Impact of Exposure Duration to High-Altitude Hypoxia on Oxidative Homeostasis in Rat Brain Regions
Abstract
1. Introduction
2. Results
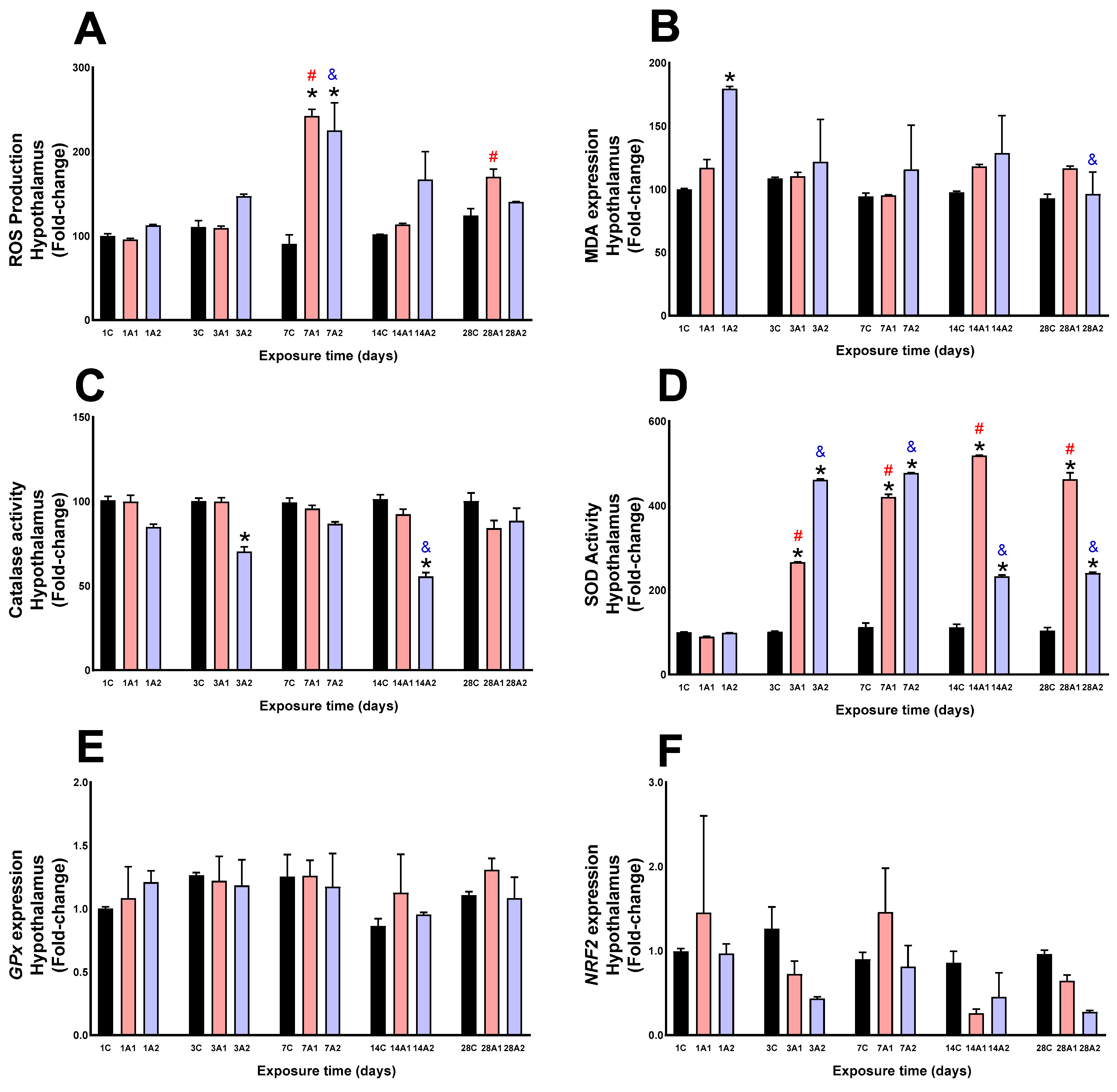
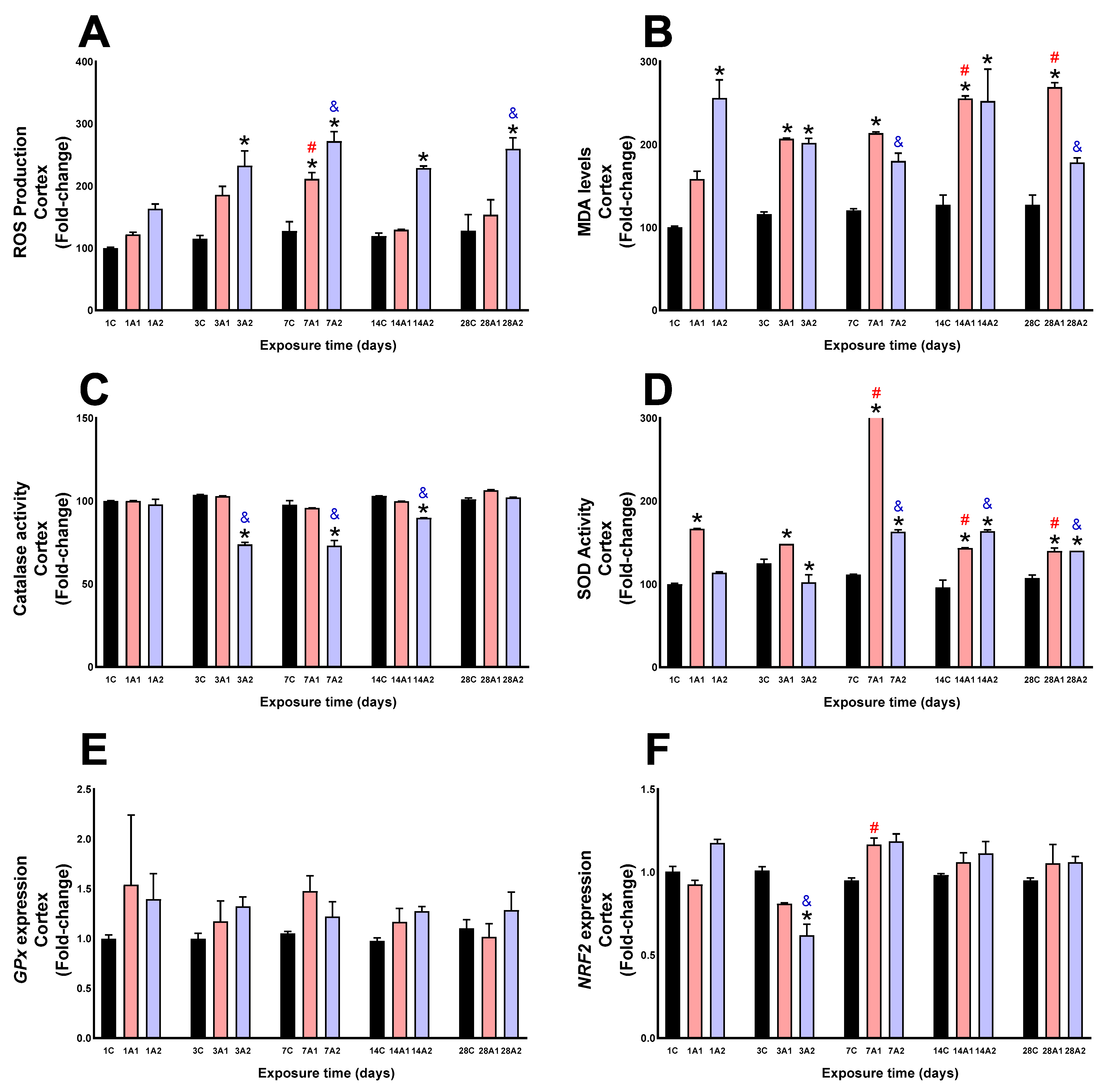
2.1. Antioxidant Function and Oxidative Stress
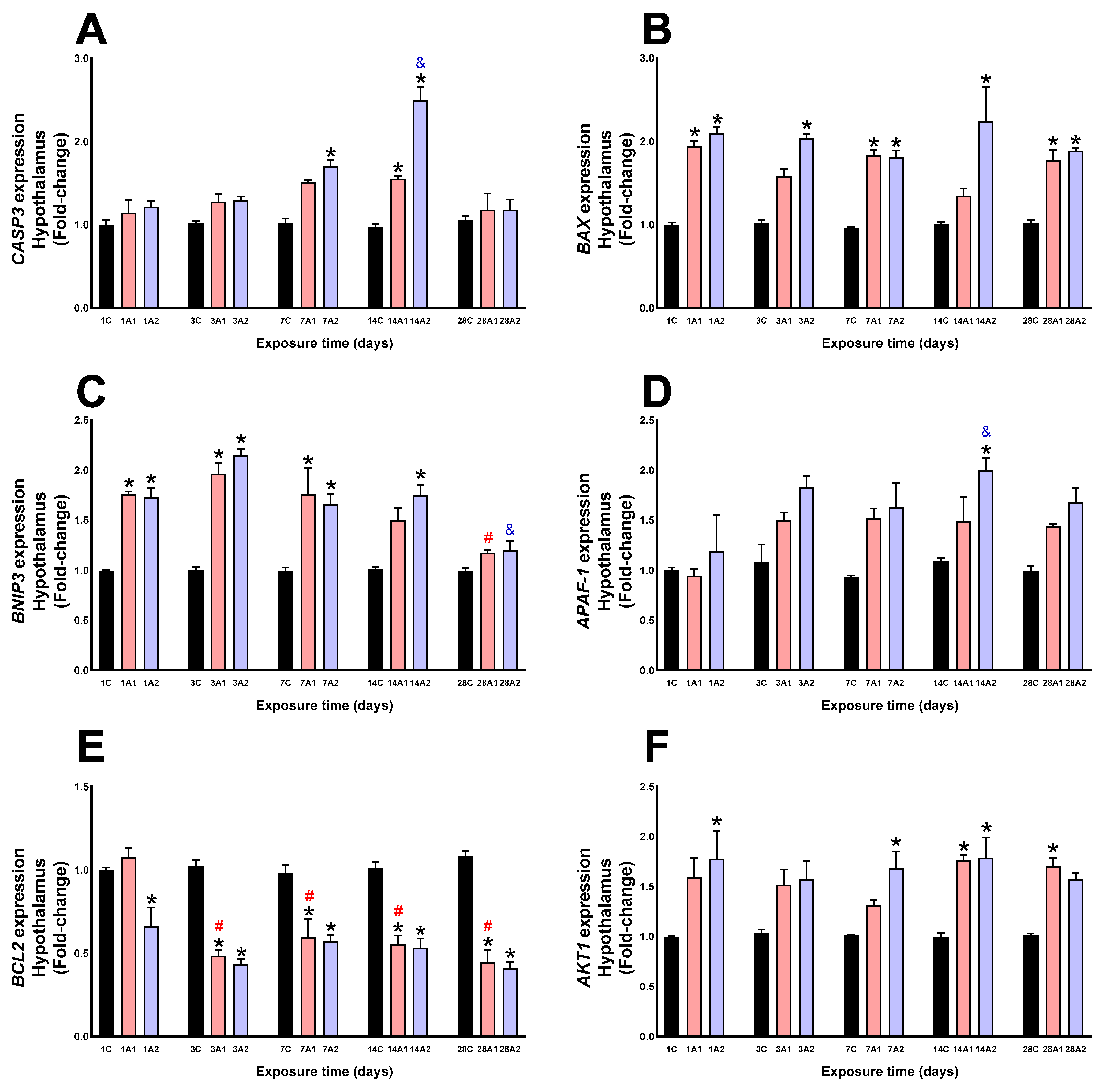
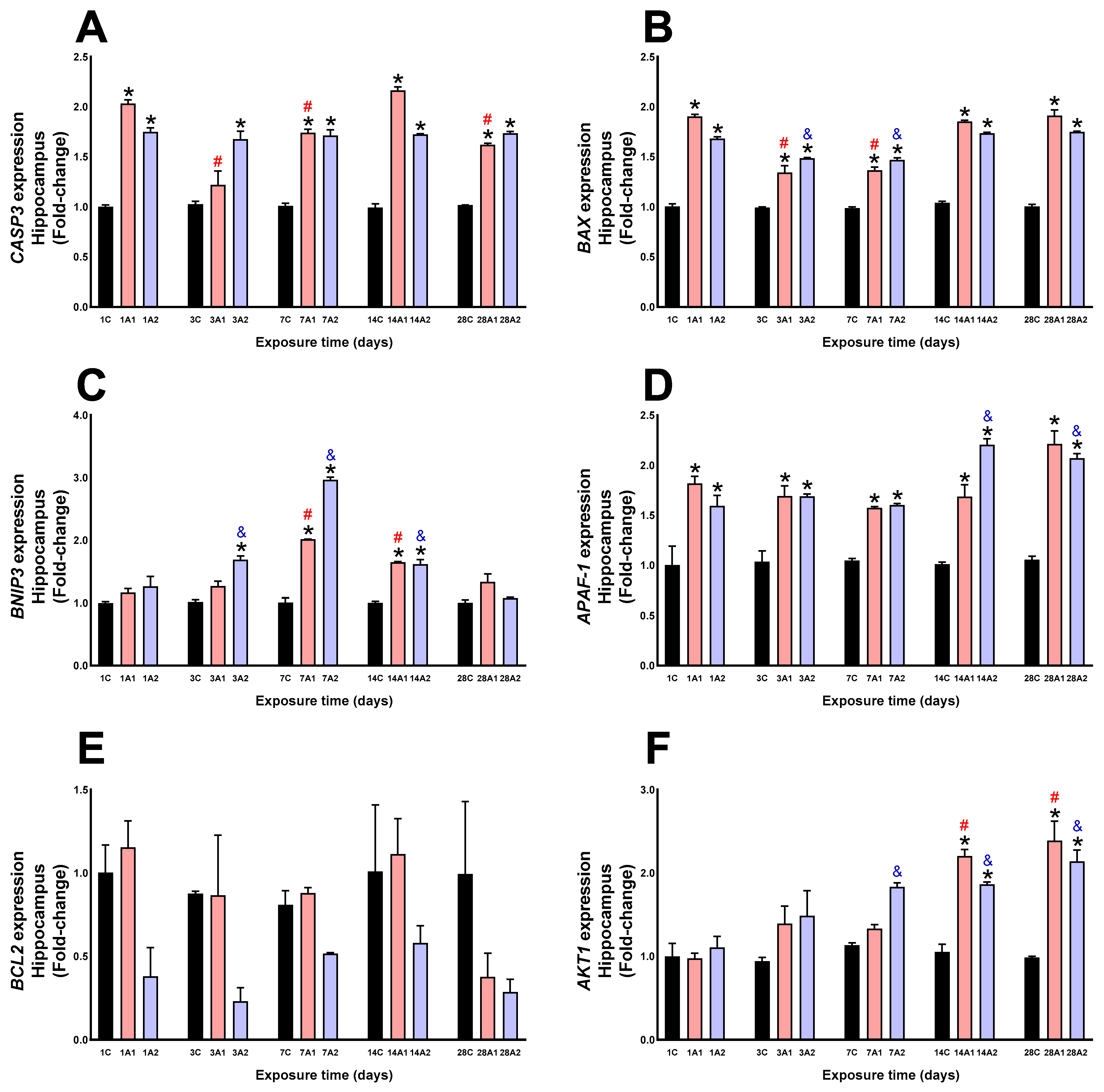
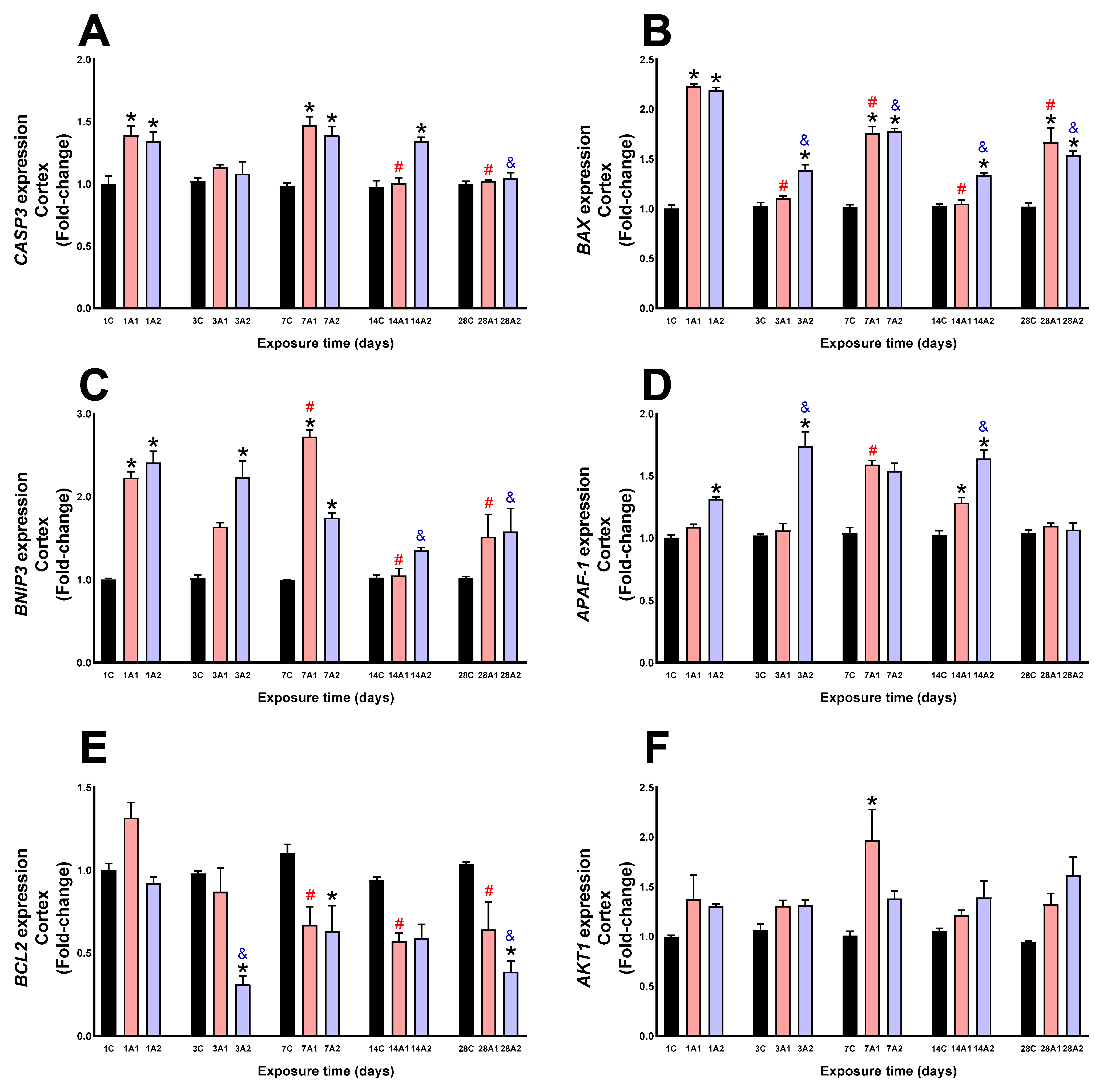
