Potential of Selected C-X-C Motif Chemokines as Biomarkers in Colorectal Cancer Diagnosis
Abstract
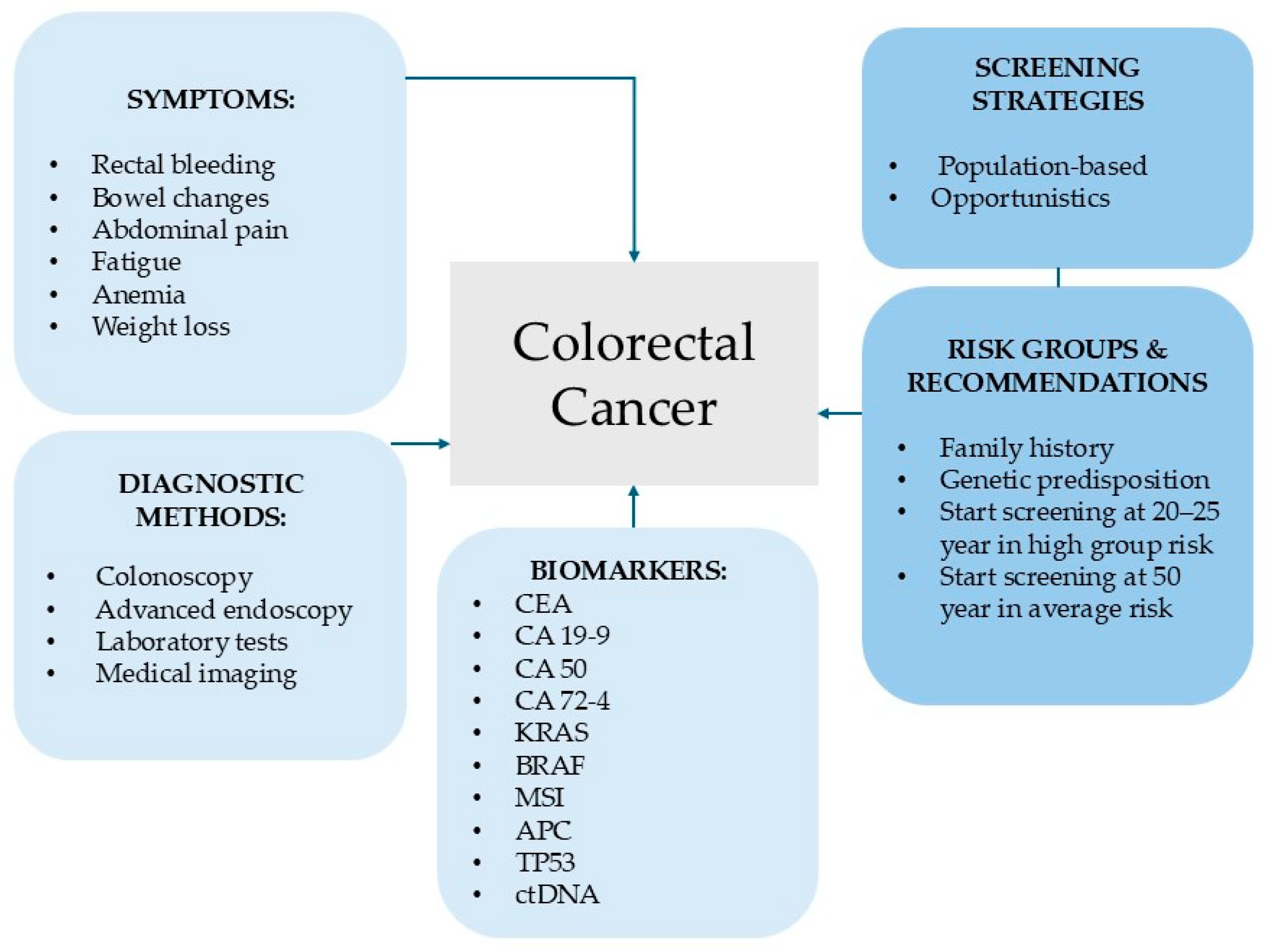
1. Colorectal Cancer—Epidemiology, Mortality and Risk Factors
An Overview of Current Diagnostic and Screening Strategies for Colorectal Cancer
2. Inflammation and Chemokines in Cancer Progression
| Lineage | Cell Type | CXC Receptor/Ligand | Role in CRC | References |
|---|---|---|---|---|
| Myeloid | Neutrophils (TANs) | CXCR1/2, CXCL8 |
| [51] |
| Macrophages (TAMs) | CXCL12, CXCL1 |
| [52,53] | |
| Lymphoid | T cells (CD8+/Th1) | CXCR3, CXCL9, CXCL10, CXCL11 |
| [54] |
| B cells (TLS) | CXCL13 |
| [55] |
3. Functional Roles of Chemokines in Colorectal Cancer Pathogenesis
3.1. ELR+ Chemokines
3.1.1. CXCL8, Its Receptors, and the Related Signaling Axis in Colorectal Cancer
3.1.2. CXCL1, Its Receptor, and Related Signaling Pathways in Colorectal Cancer
3.2. ELR- Chemokines
3.2.1. CXCL12, Its Receptors, and Related Signaling Pathways in Colorectal Cancer
3.2.2. CXCL13, Its Receptors, and Related Signaling Pathways in Colorectal Cancer
3.2.3. CXCL14, Its Receptors, and Related Signaling Pathways in Colorectal Cancer
3.2.4. CXCL16, Its Receptor, and Related Signaling Pathways in Colorectal Cancer
3.3. Cross-Talk and Subtype-Specific Roles of CXC Chemokines
4. Advanced Technologies and Clinical Perspectives in Colorectal Cancer
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Torre, L.A.; Soerjomataram, I.; Hayes, R.B.; Bray, F.; Weber, T.K.; Jamal, A. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019, 68, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
- Mármol, I.; Sánchez-De-Diego, C.; Dieste, A.P.; Cerrada, E.; Yoldi, M.J.R. Colorectal carcinoma: A general overview and future perspectives in colorectal cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef]
- Binefa, G.; Rodríguez-Moranta, F.; Teule, A.; Medina-Hayas, M. Colorectal cancer: From prevention to personalized medicine. World J. Gastroenterol. 2014, 20, 6786–6808. [Google Scholar] [CrossRef]
- Gonzalez, C.A.; Riboli, E. Diet and cancer prevention: Contributions from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Cancer 2010, 46, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Farrington, S.M.; Tenesa, A.; Barnetson, R.; Wiltshire, A.; Prendergast, J.; Porteous, M.; Campbell, H.; Dunlop, M.G. Germline susceptibility to colorectal cancer due to base-excision repair gene defects. Am. J. Hum. Genet. 2005, 77, 112–119. [Google Scholar] [CrossRef]
- Piñol, V.; Castells, A.; Andreu, M.; Castellví-Bel, S.; Alenda, C.; Llor, X.; Xicola, R.M.; Rodríguez-Moranta, F.; Payá, A.; Jover, R.; et al. Accuracy of Revised Bethesda Guidelines, microsatellite instability, and immunohistochemistry for the identification of patients with hereditary nonpolyposis colorectal cancer. JAMA 2005, 293, 1986–1994. [Google Scholar] [CrossRef]
- Verdecchia, A.; Francisci, S.; Brenner, H.; Gatta, G.; Micheli, A.; Mangone, L.; Kunkler, I. EUROCARE-4 Working GroupRecent cancer survival in Europe: A 2000–02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007, 8, 784–796. [Google Scholar] [CrossRef]
- Ciccolallo, L.; Capocaccia, R.; Coleman, M.P.; Berrino, F.; Coebergh, J.W.; Damhuis, R.A.; Faivre, J.; Martinez-Garcia, C.; Møller, H.; Ponz de Leon, M.; et al. Survival differences between European and US patients with colorectal cancer: Role of stage at diagnosis and surgery. Gut 2005, 54, 268–273. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef]
- Kawahara, M.; Chia, D.; Terasaki, P.I.; Roumanas, A.; Sugich, L.; Hermes, M.; Iguro, T. Detection of sialylated LewisX antigen in cancer sera using a sandwich radioimmunoassay. Int. J. Cancer 1985, 36, 421–425. [Google Scholar] [CrossRef] [PubMed]
- de Assis, J.V.; Coutinho, L.A.; Oyeyemi, I.T.; Oyeyemi, O.T. Diagnostic and therapeutic biomarkers in colorectal cancer: A review. Am. J. Cancer Res. 2022, 12, 661–680. [Google Scholar]
- Bellcross, C.A.; Bedrosian, S.R.; Daniels, E.; Duquette, D.; Hampel, H.; Jasperson, K.; Joseph, D.A.; Kaye, C.; Lubin, I.; Meyer, L.J.; et al. Implementing screening for Lynch syndrome among patients with newly diagnosed colorectal cancer: Summary of a public health/clinical collaborative meeting. Genet. Med. 2012, 14, 152–162. [Google Scholar] [CrossRef]
