Abstract
Striatum can be described as a brain region containing a general neuronal mechanism to associate actions or events with reward. In particular, neural activity in the human striatum is modulated by social actions and, critically, by the conjunction of social actions and own reward. To perform this function, dopamine and oxytocin signaling reaching the striatum represent a key factor. These neurotransmitters, in both humans and animals, are released in response to afferent vagal and sensory stimulation, as well as sexual and social interactions, conveying information related to reward and pleasure associated with an event. Dopamine and oxytocin have several effects in common, but of particular interest is evidence indicating that they can mutually modulate their action. The present review focuses on available data delineating interactions between dopaminergic and oxytocinergic signaling in the striatum. In this context, recent data on the possible role played by striatal astrocytes and microglia as key modulators of this crosstalk will be briefly discussed.
1. Introduction
The monoamine dopamine is a well-known very important central neurotransmitter with a widespread receptor distribution in the brain and representing the signaling molecule of an extensive neural system (see [1]), whose basic architecture was for the first time described in the 1960s [2,3]. Dopaminergic neuronal cell bodies located in the substantia nigra, hypothalamus, ventral tegmental area, arcuate nucleus, and the zona incerta project to various brain structures forming five main pathways [4,5,6]. They include the nigrostriatal pathway, running from the substantia nigra to the striatum, the mesolimbic/mesocortical pathways, originating in the ventral tegmental area and projecting to ventral striatum and prefrontal cortex, respectively, the tubero-infundibular pathway, from the hypothalamus to the pituitary gland, and the incerto-hypothalamic pathway, reaching the lateral septum and other areas within the hypothalamus. In addition to these pathways within the brain, a pathway from the hypothalamus to the spinal cord is also present (see [7] for a review). Furthermore, there are dopaminergic neurons locally spread, for instance in the paraventricular nucleus of the hypothalamus.
Over the last 30 years, the peptide oxytocin (a classical neurohypophysial hormone) has emerged as an influential signaling molecule released in the brain [8]. It is primarily synthesized in the supraoptic nucleus (SON) and in the paraventricular nucleus (PVN) of the hypothalamus [7]. Axons of the magnocellular neurons in SON and PVN reach the posterior pituitary to release the peptide into the blood, while parvocellular neurons in the PVN project to extrahypothalamic regions of the central nervous system, such as prefrontal cortex, striatum, ventral tegmental area, hippocampus, amygdala, olfactory bulb, brain stem, and spinal cord [9]. Of interest, is also evidence indicating that oxytocin can be released from the dendrites of both the parvo- and the magnocellular neurons in the PVN and SON, allowing for a widespread diffusion of the peptide within the central nervous system [10]. Consistently, oxytocin receptors are also abundantly expressed throughout the CNS. In addition to their expression in the SON and PVN, oxytocin receptors are also found in the abovementioned regions reached by oxytocin fibers [11]. However, some brain areas (e.g., olfactory bulb, ventral pallidum, medial preoptic area and ventromedial nucleus of the hypothalamus) exhibit a distinct mismatch between oxytocin fiber distribution and oxytocin receptor expression [7,12,13], suggesting that centrally released oxytocin can also act as a neuromodulator operating by volume transmission [14,15,16]. Oxytocin receptors are present in the presynaptic and postsynaptic membranes at both excitatory and inhibitory synapses, and the molecular effect of oxytocin on neuronal excitability is the regulation of the activity of ion channels in the cell membrane (see [17] for a review).
The main characteristics of the two signaling systems are summarized in Table 1. As shown, despite their molecular differences, dopamine and oxytocin signaling pathways exhibit a range of functional effects and characteristics in common, being both involved in the regulation of social behaviors, feeding, and reward mechanisms (see [18] for a recent review). Disturbances of these signals are known to be implicated in behavioral disorders including anxiety, depression, autism spectrum disorders (ASD), obsessive–compulsive disorders (OCD), attention deficit hyperactivity disorders (ADHD), and schizophrenia [7,18].
Furthermore, evidence for the existence of dopamine–oxytocin positive interaction in social behavioral paradigms and associated disorders has been reported (see [7] for a review), and anatomical and immunocytochemical studies provided some evidence of possible molecular mechanisms involved in this crosstalk. Receptor binding sites and neuronal fibers of the two neurotransmitters often exist in the same CNS regions of the brain [19,20]. Dopamine receptors, for instance, have been demonstrated in oxytocin neurons, and dopamine signaling influences oxytocin release [21,22]. Conversely, oxytocin has been found to increase dopamine in regions, such as the medial preoptic area, the amygdala, and the ventral tegmental area, where oxytocin receptors are also located [23,24]. Besides modulating each other’s release, the oxytocin receptor and dopamine receptors can also undergo direct receptor–receptor interactions. For instance, both oxytocin and dopamine receptors have been demonstrated in the neurons of nucleus accumbens, and the amygdala [21,23,24], where oxytocin and dopamine D2-receptors have been shown to form heteroreceptor complexes leading to changes in the G protein-mediated signal transduction [25,26].

Table 1.
Main features of dopamine and oxytocin in the brain.
Table 1.
Main features of dopamine and oxytocin in the brain.
| Dopamine | Oxytocin | |||
|---|---|---|---|---|
| Chemical identity | Monoamine | Nonapeptide | ||
| Main sources | Substantia nigra Ventral tegmental area Zona incerta | [1] | Supraoptic nucleus and Paraventricular nucleus of the hypothalamus | [7] |
| Main target regions | Dorsal and ventral striatum, Cortex, Hippocampus, Amigdala, Pituitary gland, Hypothalamus, Spinal cord | [4] | Striatum, Prefrontal cortex, Ventral tegmental area, Hippocampus, Amygdala, Brain stem, Spinal cord | [9] |
| Receptors | D1-like (D1, D5) D2-like (D2, D3, D4) | [1,27] | OTR | [22] |
| Signaling pathway | Gs and Gq proteins (D1-like) Gi/0 protein (D2-like) | [5,28] | Gq protein Gi protein | [22] |
| Main effects | Control of organic functions: | Control of organic functions: | ||
| ● Cardiovascular | [29] | ● Cardiovascular | [30] | |
| ● Renal | [31] | ● Respiration | [7] | |
| ● Penile striated muscles | [32] | ● Penile erection | [32] | |
| Rewarding | [18] | Rewarding | [18] | |
The present review will focus on available data delineating interactions between dopaminergic and oxytocinergic signaling in the striatum, a region that receives significant dopaminergic and oxytocinergic innervation, and that contributes not only to motor control but also to a broad range of behavioral domains in humans, ranging from basic salience/reward and novelty processing to complex decision making [33,34]. In this context, recent data on the possible role played by striatal astrocytes and microglia as key modulators of this crosstalk will be briefly discussed.
2. Dopamine and Oxytocin in the Striatum
The main structure of the basal ganglia was defined as ‘corpus striatum’ in the 17th century because of the mixture of gray matter and fiber tracts it exhibits, and in 1786 Vicq d’Azir was the first who realized it was composed of two main structures, the caudate nucleus and the putamen (see [1]).
From the neuroanatomical standpoint, the striatum is classically divided into (i) dorsal striatum or neostriatum, including most of the caudate and putamen, and (ii) ventral striatum, including the nucleus accumbens, the ventromedial parts of the caudate and the putamen, and the striatal portion of the olfactory tubercle.
2.1. Dopamine
Both regions of the striatum receive massive dopamine signals from the midbrain and the understanding of the role of striatal dopamine has developed significantly in recent years (see [35]).
It has been known for a quite long time that the nigrostriatal pathway, running from the substantia nigra (SN) to the dorsal striatum is mainly associated with motor activity, as demonstrated by studies involving lesions [36] or unilateral stimulation [37] of the substantia nigra, and by the motor symptoms associated with Parkinson’s disease. On the other end, ventral striatum receives the mesolimbic pathway, originating in the ventral tegmental area (VTA), projecting to the nucleus accumbens and olfactory tubercle, and involved in reward-related motivation and learning [38]. The claim that dopamine subserves different functions in different striatal regions is also supported by more recent findings [39] showing that dopamine axons in the dorsal striatum preferentially signal locomotion, and those in progressively ventral regions preferentially signal reward. However, a quite complex relationship appears to exist between these two faces of dopaminergic modulation of striatal circuit function. For instance, data exist showing that motor function can still be achieved under conditions of high motivational drive despite dopamine depletion [40], suggesting the dorsal striatum as preferentially stimulating the execution of learned responses rather than motor movements per se. Moreover, recent evidence (see [35,41] for specific reviews) points to dopamine activity in the striatum as mainly conveying information regarding the value of an action, the outcome, and the strength of the outcome–action relationship during goal-directed learning [42,43].
As indicated in Table 1, five different subtypes of dopamine receptors (D1, D2, D3, D4, and D5) have been identified in brain tissue (see [5] for a review). They belong to the G protein-coupled receptors family, and based on their structure and pharmacological properties, they can be classified into two major groups [27]: D1-like receptors (including D1 and D5) and D2-like receptors (comprising D2, D3, and D4). Binding studies have demonstrated some differences between the two groups in terms of affinity to dopamine, with D2-like receptors exhibiting a 10- to 100-fold greater affinity to dopamine than D1-like receptors [44]. D1- and D2-like receptors also differ in their genetic structure. D2-like receptor genes have introns in their coding regions, while D1-like receptor genes do not exhibit this feature [45]. This genetic organization, therefore, enables the generation of D2-like receptor splice variants [46]. Concerning signal transduction, it is commonly accepted that the receptors of the D1-like group mainly mediate the stimulation of the second messenger adenylyl cyclase (cAMP) by coupling to the GS protein, whereas receptors of the D2-like group mainly exert inhibitory effects on this enzyme by coupling to Gi/0 protein [5,47]. In addition to the main pathway just mentioned, D1-like receptors may also couple to the Gq protein and modulate phospholipase C [28,48], leading to an increase in intracellular calcium levels and activation of protein kinase C.
