Lignin-Derived Oligomers as Promising mTOR Inhibitors: Insights from Dynamics Simulations
Abstract
1. Introduction
2. Results and Discussion
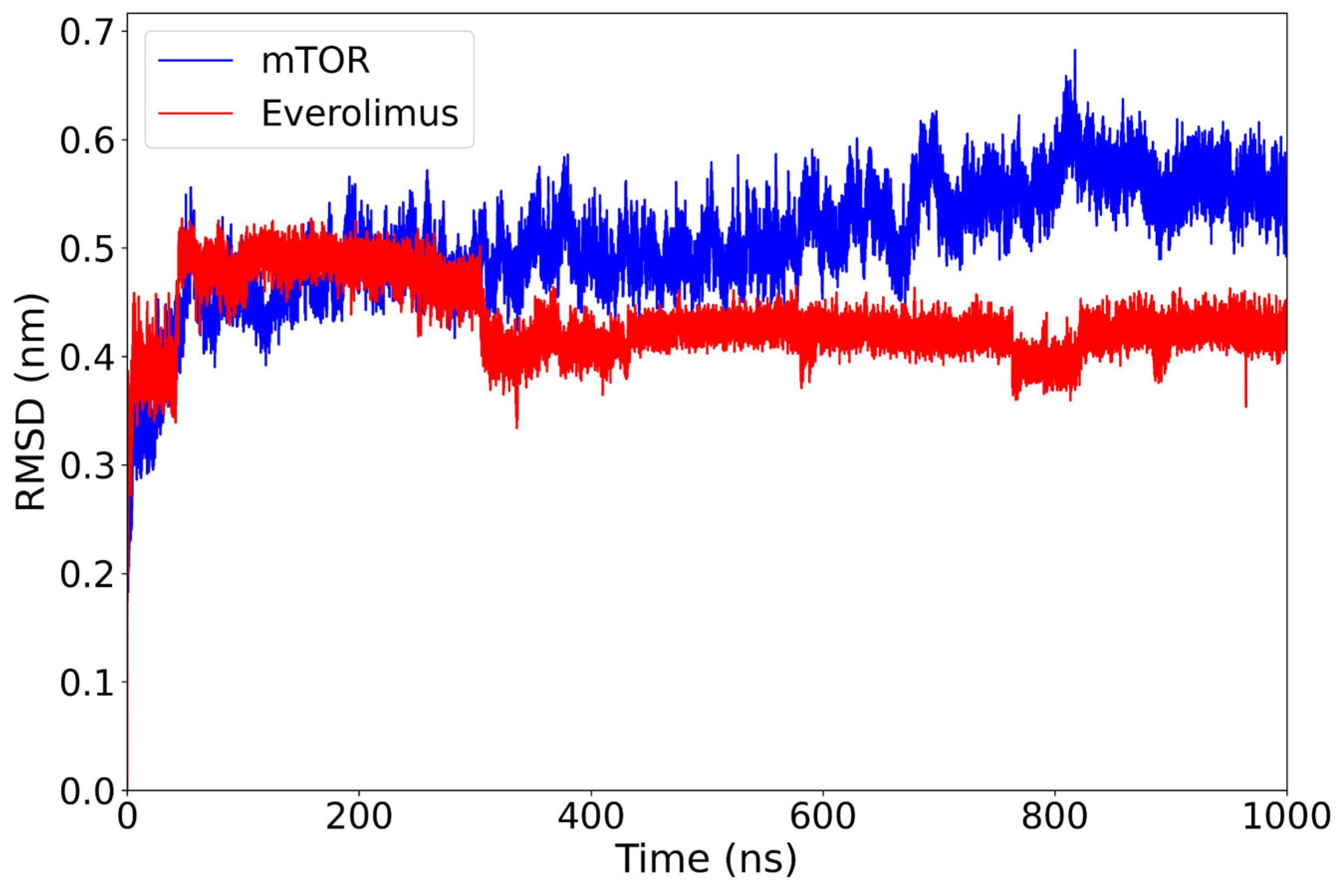
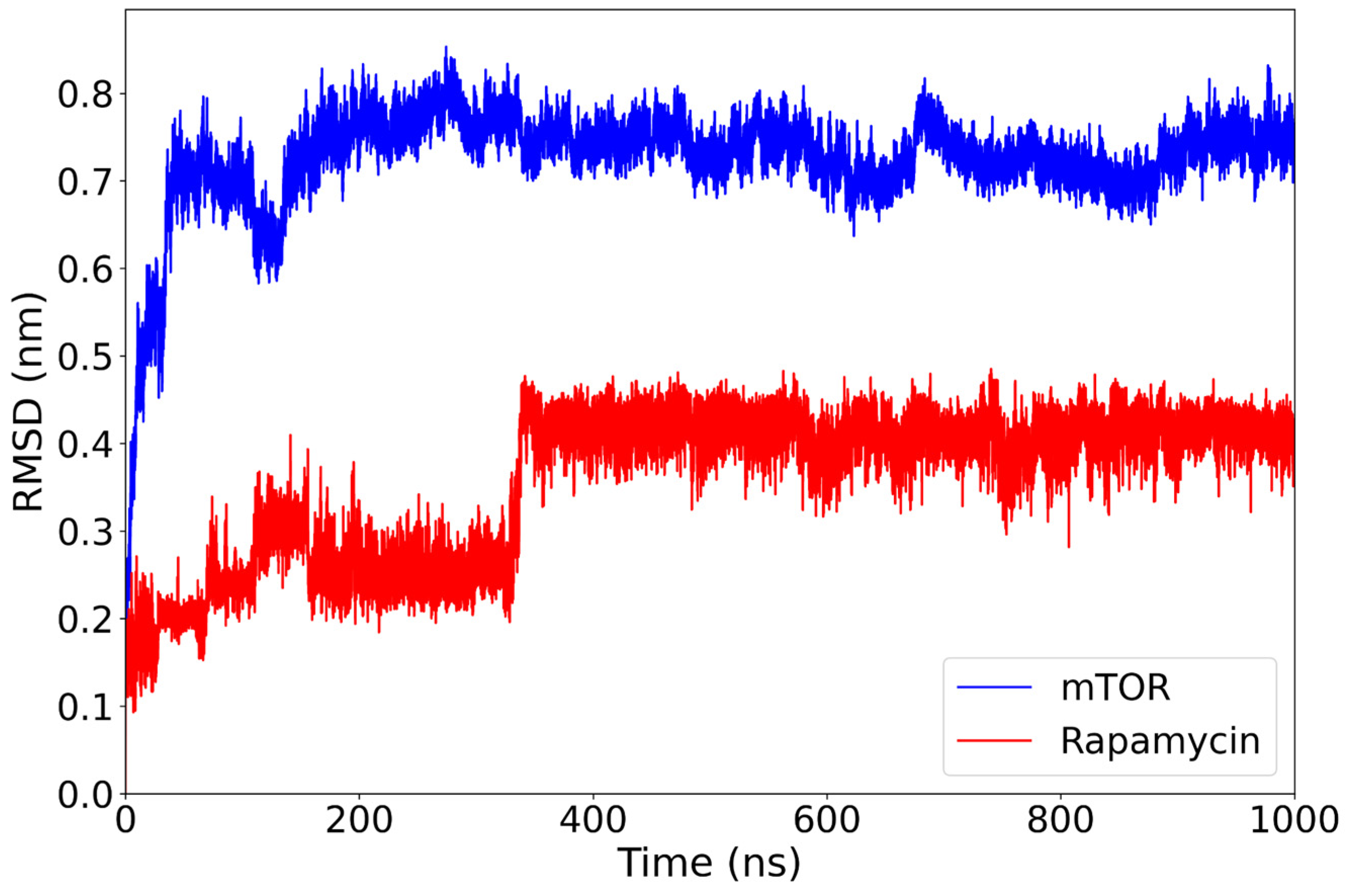
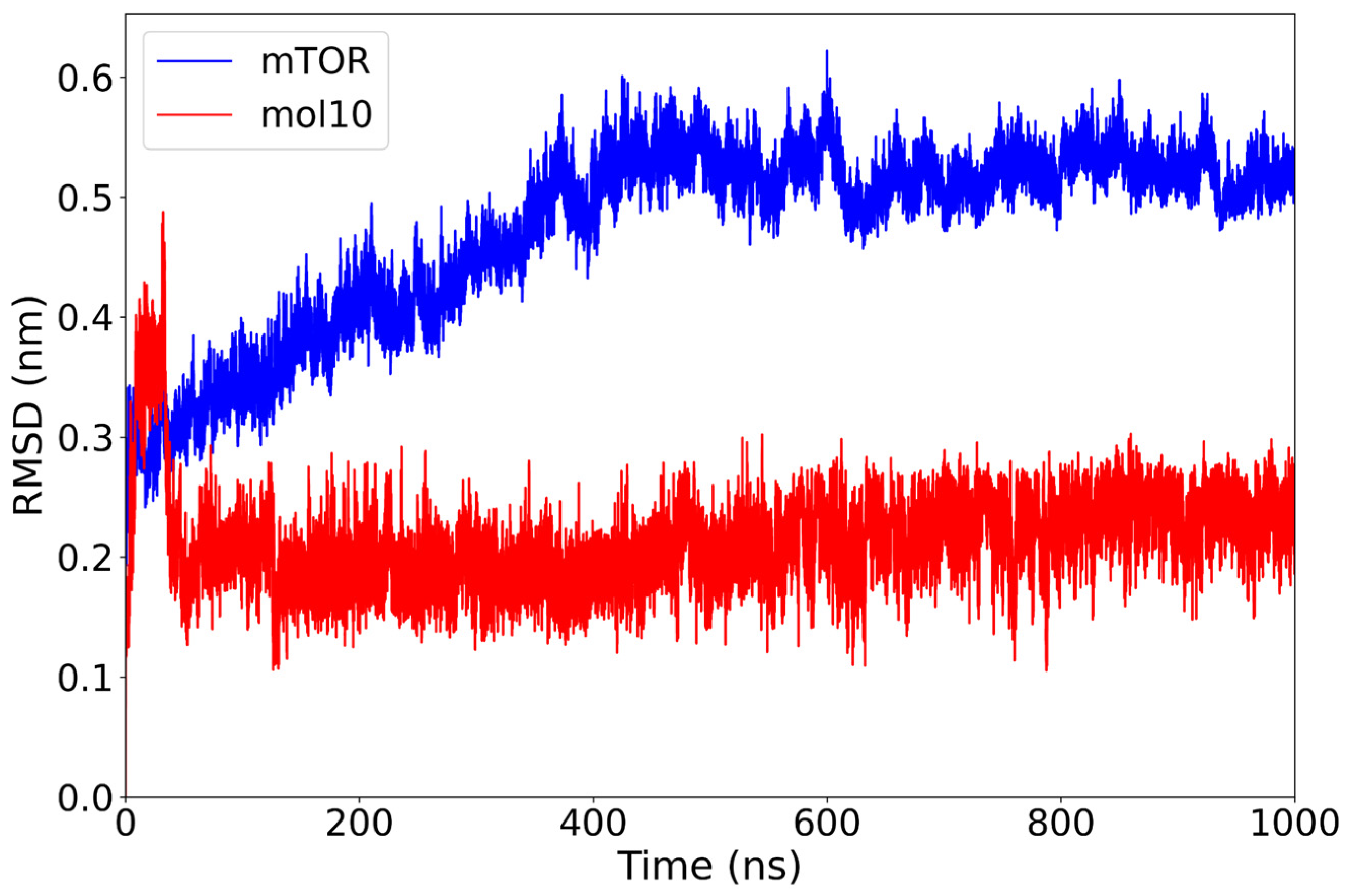
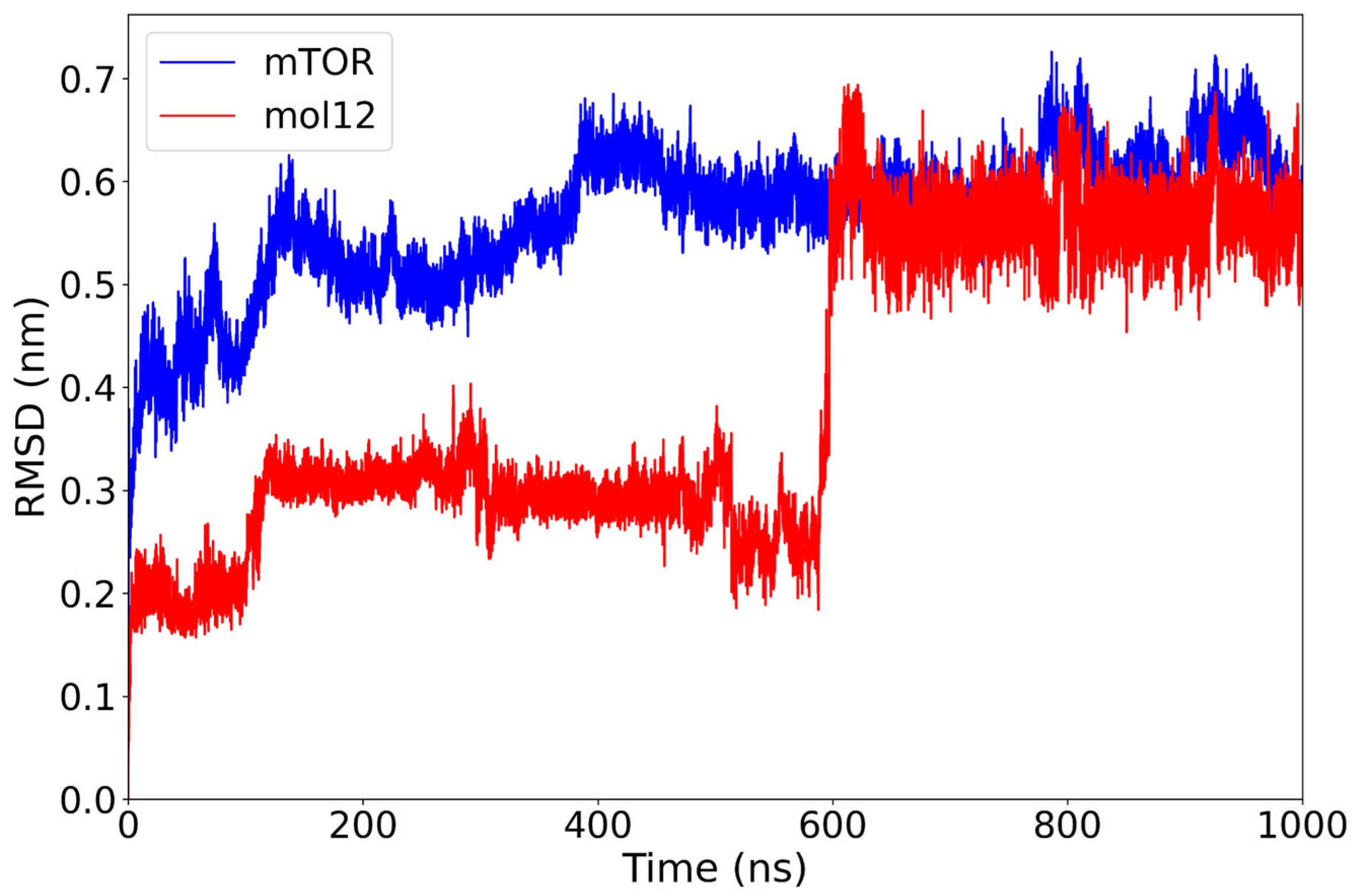
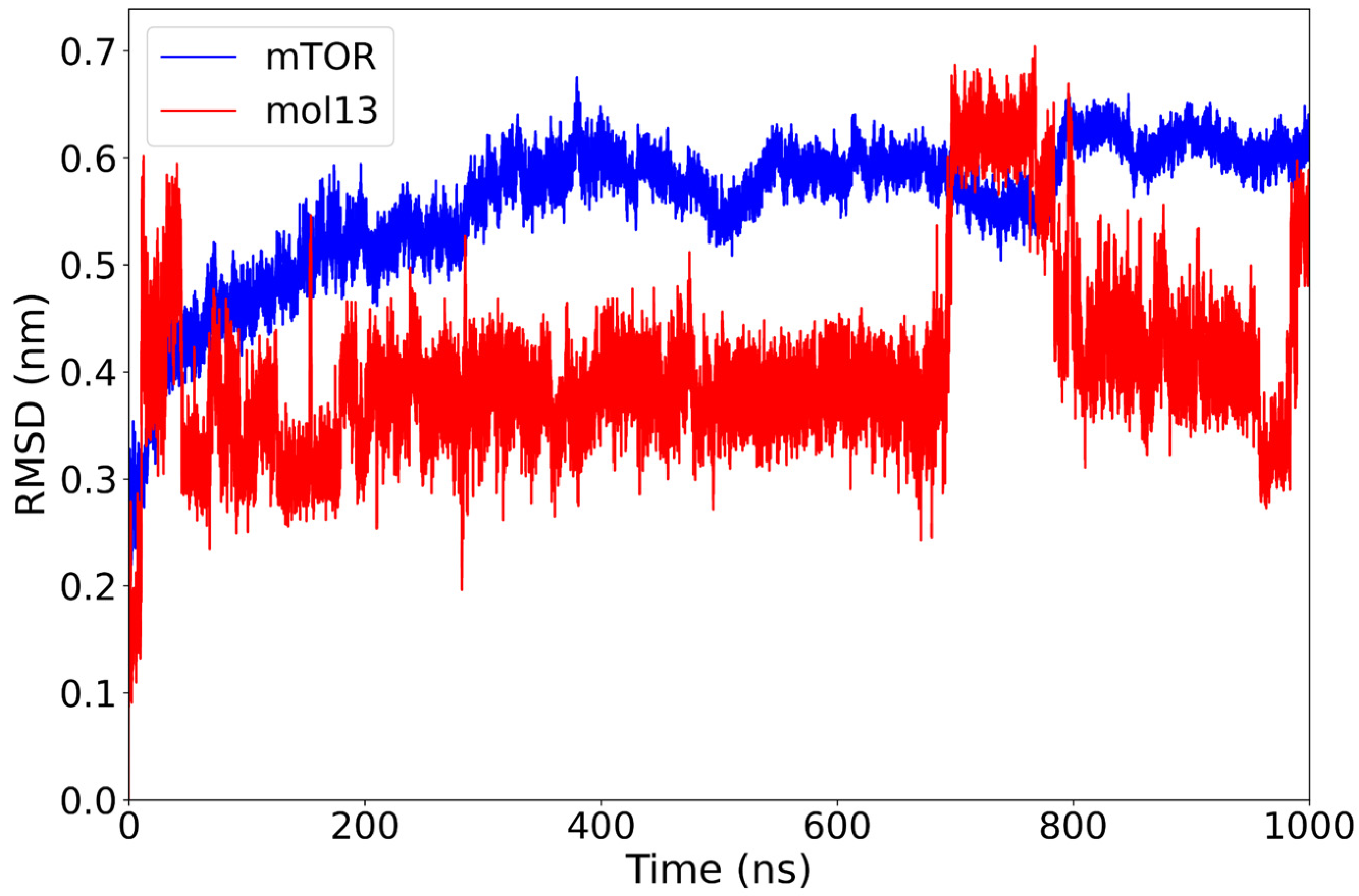
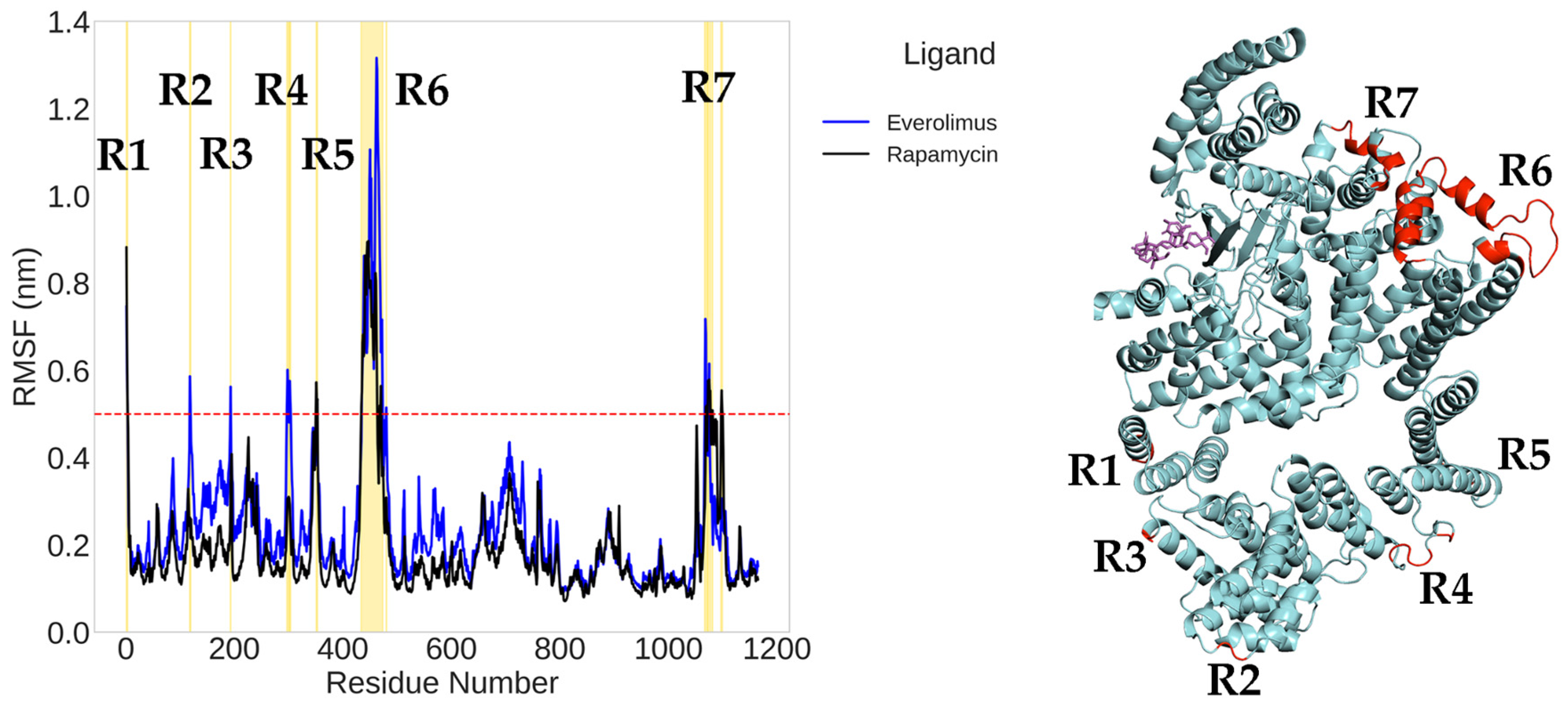
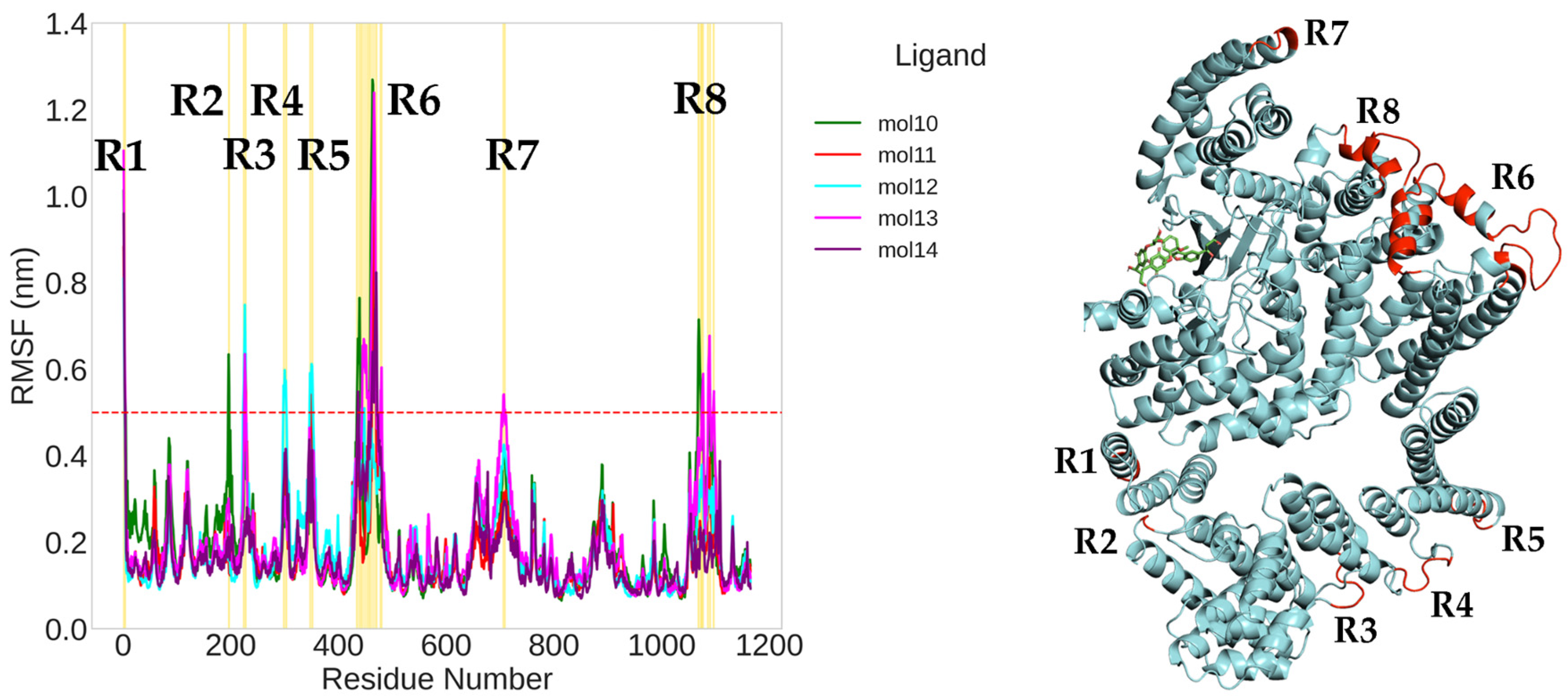
2.1. Classical Molecular Dynamics Simulation
2.2. MM/PBSA Binding Free Energy Analysis
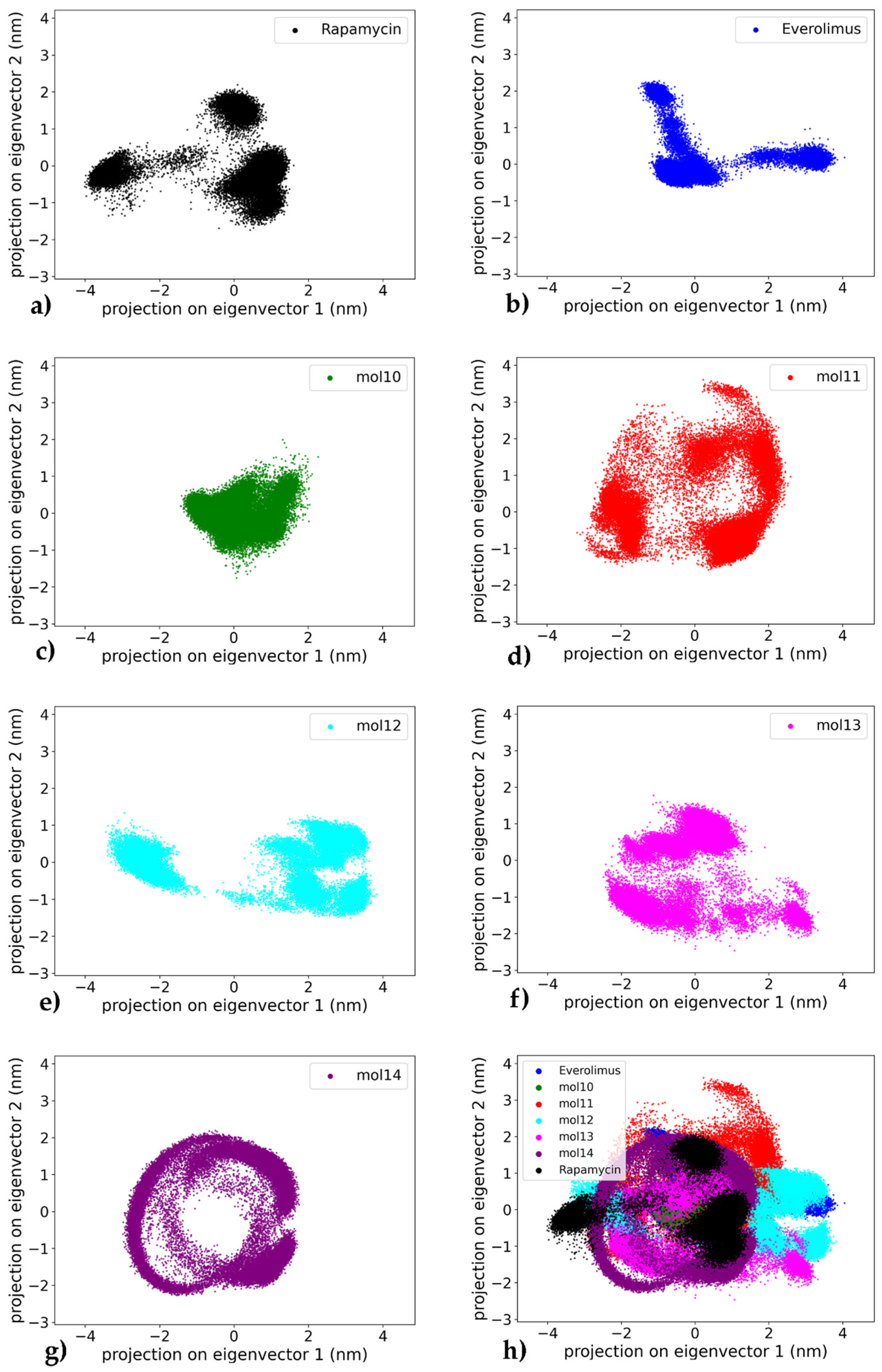
2.3. Principal Component Analysis
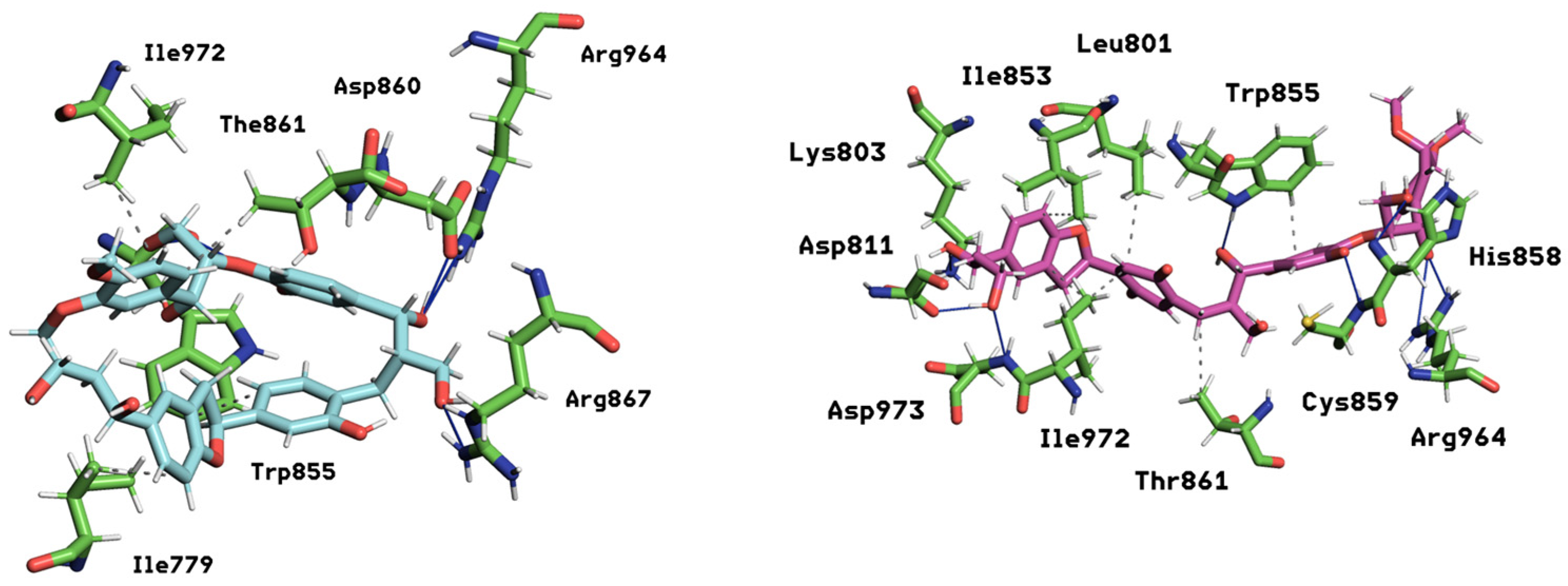
2.4. Dynamics of Ligand–Receptor Binding over Time
2.5. Results of ADME Filters
2.6. Multi-Observable Prioritization of Lignin-Derived Ligands
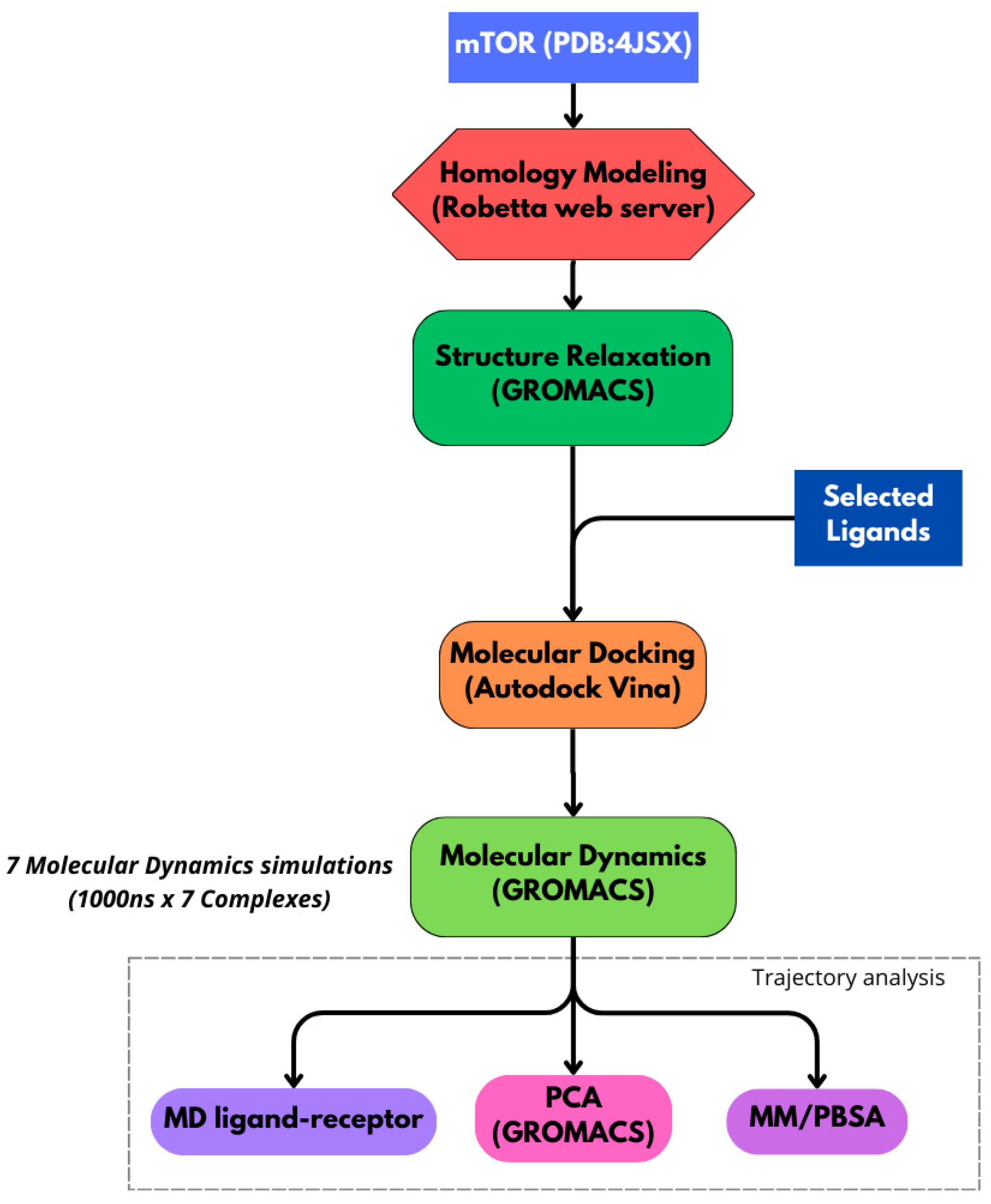
3. Materials and Methods
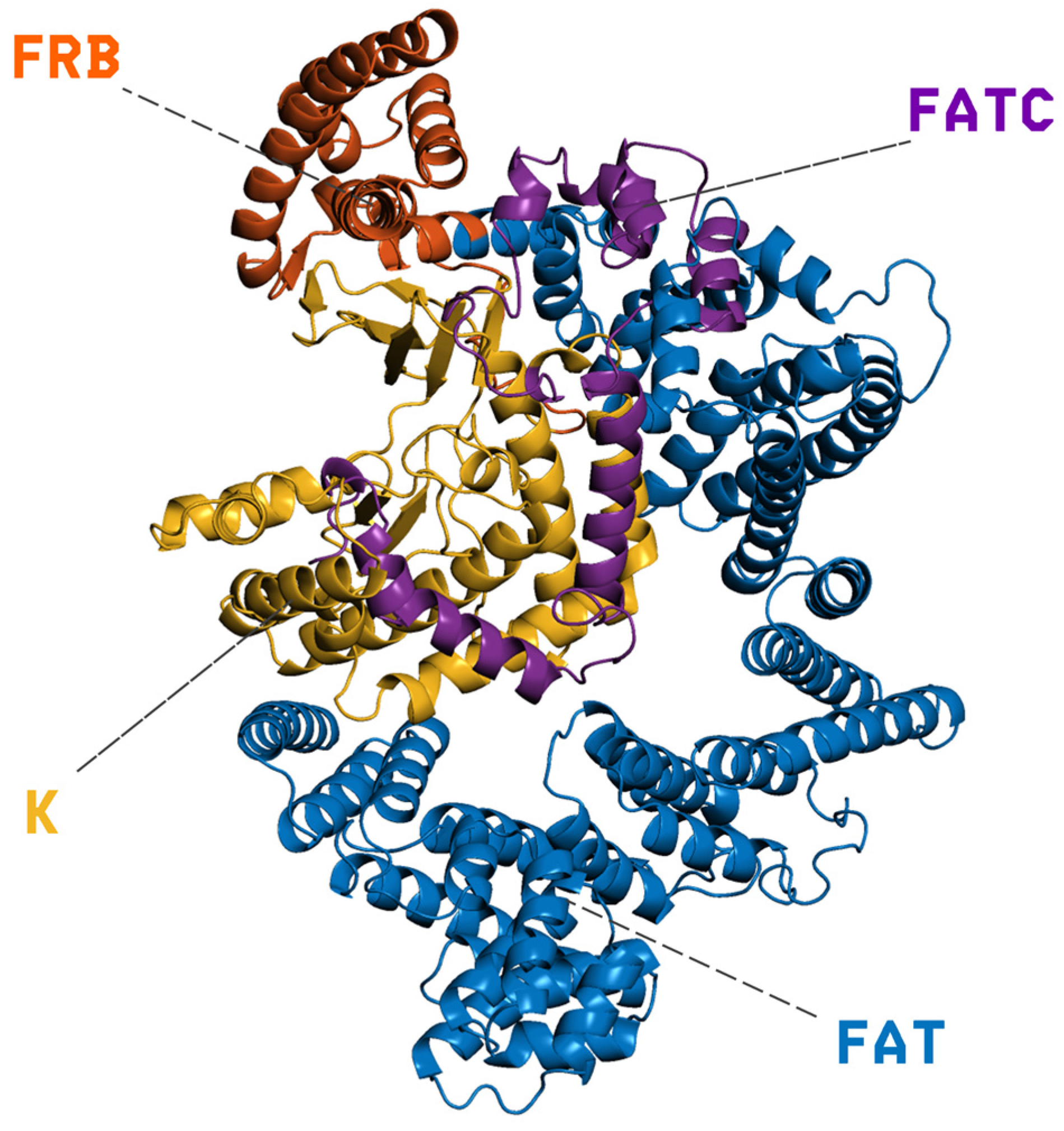
3.1. Protein Structure Preparation
3.2. Preprocessing of Molecular Dynamics Simulations
3.3. Molecular Dynamics Simulations
3.4. Binding Free Energy Estimation and Principal Component Analysis
3.5. Ligand–Receptor Interaction Analysis
3.6. Drug-Likeness and ADME Filters
3.7. Computational Resources and Infrastructure
3.8. Limitations of the Computational Approach
3.9. Data Availability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPC | High-Performance Computing |
| RMSD | Root Mean Square Deviation |
| RMSF | Root Mean Square Fluctuation |
| MD | Molecular Dynamics |
| MM/PBSA | Molecular Mechanics Poisson–Boltzmann Surface Area |
| PCA | Principal Component Analysis |
| HEAT | a type of protein structural motif composed of two antiparallel α-helices that stack together in tandem arrays, forming a solenoidal, superhelical scaffold |
| FAT | FRAP–ATM–TRRAP domain, a large α-helical region found in all members of the PIKK |
| PIKK | Phosphatidylinositol 3-kinase–related kinase family |
| FRB | FKBP12–Rapamycin Binding Domain |
| FATC | FAT C-Terminal Domain |
| FKBP12 | FK506-Binding Protein 12 |
| mTORC1 | Mechanistic Target of Rapamycin Complex 1 |
| mTORC2 | Mechanistic Target of Rapamycin Complex 2 |
References
- Ballou, L.M.; Lin, R.Z. Rapamycin and mTOR kinase inhibitors. J. Chem. Biol. 2008, 1, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Martini, M.; De Santis, M.C.; Braccini, L.; Gulluni, F.; Hirsch, E. PI3K/AKT signaling pathway and cancer: An updated review. Ann. Med. 2014, 46, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct. Target. Ther. 2023, 8, 375. [Google Scholar] [CrossRef]
