic, M.; Osei, J.S.; Hordinsky, M.K.; Norris, D.A.; Price, V.H.; Amos, C.I.; Christiano, A.M.; Mendoza, T.R. Health-Related Quality of Life (HRQoL) in alopecia areata patients-a secondary analysis of the National Alopecia Areata Registry Data. J. Investig. Dermatol. Symp. Proc. 2013, 16, S49–S50. [Google Scholar] [CrossRef]
- Chu, S.Y.; Chen, Y.J.; Tseng, W.C.; Lin, M.W.; Chen, T.J.; Hwang, C.Y.; Chen, C.C.; Lee, D.D.; Chang, Y.T.; Wang, W.J.; et al. Comorbidity profiles among patients with alopecia areata: The importance of onset age, a nationwide population-based study. J. Am. Acad. Dermatol. 2011, 65, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Wu, H.J.; Ivanov, I.I.; Darce, J.; Hattori, K.; Shima, T.; Umesaki, Y.; Littman, D.R.; Benoist, C.; Mathis, D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 2010, 32, 815–827. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009, 139, 485–498. [Google Scholar] [CrossRef]
- Gao, L.; Li, W.; Song, Q.; Gao, H.; Chen, M. The genetic link between thyroid dysfunction and alopecia areata: A bidirectional two-sample Mendelian randomization study. Front. Endocrinol. 2024, 15, 1440941. [Google Scholar] [CrossRef]
- Simakou, T.; Butcher, J.P.; Reid, S.; Henriquez, F.L. Alopecia areata: A multifactorial autoimmune condition. J. Autoimmun. 2019, 98, 74–85. [Google Scholar] [CrossRef]
- Li, D.; Liang, G.; Calderone, R.; Bellanti, J.A. Vitiligo and Hashimoto’s thyroiditis: Autoimmune diseases linked by clinical presentation, biochemical commonality, and autoimmune/oxidative stress-mediated toxicity pathogenesis. Med. Hypotheses 2019, 128, 69–75. [Google Scholar] [CrossRef]
- Chang, C.C.; Sia, K.C.; Chang, J.F.; Lin, C.M.; Yang, C.M.; Huang, K.Y.; Lin, W.N. Lipopolysaccharide promoted proliferation and adipogenesis of preadipocytes through JAK/STAT and AMPK-regulated cPLA2 expression. Int. J. Med. Sci. 2019, 16, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Erturk-Hasdemir, D.; Kasper, D.L. Finding a needle in a haystack: Bacteroides fragilis polysaccharide A as the archetypical symbiosis factor. Ann. N. Y. Acad. Sci. 2018, 1417, 116–129. [Google Scholar] [CrossRef]
- English, J.; Patrick, S.; Stewart, L.D. The potential role of molecular mimicry by the anaerobic microbiota in the aetiology of autoimmune disease. Anaerobe 2023, 80, 102721. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.; Edgar, J.D.M.; Blakely, G.; Patrick, S. Antigenic mimicry of ubiquitin by the gut bacterium Bacteroides fragilis: A potential link with autoimmune disease. Clin. Exp. Immunol. 2018, 194, 153–165. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Fröhlich, E.; Wahl, R. Microbiota and Thyroid Interaction in Health and Disease. Trends Endocrinol. Metab. 2019, 30, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Harwood, C.S.; Parales, R.E. The beta-ketoadipate pathway and the biology of self-identity. Annu. Rev. Microbiol. 1996, 50, 553–590. [Google Scholar] [CrossRef]
- Díaz, E.; Ferrández, A.; Prieto, M.A.; García, J.L. Biodegradation of aromatic compounds by Escherichia coli. Microbiol. Mol. Biol. Rev. 2001, 65, 523–569. [Google Scholar] [CrossRef] [PubMed]
- Kenealy, W.R.; Waselefsky, D.M. Studies on the substrate range of Clostridium kluyveri; the use of propanol and succinate. Arch. Microbiol. 1985, 141, 187–194. [Google Scholar] [CrossRef]
