Coptidis Rhizoma Water Extract Attenuates RANKL-Induced Osteoclast Differentiation via MAPK, Akt, and NF-κB Pathways and Prevents Ovariectomy (OVX)-Mediated Bone Loss
Abstract
1. Introduction
2. Results
2.1. Determination of Coptisine and Berberine Concentration in CRW
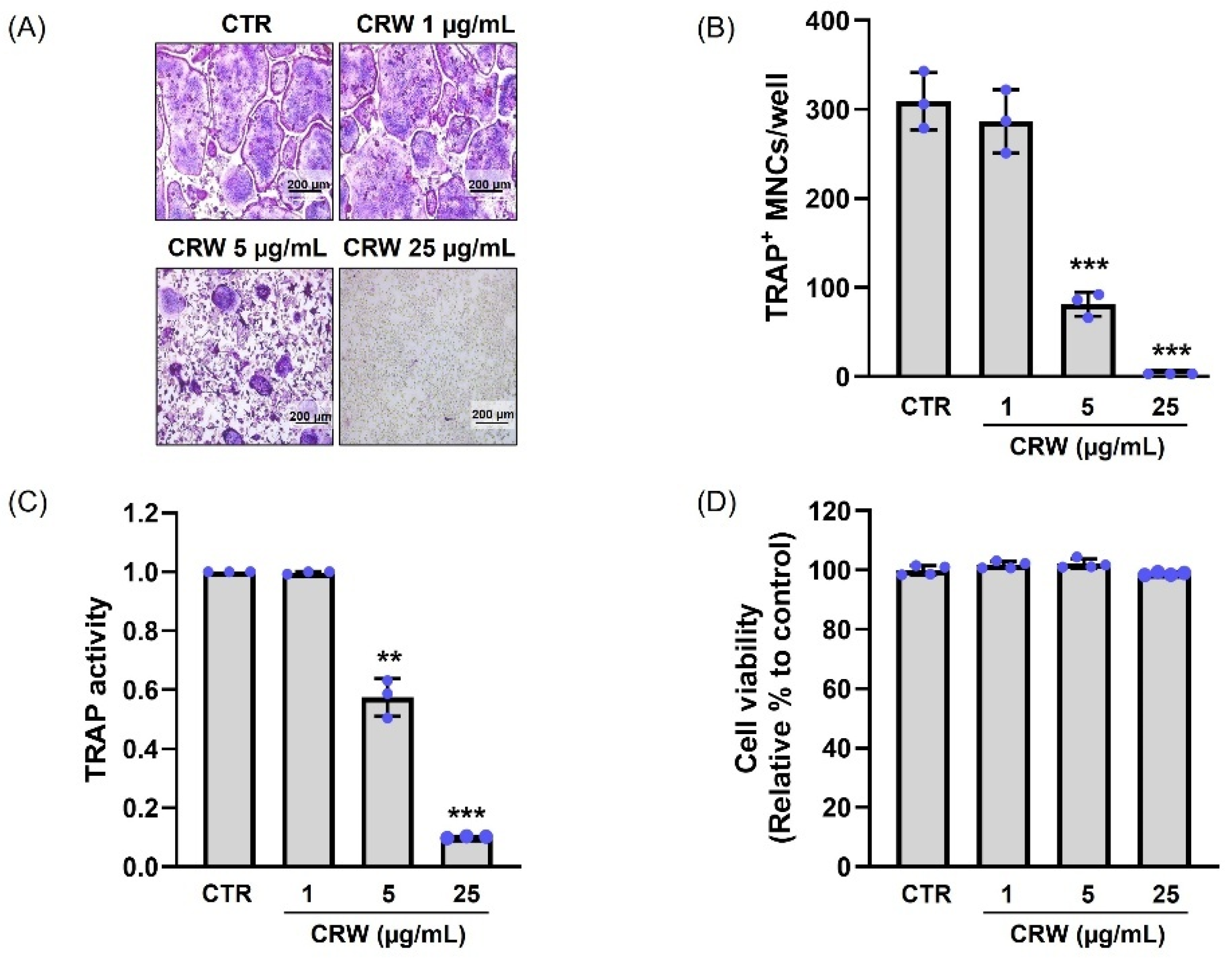
2.2. CRW Blocks RANKL-Activated Osteoclast Differentiation Without Cytotoxicity
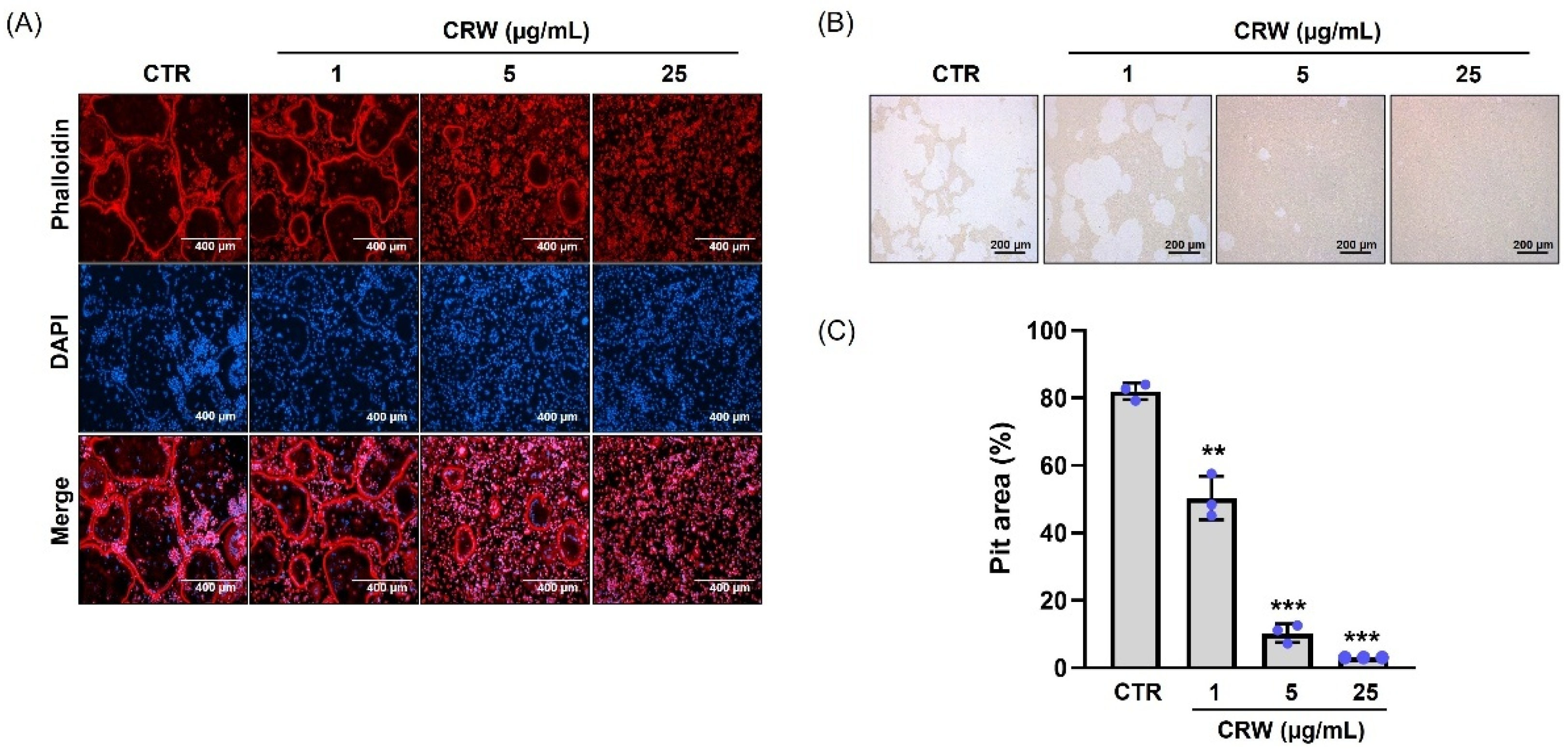
2.3. CRW Suppresses F-Actin Ring and Bone Resorption Activated by RANKL
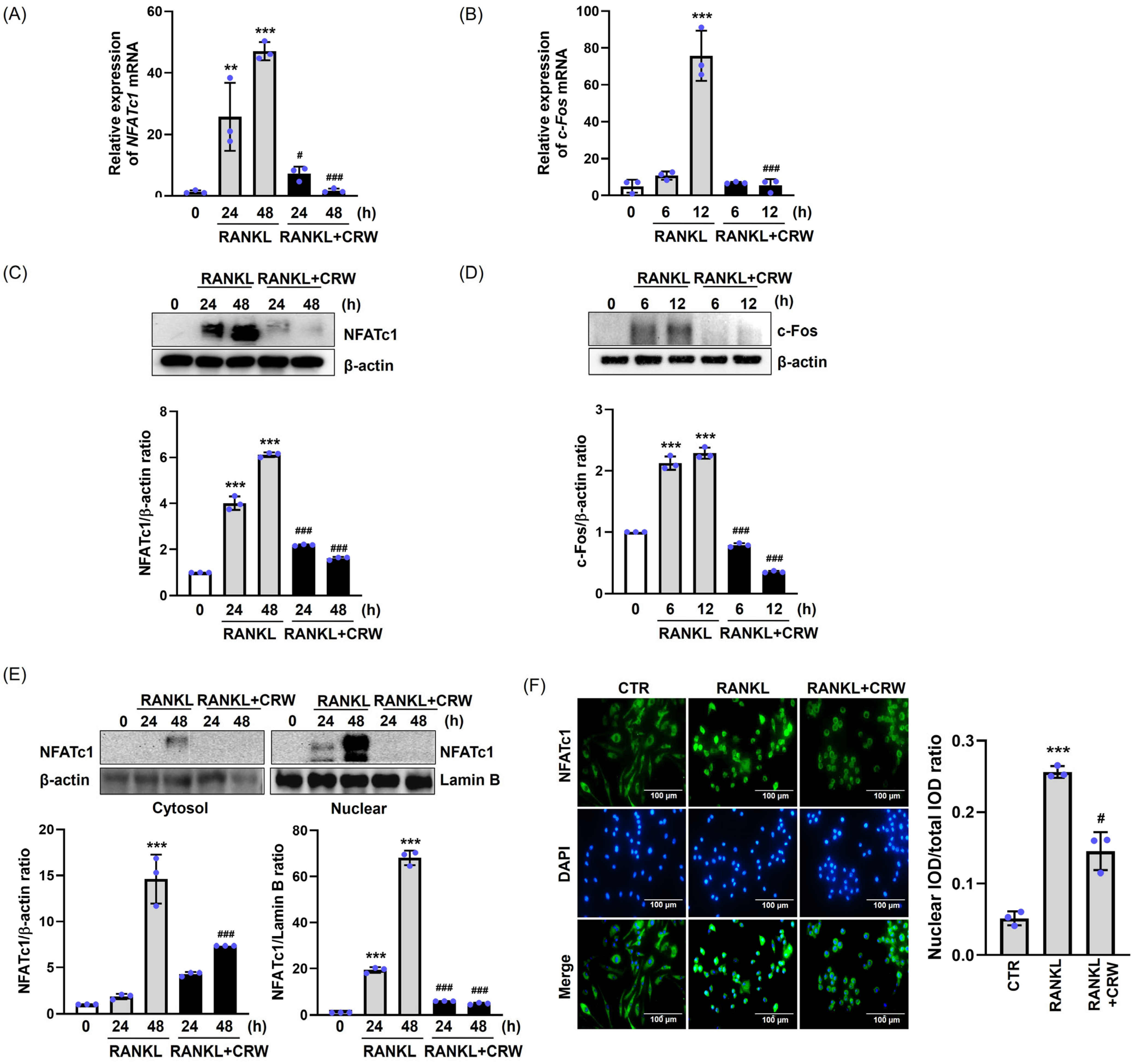
2.4. CRW Downregulates RANKL-Mediated Induction of NFATc1 and c-Fos
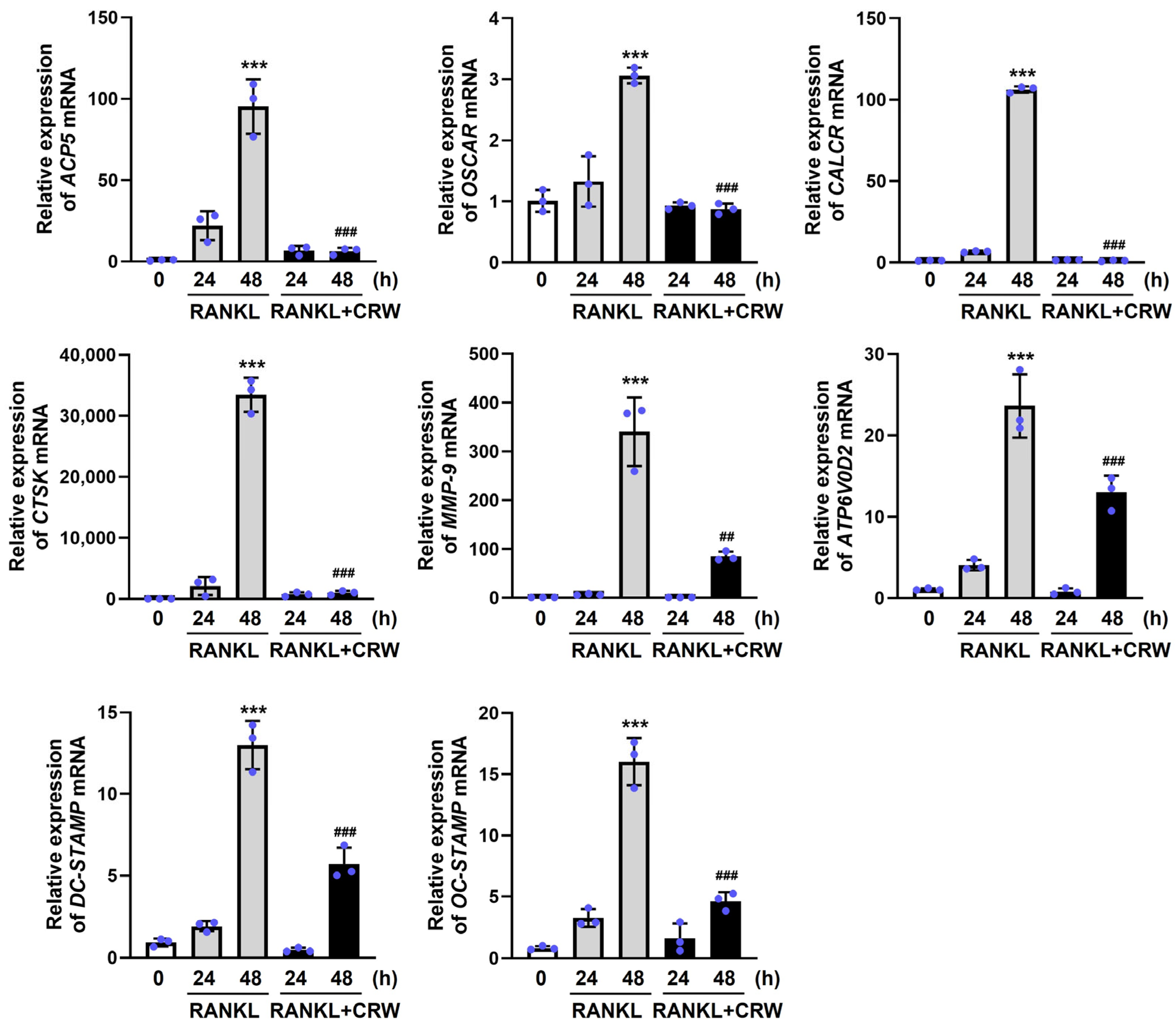
2.5. CRW Attenuates RANKL-Activated Induction of Osteoclast Marker Genes
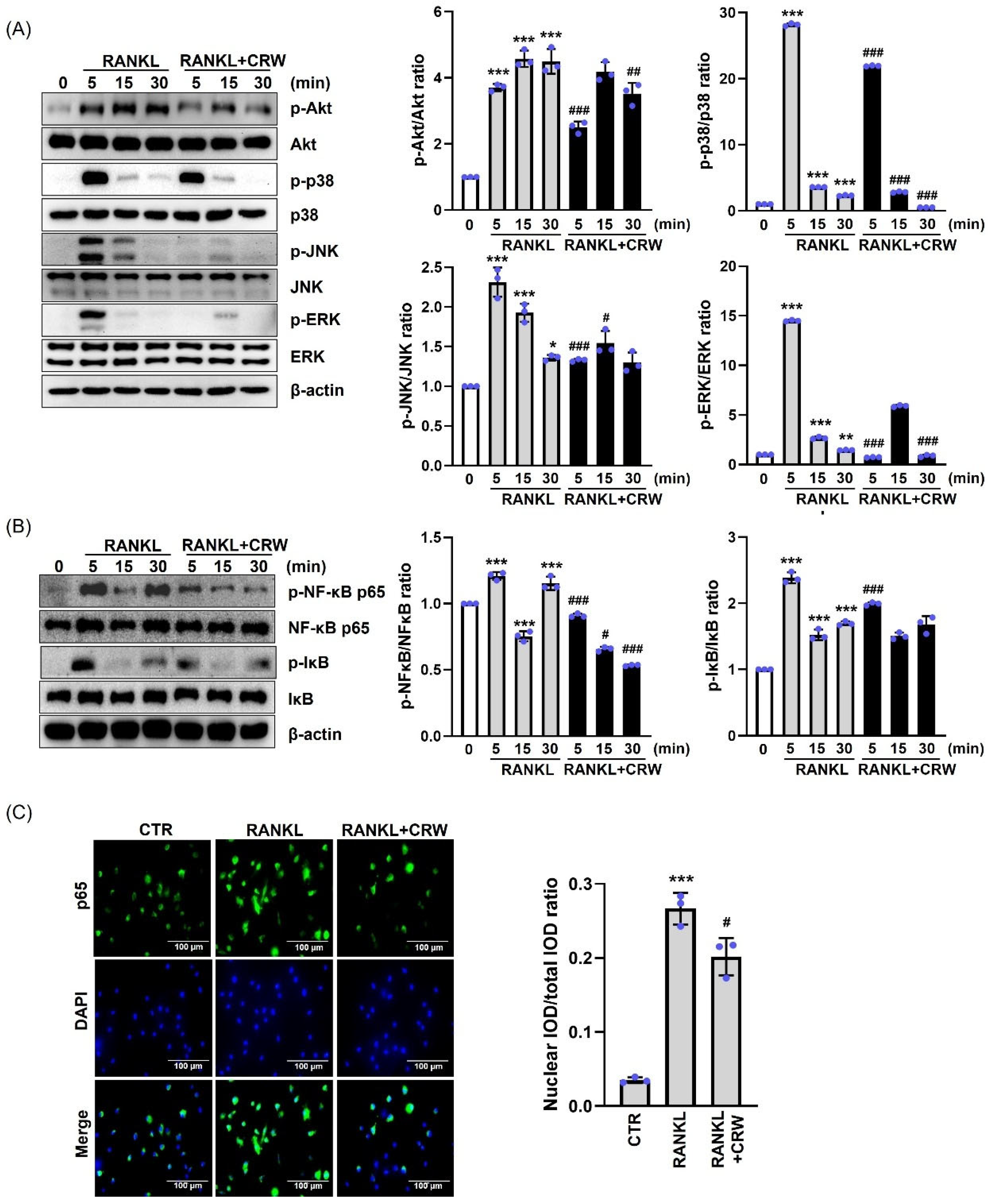
2.6. CRW Inhibited Early Signaling Pathway
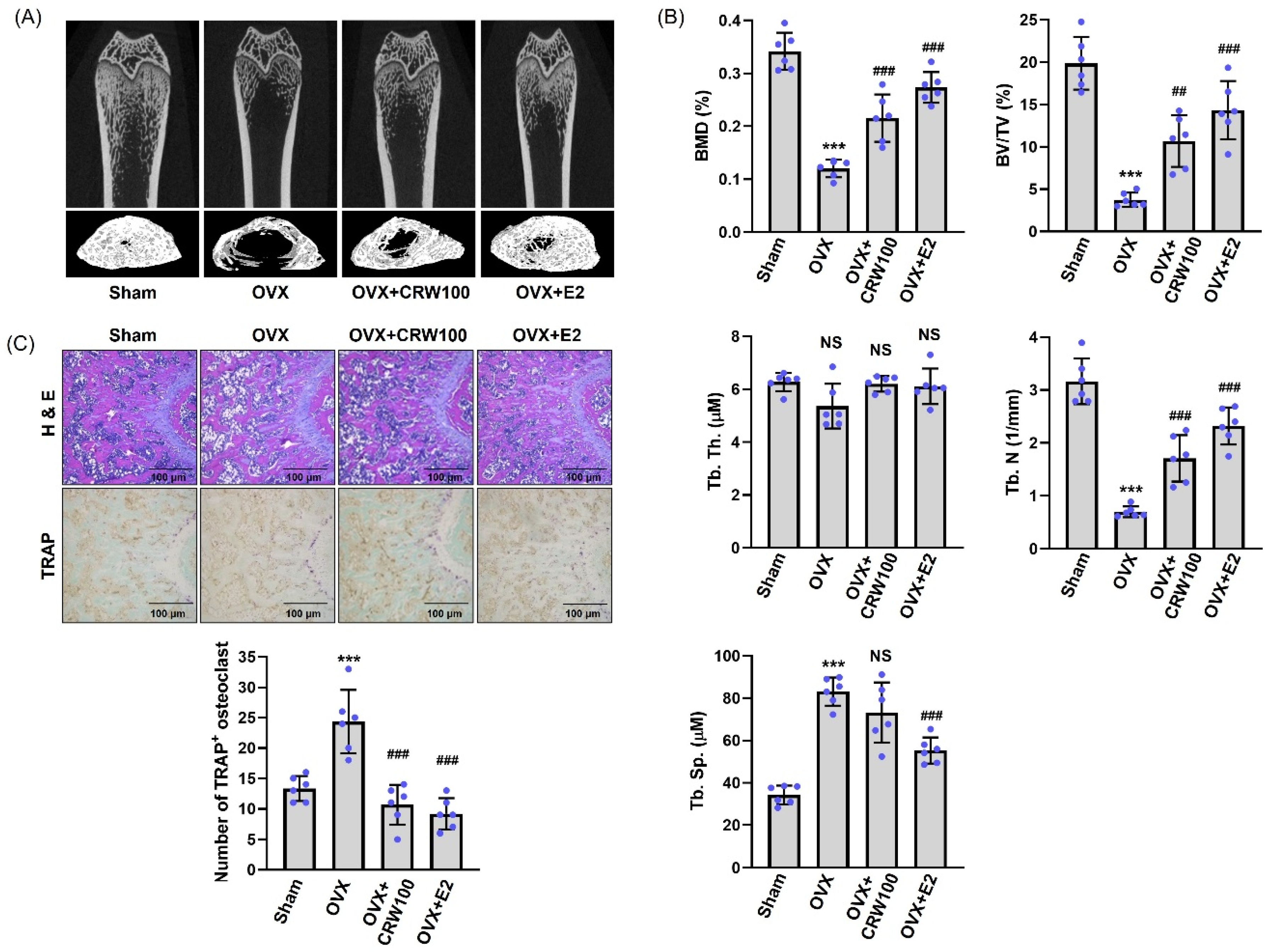
2.7. CRW Recovered Bone Destruction in Ovariectomized Rats
3. Discussion
4. Materials and Methods
4.1. Animals and Reagents
4.2. Ethics Statement
4.3. High-Performance Liquid Chromatography (HPLC) Analysis
4.4. Preparation of Coptidis Rhizoma Water Extract (CRW)
4.5. Isolation of Bone Marrow Macrophages (BMMs)
4.6. Cell Viability Assay
4.7. Osteoclast Differentiation and TRAP Assay
4.8. F-Acting Ring Staining
4.9. Western Blotting
4.10. Quantitative qPCR
4.11. Immunocytochemistry
4.12. Bone Resorption Assay
4.13. Animal Bone-Loss Model
4.14. Micro-CT and Histology
4.15. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Kong, L.; Wang, Y.; Smith, W.; Hao, D. Macrophages in Bone Homeostasis. Curr. Stem Cell Res. Ther. 2019, 14, 474–481. [Google Scholar] [CrossRef]
- Nedeva, I.R.; Vitale, M.; Elson, A.; Hoyland, J.A.; Bella, J. Role of OSCAR Signaling in Osteoclastogenesis and Bone Disease. Front. Cell Dev. Biol. 2021, 9, 641162. [Google Scholar] [CrossRef]
- Veis, D.J.; O’Brien, C.A. Osteoclasts, Master Sculptors of Bone. Annu. Rev. Pathol. 2023, 18, 257–281. [Google Scholar] [CrossRef]