All dopamine receptors are expressed in the striatum with abundant levels of D1 and D2 receptors and moderate expression of D3, D4, and D5 receptors [7]. D4 receptors are mainly localized in striatal nerve terminals of glutamatergic neurons localized in the cortex [49,50], and D5 receptors are mainly expressed by striatal cholinergic interneurons [51]. In the ventral striatum, striatal efferent medium spiny neurons (MSN) of the nucleus accumbens and the olfactory tubercle express quite high levels of the D3 receptor [52]. Very recent data [53] support an important role of this receptor in facilitating memory consolidation following a conditioned stimulus, a function having relevance to conditions (such as generalized anxiety or post-traumatic stress) characterized by a progressive worsening of responses to environmental cues.
D1 and D2, however, represent the dopamine receptors most abundantly expressed by striatal MSN. D1 receptors are mainly expressed by MSN forming the so-called “direct pathway”, while the MSN involved in the so-called “indirect pathway” mainly express D2 receptors [54]. Traditionally these two populations are considered to exhibit opposing control over striatal output. This functional segregation, however, is only approximate [55,56]. Despite the distinctive molecular fingerprinting of the two MSN populations, it has been observed that about 10% of the MSN express both D1 and D2 receptors [54]. Interestingly, it has also been suggested that many of these MSN (more than 90% in the nucleus accumbens and about 25% in dorsal striatum) present D1–D2 heterodimers, exhibiting pharmacological and signaling properties distinct from the constituent protomers [54,57,58]. Furthermore, D1 and D2 receptors can also be found pre-synaptically, where they modulate the nerve terminals activity [7]. In this context, of interest is also evidence showing that both D1 and D2 receptors are often co-expressed with D4 or D5 receptors [59], and with D3 receptors in the ventral striatum [60], where D1-D3 heterodimers [61] and D2–D3 heterodimers [62] have also been detected. These findings support the notion that the dichotomous division of MSN according to their expression of D1 or D2 receptors is not clear-cut [63,64,65].
2.2. Oxytocin
Oxytocin parvocellular neurons of the PVN provide the major ascending oxytocinergic pathways reaching forebrain areas, including ventral and dorsal striatum [66,67,68,69]. In these regions, oxytocin terminals appear very sparse when compared to the high density of dopamine terminal networks [15], the highest density of oxytocinergic fibers being observed in the nucleus accumbens [70]. Evidence from animal models (see [71] for a recent review) emphasizes the key role of ventral striatum in behavioral effects of oxytocin, such as social bonding and reward [72,73,74]. Consistently, a reduction in oxytocin receptors in the nucleus accumbens was reported to be associated with aggressive behavior in many species [75,76,77,78]. In humans, the modulatory role of oxytocin on the major striatal pathways has been explored by functional magnetic resonance imaging [79]. This study revealed that oxytocin specifically increased the connectivity between ventral striatum and upstream contralateral cingulate cortex, a circuit involved in reward and motivational processing [33,80,81]. At the same time, however, oxytocin was found to decrease the functional connectivity between putamen and the ipsilateral posterior cerebellar downstream regions, a circuit implicated in motor behavioral control and habit formation [82,83]. These findings, therefore, provide support for the notion that oxytocin in the striatum might shift processing from cerebellar regions involved in habitual responses to frontal pathways involved in context-dependent goal-directed behavior [80].
The oxytocin receptor (OTR) is a 389-amino acid polypeptide belonging to the G protein-coupled receptor family. For signal transduction OTR couples to the Gq protein that stimulates the activity of phospholipace C-β isoforms, leading to the generation of inositol trisphosphate (triggering Ca2+ release from intracellular stores) and 1,2-diacyl-glicerol (stimulating protein kinase C) [22]. This primary transduction pathway, however, is not the only one triggered by OTR. It is now established that the receptor can also couple to the Gi protein [22], leading to different cell responses [84].
OTRs exhibit a variable distribution in the brain of mammals [22] and they have been found in both dorsal and ventral striatum, where a basically complete overlap was observed between OTRs and oxytocin fibers with no significant mismatch [13]. Moreover, in these regions they clearly overlap with the distribution of dopamine D2-like receptors [15].
3. Oxytocin–Dopamine Interaction in the Striatum
As mentioned before, in both humans and animals, oxytocin and dopamine signals often act together to regulate a variety of social behaviors and habits [15,18,85]. Some of these functional processes involve the striatal circuitry (see [86] for a review).
From the neurophysiological standpoint, striatum is characterized by neuronal activity related to movements, rewards, and the conjunction of both movement and reward [87]. Thus, its neuronal activity is modulated by reward expectation concerning different stimuli, including feeding, sex, pleasure, and social situations [86,88]. In this respect, oxytocin and dopamine play a significant role, being both released in response to these stimuli [89,90,91] and appearing also involved in the mechanisms triggering the escalation of normal behaviors into addiction or abuse, as suggested by studies on side effects of L-dopa [92]. In this context, oxytocin and dopamine are also the most important signals that, acting together, trigger parental behavior. As a matter of fact, after childbirth, an increase in oxytocin receptor density occurs in areas of the brain reached by oxytocin neurons, including ventral striatum (nucleus accumbens in particular) [93,94]. At the same time, dopamine D2 receptors increase in the nucleus accumbens [95], and stimulation of D1 receptors in this region has been shown to stimulate the onset of maternal behavior in rats [96]. Correlations between maternal behavior, oxytocin, and dopamine levels have also been reported [97]. Of significant interest are also studies [95] in prairie voles (Microtus ochrogaster) indicating that the striatum is part of the neural circuitry underlying monogamous pair-bond formation, and that oxytocin and dopamine play a key role in this circuit. Stimulation of D2-like receptors in caudate-putamen induces partner preference, while D1-like activation prevents pair-bond formation [98,99,100]. Moreover, overexpression of oxytocin receptors in nucleus accumbens was shown to facilitate partner preference [101].
Apart from acting together, oxytocin and dopamine also appear to interact and modulate each other’s effects on striatal activity [7,102]. As indicated by Shahrock et al. [97], for instance, rat dams with a high degree of maternal behavior showed higher levels of dopamine in the nucleus accumbens. Such a condition was abolished following administration of an oxytocin antagonist, suggesting that the oxytocinergic system can regulate the release of dopamine. Consistent with these findings are also human studies based on the application of functional magnetic resonance imaging (fMRI), showing that fMRI signal intensity in the VTA and SN upon viewing a loved partner correlated with the distribution of oxytocin receptor expression [103]. On the other side, studies on drugs of abuse such as cocaine, known to target the mesolimbic dopaminergic system, showed that they significantly reduce the levels of oxytocin in the nucleus accumbens when taken repeatedly over time [104].
Of interest in this context are also available studies on the relationship between striatal oxytocin and dopamine in maladaptive social behavior conditions. Spontaneously hypertensive rats (SHR) have been used as animal models of ADHD [105]. In the nucleus, accumbens of these rats decreased levels of dopamine and changes in dopamine receptors have been reported [106]. Interestingly, SHR also exhibit lower oxytocin levels [107], and pretreatment with oxytocin was able to induce a larger increase in dopamine following methylphenidate treatment, an effect abolished administering an oxytocin antagonist [108]. Animal models of schizophrenia have also shown that oxytocin can modulate the activity in the mesolimbic dopamine pathway. However, in this case oxytocin would decrease the activity, having the capacity to inhibit the hyperactivity in the nucleus accumbens induced by drugs [109]. Several human studies have investigated the interaction between oxytocin and dopamine in the context of ASD, showing that there are links between oxytocin and dopamine also in this condition (see [18,85]). The relationship, however, is not well defined. In a study where improved learning in young adults with ASD was observed as a response to oxytocin, an increase in dopamine signaling in the nucleus accumbens was reported [110]. Studies however are available, reporting both increases and decreases [111,112].
3.1. Mechanisms of Interaction
Concerning the mechanisms underlying this mutual modulation of the two signals, different mechanisms can be identified. They will be briefly described here.
3.1.1. Network-Level Mechanism via Interconnected Brain Regions
As schematically illustrated in Figure 1a, first mechanism can be identified in the network of connections between dopaminergic and oxytocinergic regions projecting to the striatum. The dopaminergic incerto-hypothalamic pathway, for instance, projects to the SON and PVN, where oxytocin is produced [113], and dopamine receptors have been demonstrated in these oxytocin neurons [11]. The molecular mechanism behind the modulation of oxytocin release by dopamine is complex and is likely linked to the expression levels of the different dopamine receptor subtypes [18]. Oxytocin neurons mainly express dopamine D2 receptors, but D1 and D3 receptors have also been identified [32], and specific ligands targeting the different subtypes can differently influence the depolarization of the cells [114]. Oxytocin neurons, in turn, project to many areas with dopaminergic neurons, including SN [7,115] and VTA [7,116], where oxytocin receptors have also been identified [22,117].
