Advancing Gene Therapy for Phenylketonuria: From Precision Editing to Clinical Translation
Abstract
1. Introduction
2. Pathophysiology and Genetic Basis of PKUs
2.1. PAH Gene Location and Enzyme Function
2.2. Clinical Manifestations
2.3. Limitations of Current Therapies
3. Gene Therapy Approaches for PKU
3.1. rAAV-Mediated Gene Therapy
3.2. Base Editing Strategies Using CRISPR-Cas9
3.3. LNP Delivery Systems
4. Animal Models of PKU
4.1. Germline Mutagenesis for PKU Models
4.2. CRISPR/Cas9 Knockout (KO) Models
4.3. CRISPR-Based Pah-R261Q KI Mouse
4.4. Prime Editing in Humanized PKU Models
4.5. CRISPR/Cas9-Mediated Pah Exon 1 Deletion Model
4.6. Rat Models
4.7. Pig Models
5. Clinical and Trial Medicine
5.1. SAR444836
5.2. NGGT002
5.3. HMI-102
5.4. BMN 307
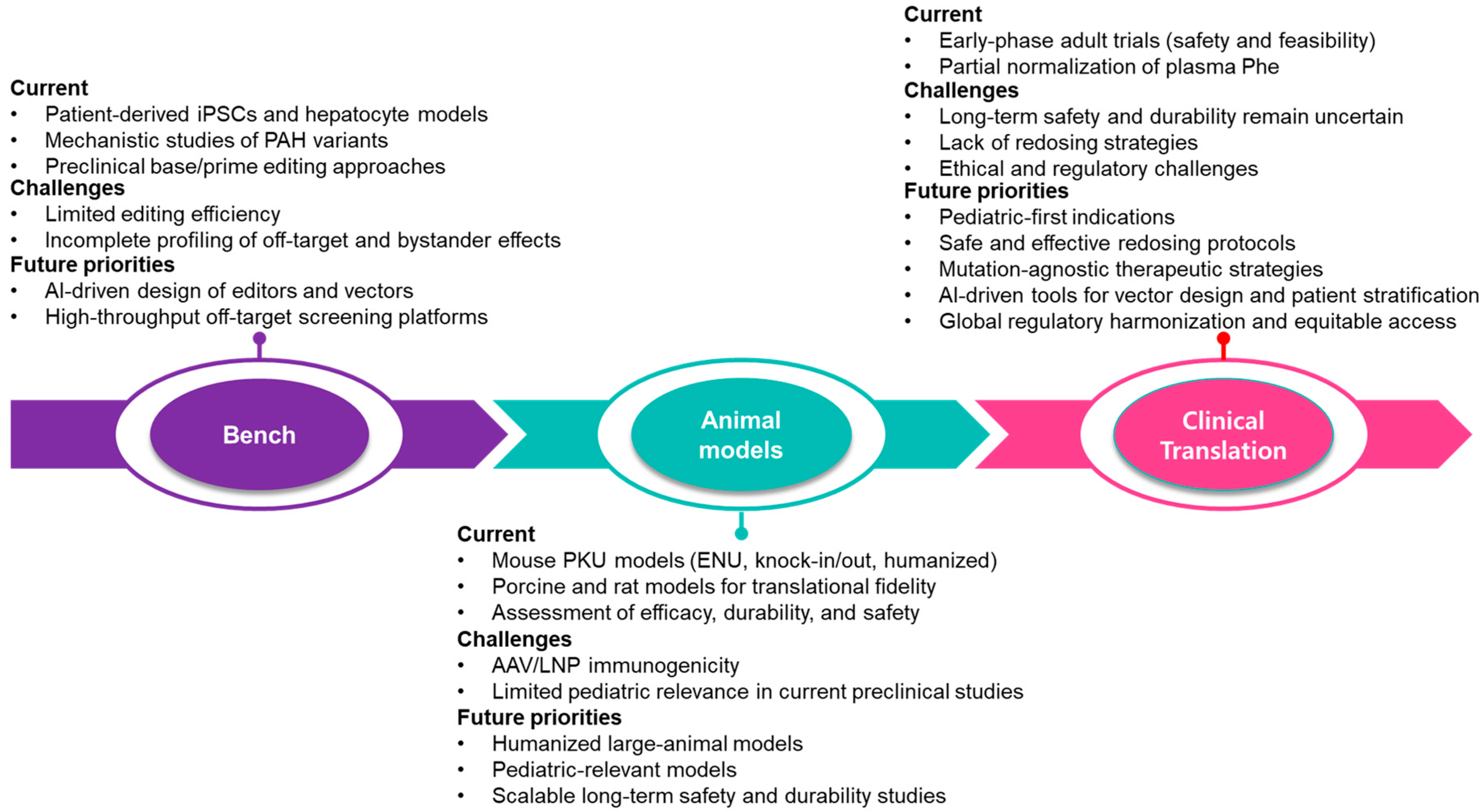
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABEs | adenine base editors |
| AAV | adeno-associated virus |
| AF | allele frequency |
| CNS | central nervous system |
| CBEs | cytosine base editors |
| enu | N-ethyl-N-nitrosourea |
| KI | Knock-in |
| KO | Knockout |
| LNPs | lipid nanoparticles |
| Phe | phenylalanine |
| PAH | phenylalanine hydroxylase |
| PKU | Phenylketonuria |
| rAAVs | recombinant adeno-associated viruses |
| TALENs | transcription activator-like effector nucleases |
| Tyr | tyrosine |
References
- Vargas, P.R.; Poubel, M.; Martins, B.; Velez, P.; Vilela, D.; Mesojedovas, D.; Magalhães, T.S.P.C.; Louie, K.S.; Pessoa, A. Patient journey and disease burden characterization of the population with phenylketonuria (PKU) in Brazil: A retrospective analysis through data reported in the public health system administrative database (DATASUS). Lancet Reg. Health Am. 2025, 47, 101134. [Google Scholar] [CrossRef]
- Fölling, A. Excretion of phenylpyruvic acid in urine as a metabolic anomaly in connection with imbecility. Nord. Med. Tidskr. 1934, 8, 1054–1059. [Google Scholar]
- Blau, N.; van Spronsen, F.J.; Levy, H.L. Phenylketonuria. Lancet 2010, 376, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Enns, G.M.; Koch, R.; Brumm, V.; Blakely, E.; Suter, R.; Jurecki, E. Suboptimal outcomes in patients with PKU treated early with diet alone: Revisiting the evidence. Mol. Genet. Metab. 2010, 101, 99–109. [Google Scholar] [CrossRef]
- Vockley, J.; Andersson, H.C.; Antshel, K.M.; Braverman, N.E.; Burton, B.K.; Frazier, D.M.; Mitchell, J.; Smith, W.E.; Thompson, B.H.; Berry, S.A. Phenylalanine hydroxylase deficiency: Diagnosis and management guideline. Genet. Med. 2014, 16, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, S. Upcoming market catalysts in Q3 2023. Nat. Rev. Drug Discov. 2023, 22, 528. [Google Scholar] [CrossRef]
- Bier, C.; Dickey, K.; Bibb, B.; Crutcher, A.; Sponberg, R.; Chang, R.; Boyer, M.; Davis-Keppen, L.; Matthes, C.; Tharp, M.; et al. Outcomes in 14 live births resulting from Pegvaliase-treated pregnancies in PKU-affected females. Mol. Genet. Metab. 2024, 141, 108152. [Google Scholar] [CrossRef]
- Louie, K.; Vedantham, B.; Wadhwa, K.; Marwah, R.; Khan, S.; Jones, S. P046: Occurrence of anaphylaxis in adult incident pegvaliase-treated PKU patients in a post-marketing safety analysis in the United States. Genet. Med. Open 2025, 3, 102890. [Google Scholar] [CrossRef]