- Liu, C.; He, Y.; Xu, X.; He, B. Phospholipase Cγ Signaling in Bone Marrow Stem Cell and Relevant Natural Compounds Therapy. Curr. Stem Cell Res. Ther. 2020, 15, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Zhang, B.; Kong, L.; Gong, Y.; Feng, M.; Gao, X.; Wang, D.; Yan, L. Receptor activator of nuclear factor-κB ligand-mediated osteoclastogenesis signaling pathway and related therapeutic natural compounds. Front. Pharmacol. 2022, 13, 1043975. [Google Scholar] [CrossRef]
- Feng, X.; Teitelbaum, S.L. Osteoclasts: New Insights. Bone Res. 2013, 1, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, H.; Marahleh, A.; Ohori, F.; Noguchi, T.; Shen, W.R.; Qi, J.; Nara, Y.; Pramusita, A.; Kinjo, R.; Mizoguchi, I. Osteocyte-Related Cytokines Regulate Osteoclast Formation and Bone Resorption. Int. J. Mol. Sci. 2020, 21, 5169. [Google Scholar] [CrossRef]
- Asagiri, M.; Sato, K.; Usami, T.; Ochi, S.; Nishina, H.; Yoshida, H.; Morita, I.; Wagner, E.F.; Mak, T.W.; Serfling, E.; et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 2005, 202, 1261–1269. [Google Scholar] [CrossRef]
- Barrow, A.D.; Raynal, N.; Andersen, T.L.; Slatter, D.A.; Bihan, D.; Pugh, N.; Cella, M.; Kim, T.; Rho, J.; Negishi-Koga, T.; et al. OSCAR is a collagen receptor that costimulates osteoclastogenesis in DAP12-deficient humans and mice. J. Clin. Investig. 2011, 121, 3505–3516. [Google Scholar] [CrossRef]
- Kim, K.; Lee, S.H.; Ha Kim, J.; Choi, Y.; Kim, N. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP). Mol. Endocrinol. 2008, 22, 176–185. [Google Scholar] [CrossRef]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Lou, G.H.; Zeng, H.R.; Hu, J.; Huang, Q.W.; Peng, W.; Yang, X.B. Coptidis Rhizoma: A comprehensive review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. Pharm. Biol. 2019, 57, 193–225. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, Y.; Ren, D.; Li, J.; Yuan, G.; An, L.; Du, P.; Ma, J. Antidiabetic mechanism of Coptis chinensis polysaccharide through its antioxidant property involving the JNK pathway. Pharm. Biol. 2015, 53, 1022–1029. [Google Scholar] [CrossRef]