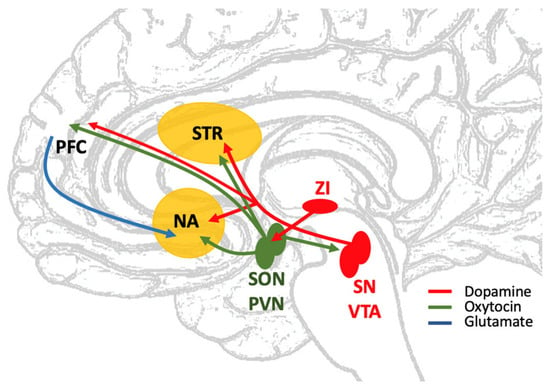
Figure 1.
Schematic view of dopaminergic and oxytocinergic pathways involving the striatum [7,15]. STR: dorsal striatum; NA: nucleus accumbens; SN: substantia nigra; VTA: ventral tegmental area; ZI: zona incerta; SON: supraoptic nucleus of the hypothalamus; PVN: paraventricular nucleus of the hypothalamus; PFC: prefrontal cortex. Dopaminergic fibers (red) of the nigro-striatal and mesolimbic pathways coming from SN and VTA target dorsal and ventral striatum. Mesocortical dopaminergic fibers run from VTA to PFC. Oxytocin fibers (green) from parvocellular neurons of PVN also reach the striatum (NA in particular). SON and PVN receive dopaminergic innervation from ZI, and, in turn, send oxytocinergic fibers to SN and VTA. Oxytocinergic fibers also reach neurons of the prefrontal cortex, that send axons (blue) to NA. Their glutamatergic signal could modulate the release of dopamine in this region of the ventral striatum [85].
3.1.2. Indirect Mechanism via Other Neurotransmitters
A second possible mechanism of interaction between oxytocin and dopamine signaling could be mediated by other signaling pathways. Concerning the striatum (see Figure 1), it has been suggested [85] that VTA dopaminergic terminals within the nucleus accumbens express glutamatergic receptors, and that glutamate release from the prefrontal cortex (a region receiving oxytocin fibers [23]) may modulate dopamine release in the accumbens. Supporting the hypothesis, the glutamate-induced release of dopamine has been demonstrated in vitro [118,119], and in vivo [120,121]. Furthermore, oxytocin administration into the prefrontal cortex was shown to induce an increased dopamine release in the nucleus accumbens [122].
3.1.3. Direct Molecular Mechanism via Receptor Complexes
A third and more direct mechanism of interaction is based on the existence of heteroreceptor complexes between dopamine D2 and oxytocin receptors (D2-OTR) in the neurons of the nucleus accumbens and the caudate putamen [25,123]. The basic molecular mechanism underlying the formation and the dynamics of these receptor assemblies are allosteric interactions (see [6] for a review). Allostery is a mode of communication between distant sites in a protein, in which the energy associated with dynamic or conformational changes at one site can be transferred (along specific pathways within the protein structure) to other sites, that, in turn, will change their conformational or dynamic features. Thus, when a quaternary structure is established via direct receptor–receptor interactions between protomers, energy perturbations at some site of one protomer can propagate into the nearby protomers and change their conformational and functional properties, leading to a cooperative behavior of the whole complex. Bidirectional facilitatory allosteric receptor–receptor interactions occur in the D2-OTR heteromer [26]. In particular, oxytocin-induced OTR activation involves enhancement of D2 recognition and signaling [15,26,123]. Figure 2 shows the structure of the heteroreceptor complex as obtained by molecular modeling methods [124]. The residues predicted to be mainly involved in the heteromerization interface are in the transmembrane domains four and five (TM4 and TM5) of both D2 and OTR.
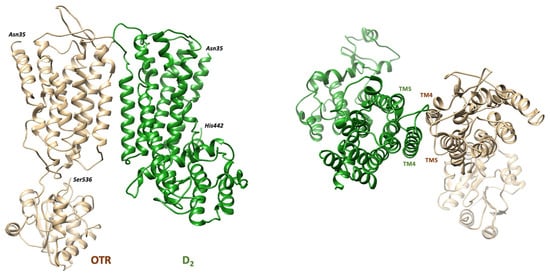
Figure 2.
Estimated model of the D2-OTR heterodimer. The structure of the complex as predicted by molecular modeling procedures [124] is shown on the (left) panel. On the (right), a view of the extracellular side is shown to indicate the TM4 and TM5 domains forming the interface between the two protomers.
Evidence, demonstrating that striatal astrocytes and microglia may represent a key element regulating the interaction between dopamine and oxytocin signaling in the striatum, is a further element of interest. This aspect will be briefly discussed in the section that follows.
4. Oxytocin–Dopamine Interactions Mediated by Striatal Astrocytes and a Potential Role for Microglia
In the dorsal striatum, distinct subpopulations of astrocytes, in response to cortical stimulation [125], release glutamate that activates N-methyl-D-aspartate (NMDA) receptors on specific medium spiny neurons and metabotropic glutamate receptors at distal synapses [126]. In the ventral striatum, astrocyte–neuron signaling is also well established as follows: astrocytes respond to neurotransmitters with Ca2+ increases and release of gliotransmitters (including glutamate and ATP/adenosine), thereby modulating neuronal activity and synaptic transmission [127]. Reported examples show that D2 receptors for dopamine, as well as OTR for oxytocin, may regulate the release of glutamate from the striatal astrocyte processes [128,129].
In this context, of particular interest are quite recent findings demonstrating that D2-OTR receptor–receptor interactions (RRI) can be established at the level of the plasma membrane of striatal astrocytes [124]. In this study, confocal imaging showed that both the receptors were expressed on the same astrocyte and astrocyte processes and that activation of either the D2 receptor or the OTR could inhibit the evoked glutamate release from the cell, suggesting that both oxytocin and dopamine (through OTR and D2 receptors, respectively) possess the capability to regulate glutamatergic transmission in striatal neuron–astrocyte networks. Furthermore, dopamine D2 and OTR were found to interact functionally. When oxytocin was bound to its receptor, the affinity of D2 receptors increased, allowing to make effective agonist subthreshold concentrations, otherwise too low, to activate the astrocytic D2 receptor. As demonstrated by co-immunoprecipitation experiments, this facilitatory RRI was based on receptor heteromerization, a result confirmed by proximity ligation assay [130] tests. Concerning the distribution in the astrocytic cell membrane, lipid rafts appear particularly enriched of these receptor complexes, as suggested by co-immunoprecipitation of oxytocin and dopamine D2 receptors with the membrane lipid rafts marker flotillin-1 [131].
The control of glutamatergic transmission in striatum, however, is not the only astrocyte function leading to an interaction between oxytocin and dopamine signaling. Oxytocin was reported to affect the perisynaptic astrocyte processes (PAPs) motility by retracting PAPs and regulating coverage of the synapse [132,133,134], allowing a shift from high privacy of the synaptic transmission (close enwrap of the synapse) to a broad opening of the enwrapping. This would lead to transmitter diffusion (by extra-synaptic volume transmission [135]) to neighboring synapses. Thus, the presence of OTR and D2-OTR heteromers on striatal PAPs suggests that oxytocin can be involved in the control of this process. Interestingly, it is known that in striatum, dopamine can also be released non-synaptically and act through volume transmission [136,137,138]. Therefore, the oxytocin’s ability to control astrocytic coverage of the synapses could represent a significant mechanism to modulate dopamine diffusion in striatum, potentially relevant in physiological and pathological conditions.
Available data suggest that also microglial cells are responsive to both oxytocin [139] and dopamine [140], leading to a reduction in the proinflammatory activity of microglia. Dopamine stimulation was found to decrease LPS-induced microglial nitric-oxide production [140], and oxytocin decreases proinflammatory factor levels of TNF-α and IL1-β [141]. In this respect, quite recent functional studies have shown that microglia help organize social circuits and shape social behavior associated with dopamine and oxytocin signaling, as, for instance, pair bonding (see [142] for a specific review). Consistently, neuroinflammation is a component of many disorders associated (see Section 3) with unbalanced dopamine–oxytocin interaction. This process is triggered by microglia in the classical M1 phenotype (see [143]) characterized by the production of proinflammatory factors and the release of glutamate [144]. Altogether these observations may suggest a possible involvement of microglia in the dynamics of the relationship between dopamine and oxytocin. Many aspects of these processes, however, would require additional research to be fully elucidated.
5. Concluding Remarks
Striatum can be described as a brain region containing a general neuronal mechanism to associate actions or events with reward. In particular, neural activity in the human striatum is modulated by social actions and, critically, by the conjunction of social actions and own reward [86]. Consistently, striatum is the target of a dense dopaminergic innervation from SN and VTA and of oxytocinergic fibers coming from parvocellular neurons of the hypothalamus. Dopamine and oxytocin are two key neurotransmitters with widespread functions in the brain. They control organic functions, as in the cardiovascular [29,30] system, but in both humans and animals, they are also released in response to afferent vagal and sensory stimulation, as well as sexual and social interactions, representing signals related to reward and pleasure associated with an event [18,102,145,146].