- Yang, H.; Rudge, D.G.; Koos, J.D.; Vaidialingam, B.; Yang, H.J.; Pavletich, N.P. mTOR kinase structure, mechanism and regulation. Nature 2013, 497, 217–223. [Google Scholar] [CrossRef]
- Baretić, D.; Williams, R.L. The structural basis for mTOR function. Semin. Cell Dev. Biol. 2014, 36, 91–101. [Google Scholar] [CrossRef]
- Jhanwar-Uniyal, M.; Wainwright, J.V.; Mohan, A.L.; Tobias, M.E.; Murali, R.; Gandhi, C.D.; Schmidt, M.H. Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship. Adv. Biol. Regul. 2019, 72, 51–62, Erratum in Adv. Biol. Regul. 2021, 82, 100846. [Google Scholar] [CrossRef]
- Morita, M.; Gravel, S.-P.; Chénard, V.; Sikström, K.; Zheng, L.; Alain, T.; Gandin, V.; Avizonis, D.; Arguello, M.; Zakaria, C.; et al. mTORC1 Controls Mitochondrial Activity and Biogenesis through 4E-BP-Dependent Translational Regulation. Cell Metab. 2013, 18, 698–711. [Google Scholar] [CrossRef]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef]
- Marafie, S.K.; Al-Mulla, F.; Abubaker, J. mTOR: Its Critical Role in Metabolic Diseases, Cancer, and the Aging Process. Int. J. Mol. Sci. 2024, 25, 6141. [Google Scholar] [CrossRef] [PubMed]
- Hare, S.H.; Harvey, A.J. mTOR function and therapeutic targeting in breast cancer. Am. J. Cancer Res. 2017, 7, 383–404. [Google Scholar]
- Ma, Y.; Guan, R.; Gao, S.; Song, W.; Liu, Y.; Yang, Y.; Liu, H. Designing orodispersible films containing everolimus for enhanced compliance and bioavailability. Expert. Opin. Drug Deliv. 2020, 17, 1499–1508. [Google Scholar] [CrossRef]
- Piccinino, D.; Capecchi, E.; Trifero, V.; Tomaino, E.; Marconi, C.; Del Giudice, A.; Galantini, L.; Poponi, S.; Ruggieri, A.; Saladino, R. Lignin Nanoparticles as Sustainable Photoprotective Carriers for Sunscreen Filters. ACS Omega 2022, 7, 37070–37077. [Google Scholar] [CrossRef]
- Piccinino, D.; Capecchi, E.; Tomaino, E.; Gabellone, S.; Gigli, V.; Avitabile, D.; Saladino, R. Nano-Structured Lignin as Green Antioxidant and UV Shielding Ingredient for Sunscreen Applications. Antioxidants 2021, 10, 274. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Liu, Y. A Review of Lignocellulosic-Derived Nanoparticles for Drug Delivery Applications: Lignin Nanoparticles, Xylan Nanoparticles, and Cellulose Nanocrystals. Molecules 2021, 26, 676. [Google Scholar] [CrossRef]