- Brooks, D.L.; Whittaker, M.N.; Said, H.; Dwivedi, G.; Qu, P.; Musunuru, K.; Ahrens-Nicklas, R.C.; Alameh, M.-G.; Wang, X. A base editing strategy using mRNA-LNPs for in vivo correction of the most frequent phenylketonuria variant. Hum. Genet. Genom. Adv. 2024, 5, 100253. [Google Scholar] [CrossRef]
- Diaz-Trelles, R.; Perez-Garcia, C.G. Chapter Four—Present and future of lipid nanoparticle-mRNA technology in phenylketonuria disease treatment. In International Review of Cell and Molecular Biology; Aranda, F., Berraondo, P., Galluzzi, L., Eds.; Academic Press: Cambridge, MA, USA, 2022; Volume 372, pp. 159–174. [Google Scholar]
- Baruteau, J.; Brunetti-Pierri, N.; Gissen, P. Liver-directed gene therapy for inherited metabolic diseases. J. Inherit. Metab. Dis. 2024, 47, 9–21. [Google Scholar] [CrossRef]
- Arjunan, P.; Kathirvelu, D.; Mahalingam, G.; Goel, A.K.; Zacharaiah, U.G.; Srivastava, A.; Marepally, S. Lipid-nanoparticle-enabled nucleic acid therapeutics for liver disorders. Acta Pharm. Sin. B 2024, 14, 2885–2900. [Google Scholar] [CrossRef]
- Ding, Z.; Harding, C.O.; Rebuffat, A.; Elzaouk, L.; Wolff, J.A.; Thöny, B. Correction of Murine PKU Following AAV-mediated Intramuscular Expression of a Complete Phenylalanine Hydroxylating System. Mol. Ther. 2008, 16, 673–681. [Google Scholar] [CrossRef]
- Brooks, D.L.; Whittaker, M.N.; Qu, P.; Musunuru, K.; Ahrens-Nicklas, R.C.; Wang, X. Efficient in vivo prime editing corrects the most frequent phenylketonuria variant, associated with high unmet medical need. Am. J. Hum. Genet. 2023, 110, 2003–2014. [Google Scholar] [CrossRef]
- Shao, A.B.; Liu, J.; Zhang, Y.; Wang, F.; Qiao, C.; Zhang, Y.; Zhu, Y.; Lin, P.; Hu, T.; Tao, Z.; et al. A capillary electrophoresis-based variant hotspot genotyping method for rapid and reliable analysis of the phenylalanine hydroxylase gene in the Chinese Han population. Clin. Chim. Acta 2021, 523, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Himmelreich, N.; Blau, N.; Thöny, B. Molecular and metabolic bases of tetrahydrobiopterin (BH(4)) deficiencies. Mol. Genet. Metab. 2021, 133, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Tebieva, I.S.; Mishakova, P.V.; Gabisova, Y.V.; Khokhova, A.V.; Kaloeva, T.G.; Marakhonov, A.V.; Shchagina, O.A.; Polyakov, A.V.; Ginter, E.K.; Kutsev, S.I.; et al. Genetic Landscape and Clinical Features of Hyperphenylalaninemia in North Ossetia-Alania: High Frequency of P281L and P211T Genetic Variants in the PAH Gene. Int. J. Mol. Sci. 2024, 25, 4598. [Google Scholar] [CrossRef]
- Hillert, A.; Anikster, Y.; Belanger-Quintana, A.; Burlina, A.; Burton, B.K.; Carducci, C.; Chiesa, A.E.; Christodoulou, J.; Đorđević, M.; Desviat, L.R.; et al. The Genetic Landscape and Epidemiology of Phenylketonuria. Am. J. Hum. Genet. 2020, 107, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Michals-Matalon, K.; Bhatia, G.; Guttler, F.; Tyring, S.K.; Matalon, R. Response of Phenylketonuria to Tetrahydrobiopterin123. J. Nutr. 2007, 137, 1564S–1567S. [Google Scholar] [CrossRef]
- Yılmaz, B.K.; Bağcı, Z. Delays in Newborn Screening for Phenylketonuria from Birth to Diagnosis and Factors Affecting This. Children 2024, 11, 571. [Google Scholar] [CrossRef]
- Waisbren, S.E.; Noel, K.; Fahrbach, K.; Cella, C.; Frame, D.; Dorenbaum, A.; Levy, H. Phenylalanine blood levels and clinical outcomes in phenylketonuria: A systematic literature review and meta-analysis. Mol. Genet. Metab. 2007, 92, 63–70. [Google Scholar] [CrossRef]
- Rovelli, V.; Longo, N. Phenylketonuria and the brain. Mol. Genet. Metab. 2023, 139, 107583. [Google Scholar] [CrossRef]