- Stone, J.K.; Mehta, N.A.; Singh, H.; El-Matary, W.; Bernstein, C.N. Endoscopic and chemopreventive management of familial adenomatous polyposis syndrome. Fam. Cancer 2023, 22, 413–422. [Google Scholar] [CrossRef]
- Jung, Y.S.; Lee, J.; Lee, H.A.; Moon, C.M. Interval cancer rate and diagnostic performance of fecal immunochemical test according to family history of colorectal cancer. J. Clin. Med. 2020, 9, 3302. [Google Scholar] [CrossRef]
- He, X.X.; Yuan, S.Y.; Li, W.B.; Yang, H.; Ji, W.; Wang, Z.Q.; Hao, J.Y.; Chen, C.; Chen, W.Q.; Gao, Y.X.; et al. Improvement of Asia-Pacific colorectal screening score and evaluation of its use combined with fecal immunochemical test. BMC Gastroenterol. 2019, 19, 226. [Google Scholar] [CrossRef] [PubMed]
- Zlotnik, A.; Yoshie, O. Chemokines: A new classification system and their role in immunity. Immunity 2000, 12, 121–127. [Google Scholar] [CrossRef]
- Balkwill, F.R. The chemokine system and cancer. J. Pathol. 2011, 226, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Palomino, D.C.T.; Marti, L.C. Chemokines and immunity. Einstein 2015, 13, 469–473. [Google Scholar] [CrossRef]
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and chemokine receptors: Positioning cells for host defense and immunity. Annu. Rev. Immunol. 2014, 32, 659–702. [Google Scholar] [CrossRef]
- Miller, M.C.; Mayo, K.H. Chemokines from a structural perspective. Int. J. Mol. Sci. 2017, 18, 2088. [Google Scholar] [CrossRef]
- Jiang, S.; Liang, J.; Li, W.; Wang, L.; Song, M.; Xu, S.; Liu, G.; Du, Q.; Zhai, D.; Tang, L.; et al. The role of CXCL1/CXCR2 axis in neurological diseases. Int. Immunopharmacol. 2023, 120, 110330. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Gao, Y.; Ding, P.; Wu, T.; Ji, G. The role of CXCL family members in different diseases. Cell Death Discov. 2023, 9, 212. [Google Scholar] [CrossRef]
- Hoellwerth, M.; Koelblinger, P.; Lang, R.; Harrer, A. Revisiting the role of the CXCL13/CXCR5-associated immune axis in melanoma: Potential implications for anti-PD-1-related biomarker research. Life 2023, 13, 553. [Google Scholar] [CrossRef]
- Gowhari Shabgah, A.; Haleem Al-Qaim, Z.; Markov, A.; Valerievich Yumashev, A.; Ezzatifar, F.; Ahmadi, M.; Mohammad Gheibihayat, S.; Gholizadeh Navashenaq, J. Chemokine CXCL14: A double-edged sword in cancer development. Int. Immunopharmacol. 2021, 97, 107681. [Google Scholar] [CrossRef]
- Korbecki, J.; Bajdak-Rusinek, K.; Kupnicka, P.; Kapczuk, P.; Siminska, D.; Chlubek, D.; Baranowska-Bosiacka, I. The role of CXCL16 in the pathogenesis of cancer and other diseases. Int. J. Mol. Sci. 2021, 22, 3490. [Google Scholar] [CrossRef]
- Koenen, R.R.; Weber, C. Therapeutic targeting of chemokine interactions in atherosclerosis. Nat. Rev. Drug Discov. 2010, 9, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Raman, D.; Sobolik-Delmaire, T.; Richmond, A. Chemokines in health and disease. Exp. Cell Res. 2011, 317, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Karin, N. Chemokines and cancer: New immune checkpoints for cancer therapy. Curr. Opin. Immunol. 2018, 51, 140–145. [Google Scholar] [CrossRef]
- Sethi, G.; Shanmugam, M.K.; Ramachandran, L.; Kumar, A.P.; Tergaonkar, V. Multifaceted link between cancer and inflammation. Biosci. Rep. 2012, 32, 1–15. [Google Scholar] [CrossRef]
- Lazennec, G.; Richmond, A. Chemokines and chemokine receptors: New insights into cancer-related inflammation. Trends Mol. Med. 2010, 16, 133–144. [Google Scholar] [CrossRef]
- Ransohoff, R.M. Chemokines and chemokine receptors: Standing at the crossroads of immunobiology and neurobiology. Immunity 2009, 31, 711–721. [Google Scholar] [CrossRef]
- Qin, R.; Ren, W.; Ya, G.; Wang, B.; He, J.; Ren, S.; Jiang, L.; Zhao, S. Role of chemokines in the crosstalk between tumor and tumor-associated macrophages. Clin. Exp. Med. 2023, 23, 1359–1373. [Google Scholar] [CrossRef]
- Vindrieux, D.; Escobar, P.; Lazennec, G. Emerging roles of chemokines in prostate cancer. Endocr. Relat. Cancer 2009, 16, 663–673. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Muzio, M.; Garlanda, C.; Sozzani, S.; Allavena, P. Macrophage control of inflammation: Negative pathways of regulation of inflammatory cytokines. Novartis Found. Symp. 2001, 234, 120–131. [Google Scholar]