- Wu, W.; Dutta, T.; Varman, A.M.; Eudes, A.; Manalansan, B.; Loqué, D.; Singh, S. Lignin Valorization: Two Hybrid Biochemical Routes for the Conversion of Polymeric Lignin into Value-added Chemicals. Sci. Rep. 2017, 7, 8420. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Lv, Q.; Liu, X.; Ye, X.; Cao, L.; Wang, M.; Li, J.; Yang, Y.; Li, L.; Wang, S. Natural compounds from botanical drugs targeting mTOR signaling pathway as promising therapeutics for atherosclerosis: A review. Front. Pharmacol. 2023, 14, 1083875. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Li, R.; Wei, P.F.; Ou, L.; Li, M.; Bai, Y.; Luo, W.J.; Fan, Z. Synergistic anticancer effects of everolimus (RAD001) and Rhein on gastric cancer cells via phosphoinositide-3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway. Bioengineered 2022, 13, 6332–6342. [Google Scholar] [CrossRef]
- Kumar, A.; Biswas, B.; Saini, K.; Kumar, A.; Kumar, J.; Krishna, B.B.; Bhaskar, T. Effect of hydrogen peroxide on the depolymerization of prot lignin-Industrial Crops and Products. Ind. Crops Prod. 2020, 150, 112355. [Google Scholar] [CrossRef]
- Ahmad, Z.; Al Dajani, W.W.; Paleologou, M.; Xu, C. Sustainable Process for the Depolymerization/Oxidation of Softwood and Hardwood Kraft Lignins Using Hydrogen Peroxide under Ambient Conditions. Molecules 2020, 25, 2329. [Google Scholar] [CrossRef]
- More, A.; Elder, T.; Jiang, Z. A review of lignin hydrogen peroxide oxidation chemistry with emphasis on aromatic aldehydes and acids. Holzforschung 2021, 75, 806–823. [Google Scholar] [CrossRef]
- Ye, Z.; Deng, Z.; Zhang, L.; Chen, J.; Wang, G.; Wu, Z. The structure of copper ferrite prepared by five methods and its catalytic activity on lignin oxidative degradation. Mater. Res. Express. 2020, 7, 035007. [Google Scholar] [CrossRef]
- Han, H.; Li, J.; Santos, H.A. Recent advances in Fenton and Fenton-like reactions mediated nanoparticles in cancer therapy. Biomed. Technol. 2023, 3, 40–51. [Google Scholar] [CrossRef]
- Wang, X.; Yao, B.; Su, X. Linking Enzymatic Oxidative Degradation of Lignin to Organics Detoxification. Int. J. Mol. Sci. 2018, 19, 3373. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Kelkar, A.; Baraniya, D.; Molaei, A.; Moulick, A.; Meena, R.S.; Formanek, P. Enzymatic Degradation of Lignin in Soil: A Review. Sustainability 2017, 9, 1163. [Google Scholar] [CrossRef]