- Nulmans, I.; Lequeue, S.; Desmet, L.; Neuckermans, J.; De Kock, J. Current state of the treatment landscape of phenylketonuria. Orphanet J. Rare Dis. 2025, 20, 281. [Google Scholar] [CrossRef] [PubMed]
- Jahja, R.; Huijbregts, S.C.; de Sonneville, L.M.; van der Meere, J.J.; van Spronsen, F.J. Neurocognitive evidence for revision of treatment targets and guidelines for phenylketonuria. J. Pediatr. 2014, 164, 895–899.e2. [Google Scholar] [CrossRef] [PubMed]
- Bailey, N.A.; Mackay, L. Phenylketonuria—Past, Present, and Future Directions. OBM Genet. 2024, 8, 256. [Google Scholar] [CrossRef]
- van Calcar, S.C.; Ney, D.M. Food Products Made with Glycomacropeptide, a Low-Phenylalanine Whey Protein, Provide a New Alternative to Amino Acid–Based Medical Foods for Nutrition Management of Phenylketonuria. J. Acad. Nutr. Diet. 2012, 112, 1201–1210. [Google Scholar] [CrossRef]
- Al Hafid, N.; Christodoulou, J. Phenylketonuria: A review of current and future treatments. Transl. Pediatr. 2015, 4, 304–317. [Google Scholar] [CrossRef]
- Anton-Păduraru, D.-T.; Trofin, F.; Chis, A.; Sur, L.M.; Streangă, V.; Mîndru, D.E.; Dorneanu, O.S.; Păduraru, D.; Nastase, E.V.; Vulturar, R. Current Insights into Nutritional Management of Phenylketonuria: An Update for Children and Adolescents. Children 2025, 12, 199. [Google Scholar] [CrossRef]
- Heintz, C.; Cotton, R.G.; Blau, N. Tetrahydrobiopterin, its Mode of Action on Phenylalanine Hydroxylase, and Importance of Genotypes for Pharmacological Therapy of Phenylketonuria. Hum. Mutat. 2013, 34, 927–936. [Google Scholar] [CrossRef]
- Adams, A.D.; Fiesco-Roa, M.Ó.; Wong, L.; Jenkins, G.P.; Malinowski, J.; Demarest, O.M.; Rothberg, P.G.; Hobert, J.A. Phenylalanine hydroxylase deficiency treatment and management: A systematic evidence review of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2023, 25, 100358. [Google Scholar] [CrossRef] [PubMed]
- Doyle, S.; O’Regan, M.; Stenson, C.; Bracken, J.; Hendroff, U.; Agasarova, A.; Deverell, D.; Treacy, E.P. Extended Experience of Lower Dose Sapropterin in Irish Adults with Mild Phenylketonuria. JIMD Rep. 2018, 40, 71–76. [Google Scholar] [CrossRef]
- Thomas, J.; Levy, H.; Amato, S.; Vockley, J.; Zori, R.; Dimmock, D.; Harding, C.O.; Bilder, D.A.; Weng, H.H.; Olbertz, J.; et al. Pegvaliase for the treatment of phenylketonuria: Results of a long-term phase 3 clinical trial program (PRISM). Mol. Genet. Metab. 2018, 124, 27–38. [Google Scholar] [CrossRef]
- Yan, K.; Zhang, L.; Yang, G.; Yan, L.; Wu, L.; He, P.; Yu, Q. Evaluating adverse events of pegvaliase-pqpz in phenylketonuria treatment: A comprehensive safety assessment. SAGE Open Med. 2025, 13, 20503121251330187. [Google Scholar] [CrossRef]
- Longo, N.; Zori, R.; Wasserstein, M.P.; Vockley, J.; Burton, B.K.; Decker, C.; Li, M.; Lau, K.; Jiang, J.; Larimore, K.; et al. Long-term safety and efficacy of pegvaliase for the treatment of phenylketonuria in adults: Combined phase 2 outcomes through PAL-003 extension study. Orphanet J. Rare Dis. 2018, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- A Phase 3 Multi-Center Study to Evaluate the Safety and Efficacy of Subcutaneous Injections of Pegvaliase in Adolescent Subjects (Ages 12-17) with Phenylketonuria Featuring an Open-Label Randomized Two-Arm (Active vs Diet-Only Control) Design. Genet. Metab. 2020.
