A Plasma Extracellular Vesicle-Derived microRNA Signature as a Potential Biomarker for Subclinical Coronary Atherosclerosis
Abstract
1. Introduction
2. Results
2.1. Discovery Phase
2.1.1. Main Clinical Features of Study Participants
2.1.2. Characterization of EVs
2.1.3. Identification of Candidate miRNAs and Target Genes
2.1.4. Pathway Enrichment Analysis and Interaction Networks
2.2. Validation Phase
2.2.1. Main Clinical Features of Study Participants
2.2.2. Validation of Differentially Expressed miRNAs by RT-qPCR
2.2.3. Identification of a miRNA Signature Through ROC Curve Analysis
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Subjects
4.3. Sample Collection
4.4. Discovery Phase
4.4.1. Isolation of EVs
4.4.2. EV Characterization by Transmission Electron Microscopy (TEM) and Western Blot (WB)
4.4.3. Total RNA Isolation
4.4.4. miRNA Expression Profiling
4.4.5. Bioinformatic Analysis of Differentially Expressed miRNAs
Selection of Candidate miRNAs
Prediction of miRNA Target Genes
Selection of Candidate Genes and Pathway Enrichment Analysis
4.5. Validation Phase
Quantification of miRNA Expression by RT-qPCR
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCA | Subclinical Coronary Atherosclerosis |
| EVs | Extracellular Vesicles |
| miRNA | microRNA |
| RT-qPCR | Reverse Transcription Quantitative Polymerase Chain Reaction |
| AUC | Area Under the Curve |
| ROC | Receiver Operating Characteristic |
| CACS | Coronary Artery Calcium Score |
| BMI | Body Mass Index |
| HDL-c | High-Density Lipoprotein Cholesterol |
| LDL-c | Low-Density Lipoprotein Cholesterol |
| DE-miRNAs | Differentially Expressed microRNAs |
| DEGs | Differentially Expressed Genes |
| GEO | Gene Expression Omnibus |
| RMA | Robust Multi-array Average |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| PBS | Phosphate-Buffered Saline |
| RIPA | Radioimmunoprecipitation Assay Buffer |
| PEG | Polyethylene Glycol |
| TEM | Transmission Electron Microscopy |
| FDR | False Discovery Rate |
| SD | Standard Deviation |
| IQR | Interquartile Range |
| SPSS | Statistical Package for the Social Sciences |
References
- Tewma, C.; Mifsud, J.L. The Impact of Air Pollution on Atherosclerotic Cardiovascular Disease Development. Br. J. Cardiol. 2024, 31, 013. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Pratico, D.; Lin, L.; Mantzoros, C.S.; Bahijri, S.; Tuomilehto, J.; Ren, J. Inflammation in Atherosclerosis: Pathophysiology and Mechanisms. Cell Death Dis. 2024, 15, 817. [Google Scholar] [CrossRef]
- Kong, P.; Cui, Z.Y.; Huang, X.F.; Zhang, D.D.; Guo, R.J.; Han, M. Inflammation and Atherosclerosis: Signaling Pathways and Therapeutic Intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef]