- Berndt, C.; Lillig, C.H.; Wollenberg, M.; Bill, E.; Mansilla, M.C.; de Mendoza, D.; Seidler, A.; Schwenn, J.D. Characterization and reconstitution of a 4Fe-4S adenylyl sulfate/phosphoadenylyl sulfate reductase from Bacillus subtilis. J. Biol. Chem. 2004, 279, 7850–7855. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X. Gut microbiome, metabolome and alopecia areata. Front. Microbiol. 2023, 14, 1281660. [Google Scholar] [CrossRef]
- Soda, K. Microbial sulfur amino acids: An overview. Methods Enzymol. 1987, 143, 453–459. [Google Scholar] [CrossRef]
- Doherty, N.C.; Shen, F.; Halliday, N.M.; Barrett, D.A.; Hardie, K.R.; Winzer, K.; Atherton, J.C. In Helicobacter pylori, LuxS is a key enzyme in cysteine provision through a reverse transsulfuration pathway. J. Bacteriol. 2010, 192, 1184–1192. [Google Scholar] [CrossRef]
- Samuel, G.; Reeves, P. Biosynthesis of O-antigens: Genes and pathways involved in nucleotide sugar precursor synthesis and O-antigen assembly. Carbohydr. Res. 2003, 338, 2503–2519. [Google Scholar] [CrossRef] [PubMed]
- Bain, K.A.; Nichols, B.; Moffat, F.; Kerbiriou, C.; Ijaz, U.Z.; Gerasimidis, K.; McInnes, I.B.; Åstrand, A.; Holmes, S.; Milling, S.W.F. Stratification of alopecia areata reveals involvement of CD4 T cell populations and altered faecal microbiota. Clin. Exp. Immunol. 2022, 210, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Salter, S.J.; Cox, M.J.; Turek, E.M.; Calus, S.T.; Cookson, W.O.; Moffatt, M.F.; Turner, P.; Parkhill, J.; Loman, N.J.; Walker, A.W. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 2014, 12, 87. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Alopecia Areata | Hashimoto Thyroiditis | Healthy Control |
|---|---|---|---|
| n | 17 | 16 | 18 |
| Age, years, SD | 38.47 ± 5.14 | 37.06 ± 9.15 | 28.56 ± 5.14 |
| Sex (Female/Male) | 10/7 | 14/2 | 12/6 |
| BMI | 25.43 ± 4.83 | 24.41 ± 3.18 | 22.74 ± 4.53 |
| Disease duration, years, SD | 8 ± 6.13 | 8.3 ± 4.44 | - |
| An elevated anti-TPO antibody level (>35 IU/mL) | 4 (23%) | 14 (87.5%) | - |
| Thyroid hormone replacement therapy | 3 (18%) | 9 (56%) | - |
| Family history | |||
| Alopecia areata | 5 (29%) | 2 (12.5%) | - |
| Hashimoto thyroiditis | 2 (12%) | 7 (44%) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovenskiy, A.; Katkenov, N.; Ramazanova, A.; Vinogradova, E.; Jarmukhanov, Z.; Mukhatayev, Z.; Kushugulova, A. Bacteroides fragilis and Microbacterium as Microbial Signatures in Hashimoto’s Thyroiditis. Int. J. Mol. Sci. 2025, 26, 8724. https://doi.org/10.3390/ijms26178724
Kovenskiy A, Katkenov N, Ramazanova A, Vinogradova E, Jarmukhanov Z, Mukhatayev Z, Kushugulova A. Bacteroides fragilis and Microbacterium as Microbial Signatures in Hashimoto’s Thyroiditis. International Journal of Molecular Sciences. 2025; 26(17):8724. https://doi.org/10.3390/ijms26178724
Chicago/Turabian StyleKovenskiy, Artur, Nurlubek Katkenov, Aigul Ramazanova, Elizaveta Vinogradova, Zharkyn Jarmukhanov, Zhussipbek Mukhatayev, and Almagul Kushugulova. 2025. "Bacteroides fragilis and Microbacterium as Microbial Signatures in Hashimoto’s Thyroiditis" International Journal of Molecular Sciences 26, no. 17: 8724. https://doi.org/10.3390/ijms26178724
APA StyleKovenskiy, A., Katkenov, N., Ramazanova, A., Vinogradova, E., Jarmukhanov, Z., Mukhatayev, Z., & Kushugulova, A. (2025). Bacteroides fragilis and Microbacterium as Microbial Signatures in Hashimoto’s Thyroiditis. International Journal of Molecular Sciences, 26(17), 8724. https://doi.org/10.3390/ijms26178724