- Raman, D.; Baugher, P.J.; Thu, Y.M.; Richmond, A. Role of chemokines in tumor growth. Cancer Lett. 2007, 256, 137–165. [Google Scholar] [CrossRef]
- Rot, A.; von Andrian, U.H. Chemokines in innate and adaptive host defense: Basic chemokinese grammar for immune cells. Annu. Rev. Immunol. 2004, 22, 891–928. [Google Scholar] [CrossRef] [PubMed]
- Charo, I.; Ransohoff, R. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 2006, 354, 610–621. [Google Scholar] [CrossRef]
- Chow, M.T.; Luster, A.D. Chemokines in cancer. Cancer Immunol. Res. 2014, 2, 1125–1131. [Google Scholar] [CrossRef]
- Payne, A.S.; Cornelius, L.A. The role of chemokines in melanoma tumor growth and metastasis. J. Investig. Dermatol. 2002, 118, 915–922. [Google Scholar] [CrossRef]
- Keeley, E.C.; Mehrad, B.; Strieter, R.M. CXC chemokines in cancer angiogenesis and metastases. Adv. Cancer Res. 2010, 106, 91–111. [Google Scholar]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N.; et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Zlotnik, A.; Burkhardt, A.M.; Homey, B. Homeostatic chemokine receptors and organ-specific metastasis. Nat. Rev. Immunol. 2011, 11, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Darash-Yahana, M.; Pikarsky, E.; Abramovitch, R.; Zeira, E.; Pal, B.; Karplus, R.; Beider, K.; Avniel, S.; Kasem, S.; Galun, E.; et al. Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. FASEB J. 2004, 18, 1240–1242. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Kawada, K.; Iwamoto, M.; Akagami, M.; Hida, K.; Nakanishi, Y.; Kanda, K.; Kawada, M.; Seno, H.; Taketo, M.M.; et al. The role of CXCR3 and CXCR4 in colorectal cancer metastasis. Int. J. Cancer 2013, 132, 276–287. [Google Scholar] [CrossRef]
- Vicinus, B.; Rubie, C.; Stegmaier, N.; Frick, V.O.; Kölsch, K.; Kauffels, A.; Ghadjar, P.; Wagner, M.; Glanemann, M. miR-21 and its target gene CCL20 are both highly overexpressed in the microenvironment of colorectal tumors: Significance of their regulation. Oncol. Rep. 2013, 30, 1285–1292. [Google Scholar] [CrossRef]
- Hu, D.; Du, C.; Xue, W.; Dou, F.; Yao, Y.; Gu, J. The expression of chemokine receptors CCR6, CXCR2 and CXCR4 is not organ-specific for distant metastasis in colorectal cancer: A comparative study. Histopathology 2013, 63, 167–173. [Google Scholar] [CrossRef]
- Masui, H.; Kawada, K.; Obama, K. Neutrophil and Colorectal Cancer. Int. J. Mol. Sci. 2024, 26, 6. [Google Scholar] [CrossRef] [PubMed]
- Daniel, S.K.; Seo, Y.D.; Pillarisetty, V.G. The CXCL12-CXCR4/CXCR7 Axis as a Mechanism of Immune Resistance in Gastrointestinal Malignancies. Semin. Cancer Biol. 2020, 65, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sun, H.; Wei, J.; Cen, B.; DuBois, R.N. CXCL1 is critical for premetastatic niche formation and metastasis in colorectal cancer. Cancer Res. 2017, 77, 3655–3665. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Wieder, T.; Mauerer, B.; Schäfer, L.; Kesselring, R.; Braumüller, H.T. Cells in Colorectal Cancer: Unravelling the Function of Different T Cell Subsets in the Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 11673. [Google Scholar] [CrossRef]
- Guo, F.F.; Yan, Y.Q.; Chen, W.W.; Cheng, Y.; Zhang, R.; Shen, C.Q.; Cui, Y.; Peng, Y.S.; Chen, H.Y.; Ji, L.H.; et al. CXCL13 as a Prognostic Biomarker and Modulator of the Tumor Microenvironment in Colorectal Cancer. J. Dig. Dis. 2025. [Google Scholar] [CrossRef]
- Nasser, M.W.; Raghuwanshi, S.K.; Grant, D.J.; Jala, V.R.; Rajarathnam, K.; Richardson, R. Differential activation and regulation of CXCR1 and CXCR2 by CXCL8 monomer and dimer. J. Immunol. 2009, 183, 3425–3432. [Google Scholar] [CrossRef]
- Cabrero-de Las Heras, S.; Martínez-Balibrea, E. CXC family of chemokines as prognostic or predictive biomarkers and possible drug targets in colorectal cancer. World J. Gastroenterol. 2018, 24, 4738–4749. [Google Scholar] [CrossRef]
- Baggiolini, M. Reflections on chemokines. Immunol. Rev. 2000, 177, 5–7. [Google Scholar] [CrossRef]