- Maity, S.; Daskalakis, V.; Elstner, M.; Kleinekathöfer, U. Multiscale QM/MM molecular dynamics simulations of the trimeric major light-harvesting complex II. Phys. Chem. Chem. Phys. 2021, 23, 7407–7417. [Google Scholar] [CrossRef]
- Lesgidou, N.; Vlassi, M. Community analysis of large-scale molecular dynamics simulations elucidated dynamics-driven allostery in tyrosine kinase 2. Proteins 2024, 92, 474–498. [Google Scholar] [CrossRef]
- Castrignanò, T.; Chillemi, G.; Desideri, A. Structure and hydration of BamHI DNA recognition site: A molecular dynamics investigation. Biophys. J. 2000, 79, 1263–1272. [Google Scholar] [CrossRef]
- Chillemi, G.; Castrignanò, T.; Desideri, A. Structure and hydration of the DNA-human topoisomerase I covalent complex. Biophys. J. 2001, 81, 490–500. [Google Scholar] [CrossRef]
- Castrignanò, T.; Chillemi, G.; Varani, G.; Desideri, A. Molecular dynamics simulation of the RNA complex of a double-stranded RNA-binding domain reveals dynamic features of the intermolecular interface and its hydration. Biophys. J. 2002, 83, 3542–3552. [Google Scholar] [CrossRef][Green Version]
- Ubertini, V.; Capecchi, E.; Tomaino, E.; Piccinino, D.; De Marchi, E.; Bizzarri, B.M.; Carotenuto, G. One-Pot Synthesis of (S)-Flavanones by a Double-Face Promiscuous Chemo-Enzymatic Cascade of Lipases. ChemCatChem 2024, 16, e202400974. [Google Scholar] [CrossRef]
- Madeddu, F.; Di Martino, J.; Pieroni, M.; Del Buono, D.; Bottoni, P.; Botta, L.; Castrignanò, T.; Saladino, R. Molecular Docking and Dynamics Simulation Revealed the Potential Inhibitory Activity of New Drugs against Human Topoisomerase I Receptor. Int. J. Mol. Sci. 2022, 23, 14652. [Google Scholar] [CrossRef]
- Gabellone, S.; Piccinino, D.; Filippi, S.; Castrignanò, T.; Zippilli, C.; Del Buono, D.; Saladino, R. Lignin Nanoparticles Deliver Novel Thymine Biomimetic Photo-Adducts with Antimelanoma Activity. Int. J. Mol. Sci. 2022, 23, 915. [Google Scholar] [CrossRef]
- Di Martino, J.; Arcieri, M.; Madeddu, F.; Pieroni, M.; Carotenuto, G.; Bottoni, P.; Botta, L.; Castrignanò, T.; Gabellone, S.; Saladino, R. Molecular Dynamics Investigations of Human DNA-Topoisomerase I Interacting with Novel Dewar Valence Photo-Adducts: Insights into Inhibitory Activity. Int. J. Mol. Sci. 2023, 25, 234. [Google Scholar] [CrossRef] [PubMed]