- Hasbani, N.R.; Ligthart, S.; Brown, M.R.; Heath, A.S.; Bebo, A.; Ashley, K.E.; Boerwinkle, E.; Morrison, A.C.; Folsom, A.R.; Aguilar, D.; et al. American Heart Association’s Life’s Simple 7: Lifestyle Recommendations, Polygenic Risk, and Lifetime Risk of Coronary Heart Disease. Circulation 2022, 145, 808–818. [Google Scholar] [CrossRef]
- Robinson, J.G.; Farnier, M.; Krempf, M.; Bergeron, J.; Luc, G.; Averna, M.; Stroes, E.S.; Langslet, G.; Raal, F.J.; El Shahawy, M.; et al. Efficacy and Safety of Alirocumab in Reducing Lipids and Cardiovascular Events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef]
- Chaudhary, R.; Garg, J.; Shah, N.; Sumner, A. PCSK9 Inhibitors: A New Era of Lipid Lowering Therapy. World J. Cardiol. 2017, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Eloso, J.; Awad, A.; Zhao, X.; Cunningham, F.E.; Zhang, R.; Dong, D.; Kelley, C.; Glassman, P.A.; Aspinall, S.L. PCSK9 Inhibitor Use and Outcomes Using Concomitant Lipid-Lowering Therapies in the Veterans Health Administration. Am. J. Med. Open 2023, 9, 100035. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e21–e129. [Google Scholar] [CrossRef]
- Fuchs, A.; Kühl, J.T.; Sigvardsen, P.E.; Afzal, S.; Knudsen, A.D.; Møller, M.B.; De Knegt, M.C.; Sørgaard, M.H.; Nordestgaard, B.G.; Køber, L.V.; et al. Subclinical Coronary Atherosclerosis and Risk for Myocardial Infarction in a Danish Cohort: A Prospective Observational Cohort Study. Ann. Intern. Med. 2023, 176, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Morddarvanjoghi, F.; Abdolmaleki, A.; Rasoulpoor, S.; Khaleghi, A.A.; Hezarkhani, L.A.; Shohaimi, S.; Mohammadi, M. The Global Prevalence of Myocardial Infarction: A Systematic Review and Meta-Analysis. BMC Cardiovasc. Disord. 2023, 23, 206. [Google Scholar] [CrossRef]
- Borrayo-Sánchez, G.; Rosas-Peralta, M.; Ramírez-Arias, E.; Saturno-Chiu, G.; Estrada-Gallegos, J.; Parra-Michel, R.; Hernandez-García, H.R.; Ayala-López, E.A.; Barraza-Felix, R.; García-Rincón, A.; et al. STEMI and NSTEMI: Real-World Study in Mexico (RENASCA). Arch. Med. Res. 2018, 49, 609–619. [Google Scholar] [CrossRef]
- Pérez-Cuevas, R.; Contreras-Sánchez, S.E.; Doubova, S.V.; García-Saisó, S.; Sarabia-González, O.; Pacheco-Estrello, P.; Arias-Mendoza, A. Gaps between Supply and Demand of Acute Myocardial Infarction Treatment in Mexico. Salud. Publica. Mex. 2020, 62, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, V.; Todaro, F.; Cataldi, M.; Tuttolomondo, A. Atherosclerosis and Its Related Laboratory Biomarkers. Int. J. Mol. Sci. 2023, 24, 15546. [Google Scholar] [CrossRef]