- Yan, W.; Chen, X. Identification of GRO1 as a critical determinant for mutant p53 gain of function. J. Biol. Chem. 2009, 284, 12178–12187. [Google Scholar] [CrossRef]
- Chang, W.J.; Du, Y.; Zhao, X.; Ma, L.Y.; Cao, G.W. Inflammation-related factors predicting prognosis of gastric cancer. World J. Gastroenterol. 2014, 20, 4586–4596. [Google Scholar] [CrossRef]
- Oladipo, O.; Conlon, S.; O’Grady, A.; Purcell, C.; Wilson, C.; Maxwell, P.J.; Johnston, P.G.; Stevenson, M.; Kay, E.W.; Wilson, R.H.; et al. The expression and prognostic impact of CXC-chemokines in stage II and III colorectal cancer epithelial and stromal tissue. Br. J. Cancer 2011, 104, 480–487. [Google Scholar] [CrossRef]
- Wu, W.; Sun, C.; Xu, D.; Zhang, X.; Shen, W.; Lv, Y.; Ma, T. Expression of CXCR2 and its clinical significance in human colorectal cancer. Int. J. Clin. Exp. Med. 2015, 8, 5883–5890. [Google Scholar]
- Lee, Y.S.; Choi, I.; Ning, Y.; Kim, N.Y.; Khatchadourian, V.; Yang, D.; Chung, H.K.; Choi, D.; LaBonte, M.J.; Ladner, R.D.; et al. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br. J. Cancer 2012, 106, 1833–1841. [Google Scholar] [CrossRef]
- Erreni, M.; Bianchi, P.; Laghi, L.; Mirolo, M.; Fabbri, M.; Locati, M.; Mantovani, A.; Allavena, P. Expression of chemokines and chemokine receptors in human colon cancer. Methods Enzymol. 2009, 460, 105–121. [Google Scholar] [PubMed]
- Pennel, K.A.; Quinn, J.A.; Nixon, C.; Inthagard, J.; van Wyk, H.C.; Chang, D.; Rebus, S.; GPOL Group; Hay, J.; Maka, N.N.; et al. CXCL8 expression is associated with advanced stage, right sidedness, and distinct histological features of colorectal cancer. J. Pathol. Clin. Res. 2022, 8, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Bazzichetto, C.; Milella, M.; Zampiva, I.; Simionato, F.; Amoreo, C.A.; Buglioni, S.; Pacelli, C.; Le Pera, L.; Colombo, T.; Bria, E.; et al. Interleukin-8 in Colorectal Cancer: A Systematic Review and Meta-Analysis of Its Potential Role as a Prognostic Biomarker. Biomedicines 2022, 10, 2631. [Google Scholar] [CrossRef] [PubMed]
- Conciatori, F.; Bazzichetto, C.; Amoreo, C.A.; Sperduti, I.; Donzelli, S.; Diodoro, M.G.; Buglioni, S.; Falcone, I.; Shirasawa, S.; Blandino, G.; et al. BRAF status modulates Interelukin-8 expression through a CHOP-dependent mechanism in colorectal cancer. Commun. Biol. 2020, 3, 546. [Google Scholar] [CrossRef]
- Ogawa, R.; Yamamoto, T.; Hirai, H.; Hanada, K.; Kiyasu, Y.; Nishikawa, G.; Mizuno, R.; Inamoto, S.; Itatani, Y.; Sakai, Y.; et al. Loss of SMAD4 Promotes Colorectal Cancer Progression by Recruiting Tumor-Associated Neutrophils via the CXCL1/8-CXCR2 Axis. Clin. Cancer Res. 2019, 25, 2887–2899. [Google Scholar] [CrossRef] [PubMed]
- Pączek, S.; Łukaszewicz-Zając, M.; Gryko, M.; Mroczko, P.; Kulczyńska-Przybik, A.; Mroczko, B. CXCL-8 in preoperative colorectal cancer patients: Significance for diagnosis and cancer progression. Int. J. Mol. Sci. 2020, 21, 2040. [Google Scholar] [CrossRef]
- Kaminska, J.; Nowacki, M.P.; Kowalska, M.; Rysinska, A.; Chwalinski, M.; Fuksiewicz, M.; Michalski, W.; Chechlinska, M. Clinical significance of serum cytokine measurements in untreated colorectal cancer patients: Soluble tumor necrosis factor receptor type I—an independent prognostic factor. Tumor Biol. 2005, 26, 186–194. [Google Scholar] [CrossRef]
- Becker, S.; Quay, J.; Koren, H.S.; Haskill, J.S. Constitutive and stimulated MCP-1, GRO alpha, beta, and gamma expression in human airway epithelium and bronchoalveolar macrophages. Am. J. Physiol. 1994, 266, L278–L286. [Google Scholar] [CrossRef] [PubMed]
- Talmadge, J.E. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin. Cancer Res. 2007, 13, 5243–5248. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012, 12, 253–268. [Google Scholar] [CrossRef]
- Ogata, H.; Sekikawa, A.; Yamagishi, H.; Ichikawa, K.; Tomita, S.; Imura, J.; Ito, Y.; Fujita, M.; Tsubaki, M.; Kato, H.; et al. GRO alpha promotes invasion of colorectal cancer cells. Oncol. Rep. 2010, 24, 1479–1486. [Google Scholar]
- Baier, P.K.; Eggstein, S.; Wolff-Vorbeck, G.; Baumgartner, U.; Hopt, U.T. Chemokines in human colorectal carcinoma. Anticancer Res. 2005, 25, 3581–3584. [Google Scholar]