- Harding, C.O.; Gibson, K.M. Therapeutic liver repopulation for phenylketonuria. J. Inherit. Metab. Dis. 2010, 33, 681–687. [Google Scholar] [CrossRef]
- Li, C.; Samulski, R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Greig, J.A.; Martins, K.M.; Breton, C.; Lamontagne, R.J.; Zhu, Y.; He, Z.; White, J.; Zhu, J.-X.; Chichester, J.A.; Zheng, Q.; et al. Integrated vector genomes may contribute to long-term expression in primate liver after AAV administration. Nat. Biotechnol. 2024, 42, 1232–1242. [Google Scholar] [CrossRef]
- Gao, G.; Vandenberghe, L.H.; Alvira, M.R.; Lu, Y.; Calcedo, R.; Zhou, X.; Wilson, J.M. Clades of Adeno-Associated Viruses Are Widely Disseminated in Human Tissues. J. Virol. 2004, 78, 6381–6388. [Google Scholar] [CrossRef]
- Tao, R.; Xiao, L.; Zhou, L.; Zheng, Z.; Long, J.; Zhou, L.; Tang, M.; Dong, B.; Yao, S. Long-Term Metabolic Correction of Phenylketonuria by AAV-Delivered Phenylalanine Amino Lyase. Mol. Ther. Methods Clin. Dev. 2020, 19, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.-J.; Park, E.-S.; Kang, S.; Jo, I.; Jung, S.-C. Long-term enzymatic and phenotypic correction in the phenylketonuria mouse model by adeno-associated virus vector-mediated gene transfer. Pediatr. Res. 2004, 56, 278–284. [Google Scholar] [CrossRef]
- Kaiser, R.A.; Weber, N.D.; Trigueros-Motos, L.; Allen, K.L.; Martinez, M.; Cao, W.; VanLith, C.J.; Hillin, L.G.; Douar, A.; González-Aseguinolaza, G.; et al. Use of an adeno-associated virus serotype Anc80 to provide durable cure of phenylketonuria in a mouse model. J. Inherit. Metab. Dis. 2021, 44, 1369–1381. [Google Scholar] [CrossRef]
- Ding, Z.; Georgiev, P.; Thöny, B. Administration-route and gender-independent long-term therapeutic correction of phenylketonuria (PKU) in a mouse model by recombinant adeno-associated virus 8 pseudotyped vector-mediated gene transfer. Gene Ther. 2006, 13, 587–593. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Rubin, H.; Wang, M.; Faulkner, D.; Sengooba, A.; Dollive, S.N.; Avila, N.; Ellsworth, J.L.; Lamppu, D.; Lobikin, M.; et al. Sustained Correction of a Murine Model of Phenylketonuria following a Single Intravenous Administration of AAVHSC15-PAH. Mol. Ther. Methods Clin. Dev. 2020, 17, 568–580. [Google Scholar] [CrossRef]
- Naso, M.F.; Tomkowicz, B.; Perry, W.L., 3rd; Strohl, W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 2017, 31, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Cabré-Romans, J.-J.; Cuella-Martin, R. CRISPR-dependent base editing as a therapeutic strategy for rare monogenic disorders. Front. Genome Ed. 2025, 7, 1553590. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zheng, C.; Xu, W.; Zhang, S.; Liu, S.; Chen, X.; Yao, K. Breaking genetic shackles: The advance of base editing in genetic disorder treatment. Front. Pharmacol. 2024, 15, 1364135. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, M.N.; Brooks, D.L.; Quigley, A.; Jindal, I.; Said, H.; Qu, P.; Wang, J.Z.; Ahrens-Nicklas, R.C.; Musunuru, K.; Alameh, M.G.; et al. Improved specificity and efficacy of base-editing therapies with hybrid guide RNAs. bioRxiv 2024. [Google Scholar] [CrossRef]
- Musunuru, K.; Chadwick, A.C.; Mizoguchi, T.; Garcia, S.P.; DeNizio, J.E.; Reiss, C.W.; Wang, K.; Iyer, S.; Dutta, C.; Clendaniel, V.; et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature 2021, 593, 429–434. [Google Scholar] [CrossRef]
- Jung, H.N.; Lee, S.-Y.; Lee, S.; Youn, H.; Im, H.-J. Lipid nanoparticles for delivery of RNA therapeutics: Current status and the role of in vivo imaging. Theranostics 2022, 12, 7509–7531. [Google Scholar] [CrossRef]