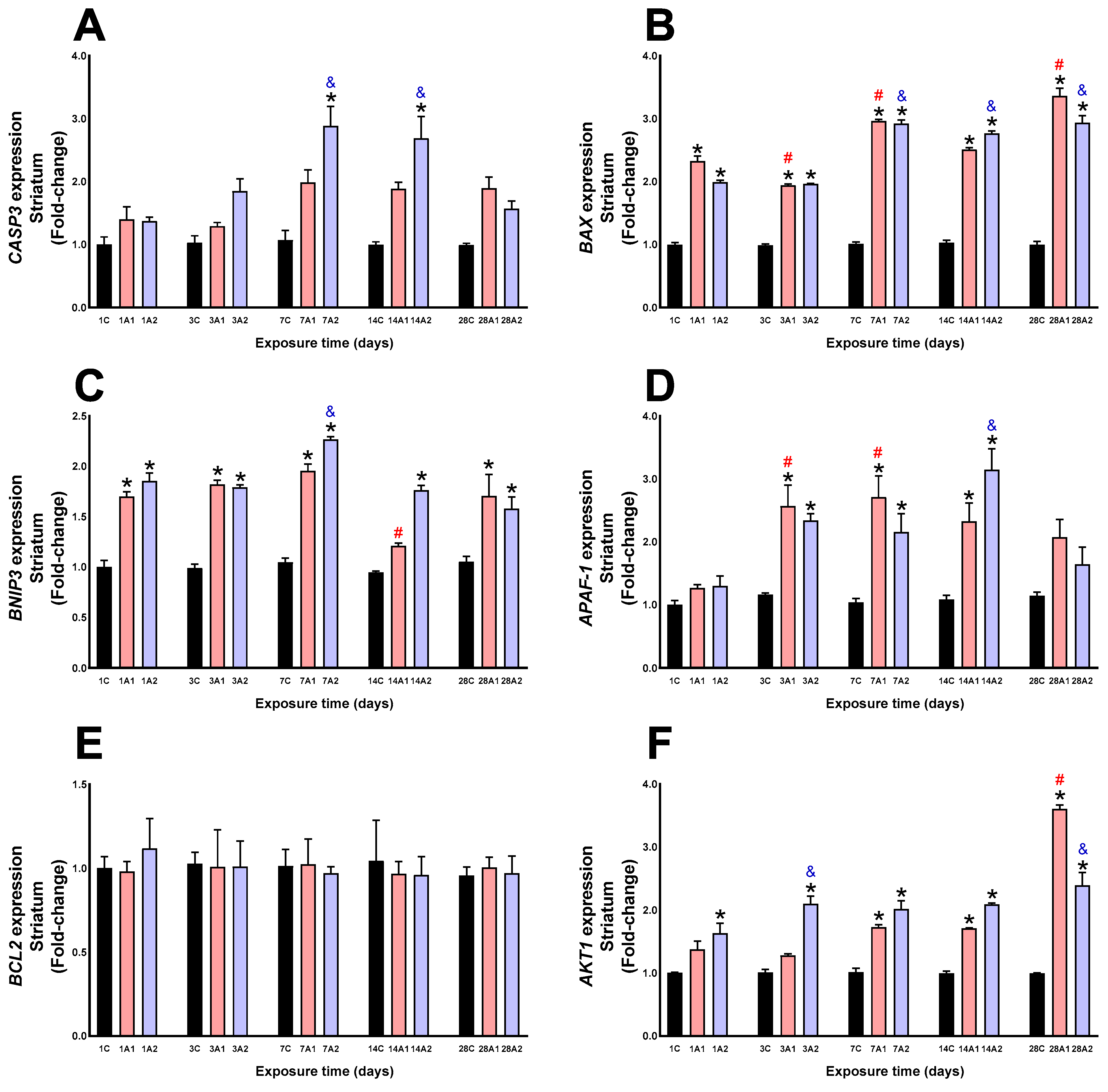
2.2. Survival/Death Cell Pathways
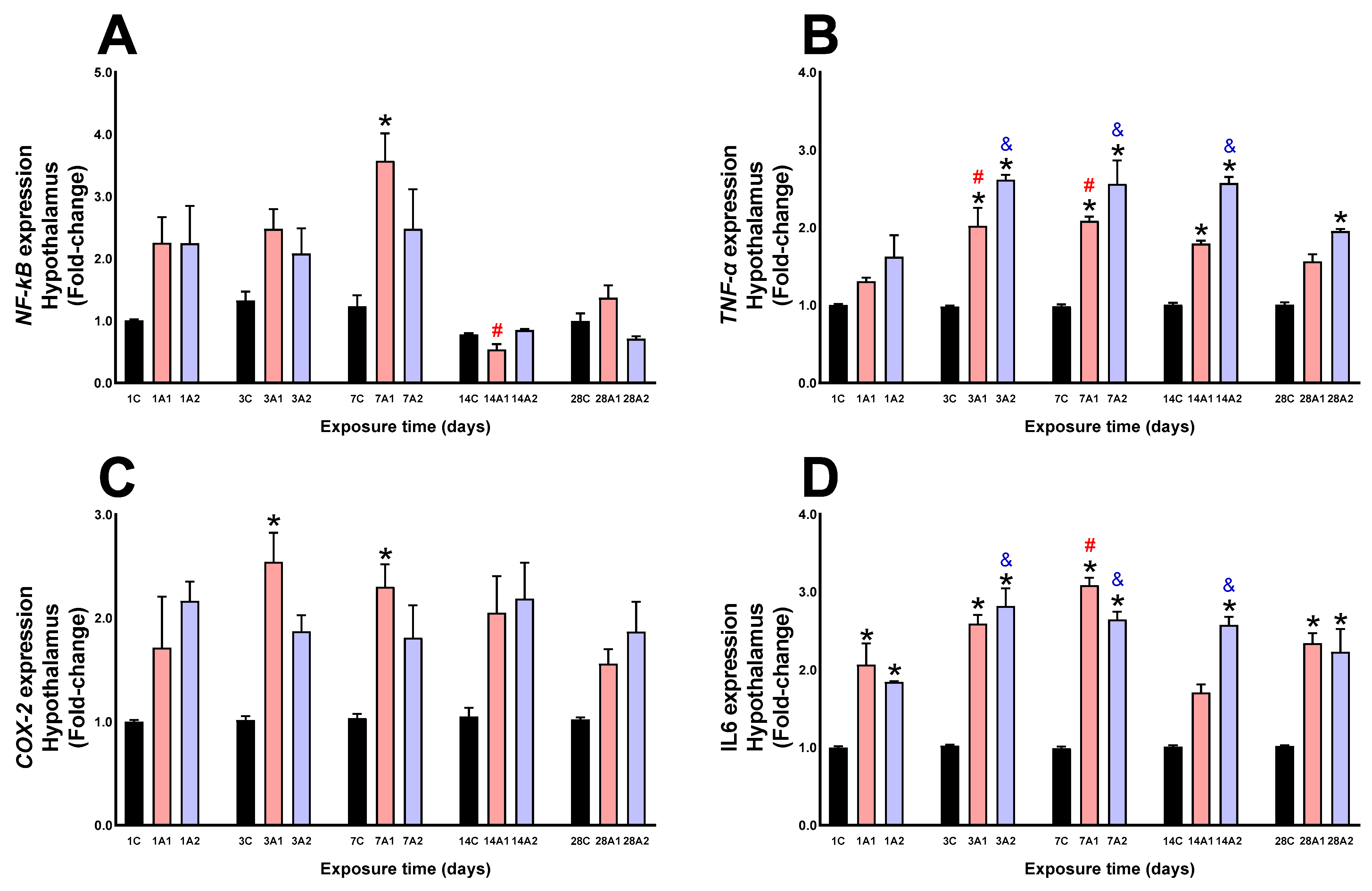
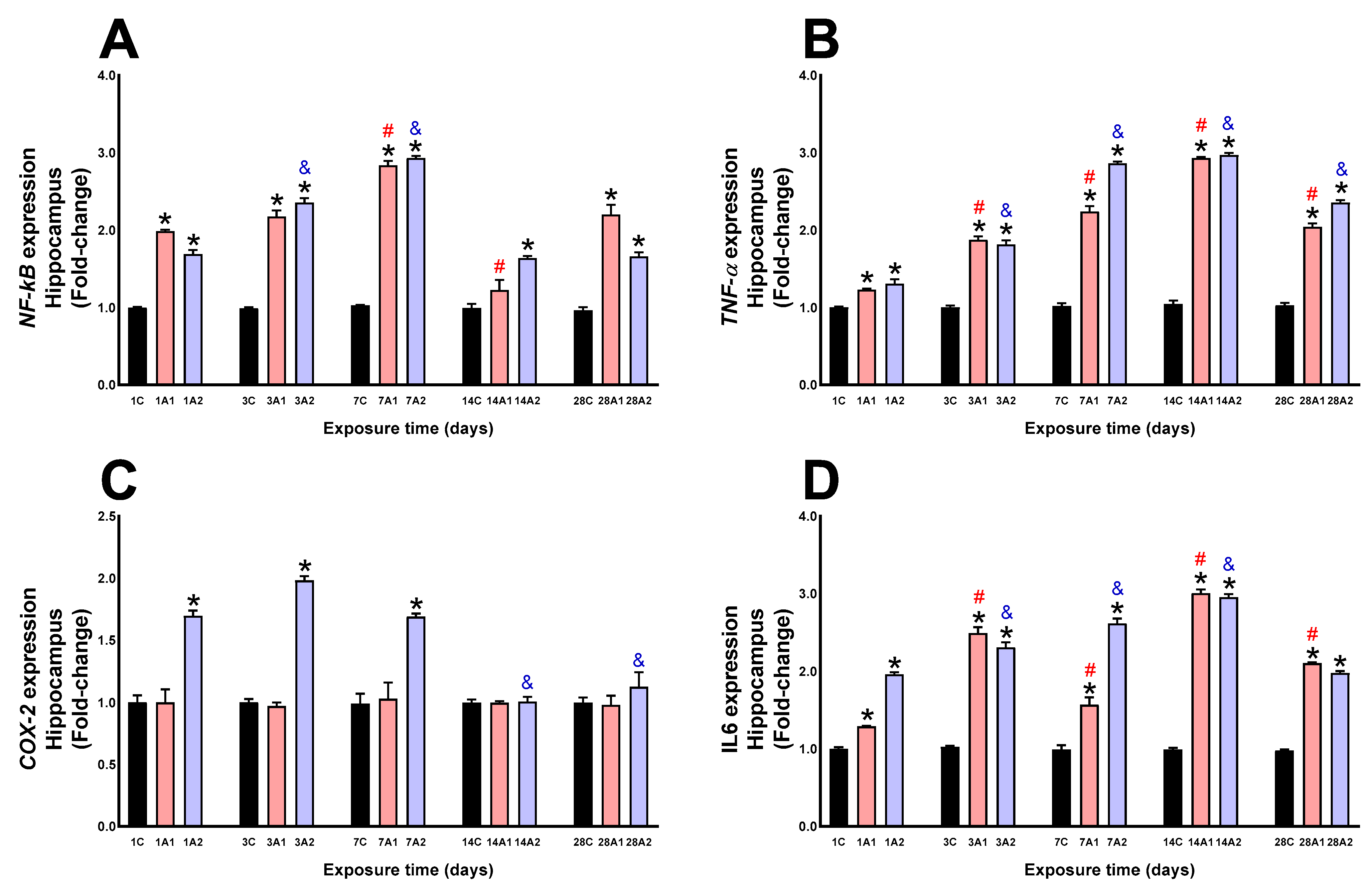
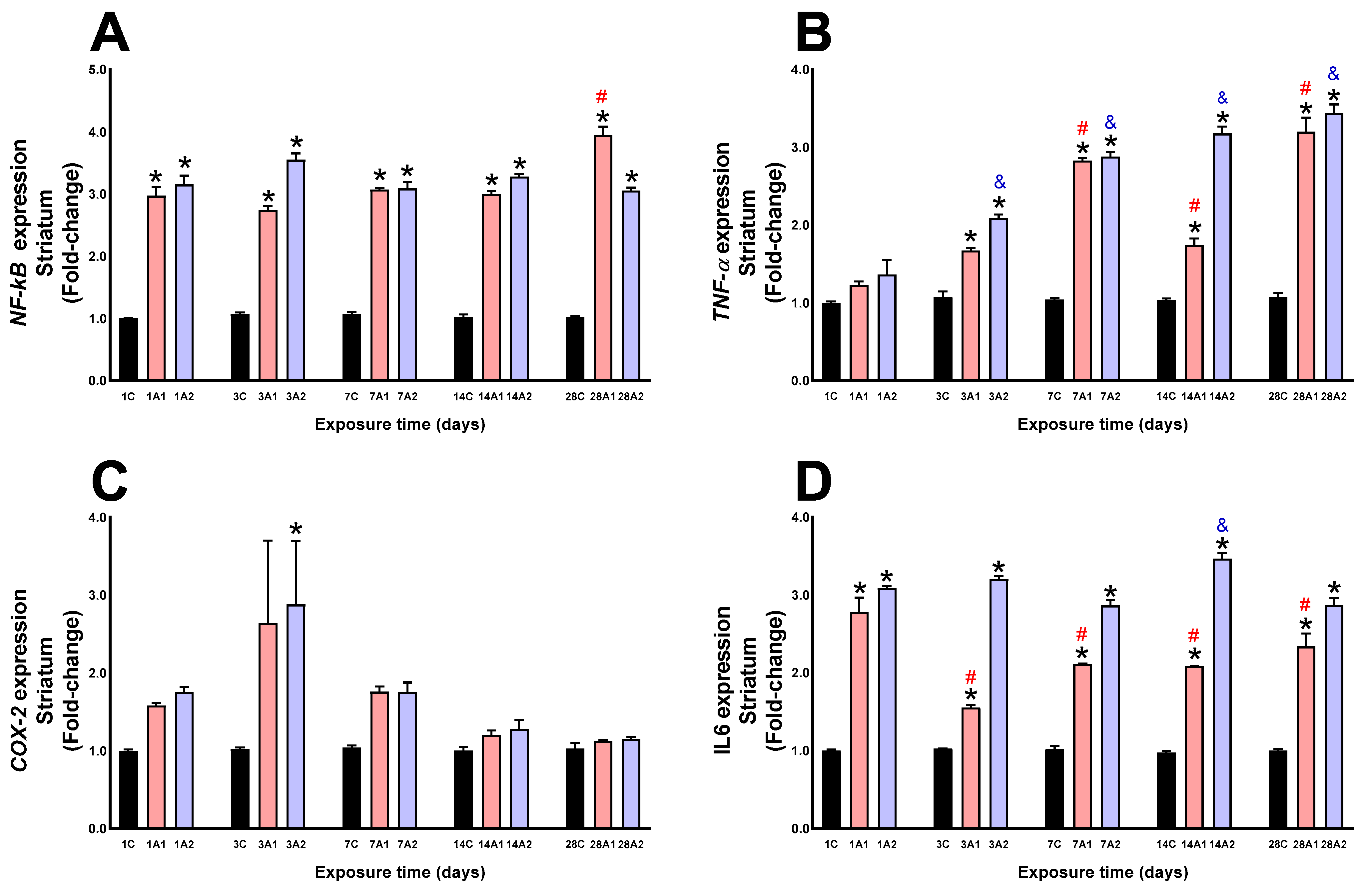
2.3. Neuroinflammation
3. Discussion
4. Materials and Methods
4.1. Chemicals
| Target Gene | Forward Primer | Reverse Primer |
| GAPDH | 5′-TCCCTGTTCTAGAGACAG-3′ | 5′-CCACTTTGTCACAAGAGA-3′ |