- Cirigliano, A.; Stirpe, A.; Menta, S.; Mori, M.; Dell’Edera, D.; Pick, E.; Negri, R.; Botta, B.; Rinaldi, T. Yeast as a tool to select inhibitors of the cullin deneddylating enzyme Csn5. J. Enzym. Inhib. Med. Chem. 2016, 31, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Bolis, M.; Garattini, E.; Paroni, G.; Zanetti, A.; Kurosaki, M.; Castrignanò, T.; Garattini, S.K.; Biancardi, F.; Barzago, M.M.; Gianni’, M.; et al. Network-guided modeling allows tumor-type independent prediction of sensitivity to all-trans-retinoic acid. Ann. Oncol. 2017, 28, 611–621. [Google Scholar] [CrossRef]
- Castrignanò, T.; Gioiosa, S.; Flati, T.; Cestari, M.; Picardi, E.; Chiara, M.; Fratelli, M.; Amente, S.; Cirilli, M.; Tangaro, M.A.; et al. ELIXIR-IT HPC@CINECA: High performance computing resources for the bioinformatics community. BMC Bioinform. 2020, 21 (Suppl. S10), 352. [Google Scholar] [CrossRef]
- Guterres, H.; Im, W. Improving Protein-Ligand Docking Results with High-Throughput Molecular Dynamics Simulations. J. Chem. Inf. Model. 2020, 60, 2189–2198. [Google Scholar] [CrossRef]
- Kutzner, C.; Miletić, V.; Palacio Rodríguez, K.; Rampp, M.; Hummer, G.; de Groot, B.L.; Grubmüller, H. Scaling of the GROMACS Molecular Dynamics Code to 65k CPU Cores on an HPC Cluster. J. Comput. Chem. 2025, 46, e70059. [Google Scholar] [CrossRef] [PubMed]
- David, C.C.; Jacobs, D.J. Principal Component Analysis: A Method for Determining the Essential Dynamics of Proteins. In Protein Dynamics. Methods in Molecular Biology; Livesay, D., Ed.; Humana Press: Totowa, NJ, USA, 2014; Volume 1084. [Google Scholar]
- Cavasotto, C.N.; Phatak, S.S. Homology modeling in drug discovery: Current trends and applications. Drug Discov. Today 2009, 14, 13–14. [Google Scholar] [CrossRef]
- Song, Y.; DiMaio, F.; Wang, R.Y.-R.; Kim, D.; Miles, C.; Brunette, T.; Thompson, J.; Baker, D. High resolution comparative modeling with RosettaCM. Structure 2013, 21, 1735–1742. [Google Scholar] [CrossRef]
- Raman, S.; Vernon, R.; Thompson, J.; Tyka, M.; Sadreyev, R.; Pei, J.; Kim, D.; Kellogg, E.; DiMaio, F.; Lange, O.; et al. Structure prediction for CASP8 with all-atom refinement using Rosetta. Proteins 2009, 77 (Suppl. S9), 89–99. [Google Scholar] [CrossRef] [PubMed]