- Perez-Garcia, C.G.; Diaz-Trelles, R.; Vega, J.B.; Bao, Y.; Sablad, M.; Limphong, P.; Chikamatsu, S.; Yu, H.; Taylor, W.; Karmali, P.P.; et al. Development of an mRNA replacement therapy for phenylketonuria. Mol. Ther. Nucleic Acids 2022, 28, 87–98. [Google Scholar] [CrossRef]
- Shedlovsky, A.; McDonald, J.D.; Symula, D.; Dove, W.F. Mouse models of human phenylketonuria. Genetics 1993, 134, 1205–1210. [Google Scholar] [CrossRef]
- Singh, K.; Cornell, C.S.; Jackson, R.; Kabiri, M.; Phipps, M.; Desai, M.; Fogle, R.; Ying, X.; Anarat-Cappillino, G.; Geller, S.; et al. CRISPR/Cas9 generated knockout mice lacking phenylalanine hydroxylase protein as a novel preclinical model for human phenylketonuria. Sci. Rep. 2021, 11, 7254. [Google Scholar] [CrossRef]
- Aubi, O.; Prestegård, K.S.; Jung-Kc, K.; Shi, T.-J.S.; Ying, M.; Grindheim, A.K.; Scherer, T.; Ulvik, A.; McCann, A.; Spriet, E.; et al. The Pah-R261Q mouse reveals oxidative stress associated with amyloid-like hepatic aggregation of mutant phenylalanine hydroxylase. Nat. Commun. 2021, 12, 2073. [Google Scholar] [CrossRef]
- Richards, D.Y.; Winn, S.R.; Dudley, S.; Fedorov, L.; Rimann, N.; Thöny, B.; Harding, C.O. A novel Pah-exon1 deleted murine model of phenylalanine hydroxylase (PAH) deficiency. Mol. Genet. Metab. 2020, 131, 306–315. [Google Scholar] [CrossRef]
- Diamond, A.; Ciaramitaro, V.; Donner, E.; Djali, S.; Robinson, M. An animal model of early-treated PKU. J. Neurosci. 1994, 14, 3072–3082. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R.A.; Carlson, D.F.; Allen, K.L.; Webster, D.A.; VanLith, C.J.; Nicolas, C.T.; Hillin, L.G.; Yu, Y.; Kaiser, C.W.; Wahoff, W.R.; et al. Development of a porcine model of phenylketonuria with a humanized R408W mutation for gene editing. PLoS ONE 2021, 16, e0245831. [Google Scholar] [CrossRef] [PubMed]
- Koppes, E.A.; Redel, B.K.; Johnson, M.A.; Skvorak, K.J.; Ghaloul-Gonzalez, L.; Yates, M.E.; Lewis, D.W.; Gollin, S.M.; Wu, Y.L.; Christ, S.E.; et al. A porcine model of phenylketonuria generated by CRISPR/Cas9 genome editing. J. Clin. Investig. 2020, 5, e141523. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. A Phase 1/Phase 2, Open-Label, Dose-Escalation, and Dose Expansion Study to Evaluate the Safety, Tolerability, and Efficacy of SAR444836, an Adeno-Associated Viral Vector-Mediated Gene Transfer of Human Phenylalanine Hydroxylase, in Adult Participants with Phenylketonuria. Available online: https://clinicaltrials.gov/study/NCT05972629 (accessed on 4 September 2025).
- ClinicalTrials.gov. AAV Gene Therapy Clinical Study in Adult Classic PKU (PHEdom). Available online: https://clinicaltrials.gov/study/NCT06332807 (accessed on 4 September 2025).
- ClinicalTrials.gov. A Clinical Study for the Safety and Efficacy of IV Infusion of NGGT002 in the Treatment of Phenylketonuria. Available online: https://www.clinicaltrials.gov/study/NCT06061614 (accessed on 4 September 2025).
- ClinicalTrials.gov. A Phase I/II Study for the Safety and Efficacy of Intravenous Infusion with NGGT002 in Adults Patients with Classic Phenylketonuria. Available online: https://clinicaltrials.gov/study/NCT06687733 (accessed on 4 September 2025).
- ClinicalTrials.gov. A Phase 1/2 Open-Label, Randomized, Concurrently-Controlled, Dose Escalation Study to Evaluate the Safety and Efficacy of HMI-102 in Adult PKU Subjects with PAH Deficiency. Available online: https://www.clinicaltrials.gov/study/NCT03952156 (accessed on 4 September 2025).
- ClinicalTrials.gov. A Phase 1/2 Open-Label, Dose Escalation Study to Determine the Safety and Efficacy of BMN 307, an Adeno-Associated Virus Vector-Mediated Gene Transfer of Human Phenylalanine Hydroxylase in Subjects with Phenylketonuria. Available online: https://clinicaltrials.gov/study/NCT04480567 (accessed on 4 September 2025).