| BCL2 | 5′-GTACCTGAACCGGCATCTG-3′ | 5′-GGGGCCATATAGTTCCACAA-3′ |
| CASP3 | 5′-AATTCAAGGGACGGGTCATG-3′ | 5’-GCTTGTGCGCGTACAGTTTC-3′ |
| BAX | 5′-CTGCAGAGGATGATTGCTGA-3′ | 5′-GATCAGCTCGGGCACTTTAG-3′ |
| BNIP3 | 5′-TTTTAAACACCCGAAGCGCA-3′ | 5′-TGAGCAGAAGGCAGATCCAA-3′ |
| APAF1 | 5′-TTCAGGTTTGTAGCTCGGCA-3′ | 5′-ACCCAAGGATCCCAAACGTC-3′ |
| AKT1 | 5′-CACCGCTTCTTTGCCAACAT-3′ | 5′-CACACACTCCATGCTGTCATCT-3′ |
| NRF2 | 5′-TGTAGATGACCATGAGTCGC-3′ | 5′- TGTCCTGCTGTATGCTGCTT-3′ |
| GPx | 5′-TCAGTTCGGACATCAGGAGA-3′ | 5′-GAAGGTAAAGAGCGGGTGAG-3′ |
| COX2 | 5′-CAGGAGAGAAAGAAATGGCTGC-3′ | 5′-TGGTCTCCCCAAAGATAGCATC-3′ |
| TNF-α | 5′-ATCCGAGATGTGGAACTGGC-3′ | 5′-AAATGGCAAATCGGCTGACG-3′ |
| NFκB | 5′-ATATTCACCTGCACGCCCAC-3′ | 5′-GGTTTGCAAAGCCAACCACC-3′ |
4.2. Animals and Sample Collection
- Five control groups (C; 1, 3, 7, 14 and 28 days of exposure) maintained at sea level (170 m, PO2: 159 mmHg, Lima, Peru).
- Five groups exposed to high altitude A1 (3151 m, PO2: 110 mmHg, Ayauca-Yauyos, Peru) for 1, 3, 7, 14, or 28 days.
- Five groups exposed to very high altitude A2 (4214 m, PO2: 96 mmHg, Casapalca-Huarochirí, Peru) for the same time periods (Figure 13).