In this respect, dopamine and oxytocin have several effects in common, but of particular interest is evidence indicating that they can mutually modulate their action [7,18]. As discussed in the previous sections, in striatum different mechanisms mediating this interaction between the two signaling systems can be identified. Some of these processes involve dopaminergic or oxytocinergic cell groups projecting to the striatum, whose activity is modulated by signals based on the other neurotransmitter [113]. Other indirect processes involve a different signaling pathway, as in the abovementioned example of the glutamatergic cortico-striatal pathway, modulated by oxytocin signals and regulating dopamine release in the striatum [85]. Direct processes also exist, involving striatal cells and synapses. Examples include the formation of D2-OTR heteroreceptor complexes in striatal neurons and astrocytes [123,124], and the regulation by striatal astrocytes of dopaminergic volume transmission [124,132].
These mechanisms attracted attention also from a therapeutic standpoint. Of particular interest in this field are studies exploring strategies targeting oxytocinergic signaling to regulate dopaminergic transmission when altered. The dynamics of the D2-OTR heteroreceptor complex provides an example (see [134]). In this, heteromer oxytocin has a facilitatory effect on D2 receptor activation. Then, it might help to maintain dopaminergic neuron function in early Parkinson’s disease (PD), to delay the onset of PD symptoms related to defective dopamine receptor activation, and to make effective low doses of PD medications. Thus, the potential clinical usefulness of oxytocin as adjunctive drug therapy in PD patients would be based also on the possibility to reduce the dopaminergic therapy side effects. As a matter of fact, some evidence exists that after intranasal administration, oxytocin concentration increased in the striatum [147], and dopamine levels also increased [148], improving locomotor disabilities and anxiety-like behavior [149]. However, although in many studies oxytocin was shown to increase dopaminergic activity, evidence also exist identifying conditions in which oxytocin treatment has the capacity to decrease dopaminergic activity, as, for instance, in animal models of schizophrenia, where oxytocin seems to decrease dopaminergic activity in the nucleus accumbens [109].
Various mechanisms have been proposed to explain the observed opposite effects of oxytocin on dopaminergic activity. The variability in the oxytocin gene, as suggested by Love et al. [150], is a first possibility. In addition, very recently it has been reported that in striatal astrocytes oxytocin could induce dual responses, namely the inhibition and facilitation of both Ca2+ signals and glutamate release, and that the inhibitory and the facilitatory response appear dependent on activation by OTR of different transduction pathways, the Gi and the Gq pathway, respectively [151]. It is also possible that the many and sometime opposite effects might be explained by the expression levels of the different subtypes of dopamine receptors, and the possible different receptor complexes they could form [25,26]. The different effects, of course, may also be a consequence of the complex network of integrative processes [152] involving oxytocin and dopamine systems in the brain, and of the interactions they may have with other signaling lines [18,26]. Thus, this complex pattern of different and sometimes opposite effects makes it presently difficult to use dopamine and oxytocin (or analogs) as treatments.
The challenge is to find more specific agents acting on specific elements and processes mediating dopamine–oxytocin interactions. In this respect, receptor complexes involving dopamine receptors and OTR may represent a promising research line [26]. In view of the demonstrated existence of many heterodimers involving dopamine D3 receptor [61,153,154,155], of particular interest may be to test if also D3-OTR complexes exist in ventral striatum, and their involvement in striatal functional processes. The OTR transduction pathways certainly represent a second research objective. In view of the diverse effects the oxytocin receptor can induce [151] depending on the G protein it exploits, exploring possible strategies to modulate the transduction pathway may be a target of pharmacological interest. Finally, to expand our understanding of dopamine–oxytocin interaction, it is important to consider not only neuronal dynamics, but also the role and the contribution of glial cells. As briefly outlined here, recent data support a significant role of striatal astrocytes in the regulation of the interaction between dopamine and oxytocin signaling, and functional studies have been reported [142] suggesting that also microglial cells may have the capacity to be regulators of these processes. Specific studies on microglia, however, are still at the beginning and more research activity would be needed to characterize the potential regulatory mechanisms associated with these cells. A research effort applied to glial cells, however, may represent a topic of particular interest, not only to reach a better understanding of the role they have in the regulation of dopaminergic and oxytocinergic systems, but also from a therapeutic standpoint. Such a research effort, indeed, may open the possibility of exploring novel, glia-mediated, strategies to address the disorders associated with unbalance of these signals [156].
Author Contributions
Conceptualization, D.G., G.M., and L.F.A.; data curation, C.T., C.C., and M.M.; writing—original draft preparation, D.G.; writing—review and editing, D.G., G.M., and L.F.A.; supervision, D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable since no new data were recorded or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SON | Supraoptic nucleus |
| PVN | Paraventricular nucleus |
| SN | Substantia nigra |
| VTA | Ventral tegmental area |
| ASD | Autism spectrum disorder |
| ADHD | Attention deficit hyperactivity disorder |
| MSN | Medium spiny neurons |
| OTR | Oxytocin receptor |
| SHR | Spontaneously hypertensive rats |
| TM | Transmembrane domain |
| RRI | Receptor-receptor interaction |
| PAP | Perisynaptic astrocyte process |
References
- Bentivoglio, M.; Morelli, M. The organization and circuits of mesencephalic dopaminergic neurons and the distribution of dopamine receptors in the brain. In Dopamine (Handbook of Chemical Neuroanatomy); Dunnet, S.B., Bentivoglio, M., Björklund, A., Hökfelt, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 21, pp. 1–107. [Google Scholar]
- Dahlström, A.; Fuxe, K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol. Scand. 1964, 62 (Suppl. 232), 1–55. [Google Scholar]
- Fuxe, K. Evidence for the existence of monoamine neurons in the central nervous system. IV. Distribution of monoamine nerve terminals in the central nervous system. Acta Physiol. Scand. 1965, 247, 37. [Google Scholar]
- Albanese, A.; Altavista, M.C.; Rossi, P. Organization of central nervous system dopaminergic pathways. J. Neural Transm. 1986, 22, 3–17. [Google Scholar]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Function, signaling, and association with neurological diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef]
- Guidolin, D.; Tortorella, C.; Marcoli, M.; Cervetto, C.; De Caro, R.; Maura, G.; Agbati, L.F. Modulation of neurons and astrocyte dopamine receptors via receptor-receptor interactions. Pharmaceuticals 2023, 16, 1427. [Google Scholar] [CrossRef]
- Baskerville, T.A.; Douglas, A.J. Dopamine and oxytocin interactions underlying behaviors: Potential contributions to behavioral disorders. CNS Neurosci. Ther. 2010, 16, e92–e123. [Google Scholar] [CrossRef]
- Florea, T.; Palimariciuc, M.; Cristofor, A.C.; Dobrin, I.; Chirită, R.; Bîrsan, M.; Dobrin, R.P.; Pădurariu, M. Oxytocin: Narrative expert review of current perspectives on the relationship with other neurotransmitters and the impact on the main psychiatric disorders. Medicina 2022, 58, 923. [Google Scholar] [CrossRef]
- Buijs, R.M.; Swaab, D.F.; Dogterom, J.; van Leeuwen, F.W. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Cell Tissue Res. 1978, 186, 423–433. [Google Scholar] [CrossRef]