- Nathwani, A.C.; Reiss, U.M.; Tuddenham, E.G.; Rosales, C.; Chowdary, P.; McIntosh, J.; Della Peruta, M.; Lheriteau, E.; Patel, N.; Raj, D.; et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014, 371, 1994–2004. [Google Scholar] [CrossRef]
- Wang, L.; Bell, P.; Somanathan, S.; Wang, Q.; He, Z.; Yu, H.; McMenamin, D.; Goode, T.; Calcedo, R.; Wilson, J.M. Comparative Study of Liver Gene Transfer With AAV Vectors Based on Natural and Engineered AAV Capsids. Mol. Ther. 2015, 23, 1877–1887. [Google Scholar] [CrossRef]
- Villiger, L.; Grisch-Chan, H.M.; Lindsay, H.; Ringnalda, F.; Pogliano, C.B.; Allegri, G.; Fingerhut, R.; Häberle, J.; Matos, J.; Robinson, M.D.; et al. Treatment of a metabolic liver disease by in vivo genome base editing in adult mice. Nat. Med. 2018, 24, 1519–1525. [Google Scholar] [CrossRef]
- Song, C.-Q.; Jiang, T.; Richter, M.; Rhym, L.H.; Koblan, L.W.; Zafra, M.P.; Schatoff, E.M.; Doman, J.L.; Cao, Y.; Dow, L.E.; et al. Adenine base editing in an adult mouse model of tyrosinaemia. Nat. Biomed. Eng. 2020, 4, 125–130. [Google Scholar] [CrossRef]
- Nguyen, G.N.; Everett, J.K.; Kafle, S.; Roche, A.M.; Raymond, H.E.; Leiby, J.; Wood, C.; Assenmacher, C.A.; Merricks, E.P.; Long, C.T.; et al. A long-term study of AAV gene therapy in dogs with hemophilia A identifies clonal expansions of transduced liver cells. Nat. Biotechnol. 2021, 39, 47–55. [Google Scholar] [CrossRef]
- Palanki, R.; Bose, S.K.; Dave, A.; White, B.M.; Berkowitz, C.; Luks, V.; Yaqoob, F.; Han, E.; Swingle, K.L.; Menon, P.; et al. Ionizable Lipid Nanoparticles for Therapeutic Base Editing of Congenital Brain Disease. ACS Nano 2023, 17, 13594–13610. [Google Scholar] [CrossRef]
- Khirallah, J.; Bloomer, H.; Wich, D.; Huang, C.; Workman, J.N.; Li, Y.; Newby, G.A.; Liu, D.R.; Xu, Q. In vivo base editing of Angptl3 via lipid nanoparticles to treat cardiovascular disease. Mol. Ther. Nucleic Acids 2025, 36, 102486. [Google Scholar] [CrossRef] [PubMed]
- Bolsoni, J.; Liu, D.; Mohabatpour, F.; Ebner, R.; Sadhnani, G.; Tafech, B.; Leung, J.; Shanta, S.; An, K.; Morin, T.; et al. Lipid Nanoparticle-Mediated Hit-and-Run Approaches Yield Efficient and Safe In Situ Gene Editing in Human Skin. ACS Nano 2023, 17, 22046–22059. [Google Scholar] [CrossRef]
- Calcedo, R.; Wilson, J.M. Humoral Immune Response to AAV. Front. Immunol. 2013, 43, 341. [Google Scholar] [CrossRef] [PubMed]
- Ronzitti, G.; Gross, D.A.; Mingozzi, F. Human Immune Responses to Adeno-Associated Virus (AAV) Vectors. Front. Immunol. 2020, 11, 670. [Google Scholar] [CrossRef]
- Shirley, J.L.; de Jong, Y.P.; Terhorst, C.; Herzog, R.W. Immune Responses to Viral Gene Therapy Vectors. Mol. Ther. 2020, 28, 709–722. [Google Scholar] [CrossRef]
- Mingozzi, F.; High, K.A. Immune responses to AAV in clinical trials. Curr. Gene Ther. 2011, 11, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.; Hur, J.K.; Been, K.W.; Yoon, S.-H.; Kim, J.-S. Genome-wide analysis reveals specificities of Cpf1 endonucleases in human cells. Nat. Biotechnol. 2016, 34, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Hassett, K.J.; Benenato, K.E.; Jacquinet, E.; Lee, A.; Woods, A.; Yuzhakov, O.; Himansu, S.; Deterling, J.; Geilich, B.M.; Ketova, T.; et al. Optimization of Lipid Nanoparticles for Intramuscular Administration of mRNA Vaccines. Mol. Ther. Nucleic Acids 2019, 15, 1–11. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Paunovska, K.; Sago, C.D.; Monaco, C.M.; Hudson, W.H.; Castro, M.G.; Rudoltz, T.G.; Kalathoor, S.; Vanover, D.A.; Santangelo, P.J.; Ahmed, R.; et al. A Direct Comparison of in Vitro and in Vivo Nucleic Acid Delivery Mediated by Hundreds of Nanoparticles Reveals a Weak Correlation. Nano Lett. 2018, 18, 2148–2157. [Google Scholar] [CrossRef]