- Bordeianu, G.; Mitu, I.; Stanescu, R.S.; Ciobanu, C.P.; Petrescu-Danila, E.; Marculescu, A.D.; Dimitriu, D.C. Circulating Biomarkers for Laboratory Diagnostics of Atherosclerosis—Literature Review. Diagnostics 2022, 12, 3141. [Google Scholar] [CrossRef]
- Li, S.; Guo, Y.L.; Zhao, X.; Zhang, Y.; Zhu, C.G.; Wu, N.Q.; Xu, R.X.; Qing, P.; Gao, Y.; Li, X.L.; et al. Novel and Traditional Lipid-Related Biomarkers and Their Combinations in Predicting Coronary Severity. Sci. Rep. 2017, 7, 360. [Google Scholar] [CrossRef]
- Ezekwueme, F.; Tolu-Akinnawo, O.; Smith, Z.; Ogunniyi, K.E. Non-Invasive Assessment of Coronary Artery Disease: The Role of AI in the Current Status and Future Directions. Cureus 2025, 17, e78994. [Google Scholar] [CrossRef]
- Won, K.B.; Park, G.M.; Yang, Y.J.; Ann, S.H.; Kim, Y.G.; Yang, D.H.; Kang, J.W.; Lim, T.H.; Kim, H.K.; Choe, J.; et al. Independent Role of Low-Density Lipoprotein Cholesterol in Subclinical Coronary Atherosclerosis in the Absence of Traditional Cardiovascular Risk Factors. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jeon, Y.J.; Ann, S.H.; Kim, Y.G.; Lee, Y.; Choi, S.H.; Han, S.; Park, G.M. Comprehensive Prediction of Subclinical Coronary Atherosclerosis in Subjects Without Traditional Cardiovascular Risk Factors. Am. J. Cardiol. 2023, 198, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wu, L.; Wang, L.; Reiter, R.J.; Lip, G.Y.H.; Ren, J. Extracellular Vesicles in Cardiovascular Diseases: From Pathophysiology to Diagnosis and Therapy. Cytokine Growth Factor Rev. 2023, 74, 40–55. [Google Scholar] [CrossRef]
- Eichner-Seitz, N. Diagnosis of Extracellular Vesicles in Cardiovascular and Metabolic Diseases. Adv. Exp. Med. Biol. 2023, 1418, 171–185. [Google Scholar] [CrossRef]
- Han, C.; Yang, J.; Sun, J.; Qin, G. Extracellular Vesicles in Cardiovascular Disease: Biological Functions and Therapeutic Implications. Pharmacol. Ther. 2022, 233, 108025. [Google Scholar] [CrossRef] [PubMed]
- Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-George, F.; Drees, E.E.E.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F.; et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017, 120, 1632–1648. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.Y.; Lee, C.K.; Huang, C.; Ou, Y.H.; Charles, C.J.; Richards, A.M.; Neupane, Y.R.; Pavon, M.V.; Zharkova, O.; Pastorin, G.; et al. Extracellular Vesicles in Cardiovascular Diseases: Alternative Biomarker Sources, Therapeutic Agents, and Drug Delivery Carriers. Int. J. Mol. Sci. 2019, 20, 3272. [Google Scholar] [CrossRef]