- Ludwig, M.; Leng, G. Dendritic peptide release and peptide-dependent behaviors. Nat. Rev. Neurosci. 2006, 7, 126–136. [Google Scholar] [CrossRef]
- Freundmercier, M.J.; Stoeckel, M.E.; Klein, M.J. Oxytocin receptors on oxytocin neurons—Histoautoradiographic detection in the lactating rat. J. Physiol. 1994, 480, 155–161. [Google Scholar] [CrossRef]
- Ferguson, J.N.; Aldag, J.M.; Insel, T.R.; Young, L.J. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J. Neurosci. 2001, 21, 8278–8285. [Google Scholar] [CrossRef]
- Grinevich, V.; Knobloch-Bollmann, H.S.; Eliava, M.; Busnelli, M.; Chini, B. Assembling the puzzle: Pathways of oxytocin signaling in the brain. Biol. Psych. 2016, 79, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Agnati, L.F.; Fuxe, K.; Zoli, M.; Ozini, I.; Toffano, G.; Ferraguti, F. A correlation analysis of the regional distribution of central enkephalin and beta endorphin immunoreactive terminals and of opiate receptors in adult and old male rats. Evidence for the existence of two main types of communication in the central nervous system: The volume transmission and the wiring transmission. Acta Physiol. Scand. 1986, 28, 201–207. [Google Scholar]
- Fuxe, K.; Borroto-Escuela, D.O.; Romero-Fernandez, W.; Ciruela, F.; Manger, P.; Leo, G.; Diaz-Cabiale, Z.; Agnati, L.F. On the role of volume transmission and receptor-receptor interactions in social behavior: Focus on central catecholamine and oxytocin neurons. Brain Res. 2012, 1476, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, D.; Marcoli, M.; Maura, G.; Agnati, L.F. New dimensions of connectomics and network plasticity in the central nervous system. Rev. Neurosci. 2017, 28, 113–132. [Google Scholar] [CrossRef]
- Bakos, J.; Srancikova, A.; Havranek, T.; Bacova, Z. Molecular mechanisms of oxytocin signaling at the synaptic connection. Neural Plast. 2018, 2018, 4864107. [Google Scholar] [CrossRef]
- Petersson, M.; Uvnäs-Moberg, K. Interactions of oxytocin and dopamine—Effects on behavior in health and disease. Biomedicines 2024, 12, 2440. [Google Scholar] [CrossRef]
- Veronneau-Longueville, F.; Rampin, O.; Freund-Mercier, M.J.; Tang, Y.; Calas, A.; Marson, L.; McKenna, K.E.; Stoeckel, M.E.; Benoît, G.; Giuliano, F. Oxytocinergic innervation of autonomic nuclei controlling penile erection in the rat. Neuroscience 1999, 93, 1437–1447. [Google Scholar] [CrossRef]
- Smeltzer, M.D.; Curtis, J.T.; Aragona, B.J.; Wang, Z.X. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci. Lett. 2006, 394, 146–151. [Google Scholar] [CrossRef]
- Buijs, R.M.; Geffard, M.; Pool, C.W.; Hoorneman, E.M. The dopaminergic innervation of the supraoptic and paraventricular nucles. A light and electron microscopical study. Brain Res. 1984, 323, 65–72. [Google Scholar] [CrossRef]
- Gimpl, G.; Fahrenholz, F. The oxytocin receptor system: Structure, function and regulation. Physiol. Rev. 2001, 81, 629–683. [Google Scholar] [CrossRef] [PubMed]
- Roeling, T.A.; Veening, J.G.; Peters, J.P.; Vermelis, M.E.; Nieuwenhuys, R. Efferent connections of the hypothalamic “grooming area” in the rat. Neuroscience 1993, 56, 199–225. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.R.; Succu, S.; Sanna, F.; Boi, A.; Argiolas, A. Oxytocin injected into the ventral subiculum or the posteromedial cortical nucleus of the amygdala induces penile erection and increases extracellular dopamine levels in the nucleus accumbens of male rats. Eur. J. Neurosci. 2009, 30, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- de La Mora, M.; Perez-Carrera, D.; Crespo-Ramirez, M.; Tarakanov, A.; Fuxe, K.; Borroto-Escuela, D.O. Signaling in dopamine D2 receptor-oxytocin receptor heterocompexes and its relevance for the anxiolytic effects of dopamine and oxytocin interactions in the amygdala of the rat. Biochim. Biophys. Acta 2016, 1862, 2075–2085. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.; Cuesta-Marti, C.; Lopez-Salas, A.; Chruscicka-Smaga, B.; Crespo-Ramirez, M.; Tesoro-Cruz, E.; Palacios-Lagunas, D.A.; Perez de la Mora, M.; Schellekens, H.; Fuxe, K. The oxytocin receptor represents a key hub in the GPCR heteroceptor network: Potential relevance for brain and behavior. Front. Mol. Neurosci. 2022, 15, 1055344. [Google Scholar] [CrossRef]
- Baik, J.-H. Dopamine Signaling in reward-related behaviors. Front. Neural Circuits 2013, 7, 152. [Google Scholar] [CrossRef]
- Rashid, A.; O’Dowd, B.F.; Verma, V.; George, S.R. Neuromal Gq/11-coupled dopamine receptors: An uncharted role for dopamine. Trends Pharmacol. Sci. 2007, 28, 551–555. [Google Scholar] [CrossRef]
- Neumann, J.; Hofmann, B.; Dhein, S.; Gergs, U. Role of dopamine in the heart in health and disease. Int. J. Mol. Sci. 2023, 24, 5042. [Google Scholar] [CrossRef]
- Jankowski, M.; Broderick, T.L.; Gutkowska, J. The role of oxytocin in cardiovascular protection. Front. Psychol. 2020, 11, 2139. [Google Scholar] [CrossRef]
- Choi, M.R.; Kouyoumdzian, N.M.; Rukavina Mikusic, N.L.; Kravetz, M.C.; Rosón, M.I.; Rodríguez Fermepin, M.; Fernández, B.E. Renal dopaminergic system: Pathophysiological implications and clinical perspectives. World J. Nephrol. 2015, 4, 196–212. [Google Scholar] [CrossRef]
- Baskerville, T.A.; Allard, J.; Wayman, C.; Douglas, A.J. Dopamine-oxytocin interactions in penile erection. Eur. J. Neurosci. 2009, 30, 2151–2164. [Google Scholar] [CrossRef]
- Arsalidou, M.; Duerden, E.G.; Taylor, M.J. The centre of the brain: Topographical model of motor, cognitive, affective, and somatosensory functions of the basal ganglia. Hum. Brain Mapp. 2013, 34, 3031–3054. [Google Scholar] [CrossRef]
- Haber, S.N. Corticostriatal circuitry. Dialogs Clin. Neurosci. 2016, 18, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Hart, G.; Burton, T.J.; Balleine, B.W. What role does striatal dopamine play in goal directed action? Neuroscience 2024, 546, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Ungerstedt, U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol. Scand. 1971, 82 (Suppl. 367), 1–48. [Google Scholar] [CrossRef] [PubMed]
- Arbuthnott, G.W.; Crow, T.J. Relation of contraversive turning to unilateral release of dopamine from the nigrostriatal pathway in rats. Exp. Neurol. 1971, 30, 484–491. [Google Scholar] [CrossRef]
- Robbins, T.W.; Everitt, B.J. Functions of dopamine in the dorsal and ventral striatum. Semin. Neurosci. 1992, 4, 119–127. [Google Scholar] [CrossRef]
- Howe, M.W.; Dombeck, D.A. Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature 2016, 535, 505–510. [Google Scholar] [CrossRef]
- Glickstein, M.; Stein, J. Paradoxical movement in Parkinson’s disease. Trends Neurosci. 1991, 14, 480–482. [Google Scholar] [CrossRef]
- Long, C.; Masmanidis, S.C. The learning primacy hypothesis of dopamine: Reconsidering dopamine’s dual functions. Front. Cell Neurosci. 2025, 19, 1538500. [Google Scholar] [CrossRef]
- Balleine, B.W.; Peak, J.; Matamales, M.; Bertran-Gonzalez, J.; Hart, G. The dorsomedial striatum: An optimal cellular environment for encoding and updating goal-directed learning. Curr. Opin. Behav. Sci. 2021, 41, 38–44. [Google Scholar] [CrossRef]
- Hart, G.; Burton, T.J.; Nolan, C.; Balleine, B.W. Striatal dopamine release tracks the relationship between actions and their consequences. Cell Rep. 2024, 43, 113828. [Google Scholar] [CrossRef]
- Tritsch, N.X.; Sabatini, B.L. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron 2012, 76, 33–50. [Google Scholar] [CrossRef]
- Gingrich, J.A.; Caron, M.G. Recent advances in the molecular biology of dopamine receptors. Annu. Rev. Neurosci. 1993, 16, 299–321. [Google Scholar] [CrossRef] [PubMed]
- Dal Toso, R.; Sommer, B.; Ewert, M.; Herb, A.; Pritchett, D.B.; Bach, A.; Shivers, B.D.; Seeburg, P.H. The dopamine D2 receptor: Two molecular forms generated by alternative splicing. EMBO J. 1989, 8, 4025–4034. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-M. Unveiling the differences in signaling and regulatory mechanisms between dopamine D2 and D3 receptors and their impact on behavioral sensitization. Int. J. Mol. Sci. 2023, 24, 6742. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Tyeryar, K.R.; Vongtau, H.O.; Sibley, D.R.; Undieh, A.S. D5 dopamine receptors are required for dopaminergic activation of phospholipase C. Mol. Pharmacol. 2009, 75, 447–453. [Google Scholar] [CrossRef]