- Bandapalli, O.R.; Ehrmann, F.; Ehemann, V.; Gaida, M.; Macher-Goeppinger, S.; Wente, M.; Schirmacher, P.; Brand, K. Downregulation of CXCL1 inhibits tumor growth in colorectal liver metastasis. Cytokine 2012, 57, 46–53. [Google Scholar] [CrossRef]
- Zhuo, C.; Wu, X.; Li, J.; Hu, D.; Jian, J.; Chen, C.; Zheng, X.; Yang, C. Chemokine (C-X-C motif) ligand 1 is associated with tumor progression and poor prognosis in patients with colorectal cancer. Biosci. Rep. 2018, 38, BSR20180580. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.A.; Vousden, K.H. Mutant p53 in cancer: New functions and therapeutic opportunities. Cancer Cell 2014, 25, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Soussi, T. The p53 tumor suppressor gene: From molecular biology to clinical investigation. Ann. N. Y. Acad. Sci. 2000, 910, 121–139. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kikuchi, H.; Ohta, M.; Kawabata, T.; Hiramatsu, Y.; Kondo, K.; Baba, M.; Kamiya, K.; Tanaka, T.; Kitagawa, M.; et al. TSU68 prevents liver metastasis of colon cancer xenografts by modulating the premetastatic niche. Cancer Res. 2008, 68, 9754–9762. [Google Scholar] [CrossRef]
- Kong, X.; Li, Y.; Zhang, M.; Wang, J.; Liu, Z. CXCL1 promotes immune escape in colorectal cancer by autophagy-mediated MHC-I degradation. Hum. Immunol. 2023, 84, 110716. [Google Scholar] [CrossRef]
- Zhuo, C.; Ruan, Q.; Zhao, X.; Shen, Y.; Lin, R. CXCL1 promotes colon cancer progression through activation of NF-κB/P300 signaling pathway. Biol. Direct 2022, 17, 34. [Google Scholar] [CrossRef]
- Miao, Z.; Luker, K.E.; Summers, B.C.; Berahovich, R.; Bhojani, M.S.; Rehemtulla, A.; Kleer, C.G.; Essner, J.J.; Nasevicius, A.; Luker, G.D.; et al. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc. Natl. Acad. Sci. USA 2007, 104, 15735–15740. [Google Scholar] [CrossRef]
- Teicher, B.A.; Fricker, S.P. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin. Cancer Res. 2010, 16, 2927–2931. [Google Scholar] [CrossRef]
- Bleul, C.C.; Farzan, M.; Choe, H.; Parolin, C.; Clark-Lewis, I.; Sodroski, J.; Springer, T.A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/Fusin and blocks HIV-1 entry. Nature 1996, 382, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Balabanian, K.; Lagane, B.; Infantino, S.; Chow, K.Y.; Harriague, J.; Moepps, B.; Arenzana-Seisdedos, F.; Thelen, M.; Bachelerie, F. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J. Biol. Chem. 2005, 280, 35760–35766. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, S.; Kim, J.; Ahn, S.; Craig, S.; Lam, C.M.; Gerard, N.P.; Gerard, C.; Lefkowitz, R.J. Beta-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc. Natl. Acad. Sci. USA 2010, 107, 628–632. [Google Scholar] [CrossRef]
- Akishima-Fukasawa, Y.; Nakanishi, Y.; Ino, Y.; Moriya, Y.; Kanai, Y.; Hirohashi, S. Prognostic significance of CXCL12 expression in patients with colorectal carcinoma. Am. J. Clin. Pathol. 2009, 132, 202–210. [Google Scholar] [CrossRef]
- Yoshitake, N.; Fukui, H.; Yamagishi, H.; Sekikawa, A.; Fujii, S.; Tomita, S.; Ichikawa, K.; Imura, J.; Hiraishi, H.; Fujimori, T. Expression of SDF-1 alpha and nuclear CXCR4 predicts lymph node metastasis in colorectal cancer. Br. J. Cancer 2008, 98, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, X.; Wei, M.; Wang, Z. The role of CXCL12 axis in lung metastasis of colorectal cancer. J. Cancer 2018, 9, 3898–3903. [Google Scholar] [CrossRef]
- Zhang, S.; Li, G. Tumor expression of CXCL12 and survival of patients with colorectal cancer: A meta-analysis. Oncol. Lett. 2022, 24, 436. [Google Scholar] [CrossRef] [PubMed]
- Zengin, M.; Dursun, N.; Behzatoğlu, K.; Paşaoğlu, H.E.; Benek, S. Evaluation of CXCL12 and CXCR4 to predict poor survival in lymph node-positive colorectal cancer patients. Pol. J. Pathol. 2020, 71, 328–338. [Google Scholar] [CrossRef]
- Ottaiano, A.; Scala, S.; Normanno, N.; Botti, G.; Tatangelo, F.; Di Mauro, A.; Capozzi, M.; Facchini, S.; Tafuto, S.; Nasti, G. Prognostic and predictive role of CXC chemokine receptor 4 in metastatic colorectal cancer patients. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 755–760. [Google Scholar] [CrossRef]
- Romain, B.; Hachet-Haas, M.; Rohr, S.; Brigand, C.; Galzi, J.L.; Gaub, M.P.; Pencreach, E.; Guenot, D. Hypoxia differentially regulates CXCR4 and CXCR7 signaling in colon cancer. Mol. Cancer 2014, 13, 58. [Google Scholar] [CrossRef]