- Le Guilloux, V.; Schmidtke, P.; Tuffery, P. Fpocket: An open source platform for ligand pocket detection. BMC Bioinform. 2009, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminformatics 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminformatics 2012, 4, 17. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexiblity. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Modeling. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Blanes-Mira, C.; Fernández-Aguado, P.; de Andrés-López, J.; Fernández-Carvajal, A.; Ferrer-Montiel, A.; Fernández-Ballester, G. Comprehensive Survey of Consensus Docking for High-Throughput Virtual Screening. Molecules 2023, 28, 175. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh ewald potential. J. Chem. Phys. 1995, 103, 8577–8592. [Google Scholar] [CrossRef]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. “Improved side-chain torsion potentials for the AMBER ff99SB protein force field,” PROTEINS: Struct. Funct. Gen. 2010, 78, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Sousa da Silva, A.W.; Vranken, W.F. ACPYPE–AnteChamber PYthon Parser interfacE. BMC Res. Notes 2012, 5, 367. [Google Scholar] [CrossRef]
- Mark, P.; Nilsson, L. Structure and Dynamics of the TIP3P, SPC, and SPC/E Water Models at 298 K. J. Phys. Chem. A 2001, 105, 9954–9960. [Google Scholar] [CrossRef]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. gmx_MMPBSA: A New Tool to Perform End-State Free Energy Calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef]
- Roux, B.; Chipot, C. Editorial Guidelines for Computational Studies of Ligand Binding Using MM/PBSA and MM/GBSA Approximations Wisely. J. Phys. Chem. B 2024, 128, 12027–12029. [Google Scholar] [CrossRef]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef]
- Homeyer, N.; Gohlke, H. Free Energy Calculations by the Molecular Mechanics Poisson−Boltzmann Surface Area Method. Mol. Inf. 2012, 31, 114–122. [Google Scholar] [CrossRef]
- Pieroni, M.; Madeddu, F.; Di Martino, J.; Arcieri, M.; Parisi, V.; Bottoni, P.; Castrignanò, T. MD–Ligand–Receptor: A High-Performance Computing Tool for Characterizing Ligand–Receptor Binding Interactions in Molecular Dynamics Trajectories. Int. J. Mol. Sci. 2023, 24, 11671. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]


| Ligand Name | Ligand Structure | SMILES String |
|---|---|---|
| mol10 | 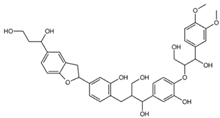 | OCC(C(O)C1=CC=C(OC(CO)C(O)C2= CC=C(OC)C(OC)=C2)C(O)=C1)CC(C(O)= C3)=CC=C3C4CC5=CC(C(O)CCO)=CC=C5O4 |