- Tarazi, F.I.; Campbell, A.; Yeghiayan, S.K.; Baldessarini, R.J. Localization of dopamine receptor subtypes in corpus striatum and nucleus accumbens septi of rat brain: Comparison of D1-, D2- and D4-like receptors. Neuroscience 1997, 83, 169–176. [Google Scholar] [CrossRef]
- Ferré, S.; Belcher, A.M.; Bonaventura, J.; Quiroz, C.; Sánchez-Soto, M.; Casadó-Anguera, V.; Cai, N.S.; Moreno, E.; Boateng, C.A.; Keck, T.M.; et al. Functional and pharmacological role of the dopamine D4 receptor and its polymorphic variants. Front. Endocrinol. 2022, 13, 1014678. [Google Scholar] [CrossRef]
- Castello, J.; Cortés, M.; Malave, L.; Kottmann, A.; Sibley, D.R.; Friedman, E.; Rebholz, H. The dopamine D5 receptor contributes to activation of cholinergic interneurons during L-DOPA induced dyskinesia. Sci. Rep. 2020, 10, 2542, Erratum in Sci. Rep. 2020, 10, 4917. [Google Scholar] [CrossRef]
- Khan, Z.U.; Gutierrez, A.; Martin, R.; Penafiel, A.; Rivera, A.; De La Calle, A. Differential regional and cellular distribution of dopamine D2-like receptors: An immunocytochemical study of subtype-specific antibodies in rat and human brain. J. Comparat. Neurol. 1998, 402, 353–371. [Google Scholar] [CrossRef]
- Lapointe, T.; Baidoo, N.; Renda, B.; Leri, F. Role of the dopamine D3 receptor in the core and shell of the nucleus accumbens in conditioned modulation of memory consolidation. Neuropharmacology 2025, in press. [Google Scholar] [CrossRef]
- Soares-Cunha, C.; Coimbra, B.; Sousa, N.; Rodrigues, A.J. Reappraising striatal D1- and D2-neurons in reward and aversion. Neurosci. Biobehav. Rev. 2016, 68, 370–386. [Google Scholar] [CrossRef]
- Aizman, O.; Brismar, H.; Uhlen, P.; Zettergren, E.; Levey, A.I.; Forssberg, H.; Greengard, P.; Aperia, A. Anatomical and physiological evidence for D1 and D2 dopamine receptor co-localization in neostriatal neurons. Nat. Neurosci. 2000, 3, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Nieoullon, A. Dopamine and the regulation of cognition and attention. Prog. Neurobiol. 2002, 67, 53–83. [Google Scholar] [CrossRef] [PubMed]
- Perreault, M.L.; Hasbi, A.; O’Dowd, B.F.; George, S.R. The dopamine D1-D2 receptor heteromer in striatal medium spiny neurons: Evidence for a third distinct neuronal pathway in basal ganglia. Front. Neuroanat. 2011, 5, 31. [Google Scholar] [CrossRef]
- Perreault, M.L.; Hasbi, A.; O’Dowd, B.F.; George, S.R. Heteromeric dopamine receptor signaling complexes: Emerging neurobiology and disease relevance. Neuropsychopharmacology 2014, 39, 156–168. [Google Scholar] [CrossRef]
- Smith, Y.; Kieval, J.Z. Anatomy of the dopamine system in the basal ganglia. TINS 2000, 23 (Suppl. 1), S28–S33. [Google Scholar] [CrossRef]
- Lemoine, C.; Bloch, B. Expression of the D3 dopamine receptor in peptidergic neurons of the nucleus accumbens: Comparison with the D1 and D2 dopamine receptor. Neuroscience 1996, 73, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Bono, F.; Mutti, V.; Tomasoni, Z.; Sbrini, G.; Missale, C.; Fiorentini, C. Recent advances in dopamine D3 receptor heterodimers: Focus on dopamine D3 and D1 receptor-receptor interaction and striatal function. Curr. Top. Behav. Neurosci. 2023, 60, 47–72. [Google Scholar]
- Maggio, R.; Millan, M.J. Dopamine D2-D3 receptor heteromers: Pharmacological properties and theraputic significance. Curr. Opin. Pharmacol. 2010, 10, 100–107. [Google Scholar] [CrossRef]
- Thibault, D.; Loustalot, F.; Fortin, G.M.; Bourque, M.J.; Trudeau, L. Evaluation of D1 and D2 dopamine receptor segregation in the developing striatum using BAC transgenic mice. PLoS ONE 2013, 8, e67219. [Google Scholar] [CrossRef]
- Zinsmaier, A.K.; Dong, Y.; Huang, Y.H. Cocaine-induced projection-specific and cell type-specific adaptations in the nucleus accumbens. Mol. Psychiatry 2022, 27, 669–686. [Google Scholar] [CrossRef]
- Gayden, J.; Puig, S.; Srinivasan, C.; Phan, B.N.; Abdelhady, G.; Buck, S.A.; Gamble, M.C.; Tejeda, H.A.; Dong, Y.; Pfenning, A.R.; et al. Integrative multi-dimensional characterization of striatal projection neuron heterogeneity in adult brain. bioRxiv 2023, preprint. [Google Scholar] [CrossRef] [PubMed]
- De Vries, G.J.; Buijs, R.M. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983, 273, 307–317. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.B. General introduction to vasopressin and oxytocin: Structure/metabolism, evolutionary aspects, neural pathway/receptor distribution, and functional aspects relevant to memory processing. Adv. Pharmacol. 2004, 50, 655–708. [Google Scholar]
- Liao, P.-Y.; Chlu, Y.-M.; Ju, J.-H.; Chen, S.-K. Mapping central projections of oxytocin neurons in unmated mice using cre and alkaline phosphatase reporter. Front. Neuroanat. 2020, 14, 559402. [Google Scholar] [CrossRef]
- Froemke, R.C.; Young, L.J. Oxytocin, neural plasticity and social behavior. Annu. Rev. Neurosci. 2021, 44, 359–381. [Google Scholar] [CrossRef]
- Knobloch, H.S.; Charlet, A.; Hoffmann, L.C.; Eliava, M.; Khrulev, S.; Cetin, A.H.; Osten, P.; Schwarz, M.K.; Seeburg, P.H.; Stoop, R.; et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 2012, 73, 553–566. [Google Scholar] [CrossRef]
- Borland, J.M. A review of the effects of different types of social behaviors on the recruitment of neuropeptides and neurotransmitters in the nucleus accumbens. Front. Neuroendocrinol. 2025, 77, 101175. [Google Scholar] [CrossRef]
- Moaddab, M.; Hyland, B.I.; Brown, C.H. Oxytocin excites nucleus accumbens shell neurons in vivo. Mol. Cell. Neurosci. 2015, 68, 323–330. [Google Scholar] [CrossRef]
- King, L.B.; Walum, H.; Inoue, K.; Eyrich, N.W.; Young, L.J. Variation in the oxytocin receptor gene predicts brain region-specific expression and social attachment. Biol. Psychiatr. 2016, 80, 160–169. [Google Scholar] [CrossRef]
- Amadei, E.A.; Johnson, Z.V.; Jun Kwon, Y.; Shpiner, A.C.; Saravanan, V.; Mays, W.D.; Ryan, S.J.; Walum, H.; Rainnie, D.G.; Young, L.J.; et al. Dynamic corticostriatal activity biases social bonding in monogamous female prairie voles. Nature 2017, 546, 297–301. [Google Scholar] [CrossRef]
- Beery, A.K.; Zucker, I. Oxytocin and same-sex social behavior in female meadow voles. Neuroscience 2010, 169, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.E.M.; Neumann, I.D.; de Jong, T.R. Post-weaning social isolation exacerbates aggression in both sexes and affects the vasopressin and oxytocin system in a sex-specific manner. Neuropharmacology 2019, 156, 107504. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Sun, X.; Wang, Z.; Song, M.; Zhang, Z. Regulation of social behaviors by p-Stat3 via oxytocin and its receptor in the nucleus accumbens of male Brandt’s voles (Lasiopodomys brandtii). Horm Behav. 2020, 119, 104638. [Google Scholar] [CrossRef] [PubMed]
- Grieb, Z.A.; Ross, A.P.; McCann, K.E.; Lee, S.; Welch, M.; Gomez, M.G.; Norvelle, A.; Michopoulos, V.; Huhman, K.L.; Albers, H.E. Sex-Dependent Effects of Social Status on the Regulation of Arginine-Vasopressin (AVP) V1a, Oxytocin (OT), and Serotonin (5-HT) 1A Receptor Binding and Aggression in Syrian Hamsters (Mesocricetus auratus). Horm. Behav. 2021, 127, 104878. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, X.; Geng, Y.; Zhao, W.; Zhou, F.; Wang, J.; Markett, S.; Biswal, B.B.; Ma, Y.; Kendrick, K.M.; et al. Oxytocin differentially modulates specific dorsal and ventral striatal functional connections with frontal and cerebellar regions. Neuroimage 2019, 184, 781–789. [Google Scholar] [CrossRef]
- O’Doherty, J.P. Reward representations and reward-related learning in the human brain: Insights from neuroimaging. Curr. Opin. Neurobiol. 2004, 14, 769–776. [Google Scholar] [CrossRef]
- Kringelbach, M.L.; Berridge, K.C. Towards a functional neuroanatomy of pleasure and happiness. Trends Cognit. Sci. 2009, 13, 479–487. [Google Scholar] [CrossRef]
- Yin, H.H.; Knowlton, B.J.; Balleine, B.W. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur. J. Neurosci. 2004, 19, 181–189. [Google Scholar] [CrossRef]
- Habas, C.; Kamdar, N.; Nguyen, D.; Prater, K.; Beckmann, C.F.; Menon, V.; Greicius, M.D. Distinct cerebellar contributions to intrinsic connectivity networks. J. Neurosci. 2009, 29, 8586–8594. [Google Scholar] [CrossRef] [PubMed]
- Busnelli, M.; Saulière, A.; Manning, M.; Bouvier, M.; Galés, C.; Chini, B. Functional selective oxytocin-derived agonists discriminate between individual G protein family subtypes. J. Biol. Chem. 2012, 287, 3617–3629. [Google Scholar] [CrossRef] [PubMed]