- Hafiane, A.; Daskalopoulou, S.S. Extracellular Vesicles Characteristics and Emerging Roles in Atherosclerotic Cardiovascular Disease. Metabolism 2018, 85, 213–222. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, S.; Chen, X. Extracellular Vesicles and Ischemic Cardiovascular Diseases. Adv. Exp. Med. Biol. 2023, 1418, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, Y.Y.; Kim, V.N. The Biogenesis and Regulation of Animal MicroRNAs. Nat. Rev. Mol. Cell Biol. 2024, 26, 276–296. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhou, Y.; Gao, S.; Cheng, Y.; Li, Z. Homogeneous and Sensitive Detection of MicroRNA with Ligase Chain Reaction and Lambda Exonuclease-Assisted Cationic Conjugated Polymer Biosensing. ACS Appl. Mater. Interfaces 2014, 6, 6181–6185. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An Overview of MicroRNAs: Biology, Functions, Therapeutics, and Analysis Methods. J. Cell Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef]
- Malik, R.; Mushtaque, R.S.; Siddiqui, U.A.; Younus, A.; Aziz, M.A.; Humayun, C.; Mansoor, K.; Latif, M.A.; Waheed, S.; Assad, S.; et al. Association Between Coronary Artery Disease and MicroRNA: Literature Review and Clinical Perspective. Cureus 2017, 9, e1188. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Li, J.; Chen, J.Y.; Zhou, Y.L.; Cai, A.P.; Huang, C.; Feng, Y.Q. The Association of Circulating MiR-29b and Interleukin-6 with Subclinical Atherosclerosis. Cell Physiol. Biochem. 2017, 44, 1537–1544. [Google Scholar] [CrossRef]
- Kiełbowski, K.; Żychowska, J.; Bakinowska, E.; Pawlik, A. Non-Coding RNA Involved in the Pathogenesis of Atherosclerosis—A Narrative Review. Diagnostics 2024, 14, 1981. [Google Scholar] [CrossRef]
- Šatrauskienė, A.; Navickas, R.; Laucevičius, A.; Krilavičius, T.; Užupytė, R.; Zdanytė, M.; Ryliškytė, L.; Jucevičienė, A.; Holvoet, P. MiR-1, MiR-122, MiR-132, and MiR-133 Are Related to Subclinical Aortic Atherosclerosis Associated with Metabolic Syndrome. Int. J. Environ. Res. Public Health 2021, 18, 1483. [Google Scholar] [CrossRef]
- Olejarz, W.; Sadowski, K.; Radoszkiewicz, K. Extracellular Vesicles in Atherosclerosis: State of the Art. Int. J. Mol. Sci. 2023, 25, 388. [Google Scholar] [CrossRef]
- Raitoharju, E.; Lyytikäinen, L.P.; Levula, M.; Oksala, N.; Mennander, A.; Tarkka, M.; Klopp, N.; Illig, T.; Kähönen, M.; Karhunen, P.J.; et al. MiR-21, MiR-210, MiR-34a, and MiR-146a/b Are up-Regulated in Human Atherosclerotic Plaques in the Tampere Vascular Study. Atherosclerosis 2011, 219, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Mei, S.; He, Y.; Wuyun, Q.; Zhou, L.; Cai, Z.; Luo, Q.; Wen, Y.; Yan, J. Unraveling the Association and Regulatory Role of MiR-146b-5p in Coronary Artery Disease. BMC Cardiovasc. Disord. 2025, 25, 81. [Google Scholar] [CrossRef]