- Yang, D.; Dai, T.; Xue, L.; Liu, X.; Wu, B.; Geng, J.; Mao, X.; Wang, R.; Chen, L.; Chu, X. Expression of chemokine receptor CXCR7 in colorectal carcinoma and its prognostic significance. Int. J. Clin. Exp. Pathol. 2015, 8, 13051–13058. [Google Scholar]
- Sherif, M.F.; Ismail, I.M.; Ata, S.M.S. Expression of CXCR7 in colorectal adenoma and adenocarcinoma: Correlation with clinicopathological parameters. Ann. Diagn. Pathol. 2020, 49, 151621. [Google Scholar] [CrossRef]
- Kheirelseid, E.A.; Miller, N.; Chang, K.H.; Nugent, M.; Kerin, M.J. Clinical applications of gene expression in colorectal cancer. J. Gastrointest. Oncol. 2013, 4, 144–157. [Google Scholar] [PubMed]
- Stanisavljevic, L.; Assmus, J.; Storli, K.E.; Leh, S.M.; Dahl, O.; Myklebust, M.P. CXCR4, CXCL12 and the relative CXCL12-CXCR4 expression as prognostic factors in colon cancer. Tumour Biol. 2016, 37, 7441–7452. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Lei, W.; Wang, H.; Ni, Y.; Liu, Y.; Yan, H.; Tian, Y.; Wang, Z.; Yang, Z.; et al. CXCL12-CXCR4/CXCR7 Axis in Cancer: From Mechanisms to Clinical Applications. Int. J. Biol. Sci. 2023, 19, 3341–3359. [Google Scholar] [CrossRef]
- Peng, S.B.; Zhang, X.; Paul, D.; Kays, L.M.; Gough, W.; Stewart, J.; Uhlik, M.T.; Chen, Q.; Hui, Y.H.; Zamek-Gliszczynski, M.J.; et al. Identification of LY2510924, a novel cyclic peptide CXCR4 antagonist that exhibits antitumor activities in solid tumor and breast cancer metastatic models. Mol. Cancer Ther. 2015, 14, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Vogelzang, N.J.; Conkling, P.; Raddad, E.; Polzer, J.; Roberson, S.; Stille, J.R.; Saleh, M.; Thornton, D. A phase I trial of LY2510924, a CXCR4 peptide antagonist, in patients with advanced cancer. Clin. Cancer Res. 2014, 20, 3581–3588, Erratum in Clin. Cancer Res. 2014, 20, 4414. [Google Scholar] [CrossRef]
- Zhang, G.; Luo, X.; Zhang, W.; Chen, E.; Xu, J.; Wang, F.; Cao, G.; Ju, Z.; Jin, D.; Huang, X.; et al. CXCL13 regulates resistance to 5-fluorouracil in colorectal cancer. Cancer Res. Treat. 2020, 52, 622–633. [Google Scholar] [CrossRef]
- Huber, A.K.; Irani, D.N. Targeting CXCL13 during neuroinflammation. Adv. Neuroimmune Biol. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, Z.; Yao, G.; Lyu, X.; Li, J.; Hu, X.; Cai, Y.; Li, W.; Li, X.; Ye, C. The expression of CXCL13 and its relation to unfavorable clinical characteristics in young breast cancer. J. Transl. Med. 2016, 13, 168, Erratum in J. Transl. Med. 2016, 14, 318. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Tai, J.; Zhao, Y.; Zhao, P.; Sun, D.; Wang, L. Associations of C-X-C motif chemokine ligands 1/2/8/13/14 with clinicopathological features and survival profile in patients with colorectal cancer. Oncol. Lett. 2022, 24, 348. [Google Scholar] [CrossRef]
- Li, C.; Kang, D.; Sun, X.; Liu, Y.; Wang, J.; Gao, P. The effect of C X C motif chemokine 13 on hepatocellular carcinoma associates with Wnt signaling. BioMed Res. Int. 2015, 2015, 345413. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Porras, V.; Bystrup, S.; Martinez Cardus, A.; Pluvinet, R.; Sumoy, L.; Howells, L.; James, M.I.; Iwuji, C.; Manzano, J.L.; Layos, L.; et al. Curcumin mediates oxaliplatin acquired resistance reversion in colorectal cancer cell lines through modulation of CXC Chemokine/NF κB signalling pathway. Sci. Rep. 2016, 6, 24675. [Google Scholar] [CrossRef]
- Tong, G.J.; Zhang, G.Y.; Liu, J.; Zheng, Z.Z.; Chen, Y.; Niu, P.P.; Xu, X.T. Comparison of the eighth version of the American Joint Committee on Cancer manual to the seventh version for colorectal cancer: A retrospective review of our data. World J. Clin. Oncol. 2018, 9, 148–161. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, X.; Guo, H.; Fu, L.; Pan, G.; Sun, Y. CXCL13 CXCR5 axis promotes the growth and invasion of colon cancer cells via PI3K/AKT pathway. Mol. Cell Biochem. 2015, 400, 287–295. [Google Scholar] [CrossRef]
- Gu-Trantien, C.; Migliori, E.; Buisseret, L.; de Wind, A.; Brohee, S.; Garaud, S.; Noël, G.; Dang Chi, V.L.; Lodewyckx, J.N.; Naveaux, C.; et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight 2017, 2, e91487. [Google Scholar] [CrossRef]