- Zong, Y.; Lin, Y.; Wei, T.; Cheng, Q. Lipid Nanoparticle (LNP) Enables mRNA Delivery for Cancer Therapy. Adv. Mater. 2023, 35, e2303261. [Google Scholar] [CrossRef]
- Blau, N. Genetics of Phenylketonuria: Then and Now. Hum. Mutat. 2016, 37, 508–515. [Google Scholar] [CrossRef]
- Bonfim-Freitas, P.E.; Andrade, R.S.; Ribeiro-Dos-Santos, Â.K.; Silva, L.C.S. Molecular characterization of phenylketonuria patients from the North Region of Brazil: State of Pará. Mol. Genet. Genom. Med. 2023, 11, e2224. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jia, H.; Liu, Z.; Tao, J.; Chen, S.; Li, X.; Deng, Y.; Jin, X.; Song, J.; Zhang, L.; et al. Molecular characterisation of phenylketonuria in a Chinese mainland population using next-generation sequencing. Sci. Rep. 2015, 5, 15769. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Dong, Y.; Qi, J.; Yu, W.; Chai, R. Artificial Intelligence-Based Approaches for AAV Vector Engineering. Adv. Sci. 2025, 12, e2411062. [Google Scholar] [CrossRef]
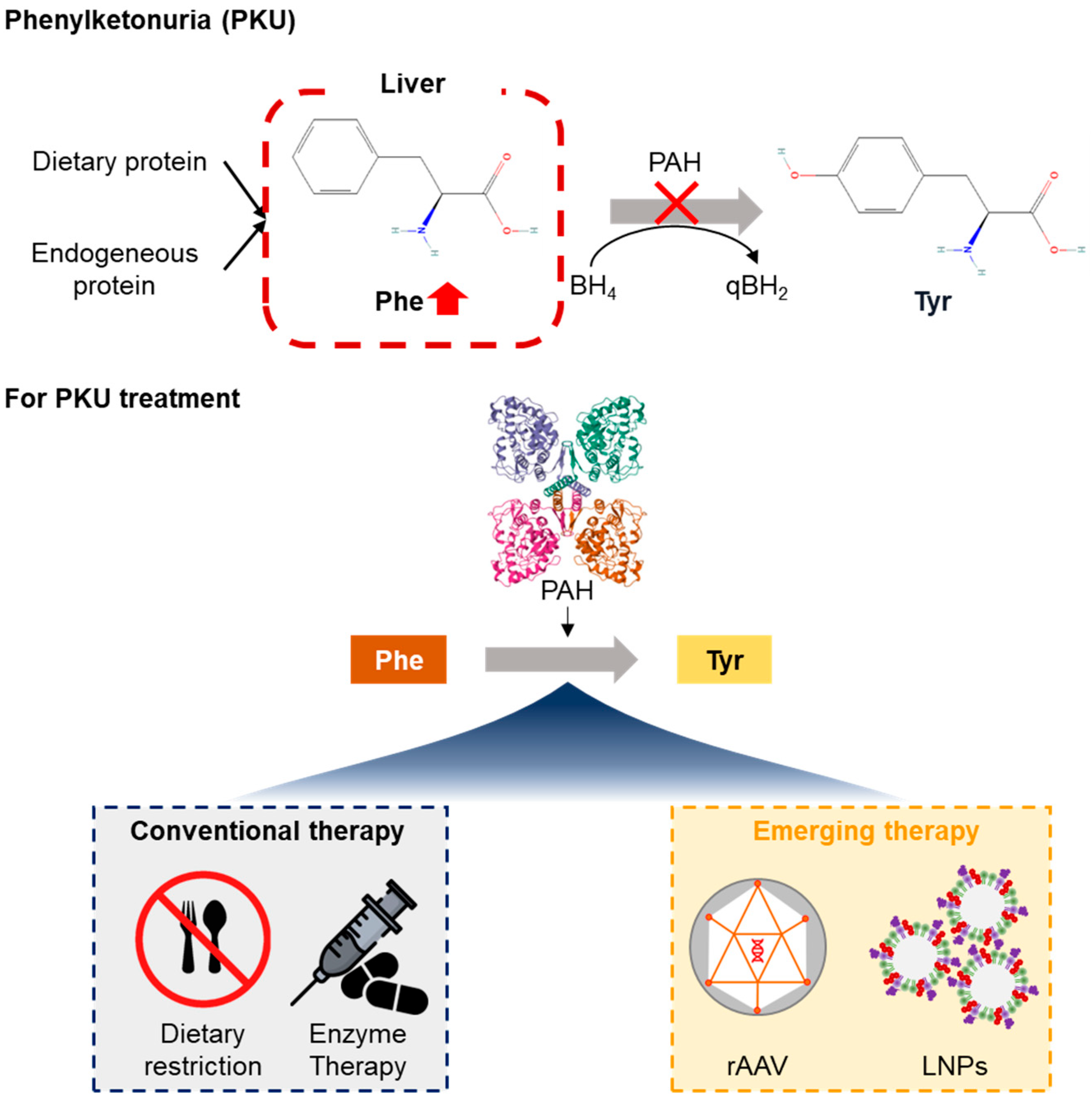
| Therapy | Mechanism | Administration | Durability | Limitations |
|---|---|---|---|---|
| Dietary Restriction | Limit Phe intake | Daily diet | Lifelong | Poor adherence |
| Sapropterin | Cofactor supplementation | Oral tablet | Limited, mutation-specific | Only for mild cases |
| Pegvaliase | Enzyme substitution | Subcutaneous injection | Variable | Immunogenicity |
| AAV Gene Therapy | Gene addition (PAH) | IV infusion (1×) | Potentially long-term | Redosing issues |
| Base Editing (LNP) | Gene correction (PAH) | IV injection (1× or repeated) | Potentially permanent | Still under investigation |
| Mutation Name | Example Genotype(s) | Responsiveness | Notes/Description |
|---|---|---|---|
| R408W | [R408W]; [R408W], [L348V]; [R408W] | Very low/None | Most common nonresponsive mutation; catalytic domain |
| IVS12+1G>A | [IVS12+1G>A]; [IVS12+1G>A] | None | Splicing mutation; almost no residual enzyme activity |
| P281L | [P281L]; [P281L] | None | Catalytic domain; very low enzyme activity |
| Null mutations (e.g., large deletions) | [Null]; [Null] | None | No enzyme activity |
| R158Q | [R158Q]; [R408W] | None | Nonresponsive when combined with R408W |
| L348V | [L348V]; [R408W] | None | Nonresponsive when combined with R408W |
| R261Q | [R261Q]; [R408W] | None | Nonresponsive when combined with R408W |
| Y414C | [Y414C]; [?] | Responsive | Known to be sapropterin-responsive |
| L48S | [L48S]; [?] | Responsive | Known to be sapropterin-responsive |
| Model | Species | Key Mutations | Phenotype | Applications | References |
|---|---|---|---|---|---|
| Germline mutagenesis | Mouse | ENU-induced point mutation in PAH | Hyperphenylalaninemia, reversible with diet | Maternal PKU, gene therapy safety | [52] |
| Pah-KO (C57BL/6) | Mouse | Exon 7 stop codon | Classic PKU symptoms, hypomyelination | Preclinical drug testing | [53] |
| Pah-R261Q | Mouse | R261Q KI | Oxidative stress, protein aggregation | Mutation-specific biomarker discovery | [54] |
| R408W | Mouse | Humanized PAH exon 12 (c.1222C>T; R408W) KI | Elevated blood Phe, hypopigmentation, and reduced body weight | Model for testing gene editing strategies | [14] |
| ΔExon1 | Mouse | CRISPR/Cas9-mediated deletion of exon 1 in PAH | Severe hyperphenylalaninemia, hypopigmentation, reduced serotonin and 5-HIAA in brain, undetectable PAH activity, partial perinatal | Preclinical evaluation of gene therapy; assessment of severe PKU pathophysiology and neurochemical imbalance | [55] |