- Hulsmans, M.; van Dooren, E.; Mathieu, C.; Holvoet, P. Decrease of MiR-146b-5p in Monocytes during Obesity Is Associated with Loss of the Anti-Inflammatory but Not Insulin Signaling Action of Adiponectin. PLoS ONE 2012, 7, e32794. [Google Scholar] [CrossRef]
- Chang, T.Y.; Tsai, W.C.; Huang, T.S.; Su, S.H.; Chang, C.Y.; Ma, H.Y.; Wu, C.H.; Yang, C.Y.; Lin, C.H.; Huang, P.H.; et al. Dysregulation of Endothelial Colony-Forming Cell Function by a Negative Feedback Loop of Circulating MiR-146a and -146b in Cardiovascular Disease Patients. PLoS ONE 2017, 12, e0181562. [Google Scholar] [CrossRef]
- Pérez-Sánchez, L.; Patiño-Trives, A.M.; Aguirre-Zamorano, M.Á.; Luque-Tévar, M.; Ábalos-Aguilera, M.C.; Arias-De La Rosa, I.; Seguí, P.; Velasco-Gimena, F.; Barbarroja, N.; Escudero-Contreras, A.; et al. Characterization of Antiphospholipid Syndrome Atherothrombotic Risk by Unsupervised Integrated Transcriptomic Analyses. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, M.; Xu, Z.; Huang, H.; Gong, P.; Zhu, H.; Ruan, C. MiR-146b-5p Promotes VSMC Proliferation and Migration. Int. J. Clin. Exp. Pathol. 2015, 8, 12901. [Google Scholar]
- Lee, S.M.; Yoon, B.H.; Lee, J.W.; Jeong, I.J.Y.; Kim, I.; Pack, C.G.; Kim, Y.H.; Ha, C.H. Circulating MiRNA-4701-3p as a Predictive Biomarker of Cardiovascular Disease Which Induces Angiogenesis by Inhibition of TOB2. Microvasc. Res. 2024, 155, 104698. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fan, B.; Wang, F.; Song, Y.; Wang, X.; Meng, Y.; Chen, Y.; Xia, Q.; Sun, J. Research Progress in Pharmacological Effects and Mechanisms of Angelica Sinensis against Cardiovascular and Cerebrovascular Diseases. Molecules 2024, 29, 2100. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, R.C.C.; Bortolin, R.H.; Lopes, M.B.; Hirata, M.H.; Hirata, R.D.C.; Silbiger, V.N.; Luchessi, A.D. Integrated Analysis of MiRNA and MRNA Gene Expression Microarrays: Influence on Platelet Reactivity, Clopidogrel Response and Drug-Induced Toxicity. Gene 2016, 593, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zheng, C.; Pu, Y.; Zhou, H.; Li, Y.; Wang, W.; Chen, X.; Zhang, C.; Chen, Y. Vericiguat Enhances the Therapeutic Efficacy of Mesenchymal Stem Cells-Derived Exosomes in Acute Myocardial Infarction through MicroRNA-1180-3p/ETS1 Pathway. Cell Signal. 2025, 125, 111512. [Google Scholar] [CrossRef]
- Pedersen, O.B.; Hvas, A.M.; Grove, E.L.; Larsen, S.B.; Pasalic, L.; Kristensen, S.D.; Nissen, P.H. Association of Whole Blood MicroRNA Expression with Platelet Function and Turnover in Patients with Coronary Artery Disease. Thromb. Res. 2022, 211, 98–105. [Google Scholar] [CrossRef]