| mol11 | 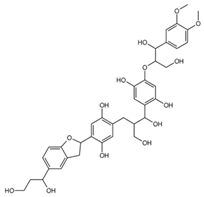 | OCC(C(O)C1=C(O)C=C(OC(CO)C(O)C2= CC=C(OC)C(OC)=C2)C(O)=C1)CC(C(O)= C3)=CC(O)=C3C4CC5=CC(C(O) CCO)=CC=C5O4 |
| mol12 | 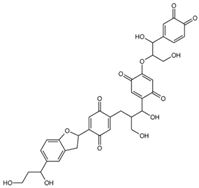 | OCC(C(O)C(C(C=C1OC(CO)C(O)C(C= CC2=O)=CC2=O)=O)=CC1=O)CC3=CC(C (C4CC5=CC(C(O)CCO)=CC=C5O4)= CC3=O)=O |
| mol13 | 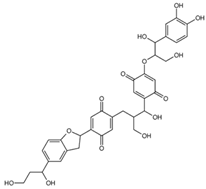 | OCC(C(O)C(C(C=C1OC(CO)C(O)C2= CC(O)=C(O)C=C2)=O)=CC1=O)CC3=CC(C (C4CC5=CC(C(O)CCO)=CC=C5O4)= CC3=O)=O |
| mol14 | 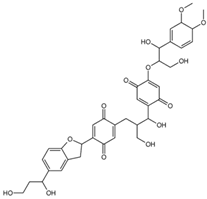 | OCC(C(O)C(C(C=C1OC(CO)C(O)C2=CC (OC)C(OC)C=C2)=O)=CC1=O)CC3= CC(C(C4CC5=CC(C(O)CCO)=CC=C5O4)= CC3=O)=O |
| Rapamycin |  | C[C@@H]1CCC2C[C@@H](/C(=C/C= C/C=C/[C@H](C[C@H](C(=O)[C@@H] ([C@@H](/C(=C/[C@H](C(=O)C[C@H](OC(=O) [C@@H]3CCCCN3C(=O)C(=O)[C@@]1(O2)O) [C@H](C)C[C@@H]4CC[C@H]([C@@H](C4) OC)O)C)/C)O)OC)C)C)/C)OC |
| Everolimus | 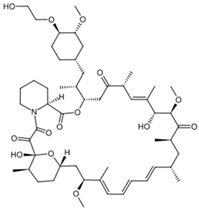 | C[C@@H]1CC[C@H]2C[C@@H](/C(=C/C= C/C=C/[C@H](C[C@H](C(=O)[C@@H]([C@@H] (/C(=C/[C@H](C(=O)C[C@H](OC(=O)[C@@H] 3CCCCN3C(=O)C(=O)[C@@]1(O2)O)[C@H](C) C[C@@H]4CC[C@H]([C@@H](C4)OC)OCCO)C)/ C)O)OC)C)C)/C)OC |
| Ligand | MM/PBSA ΔG (kcal/mol) | Ligand RMSD (1 µs) | PCA (Ligand Heavy Atoms) | Most Persistent Interactions (Occupancy, %) |
|---|---|---|---|---|
| Rapamycin | −26.39 ± 6.14 | Stable plateau ~0.40 nm after ~350 ns | Dense core, limited spread | Hydrophobics: ILE801 (8.1%), THR861 (6.9%), ILE972 (14.0%); H-bonds: THR780 (27.1%), GLN777 (28.0%). |
| Everolimus | −31.51 ± 9.61 | Stable (~0.43 nm); protein ~0.47 nm | Compact clustering | Hydrophobics: LEU877 (15.4%), TYR1158 (21.8%); H-bond: LYS986 (26.1%). |
| mol10 | −26.37 ± 4.84 | Very low and flat (~0.20–0.25 nm) | Narrow/compact | TRP855 (20.7%) hydroph./H-bond; H-bonds: ASP860 (38.4%), ARG964 (33.4%). |
| mol11 | −25.93 ± 6.44 | High variability, no plateau (>0.6 nm) | Widest dispersion | Hydrophobics: LYS782 (13.2%), TYR1158 (11.8%); H-bond: LYS782 (13.1%). |
| mol12 | −25.12 ± 7.54 | One transition ~600 ns → stable plateau ~0.55 nm | Two moderately compact clusters | Hydrophobics: TRP855 (17.5%), ILE1159A (8.1%); H-bond: VAL856 (21.5%). |
| mol13 | −31.02 ± 7.51 | Multiple late rearrangements (0.35→0.6→0.4–0.5 nm) | Wide/fragmented cluster | Hydrophobics: LEU801A (15.2%), ALA864A (17.8%); H-bonds: LYS782A (19.0%), GLN783A (13.4%), LYS803A (12.7%), VAL856A (10.6%). |