4.3. Oxidative Stress Assay (ROS Production)
4.4. Malondialdehyde (MDA) Assay
4.5. Catalase Enzyme Activity Assay
4.6. Superoxide Dismutase (SOD) Enzyme Activity Assay
4.7. Molecular Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A1 | exposed to high altitude 3151 m |
| A2 | exposed to high altitude 4214 m |
| ROS | reactive oxygen species |
| MDA | malondialdehyde |
| SOD | superoxide dismutase |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| BCL2 | B-cell lymphoma 2 |
| CASP3 | caspase 3 |
| BAX | BCL2 associated X |
| BNIP3 | BCL2 interacting protein 3 |
| APAF-1 | apoptotic protease activating factor 1 |
| NRF2 | nuclear erythroid 2- related factor 2 |
| GPx | glutathione peroxidase |
| COX2 | cyclooxygenase 2 |
| TNF-α | tumor necrosis factor alpha |
| NF-kB | nuclear factor kappa B |
References
- Mukandala, G.; Tynan, R.; Lanigan, S.; O’Connor, J.J. The Effects of Hypoxia and Inflammation on Synaptic Signaling in the CNS. Brain Sci. 2016, 6, 6. [Google Scholar] [CrossRef]
- Sarkar, S.; Banerjee, P.K.; Selvamurthy, W. High altitude hypoxia: An intricate interplay of oxygen responsive macroevents and micromolecules. Mol. Cell. Biochem. 2003, 253, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, J.; Zhang, X.; Jin, Q.; Zheng, X.; Mo, L.; Da, Z. Oxygen metabolism abnormalities and high-altitude cerebral edema. Front. Immunol. 2025, 16, 1555910. [Google Scholar] [CrossRef]
- Zhang, Z.A.; Sun, Y.; Yuan, Z.; Wang, L.; Dong, Q.; Zhou, Y.; Zheng, G.; Aschner, M.; Zou, Y.; Luo, W. Insight into the Effects of High-Altitude Hypoxic Exposure on Learning and Memory. Oxidative Med. Cell. Longev. 2022, 2022, 4163188. [Google Scholar] [CrossRef]
- Pena, E.; El Alam, S.; Siques, P.; Brito, J. Oxidative Stress and Diseases Associated with High-Altitude Exposure. Antioxidants 2022, 11, 267. [Google Scholar] [CrossRef]
- Maiti, P.; Singh, S.B.; Sharma, A.K.; Muthuraju, S.; Banerjee, P.K.; Ilavazhagan, G. Hypobaric hypoxia induces oxidative stress in rat brain. Neurochem. Int. 2006, 49, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Coimbra-Costa, D.; Alva, N.; Duran, M.; Carbonell, T.; Rama, R. Oxidative stress and apoptosis after acute respiratory hypoxia and reoxygenation in rat brain. Redox Biol. 2017, 12, 216–225. [Google Scholar] [CrossRef]
- Ionescu, C.; Ghidersa, M.; Ciobica, A.; Mavroudis, I.; Kazis, D.; Petridis, F.E.; Gorgan, D.L.; Balmus, I.M. Potential Correlation Between Molecular Biomarkers and Oxidative Stress in Traumatic Brain Injury. Int. J. Mol. Sci. 2025, 26, 3858. [Google Scholar] [CrossRef]
- Banasiak, K.J.; Xia, Y.; Haddad, G.G. Mechanisms underlying hypoxia-induced neuronal apoptosis. Prog. Neurobiol. 2000, 62, 215–249. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Jiang, X.; Wang, Y.; Li, C.; Lin, Z.; Wei, Y.; Ni, Q. Cerebral Hypoxia-Induced Molecular Alterations and Their Impact on the Physiology of Neurons and Dendritic Spines: A Comprehensive Review. Cell. Mol. Neurobiol. 2024, 44, 58. [Google Scholar] [CrossRef]
- Basheeruddin, M.; Qausain, S. Hypoxia-Inducible Factor 1-Alpha (HIF-1α): An Essential Regulator in Cellular Metabolic Control. Cureus 2024, 16, e63852. [Google Scholar] [CrossRef] [PubMed]
- Puchowicz, M.A.; Parveen, K.; Sethuraman, A.; Ishrat, T.; Xu, K.; LaManna, J. Pro-survival Phenotype of HIF-1α: Neuroprotection Through Inflammatory Mechanisms. Adv. Exp. Med. Biol. 2023, 1438, 33–36. [Google Scholar] [CrossRef]
- Ostrowski, R.P.; Zhang, J.H. The insights into molecular pathways of hypoxia-inducible factor in the brain. J. Neurosci. Res. 2020, 98, 57–76. [Google Scholar] [CrossRef]
- Guo, L.; Zhu, L. Multiple Roles of Peripheral Immune System in Modulating Ischemia/Hypoxia-Induced Neuroinflammation. Front. Mol. Biosci. 2021, 8, 752465. [Google Scholar] [CrossRef]
- Muraoka, K.; Shimizu, K.; Sun, X.; Zhang, Y.K.; Tani, T.; Hashimoto, T.; Yagi, M.; Miyazaki, I.; Yamamoto, K. Hypoxia, but not reoxygenation, induces interleukin 6 gene expression through NF-kappa B activation. Transplantation 1997, 63, 466–470. [Google Scholar] [CrossRef]
- Fitzpatrick, S.F.; Tambuwala, M.M.; Bruning, U.; Schaible, B.; Scholz, C.C.; Byrne, A.; O’Connor, A.; Gallagher, W.M.; Lenihan, C.R.; Garvey, J.F.; et al. An intact canonical NF-κB pathway is required for inflammatory gene expression in response to hypoxia. J. Immunol. 2011, 186, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Schmedtje, J.F.; Ji, Y.S.; Liu, W.L.; DuBois, R.N.; Runge, M.S. Hypoxia induces cyclooxygenase-2 via the NF-kappaB p65 transcription factor in human vascular endothelial cells. J. Biol. Chem. 1997, 272, 601–608. [Google Scholar] [CrossRef]
- Gross, A.; McDonnell, J.M.; Korsmeyer, S.J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999, 13, 1899–1911. [Google Scholar] [CrossRef]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ney, P.A. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 2009, 16, 939–946. [Google Scholar] [CrossRef]
- Aboouf, M.A.; Thiersch, M.; Soliz, J.; Gassmann, M.; Schneider Gasser, E.M. The Brain at High Altitude: From Molecular Signaling to Cognitive Performance. Int. J. Mol. Sci. 2023, 24, 10179. [Google Scholar] [CrossRef] [PubMed]
- Carreau, A.; El Hafny-Rahbi, B.; Matejuk, A.; Grillon, C.; Kieda, C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell. Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, Y.; Zhao, T.; Wu, L.; Huang, X.; Wu, K.; Xu, L.; Li, D.; Liu, S.; Zhao, Y.; et al. Reduced Cerebral Oxygen Content in the DG and SVZ In Situ Promotes Neurogenesis in the Adult Rat Brain In Vivo. PLoS ONE 2015, 10, e0140035. [Google Scholar] [CrossRef] [PubMed]
- Hultgren, H.N. High Altitude Medicine; Hultgren Publications: Stanford, CA, USA, 1997. [Google Scholar]
- Garrido, E.; Segura, R.; Capdevila, A.; Pujol, J.; Javierre, C.; Ventura, J.L. Are Himalayan Sherpas better protected against brain damage associated with extreme altitude climbs? Clin. Sci. 1996, 90, 81–85. [Google Scholar] [CrossRef]
- Kottke, R.; Pichler Hefti, J.; Rummel, C.; Hauf, M.; Hefti, U.; Merz, T.M. Morphological Brain Changes after Climbing to Extreme Altitudes—A Prospective Cohort Study. PLoS ONE 2015, 10, e0141097. [Google Scholar] [CrossRef][Green Version]