- Flinn, B.; Adams, C.; Chowdhury, N.; Gress, T.; Santanam, N. Profiling of Non-Coding Regulators and Their Targets in Epicardial Fat from Patients with Coronary Artery Disease. Int. J. Mol. Sci. 2022, 23, 5297. [Google Scholar] [CrossRef]
- Posadas-Sánchez, R.; Pérez-Hernández, N.; Rodríguez-Pérez, J.M.; Coral-Vázquez, R.M.; Roque-Ramírez, B.; Llorente, L.; Lima, G.; Flores-Dominguez, C.; Villarreal-Molina, T.; Posadas-Romero, C.; et al. Interleukin-27 Polymorphisms Are Associated with Premature Coronary Artery Disease and Metabolic Parameters in the Mexican Population: The Genetics of Atherosclerotic Disease (GEA) Mexican Study. Oncotarget 2017, 8, 64459–64470. [Google Scholar] [CrossRef]
- Mócsai, A.; Ruland, J.; Tybulewicz, V.L.J. The SYK Tyrosine Kinase: A Crucial Player in Diverse Biological Functions. Nat. Rev. Immunol. 2010, 10, 387–402. [Google Scholar] [CrossRef]
- Domingues-Montanari, S.; Fernández-Cadenas, I.; del Río-Espinola, A.; Mendioroz, M.; Fernandez-Morales, J.; Corbeto, N.; Delgado, P.; Ribó, M.; Rubiera, M.; Obach, V.; et al. KCNK17 Genetic Variants in Ischemic Stroke. Atherosclerosis 2010, 208, 203–209. [Google Scholar] [CrossRef]
- Kinoshita, T.; Yip, K.W.; Spence, T.; Liu, F.F. MicroRNAs in Extracellular Vesicles: Potential Cancer Biomarkers. J. Hum. Genet. 2016, 62, 67–74. [Google Scholar] [CrossRef]
- Nobrega, M.; dos Reis, M.B.; de Souza, M.F.; Furini, H.H.; Costa Brandão Berti, F.; Souza, I.L.M.; Mingorance Carvalho, T.; Zanata, S.M.; Fuganti, P.E.; Malheiros, D.; et al. Comparative Analysis of Extracellular Vesicles MiRNAs (EV-MiRNAs) and Cell-Free MicroRNAs (Cf-MiRNAs) Reveals That EV-MiRNAs Are More Promising as Diagnostic and Prognostic Biomarkers for Prostate Cancer. Gene 2025, 939, 149186. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Molina, T.; Posadas-Romero, C.; Romero-Hidalgo, S.; Antúnez-Argüelles, E.; Bautista-Grande, A.; Vargas-Alarcón, G.; Kimura-Hayama, E.; Canizales-Quinteros, S.; Juárez-Rojas, J.G.; Posadas-Sánchez, R.; et al. The ABCA1 Gene R230C Variant Is Associated with Decreased Risk of Premature Coronary Artery Disease: The Genetics of Atherosclerotic Disease (GEA) Study. PLoS ONE 2012, 7, e49285. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Greene, J.A.; Hernández-Ortega, K.; Quiroz-Baez, R.; Resendis-Antonio, O.; Pichardo-Casas, I.; Sinclair, D.A.; Budnik, B.; Hidalgo-Miranda, A.; Uribe-Querol, E.; Ramos-Godínez, M.D.P.; et al. Quantitative Proteomic Analysis of Extracellular Vesicle Subgroups Isolated by an Optimized Method Combining Polymer-Based Precipitation and Size Exclusion Chromatography. J. Extracell. Vesicles 2021, 10, e12087. [Google Scholar] [CrossRef] [PubMed]