- Luo, T.; Zhang, H.; Zhang, W.W.; Huang, J.T.; Song, E.L.; Chen, S.G.; He, F.; Xu, J.; Wang, H.Q. Neuroprotective effect of Jatrorrhizine on hydrogen peroxide-induced cell injury and its potential mechanisms in PC12 cells. Neurosci. Lett. 2011, 498, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tan, H.Y.; Li, L.; Yuen, M.F.; Feng, Y. Berberine and Coptidis Rhizoma as potential anticancer agents: Recent updates and future perspectives. J. Ethnopharmacol. 2015, 176, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Deng, Y.; Zhou, H.; Jiang, H.; Li, Y.; Chu, Y.; Wang, X.; Gong, L. Metabolic characteristics of Rhizoma Coptidis intervention in spontaneously hypertensive rats: Insights gained from metabolomics analysis of serum. Mol. Med. Rep. 2017, 16, 4301–4308. [Google Scholar] [CrossRef]
- Yokozawa, T.; Ishida, A.; Kashiwada, Y.; Cho, E.J.; Kim, H.Y.; Ikeshiro, Y. Coptidis Rhizoma: Protective effects against peroxynitrite-induced oxidative damage and elucidation of its active components. J. Pharm. Pharmacol. 2004, 56, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.C.; Wu, Z.F.; Yin, Z.Q.; Lin, L.G.; Wang, R.; Zhang, Q.W. Coptidis rhizoma and its main bioactive components: Recent advances in chemical investigation, quality evaluation and pharmacological activity. Chin. Med. 2018, 13, 13. [Google Scholar] [CrossRef]
- Han, S.Y.; Kim, Y.K. Berberine Suppresses RANKL-Induced Osteoclast Differentiation by Inhibiting c-Fos and NFATc1 Expression. Am. J. Chin. Med. 2019, 47, 439–455. [Google Scholar] [CrossRef]
- Lee, J.W.; Iwahashi, A.; Hasegawa, S.; Yonezawa, T.; Jeon, W.B.; Cha, B.Y.; Nagai, K.; Woo, J.T. Coptisine inhibits RANKL-induced NF-κB phosphorylation in osteoclast precursors and suppresses function through the regulation of RANKL and OPG gene expression in osteoblastic cells. J. Nat. Med. 2012, 66, 8–16. [Google Scholar] [CrossRef]