| Early-treated PKU | Rat | Pharmacological Phe | Cognitive deficits, neurotransmitter loss | Neurodevelopmental studies | [56] |
| Humanized R408W | Pig | TALEN-mediated R408W KI | Human-like PAH dysfunction | Gene editing platform validation | [57] |
| CRISPR/Cas9 PAH-null pig | Pig (domestic sow × Yucatan minipig) | Biallelic exon 6 deletion in PAH via CRISPR/Cas9 injection into zygotes | Severe hyperphenylalaninemia, hypopigmentation, growth retardation, high urinary phenylacetate, responsive to dietary Phe restriction, reduced cortical and cerebellar brain volume, no overt neurological deficits | Preclinical testing of PKU therapeutics, dietary treatment validation, neurocognitive and MRI endpoint assessment, maternal PKU studies | [58] |
| Therapy | Vector /Delivery | Clinical Stage | Patient Population | Administration | Outcomes | Limitations/ Current Status | Reference |
|---|---|---|---|---|---|---|---|
| SAR444836 (Sanofi) | AAV-based (PAH transgene); IV single dose | Phase 1/2 (NCT05972629) | Adults with classic PKU | One-time IV infusion | Safe and well tolerated; transient liver enzyme elevations only | Long-term durability of transgene expression and AAV immunogenicity remain concerns; ongoing 96-week study | [59] |
| NGGT002 | Recombinant AAV8; IV single dose | Phase 1/2 (NCT06687733, NCT06061614, NCT06332807) | Adults 18–55 yrs with classic PKU, severe PAH deficiency | Single IV infusion; dose-escalation cohorts | High-dose cohort: 5/6 patients normalized Phe within 3 weeks; durable response in some up to 40 weeks; designed for severe PAH deficiency | Need to confirm durability in long-term (5 years follow-up); safety monitoring for immune response and vector shedding; pediatric expansion planned | [60,61,62] |
| HMI-102 (Homology Medicines) | AAVHSC15 vector; IV single dose | Phase 1/2 pheNIX trial (terminated) (NCT03952156) | Adults with PKU | Single IV administration | Preclinical: sustained Phe correction, restored Tyr and neurotransmitters; Early trial: clinically meaningful Phe reduction | FDA hold (2022) due to ALT/AST elevations; development halted (company shifted to HMI-103 gene editing) | [63] |
| BMN 307 (BioMarin) | AAV-based; IV single dose | Phase 1/2 (NCT04480567) | Adults with classical PKU | One-time IV infusion | Generally well tolerated; transient liver enzyme increases; aimed at long-term Phe correction | Long-term persistence of effect still under evaluation; ongoing follow-up for efficacy and safety | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, I.; Jeong, J. Advancing Gene Therapy for Phenylketonuria: From Precision Editing to Clinical Translation. Int. J. Mol. Sci. 2025, 26, 8722. https://doi.org/10.3390/ijms26178722
Yu I, Jeong J. Advancing Gene Therapy for Phenylketonuria: From Precision Editing to Clinical Translation. International Journal of Molecular Sciences. 2025; 26(17):8722. https://doi.org/10.3390/ijms26178722
Chicago/Turabian StyleYu, Inseon, and Jaemin Jeong. 2025. "Advancing Gene Therapy for Phenylketonuria: From Precision Editing to Clinical Translation" International Journal of Molecular Sciences 26, no. 17: 8722. https://doi.org/10.3390/ijms26178722
APA StyleYu, I., & Jeong, J. (2025). Advancing Gene Therapy for Phenylketonuria: From Precision Editing to Clinical Translation. International Journal of Molecular Sciences, 26(17), 8722. https://doi.org/10.3390/ijms26178722