- Rappenau, V.; Castillo Dìaz, F. Convergence of oxytocin and dopamine signaling in neuronal circuits: Insight into the neurobiology of social interactions across species. Neurosci. Biobehav. Rev. 2024, 161, 105675. [Google Scholar] [CrossRef]
- Bàez-Mendoza, R.; Schultz, W. The role of the striatum in social behavior. Front. Neurosci. 2013, 7, 233. [Google Scholar] [CrossRef] [PubMed]
- Hollerman, J.R.; Tremblay, L.; Schultz, W. Influence of reward expectation on behavior-related neuronal activity in primate striatum. J. Neurophysiol. 1998, 80, 947–963. [Google Scholar] [CrossRef]
- Schultz, W.; Apicella, P.; Scarnati, E.; Ljungberg, T. Neuronal activity in monkey ventral striatum related to the expectation of reward. J. Neurosci. 1992, 12, 4595–4610. [Google Scholar] [CrossRef]
- Koepp, M.J.; Gunn, R.N.; Lawrence, A.D.; Cunningham, V.J.; Dagher, A.; Jones, T.; Brooks, D.J.; Bench, C.J.; Grasby, P.M. Evidence for striatal dopamine release during a video game. Nature 1998, 393, 266–268. [Google Scholar] [CrossRef]
- Blood, A.J.; Zatorre, R.J. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotions. Proc. Natl. Acad. Sci. USA 2001, 98, 11818–11823. [Google Scholar] [CrossRef]
- Ooishi, Y.; Mukai, H.; Watanabe, K.; Kawato, S.; Kashino, M. Increase in salivary oxytocin and decrease in salivary cortisol after listening to relaxing slow-tempo and exciting fast-tempo music. PLoS ONE 2017, 12, e0189075. [Google Scholar] [CrossRef]
- Friedman, J.H. Punding on levodopa. Biol. Psychiatry 1994, 36, 350–351. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, K.M. Oxytocin, motherhood and bonding. Exp. Physiol. 2000, 85, 111S–124S. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Andrade, G.; Kendrick, K.M. The main olfactory system and social learning in mammals. Behav. Brain Res. 2009, 200, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Z. Nucleus Accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 2003, 121, 537–544. [Google Scholar] [CrossRef]
- Stolzenberg, D.S.; McKenna, J.B.; Keough, S.; Hancock, R.; Numan, M.J.; Numan, M. Dopamine D1 receptor stimulation of the nucleus accumbens or the medial preoptic area promotes the onset of maternal behavior in pregnancy-terminated rats. Behav. Neurosci. 2007, 121, 907–919. [Google Scholar] [CrossRef]
- Shahrokh, D.K.; Zhang, T.Y.; Diorio, J.; Gratton, A.; Meaney, M.J. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology 2010, 151, 2276–2286. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, G.; Cascio, C.; Liu, Y.; Gingrich, B.; Insel, T.R. Dopamine D2 receptor-mediated regulation of partner preferences in female prairie voles (Microtus ochrogaster): A mechanism for pair bonding? Behav. Neurosci. 1999, 113, 602–611. [Google Scholar] [CrossRef]
- Aragona, B.J.; Liu, Y.; Curtis, T.; Stephan, F.K.; Wang, Z.X. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J. Neurosci. 2003, 23, 3483–3490. [Google Scholar] [CrossRef]
- Aragona, B.J.; Liu, Y.; Yu, Y.J.; Curtis, J.T.; Detwiler, J.M.; Insel, T.R.; Wang, Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat. Neurosci. 2006, 9, 133–139. [Google Scholar] [CrossRef]
- Ross, H.E.; Freeman, S.M.; Spiegel, L.L.; Ren, X.; Terwilliger, E.F.; Young, L.J. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J. Neurosci. 2009, 29, 1312–1318. [Google Scholar] [CrossRef]
- Love, T.M. Oxytocin, motivation and the role of dopamine. Pharmacol. Biochem. Behav. 2014, 119, 49–60. [Google Scholar] [CrossRef]
- Bartels, A.; Zeki, S. The chronoarchitecture of the human brain—Natural viewing conditions reveal a time-based anatomy of the brain. Neuroimage 2004, 22, 419–433. [Google Scholar] [CrossRef]
- Sarnyai, Z.; Vecsernyes, M.; Laczi, F.; Biro, E.; Szabo, G.; Kovacs, G.L. Effects of cocaine on the contents of neurohypophyseal hormones in the plasma and in different brain structures in rats. Neuropeptides 1992, 23, 27–31. [Google Scholar] [CrossRef]
- Kim, D.; Yaday, D.; Song, M. An updated review on animal models to study attention-deficit hyperactivity disorder. Transl. Psychiatry 2024, 14, 187. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lu, G.; Antonio, G.; Mak, Y.; Rudd, J.A.; Fan, M.; Yew, D.T. The usefulness of the spontaneously hypertensive rat to model attention-deficit/hyperactivity disorder (ADHD) may be explained by the differential expression of dopamine-related genes in the brain. Neurochem. Int. 2007, 50, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Van Tol, H.H.; van den Buuse, M.; de Jong, W.; Burbach, J.P. Vasopressin and oxytocin gene expression in the supraoptic and paraventricular nucleus of the spontaneously hypertensive rat (SHR) during development of hypertension. Brain Res. 1988, 464, 303–311. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hersey, M.; Bacon, A.K.; Bailey, L.G.; Lee, M.R.; Chen, A.Y.; Leggio, L.; Tanda, G. Oxytocin receptors mediate oxytocin potentiation of methylphenidate-induced stimulation of accumbens dopamine in rats. J. Neurochem. 2023, 164, 613–623. [Google Scholar] [CrossRef]
- Jones, C.A.; Watson, D.J.G.; Fone, K.C.R. Animal models of schizophrenia. Br. J. Pharmacol. 2011, 164, 1162–1194. [Google Scholar] [CrossRef]
- Kruppa, J.A.; Gossen, A.; Oberwelland, E.W.; Kohls, G.; Grosheinrich, N.; Cholemkery, H.; Freitag, C.M.; Karges, W.; Wölfle, E.; Sinzig, J.; et al. Neural modulation of social reinforcement learning by intranasal oxytocin in male adults with high-functioning autism spectrum disorder: A randomized trial. Neuropsychopharmacology 2019, 44, 749–756. [Google Scholar] [CrossRef]
- El-Ansary, A.K.; Bacha, A.B.; Al-Ayahdi, L. Relationship between chronic lead toxicity and plasma neurotransmitters in autistic patients from Saudi Arabia. Clin. Biochem. 2011, 44, 1116–1120. [Google Scholar] [CrossRef]
- Alabdali, A.; Al-Ayadhi, L.; El-Ansary, A. Association of social and cognitive impairment and biomarkers in autisms pectrum disorders. J. Neuroinflamm. 2014, 11, 4. [Google Scholar] [CrossRef]
- Björklund, A.; Lindvall, O.; Nobin, A. Evidence of an incertohypothalamic dopamine neurone system in the rat. Brain Res. 1975, 89, 29–42. [Google Scholar] [CrossRef]
- Uvnäs-Moberg, K.; Alster, P.; Svensson, T.H. Amperozide and clozapine but not haloperidol or raclopride increase the secretion of oxytocin in rats. Psychopharmacology 1992, 109, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Sawchenko, P.E.; Swanson, L.W. Relationship of oxytocin pathways to the control of neuroendocrine and autonomic function. J. Steroid Biochem. Mol. Biol. 1984, 20, 87–103. [Google Scholar] [CrossRef]
- Whitman, D.C.; Albers, H.E. Oxytocin immunoreactivity in the hypothalamus of female hamsters. Cell Tissue Res. 1998, 291, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Warfvinge, K.; Krause, D.; Edvinsson, L. The distribution of oxytocin and the oxytocin receptor in rat brain: Relation to regions active in migraine. J. Headache Pain 2020, 21, 10. [Google Scholar] [CrossRef]
- Clow, D.W.; Jhamandas, K. Characterization of L-glutamate action on the release of endogenous dopamine from the rat caudate-putamen. J. Pharmacol. Exp. Ther. 1989, 248, 722–728. [Google Scholar] [CrossRef]
- Desce, J.M.; Godeheu, G.; Galli, T.; Artaud, F.; Ch’eramy, A.; Glowinski, J. L-glutamate-evoked release of dopamine from synaptosomes of the rat striatum: Involvement of AMPA and N-methyl-D-aspartate receptors. Neuroscience 1992, 47, 333–339. [Google Scholar] [CrossRef]
- Krebs, M.O.; Kemel, M.L.; Gauchy, C.; Desban, M.; Glowinski, J. Glycine potentiates the NMDA-induced release of dopamine through a strychnine-insensitive site in the rat striatum. Eur. J. Pharmacol. 1989, 166, 567–570. [Google Scholar] [CrossRef]
- Galli, T.; Godeheu, G.; Artaud, F.; Desce, J.M.; Pittaluga, A.; Barbeito, L.; Glowsinki, J.; Chéramy, A. Specific role of N-acetyl-aspartyl-glutamate in the in vivo regulation of dopamine release from dendrites and nerve terminals of nigrostriatal dopaminergic neurons in the cat. Neuroscience 1991, 42, 19–28. [Google Scholar] [CrossRef]
- Young, K.A.; Liu, Y.; Gobrogge, K.L.; Wang, H.; Wang, Z. Oxytocin reverses amphetamine-induced deficits in social bonding: Evidence for an interaction with nucleus accumbens dopamine. J. Neurosci. 2014, 34, 8499–8506. [Google Scholar] [CrossRef]