- Smith, J.K. Osteoclasts and Microgravity. Life 2020, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Bruzzaniti, A.; Baron, R. Molecular regulation of osteoclast activity. Rev. Endocr. Metab. Disord. 2006, 7, 123–139. [Google Scholar] [CrossRef]
- Kim, E.J.; Lee, H.; Kim, M.H.; Yang, W.M. Inhibition of RANKL-stimulated osteoclast differentiation by Schisandra chinensis through down-regulation of NFATc1 and c-fos expression. BMC Complement. Altern. Med. 2018, 18, 270. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, D.; Lee, M.J.; Kim, M.J.; Jang, H.S.; Park, S.H.; Lee, J.M.; Lee, H.Y.; Han, B.S.; Son, W.C.; et al. Subchronic toxicity study of Coptidis rhizoma in rats. J. Ethnopharmacol. 2014, 152, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Ishida, A.; Cho, E.J.; Nakagawa, T. The effects of Coptidis Rhizoma extract on a hypercholesterolemic animal model. Phytomedicine 2003, 10, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, J.; Zhang, J.; Xue, Q.; Qin, R.; Wang, R.; Goltzman, D.; Miao, D. 17β-estradiol plays the anti-osteoporosis role via a novel ESR1-Keap1-Nrf2 axis-mediated stress response activation and Tmem119 upregulation. Free Radic. Biol. Med. 2023, 195, 231–244. [Google Scholar] [CrossRef]
- Lee, G.H.; Hoang, T.H.; Lee, H.Y.; Lim, Y.J.; Kim, J.H.; Jung, S.J.; Chae, S.W.; Rashid, M.M.U.; Chae, H.J.; Yoon, S.J. Ramie leaf Extract Alleviates Bone Loss in Ovariectomized Rats-The Involvement of ROS and Its Associated Signalings. Nutrients 2023, 15, 745. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, Z.; Xiong, Z.; Liu, X. Ferroptosis—A new target of osteoporosis. Exp. Gerontol. 2022, 165, 111836. [Google Scholar] [CrossRef]
- Song, S.; Guo, Y.; Yang, Y.; Fu, D. Advances in pathogenesis and therapeutic strategies for osteoporosis. Pharmacol. Ther. 2022, 237, 108168. [Google Scholar] [CrossRef]