- Fan, L.; Zhu, Q.; Liu, L.; Zhu, C.; Huang, H.; Lu, S.; Liu, P. CXCL13 is androgen-responsive and involved in androgen induced prostate cancer cell migration and invasion. Oncotarget 2017, 8, 53244–53261. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef]
- Zheng, Z.; Cai, Y.; Chen, H.; Chen, Z.; Zhu, D.; Zhong, Q.; Xie, W. CXCL13/CXCR5 axis predicts poor prognosis and promotes progression through PI3K/AKT/mTOR pathway in clear cell renal cell carcinoma. Front. Oncol. 2019, 8, 682. [Google Scholar] [CrossRef]
- Qi, X.W.; Xia, S.H.; Yin, Y.; Jin, L.F.; Pu, Y.; Hua, D.; Wu, H.R. Expression features of CXCR5 and its ligand, CXCL13 associated with poor prognosis of advanced colorectal cancer. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1916–1924. [Google Scholar]
- Shen, Y.; Tong, M.; Liang, Q.; Guo, Y.; Sun, H.Q.; Zheng, W.; Ao, L.; Guo, Z.; She, F. Epigenomics alternations and dynamic transcriptional changes in responses to 5-fluorouracil stimulation reveal mechanisms of acquired drug resistance of colorectal cancer cells. Pharmacogenomics J. 2018, 18, 23–28. [Google Scholar] [CrossRef]
- Xie, T.; Huang, M.; Wang, Y.; Wang, L.; Chen, C.; Chu, X. MicroRNAs as regulators, biomarkers and therapeutic targets in the drug resistance of colorectal cancer. Cell Physiol. Biochem. 2016, 40, 62–76. [Google Scholar] [CrossRef]
- Lin, K.; Zou, R.; Lin, F.; Zheng, S.; Shen, X.; Xue, X. Expression and effect of CXCL14 in colorectal carcinoma. Mol. Med. Rep. 2014, 10, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, P.; Zhang, L.; Wei, J.; Xie, Q.; Sun, Q.; Zhou, X.; Xie, H.; Zhou, L.; Zheng, S. Antitumor efficacy of CXC motif chemokine ligand 14 in hepatocellular carcinoma in vitro and in vivo. Cancer Sci. 2013, 104, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Yang, X.; Cheng, L.; Liu, R.; Lei, Y.; Dong, D.; Li, F.; Lau, Q.C.; Deng, L.; Nice, E.C.; et al. Chemokine CXCL14 Is Associated with Prognosis in Patients with Colorectal Carcinoma after Curative Resection. J. Transl. Med. 2013, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Yang, Y.; Pan, Y.; Jia, Y.; Brock, M.V.; Herman, J.G.; Guo, M. Epigenetic silencing of CXCL14 induced colorectal cancer migration and invasion. Discov. Med. 2013, 16, 137–147. [Google Scholar]
- Abel, S.; Hundhausen, C.; Mentlein, R.; Schulte, A.; Berkhout, T.A.; Broadway, N.; Hartmann, D.; Sedlacek, R.; Dietrich, S.; Muetze, B.; et al. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J. Immunol. 2004, 172, 6362–6372. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Dai, W.; Yang, L.; Yang, H.; Ding, L.; He, Y.; Song, X.; Cui, J. Elevated expression of CXCL16 correlates with poor prognosis in patients with colorectal cancer. Cancer Manag. Res. 2019, 11, 4691–4697. [Google Scholar] [CrossRef]
- Deng, W.; Liu, X.; Huang, S.; Wu, Z.; Alessandro, F.; Lin, Q.; Cai, Z.; Zhang, Z.; Huang, Y.; Wang, H.; et al. CXCL16 promotes tumor metastasis by regulating angiogenesis in the tumor micro-environment of BRAF V600E mutant colorectal cancer. Transl. Oncol. 2024, 41, 101854. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Y.; Sun, X.; Deng, G.; Huang, W.; Wu, X.; Gu, Y.; Tian, Z.; Fan, Z.; Xu, Q.; et al. CXCR6 is required for antitumor efficacy intratumoral CD8+ T cell. J. Immunother. Cancer 2021, 9, e003100. [Google Scholar] [CrossRef]
- Liu, Z.; Tao, J.; Zhu, Y.; Li, D.; Teng, L. Silencing CXCR6 promotes epithelial-mesenchymal transition and invasion in colorectal cancer by activating the VEGFA/PI3K/AKT/mTOR pathway. Int. Immunopharmacol. 2024, 143 Pt 3, 113529. [Google Scholar] [CrossRef]
- Yue, M.; Chen, M.-M.; Zhang, B.; Wang, Y.; Li, P.; Zhao, Y. The Functional Roles of Chemokines and Chemokine Receptors in Colorectal Cancer Progression. Biomedicines 2024, 170, 116040. [Google Scholar] [CrossRef]
- Bogomolova, I.A.; Antoneeva, I.I.; Dolgova, D.R.; Abakumova, T.V.; Gening, T.P. Chemokine CXCL8 and its receptors as markers of colorectal cancer. Kazan Med. J. 2024, 105, 424–432. [Google Scholar] [CrossRef]
- Braumüller, H.; Mauerer, B.; Andris, J.; Berlin, C.; Wieder, T.; Kesselring, R. The Cytokine Network in Colorectal Cancer: Implications for New Treatment Strategies. Cells 2023, 12, 138. [Google Scholar] [CrossRef]