- An, X.; Tao, G.; Zhang, X.; Ma, H.; Wang, Y. Attention Network Changes of High-Altitude Migrants. Aerosp. Med. Hum. Perform. 2022, 93, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, R.; Matsuzawa, Y.; Kubo, K.; Kobayashi, T. Effects of simulated high altitude on event-related potential (P300) and auditory brain-stem responses. Clin. Neurophysiol. 2005, 116, 1471–1476. [Google Scholar] [CrossRef]
- Schönenberger, M.J.; Kovacs, W.J. Hypoxia signaling pathways: Modulators of oxygen-related organelles. Front. Cell Dev. Biol. 2015, 3, 42. [Google Scholar] [CrossRef]
- Turrens, J.F. Superoxide production by the mitochondrial respiratory chain. Biosci. Rep. 1997, 17, 3–8. [Google Scholar] [CrossRef]
- Genova, M.L.; Ventura, B.; Giuliano, G.; Bovina, C.; Formiggini, G.; Parenti Castelli, G.; Lenaz, G. The site of production of superoxide radical in mitochondrial Complex I is not a bound ubisemiquinone but presumably iron-sulfur cluster N2. FEBS Lett. 2001, 505, 364–368. [Google Scholar] [CrossRef]
- Alvarez, S.; Valdez, L.B.; Zaobornyj, T.; Boveris, A. Oxygen dependence of mitochondrial nitric oxide synthase activity. Biochem. Biophys. Res. Commun. 2003, 305, 771–775. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S.; Maltepe, E.; Goldwasser, E.; Mathieu, C.E.; Simon, M.C.; Schumacker, P.T. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. USA 1998, 95, 11715–11720. [Google Scholar] [CrossRef] [PubMed]
- Waypa, G.B.; Schumacker, P.T. O(2) sensing in hypoxic pulmonary vasoconstriction: The mitochondrial door re-opens. Respir. Physiol. Neurobiol. 2002, 132, 81–91. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Storey, K.B. Oxidative stress concept updated: Definitions, classifications, and regulatory pathways implicated. EXCLI J. 2021, 20, 956–967. [Google Scholar] [CrossRef]
- Leveque, C.; Mrakic Sposta, S.; Theunissen, S.; Germonpré, P.; Lambrechts, K.; Vezzoli, A.; Gussoni, M.; Levenez, M.; Lafère, P.; Guerrero, F.; et al. Oxidative Stress Response Kinetics after 60 Minutes at Different Levels (10% or 15%) of Normobaric Hypoxia Exposure. Int. J. Mol. Sci. 2023, 24, 10188. [Google Scholar] [CrossRef] [PubMed]
- Guzy, R.D.; Schumacker, P.T. Oxygen sensing by mitochondria at complex III: The paradox of increased reactive oxygen species during hypoxia. Exp. Physiol. 2006, 91, 807–819. [Google Scholar] [CrossRef]
- Chakraborty, S.; Roychoudhury, S. Pathological Roles of Reactive Oxygen Species in Male Reproduction. Adv. Exp. Med. Biol. 2022, 1358, 41–62. [Google Scholar] [CrossRef]
- Himadri, P.; Kumari, S.S.; Chitharanjan, M.; Dhananjay, S. Role of oxidative stress and inflammation in hypoxia-induced cerebral edema: A molecular approach. High Alt. Med. Biol. 2010, 11, 231–244. [Google Scholar] [CrossRef]
- Agrawal, A.; Rathor, R.; Suryakumar, G. Oxidative protein modification alters proteostasis under acute hypobaric hypoxia in skeletal muscles: A comprehensive in vivo study. Cell Stress Chaperones 2017, 22, 429–443. [Google Scholar] [CrossRef]
- Wang, Y.P.; Yang, Y.Z.; Ma, L.; Zhao, Y.X.; Ge, R.L. Effect of different altitudes on telomere length of rat peripheral blood leukocyte. Sheng Li Xue Bao Acta Physiol. Sin. 2013, 65, 540–546. [Google Scholar]
- Esteva, S.; Pedret, R.; Fort, N.; Torrella, J.R.; Pagès, T.; Viscor, G. Oxidative stress status in rats after intermittent exposure to hypobaric hypoxia. Wilderness Environ. Med. 2010, 21, 325–331. [Google Scholar] [CrossRef]
- Aguilar, M.; González-Candia, A.; Rodríguez, J.; Carrasco-Pozo, C.; Cañas, D.; García-Herrera, C.; Herrera, E.A.; Castillo, R.L. Mechanisms of Cardiovascular Protection Associated with Intermittent Hypobaric Hypoxia Exposure in a Rat Model: Role of Oxidative Stress. Int. J. Mol. Sci. 2018, 19, 366. [Google Scholar] [CrossRef]
- Pu, X.; Lin, X.; Duan, X.; Wang, J.; Shang, J.; Yun, H.; Chen, Z. Oxidative and Endoplasmic Reticulum Stress Responses to Chronic High-Altitude Exposure During the Development of High-Altitude Pulmonary Hypertension. High Alt. Med. Biol. 2020, 21, 378–387. [Google Scholar] [CrossRef]
- Purushothaman, J.; Suryakumar, G.; Shukla, D.; Jayamurthy, H.; Kasiganesan, H.; Kumar, R.; Sawhney, R.C. Modulation of Hypoxia-Induced Pulmonary Vascular Leakage in Rats by Seabuckthorn (Hippophae rhamnoides L.). Evid.-Based Complement. Altern. Med. 2011, 2011, 574524. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, J.; Suryakumar, G.; Shukla, D.; Malhotra, A.S.; Kasiganesan, H.; Kumar, R.; Sawhney, R.C.; Chami, A. Modulatory effects of seabuckthorn (Hippophae rhamnoides L.) in hypobaric hypoxia induced cerebral vascular injury. Brain Res. Bull. 2008, 77, 246–252. [Google Scholar] [CrossRef]
- Gong, G.; Yin, L.; Yuan, L.; Sui, D.; Sun, Y.; Fu, H.; Chen, L.; Wang, X. Ganglioside GM1 protects against high altitude cerebral edema in rats by suppressing the oxidative stress and inflammatory response via the PI3K/AKT-Nrf2 pathway. Mol. Immunol. 2018, 95, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Hota, S.K.; Barhwal, K.; Singh, S.B.; Ilavazhagan, G. Chronic hypobaric hypoxia induced apoptosis in CA1 region of hippocampus: A possible role of NMDAR mediated p75NTR upregulation. Exp. Neurol. 2008, 212, 5–13. [Google Scholar] [CrossRef]
- Yin, J.; Wang, Y.; Li, B.; Hu, X.; Ma, Y.; Zhang, C.; Ha, X.; Wang, L.; Pan, Y. Hypobaric Hypoxia Increased Autophagy and Apoptosis in PC12 Rat Pheochromocytoma Cells More Than Normobaric Hypoxia. High Alt. Med. Biol. 2025, 26, 301–307. [Google Scholar] [CrossRef]
- Huan, Y.; Quan, H.; Jia, B.; Hao, G.; Shi, Z.; Zhao, T.; Yuan, Y.; Yuan, F.; Dong, Y.; Liang, G. High-altitude cerebral hypoxia promotes mitochondrial dysfunction and apoptosis of mouse neurons. Front. Mol. Neurosci. 2023, 16, 1216947. [Google Scholar] [CrossRef]
- Jing, L.; Da, Q.; Zhang, S.; Zhang, J.; Ma, H.; Luo, H. Nitronyl Nitroxide Ameliorates Hypobaric Hypoxia-Induced Cognitive Impairment in Mice by Suppressing the Oxidative Stress, Inflammatory Response and Apoptosis. Neurochem. Res. 2024, 49, 785–799. [Google Scholar] [CrossRef] [PubMed]
- Shushanyan, R.; Grigoryan, A.; Abgaryan, T.; Karapetyan, A. Histological and cytochemical analysis of the brain under conditions of hypobaric hypoxia-induced oxygen deficiency in albino rats. Acta Histochem. 2023, 125, 152114. [Google Scholar] [CrossRef]