- Li, H.; Xiao, Z.; Quarles, L.D.; Li, W. Osteoporosis: Mechanism, Molecular Target and Current Status on Drug Development. Curr. Med. Chem. 2021, 28, 1489–1507. [Google Scholar] [CrossRef]
- Martiniakova, M.; Babikova, M.; Omelka, R. Pharmacological agents and natural compounds: Available treatments for osteoporosis. J. Physiol. Pharmacol. 2020, 71, 307–320. [Google Scholar]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Khan, M.S.A.; Ahmad, I. Herbal Medicine: Current Trends and Future Prospects. New Look to Phytomedicine; Academic Press: Cambridge, MA, USA, 2019; pp. 3–13. [Google Scholar]
- Zhou, S.; Huang, J.; Chen, K.; Wang, Q.; Liu, Z.; Sun, Y.; Yin, F.; Wang, S.; Pang, Z.; Ma, M. Attenuating bone loss in osteoporosis: The potential of corylin (CL) as a therapeutic agent. Aging 2024, 16, 9569–9583. [Google Scholar] [CrossRef]
- Gan, Z.; Huang, J.; Xu, M.; Yuan, X.; Shang, X.; Chen, X.; Chen, K. Micheliolide prevents estrogen deficiency-induced bone loss via inhibiting osteoclast bone resorption. Aging 2023, 15, 10732–10745. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.M.; Wang, Z.; Qi, G.B.; Lai, Q.; Jiang, A.L.; Zhang, Y.Q.; Chen, K.; Wang, X.H. Icaritin ameliorates RANKL-mediated osteoclastogenesis and ovariectomy-induced osteoporosis. Aging 2023, 15, 10213–10236. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, N. Regulation of NFATc1 in Osteoclast Differentiation. J. Bone Metab. 2014, 21, 233–241. [Google Scholar] [CrossRef]
- Hayman, A.R.; Bune, A.J.; Bradley, J.R.; Rashbass, J.; Cox, T.M. Osteoclastic tartrate-resistant acid phosphatase (Acp 5): Its localization to dendritic cells and diverse murine tissues. J. Histochem. Cytochem. 2000, 48, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Gingery, A.; Bradley, E.; Shaw, A.; Oursler, M.J. Phosphatidylinositol 3-kinase coordinately activates the MEK/ERK and AKT/NFkappaB pathways to maintain osteoclast survival. J. Cell. Biochem. 2003, 89, 165–179. [Google Scholar] [CrossRef]