- Sulkava, M.; Raitoharju, E.; Levula, M.; Seppälä, I.; Lyytikaïnen, L.P.; Mennander, A.; Järvinen, O.; Zeitlin, R.; Salenius, J.P.; Illig, T.; et al. Differentially Expressed Genes and Canonical Pathway Expression in Human Atherosclerotic Plaques-Tampere Vascular Study. Sci. Rep. 2017, 7, 41483. [Google Scholar] [CrossRef] [PubMed]
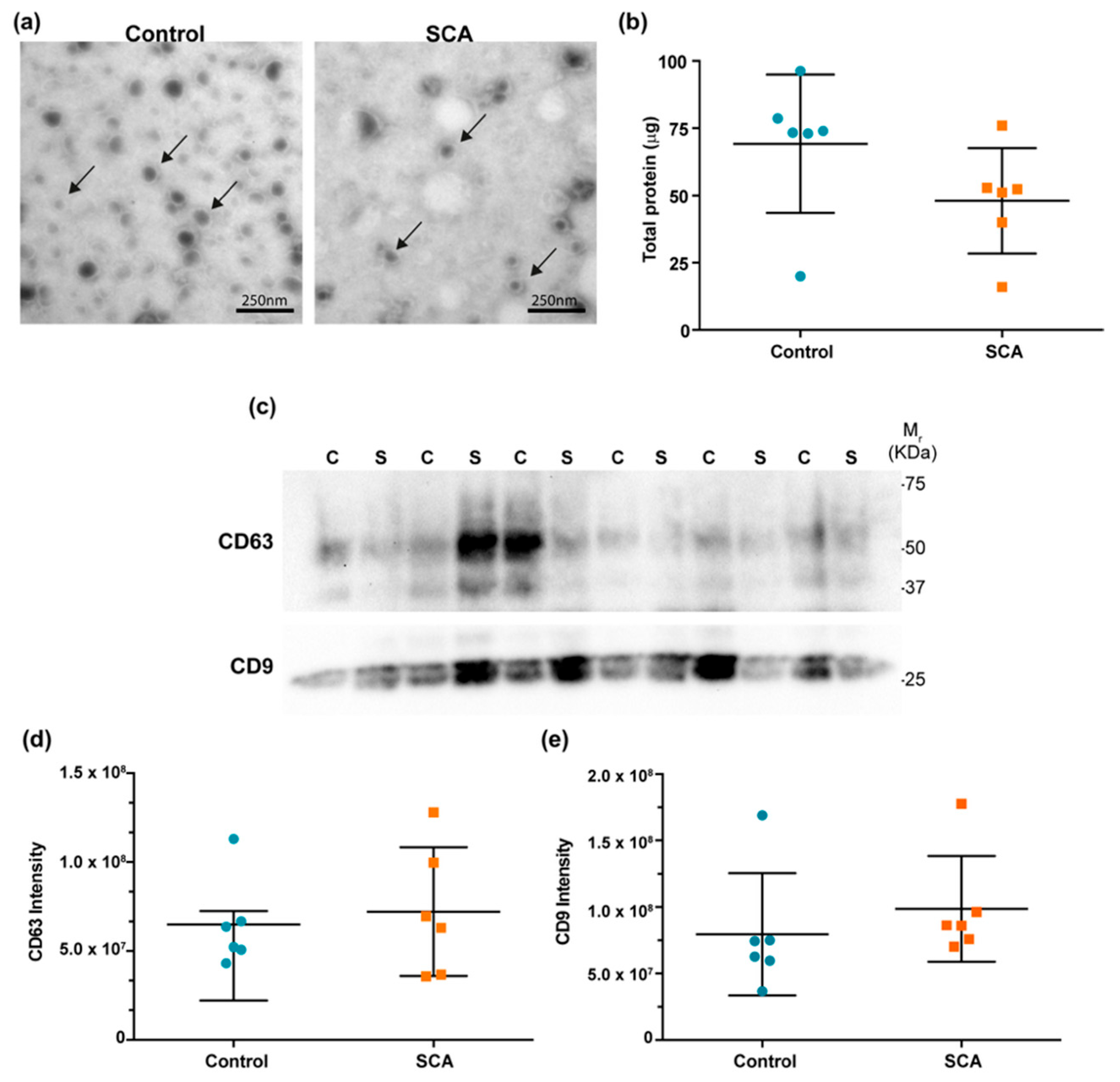

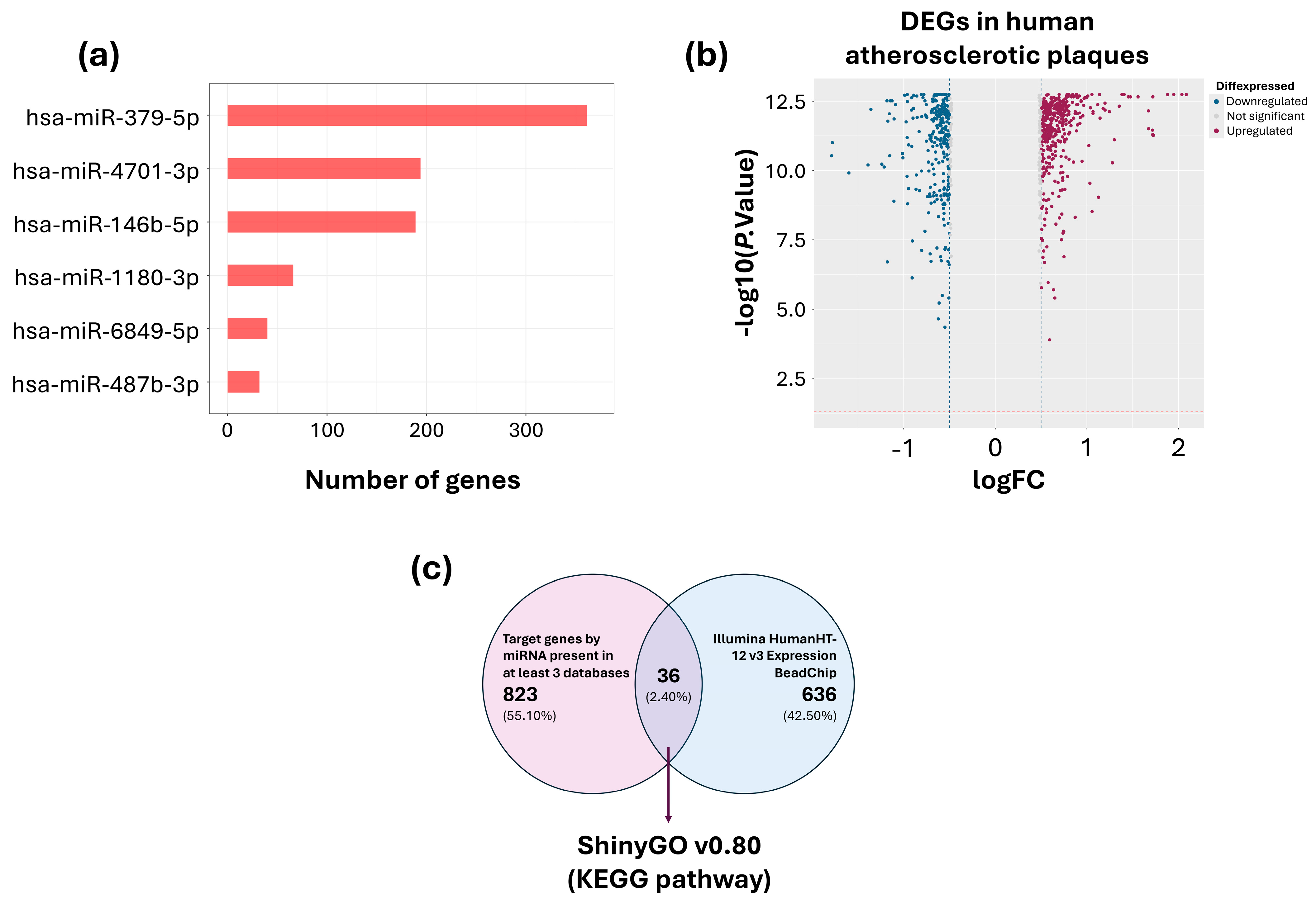
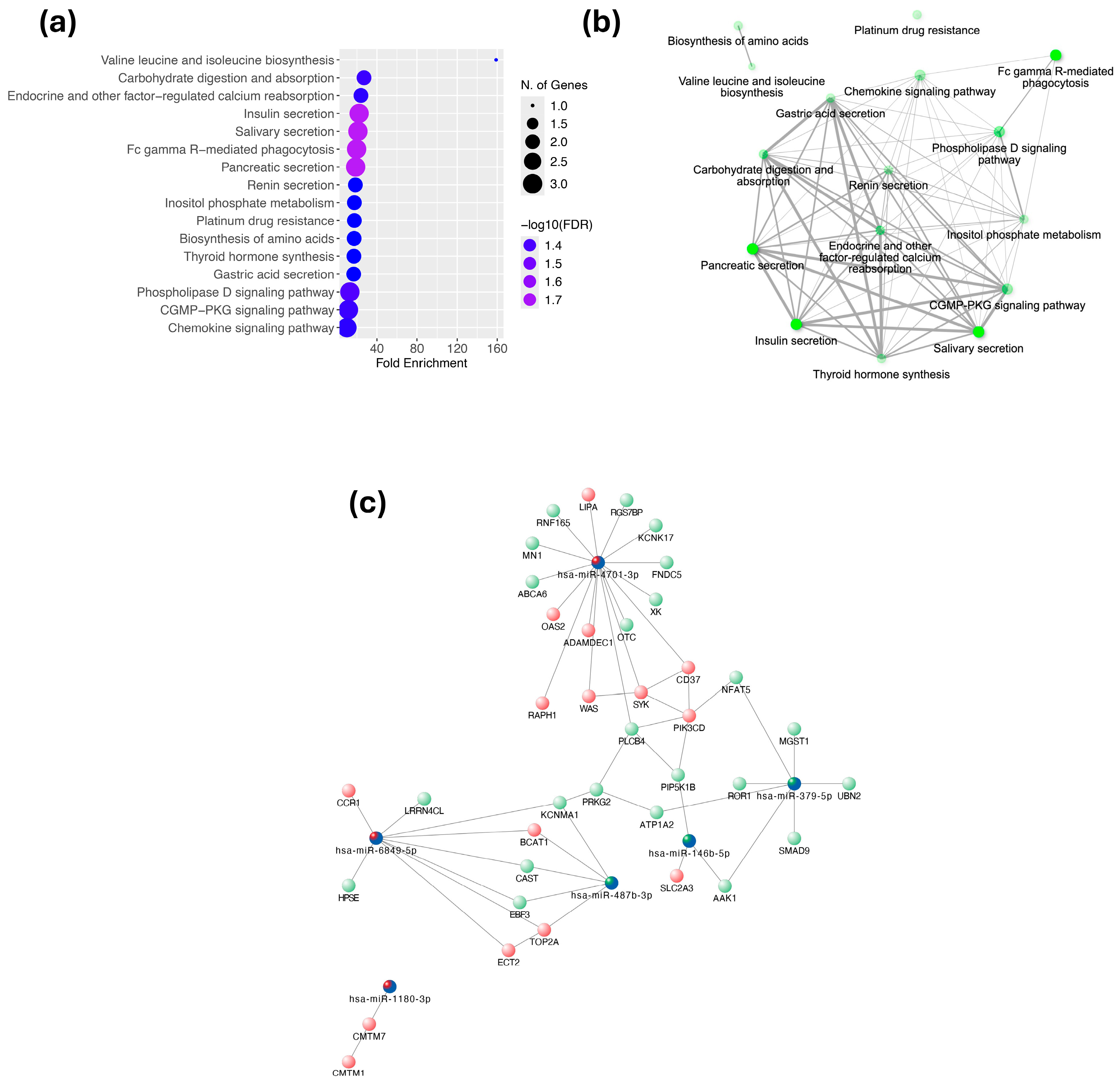

| miRNA | Control (n = 17) | SCA (n = 17) | p |
|---|---|---|---|
| miR-146b-5p | 14.04 (5.850–25.663) | 4.928 (1.806–16.079) | 0.006 |
| miR-4701-3p | 0.054 (0.025–0.077) | 0.083 (0.059–0.108) | 0.014 |
| miR-487b-3p | 1.942 (1.497–2.094) | 0.526 (0.274–1.573) | 0.034 |
| miR-379-5p | 1.341 (0.807–1.424) | 3.260 (2.109–8.056) | 0.036 |
| miR-1180-3p | 0.028 (0.002–0.045) | 0.084 (0.046–0.200) | 0.036 |
| miR-6849-5p | 0.007 (0.006–0.017) | 0.012 (0.006–0.067) | 0.048 |
| miRNA | AUC | p | Sensitivity% | Specificity% |
|---|---|---|---|---|
| miR-146b-5p | 0.7543 | 0.0106 | 88.24 | 58.82 |
| miR-4701-3p | 0.7404 | 0.0283 | 62.5 | 92.31 |
| miR-487b-3p | 0.6902 | 0.067 | 66.67 | 76.47 |
| miR-379-5p | 0.6863 | 0.0729 | 86.67 | 52.94 |
| miR-1180-3p | 0.6863 | 0.0757 | 76.47 | 60 |
| miR-6849-5p | 0.6765 | 0.0955 | 41.18 | 100 |
| miR-146b-5p + miR-4701-3p | 0.8413 | 0.0018 | 87.5 | 69.23 |
| miR-146b-5p + miR-487b-3p | 0.8693 | 0.0013 | 93.75 | 63.64 |
| miR-146b-5p + miR-4701-3p + miR-487b-3p | 0.8846 | 0.0007 | 100 | 69.23 |
| miR-146b-5p + miR-4701-3p + miR-379-5p | 0.8791 | 0.0008 | 100 | 69.23 |
| miR-146b-5p + miR-4701-3p + miR-1180-5p | 0.8281 | 0.0026 | 93.75 | 93.75 |
| miR-146b-5p + miR-4701-3p + miR-487b-3p + miR-379-5p | 0.8791 | 0.0008 | 100 | 69.23 |
| miR-146b-5p + miR-4701-3p + miR-487b-3p + miR-1180-3p | 0.869 | 0.0014 | 100 | 66.67 |
| Control (n = 8) | SCA (n = 8) | p | |
|---|---|---|---|
| Age (years) | 66.5 (63.75–69.5) | 68.5 (66–72.5) | 0.594 |
| Weight (kg) | 71.35 (67.425–86.45) | 80.25 (65.05–95.15) | 0.529 |
| Height (m) | 1.655 (1.575–1.715) | 1.655 (1.640–1.772) | 0.526 |
| BMI (kg/m2) | 28.23 (24.707–30.45) | 29.61 (24.00–30.75) | 0.875 |
| Glucose (mg/dL) | 101.8 (90.725–113.6) | 96.30 (91.15–103.1) | 0.529 |
| Total cholesterol (mg/dL) | 162.2 (144.15–176.4) | 165.7 (110.4–197.5) | 1.000 |
| HDL-c (mg/dL) | 40.20 (32.150–45.07) | 40.35 (30.70–45.15) | 1.000 |
| LDL-c (mg/dL) | 92.80 (69.650–109.9) | 113.3 (50.75–126.4) | 0.462 |
| Triglycerides (mg/dL) | 133.8 (72.175–216.1) | 165.1 (119.4–296.7) | 0.401 |
| CACS (AU) | 0.0 (0.0–0.0) | 483.4 (436.9–770.1) | <0.0001 |
| Control (n = 17) | CSA (n = 17) | p | |
|---|---|---|---|
| Age (years) | 67.0 (57.0–72.5) | 67.0 (61.0–72.0) | 0.666 |