| mol14 | −37.76 ± 3.41 | Reaches plateau ~0.48 nm by ~400 ns | Most compact; ring-like, restricted drift | Hydrophobics: LEU801 (35.8%), ILE853 (23.0%), TRP855 (18.0%), THR861 (15.1%), ILE972 (16.8%); H-bonds: ASP811 (40.0%), ASP973 (40.0%), LYS803 (27.4%), TRP855 (18.0%), ARG964 (16.0%). |
| Item | Data Type | Accession |
|---|---|---|
| Ligands | PDB files | https://doi.org/10.6084/m9.figshare.29598395, accessed on 18 August 2025 |
| Protein/MD starting coordinates | mTOR_protein.pdb file | https://doi.org/10.6084/m9.figshare.29646449, accessed on 18 August 2025 |
| Ligand–receptor analysis | CSV and PNG files | https://doi.org/10.6084/m9.figshare.29598389, accessed on 18 August 2025 |
| PCA plots | PNG files | https://doi.org/10.6084/m9.figshare.29598419, accessed on 18 August 2025 |
| RMSD and RMSF plots | PNG files | https://doi.org/10.6084/m9.figshare.29598422, accessed on 18 August 2025 |
| MM/PBSA free binding energy | CSV file | https://doi.org/10.6084/m9.figshare.29603588, accessed on 18 August 2025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabellone, S.; Carotenuto, G.; Arcieri, M.; Bottoni, P.; Sbanchi, G.; Castrignanò, T.; Piccinino, D.; Liverani, C.; Saladino, R. Lignin-Derived Oligomers as Promising mTOR Inhibitors: Insights from Dynamics Simulations. Int. J. Mol. Sci. 2025, 26, 8728. https://doi.org/10.3390/ijms26178728
Gabellone S, Carotenuto G, Arcieri M, Bottoni P, Sbanchi G, Castrignanò T, Piccinino D, Liverani C, Saladino R. Lignin-Derived Oligomers as Promising mTOR Inhibitors: Insights from Dynamics Simulations. International Journal of Molecular Sciences. 2025; 26(17):8728. https://doi.org/10.3390/ijms26178728
Chicago/Turabian StyleGabellone, Sofia, Giovanni Carotenuto, Manuel Arcieri, Paolo Bottoni, Giulia Sbanchi, Tiziana Castrignanò, Davide Piccinino, Chiara Liverani, and Raffaele Saladino. 2025. "Lignin-Derived Oligomers as Promising mTOR Inhibitors: Insights from Dynamics Simulations" International Journal of Molecular Sciences 26, no. 17: 8728. https://doi.org/10.3390/ijms26178728
APA StyleGabellone, S., Carotenuto, G., Arcieri, M., Bottoni, P., Sbanchi, G., Castrignanò, T., Piccinino, D., Liverani, C., & Saladino, R. (2025). Lignin-Derived Oligomers as Promising mTOR Inhibitors: Insights from Dynamics Simulations. International Journal of Molecular Sciences, 26(17), 8728. https://doi.org/10.3390/ijms26178728