- Lee, K.; Seo, I.; Choi, M.H.; Jeong, D. Roles of Mitogen-Activated Protein Kinases in Osteoclast Biology. Int. J. Mol. Sci. 2018, 19, 3004. [Google Scholar] [CrossRef]
- Fumimoto, R.; Sakai, E.; Yamaguchi, Y.; Sakamoto, H.; Fukuma, Y.; Nishishita, K.; Okamoto, K.; Tsukuba, T. The coffee diterpene kahweol prevents osteoclastogenesis via impairment of NFATc1 expression and blocking of Erk phosphorylation. J. Pharmacol. Sci. 2012, 118, 479–486. [Google Scholar] [CrossRef][Green Version]
- Wagner, E.F.; Eferl, R. Fos/AP-1 proteins in bone and the immune system. Immunol. Rev. 2005, 208, 126–140. [Google Scholar] [CrossRef]
- Iotsova, V.; Caamaño, J.; Loy, J.; Yang, Y.; Lewin, A.; Bravo, R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat. Med. 1997, 3, 1285–1289. [Google Scholar] [CrossRef]
- Jimi, E.; Katagiri, T. Critical Roles of NF-κB Signaling Molecules in Bone Metabolism Revealed by Genetic Mutations in Osteopetrosis. Int. J. Mol. Sci. 2022, 23, 7995. [Google Scholar] [CrossRef]
- Zhou, L.; Song, F.; Liu, Q.; Yang, M.; Zhao, J.; Tan, R.; Xu, J.; Zhang, G.; Quinn, J.M.; Tickner, J.; et al. Berberine Sulfate Attenuates Osteoclast Differentiation through RANKL Induced NF-κB and NFAT Pathways. Int. J. Mol. Sci. 2015, 16, 27087–27096. [Google Scholar] [CrossRef]
- Hu, J.P.; Nishishita, K.; Sakai, E.; Yoshida, H.; Kato, Y.; Tsukuba, T.; Okamoto, K. Berberine inhibits RANKL-induced osteoclast formation and survival through suppressing the NF-kappaB and Akt pathways. Eur. J. Pharmacol. 2008, 580, 70–79. [Google Scholar] [CrossRef]