| Weight (kg) | 77.0 (67.45–81.8) | 77.6 (72.15–88.90) | 0.491 |
| Height (m) | 1.67 (1.635–1.719) | 1.63 (1.60–1.73) | 0.73 |
| BMI (kg/m2) | 28.50 (23.98–29.86) | 28.97 (27.16–30.27) | 0.38 |
| Body fat (%) | 753.0 (432.0–1315.0) | 654.0 (442.0–1121.0) | 0.796 |
| Glucose (mg/dL) | 95.8 (90.8–106.0) | 97.1 (89.5–113.2) | 0.931 |
| Total cholesterol (mg/dL) | 185.7 (165.2–205.1) | 176.0 (151.8–196.4) | 0.418 |
| HDL-c (mg/dL) | 38.8 (34.8–47.9) | 44.8 (35.4–50.9) | 0.558 |
| LDL-c (mg/dL) | 117.0 (101.7–131.4) | 111.3 (87.0–130.3) | 0.667 |
| Triglycerides (mg/dL) | 149.8 (117.4–201.9) | 130.8 (103.2–198.5) | 0.547 |
| CACS (AU) | 0.0 (0.0–0.0) | 200.5 (118.2–293.9) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña-Peña, M.; Zepeda-García, Ó.; Posadas-Sánchez, R.; Sánchez-Muñoz, F.; Domínguez-Pérez, M.; Martínez-Greene, J.A.; López-Bautista, F.; Hernández-Díazcouder, A.; Jiménez-Ortega, R.F.; Valencia-Cruz, A.I.; et al. A Plasma Extracellular Vesicle-Derived microRNA Signature as a Potential Biomarker for Subclinical Coronary Atherosclerosis. Int. J. Mol. Sci. 2025, 26, 8727. https://doi.org/10.3390/ijms26178727
Peña-Peña M, Zepeda-García Ó, Posadas-Sánchez R, Sánchez-Muñoz F, Domínguez-Pérez M, Martínez-Greene JA, López-Bautista F, Hernández-Díazcouder A, Jiménez-Ortega RF, Valencia-Cruz AI, et al. A Plasma Extracellular Vesicle-Derived microRNA Signature as a Potential Biomarker for Subclinical Coronary Atherosclerosis. International Journal of Molecular Sciences. 2025; 26(17):8727. https://doi.org/10.3390/ijms26178727
Chicago/Turabian StylePeña-Peña, Mario, Óscar Zepeda-García, Rosalinda Posadas-Sánchez, Fausto Sánchez-Muñoz, Mayra Domínguez-Pérez, Juan Alfonso Martínez-Greene, Fabiola López-Bautista, Adrián Hernández-Díazcouder, Rogelio F. Jiménez-Ortega, Alejandra Idan Valencia-Cruz, and et al. 2025. "A Plasma Extracellular Vesicle-Derived microRNA Signature as a Potential Biomarker for Subclinical Coronary Atherosclerosis" International Journal of Molecular Sciences 26, no. 17: 8727. https://doi.org/10.3390/ijms26178727
APA StylePeña-Peña, M., Zepeda-García, Ó., Posadas-Sánchez, R., Sánchez-Muñoz, F., Domínguez-Pérez, M., Martínez-Greene, J. A., López-Bautista, F., Hernández-Díazcouder, A., Jiménez-Ortega, R. F., Valencia-Cruz, A. I., Nuñez-Salgado, A., Mani-Arellano, I. E., Martínez-Flores, K., Villarreal-Molina, T., Martínez-Martínez, E., & Jacobo-Albavera, L. (2025). A Plasma Extracellular Vesicle-Derived microRNA Signature as a Potential Biomarker for Subclinical Coronary Atherosclerosis. International Journal of Molecular Sciences, 26(17), 8727. https://doi.org/10.3390/ijms26178727