- Cui, J.; Ma, Q.; Zhang, C.; Li, Y.; Liu, J.; Xie, K.; Luo, E.; Zhai, M.; Tang, C. Keratin 18 Depletion as a Possible Mechanism for the Induction of Apoptosis and Ferroptosis in the Rat Hippocampus After Hypobaric Hypoxia. Neuroscience 2023, 513, 64–75. [Google Scholar] [CrossRef]
- Shi, H.; Li, P.; Zhou, H.; Nie, Z.; Zhang, J.; Sui, X.; Guo, J.; Wang, Y.; Wang, L. Dynamic cerebral blood flow changes with FOXOs stimulation are involved in neuronal damage associated with high-altitude cerebral edema in mice. Brain Res. 2022, 1790, 147987. [Google Scholar] [CrossRef]
- Guo, K.; Searfoss, G.; Krolikowski, D.; Pagnoni, M.; Franks, C.; Clark, K.; Yu, K.T.; Jaye, M.; Ivashchenko, Y. Hypoxia induces the expression of the pro-apoptotic gene BNIP3. Cell Death Differ. 2001, 8, 367–376. [Google Scholar] [CrossRef]
- Snyder, C.M.; Chandel, N.S. Mitochondrial regulation of cell survival and death during low-oxygen conditions. Antioxid. Redox Signal. 2009, 11, 2673–2683. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Liu, Z.; Mei, Y.; Liu, Y.; Wei, X.; Xiao, C.; Gao, Y.; Ma, Z. Time-course effects and mechanisms of hypobaric hypoxia on nervous system in mice. Neurosci. Lett. 2023, 801, 137163. [Google Scholar] [CrossRef]
- Jiang, S.; Fan, F.; Yang, L.; Chen, K.; Sun, Z.; Zhang, Y.; Cairang, N.; Wang, X.; Meng, X. Salidroside attenuates high altitude hypobaric hypoxia-induced brain injury in mice via inhibiting NF-κB/NLRP3 pathway. Eur. J. Pharmacol. 2022, 925, 175015. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ma, S.; Yang, J.; Luo, K.; Qian, Q.; Pan, J.; Liang, K.; Wang, Y.; Gao, Y.; Li, M. Sodium Hydrosulfide Protects Rats from Hypobaric-Hypoxia-Induced Acute Lung Injury. Int. J. Mol. Sci. 2024, 25, 10734. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, J.; Ma, Y.; Dong, X.; Qu, J.; Liang, F.; Liu, J. Curcumin-mediated enhancement of lung barrier function in rats with high-altitude-associated acute lung injury via inhibition of inflammatory response. Respir. Res. 2024, 25, 354. [Google Scholar] [CrossRef]
- Liu, J.; Pei, C.; Jia, N.; Han, Y.; Zhao, S.; Shen, Z.; Huang, D.; Chen, Q.; Wu, Y.; Shi, S.; et al. Preconditioning with Ginsenoside Rg3 mitigates cardiac injury induced by high-altitude hypobaric hypoxia exposure in mice by suppressing ferroptosis through inhibition of the RhoA/ROCK signaling pathway. J. Ethnopharmacol. 2025, 337 Pt 2, 118861. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, J.; Ma, H.; Jing, L. Network pharmacology and in vivo experimental studies reveal the protective effects of 6-hydroxygenistein against hypobaric hypoxia-induced brain injury. Heliyon 2024, 10, e36241. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Allan, S.M.; Rothwell, N.J. Cytokines and acute neurodegeneration. Nat. Rev. Neurosci. 2001, 2, 734–744. [Google Scholar] [CrossRef]
- Nakayama, M.; Uchimura, K.; Zhu, R.L.; Nagayama, T.; Rose, M.E.; Stetler, R.A.; Isakson, P.C.; Chen, J.; Graham, S.H. Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc. Natl. Acad. Sci. USA 1998, 95, 10954–10959. [Google Scholar] [CrossRef] [PubMed]
- McMorris, T.; Hale, B.J.; Barwood, M.; Costello, J.; Corbett, J. Effect of acute hypoxia on cognition: A systematic review and meta-regression analysis. Neurosci. Biobehav. Rev. 2017, 74, 225–232, Erratum in Neurosci. Biobehav. Rev. 2019, 98, 333. https://doi.org/10.1016/j.neubiorev.2019.01.017. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Lin, Y.; Li, Y.; Wang, H.; Wang, Z.; Liu, H.; Hu, Y.; Liu, L. Mechanism, prevention and treatment of cognitive impairment caused by high altitude exposure. Front. Physiol. 2023, 14, 1191058. [Google Scholar] [CrossRef]
- Lu, G.; Rili, G.; Shuang, M. Impact of hypoxia on the hippocampus: A review. Medicine 2025, 104, e41479. [Google Scholar] [CrossRef]
- Virués-Ortega, J.; Buela-Casal, G.; Garrido, E.; Alcázar, B. Neuropsychological functioning associated with high-altitude exposure. Neuropsychol. Rev. 2004, 14, 197–224. [Google Scholar] [CrossRef]
- Glowinski, J.; Iversen, L.L. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J. Neurochem. 1966, 13, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Lebel, C.P.; Bondy, S.C. Sensitive and rapid quantitation of oxygen reactive species formation in rat synaptosomes. Neurochem. Int. 1990, 17, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Klein, K. (Eds.) Chapter 2—Methods for Measuring Oxidative Stress in the Laboratory. In Antioxidants in Food, Vitamins and Supplements; Elsevier: Amsterdam, The Netherlands, 2014; pp. 19–40. ISBN 9780124058729. [Google Scholar] [CrossRef]
- Barrios-Arpi, L.; Arias, Y.; Lopez-Torres, B.; Ramos-Gonzalez, M.; Ticli, G.; Prosperi, E.; Rodríguez, J.L. In Vitro Neurotoxicity of Flumethrin Pyrethroid on SH-SY5Y Neuroblastoma Cells: Apoptosis Associated with Oxidative Stress. Toxics 2022, 10, 131. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lira-Mejía, B.; Calderon-Romero, R.; Ordaya-Fierro, J.; Medina, C.; Rodríguez, J.-L.; Romero, A.; Dávila, R.; Ramos-Gonzalez, M. Impact of Exposure Duration to High-Altitude Hypoxia on Oxidative Homeostasis in Rat Brain Regions. Int. J. Mol. Sci. 2025, 26, 8714. https://doi.org/10.3390/ijms26178714
Lira-Mejía B, Calderon-Romero R, Ordaya-Fierro J, Medina C, Rodríguez J-L, Romero A, Dávila R, Ramos-Gonzalez M. Impact of Exposure Duration to High-Altitude Hypoxia on Oxidative Homeostasis in Rat Brain Regions. International Journal of Molecular Sciences. 2025; 26(17):8714. https://doi.org/10.3390/ijms26178714
Chicago/Turabian StyleLira-Mejía, Boris, Roger Calderon-Romero, Jorge Ordaya-Fierro, Cristian Medina, José-Luis Rodríguez, Alejandro Romero, Roberto Dávila, and Mariella Ramos-Gonzalez. 2025. "Impact of Exposure Duration to High-Altitude Hypoxia on Oxidative Homeostasis in Rat Brain Regions" International Journal of Molecular Sciences 26, no. 17: 8714. https://doi.org/10.3390/ijms26178714
APA StyleLira-Mejía, B., Calderon-Romero, R., Ordaya-Fierro, J., Medina, C., Rodríguez, J.-L., Romero, A., Dávila, R., & Ramos-Gonzalez, M. (2025). Impact of Exposure Duration to High-Altitude Hypoxia on Oxidative Homeostasis in Rat Brain Regions. International Journal of Molecular Sciences, 26(17), 8714. https://doi.org/10.3390/ijms26178714