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T. RANKL biology: Bone metabolism, the immune system, and beyond. Inflamm. Regen. 2020, 40, 2. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.C.; Kim, J.H.; Kim, K.; Kim, I.; Seong, S.; Kim, N. ATF3 modulates calcium signaling in osteoclast differentiation and activity by associating with c-Fos and NFATc1 proteins. Bone 2017, 95, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Suh, J.H.; Kim, H.N.; Kim, A.Y.; Park, S.Y.; Shin, C.S.; Choi, J.Y.; Kim, J.B. Berberine promotes osteoblast differentiation by Runx2 activation with p38 MAPK. J. Bone Miner. Res. 2008, 23, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, N.; Kashfi, K.; Jeddi, S.; Ghasemi, A. Ovariectomized rat model of osteoporosis: A practical guide. EXCLI J. 2020, 19, 89–107. [Google Scholar] [PubMed]
- Han, S.Y.; Kim, J.H.; Jo, E.H.; Kim, Y.K. Eleutherococcus sessiliflorus Inhibits Receptor Activator of Nuclear Factor Kappa-B Ligand (RANKL)-Induced Osteoclast Differentiation and Prevents Ovariectomy (OVX)-Induced Bone Loss. Molecules 2021, 26, 1886. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
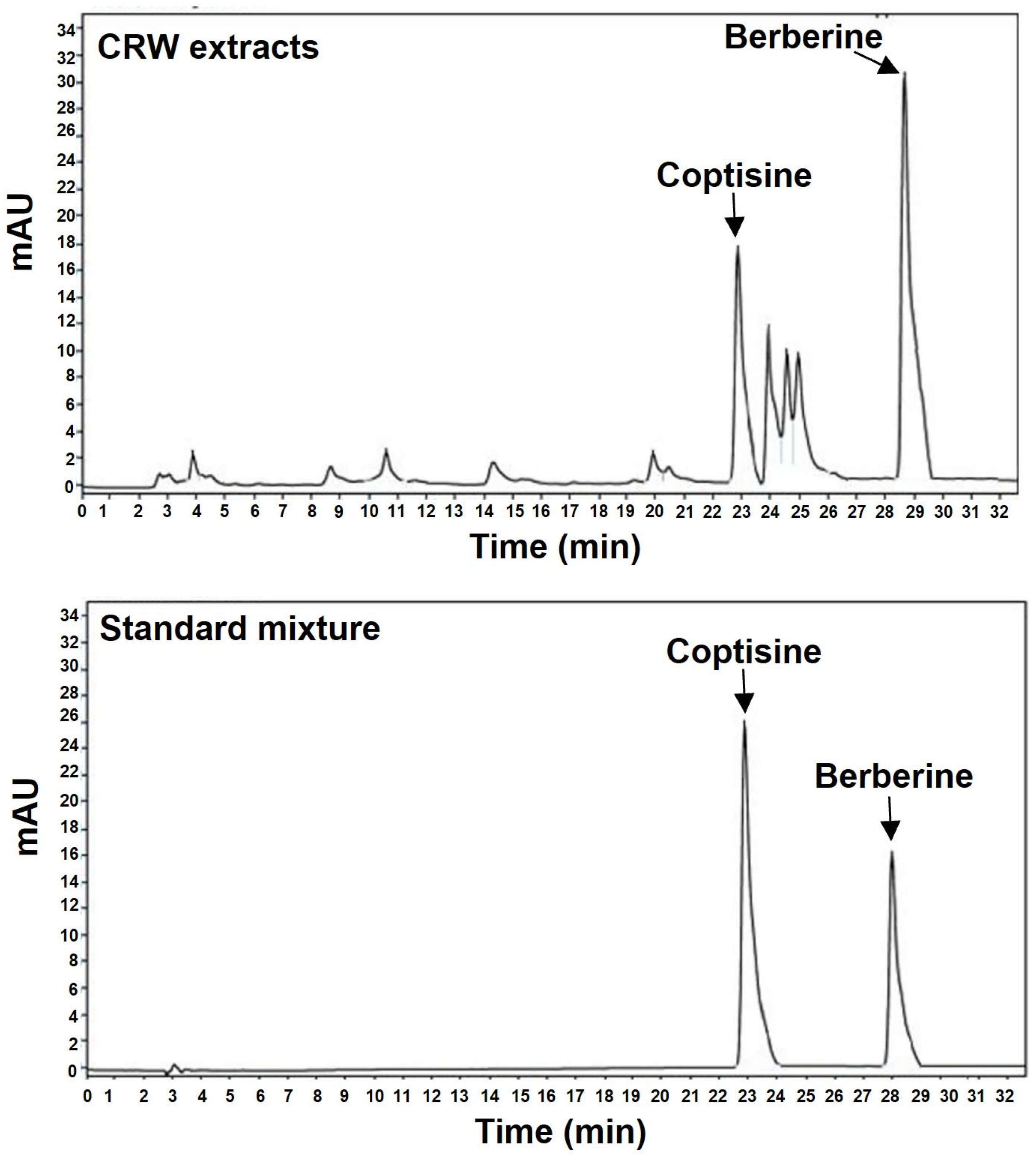
| Parameters | Condition | ||
|---|---|---|---|
| Instrument | Agilent 1200 series HPLC system (Agilent Technologies, Santa Clara, CA, USA) | ||
| Column | C18 4.6 mm × 150 mm, 4.0 μm | ||
| Column temp. | 30 °C | ||
| Mobile phase | Time (min) | A (%) | B (%) |
| 0 | 85 | 15 | |
| 20 | 60 | 40 | |
| A: 0.5% formic acid in DW (v/v, %) B: 0.5% formic acid in ACN:MeOH (3:7) (v/v, %) | |||
| Detector | Waters 996 Photodiode Array Detector 280 nm | ||
| Flow rate | 0.5 mL/min | ||
| Injection volume | 10 μL | ||
| Run time | 35min | ||
| Target Gene | Gene Accession Number | Primer Sequence (5′-3′) | |
|---|---|---|---|
| c-Fos | NM_010234.3 | Forward | CTGGTGCAGCCCACTCTGGTC |
| Reverse | CTTTCAGCAGATTGGCAATCTC | ||
| NFATc1 | NM_198429.2 | Forward | CAACGCCCTGACCACCGATAG |
| Reverse | GGCTGCCTTCCGTCTCATAGT | ||
| ACP5 | NM_007388 | Forward | ACTTCCCCAGCCCTTACTAC |
| Reverse | TCAGCACATAGCCCACACCG | ||
| OSCAR | NM_175632.3 | Forward | CTGCTGGTAACGGATCAGCTCCCCAGA |
| Reverse | CCAAGGAGCCAGAACCTTCGAAACT | ||