- Romero-Fernandez, W.; Borroto-Escuela, D.O.; Agnati, L.F.; Fuxe, K. Evidence for the existence of dopamine D2-oxytocin receptor heteromers in the ventral and dorsal striatum with facilitatory receptor-receptor interactions. Mol. Psychiatry 2013, 18, 849–850. [Google Scholar] [CrossRef]
- Amato, S.; Averna, M.; Guidolin, D.; Ceccoli, C.; Gatta, E.; Candiani, S.; Pedrazzi, M.; Capraro, M.; Maura, G.; Agnati, L.F.; et al. Heteromerization of Dopamine D2 and Oxytocin Receptor in Adult Striatal Astrocytes. Int. J. Mol. Sci. 2023, 24, 4677. [Google Scholar] [CrossRef]
- Cavaccini, A.; Durkee, C.; Kofuji, P.; Tonini, R.; Araque, A. Astrocyte signaling gates long-term depression at corticostriatal synapses of the direct pathway. J. Neurosci. 2020, 40, 5757–5768. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Bajo-Grañeras, R.; Moratalla, R.; Perea, G.; Araque, A. circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 2015, 349, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Corkrum, M.; Araque, A. Astrocyte-neuron signaling in the mesolimbic dopamine system: The hidden stars of dopamine signaling. Neuropsychopharmacology 2021, 46, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Cervetto, C.; Venturini, A.; Passalacqua, M.; Guidolin, D.; Genedani, S.; Fuxe, K.; Borroto-Esquela, D.O.; Cortelli, P.; Woods, A.; Maura, G.; et al. A2A-D2 receptor–receptor interaction modulates gliotransmitter release from striatal astrocyte processes. J. Neurochem. 2017, 140, 268–279. [Google Scholar] [CrossRef]
- Cervetto, C.; Venturini, A.; Guidolin, D.; Maura, G.; Passalacqua, M.; Tacchetti, C.; Cortelli, P.; Genedani, S.; Candiani, S.; Ramoino, P.; et al. Homocysteine and A2A-D2 receptor-receptor interaction at striatal astrocyte processes. J. Mol. Neurosci. 2018, 65, 456–466. [Google Scholar] [CrossRef]
- Trifilieff, P.; Rives, M.-L.; Urizar, E.; Piskorowski, R.A.; Vishwasrao, H.D.; Castrillon, J.; Schmauss, C.; Slättman, M.; Gullberg, M.; Javitch, J.A. Detection of antigen interactions Ex Vivo by proximity ligation assay: Endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. Biotechniques 2011, 51, 111–118. [Google Scholar] [CrossRef]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef]
- Theodosis, D.T.; Poulain, D.A. Activity-dependent neuronal-glial and synaptic plasticity in the adult mammalian hypothalamus. Neuroscience 1993, 57, 501–535. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Hatton, G.I. Astrocytic plasticity and patterned oxytocin neuronal activity: Dynamic interactions. J. Neurosci. 2009, 29, 1743–1754. [Google Scholar] [CrossRef]
- Meinung, C.-P. Oxytocin Receptor-Mediated Signaling in Astrocytes. Ph.D. Thesis, Universitat Regensburg, Regensburg, Germany, 2020. [Google Scholar]
- Agnati, L.F.; Genedani, S.; Spano, P.F.; Guidolin, D.; Fuxe, K. Volume Transmission and the Russian-doll Organization of Brain Cell Networks: Aspects of their Integrative Actions. Neuronal Networks. In Brain Function, CNS Disorders and Therapeutics; Faingold, C.L., Blumenfeld, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 103–119. [Google Scholar]
- Bjelke, B.; Stromberg, I.; O’Connor, W.T.; Andbjer, B.; Agnati, L.F.; Fuxe, K. Evidence for volume transmission in the dopamine denervated neostriatum of the rat after a unilateral nigral 6-OHDA microinjection. Studies with systemic D-amphetamine treatment. Brain Res. 1994, 662, 11–24. [Google Scholar] [CrossRef]
- Jansson, A.; Goldstein, M.; Tinner, B.; Zoli, M.; Meador-Woodruff, J.H.; Lew, J.Y.; Levey, A.I.; Watson, S.; Agnati, L.F.; Fuxe, K. On the distribution patterns of D1, D2, tyrosine hydroxylase and dopamine transporter immunoreactivities in the ventral striatum of the rat. Neuroscience 1999, 89, 473–489. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, K.; Agnati, L.F.; Marcoli, M.; Borroto-Escuela, D.O. Volume Transmission in central dopamine and noradrenaline neurons and its astroglial targets. Neurochem. Res. 2015, 40, 2600–2614. [Google Scholar] [CrossRef]
- Mairesse, J.; Zinni, M.; Pansiot, J.; Hassan-Abdi, R.; Demene, C.; Colella, M.; Charriaut-Marlangue, C.; Rideau Batista Novais, A.; Tanter, M.; Maccari, S.; et al. Oxytocin receptor agonist reduces perinatal brain damage by targeting microglia. Glia 2019, 67, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Färber, K.; Pannasch, U.; Kettenmann, H. Dopamine and noradrenaline control distinct functions in rodent microglial cells. Mol. Cell Neurosci. 2005, 29, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Liu, S.; Bai, X.; Gao, Y.; Liu, G.; Wang, X.; Liu, D.; Li, T.; Hao, A.; Wang, Z. Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. J. Neuroinflamm. 2016, 13, 77. [Google Scholar] [CrossRef]
- Loth, M.K.; Donaldson, Z.R. Oxytocin, dopamine, and opioid interactions underlying pair bonding: Highlighting a potential role for microglia. Endocrinology 2021, 162, bqaa223. [Google Scholar] [CrossRef]
- Eggen, B.J.L.; Raj, D.; Hanisch, U.K.; Boddeke, H.W. Microglial phenotype and adaptation. J. Neuroimmune Pharmacol. 2013, 8, 807–823. [Google Scholar] [CrossRef]
- Guidolin, D.; Tortorella, C.; Marcoli, M.; Cervetto, C.; De Caro, R.; Maura, G.; Agnati, L.F. Possible roles of heteroreceptor complexes in exitotoxic processes. Exlor. Neuroprot. Ther. 2024, 4, 366–391. [Google Scholar] [CrossRef]
- Uvnas-Moberg, K. Role of efferent and afferent vagal nerve activity during reproduction: Integrating function of oxytocin on metabolism and behavior. Psychoneuroendocrinology 1994, 19, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Field, T.; Hernandez-Reif Diego, M.; Schanberg, S.; Kuhn, C. Cortisol decreases and serotonin and dopamine increase following massage therapy. Int. J. Neurosci. 2005, 115, 1397–1413. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, D.C.; Hanson, L.R.; Carson, D.S.; Tunstall, B.J.; Lee, M.R.; Tzabazis, A.Z.; Jacobs, D.; Frey, W.H. Nasal oxytocin for the treatment of psychiatric disorders and pain: Achieving meaningful brain concentrations. Transl. Psychiatry 2021, 11, 388. [Google Scholar] [CrossRef]
- Gamal-Eltrabily, M.; Manzano-García, A. Role of central oxytocin and dopamine systems in nociception and their POSSIBLE interactions: Suggested hypotheses. Rev. Neurosci. 2018, 29, 377–386. [Google Scholar] [CrossRef]
- Almansoub, H.A.M.M.; Tang, H.; Wu, Y.; Wang, D.-Q.; Mahaman, Y.A.R.; Salissou, M.T.M.; Lu, Y.; Hu, F.; Zhou, L.-T.; Almansob, Y.A.M.; et al. Oxytocin alleviates MPTP-induced neurotoxicity in mice by targeting MicroRNA-26a/death-associated protein kinase 1 pathway. J. Alzheimers Dis. 2020, 74, 883–901. [Google Scholar] [CrossRef]
- Love, T.M.; Enoch, M.A.; Hodgkinson, C.A.; Pecina, M.; Mickey, B.; Koeppe, R.A.; Stohler, C.S.; Goldman, D.; Zubieta, J.K. Oxytocin gene polymorphisms influence human dopaminergic function in a sex-dependent manner. Biol. Psychiatry 2012, 72, 198–206. [Google Scholar] [CrossRef]
- Farsetti, E.; Amato, S.; Averna, M.; Guidolin, D.; Pedrazzi, M.; Maura, G.; Agnati, L.F.; Cervetto, C.; Marcoli, M. Dual oxytocin signals in striatal astrocytes. Biomolecules 2025, 15, 1122. [Google Scholar] [CrossRef]
- Agnati, L.F.; Marcoli, M.; Maura, G.; Woods, A.; Guidolin, D. The brain as a “hyper-network”: The key role of neural networks as main producers of the integrated brain actions especially via the “broadcasted” connectomics. J. Neural Transm. 2018, 125, 883–897. [Google Scholar] [CrossRef]
- Scarselli, M.; Novi, F.; Schallmach, E.; Lin, R.; Baragli, A.; Colzi, A.; Griffon, N.; Corsini, G.U.; Sokoloff, P.; Levenson, R.; et al. D2/D3 dopamine receptor heterodimers exhibit unique functional properties. J. Biol. Chem. 2001, 276, 30308–30314. [Google Scholar] [CrossRef]
- Torvinen, M.; Marcellino, D.; Canals, M.; Agnati, L.F.; Lluis, C.; Franco, R.; Fuxe, K. Adenosine A2A receptor and dopamine D3 receptor interactions: Evidence of functional A2A/D3 heteromeric complexes. Mol. Pharmacol. 2005, 67, 400–407. [Google Scholar] [CrossRef]
- Fiorentini, C.; Busi, C.; Gorruso, E.; Gotti, C.; Spano, P.; Missale, C. Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization. Mol. Pharmacol. 2008, 74, 59–69. [Google Scholar] [CrossRef]
- Guidolin, D.; Tortorella, C.; Marcoli, M.; Cervetto, C.; Maura, G.; Agnati, L.F. Receptor-receptor interactions and glial cell functions with a special focus on G protein-coupled receptors. Int. J. Mol. Sci. 2021, 22, 8656. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).