- Lyu, N.; Pedersen, B.; Shklovskaya, E.; Rizos, H.; Molloy, M.P.; Wang, Y. SERS characterization of colorectal cancer cell surface markers upon anti-EGFR treatment. Exploration 2022, 2, 20210176. [Google Scholar] [CrossRef]
- Li, P.; Shang, X.; Jiao, Q.; Mi, Q.; Zhu, M.; Ren, Y.; Li, J.; Li, L.; Liu, J.; Wang, C.; et al. Alteration of chromatin high-order conformation associated with oxaliplatin resistance acquisition in colorectal cancer cells. Exploration 2023, 3, 20220136. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, Y.; Dong, A.; Elsabahy, M.; Yang, Y.W.; Gao, H. Emerging strategies for combating Fusobacterium nucleatum in colorectal cancer treatment: Systematic review, improvements and future challenges. Exploration 2023, 4, 20230092. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Duan, Y.; Li, Y.; Gu, Y.; Zhou, L.; Xiao, Z.; Yu, X.; Cai, Y.; Cheng, E.; Liu, Q.; et al. Cascade loop of ferroptosis induction and immunotherapy based on metal-phenolic networks for combined therapy of colorectal cancer. Exploration 2025, 5, 20230117. [Google Scholar] [CrossRef] [PubMed]

| Chemokine | Receptor(s) | Role in CRC | Expression: Tissues/Serum | Biomarker Potential | References |
|---|---|---|---|---|---|
| CXCL8 | CXCR1 and CXCR2 | Promotes proliferation, invasion, angiogenesis, immune evasion | Elevated serum levels in CRC patients and overexpressed in CRC tissue | Diagnostic and prognostic marker—elevated tissue and serum levels correlate with advanced tumor stage, presence of distant metastases, and shorter OS and RFS. | [56,57,58,59,60,61,62,63,64,65,66,67,68,69,70] |
| CXCL1 | CXCR2 | Promotes proliferation, migration, angiogenesis | Overexpressed in CRC tissue | Prognostic marker—associated with poor survival, larger tumor size, deeper invasion, lymph node metastasis, and advanced clinical stage. | [71,72,73,74,75,76,77,78,79,80,81,82] |
| CXCL12 | CXCR4 and CXCR7 | Enhances metastasis, chemoresistance | Overexpressed in CRC tissue | Prognostic marker—tissue expression linked to lymphatic and distant metastasis and reduced DFS. | [83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101] |
| CXCL13 | CXCR5 | Promotes immune cell recruitment, tumor progression | Elevated serum levels in CRC patients and overexpressed in CRC tissue | Prognostic marker—associated with advanced TNM stage, lymph node involvement, and poor OS and DFS; elevated serum levels also predict 5-Fu resistance and worse clinical outcome. | [102,103,104,105,106,107,108,109,110,111,112,113,114,115,116] |
| CXCL14 | Unknown | Promotes or suppresses tumor progression | Overexpressed in CRC tissue | Prognostic marker—reduced expression in CRC tissues associated with advanced stage and decreased OS; may function as a tumor suppressor depending on cellular source. | [117,118,119,120] |
| CXCL16 | CXCR6 | Promotes migration, invasion, immune modulation | Overexpressed in CRC tissue | High expression linked to poor OS in stage III/IV CRC; associated with angiogenesis and unfavorable outcomes in BRAF-mutant tumors. | [121,122,123,124,125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanowicz, A.; Łukaszewicz-Zając, M.; Mroczko, B. Potential of Selected C-X-C Motif Chemokines as Biomarkers in Colorectal Cancer Diagnosis. Int. J. Mol. Sci. 2025, 26, 8715. https://doi.org/10.3390/ijms26178715
Romanowicz A, Łukaszewicz-Zając M, Mroczko B. Potential of Selected C-X-C Motif Chemokines as Biomarkers in Colorectal Cancer Diagnosis. International Journal of Molecular Sciences. 2025; 26(17):8715. https://doi.org/10.3390/ijms26178715
Chicago/Turabian StyleRomanowicz, Adrianna, Marta Łukaszewicz-Zając, and Barbara Mroczko. 2025. "Potential of Selected C-X-C Motif Chemokines as Biomarkers in Colorectal Cancer Diagnosis" International Journal of Molecular Sciences 26, no. 17: 8715. https://doi.org/10.3390/ijms26178715
APA StyleRomanowicz, A., Łukaszewicz-Zając, M., & Mroczko, B. (2025). Potential of Selected C-X-C Motif Chemokines as Biomarkers in Colorectal Cancer Diagnosis. International Journal of Molecular Sciences, 26(17), 8715. https://doi.org/10.3390/ijms26178715