| ATP6V0D2 | NM_175406.3 | Forward | TCAGATCTCTTCAAGGCTGTGCTG |
| Reverse | GTGCCAAATGAGTTCAGAGTGATG | ||
| CTSK | NM_007802.4 | Forward | ACGGAGGCATTGACTCTGAAGATG |
| Reverse | GTTGTTCTTATTCCGAGCCAAGAG | ||
| MMP-9 | NM_013599.4 | Forward | TCCAACCTCACGGACACCC |
| Reverse | AGCAAAGCCGGCCGTAGA | ||
| CALCR | NM_001377018.1 | Forward | TCCAACAAGGTGCTTGGGAA |
| Reverse | CTTGAACTGCGTCCACTGGG | ||
| DC-STAMP | NM_001289506.1 | Forward | TCCTCCATGAACAAACAGTTCCA |
| Reverse | AGACGTGGTTTAGGAATGCAGCTC | ||
| OC-STAMP | NM_029021.1 | Forward | ATGAGGACCATCAGGGCAGCCACG |
| Reverse | GGAGAAGCTGGGTCAGTAGTTCGT | ||
| GAPDH | NM_001289726.1 | Forward | ACCACAGTCCATGCCATCAC |
| Reverse | TCCACCACCCTGTTGCTGTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.-Y.; Kim, Y.-K. Coptidis Rhizoma Water Extract Attenuates RANKL-Induced Osteoclast Differentiation via MAPK, Akt, and NF-κB Pathways and Prevents Ovariectomy (OVX)-Mediated Bone Loss. Int. J. Mol. Sci. 2025, 26, 8707. https://doi.org/10.3390/ijms26178707
Han S-Y, Kim Y-K. Coptidis Rhizoma Water Extract Attenuates RANKL-Induced Osteoclast Differentiation via MAPK, Akt, and NF-κB Pathways and Prevents Ovariectomy (OVX)-Mediated Bone Loss. International Journal of Molecular Sciences. 2025; 26(17):8707. https://doi.org/10.3390/ijms26178707
Chicago/Turabian StyleHan, Sang-Yong, and Yun-Kyung Kim. 2025. "Coptidis Rhizoma Water Extract Attenuates RANKL-Induced Osteoclast Differentiation via MAPK, Akt, and NF-κB Pathways and Prevents Ovariectomy (OVX)-Mediated Bone Loss" International Journal of Molecular Sciences 26, no. 17: 8707. https://doi.org/10.3390/ijms26178707
APA StyleHan, S.-Y., & Kim, Y.-K. (2025). Coptidis Rhizoma Water Extract Attenuates RANKL-Induced Osteoclast Differentiation via MAPK, Akt, and NF-κB Pathways and Prevents Ovariectomy (OVX)-Mediated Bone Loss. International Journal of Molecular Sciences, 26(17), 8707. https://doi.org/10.3390/ijms26178707





